Abstract
Objective:
To examine frequent snoring, sleepiness, and sleep duration with baseline and longitudinal performance on neuropsychological (NP) battery.
Methods:
The analysis consists of 711 participants of the Northern Manhattan Study (NOMAS) with sleep data and NP assessment (age 63 ± 8 years, 62% women, 18% white, 17% black, 67% Hispanic) and 687 with repeat NP testing (at a mean of 6 ± 2 years). The main exposures were snoring, sleepiness, and sleep duration obtained during annual follow-up. Using factor analysis–derived domain-specific Z scores for episodic memory, language, executive function, and processing speed, we constructed multivariable regression models to evaluate sleep symptoms with baseline NP performance and change in performance in each NP domain.
Results:
In the cross-sectional analysis, adjusting for demographics and the NOMAS vascular risk score, participants with frequent snoring had worse executive function (β = −12; p = 0.04) and processing speed (β = −13; p = 0.02), but no difference in with episodic memory or language. Those with severe daytime sleepiness (β = −26; p = 0.009) had worse executive function, but no changes in the other NP domains. There was no cross-sectional association between sleep duration and NP performance. Frequent snoring (β = −29; p = 0.0007), severe daytime sleepiness (β = −29; p = 0.05), and long sleep duration (β = −29; p = 0.04) predicted decline in executive function, adjusting for demographic characteristics and NOMAS vascular risk score. Sleep symptoms did not explain change in episodic memory, language, or processing speed.
Conclusions:
In this race-ethnically diverse community-based cohort, sleep symptoms led to worse cognitive performance and predicted decline in executive function.
Sleep disturbances are common in cognitive impairment and dementia.1 Observational studies suggest that sleep duration, daytime sleepiness, and sleep-disordered breathing (SDB), including frequent snoring, increases the risk of cognitive impairment.2,3 Improving sleep might be an avenue for early intervention in those at risk of cognitive decline and dementia.1 However, there are little data evaluating self-reported sleep symptoms and cognitive decline across different cognitive domains.
The burden of dementia appears to be borne disproportionately by minorities such as non-Hispanic blacks and Hispanic/Latinos. Compared to non-Hispanic whites, non-Hispanic blacks and Hispanic/Latinos have a 1.5- to 2-fold increased risk of dementia.4 In addition, studies suggest that non-Hispanic blacks and Hispanic/Latinos have an increased frequency of snoring, daytime sleepiness, SDB, and short and long sleep durations compared to non-Hispanic whites.5–8 However, data are sparse on sleep and cognitive function in multiethnic populations.
In the Northern Manhattan Study (NOMAS), sleep disturbances are associated with worse cognitive performance in the Mini-Mental State Examination score and subclinical markers of vascular disease,9–11 known contributors to cognitive decline and dementia.12 It is unclear if specific cognitive domains are specifically affected by these sleep disturbances, accounting for confounders such as vascular risk factors, which have strong associations with poor sleep. The aim of our study was to examine the effect of sleep disturbances on cognitive processes and their change over time in NOMAS. We hypothesized that participants with frequent snoring, daytime sleepiness, and either short or long sleep duration had worse cognitive performance and decline in the domains of language, episodic memory, executive function, and processing speed.
METHODS
Original study population.
NOMAS is a population-based study initially designed to determine stroke incidence, risk factors, and outcomes in a multiethnic cohort.13 The original NOMAS community cohort of 3,298 participants was identified from random digit dialing using dual-frame sampling with the following criteria: (1) residence in northern Manhattan for at least 3 months; (2) telephone present in household; (3) age 40 or older at the time of first in-person assessment; and (4) no baseline history of stroke. Eligible participants were recruited for in-person assessments between 1993 and 2001.13
Analytic sample.
Starting in 2003, a substudy with MRI and neuropsychological assessments was performed in participants remaining clinically free of stroke. These were recruited sequentially during annual telephone follow-up using the following criteria: (1) age older than 55; (2) no contraindications to MRI; and (3) willingness to sign written informed consent.14 Only participants with a Clinical Dementia Rating of 0, which denotes no evidence of cognitive impairment, and a detailed neuropsychological examination (described below) from 2003 to 2008 were included. The initial sample of 1,290 stroke-free participants eligible for MRI (including 199 newly enrolled NOMAS household members meeting above entry criteria) completed a neurocognitive (NC) battery. The sleep questionnaire was provided in 2003, during follow-up. The sample for the cross-sectional analysis was based on the participants with valid NC test and sleep questionnaire (n = 711). A second in-person neuropsychological examination was performed 5 years later, which was completed by 687 participants with sleep questionnaires.
Standard protocol approvals, registrations, and patient consents.
NOMAS was approved by the Columbia University Medical Center and University of Miami Institutional Review Boards. All participants gave written informed consent.
Outcomes: Cognitive assessments.
We derived our outcomes from neuropsychological testing administered in a quiet room in English or Spanish by trained research assistants. We calculated domain-specific z scores for executive function, episodic memory, processing speed, and language. The neuropsychological tests were selected for each domain based on the relationships among the tests through an exploratory factor analysis and previous studies.15,16
Executive functioning.
Executive function was assessed by computing the difference in time to complete the Color Trails Test Form 1 and Form 2, and the sum of the Odd-Man-Out subtests 2 and 4.15 Briefly, the Color Trails Test requires participants to connect numbers or numbers alternating in color in numerical order as quickly as possible. The time it takes the participants to complete this task is thought to be indicative of cognitive flexibility (ability to switch between sets). The Odd-Man-Out task consists of 4 sets of subtests and involves the participant selecting which item in a set of 3 does not belong with the other items. Scores of each of the subtests were summed to create an Odd-Man-Out total score.15
Episodic memory.
Memory was assessed with a modified California Verbal Learning Test–II, using 3 subscores on 12 words, 5 trial list-learning tasks: list-learning total score, delayed recall score, and delayed recognition score.15
Processing speed.
Processing speed was assessed with the Grooved Pegboard task in the nondominant hand, the Color Trails Test Form 1, and the Visual-Motor Integration test.17
Language was assessed with the following tests: picture naming (modified Boston Naming) test, category fluency (Animal Naming) test, and phonemic fluency (C, F, L in English speakers and F, A, S in Spanish speakers).
To evaluate cognitive decline, we computed z scores using regression-based indices for each individual test, adjusting for age and years of education.18
Main exposures: Frequent snoring, daytime sleepiness, and sleep duration.
Participants were asked about snoring symptoms, daytime sleepiness, and sleep duration during annual telephone follow-up in 2003–2004.19 Frequent snoring was assessed with the following question, “Do you know, or were you told that you snore?” Frequent snoring was dichotomized (yes vs no) based on self-reported snoring >3 times per week, consistent with prior definitions.20
Daytime sleepiness was assessed with questions derived from the Epworth Sleepiness Scale, as described elsewhere.19 We categorized sleepiness into no sleepiness (0/24), mild daytime sleepiness (1–9/24), and severe daytime sleepiness (≥10/24), based on prior definitions.19 We assessed sleep duration with the following question: “During the past 4 weeks, how many hours, on average, did you sleep each night?” Responses were noted in 30-minute increments and categorized into short sleep (<6 hours), average sleep (6–<9 hours), and long sleep duration (≥9 hours).10
Covariates.
Data were collected by trained bilingual (English and Spanish) research assistants using standard techniques to measure blood pressure, height, and weight and fasting serum glucose.13 The main covariate was the NOMAS global vascular risk score (GVRS).21 The GVRS score (range 4.4–11.6) reflects an individual's 10-year risk of a vascular event using demographic, anthropometric, behavioral, and vascular risk factors. It was developed in the NOMAS cohort in order to improve upon the traditional Framingham risk score and has been demonstrated to be an effective prediction tool to calculate the overall risk of vascular related outcomes using a single measure.21
Depressive symptoms were evaluated with the Center for Epidemiologic Studies Depression scale (CES-D). The CES-D is a 20-item scale documenting 4 factors: depressive affect, somatic complaints, positive affect, and interpersonal relations. Scores on the CES-D range from 0 to 60, with higher scores indicating more symptoms of depression. Depression was categorized as present if the sum of the CES-D scores was ≥16 or if the participant reported antidepressant medication use.10
Statistical analysis.
We calculated z scores for each cognitive domain and used these variables as our outcomes. We fitted linear regression models and entered each sleep exposure separately into each statistical model. We evaluated the effect of frequent snoring (yes vs no), daytime sleepiness (severe daytime sleepiness and mild daytime sleepiness with no sleepiness as the reference), and sleep duration (6–<9 hours as the reference) with cognitive function and decline. As domain Z scores were not strongly correlated, separate analyses were performed for each cognitive domain. In the cross-sectional analysis, the Z scores for the cognitive domains at the initial assessment were not adjusted for age and education; instead, we included age and education as covariates in the models, along with time from baseline to sleep assessments and time from sleep assessments to initial neuropsychological testing. The final model also included the NOMAS GVRS. We then evaluated cognitive decline for each domain with age- and education-adjusted Z scores as the outcomes. The Z scores that represented change in performance over time were calculated using a regression-based approach that accounted for age and education and therefore these variables were not included as covariates in the analytic models. The final model further adjusted for the time difference between the sleep and cognitive assessments, time to initial neuropsychological testing, and the GVRS. We also explored the interactions between the sleep variables and APOE status in predicting cognitive decline. Participants with an APOE ε4 genotype (4/4, 4/2, 4/3) were compared to those without an ε4 genotype (2/2, 2/3, 3/3). The number of APOE ε4 alleles carried by each subject was determined by HhaI digestion of PCR products amplified from genomic DNA.22
RESULTS
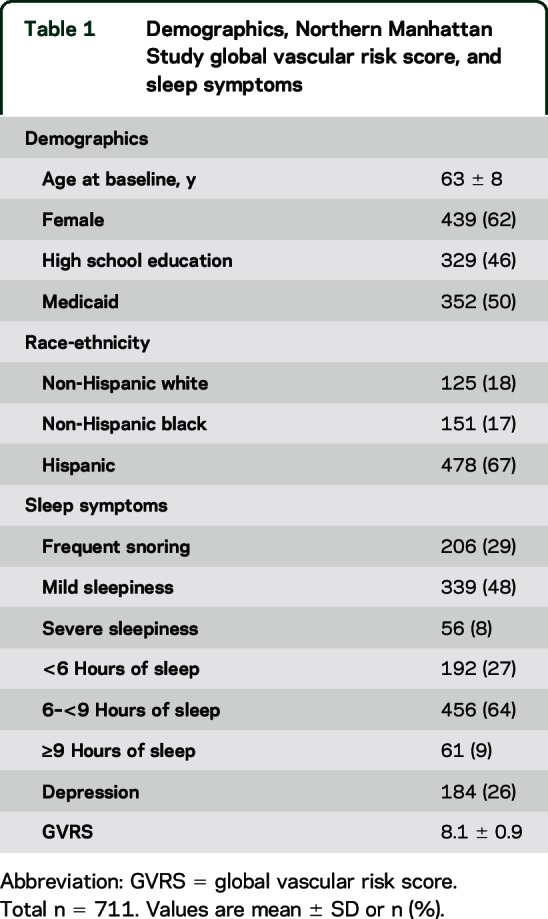
The overall descriptive statistics of the sample are presented in table 1. The mean age at NOMAS baseline recruitment was 63 years, with 62% women, and the majority was of Hispanic/Latino origin. More than half of the respondents had less than a high school education. Twenty-nine percent of the sample reported frequent snoring. Mild or severe sleepiness was reported by more than half of the cohort, and the majority reported average sleep (6–<9 hours of sleep). The mean GVRS was 8.12, SD = 0.86, with a range of 4.43–10.54. In the NOMAS baseline cohort, a GVRS of 8.2 implied a 10-year survival free of stroke, myocardial infarction, or vascular death probability of 0.10.
Table 1.
Demographics, Northern Manhattan Study global vascular risk score, and sleep symptoms
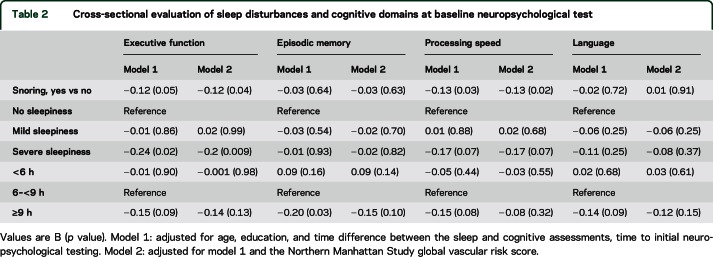
In the cross-sectional analysis, participants with frequent snoring had worse executive function and processing speed, with no differences observed in episodic memory and language (table 2). Compared to participants with no sleepiness, those with severe daytime sleepiness had worse executive function, but did not differ in episodic memory, processing speed, or language. In unadjusted models, participants with long sleep duration performed worse in episodic memory (table 2); however, in fully adjusted models (model 2), neither long nor short sleep duration led to worse cognitive function.
Table 2.
Cross-sectional evaluation of sleep disturbances and cognitive domains at baseline neuropsychological test
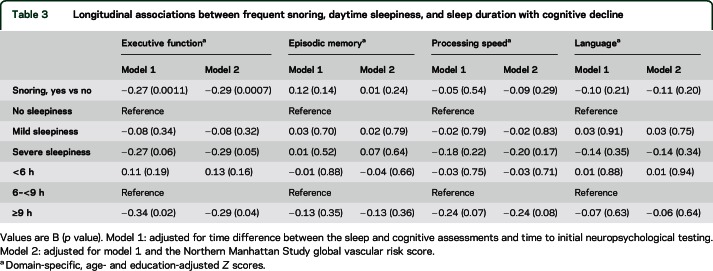
In the adjusted longitudinal analysis, participants with frequent snoring, severe daytime sleepiness, and long sleep duration had decline in executive function (table 3). There was no decline in episodic memory, processing speed, or language.
Table 3.
Longitudinal associations between frequent snoring, daytime sleepiness, and sleep duration with cognitive decline
Further adjustments for depression did not significantly change the link between frequent snoring (β = −0.13; p = 0.044) and severe daytime sleepiness (β −0.27; p = 0.0089) with executive function. Also, after adjusting for depression, frequent snoring (β = −0.29; p = 0.0010), severe daytime sleepiness (β = −0.29; p = 0.05), and long sleep duration (β =−0.29; p = 0.04) led to worse executive function. We further evaluated sleep duration and cognitive decline stratified by APOE ε4 status. There was no significant interaction between sleep duration and APOE ε4 carrier status (p > 0.05); however, the power to detect effect modification was limited. Among those with an APOE ε4 allele (n = 146), participants who reported long sleep had greater decline in episodic memory as compared to participants with normal sleep duration, but did not reach statistical significance (β = −0.44; p = 0.07). This was not observed among APOE ε4 noncarriers (β = −0.08, p = 0.67). We did not observe differences between other sleep variables and cognition in analyses stratified by APOE ε4 status.
DISCUSSION
In this diverse urban cohort of older adults, participants with frequent snoring and daytime sleepiness had worse executive function, both at baseline and over time. Further, while sleep duration did not explain baseline differences in cognitive function, those with long sleep declined in executive function. Conversely, sleep disturbances did not lead to longitudinal decline in memory or processing speed.
Frequent snoring is a common symptom of SDB, a disorder associated with increased risk of mild cognitive impairment and dementia.20,23,24 Continuous positive airway pressure, the main therapy for SDB, delayed the progression of dementia in patients with SDB.25
The NOMAS sample consists mostly of Caribbean Hispanics. Similar to ours, a cross-sectional analysis of the Hispanic Community Health Study/Study of Latinos, which consists of over 8,000 Hispanics/Latinos from 4 urban areas, described worse cognitive scores in participants with objective measures of SDB. Women, when compared to men, performed worse in the domains of executive function, memory, and language, with worse scores particularly among the younger (45–54 years) age groups.26 Future studies need to evaluate the effect and treatment of SDB on cognitive function in ethnically diverse cohorts.
Excessive daytime sleepiness and long sleep duration also are predictors of cognitive decline and dementia.27–29 In a large community-based cohort of elderly men, daytime sleepiness predicted vascular dementia, but not nonvascular dementia.30
In a different cohort, long sleepers were more likely to have dementia (hazard ratio 2.2; 95% confidence interval 1.1–4.4) after 3 years of follow-up,31 and a 58% greater risk of dementia-specific mortality up to 13 years after baseline evaluation.32
In our study, the findings between sleep disturbances and cognition, compared to others, may be partly explained by the use of sensitive neuropsychological tests. Some other studies measured global cognition using the Mini-Mental State Examination score, which may be insensitive to cognitive variability and could explain the null findings observed in other studies.33 We estimate that the prevalence of cognitive impairment at the time of the first cognitive assessment was less than 5%,34 thus our findings suggest that decline in executive function could be an early manifestation of cognitive problems in older adults with sleep disturbances. However, further studies are needed to confirm our findings.
Decreased attention and vigilance could explain declines in executive function as a consequence of poor sleep quantity, continuity, quality, sleep timing, and weaker circadian activity rhythm.33 In addition, disruptions of the sleep-wake cycle and decreased amounts of slow-wave sleep may lead to greater neuronal activity with increased deposition of amyloid-β.35 Subclinical cerebrovascular damage may also contribute to cognitive decline.36,37 Sleep disruption is linked to vascular risk factors such as hypertension and diabetes mellitus,38 which may indirectly contribute to sleep-related cognitive impairment. Sleep disruption as seen in SDB and long sleep duration are also linked to subclinical vascular disease (i.e., white matter disease).11,39 It is plausible that poor sleep differentially affects executive function by disrupting cortical-subcortical connections within the prefrontal cortex through vascular damage.3,19,30,40
This study has several important limitations. We were unable to rule out potential selection bias; it is plausible that those with the greatest cognitive decline were less likely to return for a follow-up assessment. However, the availability of follow-up cognitive data did not differ by baseline cognitive performance. In addition, reporting bias could also be an issue since our sleep measures were self-reported and we did not obtain polysomnography or actigraphy. There is also the possibility of residual confounding in any observational study such as this one.
The strength of our study is the unique diverse population of Hispanic/Latino, white, and black adults from the same community, which is often underrepresented in studies of risk factors for cognitive impairment. In addition, we systematically obtained measures of cognitive function and demographic, behavioral, and vascular risk factors.
In this multiethnic cohort, those with frequent snoring, daytime sleepiness, and long sleep duration had worse cognitive performance in the domain of executive function. Sleep disturbances represent a modifiable factor for cognitive impairment and should be examined in treatment studies for prevention of neurocognitive disorders.
GLOSSARY
- CES-D
Center for Epidemiologic Studies Depression scale
- GVRS
global vascular risk score
- NC
neurocognitive
- NOMAS
Northern Manhattan Study
- SDB
sleep-disordered breathing
AUTHOR CONTRIBUTIONS
Alberto Ramos: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis and interpretation of data. Dr. Ramos affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. Hannah Gardener: statistical analysis, drafting/revising the manuscript for content, including medical writing for content, study concept or design. Tatjana Rundek, Mitchell S.V. Elkind, Clinton B. Wright, Chuanhui Dong, Ralph L. Sacco: drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data. Bernadette Boden-Albala: study concept or design, analysis or interpretation of data. Yaakov Stern and Ying Kuen Cheung: study concept or design.
STUDY FUNDING
This work was supported by the NIH/NINDS (R37 NS 29993 and R01 NS 48134). The project was supported specifically by grant number 1KL2TR000461 (A.R.R.), Miami Clinical and Translational Science Institute, from the National Center for Advancing Translational Sciences and the National Institute on Minority Health and Health Disparities. The sponsors did not have a role in the design, interpretation, or draft of the manuscript.
DISCLOSURE
A. Ramos reports no disclosures relevant to the manuscript. H. Gardener is funded by related grants from the NIH (R01 HL 108623, R37 NS 29998). T. Rundek is funded by related grants from the NIH (R37 NS 29998, K24 NS 062737) and from the American Heart Association. M. Elkind receives research support from diaDexus, Inc., Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, and the NIH/NINDS; received compensation for consulting from Biogen IDEC, BioTelemetry, BMS-Sanofi Partnership, Boehringer-Ingelheim, Inc., Daiichi-Sankyo, and Janssen Pharmaceuticals; has given expert legal opinions on behalf of Merck/Organon (NuvaRing and stroke litigation); receives compensation from the American Academy of Neurology for service as Resident and Fellow Section Editor for Neurology®; and serves on the New York City, Founders Affiliate, and National boards of the American Heart Association/American Stroke Association. B. Boden-Albala reports no disclosures relevant to the manuscript. C. Dong is funded by a related grant from the NIH (R37 NS 29998). Y. Cheung reports no disclosures relevant to the manuscript. Y. Stern receives support from the NIH. During the last 2 years he was a consultant with Genentech with <$10,000 in honorarium. R. Sacco received research support from NINDS for the Northern Manhattan Study (R37 NS 29993) and from the American Heart Association. C. Wright is funded by related grants from the NIH (R01 HL 108623, R37 NS 29998) and from the American Heart Association. He receives royalties from UpToDate.com for chapters related to vascular dementia. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol 2014;13:1017–1028. [DOI] [PubMed] [Google Scholar]
- 2.Faubel R, Lopez-Garcia E, Guallar-Castillon P, Graciani A, Banegas JR, Rodriguez-Artalejo F. Usual sleep duration and cognitive function in older adults in Spain. J Sleep Res 2009;18:427–435. [DOI] [PubMed] [Google Scholar]
- 3.Jaussent I, Bouyer J, Ancelin ML, et al. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep 2012;35:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2014 Alzheimer's disease facts and figures. Alzheimers Dement 2014;10:e47–e92. [DOI] [PubMed] [Google Scholar]
- 5.Loredo JS, Soler X, Bardwell W, Ancoli-Israel S, Dimsdale JE, Palinkas LA. Sleep health in U.S. Hispanic population. Sleep 2010;33:962–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramos AR, Wohlgemuth WK, Dong C, et al. Race-ethnic differences of sleep symptoms in an elderly multi-ethnic cohort: the Northern Manhattan Study. Neuroepidemiology 2011;37:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Redline S, Sotres-Alvarez D, Loredo J, et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds: the Hispanic Community Health Study/Study of Latinos. Am J Respir Crit Care Med 2014;189:335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997;155:186–192. [DOI] [PubMed] [Google Scholar]
- 9.Ramos A, Zhezhen J, Rundek T, et al. Relation between long sleep and left ventricular mass from a multi-ethnic elderly cohort. Am J Cardiol 2013;111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramos AR, Dong C, Elkind MS, et al. Association between sleep duration and the mini-mental score: the Northern Manhattan Study. J Clin Sleep Med 2013;9:669–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos AR, Dong C, Rundek T, et al. Sleep duration is associated with white matter hyperintensity volume in older adults: the Northern Manhattan Study. J Sleep Res 2014;23:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacco RL, Boden-Albala B, Abel G, et al. Race-ethnic disparities in the impact of stroke risk factors: the Northern Manhattan Stroke Study. Stroke 2001;32:1725–1731. [DOI] [PubMed] [Google Scholar]
- 14.Gardener H, Scarmeas N, Gu Y, et al. Mediterranean diet and white matter hyperintensity volume in the Northern Manhattan Study. Arch Neurol 2012;69:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MS, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: the Northern Manhattan Study. J Int Neuropsychol Soc 2009;15:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquine MJ, Attix DK, Goldstein LB, et al. Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 2010;41:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry DC, Banbury S, Henry L. Transfer across form and modality in implicit and explicit memory. Q J Exp Psychol A 1997;50:1–24. [DOI] [PubMed] [Google Scholar]
- 18.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol 2012;27:248–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden-Albala B, Roberts ET, Bazil C, et al. Daytime sleepiness and risk of stroke and vascular disease: findings from the Northern Manhattan Study (NOMAS). Circ Cardiovasc Qual Outcomes 2012;5:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 2002;162:893–900. [DOI] [PubMed] [Google Scholar]
- 21.Sacco RL, Khatri M, Rundek T, et al. Improving global vascular risk prediction with behavioral and anthropometric factors: the multiethnic NOMAS (Northern Manhattan Cohort Study). J Am Coll Cardiol 2009;54:2303–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright CB, Elkind MS, Luo X, Paik MC, Sacco RL. Reported alcohol consumption and cognitive decline: the northern Manhattan study. Neuroepidemiology 2006;27:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim DC, Pack AI. Obstructive sleep apnea and cognitive impairment: addressing the blood-brain barrier. Sleep Med Rev 2014;18:35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA 2011;306:613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osorio RS, Gumb T, Pirraglia E, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology 2015;84:1964–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos AR, Tarraf W, Rundek T, et al. Obstructive sleep apnea and neurocognitive function in a Hispanic/Latino population. Neurology 2015;84:391–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohen-Zion M, Stepnowsky C, Marler, Shochat T, Kripke DF, Ancoli-Israel S. Changes in cognitive function associated with sleep disordered breathing in older people. J Am Geriatr Soc 2001;49:1622–1627. [DOI] [PubMed] [Google Scholar]
- 28.Walsleben JA, Kapur VK, Newman AB, et al. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep 2004;27:293–298. [DOI] [PubMed] [Google Scholar]
- 29.Whitney CW, Enright PL, Newman AB, Bonekat W, Foley D, Quan SF. Correlates of daytime sleepiness in 4578 elderly persons: the Cardiovascular Health Study. Sleep 1998;21:27–36. [DOI] [PubMed] [Google Scholar]
- 30.Elwood PC, Bayer AJ, Fish M, Pickering J, Mitchell C, Gallacher JE. Sleep disturbance and daytime sleepiness predict vascular dementia. J Epidemiol Community Health 2011;65:820–824. [DOI] [PubMed] [Google Scholar]
- 31.Benito-Leon J, Bermejo-Pareja F, Vega S, Louis ED. Total daily sleep duration and the risk of dementia: a prospective population-based study. Eur J Neurol 2009;16:990–997. [DOI] [PubMed] [Google Scholar]
- 32.Benito-Leon J, Louis ED, Villarejo-Galende A, Romero JP, Bermejo-Pareja F. Long sleep duration in elders without dementia increases risk of dementia mortality (NEDICES). Neurology 2014;83:1530–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scullin MK, Bliwise DL. Sleep, cognition, and normal aging: integrating a half-century of multidisciplinary research. Perspect Psychol Sci 2015;10:97–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willey JZ, Park Moon Y, Ruder R, et al. Physical activity and cognition in the Northern Manhattan Study. Neuroepidemiology 2014;42:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology: a bidirectional relationship. Nat Rev Neurol 2014;10:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke 2012;43:3137–3146. [DOI] [PubMed] [Google Scholar]
- 37.Gorelick PB, Pantoni L. Advances in vascular cognitive impairment. Stroke 2013;44:307–308. [DOI] [PubMed] [Google Scholar]
- 38.Knutson KL. Sleep duration and cardiometabolic risk: a review of the epidemiologic evidence. Best Pract Res Clin Endocrinol Metab 2010;24:731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cho ER, Kim H, Seo HS, Suh S, Lee SK, Shin C. Obstructive sleep apnea as a risk factor for silent cerebral infarction. J Sleep Res 2013;22:452–458. [DOI] [PubMed] [Google Scholar]
- 40.Foley DJ, Masaki K, White L, Larkin EK, Monjan A, Redline S. Sleep-disordered breathing and cognitive impairment in elderly Japanese-American men. Sleep 2003;26:596–599. [DOI] [PubMed] [Google Scholar]