Abstract
Recent preclinical and clinical studies demonstrate that three functionally different compounds, the NMDA receptor channel blocker ketamine, mGlu2/3 receptor antagonist LY341495, and NMDA receptor glycine site agent GLYX-13 produce rapid and long lasting antidepressant effects. Furthermore, these agents are reported to stimulate ERK and mTORC1 signaling in brain. Here we used rat primary cortical culture neurons to further examine the cellular actions of these agents. The results demonstrate that low concentrations of all three compounds rapidly increase levels of the phosphorylated and activated forms of ERK and a downstream target of mTORC1, p70S6 kinase, in a concentration and time dependent manner. In addition, each compound rapidly increases BDNF release into the culture media. Further studies demonstrate that induction of BDNF release, as well as stimulation of phospho-ERK is blocked by incubation with an AMPA receptor antagonist. The requirement for AMPA receptor stimulation suggests that the effects of these rapid agents are activity dependent. This possibility is supported by studies demonstrating that neuronal silencing, via incubation with the GABAA receptor agonist muscimol, completely blocks phospho-ERK and BDNF release by each agent. Finally, incubation with each drug for 24 hr increases the number and length of neuronal branches. Together, the results demonstrate that these three different rapid acting antidepressant agents increase ERK signaling and BDNF release in an activity dependent manner that leads to increased neuronal complexity. Further studies will be required to determine the exact mechanisms underlying these effects in cultured neurons and in rodent models.
Introduction
Major depression, characterized by sadness, anxiety, feelings of worthlessness, and an inability to experience pleasure, is a debilitating disease that affects approximately 17% of the population (Kessler et al., 2003). Current therapeutic drugs target the serotonin and norepinephrine monoaminergic systems and can take several weeks to months to produce an antidepressant response. Furthermore, about one third of depressed patients are nonresponsive to current antidepressants and are considered treatment resistant (Trivedi et al., 2006), demonstrating a serious unmet need that must be addressed with novel therapeutic agents. Toward this goal, recent clinical evidence has shown that a single, low dose of ketamine produces rapid and long lasting antidepressant effects in treatment resistant patients (Berman et al., 2000; Price et al., 2009; Zarate et al., 2006).
Ketamine is an N-methyl-D-aspartate (NMDA) receptor channel blocker that enters the open channel in an activity dependent manner and binds with low micromolar affinity (Traynelis et al., 2010). Preclinical studies have demonstrated that a subanesthetic, but not anesthetic dose of ketamine is sufficient to produce antidepressant behavioral effects in rodent models (Autry et al., 2011; Li et al., 2010; Maeng et al., 2008). Moreover, ketamine increases the number and function of spine synapses in layer V pyramidal neurons of the medial prefrontal cortex (mPFC), and activation of the mechanistic target of rapamycin complex 1 (mTORC1) pathway is necessary for the synaptogenic, as well as behavioral effects of ketamine (Li et al., 2010). Activation of mTORC1 and the subsequent behavioral effects are also dependent on activation of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptors, indicating that these effects are activity dependent (Maeng et al., 2008). This is supported by studies demonstrating that ketamine causes a rapid, transient burst of glutamate in the mPFC (Moghaddam et al., 1997). This paradoxical increase in glutamate is thought to be due to blockade of NMDA receptors on tonic firing GABA interneurons, resulting in disinhibition of glutamate transmission (Homayoun and Moghaddam, 2007). Activation of L-type calcium channels and release of brain derived neurotrophic factor (BDNF) have also been implicated in the antidepressant behavioral actions of ketamine (Autry et al., 2011; Lepack et al., 2014; Liu et al., 2012).
Several other glutamatergic rapid-acting antidepressants have been identified in clinical trials and/or preclinical models. This includes LY341495, which has subnanomolar affinity for mGluR2/3 receptor sites that control presynaptic release of glutamate (Dwyer, 2012; Koike et al., 2011; Wright et al., 2001). Another agent of interest is GLYX-13, an NMDA receptor modulator with glycine site partial agonist properties (Burgdorf et al., 2013; Preskorn et al., 2015); GLYX-13 is reported to enhance MK801 ligand binding, similar to glycine with and EC50 of approximately 5 µM (Moskal et al., 2005). Interestingly, preclinical studies demonstrate that these two drugs also stimulate mTORC1 signaling and produce antidepressant behavioral responses that are dependent on activation of AMPA receptors and mTORC1 (Burgdorf et al., 2013; Dwyer et al., 2012; Karasawa et al., 2005; Koike et al., 2011; Li et al., 2010; Lu et al., 2015). Blockade of mGlu2/3 autoreceptors, like ketamine increases glutamate release (Hascup et al., 2010; Xi et al., 2002); to date the influence of GLYX-13 on extracellular glutamate has not been reported. These data indicate that the BDNF-mTORC1 pathway and its associated signaling components, including the phosphorylated and activated forms of extracellular regulated kinase (phospho-ERK) and p70S6 kinase-1 (phospho-S6K) play a crucial role in the actions of rapid antidepressant agents. However, the cellular mechanisms underlying these effects have not been fully characterized.
The aim of this present study was to develop an in vitro system to allow for analysis of the cellular mechanisms underlying the actions of ketamine and other rapid-acting antidepressants that are difficult to measure in vivo, notably the release of BDNF and requirement for neuronal activity. The results demonstrate that incubation of cortical primary cultures with low concentrations of ketamine, LY341495, or GLYX-13 increases levels of phospho-ERK and phospho-S6K in a dose and time dependent manner. Moreover, LY341495 and GLYX-13 incubations increase BDNF release into the media similar to ketamine (Lepack et al., 2014) Finally, stimulation of phospho-ERK and BDNF release by all three agents is inhibited by AMPA receptor blockade or neuronal silencing, indicating that these effects are activity dependent. Together, these findings further characterize the cellular signaling mechanisms underlying rapid-acting antidepressants and describe an in vitro mixed cortical culture model for further mechanistic studies.
2. Methods
2.1. Primary cortical culture
Pregnant females were euthanized and cortices from E18 embryos were dissected. Following incubation in trypsin-EDTA (0.25%, Gibco) for 10 min, cortices were dissociated and neurons were plated at 0.6 million cells per well in 6-well polylysine-coated plates in DMEM (Gibco)/10% FBS (fetal bovine serum). For dentritic morphology, cells were plated on glass cover slips at 0.4 million cells per 6-well plate. For both 6-well and coverslips, medium was changed the following day to a serum-free medium containing neurobasal and B27 (Gibco) which was changed every 5 days. Cells were maintained at 37 °C, 5% CO2, and 95% humidity.
2.2. Drug treatments
On DIV 10, medium was changed four hours prior to drug treatment. For the dose response curve, cultured neurons were incubated with ketamine (10 nM – 1 µM), LY341495 (1 pM – 1 µM), or GLYX-13 (0.3 nM – 1 µM) and cells were collected 1 hr after drug treatment for western blot analysis. After establishing the optimal dose, cells were treated with ketamine (100 nM or 500 nM), LY341495 (3 nM or 10 nM), or GLYX-13 (3 nM) and collected at 5, 15, 30, 60 min, or 24 hr following treatment for analysis of phospho-ERK signaling and 15 or 60 min following treatment for phospho-S6K signaling. For blockade studies, neurons were treated with 50 µM NBQX, 500 nM K252a, or 10 µM muscimol 20 to 30 min prior to ketamine, LY 341495, or GLYX-13 treatment and cells were collected 60 min following antidepressant treatment for phospho-ERK western blot analysis and 15 or 30 min after drug treatment for BDNF analysis.
2.3. Immunoblot analysis of phospho-ERK and phospho-S6K
Levels of phospho-ERK and phospho-S6K for drug incubations were analyzed by western blot analysis of crude cell homogenates, except for ketamine regulation of phospho-S6K, which was examined in a P2 crude synaptosome fraction as previously described (Li et al., 2010). For homogenates, cells were collected into RIPA lysis buffer containing 50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 1 mM NaVO3, 5 mM NaF and 1× protease inhibitor cocktail. For P2 preparations, cells were homogenized in 0.32 M sucrose, 20 mm Hepes (pH 7.4), 1 mM EDTA, 1× protease inhibitor cocktail, 10 mM NaF and 1 mM NaVO3. Homogenates were then centrifuged at 700 × g, supernatants were collected and further centrifuged at 13,000 × g to obtain a pellet containing crude synaptosomes. Pellets were then resuspended and sonicated in RIPA lysis buffer. Total protein concentrations were measured using a BCA kit (Thermo Scientific, USA). Ten µg of protein were electrophoretically separated on an SDS-PAGE gel (7.5% Tris-HCL) and transferred to 2 µm nitrocellulose membranes. Membranes were blocked with 2% BSA in PBS+0.1% Tween 20 (PBS-T) and then incubated with phospho-ERK (Thr202/Tyr204) or phospho-S6K (Thr389) (Cell Signaling, 1:1000) antibodies overnight. Membranes were then washed in PBS-T (3 times for 10 min each) and incubated in peroxidase labeled anti-rabbit secondary at room temperature for 1 hr. Protein bands were analyzed using enhanced chemiluminescence (ECL). After detection of phospho proteins, membranes were incubated in stripping buffer (Thermo Scientific), blocked in 5% milk PBS-T for 1 hr and then reprobed with total ERK or total S6K (Cell Signaling, 1:1000) overnight. Densitometry was used to quantify protein bands using Image J Software (NIH) and phospho proteins were normalized to their respective total protein.
2.4. BDNF analysis
Measurement of BDNF was performed as previously described (Lepack et al., 2015). Briefly, medium containing an anti-BDNF antibody (sc-546, Santa Cruz, 1:100, or 2µg/mL) was carefully collected following drug treatment and the secreted BDNF captured by the antibody was immunoprecipitated using Protein G-Sepharose beads (GE Healthcare). BDNF was detected via an ELISA assay (BDNF-ELISA E-max; Promega, WI). EIA 96-well plates (Corning, NY, USA) were coated with monoclonal antibody and incubated at 4°C for 18 hours. The plates were incubated in a block and sample buffer at room temperature for 1 hr, followed by a 2 hr incubation of BDNF samples with standards and then incubated with a polyclonal antibody for 2 hrs at room temperature. After washing, the plates were incubated with a secondary anti-IgY antibody conjugated to horseradish peroxidase for 1 hr. Next the plates were incubated in peroxidase substrate and tetramethylbenzidine solution to produce the color reaction, which was stopped with 1 M hydrochloric acid. The absorbance at 450 nm was measured with an automated microplate reader and standard curves were plotted for each plate. Protein concentrations in each immunoprecipitate were measured using a BCA kit (Thermo Scientific) and values of BDNF were corrected for total amount of protein in the sample.
2.5. Immunolabeling, viral GFP infection, and Sholl analysis
Cells were plated on 22 × 22mm coverslips (0.3 million cells) and on DIV13 were fixed for immunohistochemistical analysis of MAP2 (Abcam, ab5392; 1:10,000), GAD67 (Millipore, MAB5406; 1:500), and GFAP (G3893, Sigma; 1:500). For dendritic morphology, 0.4 million cells per 6-well were plated on coverslips. On DIV 3, cells were incubated with an AAV2 viral vector that expresses green fluorescence protein (AAV2-GFP) as previously described (Dwyer et al., 2015) for 72 hr. Following viral incubation, media was changed and cells were treated with ketamine (500 nM), LY 341495 (10 nM), or GLYX-13 (3nM) at DIV 17 and then fixed 24hr after with 4% PFA. Coverslips were mounted onto slides and imaged for endogenous GFP. Dendritic branching was assessed at 50 and 100 µm away from the soma using Sholl analysis.
2.6. Data analysis
Dose response studies were analyzed using a one-way analysis of variance (ANOVA). For time course experiments comparing multiple doses (ketamine and LY 341495) were analyzed using a one-way ANOVA while time courses for GLYX-13 (vehicle vs. treatment group) were analyzed using a Students t test. For blockade experiments, a one-way ANOVA analysis was used with least significant difference post hoc tests where appropriate. For Sholl analysis experiments, a Students t test was used (vehicle vs. treatment group). Significance was determined at P < .05. All experiments were replicated at least twice and typically 3 to 4 times. All data are represented as mean ± SEM.
3. Results
3.1. Ketamine rapidly stimulates phospho-ERK and phospho-S6K signaling in primary neuronal cultures
Dissociated cortical neurons from E18 embryos were plated at a density of 0.6 million cells per well in 6-well polylysine-coated plates in DMEM (Gibco)/10% FBS (fetal bovine serum). One day later the medium was changed to serum-free conditions containing neurobasal and B27 (Gibco) and was changed every 5 days thereafter. On DIV 10, medium was changed four hours prior to drug incubation. As reported previously these conditions result in mixed neuronal cultures containing both glutamatergic and GABAergic neurons, as well as a small number of glia (Kato-Negishi et al., 2004; Weir et al., 2015) (also see below).
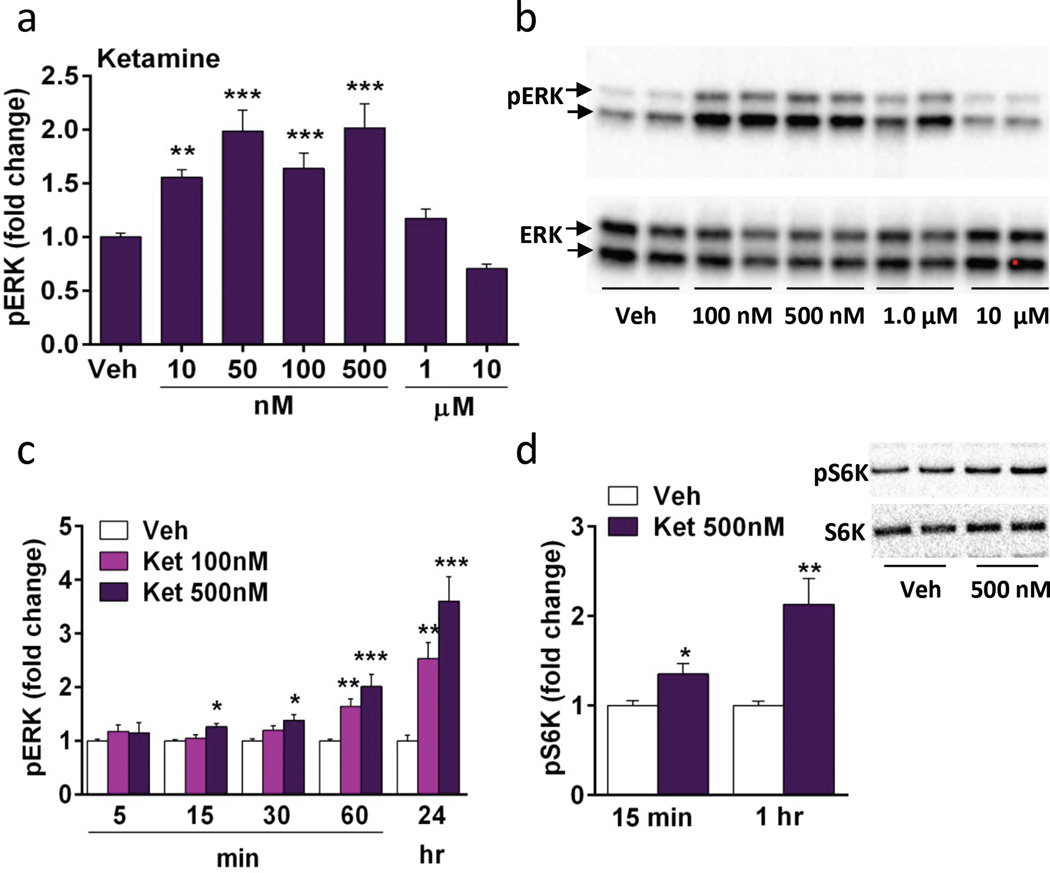
In vivo studies have shown that systemic ketamine administration rapidly activates neurotrophic factor and mTORC1 signaling and its downstream substrates in the mPFC (Li et al., 2010). In order to test the cellular mechanisms underlying these effects and to establish an in vitro model, studies were conducted in cultured cortical neurons. In preliminary studies we found that the phosphorylated and activated form of ERK was the most reliable marker of these pathways, so this phosphoprotein was used to characterize the concentration response and time course for ketamine. Concentrations of ketamine from 10 nM to 10 µM were tested with incubation time set at 1 hr, and levels of phospho-ERK were determined by western blot. Concentrations of 10, 50, 100, and 500 nM significantly increased phospho-ERK (both 42 and 44 kDa isoforms) in a dose dependent manner, while higher concentrations of 1 and 10 µM produced lower, nonsignificant responses (ANOVA, F6,59 = 15.63, p < .0001) (Fig 1a, b). A similar inverted U dose dependent response for the in vivo behavioral actions of ketamine have been reported (Li et al., 2010). Concentrations of 100 and 500 nM were chosen for time course studies and demonstrate rapid (15 min) (F2,36 = 6.8, p < 0.005; 30 min, F2,35 = 5.632, p < 0.01; 60 min, F2,39 = 22.743, p < 0.0001) and long-lasting (24 hr) (F2,36 = 20.305, p < 0.0001) stimulation of phospho-ERK (Fig 1c). We also examined phospho-mTOR, but found that levels were relatively low and inconsistent, possibly reflecting dynamic dephosphorylation of this protein. However, we found more consistent regulation of a downstream target of mTORC1 signaling, phospho-S6K, which is rapidly activated in mPFC by ketamine systemic administration (Li et al., 2010). The results demonstrate that ketamine (500 nM) incubation increases levels of phospho-S6K after incubations of 15 (t(12) = 2.766, p < 0.05) or 60 min (t(11) = 3.571, p < 0.01) (Fig. 1d). We also found that ketamine incubation increases levels of phospho-ERK and phospho-S6K in hippocampal cultures (Supplemental Figure 1).
Figure 1. Ketamine incubation increases phospho-ERK and phospho-S6K signaling in a concentration and time dependent manner in vitro.
(a) Following 1 hr of ketamine incubation, there was a concentration dependent increase in phospho-ERK (pERK) with 10–500 nM ketamine. (b) Representative immunoblots for pERK and total ERK. (c) Concentrations of 100 and 500 nM were chosen to test the time course for ketamine stimulation of pERK. (d) Ketamine (500 nM) increases phospho-S6K (pS6K) at 15 and 60 min; representative images of pS6K immunolabeling shown on the right. Levels of total ERK and S6K were determined to control for gel loading and membrane transfer. Results are presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005 compared to vehicle (ANOVA and Fisher’s post-hoc least significant difference test for results in a and c, and Students t test for d).
3.2. LY341495 and GLYX-13 stimulate phospho-ERK and phospho-S6K signaling in primary neuronal cultures
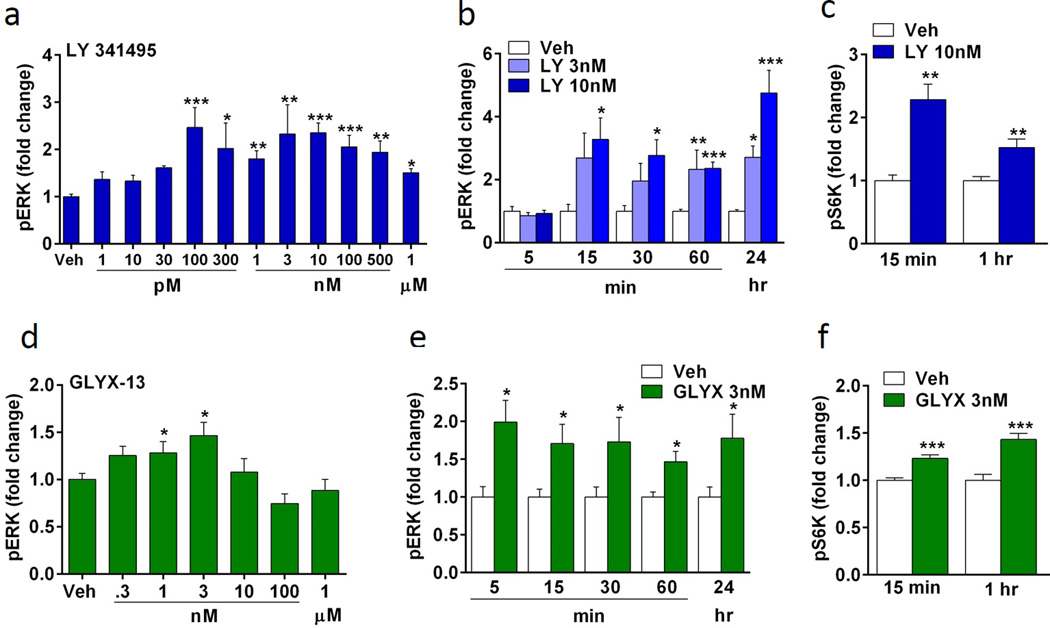
The mGluR2/3 antagonist LY341495 and the NMDA receptor modulator, glycine site partial agonist GLYX-13 are reported to increase pERK-mTORC1-S6K signaling and produce rapid antidepressant actions in vivo, and the behavioral actions of these agents require mTORC1 signaling (Dwyer et al., 2012; Koike et al., 2011; Lu et al., 2015). Here we found that both drugs increased phospho-ERK signaling in a concentration dependent manner after one hr incubation. Very low (100 pM) to higher concentrations (1 µM) of LY341495 significantly increased phospho-ERK levels in primary neuronal cultures (ANOVA, F11,100 = 5.127, p < 0.0001) (Fig. 2a), and concentrations of 3 and 10 nM produced a rapid, time dependent increase in levels of phospho-ERK, similar to ketamine (15 min, F2,21 = 3.711, p < 0.05; 30 min, F2,21 = 4.025, p < 0.05; 60 min, F2,31 = 24.378, p < 0.0001; 24 hr, F2,21 = 16.382, p < 0.0001) (Fig. 2b). GLYX-13 increased phospho-ERK levels at 1 and 3 nM following 1 hr incubation (ANOVA, F6,34 = 2.967, p < 0.05) (Fig. 2d); higher doses of GLYX-13 (10 nM to 1 µM) had no significant effect, suggesting an inverted U dose response similar to ketamine. This is also consistent with the behavioral dose response to GLYX-13 (Burgdorf et al., 2013). Time course studies show that GLYX-13 produced a rapid increase in levels of phospho-ERK after 5 min that was sustained for up to 24 hr (t(14) = 3.072, p < 0.05; 15 min, t(30) = 2.548, p < 0.05; 30 min, t(13) = 2.194, p < 0.05; 60 min, t(11) = 3.339, p < 0.05; 24hr, t(14) = 2.256, p < 0.05) (Fig. 2e). Levels of phospho-S6K were examined and both LY341495 (15 min, t(6) = −4.948, p < 0.005; 60 min, t(22) = −3.456, p < 0.005) and GLYX-13 (15 min, t(22) = 4.804, p < 0.0001; 60 min, t(22) = 4.802, p < 0.001) also increased phospho-S6K in cortical cultures (Fig. 2c,f). Together, the data indicate that these two putative rapid-acting antidepressants stimulate phospho-ERK and phospho-S6K signaling pathways similar to findings in vivo.
Figure 2. Incubations with LY 341495 or GLYX-13 increase phospho-ERK and phospho-S6K signaling in primary neuronal cultures.
(a) LY341495 increases phospho-ERK (pERK) in a concentration dependent manner. Following 1 hr incubation, 100 and 300 pM, 1 – 500 nM, and 1 uM increased pERK levels, determined by immunoblot analysis. (b) Concentrations of 3 and 10 nM LY341495 increased pERK activation in a time dependent manner. LY341495 10 nM rapidly increased pERK at 15 min with significant effects up to 24 hr. (c) LY341495 (10 nM) increased phospho-S6K (pS6K) following 15 and 60 min incubation, determined by immunoblot analysis. (d) GLYX-13 also produced a concentration dependent increase in pERK following 1 hr stimulation with 1 and 3 nM. (e) GLYX-13 (3nM) rapidly increased pERK at 5 min, and (f) increased pS6K following 15 and 60 min incubation. Levels of total ERK and S6K were determined to control for gel loading and membrane transfer. All results are presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005 compared to vehicle (ANOVA and Fisher’s post-hoc least significant difference test for results in a–c and Students t test for D, F, E).
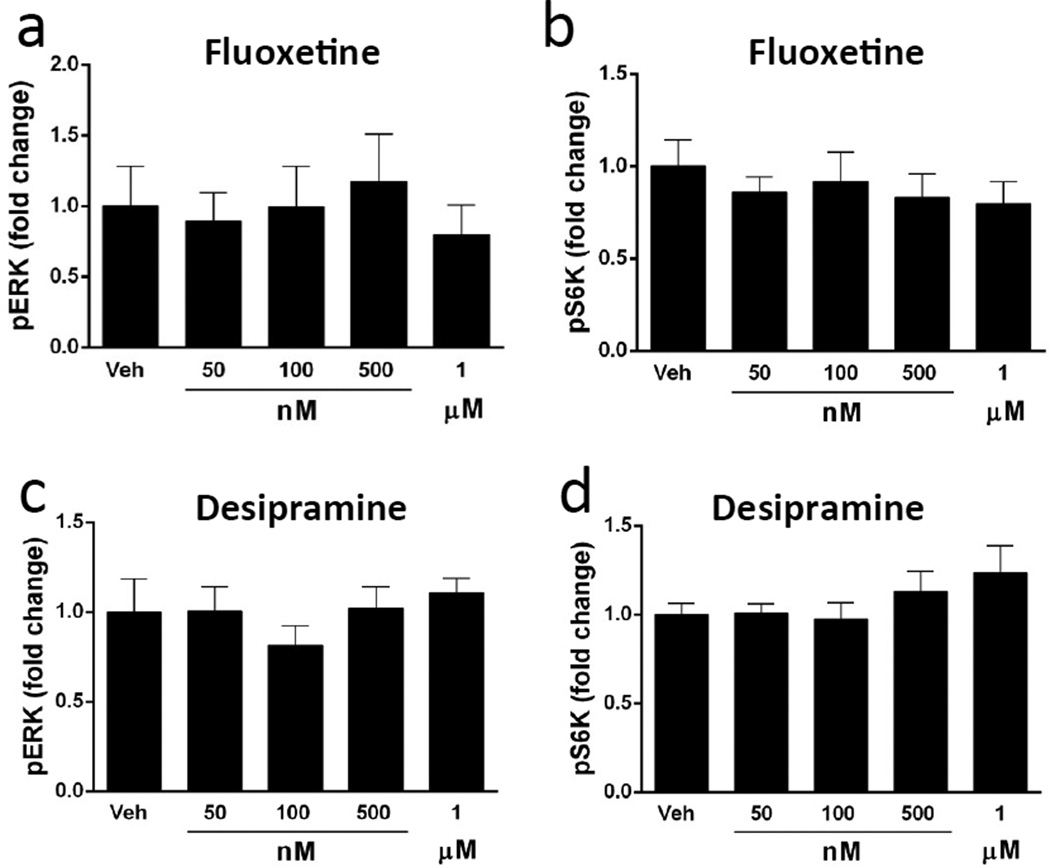
We also tested the effects of two typical antidepressants, fluoxetine and desipramine that require chronic administration to produce a therapeutic response. Fluoxetine is a selective serotonin reuptake inhibitor, and desipramine is a selective norepinephrine reuptake inhibitor and belongs to the tricyclic class of antidepressants. Several doses of these agents were tested from 0.05 to 1 µM, the upper limit of which includes the range for the therapeutic actions of these antidepressants (incubation times were set at 1 hr). In contrast to the rapid acting agents, none of the fluoxetine or desipramine doses tested increased levels of phospho-ERK or phospho-S6K in primary neuronal cultures (Figure 3a–d). Incubation with ketamine in adjacent wells significantly increased levels of phospho-ERK, serving as a positive control for this experiment (not shown).
Figure 3. Influence of fluoxetine and desipramine on levels of phospho-ERK and phospho-S6K.
Primary cultures were incubated with the typical antidepressants fluoxetine or desipramine for 1 hr at the indicated concentrations and levels of phospho-ERK (pERK) and phospho-S6K (PS6K) were determined by immunoblot analysis. Incubation with fluoxetine (a,b) or desipramine (c,d) did not influence levels of pERK or pS6K at any of the concentrations tested at this time point. Levels of total ERK and S6K were determined to control for gel loading and membrane transfer. Results are presented as mean ± SEM.
3.3 Stimulation of phospho-ERK requires activation of AMPA and TrkB receptors
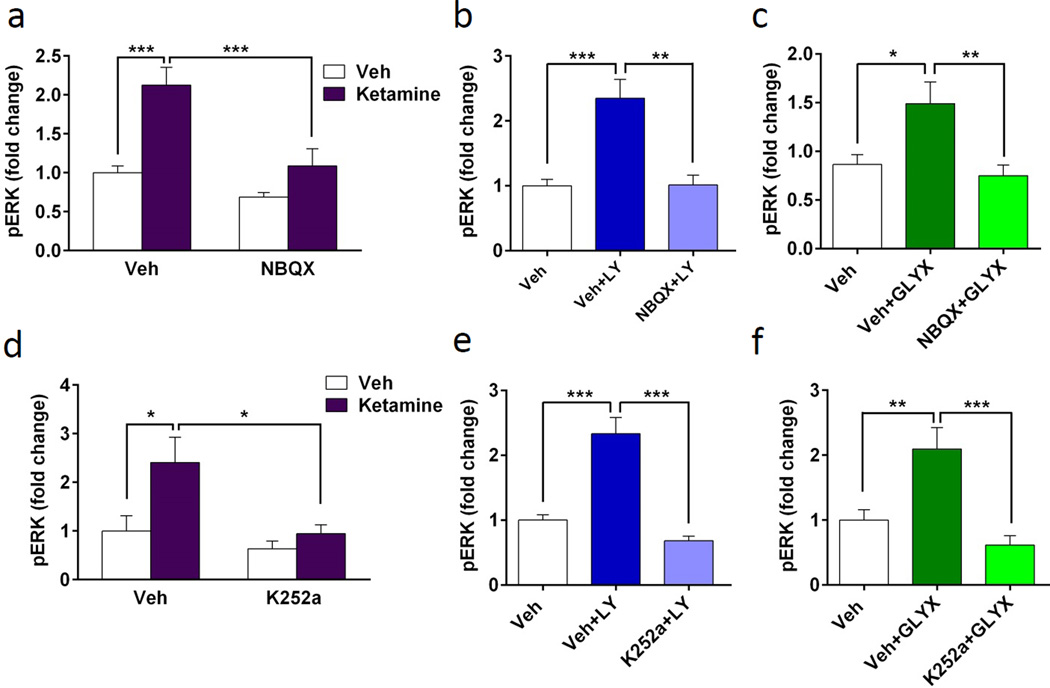
Previous in vivo studies have shown that the behavioral actions of ketamine, as well as mGluR2/3 antagonists and GLYX-13 require AMPA receptor activation (Burgdorf et al., 2013; Karasawa et al., 2005; Li et al., 2010; Lu et al., 2015). To determine if AMPA receptors are required for the activation of phospho-ERK in cultured neurons, we pretreated cells with the AMPA inhibitor NBQX for 20 min prior to addition of ketamine. After 1 hr ketamine incubation, cells were collected and phospho-ERK signaling was measured. NBQX alone had no significant effect, but completely blocked ketamine-stimulation of phospho-ERK (ANOVA, F3,36 = 12.094, p < 0.001) (Fig. 4a). Similarly, NBQX incubation completely blocked the stimulation of phospho-ERK by both LY341495 (ANOVA, F2,33 = 11.352, p < 0.001) and GLYX-13 (ANOVA, F2,32 = 6.56, p < 0.005) (Fig. 3b, c). Since NBQX alone had no effect in the ketamine experiment this condition was not included in these subsequent studies of LY341495 and GLYX-13.
Figure 4. Stimulation of phospho-ERK by rapid-acting antidepressants requires AMPA and TrkB receptors.
(a–c) Cultured neurons were incubated with 50 µM NBQX 20 min prior to addition of ketamine (a, 500 nM), LY341496 (b, 10 nM), or GLYX-13 (c, 3 nM), all for 1 hr incubation. Pretreatment with NBQX completely blocked the induction of pERK (pERK) activation by all three agents. NBQX alone did not have an effect on pERK activation (a). (d-f) Cultures were incubated with 500 nM K252a for 20 min prior to addition of ketamine (d), LY341496 (e), or GLYX-13 (f), all for 1 hr incubation. Pretreatment with K252a blocked the induction of pERK by all three agents. K252a alone did not have an effect on pERK activation (d). Levels of total ERK and S6K were determined to control for gel loading and membrane transfer. Results are presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005 compared to vehicle or blockade (ANOVA and Fisher’s post-hoc least significant difference test for results).
We have recently demonstrated that blocking BDNF with a function blocking antibody completely blocks the behavioral effects of ketamine in the forced swim test (Lepack et al., 2014). Additionally, the behavioral actions of ketamine are blocked in BDNF mutant mice with a knockin of the human ValMet66 polymorphism (the Met allele impairs activity-dependent release of BDNF), providing further evidence that BDNF release plays an important role in the effects of ketamine (Liu et al., 2012). In order to determine whether BDNF signaling is necessary for stimulation of phospho-ERK signaling, we tested the BDNF-TrkB inhibitor, K252a. Neuronal cultures were incubated with K252a (500 nM) for 20 min, and then with each of the three agents for 1 hr and cells were collected for phospho-ERK analysis. K252a incubation completely blocked the increase in phospho-ERK produced by each of the three agents (ketamine, F3,48 = 3.907, p < 0.05; LY341495, F2,37 = 22.007, p < 0.001; GLYX-13, F2,21 =11.391, p < 0.005) (Fig. 4d–f). K252a incubation alone had no effect on phospho-ERK signaling (Fig. 4d). Together, these results demonstrate that the increase in phospho-ERK signaling in response to incubation with these three agents requires activation of AMPA and TrkB receptors.
3.4. LY341495 and GLYX-13 increase BDNF release: requirement for activation of AMPA receptors
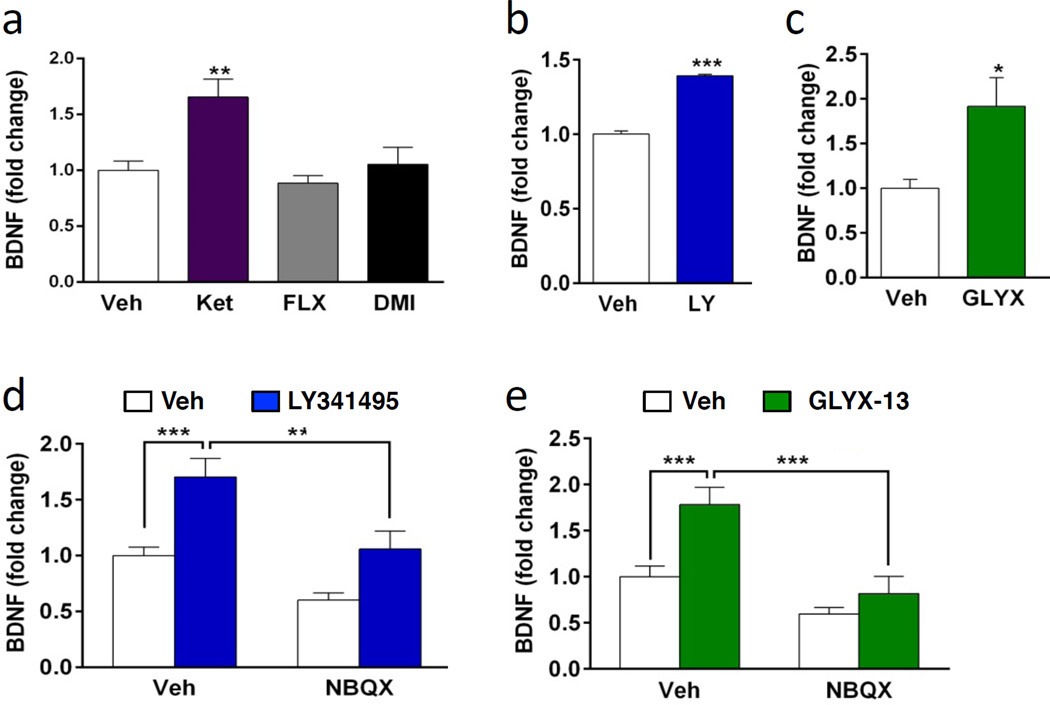
The requirement for TrkB suggests that these rapid acting antidepressants stimulate the release of BDNF, which then increases phospho-ERK signaling. In support of this possibility, we have recently reported that ketamine rapidly (15 min) increases BDNF release in cultured cells and this effect is relatively long-lasting (up to 6 hr (Lepack et al., 2014). This finding was replicated in the current study, demonstrating that incubation with ketamine significantly increases BDNF release, tested at 1 hr (Fig. 5a). To determine if LY341495 (10 nM) and GLYX-13 (3 nM) also increase BDNF release, cultured neurons were incubated with each agent and media was collected for BDNF analysis. BDNF was enriched by immunoprecipitation and assessed by ELISA as in our previous study (Lepack et al., 2014). (Incubation with LY341495 for 15 min produced inconsistent effects (not shown) but a 30 min incubation produced a significant increase (~40%) in levels of BDNF in the media (t(8) = −16.231, p < 0.001) (Fig. 5b). The results with GLYX-13 incubation for 15 min were more consistent and produced a significant increase in BDNF release of approximately 50 percent (t(16) = 2.715, p < 0.05) (Fig. 5c). In contrast to these rapid acting agents, incubation with the monoamine reuptake blockers fluoxetine or desipramine (1 hr) did not significantly influence BDNF release (Fig. 5a); the influence of ketamine was examined in the same experiment as a positive control.
Figure 5. Incubation with ketamine, LY341495, or GLYX-13 increases BDNF release: requirement for AMPA receptor activation.
(a) Primary cultures were incubated with ketamine or the typical antidepressants fluoxetine (FLX) or desipramine (DMI) for 1 hr and levels of BDNF in the media were assessed by ELISA. Ketamine, but not fluoxetine or desipramine increased levels of BDNF release. (b, c) Incubation with LY341495 (10 nM, 30 min) or GLYX-13 (3 nM, 15 min) also increased BDNF release. (d, e) Cortical cultures were pretreated with NBQX 20 min prior to treatment with LY341495 (30 min) or GLYX-13 (15 min). Following drug incubations, media was collected and BDNF release was determined. NBQX significantly blocked BDNF release induced by LY341495 or GLYX-13. NBQX on its own did not affect release of BDNF. All results are presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005 compared to vehicle or blockade (ANOVA and Fisher’s post-hoc least significant difference to test for results in a, d, e, and Students t test was used for results in b and c).
To determine if blockade of AMPA receptors also blocks BDNF release, similar to blockade of phospho-ERK stimulation (Figure 3), cells were pretreated with NBQX 20 min prior to drug treatment. ELISA analysis revealed that blockade of AMPA receptors significantly inhibits BDNF release induced by LY341495 (F3,40 = 12.939, p < 0.0001) and GLYX-13 (ANOVA, F3,44 = 11.057, p < 0.0001) (Fig. 5d,e). This extends our previous results demonstrating that NBQX blocks ketamine stimulation of BDNF release (Lepack et al., 2014), and together indicates that BDNF release by these agents is AMPA receptor dependent.
3.5. Role of GABA neurons in regulation of ERK and BDNF
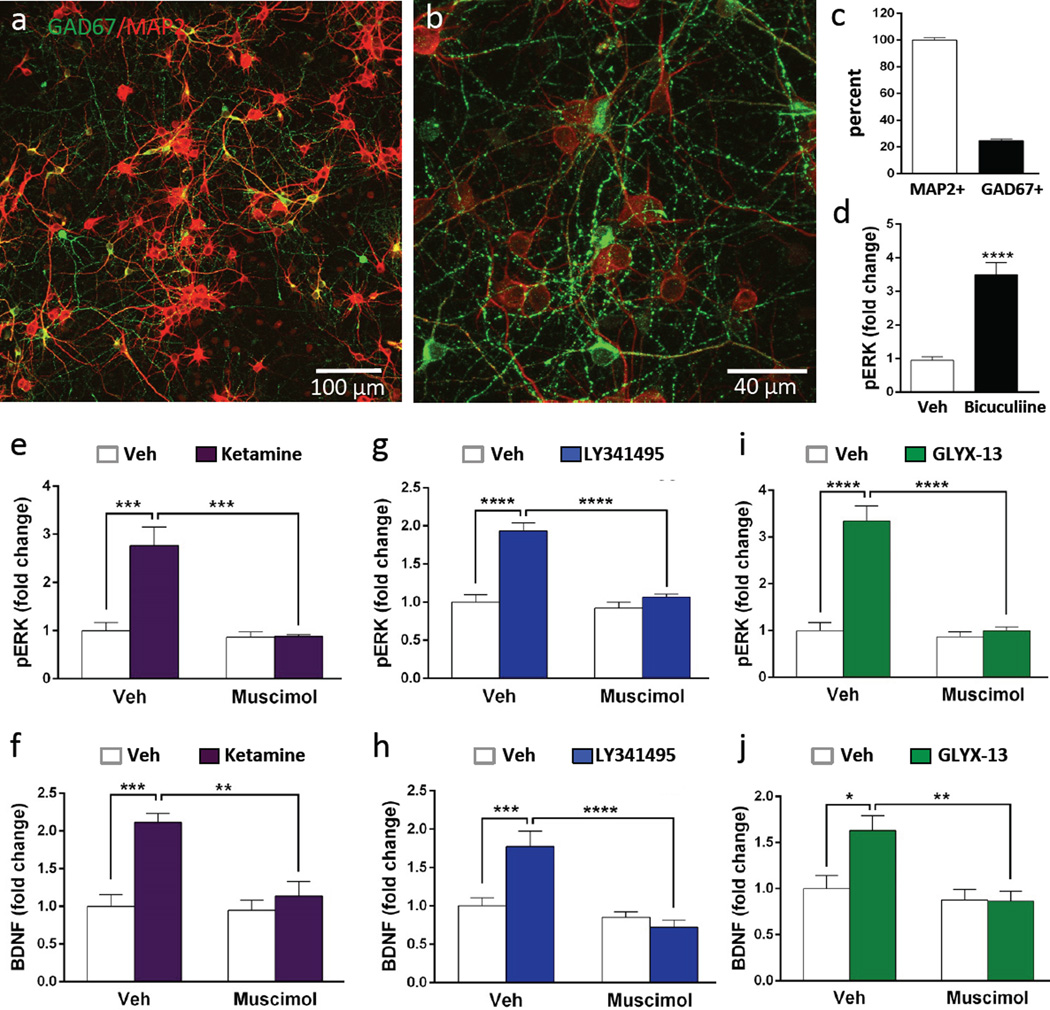
In vivo microdialysis studies demonstrate that ketamine causes a rapid, transient burst of glutamate in the mPFC that is thought to result from blockade of NMDA receptors on GABA interneurons (Homayoun and Moghaddam, 2007; Moghaddam et al., 1997). This is consistent with reports that the behavioral actions of ketamine, as well as LY341495 and GLYX-13 are dependent on AMPA receptor activation (Burgdorf et al., 2013; Dwyer, 2012; Li et al., 2010; Maeng et al., 2008). In the current study we demonstrate that the actions of ketamine, LY341495, and GLYX-13 in vitro are also dependent on AMPA receptor stimulation (Figures 4), consistent with the possibility that stimulation of phospho-ERK and release of BDNF result from increased glutamate transmission. For ketamine and possibly GLYX-13, this could occur via blockade of NMDA receptors on GABA neurons; for LY341495 blockade of mGluR2/3 autoreceptors could increase glutamate release. Primary neuronal culture systems include a mixed population of glutamatergic and GABAergic neurons. To examine the levels of GABA neurons, cells were plated on coverslips and after 10 DIV immunohistochemistry was conducted for the neuronal marker MAP2 and the GABA synthesis marker GAD67 (Fig. 6a, b). The results demonstrate that GAD67 is expressed by a subpopulation of MAP2 positive neurons; quantitative analysis demonstrates that approximately 25% of the total MAP2 positive cells are GAD67 positive (Fig. 6c), consistent with previous reports (Kato-Negishi et al., 2004; Obrietan and van den Pol, 1998; Weir et al., 2015). Examination of higher magnification images also demonstrates punctate GAD67 staining throughout the cultures (Fig. 6b). Double labeling of primary hippocampal cultures also demonstrates a subpopulation of GAD67+ neurons (approximately 10 percent) (Supplemental Figure 1). Further studies of the cell make up of the primary cultures shows a very low level (less than 1 percent) of astrocytes, immunopositive for the marker GFAP (Supplemental Figure 2)
Figure 6. Primary cortical cultures contain GABA interneurons: stimulation of phospho-ERK and BDNF release by rapid-acting antidepressants is blocked by muscimol.
(a, b) Cells plated on coverslips (DIV 10) were fixed and then GAD67 and MAP2 immunolabeling was conducted, demonstrating that a subpopulation of neurons was co-stained for the GABA synthetic marker. Representative low and high power images are shown (scale bars in lower right). (c) The total numbers of GAD67+ cells were determined on 3 separate coverslips and the percentage relative to MAP2+ cells was determined. (d) Incubation with the GABAA receptor antagonist bicuculline (10 µM, 1 hr) significantly increased levels of phospho-ERK (pERK). (e–j) Cultured cells were incubated with the GABAA receptor agonist muscimol (10 µM, 30 min) prior to addition of ketamine, LY341495, or GLYX-13 (1 hr) and levels of pERK were determined by immunoblot analysis (e, g, i), or BDNF release by ELISA (f, h, J). Levels of total ERK and S6K were determined to control for gel loading and membrane transfer. Results are presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005 compared to vehicle or blockade (ANOVA and Fisher’s post-hoc least significant difference test for results).
To examine if GABA neuronal activity influences basal levels of phospho-ERK signaling, the influence of the GABAA receptor antagonist bicuculline was determined. Incubation with bicuculline stimulated phospho-ERK levels (t(10) = 6.693, p < 0.0001) (Fig. 6d), as reported in previous studies, due to blockade of tonically active GABAA receptors that inhibit the activity of glutamatergic neurons (Hardingham et al., 2002; Jeon et al., 2011; Lee et al., 2005). To further test the role of GABA and activity dependent mechanisms in the response to rapid acting antidepressants, cells were pretreated with the GABAA receptor agonist muscimol (10 µM) for 30 min before addition of ketamine, LY341495, or GLYX-13. As shown in earlier figures, a low concentration of ketamine (500 nM, 1 hr) increased levels of phospho-ERK and BDNF release, and these effects were completely blocked by muscimol (ketamine: phospho-ERK, F1,12 = 15.94, p < 0.005; BDNF, F1,20 = 9.139, p < 0.01) (Fig. 6 e, f). Similarly, a low dose of LY341495 (10 nM, 1 hr) or GLYX-13 (3 nM, 1 hr) stimulated phospho-ERK and of BDNF release, and these effects were also completely blocked by muscimol (LY341495: phospho-ERK, F1,20 = 23.04, p < 0.0001; BDNF, F1,32 = 12.15, p < 0.005) (GLYX-13: phospho-ERK, F1,12 = 32.38, p < 0.0001; BDNF, F1,31 = 5.667, p < 0.05 (Fig. 6g–j).
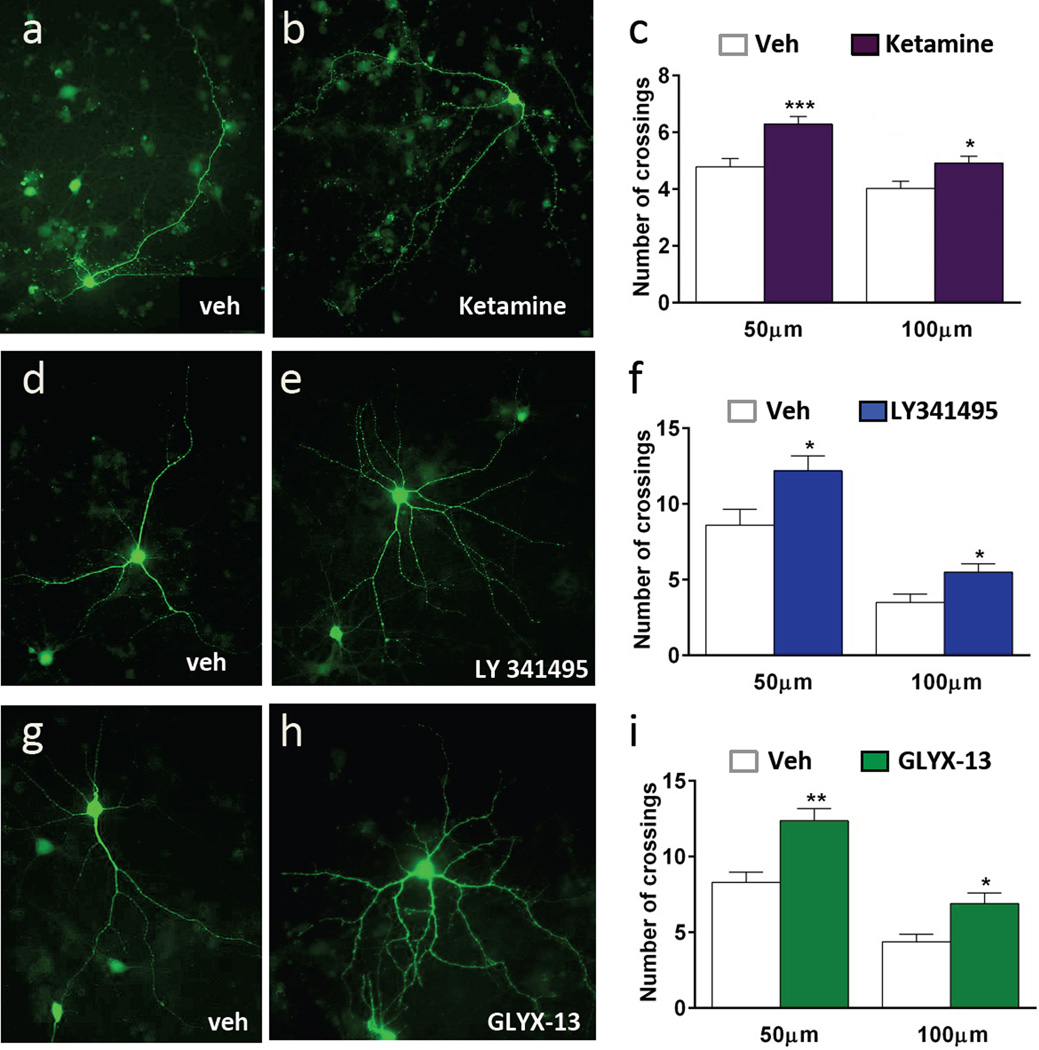
3.6. Ketamine increases neuronal complexity in vitro
Ketamine rapidly increases BDNF release, phospho-ERK and mTORC1 signaling, and this leads to increased spine density and function in vivo (Li et al., 2010). To test whether ketamine incubation increases neuronal complexity in vitro, neurons were plated on coverslips and on DIV 3 were incubated with an AAV2 viral vector that expresses GFP to label neurons. Viral expression and labeling were allowed to proceed for a two-week period and then cells were treated with vehicle, ketamine (500 nM), LY341495 (10 nM), or GLYX-13 (3 nM). After 24 hr to allow for morphological alterations the resulting labeled neurons were analyzed by Sholl analysis. Representative images of GFP labeled neurons are shown for each condition (Figure 7). Ketamine incubation resulted in greater dendrite complexity compared to vehicle treated cells, increasing the number of dendrite branch crossings compared to saline at both 50 and 100 microns from the soma (50 µm, t(79) = 3.808, p < 0.0005; 100 µm, t(79) = 2.511, p < 0.05) (Fig. 7a–c). Similarly, incubation with LY341495 (Fig. 7 d–f) (50 µm, t(18) = −2.491, p < 0.05; 100 µm, t(18) = −2.606, p < 0.05) or GLYX-13 (Fig. 7 g–i) (50 µm, t(22) = 3.906, p < 0.001; 100 µm, t(22) = 3.047, p < 0.01) for 24 hr significantly increased the dendrite complexity of cultured neurons.
Figure 7. Stimulation with rapid-acting antidepressants increases dendrite complexity in neuronal cortical culture.
Cortical cultures were incubated with AAV2-GFP on DIV3 to label neurons for morphological analysis. On DIV 17, cells were incubated with ketamine, LY341495, or GLYX-13 for 24 hr. Cells were fixed and imaged for GFP signal. Representative images of labeled cells are shown for each condition. Sholl analysis demonstrates that ketamine (a–c), LY 341495 (d–f), or GLYX-13 (g–i) increase dendrite complexity of cultured neurons. All results are presented as mean ± SEM. *p < 0.05, **p < 0.005, ***p < 0.0005 compared to vehicle (Students t test).
4. Discussion
The results demonstrate that ketamine as well as two additional functionally divergent glutamatergic rapid-acting antidepressants stimulate phospho-ERK and phospho-S6K, components of the mTORC1-signaling pathway in a concentration and time dependent manner in rat primary cortical cultures. Importantly, these effects are completely blocked by AMPA and TrkB receptor antagonists, and by the neuronal silencing agent muscimol. Furthermore, all three agents tested stimulate BDNF release in an activity dependent manner and increase the complexity of dendrites in cultured cortical neurons.
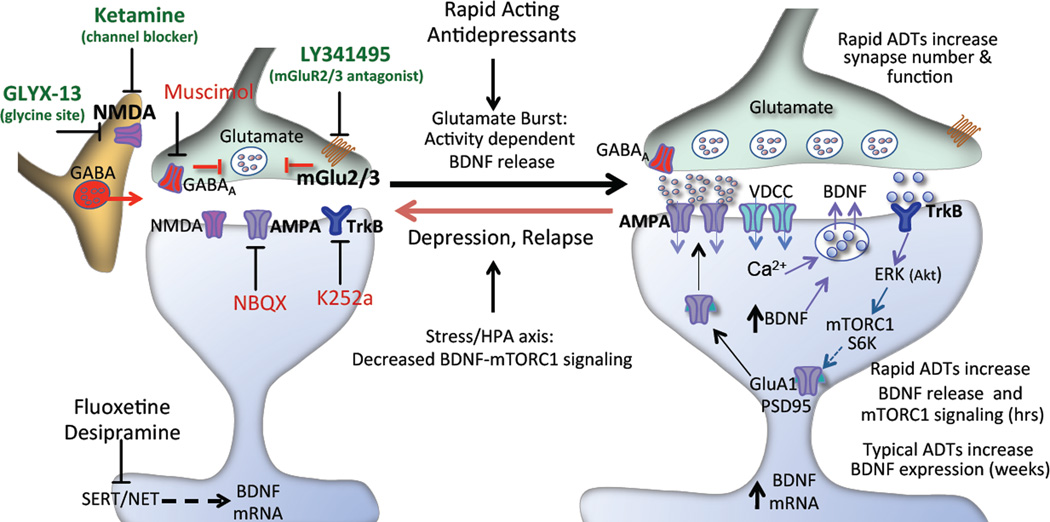
In vivo studies provide indirect evidence that ketamine stimulates a burst of glutamate release that is thought to result from disinhibition of GABAergic transmission, leading to activation of AMPA receptors and neuronal depolarization. This hypothesis is supported by studies demonstrating that ketamine rapidly increases levels of extracellular glutamate (Moghaddam et al., 1997) and decreases the firing of GABAergic interneurons in the mPFC (Homayoun and Moghaddam, 2007). There is also evidence that LY341495 increases extracellular glutamate in the mPFC by blocking presynaptic mGluR2/3 receptors that control glutamate release (Hascup et al., 2010; Xi et al., 2002) (see model, Figure 8). The influence of GLYX-13 on extracellular glutamate has not been reported, but the behavioral effects of GLYX-13, as well as ketamine and LY341495 are inhibited by AMPA receptor blockade (Dwyer et al., 2012; Karasawa et al., 2005; Li et al., 2010; Maeng et al., 2008). The results of the current in vitro study demonstrate that low concentrations of ketamine, LY341495, and GLYX-13 stimulate phospho-ERK and phospho-S6K in an activity dependent manner, as these effects are blocked by incubation with an AMPA receptor antagonist. It is also notable that higher concentrations of ketamine (1 to 10 µM) and GLYX-12 (0.01 to 1 µM) did not increase phospho-ERK and phospho-S6K. Similar biphasic dose dependent, antidepressant behavioral effects of ketamine and GLYX-13 have been reported (Burgdorf et al., 2013; Li et al., 2010), and are thought to result from blockade of postsynaptic NMDA receptors at higher doses. In contrast to the rapid effects of these agents, incubation with the typical monoaminergic antidepressants fluoxetine and desipramine for 1 hr did not influence phospho-ERK and phospho-S6K, demonstrating specificity of this signaling to the rapid acting antidepressants within this short timeframe. This is consistent with previous studies demonstrating that acute or chronic administration of fluoxetine or imipramine has no effect mTORC1 signaling in PFC (Li et al., 2010; Liu et al., 2015), although chronic fluoxetine administration reverses the down-regulation of mTORC1 signaling in mice exposed to chronic stress (Liu et al., 2015). Primary neuronal culture studies also report no effect of fluoxetine or imipramine on mTORC1 signaling, although incubation with high doses of escitalopram and paroxetine for 4 days reversed the down regulation of mTORC1 signaling caused by incubation with B27 depleted media (Park et al., 2014).
Figure 8. Schematic of the molecular mechanisms underlying rapid-acting antidepressants.
Rapid-acting antidepressants, including ketamine, LY341495, and GLYX-13 increase phospho-ERK and BDNF release via an activity dependent mechanism. This could occur via inhibition of NMDA receptors on tonic firing GABAergic neurons resulting in disinhibition of glutamatergic activity and a burst of extracellular glutamate. LY341495 blocks mGlur2/3 receptors that can be located on glutamate presynaptic terminals and regulate glutamate release. Increased release of glutamate leads to activation of AMPA receptors and depolarization of postsynaptic neurons resulting in activation of voltage dependent calcium channels (VDCCs) and subsequent BDNF release and TrkB receptor activation. TrkB receptors stimulate ERK and the mTORC1 signaling pathway, including pS6K resulting in increased translation of synaptic proteins, such as GluR1 and PSD95 required for synaptogenesis. The activity dependent action of these agents was confirmed in the current study by demonstrating that the neuronal silencing agent muscimol and the AMPA antagonist NBQX block the increase in phospho-ERK and BDNF release. A role for BDNF-TrkB signaling was demonstrated by blockade with the TrkB antagonist K252a.
Previous reports have provided evidence that the synaptic and antidepressant behavioral actions of ketamine are dependent on BDNF. This includes studies demonstrating that the responses to ketamine are blocked in BDNF conditional deletion mutant mice and BDNF Val66Met knockin mice (Autry et al., 2011; Liu et al., 2012). The BDNF Met polymorphism blocks the processing and release of BDNF, suggesting that the antidepressant actions of ketamine require BDNF release, not just synthesis (Liu et al., 2012). Clinical studies also demonstrate that patients carrying the Met polymorphism have a significantly reduced response to ketamine (Laje et al., 2012). Further support for BDNF release is provided by our recent report that infusion of a BDNF neutralizing antibody into the mPFC blocks the antidepressant behavioral actions of ketamine (Lepack et al., 2014). In the latter study we also reported that ketamine stimulates the release of BDNF in primary neuronal cultures, and that this effect was blocked by incubation with NBQX (Lepack et al., 2014). The results of the current study extend these findings and demonstrate that LY341495 and GLYX-13 also rapidly increase the release of BDNF in primary cortical cultures and that these effects are dependent on AMPA receptor activation, similar to ketamine. In contrast, incubation with the typical antidepressant fluoxetine and desipramine for a similar amount of time had no effect on BDNF release. Previous studies demonstrate that chronic administration of typical antidepressants such as fluoxetine and desipramine increase BDNF expression in rat cortical and hippocampal brain regions via up-regulation of the cAMP-CREB cascade (Carlezon et al., 2005; Duman and Monteggia, 2006). In addition, a previous study reported that incubation with fluoxetine for 4 days increased BDNF levels in cultured neurons, but only in B27 depleted media, indicating that this more long-term fluoxetine exposure accelerated the recovery from growth medium deprivation (Seo et al., 2014).
The requirement for AMPA receptor activity in the stimulation of phospho-ERK and BDNF release indicates that these rapid acting agents increase glutamate transmission and neuronal depolarization. Increased release of glutamate could occur via inhibition of tonic firing GABA neurons and/or blockade of presynaptic mGlu2/3 autoreceptors. The possibility that GABAergic neurons could exert tonic inhibitory control over glutamate signaling is supported by the presence of GABA neurons, immunostained for GAD67, in the primary cortical cultures of approximately 25 percent. Previous studies have reported a similar level of GABA neurons, although the percent varies depending on the culture conditions (Kato-Negishi et al., 2004; Obrietan and van den Pol, 1998; Weir et al., 2015). Higher magnification also shows punctate GAD67+ staining in close proximity to MAP2+ cells, suggesting extensive GABA synaptic contacts with glutamate neurons. Evidence for tonic firing of GABA neurons is provided by results demonstrating that the GABAA antagonist bicuculline increases levels of phospho- ERK, as previously reported (Hardingham et al., 2002; Jeon et al., 2011; Lee et al., 2005). In addition, the results demonstrate that the GABAA agonist muscimol completely blocks the stimulation of phospho-ERK and the release of BDNF by ketamine, LY341495, and GLYX-13. We also observed low levels of GFAP labeled astrocytes, another important source of BDNF that could contribute to the signaling effects of rapid acting antidepressants (Myamoto et al., 2015). Together these findings provide further evidence that the actions of these agents require neuronal activity, and suggest a role for GABA interneurons.
Studies in rodent models have demonstrated that ketamine rapidly increases the number and function of spine synapses in the mPFC, and that these effects are blocked in BDNF Met knockin mice (Li et al., 2010; Liu et al., 2012). A recent report demonstrates that GLYX-13 also causes a rapid increase in the number of spine synapses in the PFC (Burgdorf et al., 2015a). The ability of rapid-acting antidepressants to cause fast release of BDNF in primary neuronal cultures is consistent with the possibility that these agents stimulate dendrite complexity. The results of the current study provide direct evidence for this possibility, demonstrating that incubations with low concentrations of ketamine, LY341495 or GLYX-13 increase dendrite complexity in primary neuronal cultures.
In summary, the results of these studies of mixed glutamate/GABA neuronal cortical cultures demonstrate that low doses of ketamine, as well as LY341495 and GLYX-13, rapidly stimulate phospho- ERK, phospho-S6K, and BDNF release and that these effects are dependent on neuronal activity and AMPA receptor signaling. In contrast, the typical antidepressants fluoxetine and desipramine do not stimulate phospho-ERK, phospho-S6K, and BDNF release within this rapid time frame, providing pharmacological specificity for the rapid agents. It has also been hypothesized that the rapid actions of ketamine in vivo are activity independent and result from NMDA receptor dependent homeostatic responses that increase the expression of BDNF in the absence of neuronal activity (Autry et al., 2011; Kavalali and Monteggia, 2015; Miller et al., 2016). Further studies will be required to determine the exact role of GABAergic neurons vs. direct effects on glutamatergic neurons in the rapid AMPA-BDNF dependent actions of these agents. The in vitro cortical culture system provides a simplified, readily accessible approach to test cellular mechanisms using pharmacological, molecular or genetic tools. For example, cortical cultures derived from mouse lines with GABA vs. glutamate neuron specific promoters can be used to monitor the activity and manipulate the function of each population of neurons. In addition, the role of BDNF, mTORC1, and neuronal activity in the morphological/dendritic actions of these agents can be tested. Although the results demonstrate that mixed cortical primary cultures can be used to test certain mechanisms of rapid acting antidepressants, this is a model system and the findings will have to be confirmed by in vivo studies. Further understanding of the mechanisms specific to rapid-acting antidepressants in vitro as well as in vivo will be critical to the development of novel therapeutic agents for the treatment of depression.
Supplementary Material
(a,b) Incubation with ketamine (500 nM, 1 hr) increased levels of phosphor-ERK (pERK) and phosphor-S6K (pS6K); representative immunoblots are shown. Levels of total ERK and S6K were determined to control for gel loading and membrane transfer. Results are presented as mean ± SEM., ***p < 0.0005 compared to vehicle (Students t test). (c) Hippocampus derived cells were plated on coverslips and on DIV 10 were fixed and then GAD67 and MAP2 immunolabeling was conducted, demonstrating that a subpopulation of neurons was co-stained for the GABA synthetic marker (scale bar in lower right). (d) The total numbers of GAD67+ cells were determined on 3 separate coverslips and the percentage relative to MAP2+ cells was determined
Cortical derived cells were plated on coverslips and on DIV 10 were fixed and then GFAP and MAP2 immunolabeling was conducted, demonstrating the presence of a small population of astrocytes (less than 1 percent).
Highlights.
Rapid acting antidepressants increase ERK and BDNF release in primary cortical cultures.
Increased ERK signaling and BDNF release are dependent on neuronal activity.
Rapid acting antidepressants increase dendrite complexity of cortical neurons in culture.
Primary cortical cultures are a simple model system for studies of rapid acting antidepressants.
Acknowledgments
This work is supported by NIMH R37MH45481 (RSD), NIMH R01MH93897 (RSD), the State of Connecticut, and Yale University.
Competing financial interests
The authors list the following interests: R.S. Duman has consulted and/or received research support from Naurex, Lilly, Forest, Johnson & Johnson, Taisho, and Sunovion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural anatidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Burgdorf J, Zhang X, Nicholson K, Balster R, Leander J, Stanton P, Gross A, Kroes R, Moska lJ. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacol. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang X, Weiss C, Gross A, Boikess S, Kroes R, Khan M, Burch R, Rex C, Disterhoft J, Stanton P, Moskal J. The long-lasting antidepressant effects of rapastinel (GLYX-13) are associated with a metaplasticity process in the medial prefrontal cortex and hippocampus. Neurosci. 2015a;308:202–211. doi: 10.1016/j.neuroscience.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK, Gross AL, Kroes RA, Moskal JR. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Dwyer J, Lepack AE, Duman RS. mTOR activation is required for the antidepressants effects of mGluR2/3 blockade. Int J Neuropsychopharmacol. 2012;15:429–434. doi: 10.1017/S1461145711001702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Maldonado-Aviles JG, Lepack AE, DiLeone RJ, Duman RS. Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. PNAS. 2015;112:6188–6193. doi: 10.1073/pnas.1505289112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham G, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hascup ER, Hascup KN, Stephens M, Pomerleau F, Huettl P, Gratton A, Gerhardt GA. Rapid microelectrode measurements and the origin and regulation of extracellular glutamate in rat prefrontal cortex. J Neurochem. 2010;115:1608–1620. doi: 10.1111/j.1471-4159.2010.07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon S, Rhee S, Seo J, Bak H, Lee S, Ryu J, Cheong J, Shin C, Kim G, Lee Y, Ko K. Oroxylin A increases BDNF production by activation of MAPK-CREB pathway in rat primary cortical neuronal culture. Neurosci Res. 2011;69:214–222. doi: 10.1016/j.neures.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Karasawa J, Shimazaki T, Kawashima N, Chaki S. AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res. 2005;1042:92–98. doi: 10.1016/j.brainres.2005.02.032. [DOI] [PubMed] [Google Scholar]
- Kato-Negishi M, Muramoto K, Kawahara M, Kuroda Y, Ichikawa M. Developmental changes of GABAergic synapses formed between primarycultured cortical neurons. Brain Res Dev Brain Res. 2004;152:99–108. doi: 10.1016/j.devbrainres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM. How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol. 2015;20:35–39. doi: 10.1016/j.coph.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS National Comorbidity Survey, R. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011;61:1419–1423. doi: 10.1016/j.neuropharm.2011.08.034. [DOI] [PubMed] [Google Scholar]
- Laje G, Lally N, Mathews D, Brutsche N, Chemerinski A, Akula N, Kelmendi B, Simen A, McMahon FJ, Sanacora G, Zarate C., Jr Brain-derived neurotrophic factor Val66Met polymorphism and antidepressant efficacy of ketamine in depressed patients. Biol Psychiatry. 2012;72:e27–e28. doi: 10.1016/j.biopsych.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Butcher G, Hoyt K, Impey S, K O. Activity-dependent neuroprotection and cAMP response element-binding pro- tein (CREB): kinase coupling, stimulus intensity, and temporal regulation of CREB phosphorylation at serine 133. J Neurosci. 2005;25:1137–1148. doi: 10.1523/JNEUROSCI.4288-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack A, Fuchikami M, Dwyer J, Banasr M, Aghajanian G, Duman R. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, Duman RS. BDNF release is required for the behavioral actions of ketamine. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, Aghajanian GK. Brain-derived neurotrophic factor Val66M alllele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XL, Luo L, Mu RH, Liu BB, Geng D, Liu Q, Yi LT. Fluoxetine regulates mTOR signalling in a region-dependent manner in depression-like mice. Sci Rep. 2015;5:16024. doi: 10.1038/srep16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Wang C, Xue Z, Li C, Zhang J, Zhao X, Liu A, Wang Q, Zhou W. PI3K/AKT/mTOR signaling-mediated neuropeptide VGF in the hippocampus of mice is involved in the rapid onset antidepressant-like effects of GLYX-13. Int J Neuropsychopharmacol. 2015;18 doi: 10.1093/ijnp/pyu110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Miller O, Moran J, Hall B. Two cellular hypotheses explaining the initiation of ketamine's antidepressant actions: Direct inhibition and disinhibition. Neuropharmacol. 2016;100:17–26. doi: 10.1016/j.neuropharm.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskal J, Kuo A, Weiss C, Wood P, O'Connor Hansen A, Kelso S, Harris R, Disterhoft J. GLYX-13: a monoclonal antibody-derived peptide that acts as an N-methyl-D-aspartate receptor modulator. Neuropharmacol. 2005;49:1077–1087. doi: 10.1016/j.neuropharm.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, Itoh K, Lo EK, Lok J, Ihara M, Arai K. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J. Neurosci. 2015;35:14002–14008. doi: 10.1523/JNEUROSCI.1592-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrietan K, van den Pol A. GABAB receptor-mediated inhibition of GABAA receptor calcium elevations in developing hypothalamic neurons. J Neurophysiol. 1998;79:1360–1370. doi: 10.1152/jn.1998.79.3.1360. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee JG, Seo MK, Lee CH, Cho HY, Lee BJ, Seol W, Kim YH. Differential effects of antidepressant drugs on mTOR signalling in rat hippocampal neurons. Int. J. Neuropsychopharmacol. 2014 Nov;17(11):1831–1846. doi: 10.1017/S1461145714000534. 2014. [DOI] [PubMed] [Google Scholar]
- Preskorn S, Macaluso M, Mehra DO, Zammit G, Moskal JR, Burch RM, Group G-CS. Randomized proof of concept trial of GLYX-13, an N-methyl-D-aspartate receptor glycine site partial agonist, in major depressive disorder nonresponsive to a previous antidepressant agent. J Psychiatr Pract. 2015;21:140–149. doi: 10.1097/01.pra.0000462606.17725.93. [DOI] [PubMed] [Google Scholar]
- Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Lee C, Cho HY, Lee J, Lee B, Kim J, Seol W, Kim Y, Park S. Effects of antidepressant drugs on synaptic protein levels and dendritic outgrowth in hippocampal neuronal cultures. Neuropharmacol. 2014;79:222–233. doi: 10.1016/j.neuropharm.2013.11.019. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- Weir K, Blanquie O, Kilb W, Luhmann H, Sinning A. Comparison of spike parameters from optically identified GABAergic and glutamatergic neurons in sparse cortical cultures. Front Cell Neurosci. 2015;8:460. doi: 10.3389/fncel.2014.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R, Arnold M, Wheeler W, Ornstein P, Schoepp D. [3H]LY341495 binding to group II metabotropic glutamate receptors in rat brain. JPET. 2001;298:453–460. [PubMed] [Google Scholar]
- Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther. 2002;300:162–171. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a,b) Incubation with ketamine (500 nM, 1 hr) increased levels of phosphor-ERK (pERK) and phosphor-S6K (pS6K); representative immunoblots are shown. Levels of total ERK and S6K were determined to control for gel loading and membrane transfer. Results are presented as mean ± SEM., ***p < 0.0005 compared to vehicle (Students t test). (c) Hippocampus derived cells were plated on coverslips and on DIV 10 were fixed and then GAD67 and MAP2 immunolabeling was conducted, demonstrating that a subpopulation of neurons was co-stained for the GABA synthetic marker (scale bar in lower right). (d) The total numbers of GAD67+ cells were determined on 3 separate coverslips and the percentage relative to MAP2+ cells was determined
Cortical derived cells were plated on coverslips and on DIV 10 were fixed and then GFAP and MAP2 immunolabeling was conducted, demonstrating the presence of a small population of astrocytes (less than 1 percent).