Abstract
Objective:
To determine the association between hormone therapy (HT) and physical quality of life (QOL) in postmenopausal women with multiple sclerosis (MS).
Methods:
We included female participants from the prospective Nurses' Health Study, with a diagnosis of definite or probable MS, who had completed a physical functioning assessment (PF10; subscale of the 36-Item Short-Form Health Survey QOL survey) at a time point between 3 and 10 years after their final menstrual period (early postmenopause). We assessed the association between HT use at this time point (never vs at least 12 months of systemic estrogen with/without progestin) and both PF10 and the 36-Item Short-Form Health Survey Physical Component Scale. We used a linear regression model adjusting for age, MS duration, menopause type and duration, and further for additional covariates (only ancestry was significant).
Results:
Among 95 participants meeting all inclusion criteria at their first postmenopausal assessment, 61 reported HT use and 34 reported none. HT users differed from non–HT users in MS duration (p = 0.02) and menopause type (p = 0.01) but no other clinical or demographic characteristics. HT users had average PF10 scores that were 23 points higher than non–HT users (adjusted p = 0.004) and average Physical Component Scale scores that were 9.1 points higher in the 59 women with these available (adjusted p = 0.02). Longer duration of HT use was also associated with higher PF10 scores (p = 0.02, adjusted p = 0.06).
Conclusions:
Systemic HT use was associated with better physical QOL in postmenopausal women with MS in this observational study. Further studies are necessary to investigate causality.
Multiple sclerosis (MS) is a chronic inflammatory and neurodegenerative disease in which onset and course may be modified by hormonal exposures.1 In MS, there is an age-related increase in disability and conversion to progressive course observed at approximately age 45 years, while the incidence of new inflammatory symptoms or lesions diminishes.2 Little is known about the effect of hormonal changes occurring at menopause on MS course and whether hormone therapy (HT) modulates these changes.3,4
In healthy women, a decline in cognition has been observed after menopause, especially with the abrupt decline in hormones occurring when menopause is induced surgically,5,6 and HT, when initiated within a 5-year perimenopausal “window of opportunity,” has been reported to protect against cognitive decline.5–7 Investigating possible protective effects of HT on MS-related functional decline would have important clinical implications for women who develop MS before menopause, comprising the majority of MS cases.8
Therefore, we assessed whether HT is associated with improved physical quality of life (QOL) in the Nurses' Health Study (NHS), a large, longitudinal cohort of US women.
METHODS
Participants.
The NHS began in 1976 when 121,700 female registered nurses, aged 30 to 55 years, married, and living in 1 of 11 states, completed a lifestyle and medical history questionnaire. Women update their health behavior and medical information via questionnaire every 2 years.
Case ascertainment.
We identified 248 women with incident definite or probable MS in the NHS between 1976 and 2004, as previously described.9,10 For the current study, we included only participants with known date of MS diagnosis (figure e-1 at Neurology.org).
Outcome measure.
The 36-Item Short-Form Health Survey (SF-36)11 is a multi-item scale assessing 8 patient-reported, health-related domains that is used widely in MS research12,13 as well as in HT trials.14 It incorporates 8 subscales, including the 10-item physical functioning (PF10) scale.13 The PF10 was administered in the NHS every 4 years from 1992 to 2012, while the full SF-36 was administered in 1992, 1996, and 2000.
Our primary a priori SF-36 subscale of interest was PF10. This measure was selected because it was available for the most study participants, is decreased even in fully ambulatory patients with MS with low disability,13 and in other cohorts has shown good correlation with the primary clinical MS severity measure, Expanded Disability Status Scale (EDSS) (e.g., r = −0.7215).
The PF10 assesses performance in the following 10 activities: vigorous activities, moderate activities, lifting/carrying groceries, climbing several flights of stairs, climbing one flight of stairs, bending/kneeling/stooping, walking more than a mile, walking one block, walking several blocks, and bathing/dressing. Participants rate each domain on a 3-point scale: “Does your health now limit you in these activities? Yes, limited a lot; yes, limited a little; no, not limited at all.” The score is then summed and converted to a 100-point scale. The Physical Component Scale (PCS) is a composite score, also scaled to 100, that incorporates scores in the PF10 as well as the “role physical,” “general health,” and “bodily pain” domains of the SF-36.11
Reproductive variables.
Postmenopausal time point.
Reproductive variables were obtained from biennial questionnaires, as previously reported in NHS (e.g., reference 16). Date of menopause was defined as the date of last menstrual period beyond which no menses occurred for 1 year (natural), or date of surgery (surgical), according to the Stages of Reproductive Aging Workshop + 10 guidelines.17 Type of menopause was categorized as resulting from (1) natural physiology, (2) bilateral oophorectomy with or without hysterectomy, (3) hysterectomy with unilateral oophorectomy or hysterectomy without oophorectomy. Participants with menopause resulting from chemotherapy or radiation, or of uncertain type, were excluded.
For participants with known date of menopause, we identified the date of the first postmenopausal response for each of the SF-36 subscales. This assessment occurred at least 3 years, and fewer than 10 years, post menopause. This timeframe was selected to represent the first time point in the early postmenopausal period, but beyond the window of most pronounced perimenopausal hormonal and symptomatic fluctuations, according to the Stages of Reproductive Aging Workshop + 10 guidelines.17
HT use.
Participants were categorized based on HT exposure. We defined HT users as women who at the time of the first postmenopausal SF-36/PF10 assessment had used estrogen (conjugated estrogens [e.g., Premarin; Pfizer, New York, NY] or other estrogen) with or without progestogens in systemic administration (patch or oral) for at least 12 months. We defined non–HT users as women with no prior exposure to HT. The 22 participants who reported other formulations (black cohosh, testosterone), local application (gel or cream), or use for less than 12 months, were excluded because of heterogeneity of formulations and small sample size.
Covariates.
Every 2 years, women update their current weight and smoking status. From this information, we calculated current body mass index (BMI) in kg/m2 and smoking pack-years. Beginning in 1984, women completed a food-frequency questionnaire every 4 years (after 1986), including whether they use multivitamins or other supplements, from which we determine their intake of vitamin D per day (0, <400 IU, ≥400 IU). Women reported their ancestry (Southern European, Scandinavian, other Caucasian, other) and their state of residence at age 15 in 1992, which were categorized into tiers (north, middle, south) as previously described.18 They reported their weight at age 18 in 1990, and using the height they reported at baseline, we calculated BMI at age 18. Total physical activity is reported every 2 years as the average time spent per week performing various activities (e.g., running, swimming, etc). Each activity is assigned a metabolic equivalent (MET) score and METs are summed over all activities for a measure of total physical activity level in METs/wk. A full summary of inclusion and exclusion criteria is provided in figure e-1.
Standard protocol approvals, registrations, and patient consents.
Institutional review board approval was granted by the Partners Human Research Committee.
Statistical analysis.
We compared the demographic and disease characteristics of HT users vs non–HT users using t tests and χ2 tests.
The effect of HT use (dichotomous variable, ever/never) on postmenopausal SF-36 subscales was assessed using linear regression models. Models were adjusted for age, then further adjusted for MS duration (time since diagnosis), time since menopause, and menopausal type (natural, bilateral oophorectomy, other surgical). We also repeated the model in women with definite MS (n = 77) and in women with natural menopause only (n = 60); sample sizes were too small to examine effects in the surgical menopause groups (bilateral and unilateral oophorectomy, and hysterectomy only).
We also examined potential confounding by ancestry, smoking (cumulative pack-years), supplemental vitamin D intake, BMI at SF-36 subscale time point, BMI at age 18, latitude of residence at age 15, and physical activity at the time of the last premenopausal assessment. The effect of each covariate on the estimate of the association between HT use and PF10 and PCS scores was examined using separate linear regression models to develop the most parsimonious model; any covariate that altered the estimate size by at least 15% was retained in a further adjusted model. We then examined the effect of HT duration, as measured continuously in months, on PF10 and PCS levels at the first assessment after menopause.
To assess whether differences in PF10 observed among HT users and nonusers might reflect differences attributable to non–HT-related factors, we compared available PF10 scores for participants at a prior, premenopausal time point (i.e., 4 years before the selected postmenopausal time point, in the late perimenopausal phase17) during which time none of them were on HT. In this analysis, we compared the change in premenopausal PF10 scores for the HT “never-users” from our postmenopausal analysis, with the change in premenopausal PF10 scores for “subsequent users,” i.e., participants who did not use HT at the premenopausal time point but who subsequently began HT. We adjusted for MS duration and age, and in further sensitivity analyses, for ancestry, BMI and pack-years of smoking at assessment, BMI at age 18, and latitude at age 15. At that premenopausal time point, only a subset of women had assessments, as the first postmenopausal time point was often the first time point of inclusion in the NHS.
Finally, we assessed whether our findings in the women with MS were reflective of the broader NHS cohort. Of the 101,732 women with no MS, 31,935 had a first menopausal assessment fitting the criteria outlined above. In this sample, we repeated the regression analysis that we performed for the participants with MS (omitting MS-related variables). We also examined whether an interaction between HT use and MS diagnosis was significantly associated with PF10 in this model, adjusting for age, menopausal type, and time since menopause.
RESULTS
Demographic and disease characteristics of participants.
From a pool of 248 MS cases, 95 met all criteria for inclusion in this study (figure e-1). The participants included in this study had lower rates of smoking, and of bilateral oophorectomy, than the individuals not included, and slightly more lived in the South at age 15 (table e-1).
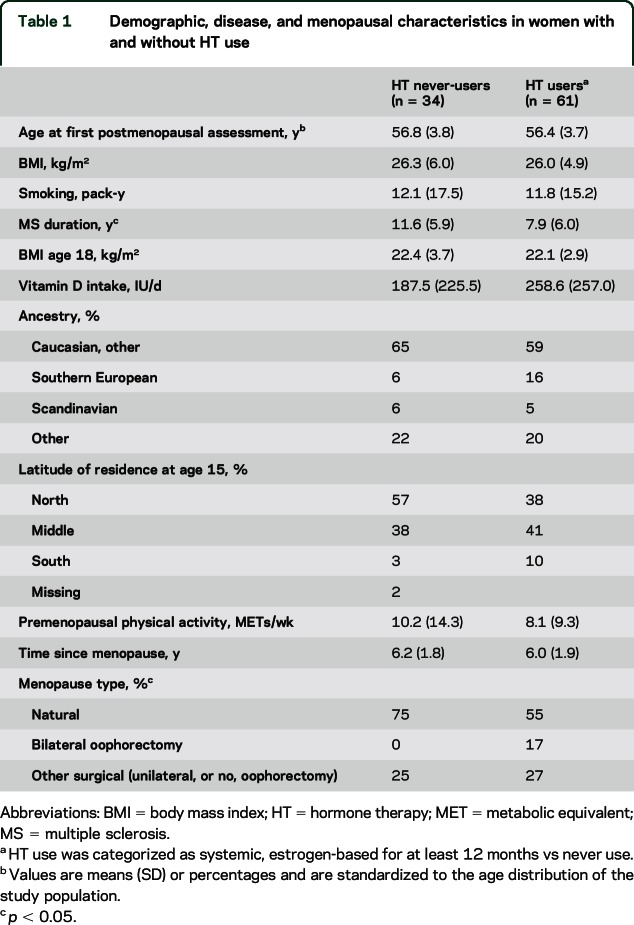
The characteristics of the 95 participants included in the current study are presented in table 1. At the first time point after menopause, HT users were of similar age at study entry as nonusers. HT users had shorter MS duration at the time of assessment. They did not differ in their BMI, smoking history, or supplemental vitamin D intake.
Table 1.
Demographic, disease, and menopausal characteristics in women with and without HT use
Association between HT and QOL measures.
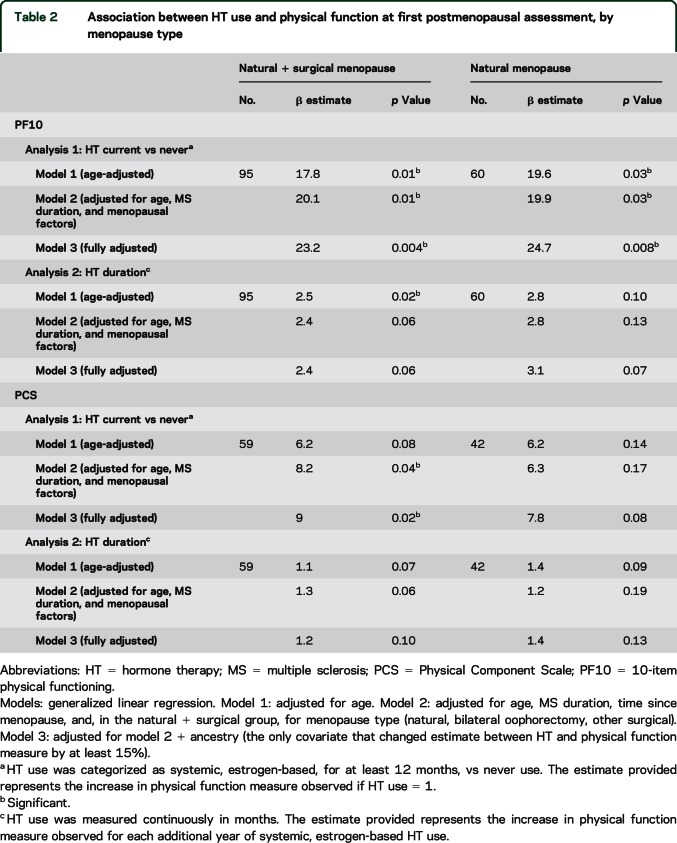
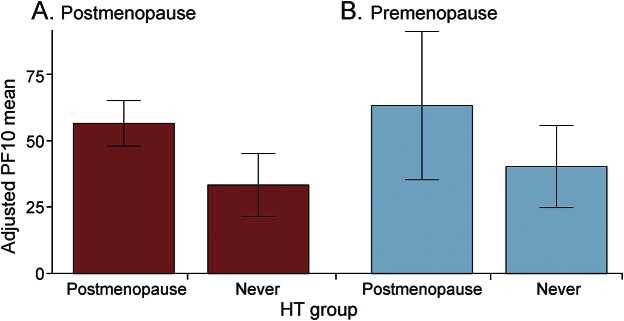
Average PF10 scores were higher in HT users (55.4) than in non–HT users (35.4) at their first postmenopausal PF10 assessment, adjusting for age, MS duration, and menopausal type and duration indicating that HT users had better physical function than non–HT users (table 2 and figure 1A). Only ancestry appeared to confound this association; however, the positive association between HT use and PF10 remained significant (HT: 56.5 vs no HT: 33.4, p = 0.004) (table e-2). When we stratified by menopausal type, these differences remained significant in the 60 women with natural menopause (HT: 56.0 vs no HT: 31.2, p = 0.008; adjusted for age, MS duration, menopausal duration, and ancestry). Results were also similar when we restricted the analysis to participants with definite MS (HT: 52.0 vs no HT: 31.0, adjusted p = 0.019).
Table 2.
Association between HT use and physical function at first postmenopausal assessment, by menopause type
Figure 1. Comparison of PF10 in women with MS according to their HT use.
(A) Comparison of PF10 in postmenopausal HT users vs never-users (n = 95, adjusted for age, MS duration, menopause type, menopause duration, and ethnicity: p = 0.004). (B) Comparison of PF10 in women at the premenopausal time point who either went on to start HT by the subsequent assessment time point or did not (n = 28, adjusted for age, MS duration, and eventual menopause type: p = 0.18). Bar plot with 95% confidence intervals. HT = hormone therapy; MS = multiple sclerosis; PF10 = 10-item physical functioning.
In addition, HT duration when measured continuously, was also associated with higher PF10 scores (p = 0.02 in the age-adjusted analysis and p = 0.06 in the analysis adjusted for age, MS duration, and menopausal type and duration) (table 2). Duration of HT use also showed the same direction of correlation with SF-36 PCS in the subset of women with values available, but the association was not significant (p = 0.13). We found no association between HT use and any other SF-36 measures (table e-2).
We further sought to determine whether the observed association between HT use and improved PF10 could be attributed to HT use itself or to uncaptured health-related behaviors (beyond physical activity, BMI, smoking, or vitamin D supplementation included in our adjusted models). A subset of 28 women had a premenopausal PF10 assessment during which they were not using HT. Here, in this restricted cohort, the adjusted means for the group of women who went on to use HT post menopause (n = 7, 63.2) was higher than for the group of women who did not use HT post menopause (n = 21, 40.3), but the difference was not statistically significant (p = 0.18, adjusting for age, MS duration, and menopausal type; figure 1B).
Association between HT use and QOL in women without MS.
Finally, we assessed whether HT use was also significantly associated with higher PF10 scores in the broader NHS cohort of 31,935 women without an MS diagnosis, who otherwise fit our inclusion criteria. In this cohort, in a fully adjusted model with all covariates, HT use was associated with significantly lower PF10 scores than was no-HT use (adjusted means = 85.9 and 86.7, respectively; p = 0.0003 [table e-3]); however, the magnitude of this difference was only 0.8 units, with the low p value being driven by the large increase in sample size. In fact, when we added each covariate sequentially into the model, it was inclusion of BMI that resulted in a positive association between HT use and PF10 becoming negative. Finally, when we also included the women with MS, the interaction term between MS diagnosis and HT use was also significant (estimate = 17.3, p < 0.0001, adjusting for age and menopausal type and duration).
DISCUSSION
Given the evidence for hormonal regulation in MS, here we hypothesized that exogenous postmenopausal HT use would mitigate the effect of MS on neurologic deterioration, as measured primarily by physical decline. In our primary analysis, HT use was associated with significantly better patient-reported physical functioning scores.
In MS, a disease characterized by both neuroinflammatory and neurodegenerative components, there is an age-related increase in disability and conversion to progressive course observed at approximately age 45 years, while the incidence of new inflammatory symptoms or lesions diminishes.2 Initial studies have suggested that there may be a worsening of MS-related disability after menopause,3,4 but further studies are needed.
Little is known about potential modulatory effects of exogenous hormones in MS. Overall, estrogens have been implicated in both shifts in immunomodulation in MS as well as purported neuroprotective effects.19 Oral contraceptives, in observational studies, have been reported to have protective,20–22 neutral,23,24 and negative effects on MS risk and course25–27; of note, the composition (estrogen and/or progestogen) and dosing may have varied according to the relevant study epochs, with potentially differing effects on risk. In addition, treatment with estriol (an estrogen markedly elevated during pregnancy, and that at lower doses has been used as HT in Europe and Asia28) for 24 months was recently reported to have beneficial effects on relapses and patient-reported fatigue.29 Given variable effects of exogenous hormones noted on MS course, the current study showing no apparent negative effects of HT use is reassuring.
Regarding HT specifically, in healthy women, in the decade since the Women's Health Initiative Memory Study raised concerns for an increased risk of stroke or cognitive decline in women starting HT at an older age,30 including in women in the NHS,16 a perimenopausal “window of opportunity” has been implicated, during which exogenous hormones may be protective against cognitive decline5–7 but beyond which treatment may be neutral or harmful.5,30–33 Given safety concerns, few women who are currently perimenopausal are treated with HT,30 limiting the power of recent or ongoing observational evaluations. In a recent clinical MS cohort study of longitudinal changes in the EDSS through the menopausal transition, less than 20% of patients were treated with HT.3,4
The PF10 was selected as our a priori primary outcome because of the following facts: it had the most available observations, it uncovers deficits in MS that reduce QOL,34 and in a prior study, it appeared to decline in women with MS relative to men at approximately the age of 50.35 The mean PF10 values in this cohort of postmenopausal women with MS are in line with those reported in several other assessments of individuals with MS34 and, specifically, in a cohort of women aged 54 to 60 years with MS (mean 45 years, SD 12).35 In separate cohorts, the PF10 subscale, which is patient-reported, has been shown to be decreased in fully ambulatory patients with MS13 and to have a good correlation with the EDSS across a range of EDSS scores.15 Furthermore, for a given EDSS score, absolute values and relative changes in PF10 are predictive of subsequent decline in the EDSS,15,36 suggesting that this test is sensitive to early patient detection of changes in physical function. Other SF-36 measures have not shown such reliable correlation with the EDSS and are influenced by factors other than physical disability, such as patient adaptations and coping with disease.36
In women without an MS diagnosis, HT was associated with slightly, but significantly, worse PF10 scores. This finding, and the significant interaction term between MS diagnosis and HT use, could be interpreted that the risks and benefits of HT in patient cohorts must be weighed carefully, and that in women with high risk of neurologic (including cognitive) deterioration, such as women with MS in the era before disease-modifying therapy, the potential benefits of HT may offset the risks to a greater extent than in women who are not at high risk of neurologic deterioration. Another interpretation is that an uncaptured bias in our MS sample belies causality. Women with physical disabilities are less likely to receive non–MS-related preventive care,37,38 and here, physical disability may have mitigated women's access to therapies seen as helpful, even when other predictors of health care utilization (education, profession, access) were similar across this cohort. We sought to partially address this by comparing QOL at a pre-HT, premenopausal time point, and the higher, but not statistically significant, PF10 score in premenopausal women who went on to use HT post menopause supports the possibility that women with less physical disability were more likely to take HT. Therefore, causality could not be established in this observational study, underscoring the importance of randomized clinical trials.
The strength of the current study includes a well-defined and validated MS cohort, as well as a large proportion of MS women treated with HT. In addition, we adjusted for other covariates that might influence MS risk and course, such as BMI, smoking, or vitamin D levels.39 Study limitations include a small sample size overall, reliance on patient-reported scores of physical function (EDSS not available in NHS), lack of information on timing of clinical MS attacks, lack of details regarding specific HT formulations, and the fact that vitamin D levels were estimated from vitamin D intake. Even though we adjusted for MS duration, it is possible that there was residual confounding given the longer MS duration in non–HT users. In addition, because physical disability accumulates with MS, it was not possible to fully separate the effects of volitional physical activity on physical function in women limited by their MS-related disability.
In this cohort of women with MS at risk of ongoing neurodegeneration, we report a positive relationship between HT use at menopause and patient-reported physical function. While the current findings do not permit a thorough assessment of causality, they suggest that HT is not harmful in women with MS. Furthermore, future interventional studies may be required to assess the protective effect of HT on neurodegeneration in MS, given the low prevalence of HT use in current observational cohorts.
Supplementary Material
GLOSSARY
- BMI
body mass index
- EDSS
Expanded Disability Status Scale
- HT
hormone therapy
- MET
metabolic equivalent
- MS
multiple sclerosis
- NHS
Nurses' Health Study
- PCS
Physical Component Scale
- PF10
10-item physical functioning
- QOL
quality of life
- SF-36
36-Item Short-Form Health Survey
Footnotes
Supplemental data at Neurology.org
Editorial, page 1430
AUTHOR CONTRIBUTIONS
Study concept and design: R.B., K.L.M., A.A. Statistical analysis and interpretation of data: R.B., C.C.W., K.C.F., K.L.M., A.A. Acquisition of data and interpretation of results: C.C.W., K.C.F., K.L.M., T.C., A.A. Manuscript drafting and revising: R.B., C.C.W., K.C.F., T.C., L.C., A.A., K.L.M.
STUDY FUNDING
This research was supported by the National Multiple Sclerosis Society Career Development Award (R.B.) and the NIH (grant 5K12HD051959-09 BIRCWH Scholar Award to R.B.; grants NS035624 and NS071082 to A.A.). The Nurses' Health Study cohort infrastructure is funded by NIH grant UM1 CA186107.
DISCLOSURE
R. Bove, C. White, and K. Fitzgerald report no disclosures relevant to the manuscript. T. Chitnis has received personal compensation for advisory board/consulting for Biogen Idec, Merck Serono, and Alexion, and has received research support from Merck Serono and Novartis Pharmaceuticals. L. Chibnik reports no disclosures relevant to the manuscript. A. Ascherio receives research grants from the NIH, the National Multiple Sclerosis Society, and the Department of Defense and served on a medical advisory board for Bayer HealthCare. K. Munger reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler 2014;20:520–526. [DOI] [PubMed] [Google Scholar]
- 2.Tutuncu M, Tang J, Zeid NA, et al. . Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult Scler 2013;19:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bove R, Healy BC, Secor E, et al. . Patients report worse MS symptoms after menopause: findings from an online cohort. Mult Scler Relat Disord 2015;4:18–24. [DOI] [PubMed] [Google Scholar]
- 4.Bove R, Healy BC, Musallam A, Glanz BI, De Jager PL, Chitnis T. Exploration of changes in disability after menopause in a longitudinal multiple sclerosis cohort. Mult Scler 2016;22:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res 2011;1379:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bove R, Secor E, Chibnik LB, et al. . Age at surgical menopause influences cognitive decline and Alzheimer pathology in older women. Neurology 2014;82:222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shao H, Breitner JC, Whitmer RA, et al. . Hormone therapy and Alzheimer disease dementia: new findings from the Cache County Study. Neurology 2012;79:1846–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bove RM, Healy B, Augustine A, Musallam A, Gholipour T, Chitnis T. Effect of gender on late-onset multiple sclerosis. Mult Scler 2012;18:1472–1479. [DOI] [PubMed] [Google Scholar]
- 9.Munger KL, Chitnis T, Ascherio A. Body size and risk of MS in two cohorts of US women. Neurology 2009;73:1543–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munger KL, Chitnis T, Frazier AL, Giovannucci E, Spiegelman D, Ascherio A. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol 2011;258:479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ware JE Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): I: conceptual framework and item selection. Med Care 1992;30:473–483. [PubMed] [Google Scholar]
- 12.Miller DM, Rudick RA, Cutter G, Baier M, Fischer JS. Clinical significance of the multiple sclerosis functional composite: relationship to patient-reported quality of life. Arch Neurol 2000;57:1319–1324. [DOI] [PubMed] [Google Scholar]
- 13.Pugliatti M, Riise T, Nortvedt MW, et al. . Self-perceived physical functioning and health status among fully ambulatory multiple sclerosis patients. J Neurol 2008;255:157–162. [DOI] [PubMed] [Google Scholar]
- 14.Walitt B, Pettinger M, Weinstein A, et al. . Effects of postmenopausal hormone therapy on rheumatoid arthritis: the Women's Health Initiative randomized controlled trials. Arthritis Rheum 2008;59:302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drulovic J, Riise T, Nortvedt M, Pekmezovic T, Manigoda M. Self-rated physical health predicts change in disability in multiple sclerosis. Mult Scler 2008;14:999–1002. [DOI] [PubMed] [Google Scholar]
- 16.Kang JH, Grodstein F. Postmenopausal hormone therapy, timing of initiation, APOE and cognitive decline. Neurobiol Aging 2012;33:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harlow SD, Gass M, Hall JE, et al. . Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernan MA, Olek MJ, Ascherio A. Geographic variation of MS incidence in two prospective studies of US women. Neurology 1999;53:1711–1718. [DOI] [PubMed] [Google Scholar]
- 19.Spence RD, Voskuhl RR. Neuroprotective effects of estrogens and androgens in CNS inflammation and neurodegeneration. Front Neuroendocrinol 2012;33:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmqvist P, Hammar M, Landtblom AM, Brynhildsen J. Age at onset of multiple sclerosis is correlated to use of combined oral contraceptives and childbirth before diagnosis. Fertil Steril 2010;94:2835–2837. [DOI] [PubMed] [Google Scholar]
- 21.Alonso A, Jick SS, Olek MJ, Ascherio A, Jick H, Hernan MA. Recent use of oral contraceptives and the risk of multiple sclerosis. Arch Neurol 2005;62:1362–1365. [DOI] [PubMed] [Google Scholar]
- 22.Villard-Mackintosh L, Vessey MP. Oral contraceptives and reproductive factors in multiple sclerosis incidence. Contraception 1993;47:161–168. [DOI] [PubMed] [Google Scholar]
- 23.Thorogood M, Hannaford PC. The influence of oral contraceptives on the risk of multiple sclerosis. Br J Obstet Gynaecol 1998;105:1296–1299. [DOI] [PubMed] [Google Scholar]
- 24.Hernan MA, Hohol MJ, Olek MJ, Spiegelman D, Ascherio A. Oral contraceptives and the incidence of multiple sclerosis. Neurology 2000;55:848–854. [DOI] [PubMed] [Google Scholar]
- 25.Sena A, Couderc R, Vasconcelos JC, Ferret-Sena V, Pedrosa R. Oral contraceptive use and clinical outcomes in patients with multiple sclerosis. J Neurol Sci 2012;317:47–51. [DOI] [PubMed] [Google Scholar]
- 26.Gava G, Bartolomei I, Costantino A, et al. . Long-term influence of combined oral contraceptive use on the clinical course of relapsing-remitting multiple sclerosis. Fertil Steril 2014;102:116–122. [DOI] [PubMed] [Google Scholar]
- 27.D'Hooghe MB, Haentjens P, Nagels G, D'Hooghe T, De Keyser J. Menarche, oral contraceptives, pregnancy and progression of disability in relapsing onset and progressive onset multiple sclerosis. J Neurol 2012;259:855–861. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi K, Manabe A, Okada M, Kurioka H, Kanasaki H, Miyazaki K. Efficacy and safety of oral estriol for managing postmenopausal symptoms. Maturitas 2000;34:169–177. [DOI] [PubMed] [Google Scholar]
- 29.Voskuhl RR, Wang H, Wu TC, et al. . Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2016;15:35–46. [DOI] [PubMed] [Google Scholar]
- 30.Rapp SR, Espeland MA, Shumaker SA, et al. . Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. JAMA 2003;289:2663–2672. [DOI] [PubMed] [Google Scholar]
- 31.Barrett-Connor E, Laughlin GA. Endogenous and exogenous estrogen, cognitive function, and dementia in postmenopausal women: evidence from epidemiologic studies and clinical trials. Semin Reprod Med 2009;27:275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Craig MC, Maki PM, Murphy DG. The Women's Health Initiative Memory Study: findings and implications for treatment. Lancet Neurol 2005;4:190–194. [DOI] [PubMed] [Google Scholar]
- 33.Henderson VW. Alzheimer's disease: review of hormone therapy trials and implications for treatment and prevention after menopause. J Steroid Biochem Mol Biol 2014;142:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dallmeijer AJ, de Groot V, Roorda LD, et al. . Cross-diagnostic validity of the SF-36 physical functioning scale in patients with stroke, multiple sclerosis and amyotrophic lateral sclerosis: a study using Rasch analysis. J Rehabil Med 2007;39:163–169. [DOI] [PubMed] [Google Scholar]
- 35.Bove R, Musallam A, Healy BC, et al. . No sex-specific difference in disease trajectory in multiple sclerosis patients before and after age 50. BMC Neurol 2013;13:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Visschedijk MA, Uitdehaag BM, Klein M, et al. . Value of health-related quality of life to predict disability course in multiple sclerosis. Neurology 2004;63:2046–2050. [DOI] [PubMed] [Google Scholar]
- 37.Horner-Johnson W, Dobbertin K, Andresen EM, Iezzoni LI. Breast and cervical cancer screening disparities associated with disability severity. Womens Health Issues 2014;24:e147–e153. [DOI] [PubMed] [Google Scholar]
- 38.Dobos K, Healy B, Houtchens M. Preventive health-care access in severely disabled women with multiple sclerosis. Int J MS Care 2015;17:200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA 2006;296:2832–2838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.