Abstract
The mechanisms underlying the postnatal maturation of micturition from a somatovesical to a vesicovesical reflex are not known but may involve neuropeptides in the lower urinary tract. A transgenic mouse model with chronic urothelial overexpression (OE) of NGF exhibited increased voiding frequency, increased number of non-voiding contractions, altered morphology and hyperinnervation of the urinary bladder by peptidergic (e.g., Sub P and CGRP) nerve fibers in the adult. In early postnatal and adult NGF-OE mice we have now examined: (1) micturition onset using filter paper void assays and open-outlet, continuous fill, conscious cystometry; (2) innervation and neurochemical coding of the suburothelial plexus of the urinary bladder using immunohistochemistry and semi-quantitative image analyses; (3) neuropeptide protein and transcript expression in urinary bladder of postnatal and adult NGF-OE mice using Q-PCR and ELISAs and (4) the effects of intravesical instillation of a neurokinin (NK)-1 receptor antagonist on bladder function in postnatal and adult NGF-OE mice using conscious cystometry. Postnatal NGF-OE mice exhibit age-dependent (R2= 0.996–0.998; p ≤ 0.01) increases in Sub and CGRP expression in the urothelium and significantly (p ≤ 0.01) increased peptidergic hyperinnervation of the suburothelial nerve plexus. By as early as P7, NGF-OE mice exhibit a vesicovesical reflex in response to intravesical instillation of saline whereas littermate WT mice require perigenital stimulation to elicit a micturition reflex until P13 when vesicovesical reflexes are first observed. Intravesical instillation of a NK-1 receptor antagonist, netupitant (0.1 μg/ml), significantly (p ≤ 0.01) increased void volume and the interval between micturition events with no effects on bladder pressure (baseline, threshold, peak) in postnatal NGF-OE mice; effects on WT mice were few. NGF-induced pleiotropic effects on neuropeptide (e.g., Sub P) expression in the urinary bladder contribute to the maturation of the micturition reflex and are excitatory to the micturition reflex in postnatal NGF-OE mice. These studies provide insight into the mechanisms that contribute to the postnatal development of the micturition reflex.
Keywords: postnatal development, Substance P, neurokinin-1 receptor antagonist, suburothelial nerve plexus
Introduction
In neonates of many species, the neural control of micturition undergoes marked changes during early postnatal development (de Groat, 1975, 1990, 1993; de Groat and Araki, 1999; de Groat and Ryall, 1969; Maggi et al., 1986). In newborn rats and cats, micturition is dependent upon a spinal reflex pathway that is activated when the mother licks the perineal region of the young animal (perineal-to-bladder reflex) (de Groat and Araki, 1999; Maggi et al., 1986). This reflex pathway consists of a somatic afferent limb in the pudendal nerve and a parasympathetic efferent limb in the pelvic nerve. Similar reflexes have been identified in human infants (Boehm and Haynes, 1966). As the CNS matures, a spinobulbospinal reflex pathway that is responsible for micturition in adult animals gradually replaces the spinal micturition reflex (de Groat, 1975, 1990, 1993; de Groat and Araki, 1999; de Groat and Ryall, 1969; Maggi et al., 1986). The spinobulbospinal micturition reflex is triggered by mechanoreceptors in the bladder and begins to elicit voiding in the rat between postnatal days (P) 16–18 (Capek and Jelinek, 1956; Maggi et al., 1986; Ng et al., 2007; Zvarova and Zvara, 2012) and continues to mature during postnatal weeks 3–6 (Capek and Jelinek, 1956; Ng et al., 2007). Studies demonstrate (Sugaya et al., 1997) that supraspinal pathways involved in adult micturition reflex patterns exist in neonatal animals but may not be functional or may function in an inhibitory manner prior to the emergence of a functional, adult micturition pattern (de Groat et al., 1998). Studies in human infants also support an anatomical connection to the cerebral cortex in advance of conscious or voluntary voiding (Sillen, 2001).
The mechanisms underlying the postnatal maturation of micturition are not known but it has been suggested that maturation of voiding function involves prominent reorganization of synaptic connections in bladder reflex pathways. This reorganization leads to down regulation of primitive spinal mechanisms and upregulation of mature supraspinal pathways (de Groat et al., 1998; de Groat and Araki, 1999). Previous studies have suggested the importance of neuroactive compounds in the process of maturation of the micturition reflexes during prenatal and early postnatal development (Ekstrom et al., 1994; Iuchi et al., 1994; Maggi et al., 1986; Sann et al., 1997). Neurochemical plasticity in central and peripheral micturition reflex pathways has been demonstrated with neural injury, disease, inflammation and during postnatal development (Arms and Vizzard, 2011; Corrow et al., 2010; de Groat et al., 1998; de Groat and Yoshimura, 2012; Merrill et al., 2013; Merrill et al., 2012; Merrill and Vizzard, 2014). For example, we have demonstrated neurochemical plasticity in neuropeptides (e.g., Sub P, CGRP, PACAP) after bladder inflammation in bladder afferent cells in DRG (Braas et al., 2006; Vizzard, 2000d, 2001) and in spinal segments involved in micturition reflexes (Vizzard, 2000a, c, d). Bladder inflammation and postnatal maturation also altered expression of neurotrophic factors (NTF), including NGF, in the urinary bladder (Vizzard, 2000b). NGF expression in the urinary bladder may directly affect urinary bladder maturation, urinary bladder function and micturition reflex pathways; however, pleiotropic changes resulting from increased NGF expression may also play a role (Girard et al., 2010; Girard et al., 2011; Zvara and Vizzard, 2007). For example, numerous studies have demonstrated the NGF-dependence of neuropeptide expression (e.g., Sub P and CGRP) in sensory neurons (Dmitrieva and McMahon, 1996; McMahon, 1996; Ribeiro-da-Silva et al., 2000; Zvara and Vizzard, 2007).
We recently characterized a transgenic mouse model of chronic, urothelium-specific NGF-overexpression that represents a novel approach to exploring the role of NGF in urinary bladder inflammation and sensory function (Schnegelsberg et al., 2010). Functionally, NGF-OE mice exhibit frequent voiding, the presence of non-voiding contractions (NVCs) during the bladder filling phase and referred somatic pelvic hypersensitivity (Schnegelsberg et al., 2010). In addition, NGF-OE mice exhibit changes in the morphology and neurochemistry of the urinary bladder and increases in inflammatory cell infiltrates to the urinary bladder (Schnegelsberg et al., 2010). Neurochemical changes include increased expression of Sub P and CGRP in the suburothelial nerve plexus of the urinary bladder (Schnegelsberg et al., 2010). Our findings support and extend many previous studies in rodents demonstrating involvement of NGF in altered bladder sensory function and the development of referred hyperalgesia in response to bladder inflammation (Dmitrieva and McMahon, 1996; Guerios et al., 2006; 2008; Hu et al., 2005; Jaggar et al., 1999; Lamb et al., 2004; Zvara and Vizzard, 2007). We now expand upon these findings and begin to identify mechanisms that drive development of bladder innervation and function. We investigated the contributions of NGF and the NGF-dependence of neuropeptide expression in the developing bladder to the maturation of voiding reflexes.
Materials and Methods
Animals
NGF-OE mice
NGF-OE transgenic mice were generated at Roche Palo Alto (material transfer agreement with Roche Palo Alto and Dr. Debra Cockayne) in collaboration with Dr. Henry Sun at New York University Medical School as previously described (Schnegelsberg et al., 2010) (Girard et al., 2011) (Girard et al., 2010). Animal genotype was confirmed by Southern and/or PCR analyses; all mice have the inbred genetic C57BL/6J background and were derived from F10 to F12 generations maintained through a hemizygous backcross strategy with C57BL/6J wildtype (WT) mice. Mice used in this study were bred locally at the University of Vermont College of Medicine. The litters were of normal size and weight and behaviors (feeding, drinking, activity patterns) appeared normal. Female and male mice of varying postnatal (P) age (P0-Adult (A)) from multiple dams were used to examine postnatal maturation of voiding reflexes. In these studies, P0 is defined as day of birth. As previously demonstrated (Girard et al., 2010; 2011; Schnegelsberg et al., 2010) and confirmed in this study, urinary bladder weight was significantly (p ≤ 0.01) increased in NGF-OE mice compared to littermate WT mice (Table 1). Wistar rats (Charles River Canada, St. Constant, Quebec) of both sexes and various postnatal ages (P0-A) were used to determine urination onset and to serve as a comparison for WT and NGF-OE mice. All experimental protocols involving animal use were approved by the University of Vermont Institutional Animal Care and Use Committee (IACUC # 08-085). Animal care was under the supervision of the University of Vermont’s Office of Animal Care Management in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress or distress. Separate groups of littermate WT and NGF-OE mice were used in the following experiments. Both male and female NGF-OE and WT mice and Wistar rats were used in these studies.
Table 1.
Urinary bladder and body weights of WT and NGF-OE transgenic mice during postnatal development. Values are mean ± S.E.M. for WT and NGF-OE male (M) and female (F) mice of both sexes from postnatal (P) day 0-Adult (A)
Urinary bladder, body weight (Wt) and bladder Wt/Body Wt of littermate WT and NGF-OE transgenic male (M) and female (F) mice during postnatal (P) (P0, P7, P14, P21, P28, P36, Adult (A) development. Values are means ± S.E.M.
| N | Sex | Age | Genotype | Bladder Wt, g | Body Wt, g | Bladder Wt/Body Wt |
|---|---|---|---|---|---|---|
| 5 | F | P0 | WT | 0.009 ± 0.001 | 1.80 ± 0.24 | 5.00 × 10–3 ± 0.42 × 10–2 |
| 5 | F | P0 | NGF-OE | 0.028 ± 0.002* | 1.92 ± 0.23 | 1.45 × 10–2 ± 0.87 × 10–2* |
| 5 | M | P0 | WT | 0.011 ± 0.001 | 1.84 ± 0.34 | 5.91 × 10–3 ± 0.29 × 10–2 |
| 5 | M | P0 | NGF-OE | 0.033 ± 0.004* | 1.91 ± 0.33 | 1.72 × 10–2 ± 1.21 × 10–2* |
| 5 | F | P7 | WT | 0.019 ± 0.001 | 3.43 ± 0.25 | 5.50 × 10–3 ± 0.40 × 10–2 |
| 5 | F | P7 | NGF-OE | 0.057 ± 0.003* | 3.45 ± 0.22 | 1.65 × 10–2 ± 1.36 × 10–2* |
| 5 | M | P7 | WT | 0.020 ± 0.002 | 3.80 ± 0.19 | 5.30 × 10–3 ± 1.05 × 10–2 |
| 5 | M | P7 | NGF-OE | 0.061 ± 0.003* | 3.51 ± 0.33 | 1.73 × 10–2 ± 0.09 × 10–3* |
| 5 | F | P14 | WT | 0.021 ± 0.001 | 7.35 ± 0.24 | 2.90 × 10–3 ± 0.42 × 10–2 |
| 5 | F | P14 | NGF-OE | 0.063 ± 0.002* | 7.24 ± 0.30 | 8.70 × 10–3 ± 0.67 × 10–2* |
| 5 | M | P14 | WT | 0.022 ± 0.001 | 7.14 ± 0.35 | 3.11 × 10–3 ± 0.29 × 10–2 |
| 5 | M | P14 | NGF-OE | 0.073 ± 0.002* | 7.66 ± 0.37 | 9.20 × 10–3 ± 0.54 × 10–2* |
| 5 | F | P21 | WT | 0.022 ± 0.002 | 11.20 ± 0.24 | 1.97 × 10–3 ± 0.83 × 10–2 |
| 5 | F | P21 | NGF-OE | 0.066 ± 0.003* | 11.22 ± 0.50 | 5.89 × 10–3 ± 0.60 × 10–2* |
| 5 | M | P21 | WT | 0.021 ± 0.002 | 11.14 ± 0.40 | 1.89 × 10–3 ± 0.50 × 10–2 |
| 5 | M | P21 | NGF-OE | 0.069 ± 0.002* | 11.35 ± 0.37 | 6.10 × 10–3 ± 0.54 × 10–2* |
| 5 | F | P28 | WT | 0.026 ± 0.001 | 14.23 ± 0.24 | 1.80 × 10–3 ± 0.41 × 10–2 |
| 5 | F | P28 | NGF-OE | 0.078 ± 0.003* | 14.92 ± 0.43 | 5.20 × 10–3 ± 0.69 × 10–2* |
| 5 | M | P28 | WT | 0.028 ± 0.002 | 14.34 ± 0.33 | 1.95 × 10–3 ± 0.61 × 10–2 |
| 5 | M | P28 | NGF-OE | 0.089 ± 0.005* | 14.95 ± 0.27 | 5.95 × 10–3 ± 1.85 × 10–2* |
| 5 | F | P36 | WT | 0.028 ± 0.001 | 20.14 ± 0.24 | 1.39 × 10–3 ± 0.42 × 10–2 |
| 5 | F | P36 | NGF-OE | 0.084 ± 0.002* | 20.12 ± 0.30 | 4.17 × 10–3 ± 0.67 × 10–2* |
| 5 | M | P36 | WT | 0.030 ± 0.002 | 20.86 ± 0.34 | 1.44 × 10–3 ± 0.59 × 10–2 |
| 5 | M | P36 | NGF-OE | 0.099 ± 0.015* | 20.25 ± 0.27 | 4.88 × 10–3 ± 0.56 × 10–1* |
| 5 | F | A | WT | 0.041 ± 0.001 | 25.30 ± 0.34 | 1.62 × 10–3 ± 0.29 × 10–3 |
| 5 | F | A | NGF-OE | 0.127 ± 0.002* | 25.92 ± 0.30 | 4.89 × 10–3 ± 0.67 × 10–2* |
| 5 | M | A | WT | 0.042 ± 0.002 | 25.14 ± 0.22 | 1.67 × 10–3 ± 0.91 × 10–2 |
| 5 | M | A | NGF-OE | 0.126 ± 0.004* | 26.22 ± 0.37 | 4.81 × 10–3 ± 0.11 × 10–1* |
Real-Time Quantitative Reverse Transcription-Polymerase Chain Reaction (Q-PCR)
Determination of NGF, Sub P, and CGRP transcript expression in the urinary bladder (urothelium, detrusor) of NGF-OE transgenic mice (n=6–8 for each age) and littermate WT mice (n=6–8 for each age) was determined using Q-PCR as previously described (Girard et al., 2010; 2011; Schnegelsberg et al., 2010). Total RNA was extracted using the STAT-60 total RNA/mRNA isolation reagent (Tel-Test‘B’, Friendswood, TX, USA) as previously described (Girard et al., 2010; 2011; Schnegelsberg et al., 2010). One μg of RNA per sample was used to synthesize complementary DNA using a mix of random hexamer and oligo dT primers with M-MLV reverse transcriptase (Promega Corp.) in a 25-μl final reaction volume. The quantitative PCR standards for all transcripts were prepared with the amplified cDNA products ligated directly into pCR2.1 TOPO vector using the TOPO TA cloning kit (Invitrogen). The nucleotide sequences of the inserts were verified by automated fluorescent dideoxy dye terminator sequencing (Vermont Cancer Center DNA Analysis Facility). To estimate the relative expression of the receptor transcripts, 10-fold serial dilutions of stock plasmids were prepared as quantitative standards. The range of standard concentrations was determined empirically. Complementary DNA templates, diluted 10-fold to minimize the inhibitory effects of the reverse transcription reaction components, were assayed using HotStart-IT SYBR Green qPCR Master Mix (USB, Cleveland, OH, USA) and 300 nM of each primer in a final 25 μl reaction volume.
Real-time quantitative PCR was performed on an Applied Biosystems 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA, USA)(Girard et al., 2002) using the following standard conditions: (1) serial heating at 94 °C for 2 min and (2) amplification over 45 cycles at 94 °C for 15 s and 60–64°C depending on primers set for 30 s. The amplified product from these amplification parameters was subjected to SYBR Green I melting analysis by ramping the temperature of the reaction samples from 60 to 95 °C. A single DNA melting profile was observed under these dissociation assay conditions demonstrating the amplification of a single unique product free of primer dimers or other anomalous products. Oligonucleotide primer sequences were: mouse NGF: forward primer (5′-AGTGAGGTGCATAGCGTAAT-3′); reverse primer (5′-CACATTGGTGGGAACAAA-3′); mouse CGRP: forward primer (5′-ATCCTGCAACACTGCCA -3′); reverse primer (5′-CACATTGGTGGGAACAAA-3′); Substance P (Sub P): forward primer (5′-CGGTGCCAACGATGATCTAAA -3′); reverse primer (5′-ACGCCTTCTTTCGTACTTCTG -3′). L32 primer sequences have been previously reported (Klinger et al., 2008).
For data analyses, a standard curve was constructed by amplification of serially diluted plasmids containing the target sequence. Data were analyzed at the termination of each assay using sequence detection software (Sequence Detection Software, version 1.3.1; Applied Biosystems, Norwalk, CT, USA). In standard assays, default baseline settings were selected. The increase in SYBR Green I fluorescence intensity (ΔRn) was plotted as a function of cycle number and the threshold cycle was determined by the software as the amplification cycle at which the ΔRn first intersects the established baseline. All data are expressed as the relative quantity of the gene of interest normalized to the relative quantity of the housekeeping gene L32.
Split bladder preparation and assessment of potential contamination of bladder layers
The urothelium + suburothelium was dissected from the detrusor smooth muscle using fine forceps under a dissecting microscope as previously described (Corrow et al., 2010; Schnegelsberg et al., 2010). To confirm the specificity of our split bladder preparations, urothelium + suburothelium and detrusor samples were examined for the presence of αsmooth muscle actin (1:1000; Abcam, Cambridge, MA) and uroplakin II (1:25; American Research Products, Belmont, MA) by western blotting or reverse transcription PCR (Corrow et al., 2010; Girard et al., 2011; Girard et al., 2013). In urothelium + suburothelium layers, only uroplakin II was present (data not shown). Conversely, in detrusor samples, only α-smooth muscle actin was present (data not shown). In these studies, the use of the term urothelium refers to the urothelium and suburothelial layers.
Measurement of urinary bladder NGF, Sub P and CGRP by ELISAs
Determination of NGF, Sub P, and CGRP protein content in the urinary bladder of NGF-OE transgenic mice and WT littermate controls was determined using enzyme-linked immunoassays (ELISAs) as previously described (Gonzalez et al., 2015; Schnegelsberg et al., 2010; Vizzard, 2000b). Individual mouse bladders were dissected, weighed, and placed in Tissue Protein Extraction Reagent (1 g tissue/20 ml; Pierce Biotechnology, Woburn, MA) with Complete protease inhibitor cocktail tablets (Roche Applied Science, Mannheim, Germany), and stored at −80 °C. On the day of assay, individual bladders were disrupted with a Polytron homogenizer until homogeneous and centrifuged (10,000 rpm for 10 min) (Gonzalez et al., 2015; Schnegelsberg et al., 2010; Vizzard, 2000b), and the supernatant was used for total protein estimation and CGRP and Sub P quantification. Total protein was determined by the Coomassie Plus Protein Assay Reagent Kit (Pierce) (Gonzalez et al., 2015), and NGF, CGRP and SP were quantified using standard 96-well ELISA plates (Phoenix Pharmaceuticals, Burlingame, CA) according to the manufacturer’s recommendations (Gonzalez et al., 2015). Microtiter plates (R & D Systems, Minneapolis, MN) were coated with an anti-rat NGF antibody, anti-rat CGRP or anti-rat Sub P antibody. Sample and standard solutions were run in duplicate. A horseradish peroxidase-streptavidin conjugate was used to detect the antibody complex. Tetramethyl benzidine was the substrate and the enzyme activity was measured by the change in optical density. The NGF standard provided with this protocol generated a linear standard curve from 15 to 1000 pg/ml (R2 = 0.998, p ≤ 0.0001) for tissue samples. The CGRP standard provided with this protocol generated a linear standard curve from 0 to 100 ng/ml (R2 = 0.996, p ≤ 0.0001) for bladder samples (Gonzalez et al., 2015). The Sub P standard provided with this protocol generated a linear standard curve from 0 to 25 ng/ml (R2 = 0.997, p ≤ 0.0001) for bladder samples (Gonzalez et al., 2015). The absorbance values of standards and samples were corrected by subtraction of the background absorbance due to nonspecific binding. No samples fell below the minimum detection limits of the assay and no samples were diluted prior to use. Curve fitting of standards and evaluation of NGF, Sub P and CGRP content of samples was performed using a least squares fit as previously described (Gonzalez et al., 2015; Schnegelsberg et al., 2010; Vizzard, 2000b).
Urinary bladder preparation, immunohistochemistry and image analysis
The urinary bladder from littermate WT (n = 6–8) and NGF-OE mice at specified postnatal ages (P7-A) (n = 6–8 each) was dissected and placed in Krebs solution. The bladder was cut open along the midline and pinned to a Sylgard-coated dish. Notches were made on one side of the bladder neck for orientation purposes. The bladder was incubated for 1.5 h at room temperature in cold fixative (2% paraformaldehyde + 0.2% picric acid). Fine-tipped forceps and iris scissors were used to dissect the urothelium + suburothelium from the underlying detrusor smooth muscle with the aid of a dissecting microscope (Schnegelsberg et al., 2010). The tissue was blocked with PBS (pH 7.4) containing 20% normal goat serum, 0.2% Triton X-100 for 2 h at room temperature and the primary antibody was applied in PBS containing 4% normal goat serum, 0.2% Triton X-100 overnight at 4° C (Arms et al., 2013; Arms et al., 2010; Schnegelsberg et al., 2010). The following primary antibodies were used: a rabbit protein gene product (PGP) 9.5 antibody (1:6,000) (Ultraclone, UK), a rabbit calcitonin gene-related peptide (CGRP) antibody (1:500) (Sigma-Aldrich, St. Louis, MO), a rabbit substance P (Sub P) antibody (1:500)(Phoenix Pharmaceuticals, Inc., Burlingame, CA), a rabbit tyrosine hydroxylase (TH) antibody (1:300) (Chemicon, Temecula, CA), and a rabbit neurofilament 200 (NF200) antibody (1:150) (Chemicon). The tissue was washed in PBS (pH 7.4) containing 0.1% BSA, 0.1% Triton-X-100, 4 X for 15 min each at room temperature. The secondary antibody used for all whole-mount staining was a Cyanine Cy3 conjugated goat anti-rabbit antibody (1:500) (Jackson ImmunoResearch, West Grove, PA) incubated for 2 h at room temperature in the same solution as the primary antibody. The tissue was washed 3 X for 10 min each in PBS and mounted with mounting medium (Polysciences, Warrington, PA).
Assessment of immunohistochemical staining in suburothelial nerve plexus
Immunohistochemistry and subsequent semi-quantification of neurochemical-IR in whole-mount preparations were performed on littermate WT and NGF-OE tissues simultaneously to reduce the incidence of staining variation that can occur between tissues processed on different days. Staining in experimental tissue was compared with that in experiment-matched negative controls. Whole mount preparations exhibiting immunoreactivity that was greater than the background level in experiment-matched negative controls were considered positively stained.
Visualization and semi-quantitative analyses of neurochemistry of suburothelial nerve plexus
Whole mounts from littermate WT (n = 6–8) and NGF-OE of various selected postnatal ages (P7-A)(n = 6–8 each) groups were examined under an Olympus fluorescence photomicroscope as described above. Cy3 was visualized with a filter with an excitation range of 560–596 nm and an emission range of 610–655 nm. Semi-quantification of neurochemical expression in the suburothelial nerve plexus was performed as previously described (Arms et al., 2013; Arms et al., 2010). Grayscale images acquired in tiff format were imported into MetaMorph image analysis software (version 4.5r4; Universal Imaging, Downingtown, PA) as previously described (Corrow et al., 2010; Corrow and Vizzard, 2007; Gonzalez et al., 2013) and images were thresholded. Images were acquired from the neck region of WT and NGF-OE mice for suburothelial nerve plexus analyses. A rectangle of fixed dimension (500 × 500 pixels) was placed on the section according to a random selection of x and y coordinates. This process was repeated seven times for each image of the suburothelial nerve plexus. The average optical density of neurochemical immunoreactivity in the suburothelial nerve plexus was then calculated. Immunoreactivity for all neurochemicals examined (PGP9.5, CGRP, Sub P, NF-200 and TH) in the suburothelial plexus was greatest in the bladder neck region. Semi-quantification of neurochemical immunoreactivity in the suburothelial plexus was determined in the bladder neck region for all WT and NGF-OE mice.
Figure preparation
Digital images were obtained using a charge-coupled device camera (MagnaFire SP, Optronics, Optical Analysis, Nashua, NH) and LG-3 frame grabber attached to an Olympus microscope (Optical Analysis). Exposure times were held constant when images were acquired from WT and NGF-OE mice processed and analyzed on the same day. Images were imported into Photoshop 7.0 (Adobe Systems, San Jose, CA), where groups of images were assembled and labeled.
Conscious, open outlet, continuous fill cystometry
Rodents (mice and rats) were anesthetized with isoflurane (3–4%), a lower midline abdominal incision was made, and polyethylene tubing (PE-10, Clay Adams, Parsippany, New Jersey) was inserted into the bladder dome and secured with a nylon purse-string sutures (6-zero) (Gonzalez et al., 2013; Schnegelsberg et al., 2010). The end of the PE tubing was heat flared, but the catheter did not extend into the bladder body or neck and it was not associated with inflammation or altered cystometric function (Gonzalez et al., 2013; Schnegelsberg et al., 2010). The distal end of the tubing was sealed, tunneled subcutaneously and externalized at the back of the neck out of reach (Gonzalez et al., 2013; Schnegelsberg et al., 2010). Abdominal and neck incisions were closed with nylon sutures (6-zero). Rodents (>P21) that had been weaned received postoperative analgesics (buprenorphine, 0.5 mg/kg, s.c.) and were maintained for 72 h after survival surgery to ensure recovery. Rodents (<P21) that had not been weaned received a single preoperative dose of analgesic (buprenorphine, 0.5 mg/kg, s.c.) and were used in conscious cystometry studies on the same day after a 2 h recovery period. Attempts to return rodents (<P21) postoperatively to the home cage with the dam and litter were not successful in pilot studies. Some rodents (>P21) were also evaluated with conscious cystometry on the same day as bladder catheter implantation and no significant differences in the cystometric parameters measured (see below) were observed; data were pooled in analyses.
For cystometry in conscious rodents (mice and rats), an unrestrained animal was placed in a Plexiglas cage with a wire bottom. Before the start of the recording, the bladder was emptied and the catheter was connected via a T-tube to a pressure transducer (Grass Model PT300, West Warwick, RI) and microinjection pump (Harvard Apparatus 22, South Natick, MA). A Small Animal Cystometry Lab Station (MED Associates, St. Albans, VT) was used for urodynamic measurements (Gonzalez et al., 2013; Schnegelsberg et al., 2010). Saline solution was infused at room temperature into the bladder at a rate of 25 μl/min (mice) or 10 ml/hr (rats) to elicit repetitive bladder contractions. At least six reproducible micturition cycles were recorded after the initial stabilization period of 25–30 min (Gonzalez et al., 2013; Schnegelsberg et al., 2010). In rodents that did not exhibit a vesicovesical reflex, voiding was elicited by perigenital stimulation to elicit a somatovesical reflex. Manual bladder emptying was accomplished by rapidly stroking the perigenital area for 60 s with a cotton swab. The following cystometric parameters were recorded in each animal: baseline pressure (BP; pressure at the beginning of the bladder filling), threshold pressure (TP; bladder pressure immediately prior to micturition), peak micturition pressure (MP), intercontraction interval (ICI; time between micturition events), bladder capacity (BC), void volume (VV), presence and amplitude of non-voiding bladder contractions (NVCs) (Gonzalez et al., 2013; Schnegelsberg et al., 2010). NVCs were defined as rhythmic intravesical pressure increases 5 cm H20 above baseline, during the filling phase, without the release of fluid from the urethra. The presence of NVCs was determined in a micturition cycle, which was defined as the duration of time that includes bladder filling, at a specified rate, resulting in a voiding reflex. Bladder capacity (BC) was measured as the volume of saline infused into the bladder at the time when micturition commenced (Gonzalez et al., 2013; Schnegelsberg et al., 2010). Mice and rats in these studies had residual volume of less than 5 μl; therefore, VV and BC were similar. At the conclusion of the experiment, the rodent was euthanized (5% isoflurane plus thoracotomy).
Conscious cystometry and effects of netupitant, a neurokinin (NK)-1 receptor antagonist
The effects of netupitant, a highly potent and selective neurokinin (NK)-1 receptor antagonist (Greenwood-Van Meerveld et al., 2014; Haab et al., 2014; Zhang et al., 2012), on urinary bladder function in littermate WT and NGF-OE mice of specified postnatal ages (P7-A; n = 6–8) were assessed using conscious, open outlet, cystometry with continuous instillation of intravesical saline (Gonzalez et al., 2013; Schnegelsberg et al., 2010). For intravesical administration of netupitant, mice were anesthetized with 2% isoflurane and netupitant (<1.0 ml) was injected through the bladder catheter; the animals were maintained under anesthesia to prevent expulsion of netupitant via a voiding reflex. In this procedure, netupitant remained in the bladder for 30 min at which time, the drug was drained, the bladder washed with saline and animals recovered from anesthesia for 20 min before experimentation. The effectiveness of intravesical netupitant (Med Chem Express (MCE), Monmouth Junction, NJ; 0.1 μg/ml) administration was evaluated in littermate WT and NGF-OE mice from various postnatal ages (P7-A). These experiments were performed in the same mice before and after treatment with netupitant. The concentration (0.1 μg/ml) of netupitant used in these studies was based upon previous studies (Greenwood-Van Meerveld et al., 2014) and pilot studies. WT and NGF-OE mice receiving intravesical administration of vehicle (0.9% saline) (n = 6–8) were also evaluated. To summarize, the experimental design involves administration of a one time, intravesical infusion of netupitant (0.1 μg/ml) with cystometric data collection occurring ~75 min after infusion. At the conclusion of the experiment, the animal was euthanized (4% isoflurane plus thoracotomy). Experiments were conducted at similar times of the day to avoid the possibility that circadian variations were responsible for changes in bladder capacity measurements. An individual blinded to genotype analyzed the cystometric data; groups were decoded after data analysis (Gonzalez et al., 2013; Schnegelsberg et al., 2010).
Exclusion Criteria
Mice were removed from the study when adverse events occurred that included a significant postoperative event, lethargy, pain, or distress not relieved by our IACUC-approved regimen of pre- and/or postoperative analgesics (Gonzalez et al., 2013; Schnegelsberg et al., 2010). In the present study, no mice or rats were excluded from the study. In addition, behavioral movements such as grooming, standing, walking, and defecation rendered bladder pressure recordings during these events unusable.
Urination Patterns
As previously described, the spinobulbospinal micturition reflex is triggered by mechanoreceptors in the bladder and begins to elicit voiding in the rat between P16–P18 (Capek and Jelinek, 1956). To determine onset of the micturition reflex in NGF-OE mice and littermate WT mice, male and female mice of both genotypes and of varying postnatal ages (P3-A) were separated from the dam (if litters were pre-weaning) and placed individually in cages lined with Whatman Grade 3 filter paper for a period of 2 hours (h) under continuous monitoring (Studeny et al., 2008). Mice were habituated to cages for 1 h in the morning (9–11 am) on each of two consecutive days prior to data accumulation (Studeny et al., 2008). As an additional comparison, the onset of the micturition reflex in individual postnatal (P3-A) Wistar rats of both sexes was also determined in the same manner. After 2 h, mice (NGF-OE and WT) or rats were returned to the litter (if pre-weaning) and the filter paper was examined under UV light to determine presence and size of urine spots. Urine spots were photographed under UV light (Studeny et al., 2008), area (cm2) of spots determined with large (0.2 – 10 cm2) (Birder et al., 2002) and small (< 0.2 cm2) (Birder et al., 2002) spots being counted. An individual blinded to genotype analyzed these data were analyzed; groups were decoded after data analysis.
Statistical Analyses
Statistical analyses did not demonstrate differences between male and female mice of either genotype. Thus, mouse data were pooled for statistical analyses of immunoassays, Q-PCR, immunohistochemical analyses and cystometry. All values are means ± S.E.M. Linear regression analyses were performed to determine the relationship between postnatal age and NGF, Sub P or CGRP mRNA or protein expression in urinary bladder. Comparisons among experimental groups were made using ANOVA. Percentage data from image analysis were arcsin transformed to meet the requirements of the statistical test. Animals, processed and analyzed on the same day, were tested as a block in the ANOVA. When F ratios exceeded the critical value (p ≤ 0.05), the Newman-Keul’s post hoc test was used to compare experimental means among groups.
Results
Bladder weight increases in NGF-OE postnatal mice
NGF-OE transgenic mice developed normally with no adverse clinical signs or altered behaviors. NGF-OE mice of both sexes had similar body weight to age-matched (P0-Adult, A), littermate WT control mice. Male and female NGF-OE mice (P0-A) exhibited a significant (p ≤ 0.01) increase in urinary bladder weight and a 2.9- to 3.2-fold increase in bladder-to-body weight ratios compared with age-matched, sex-matched, littermate WT mice (P0-A) (Table 1).
NGF transcript and protein expression in increased in urothelium of postnatal WT and NGF-OE mice with no changes in detrusor
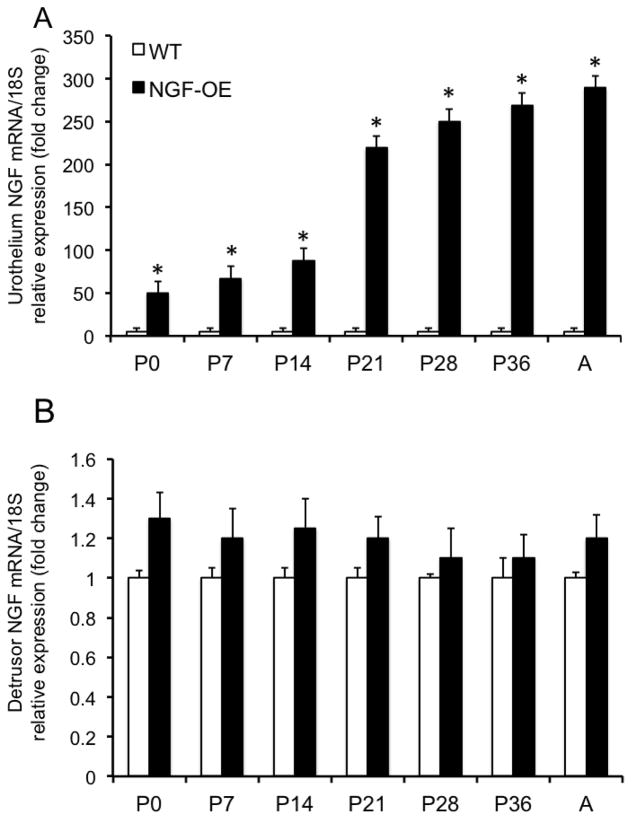
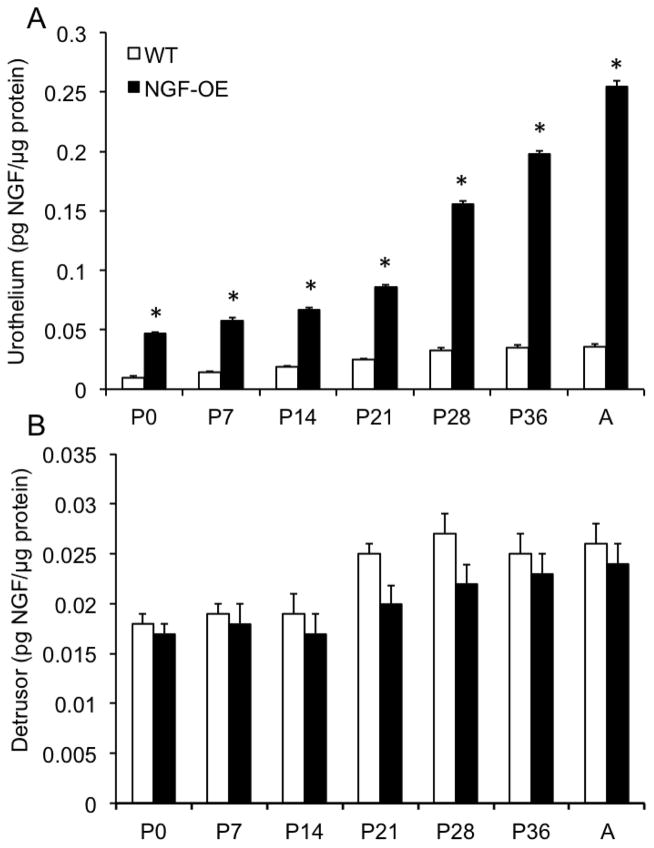
Consistent with our previous studies (Cheppudira et al., 2008; Schnegelsberg et al., 2010), NGF transcript and protein expression were significantly (p ≤ 0.01) increased in urothelium of NGF-OE mice at all postnatal (P) ages examined (P0, P7, P14, P21, P28, P36 and Adult (A)(Fig. 1A, Fig. 2A) compared to age-matched littermate WT mice. NGF transcript and protein expression in urothelium of NGF-OE mice was correlated with increasing postnatal age (R2=0.978–0.989; p ≤ 0.001)(Fig. 1A, Fig. 2A). In contrast, no changes were observed in NGF transcript and protein expression in detrusor smooth muscle between postnatal NGF-OE and littermate WT mice (Fig. 1B, Fig. 2B). No correlation with postnatal age was demonstrated for NGF transcript and protein expression in detrusor smooth muscle of postnatal NGF-OE or WT mice (Fig. 1B, Fig. 2B).
Figure 1.
Characterization of nerve growth factor (NGF) mRNA in urinary bladder from NGF-overexpressing (OE) mice at different postnatal (P) ages (P0, P7, P14, P28, P36, Adult (A)) by quantitative PCR. NGF transcript expression in urothelium/suburothelium (A) and detrusor layers (B) in NGF-OE and wildtype (WT) mice normalized to expression of the housekeeping gene 18S. Values are means ± S.E.M. (n = 6–8 for each group). *p ≤ 0.01: NGF-OE urothelium/suburothelium vs. WT urothelium/suburothelium (A).
Figure 2.
NGF content in the urinary bladders of WT and NGF-OE transgenic mice of different postnatal (P) ages. NGF content in urothelium/suburothelium (A) and detrusor (B) mice was determined in WT and NGF-OE mice of different postnatal (P) ages, including P0, P7, P14, P28, P36, and Adult (A). NGF content was significantly (*p ≤ 0.01) greater in NGF-OE vs. WT urothelium at each postnatal age examined (A). No differences in NGF content were observed in detrusor of NGF-OE and WT mice at any postnatal age examined (B). Values are means ± S.E.M. (n = 6–8 for each group).
Substance P (Sub P) transcript and protein expression in urothelium and detrusor of postnatal WT and NGF-OE mice
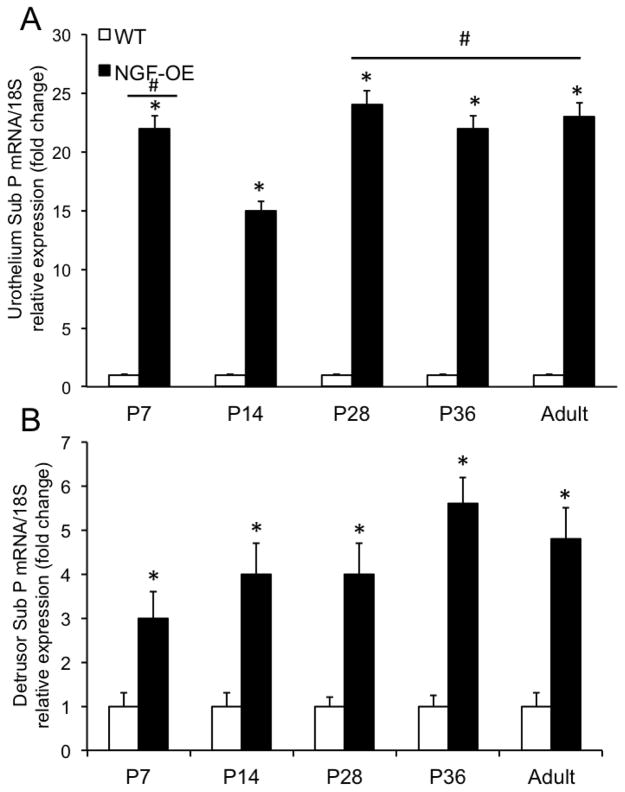
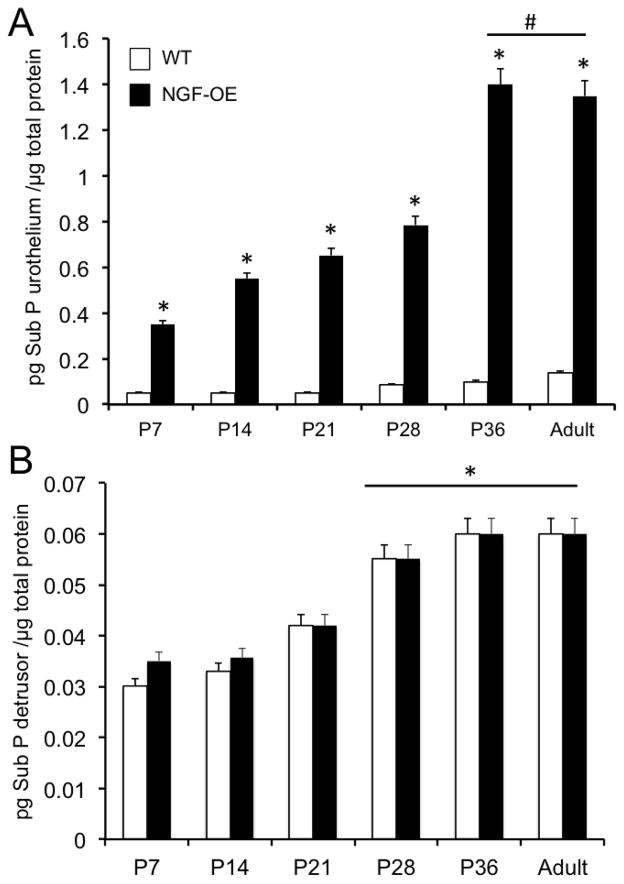
Sub P transcript and protein expression were significantly (p ≤ 0.01) increased in urothelium of NGF-OE mice compared to age-matched WT mice at all postnatal ages examined (P7, P14, P21, P28, P36, A)(Fig. 3A, Fig. 5A). Sub P transcript expression was significantly (p ≤ 0.01) greater at P7, P28, P36 and A compared to P14 in urothelium of NGF-OE mice (Fig. 3A). Sub P transcript expression was significantly (p ≤ 0.01) greater in detrusor of NGF-OE mice compared to age-matched WT mice at each postnatal age examined (Fig. 3B). Sub P protein expression in urothelium of NGF-OE mice was correlated with increasing postnatal age (R2=0.977; p ≤ 0.001)(Fig. 5A). Sub P protein expression in the urothelium of NGF-OE mice was significantly (p ≤ 0.01) greater at P36 and A compared to other postnatal ages (P7, P14, P21, P28)(Fig. 5A). Sub P protein expression in urothelium of WT mice was also correlated with increasing postnatal age (R2=0.982; p ≤ 0.001) (Fig. 5A). Although Sub P transcript was significantly increased in the detrusor of NGF-OE mice compared to WT mice at all postnatal ages examined (P7, P14, P28, P36, A)(Fig. 3B), no changes were observed in Sub P protein expression in detrusor smooth muscle (Fig. 5B) between NGF-OE mice and WT mice. Sub P protein expression in the detrusor of NGF-OE and littermate WT mice was correlated (R2=0.979; p ≤ 0.001) with increasing postnatal age (Fig. 5B). Sub P protein expression in the detrusor of NGF-OE and WT mice was greatest at P28, P36 and A (Fig. 5B).
Figure 3.
Characterization of substance P mRNA in urinary bladder from NGF-overexpressing (OE) mice at different postnatal (P) ages (P7, P14, P28, P36, Adult (A)) by quantitative PCR. Substance P transcript expression in urothelium/suburothelium (A) and detrusor layers (B) in NGF-OE and wildtype (WT) mice normalized to expression of the housekeeping gene 18S. Values are means ± S.E.M. (n = 6–8 for each group). *p ≤ 0.01: NGF-OE urothelium/suburothelium vs. WT urothelium/suburothelium (A). #, p ≤ 0.01: NGF-OE urothelium/suburothelium at P7, P28, P36 and A vs. NGF-OE urothelium/suburothelium at P14 (A). *p ≤ 0.01: NGF-OE detrusor vs. WT detrusor (B).
Figure 5.
Substance P (Sub P) content in the urinary bladders of WT and NGF-OE transgenic mice of different postnatal (P) ages. Sub P content in urothelium/suburothelium (A) and detrusor (B) mice was determined in WT and NGF-OE mice of different postnatal (P) ages, including P7, P14, P28, P36, and Adult (A). Sub P content was significantly (*p ≤ 0.01) greater in NGF-OE vs. WT urothelium at each postnatal age examined (A). #p ≤ 0.01: NGF-OE urothelium/suburothelium at P36 and A vs. NGF-OE urothelium/suburothelium at P7, P14, P21, P28. No differences in Sub P content were observed in detrusor of NGF-OE vs. WT mice at any postnatal age examined (B). *p ≤ 0.01: Sub P content in detrusor at P28, P36, A vs. P7, P14, P21 for both WT and NGF-OE. Values are means ± S.E.M. (n = 6–8 for each group).
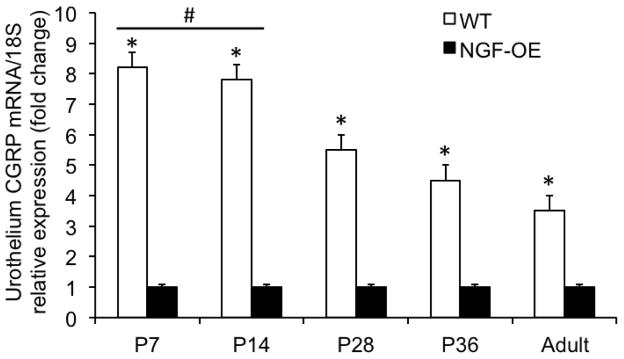
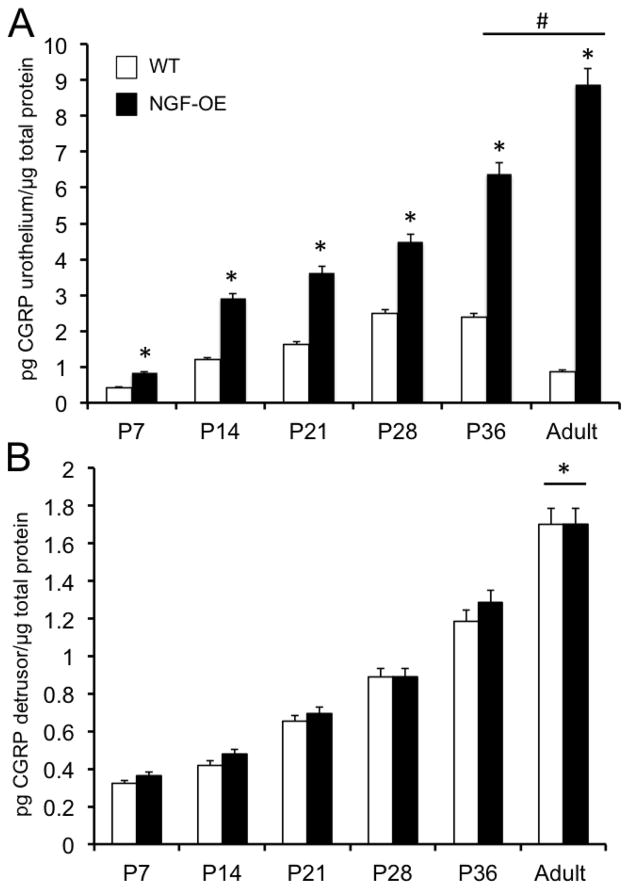
Calcitonin gene-related peptide (CGRP) transcript and protein expression in urothelium and detrusor of postnatal WT and NGF-OE mice
CGRP transcript expression was significantly (p ≤ 0.01) decreased in urothelium of WT mice compared to age-matched NGF-OE mice at all postnatal ages examined (P7, P14, P28, P36, A)(Fig. 4). CGRP transcript expression was significantly (p ≤ 0.01) greater in urothelium of P7–P28 WT mice compared to A WT mice (Fig. 4). CGRP transcript expression in urothelium of WT mice was negatively correlated with increasing postnatal age (R2=0.987; p ≤ 0.001)(Fig. 4). No changes in CGRP transcript expression in detrusor of NGF-OE mice were observed during postnatal development at the ages examined (P7, P14, P28, P36, A) (data not shown). CGRP protein expression in the urothelium of NGF-OE mice was significantly (p ≤ 0.01) greater at P36 and A compared to NGF-OE mice of other postnatal ages (P7, P14, P21, P28)(Fig. 6A). CGRP protein expression in urothelium of NGF-OE mice was significantly (p ≤ 0.01) greater than that observed in age-matched WT mice at every age examined (Fig. 6A). CGRP protein expression in urothelium of WT mice and NGF-OE mice was correlated with increasing postnatal age (R2=0.976–0.988; p ≤ 0.001)(Fig. 6A). CGRP protein content was significantly (p ≤ 0.01) greater in urothelium of NGF-OE mice at each age examined (P7, P14, P21, P28, P36, A)(Fig. 6A) compared to age-matched WT mice. No differences in CGRP protein content were demonstrated in detrusor smooth muscle between age-matched NGF-OE and WT mice at any postnatal age examined (Fig. 6B). CGRP protein content in detrusor of NGF-OE and WT mice was correlated with increasing postnatal age (R2=0.985–0.992; p ≤ 0.001)(Fig. 6B).
Figure 4.
Characterization of CGRP mRNA in urinary bladder from NGF-overexpressing (OE) mice at different postnatal (P) ages (P7, P14, P28, P36, Adult (A)) by quantitative PCR. CGRP transcript expression in urothelium/suburothelium in NGF-OE and wildtype (WT) mice normalized to expression of the housekeeping gene 18S. Values are means ± S.E.M. (n = 6–8 for each group). *p ≤ 0.01: WT urothelium/suburothelium vs. NGF-OE urothelium/suburothelium. #, p ≤ 0.01: WT urothelium/suburothelium at P7, P14 vs. WT urothelium/suburothelium at P28, P36 and A.
Figure 6.
CGRP content in the urinary bladders of WT and NGF-OE transgenic mice of different postnatal (P) ages. CGRP content in urothelium/suburothelium (A) and detrusor (B) mice was determined in WT and NGF-OE mice of different postnatal (P) ages, including P7, P14, P28, P36, and Adult (A). CGRP content was significantly (*p ≤ 0.01) greater in NGF-OE vs. WT urothelium at each postnatal age examined (A). #p ≤ 0.01: NGF-OE urothelium/suburothelium at P36 and A vs. NGF-OE urothelium/suburothelium at P7, P14, P21, P28. No differences in CGRP content were observed in detrusor of NGF-OE vs. WT mice at any postnatal age examined (B). *p ≤ 0.01: CGRP content in detrusor at A vs. P7, P14, P21, P28, P36 for both WT and NGF-OE. Values are means ± S.E.M. (n = 6–8 for each group).
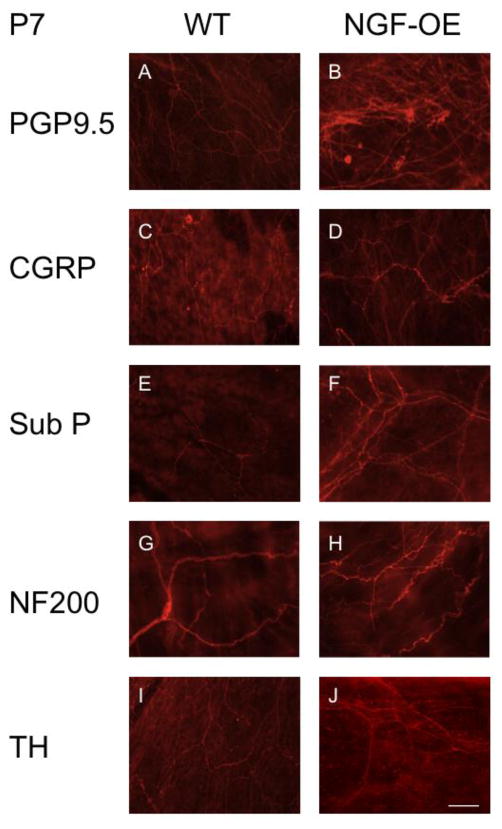
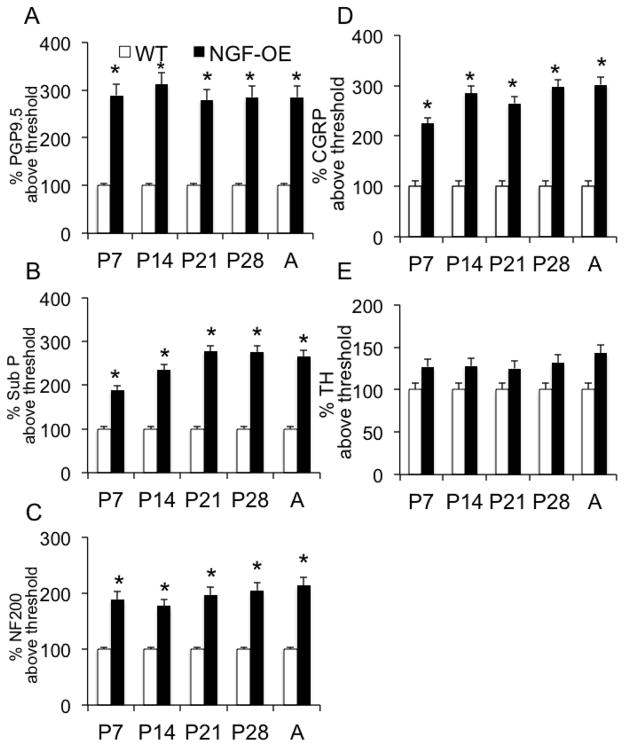
Sensory and sympathetic hyperinnervation in the urinary bladders of NGF-OE transgenic mice
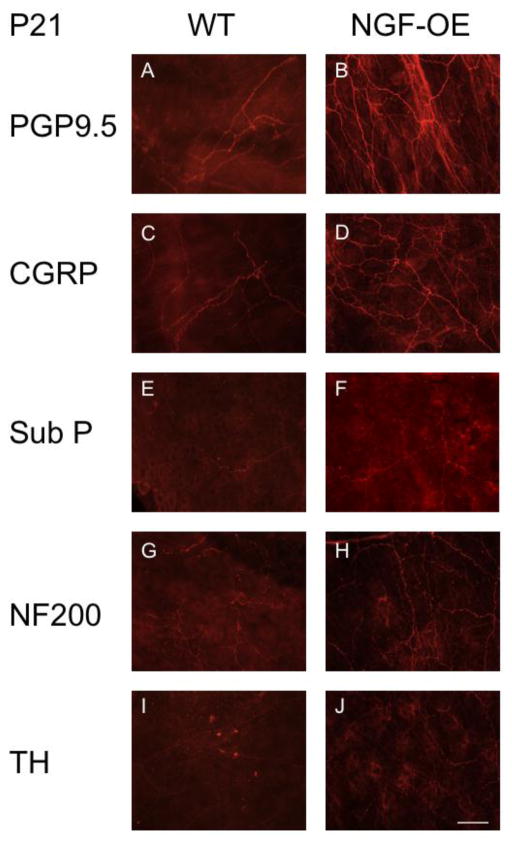
We characterized the subpopulations of neurons contributing to the generalized hyperinnervation observed in urinary bladder of postnatal NGF-OE transgenic mice at postnatal ages (P7, P14, P21, P28)(Figs. 7–9) using whole-mount immunostaining of bladder urothelium using a panel of neuronal markers. Using the pan-neuronal marker, protein gene product, PGP9.5, a significant (p ≤ 0.01) increase in total nerve fiber density was seen in the urinary bladders of NGF-OE compared to littermate WT mice (Figs. 7, 8, 9A). This dense network was composed of CGRP- (Figs. 7, 8, 9D) and Sub P-positive (Figs. 7, 8, 9B) unmyelinated C-fiber sensory afferents, NF200-positive myelinated sensory afferents (Figs. 7, 8, 9C) and TH-positive, postganglionic sympathetic nerve fibers (Figs. 7, 8, 9E). In contrast to CGRP-, Sub P- and NF-200-positive nerve fibers that exhibited increased density in NGF-OE vs. WT urinary bladders at all postnatal ages examined (P7, P14, P21, P28), no changes were observed in TH-positive nerve fibers density in NGF-OE vs. WT urinary bladders from any postnatal age examined. The increased nerve fiber density observed in the NGF-OE bladders was evident in both the neck (Figs. 7, 8) and the dome (data not shown) regions of the urinary bladder. However, consistent with published literature, we observed a higher innervation density within the urinary bladder neck region compared with the dome (Gabella and Davis, 1998; Schnegelsberg et al., 2010) in both WT and NGF-OE mice for all postnatal ages examined (data not shown).
Figure 7.
Neuronal hyperinnervation in the urinary bladders of NGF-OE mice at postnatal (P) day 7. Representative fluorescence images of PGP 9.5 (A, B), CGRP (C, D), Sub P (E, F), NF200 (G, H), and TH (I, J) immunoreactivity in the bladder neck region in urothelial whole-mount preparations of urinary bladders from P7 WT (A, C, E, G, I) and NGF-OE (B, D, F, H, J) mice (n = 6–8). Scale bar: 50 μm. Protein Gene Product (PGP 9.5), calcitonin gene-related protein (CGRP), substance P (Sub P), neurofilament (NF 200), tyrosine hydroxylase (TH).
Figure 9.
Semi-quantitative analyses of neurochemistry in urothelium of NGF-OE mice at various postnatal ages (P7, P14, P21, P28, A). Histograms of neurochemical expression in the urothelium during postnatal development expressed as a percentage of expression above threshold and averaged for all bladders from specific postnatal ages for NGF-OE and WT mice. Values are means ± S.E.M. (n = 6–8 for each group). At each postnatal age examined (P7, P14, P21, P28, Adult (A)), the percent expression of Protein Gene Produce (PGP)9.5 (A), Substance P (Sub P) (B), neurofilament (NF200) (C) and calcitonin gene-related peptide (CGRP) (D) was significantly (*p ≤ 0.01) above threshold in NGF-OE vs. WT mice. No changes were observed for tyrosine hydroxylase expression in NGF-OE vs. WT mice at any age examined (E).
Figure 8.
Neuronal hyperinnervation in the urinary bladders of NGF-OE mice at postnatal (P) day 21. Representative fluorescence images of PGP 9.5 (A, B), CGRP (C, D), Sub P (E, F), NF200 (G, H), and TH (I, J) immunoreactivity in the bladder neck region in urothelial whole-mount preparations of urinary bladders from P7 WT (A, C, E, G, I) and NGF-OE (B, D, F, H, J) mice (n = 6–8). Scale bar: 50 μm. Protein Gene Product (PGP 9.5), calcitonin gene-related protein (CGRP), substance P (Sub P), neurofilament (NF200), tyrosine hydroxylase (TH).
Onset of micturition reflex function in postnatal NGF-OE and littermate WT mice
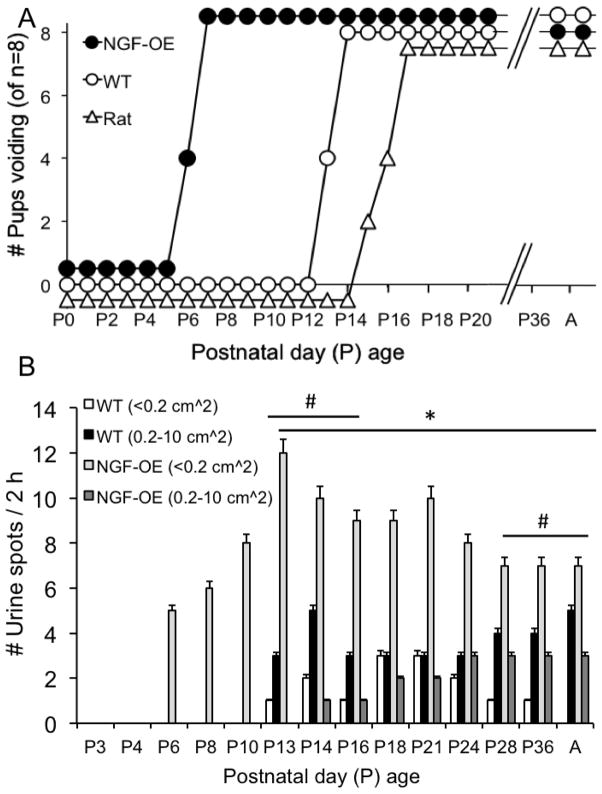
The spinobulbospinal micturition reflex is triggered by mechanoreceptors in the urinary bladder and begins to elicit voiding in the rat between P16–P18 (Capek and Jelinek, 1956). To determine the onset of the micturition reflex in postnatal NGF-OE and littermate WT mice, individual male and female mice of varying postnatal ages (P0-A) were separated from the dam (if litters were pre-weaning) and placed on filter paper for a period of 2 h under continuous monitoring. As an additional comparison, the onset of the micturition reflex in individual postnatal (P0-A) Wistar rats of both sexes was also determined in the same manner. For NGF-OE mice, the onset of the micturition reflex occurred as early as P6 with all NGF-OE mice examined exhibiting voiding behaviors by P7 (Fig. 10A). In contrast, littermate WT mice first exhibited micturition as early as P13 with all WT mice examined exhibiting voiding behaviors by P14 (Fig. 10A). Consistent with previous studies, postnatal Wistar rats first exhibited micturition as early as P15 with all Wistar rats studied exhibiting micturition behaviors by P16 (Fig. 10A). No differences in the onset of the micturition reflex were observed between sexes for postnatal NGF-OE, littermate WT or Wistar rats.
Figure 10.
Early onset of the vesicovesical reflex in postnatal NGF-OE mice. (A). Summary representation of the onset of spontaneous voiding as determined from filter paper void assays in NGF-OE mice, littermate WT mice, and Wistar rats of both sexes from various postnatal (P) ages (P0-Adult, A). NGF-OE mice exhibited spontaneous voiding by P5 with all NGF-OE mice exhibiting spontaneous voiding by P7. Littermate WT mice first exhibited spontaneous voiding on P13 with all WT mice exhibiting spontaneous voiding by P14. Wistar rats first exhibited spontaneous voiding by P15 with Wistar rats exhibiting spontaneous voiding by P17. n = 8 for NGF-OE mice, WT mice and Wistar rats. (B) Summary histogram of the number (#) and area of urine spots on filter paper over a 2 h period of time from NGF-OE mice and littermate WT mice of both sexes from various postnatal (P) ages (P3-Adult, A). NGF-OE mice exhibited an early onset of spontaneous voiding with a large number of voids of small area (≤ 0.2 cm2). For NGF-OE mice, the area of urine spots increased during postnatal development but the number of voids of small area were significantly (*p ≤ 0.01) greater than the number of voids of larger area (0.2 – 10 cm2) at all postnatal ages examined. WT mice exhibited spontaneous voiding by P13. In WT mice, the number voids of larger area (0.2 – 10 cm2) were significantly (#p ≤ 0.01) greater at P13–P16 and P24-A with other ages (P18, P21) having equal numbers of small and larger area urine spots. Values are means ± S.E.M. (n = 6–8 for each group).
Number and area of urine spots during postnatal development of NGF-OE and WT mice
The number of urine spots and the size of the urine spots varied during postnatal development between NGF-OE and littermate WT mice (Fig. 10B). For NGF-OE mice, the number of urine spots increased (p ≤ 0.01; R2=0.998) with postnatal age from P5 to P12 and then the number of urine spots remained consistent from P14-A (Fig. 10B). Up to P12, the sizes of the urine spots voided by NGF-OE mice were small in area (<0.2 cm2) (Fig. 10B). After P12, NGF-OE mice voided both small and large urine spots; however, the number of small urine spots was significantly (p ≤ 0.01) greater at each postnatal age examined (Fig. 10B). The urine spots voided by littermate WT mice were not correlated with increasing postnatal age and the number of urine spots remained consistent from P12-A. Littermate WT mice expressed both small and large size urine spots with the number of large urine spots being significantly (p ≤ 0.01) greater from P12–P16 and from P28-A (Fig. 10B). No differences in the number or area of urine spots were observed between sexes for postnatal NGF-OE or littermate WT mice.
Conscious, open-outlet cystometry in postnatal NGF-OE and WT mice
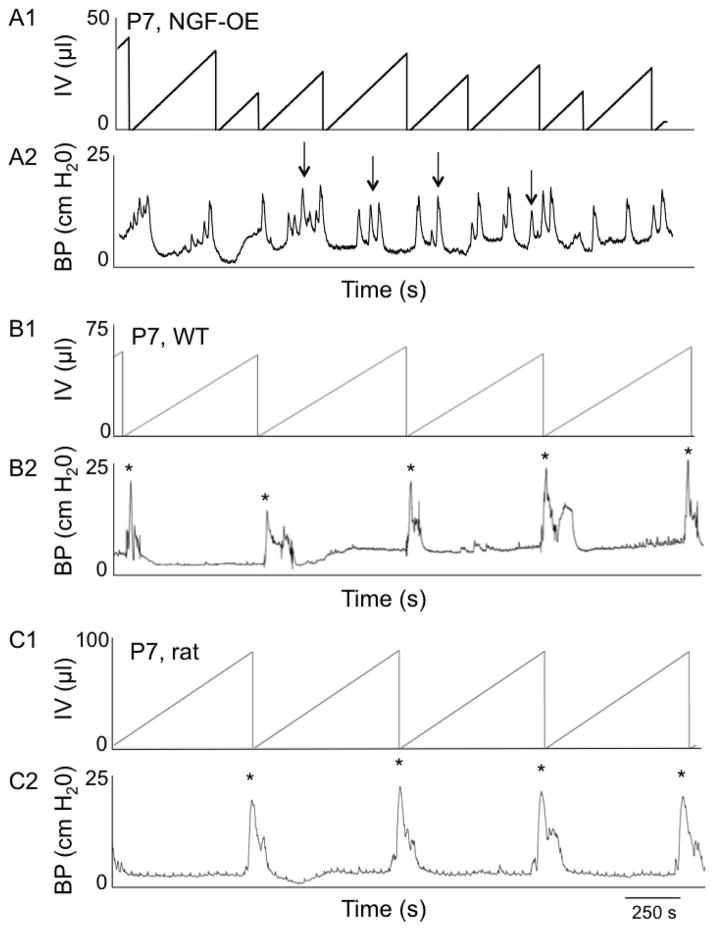
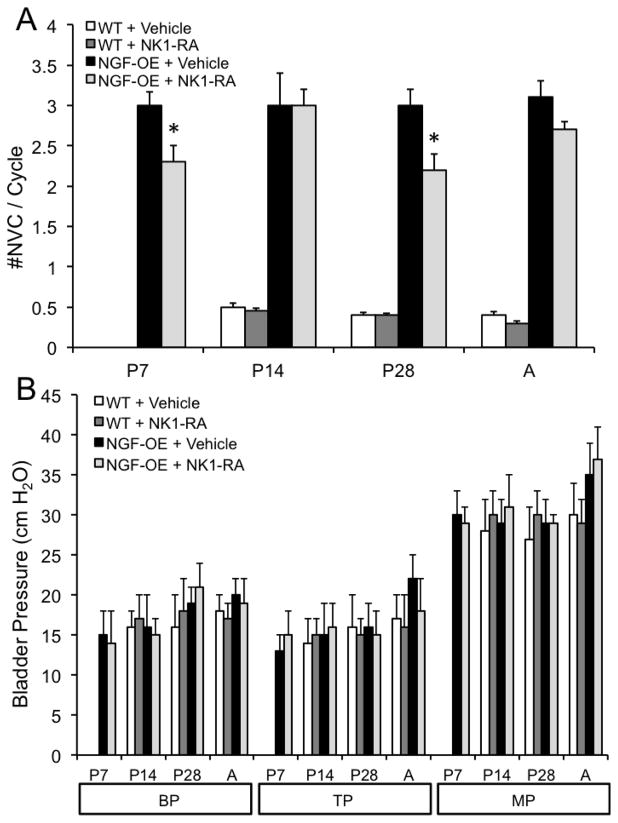
Conscious, open outlet cystometry with continuous intravesical infusion of saline was performed in separate groups (n = 6 each) of female WT and NGF-OE during postnatal development (P7, P14, P28 and A) to determine urinary bladder function (Fig. 11). In contrast to P7 NGF-OE mice that exhibited micturition reflexes with continuous intravesical infusion of saline, age-matched, littermate WT mice exhibited no micturition reflexes in the absence of perigenital stimulation (Fig. 11A, B). This finding with cystometry is consistent with the absence of urine spots from the voiding on filter paper assay. NGF-OE mice exhibited micturition reflexes with cystometry at the earliest postnatal age examined (P7) and void volumes were significantly (p ≤ 0.01) smaller than those of age-matched WT mice at all ages examined (P7, P14, P28 and A) where micturition reflexes were present in the absence of perigenital stimulation (Figs. 11, 12A). The intercontraction interval (ICI) was significantly (p ≤ 0.01) reduced in NGF-OE mice compared to littermate WT mice at postnatal ages examined (P7, P14, P28, A) (Figs. 11, 12B). NGF-OE (P7, P14, P28 and A) mice exhibited a significantly (p ≤ 0.01) greater number of non-voiding contractions (NVCs) in comparison to age-matched WT mice (Figs. 11, 13A). Baseline pressure (BP), threshold pressure (TP) and peak micturition pressure (MP) were comparable between age-matched WT and NGF-OE mice (P7, P14, P28 and A) (Fig. 13B). No differences in the cystometric parameters analyzed were observed between sexes for postnatal NGF-OE or littermate WT mice.
Figure 11.
Open outlet, continuous fill cystometry in conscious NGF-OE mice, littermate WT mice and Wistar rats at postnatal (P) day 7. Representative cystometrogram trace from a P7 conscious, unrestrained NGF-OE (A) mouse, a littermate WT (B) mouse and a Wistar rat (C) during a continuous intravesical infusion (25 μl/min for mice; 10 ml/hr for rats) of room temperature saline. A. At P7, a NGF-OE mouse exhibits micturition reflexes in the absence of perigenital stimulation. B. At P7, a WT mice (B) and a Wistar rat (C) only exhibit micturition reflexes with perigenital stimulation (*). Infused volume (IV, μl)(A1, B1, C1) and bladder pressure (BP, cm H2O) (A2, B2, C2) are shown. Arrows indicate examples of nonvoiding bladder contractions (A). seconds, s.
Figure 12.
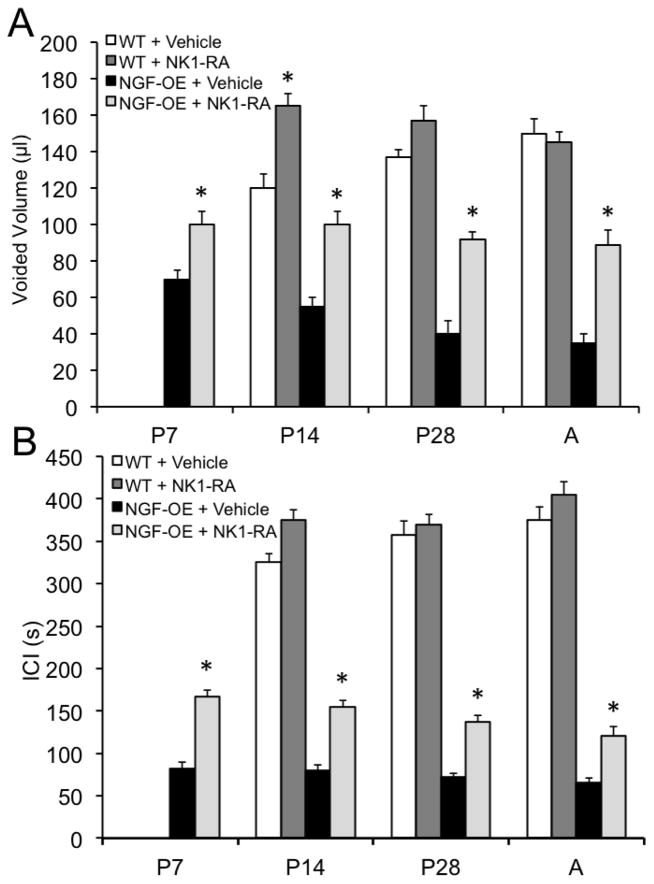
Effects of a neurokinin (NK)-1 receptor antagonist (RA), netupitant, on micturition reflex function from open outlet, continuous fill cystometry in NGF-OE mice and littermate WT mice at postnatal (P) day 7, P14, P28 and Adult (A). Intravesical netupitant (0.1 μg/ml) significantly (p ≤ 0.01) increased voided volume (A) and intercontraction interval (ICI) (B) in NGF-OE mice at all postnatal ages examined. Intravesical netupitant (0.1 μg/ml) was without effect on voided volume (A) or ICI (B) in WT mice except at P14 where there was a significant (p ≤ 0.01) increase in voided volume. *, p ≤ 0.01 compared to vehicle. Values are means ± S.E.M. (n = 6–8 for each group).
Figure 13.
Effects of a neurokinin (NK)-1 receptor antagonist (RA), netupitant, on micturition reflex function from open outlet, continuous fill cystometry in NGF-OE mice and littermate WT mice at postnatal (P) day 7, P14, P28 and Adult (A). Intravesical netupitant (0.1 μg/ml) significantly (p ≤ 0.01) decreased the number of nonvoiding contractions (NVCs) in NGF-OE mice at P7 and P28 but was without effect on NVCs in WT mice that exhibited few NVCs (A). Intravesical netupitant (0.1 μg/ml) was without effect on bladder pressures (baseline (BP), threshold pressure (TP), peak micturition pressure (MP)) in NGF-OE and WT mice at any postnatal age examined (B). *, p ≤ 0.01 compared to vehicle. Values are means ± S.E.M. (n = 6–8 for each group).
Effects of intravesical netupitant, neurokinin (NK)-1 receptor antagonist, on urinary bladder function in NGF-OE and WT mice
Intravesical netupitant (0.1 μg/ml) significantly (p ≤ 0.01) increased void volume in NGF-OE mice at each postnatal age examined (P7, P14, P28 and A) (Fig. 12A). In contrast, intravesical netupitant did not affect void volume in WT mice (P28, A) but did increase (p ≤ 0.05) void volume in P14 WT mice (Fig. 12A). Intravesical netupitant significantly (p ≤ 0.01) increased ICI in NGF-OE mice at P7, P14, P28 and A (Fig. 12B). In contrast, intravesical netupitant did not affect the ICI in age-matched WT mice (Fig. 12B). Intravesical netupitant (0.1 μg/ml) was without effect on the number of NVCs observed in postnatal (P7, P14, A) NGF-OE or WT mice except for a significant (p ≤ 0.05) reduction in NVCs in NGF-OE mice at P28 (Fig. 13A). Intravesical netupitant had no effect on BP, TP or peak MP in either NGF-OE mice or age-matched WT mice (P7, P14, P28, A)(Fig. 13B). Residual volume in NGF-OE and age-matched littermate WT mice was small (< 5 μl) and was unaffected by intravesical netupitant administration. No differences in the effects of intravesical netupitant on the cystometric parameters analyzed were observed between sexes for postnatal NGF-OE or littermate WT mice.
Discussion
These studies, involving early postnatal mice with chronic urothelial overexpression of NGF, have produced novel insights into the mechanisms that drive the development of normal bladder innervation and function. As demonstrated with filter paper void assays and open-outlet, continuous fill cystometry in conscious mice, early postnatal NGF-OE mice exhibit the onset of the micturition reflex in the absence of perigenital stimulation (i.e., vesicovesical reflex) by P6. In contrast, littermate WT mice first exhibit micturition in the absence of perigenital stimulation by P13 similar to the onset of micturition in rats by P15. In association with this early onset of micturition in postnatal NGF-OE mice, these mice also exhibit hyperinnervation of the urinary bladder and increased bladder mass at all postnatal ages examined. The neurochemical coding of bladder hyperinnervation reflected immunoreactivity (IR) for neuropeptides (i.e., Sub P and CGRP) and NF200 with no increase in TH-IR. Sub P and CGRP protein exhibited an age-dependent increase in expression in the urothelium of NGF-OE mice during postnatal development. Intravesical instillation of the neurokinin-1 (NK-1) receptor antagonist, netupitant (0.1 μg/ml) increased the void volume and the interval between micturition events in NGF-OE mice during postnatal development (P7, P21, A) with no effects of micturition pressure (baseline, threshold or peak). These studies highlight the contribution of urothelial NGF and NGF-mediated pleiotropic effects on resulting neuropeptides expression on postnatal maturation of micturition reflexes.
The maturation of peptidergic afferent pathways of the LUT of the neonatal rat may be involved in development of adult patterns of micturition (Maggi et al., 1986; 1987; 1988). In contrast, it has been demonstrated that regional noradrenergic and cholinergic innervation of the rat urinary bladder is relatively stable as the rat matures (Johnson et al., 1988). Bladder afferents contain a variety of neuropeptides, including: CGRP, Sub P, vasoactive intestinal polypeptide, pituitary adenylate cyclase-activating polypeptide, cholecystokinin and enkephalins (Arms and Vizzard, 2011; de Groat et al., 1983; Keast and De Groat, 1992; Vizzard, 2000d, 2001). The innervation of the urinary bladder arises primarily from neuronal cell bodies located at a distance from the urinary bladder; however, some neuronal cell bodies appear transiently in the bladder wall during development and early postnatal life, with few remaining by adulthood (Keast et al., 2015; Zvarova and Vizzard, 2005). The functions of these intramural ganglion neurons are not known but their chemical phenotype more closely resembles autonomic rather than sensory neurons (Forrest et al., 2014; Keast et al., 2015; Zvarova and Vizzard, 2005).
We demonstrated age-dependent increases in Sub P and CGRP protein content in the urothelium of postnatal NGF-OE mice and expression was significantly greater in the urothelium of NGF-OE mice compared to littermate WT mice at all postnatal ages examined. Sub P could be expressed by urothelial cells as well as by nerve fibers in the suburothelial nerve plexus both of which have been previously shown to express Sub P-IR (Andersson, 2002; Arms and Vizzard, 2011; Birder and Andersson, 2013; Birder et al., 2010; Heng et al., 2011; Schnegelsberg et al., 2010). Sub P and CGRP protein expression in detrusor smooth muscle also exhibited age-dependent increases in expression but no differences in protein expression were demonstrated in detrusor between NGF-OE and WT mice at any postnatal age examined. In contrast, Sub P and CGRP mRNA did not exhibit age-dependent differences in expression in urothelium or detrusor in NGF-OE mice. Sub P mRNA was consistently greater in urothelium and detrusor of NGF-OE mice at every postnatal age examined. CGRP mRNA was significantly reduced in urothelium of NGF-OE mice compared to WT and CGRP mRNA expression exhibited an age-dependent decrease in detrusor expression in WT mice. Increased Sub P and CGRP protein content in the urothelium of NGF-OE mice correlated well with increased Sub P- and CGRP-immunoreactivity (IR) in the suburothelial nerve plexus of the urinary bladder, which primarily consists of afferent nerve fibers (Birder and Andersson, 2013). Future studies should consider: (1) co-labeling of Sub P and CGRP in the suburothelial nerve plexus and urothelium and (2) differences in the degree of co-labeling in nerve fibers and urothelial cells among the various postnatal ages, between mouse strains and as a function of onset of the micturition reflex. Hyperinnervation of the urinary bladder of NGF-OE mice during early postnatal development is consistent with our previous studies in adult NGF-OE mice (Schnegelsberg et al., 2010). In addition to increased peptidergic innervation, the suburothelial nerve plexus in NGF-OE mice also exhibited increased NF-200-IR but did not exhibit increased adrenergic-IR.
To determine if increased peptidergic innervation of the urinary bladder was associated with Sub P/NK-1 receptor signaling that affected urinary bladder function, we intravesically infused a NK-1 receptor antagonist, netupitant (0.1 μg/ml) (Greenwood-Van Meerveld et al., 2014; Haab et al., 2014; Zhang et al., 2012), and determined the effects on micturition function in postnatal NGF-OE and WT mice using conscious cystometry. Intravesical infusion of a NK-1 receptor antagonist had little effect on any micturition parameter measured in conscious WT mice (P7, P28, A), which is similar to a previous report in rats (Ishizuka et al., 1994). However, there was an effect on void volume in P14 WT mice where intravesical netupitant (0.1 μg/ml) significantly increased void volume but this effect was not observed at any other age examined (P7, P28, A). In contrast, intravesical instillation of netupitant (0.1 μg/ml) significantly increased void volume and the interval between micturition events (ICI) in NGF-OE mice at P7, P14, P28 and adult. Intravesical netupitant (0.1 μg/ml) did not affect bladder pressure (baseline, threshold or peak micturition pressure) in NGF-OE, littermate WT at any postnatal age examined (P7, P14, P28, A). Intravesical instillation of netupitant significantly reduced the number of NVCs in NGF-OE mice at P7 and P28 with no effects on the number of NVCs in WT mice. The distribution of NK receptor subtypes in the urinary bladder of NGF-OE mice is not known but given the effects demonstrated via intravesical instillation of a NK-1 receptor antagonist, it is likely that NK-1 receptors are located on bladder nerve terminals and/or urothelial cells (Meini et al., 1994). However, evidence of urothelial expression of NK-1 receptors is not widely reported in rat (Heng et al., 2011; Ishizuka et al., 1995) and is unknown in mouse. Although NK receptor subtypes are likely present throughout the detrusor (Meini et al., 1994; Steinhoff et al., 2014), a lack of effect on detrusor NK receptor subtypes (as evident by the absence of effects on micturition pressures), may reflect an inability of the NK-1 receptor antagonist to reach the detrusor due to high transepithelial resistance of the urothelium or a higher concentration of the NK-1 receptor antagonist may be necessary to see an effect on micturition pressures. The results of future studies involving protamine sulfate (to disrupt the urothelial barrier) (Greenwood-Van Meerveld et al., 2015; Klinger and Vizzard, 2008) plus NK-1 receptor antagonist or intravesical instillation of a NK-2 receptor antagonist would be informative when compared to the NK-1 receptor antagonist data generated in this study.
In many species, the neuropeptide Sub P is present throughout the LUT and is released in the spinal cord or in the bladder by capsaicin-induced stimulation of nociceptive afferent nerves (Lecci and Maggi, 2001). Sub P modulates bladder activity by acting on three NK receptor subtypes (NK-1, NK-2, and NK-3) expressed by cells and tissues of the LUT including: bladder smooth muscle cells, urothelial cells, afferent nerves, or spinal cord neurons (Steinhoff et al., 2014) (Arms and Vizzard, 2011; de Groat and Yoshimura, 2009; Lecci and Maggi, 2001). However, considerable differences in the distribution of NK receptors in the LUT among species have been reported and tachykinin/receptor interactions in the LUT are complex (Regoli et al., 1994). The most consistent literature addresses the use of NK-1 receptor antagonists to reduce urinary frequency following neural injury, disease or inflammation. Clinical studies using NK-1 receptor antagonists have demonstrated a reduction in overactive bladder (OAB) symptoms (Green et al., 2006; Frenkl et al., 2010) and a reduction in hyperactive bladder reflexes induced by spinal cord injury, following cyclophosphamide-induced bladder inflammation or chemical irritation of the urinary bladder (Abdel-Gawad et al., 2001; Doi et al., 1999; 2000). It has been demonstrated that these effects reflect NK-1 receptor antagonists acting on CNS and/or PNS components of micturition reflex pathways (Lecci and Maggi, 1995; 2001; Seki et al., 2005). At the level of the urinary bladder, afferent nerves release Sub P to affect afferent nerve activity either indirectly by inducing smooth muscle contractions (Lecci and Maggi, 2001) or directly via an autofeedback mechanism (Sculptoreanu et al., 2009; Sculptoreanu et al., 2008; Sculptoreanu and de Groat, 2007). The regulation of bladder activity by Sub P/NK receptor signaling depends on species, the tissue distribution and the NK receptor subtypes expressed (Lecci and Maggi, 2001). A few studies have examined the pharmacological profiles of NK receptor subtypes in control mouse LUT (Burcher et al., 1986; Nsa Allogho et al., 1997; Regoli et al., 1994); however, no studies have examined NK receptor subtype distribution in early postnatal or adult LUT in the NGF-OE mouse. We are using Q-PCR and immunohistochemical assays on LUT tissues from NGF-OE mice to address this void in our knowledge.
Micturition in normal adult animals and humans occurs through activation of a vesicovesical reflex organized at supraspinal levels and triggered through distension of the urinary bladder and activation of mechanoreceptors (de Groat and Araki, 1999; de Groat et al., 1998; Maggi et al., 1986). It is widely accepted that the vesicovesical reflex is absent in newborn animals and that this reflex is not fully developed or functional at birth ( Maggi et al., 1986; Zvarova and Zvara, 2012). In early postnatal animals (e.g. rats, cats, guinea pigs) micturition is activated via a somatovesical reflex when the mother licks the perineal area (Capek and Jelinek, 1956; de Groat and Araki, 1999; de Groat et al., 1998; Ng et al., 2007; Maggi et al., 1986). Interestingly, transneuronal tracing studies from the urinary bladder in early postnatal rats (P2 and P12) demonstrated that the supraspinal micturition reflex pathways are intact but do not function until the third postnatal week (Sugaya et al., 1997). In this study, NGF-OE mice exhibited early onset of micturition that was demonstrated using voiding on filter paper assays and conscious cystometry. The early onset of micturition in NGF-OE mice by P5 occurred in the absence of perigenital stimulation (i.e., somatovesical reflex). In contrast, maternal separation has been shown to increase the duration of the somatovesical reflex but had no effect on the appearance of spontaneous voiding (i.e., vesicovesical reflex). In postnatal and adult NGF-OE mice, intravesical instillation of a NK-1 receptor antagonist, netupitant (0.1 μg/ml), increased void volume and ICI in NGF-OE mice; however, effects of NK-1 receptor blockade on micturition onset could not be determined. Given the chronic urothelial overexpression of NGF in the NGF-OE mice (Schnegelsberg et al., 2010), designing an experiment using pharmacological tools (NK-1 receptor antagonist) to block the long-term effects of NGF did not seem plausible. One way to address the contribution of peptidergic pathways to onset of micturition in NGF-OE mice could begin with developing new mouse lines that result from crossing TRPV1−/− mice (Birder et al., 2002) with NGF-OE mice or from crossing NK-1−/− mice (Saban et al., 2000) with NGF-OE mice.
Additional factors that may contribute to the initiation of the micturition reflex in adults include the myogenic contractile activity of the detrusor muscle and maturation of central micturition pathways (Maggi et al., 1986). The neonatal bladder exhibits high-amplitude, low-frequency, spontaneous contractions during postnatal weeks 1–3 prior to evidence of mature voiding (Kanai et al., 2007; Maggi et al., 1986; Ng et al., 2007). With the development of mature supraspinal reflex pathways, spontaneous bladder activity decreases. This spontaneous activity in neonatal bladders is highly organized and originates in the urothelium/suburothelium near the bladder dome (Kanai et al., 2007). Although the bladder exhibits myogenic activity that is independently driven by the detrusor smooth muscle, parasympathetic and sympathetic pathways are the effectors for coordinated urinary bladder storage and release. Thus, target organ (i.e., urinary bladder) changes need to be integrated with the PNS and CNS to fully understand the mechanisms underlying bladder innervation and the maturation of voiding reflexes. Maturation of central pathways may also be a factor in the postnatal development of bladder reflexes. Various transmitters including serotonin (Bregman, 1987), CRF/urocortin/receptors (CRFR1, CRFR2) (LaBerge et al., 2006; Puder and Papka, 2001; Studeny and Vizzard, 2005), somatostatin (Marti et al., 1987), NADPH-diaphorase and nNOS (Vizzard et al., 1994) appear or increase in prominence in the lumbosacral spinal cord during the second to third postnatal week.
Conclusions
These studies have begun to provide insights into some factors that contribute to the maturation of the micturition reflex from a somatovesical to a vesicovesical reflex. There is a large gap in our understanding of the mechanisms that underlie the postnatal maturation of voiding reflexes. Using a transgenic mouse model with chronic urothelial overexpression of NGF (Schnegelsberg et al., 2010) has given us the opportunity to examine the contribution of target-derived growth factors as well as secondary events resulting from NGF-OE including increased neuropeptides (e.g., Sub P and CGRP) expression in the urinary bladder (i.e., urothelium and suburothelial nerve plexus). We demonstrated an age-dependent increase in Sub P and CGRP expression in the urothelium and suburothelial nerve plexus in postnatal NGF-OE mice. In addition to an early onset of micturition in NGF-OE mice in the absence of a perineal-to-bladder reflex, postnatal NGF-OE mice exhibit increased voiding frequency, shorter intervals between void events and the presence of NVCs in comparison to littermate WT mice. Intravesical instillation of a NK-1 receptor antagonist reduced voiding frequency and increased void volume in NGF-OE mice with few effects on WT mice. In addition to providing key insights into developmental maturation of voiding reflexes, these studies may also provide important information relevant to adult OAB and neurogenic voiding disorders. For example, NK-1 receptor antagonists have been used to improve bladder dysfunction in adult overactive bladder and manage neurogenic disorders of micturition (Abdel-Gawad et al., 2001; Doi et al., 1999; 2000; Frenkl et al., 2010; Green et al., 2006). In addition, studies of the development of micturition reflexes may provide insights into the management of neurogenic disorders of micturition. For example, injuries or disorders of the adult spinal cord that lead to the reemergence of primitive functions (reflex voiding and incontinence), prominent during early development, may represent a return to early voiding patterns (de Groat et al., 1998; de Groat and Yoshimura, 2012; Keast et al., 2015). Thus, the study of the maturation of voiding reflexes not only has merit on its own but also has the potential to improve our understanding of OAB and neurogenic voiding disorders.
Acknowledgments
The authors thank Dr. Debra Cockayne, Roche Palo Alto for the generous gift of NGF-OE mouse breeders used in the present study. This work was funded by National Institutes of Health (NIH) grants DK051369 (MAV), DK060481 (MAV) and DK065989 (MAV). This publication was also supported by grants from the National Center for Research Resources (5 P30 RR 032135) and the National Institute of General Medical Sciences (8 P30 GM 103498) from the NIH.
Footnotes
Conflict of Interest
The authors declare that there is no conflict of interest in this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Gawad M, Dion SB, Elhilali MM. Evidence of a peripheral role of neurokinins in detrusor hyperreflexia: a further study of selective tachykinin antagonists in chronic spinal injured rats. J Urol. 2001;165:1739–1744. [PubMed] [Google Scholar]
- Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- Arms L, Girard BM, Malley SE, Vizzard MA. Expression and function of CCL2/CCR2 in rat micturition reflexes and somatic sensitivity with urinary bladder inflammation. Am J Physiol Renal Physiol. 2013;305:F111–122. doi: 10.1152/ajprenal.00139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arms L, Girard BM, Vizzard MA. Expression and function of CXCL12/CXCR4 in rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298:F589–600. doi: 10.1152/ajprenal.00628.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arms L, Vizzard MA. Neuropeptides in lower urinary tract function. Handb Exp Pharmacol. 2011:395–423. doi: 10.1007/978-3-642-16499-6_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder L, Andersson KE. Urothelial signaling. Physiol Rev. 2013;93:653–680. doi: 10.1152/physrev.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Birder LA, Wolf-Johnston AS, Chib MK, Buffington CA, Roppolo JR, Hanna-Mitchell AT. Beyond neurons: Involvement of urothelial and glial cells in bladder function. Neurourol Urodyn. 2010;29:88–96. doi: 10.1002/nau.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm JJ, Haynes JL. Bacteriology of “midstream catch” urines. Studies in newborn infants. Am J Dis Child. 1966;111:366–369. doi: 10.1001/archpedi.1966.02090070064007. [DOI] [PubMed] [Google Scholar]
- Braas KM, May V, Zvara P, Nausch B, Kliment J, Dunleavy JD, Nelson MT, Vizzard MA. Role for pituitary adenylate cyclase activating polypeptide in cystitis-induced plasticity of micturition reflexes. Am J Physiol Regul Integr Comp Physiol. 2006;290:R951–962. doi: 10.1152/ajpregu.00734.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bregman BS. Development of serotonin immunoreactivity in the rat spinal cord and its plasticity after neonatal spinal cord lesions. Brain Res. 1987;431:245–263. doi: 10.1016/0165-3806(87)90213-6. [DOI] [PubMed] [Google Scholar]
- Burcher E, Buck SH, Lovenberg W, O’Donohue TL. Characterization and autoradiographic localization of multiple tachykinin binding sites in gastrointestinal tract and bladder. J Pharmacol Exp Ther. 1986;236:819–831. [PubMed] [Google Scholar]
- Capek K, Jelinek J. The development of the control of water metabolism. I. The excretion of urine in young rats. Physiol Bohemoslov. 1956;5:91–96. [PubMed] [Google Scholar]
- Cheppudira BP, Girard BM, Malley SE, Schutz KC, May V, Vizzard MA. Upregulation of vascular endothelial growth factor isoform VEGF-164 and receptors (VEGFR-2, Npn-1, and Npn-2) in rats with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F826–836. doi: 10.1152/ajprenal.90305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrow K, Girard BM, Vizzard MA. Expression and response of acid-sensing ion channels in urinary bladder to cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2010;298:F1130–1139. doi: 10.1152/ajprenal.00618.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrow KA, Vizzard MA. Phosphorylation of extracellular signal-regulated kinases in urinary bladder in rats with cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2007;293:R125–134. doi: 10.1152/ajpregu.00857.2006. [DOI] [PubMed] [Google Scholar]
- De Groat WC. Nervous control of the urinary bladder of the cat. Brain Res. 1975;87:201–211. doi: 10.1016/0006-8993(75)90417-5. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Central neural control of the lower urinary tract. Ciba Found Symp. 1990;151:27–44. doi: 10.1002/9780470513941.ch3. discussion 44–56. [DOI] [PubMed] [Google Scholar]
- de Groat WC. Anatomy and physiology of the lower urinary tract. Urol Clin North Am. 1993;20:383–401. [PubMed] [Google Scholar]
- de Groat WC, Araki I. Maturation of bladder reflex pathways during postnatal development. Adv Exp Med Biol. 1999;462:253–263. doi: 10.1007/978-1-4615-4737-2_19. discussion 311–220. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behav Brain Res. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Kawatani M, Hisamitsu T, Lowe I, Morgan C, Roppolo J, Booth AM, Nadelhaft I, Kuo D, Thor K. The role of neuropeptides in the sacral autonomic reflex pathways of the cat. J Auton Nerv Syst. 1983;7:339–350. doi: 10.1016/0165-1838(83)90087-5. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Ryall RW. Reflexes to sacral parasympathetic neurones concerned with micturition in the cat. J Physiol. 1969;200:87–108. doi: 10.1113/jphysiol.1969.sp008683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Afferent nerve regulation of bladder function in health and disease. Handb Exp Pharmacol. 2009:91–138. doi: 10.1007/978-3-540-79090-7_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groat WC, Yoshimura N. Plasticity in reflex pathways to the lower urinary tract following spinal cord injury. Exp Neurol. 2012;235:123–132. doi: 10.1016/j.expneurol.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitrieva N, McMahon SB. Sensitisation of visceral afferents by nerve growth factor in the adult rat. Pain. 1996;66:87–97. doi: 10.1016/0304-3959(96)02993-4. [DOI] [PubMed] [Google Scholar]
- Doi T, Kamo I, Imai S, Okanishi S, Ikeura Y, Natsugari H. Effects of TAK-637, a tachykinin receptor antagonist, on the micturition reflex in guinea pigs. Eur J Pharmacol. 2000;395:241–246. doi: 10.1016/s0014-2999(00)00177-1. [DOI] [PubMed] [Google Scholar]
- Doi T, Kamo I, Imai S, Okanishi S, Ishimaru T, Ikeura Y, Natsugari H. Effects of TAK-637, a tachykinin receptor antagonist, on lower urinary tract function in the guinea pig. Eur J Pharmacol. 1999;383:297–303. doi: 10.1016/s0014-2999(99)00657-3. [DOI] [PubMed] [Google Scholar]
- Ekstrom J, Ekman R, Hakanson R. Ontogeny of neuropeptides in the rat urinary bladder. Regul Pept. 1994;50:23–28. doi: 10.1016/0167-0115(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Forrest SL, Osborne PB, Keast JR. Characterization of axons expressing the artemin receptor in the female rat urinary bladder: a comparison with other major neuronal populations. J Comp Neurol. 2014;522:3900–3927. doi: 10.1002/cne.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkl TL, Zhu H, Reiss T, Seltzer O, Rosenberg E, Green S. A multicenter, double-blind, randomized, placebo controlled trial of a neurokinin-1 receptor antagonist for overactive bladder. J Urol. 2010;184:616–622. doi: 10.1016/j.juro.2010.03.147. [DOI] [PubMed] [Google Scholar]
- Gabella G, Davis C. Distribution of afferent axons in the bladder of rats. J Neurocytol. 1998;27:141–155. doi: 10.1023/a:1006903507321. [DOI] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Braas KM, May V, Vizzard MA. PACAP/VIP and Receptor Characterization in Micturition Pathways in Mice with Overexpression of NGF in Urothelium. J Mol Neurosci. doi: 10.1007/s12031-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Braas KM, May V, Vizzard MA. PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium. J Mol Neurosci. 2010;42:378–389. doi: 10.1007/s12031-010-9384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Malley SE, Vizzard MA. Neurotrophin/receptor expression in urinary bladder of mice with overexpression of NGF in urothelium. Am J Physiol Renal Physiol. 2011;300:F345–355. doi: 10.1152/ajprenal.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard BM, Merrill L, Malley S, Vizzard MA. Increased TRPV4 expression in urinary bladder and lumbosacral dorsal root ganglia in mice with chronic overexpression of NGF in urothelium. J Mol Neurosci. 2013;51:602–614. doi: 10.1007/s12031-013-0033-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Girard BM, Vizzard MA. Expression and function of transforming growth factor-beta isoforms and cognate receptors in the rat urinary bladder following cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2013;305:F1265–1276. doi: 10.1152/ajprenal.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez EJ, Peterson A, Malley S, Daniel M, Lambert D, Kosofsky M, Vizzard MA. The effects of tempol on cyclophosphamide-induced oxidative stress in rat micturition reflexes. Scientific World Journal. 2015;2015:545048. doi: 10.1155/2015/545048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Alon A, Ianus J, McNaughton KS, Tozzi CA, Reiss TF. Efficacy and safety of a neurokinin-1 receptor antagonist in postmenopausal women with overactive bladder with urge urinary incontinence. J Urol. 2006;176:2535–2540. doi: 10.1016/j.juro.2006.08.018. discussion 2540. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Mohammadi E, Tyler K, Pietra C, Bee LA, Dickenson A. Synergistic effect of 5-hydroxytryptamine 3 and neurokinin 1 receptor antagonism in rodent models of somatic and visceral pain. J Pharmacol Exp Ther. 2014;351:146–152. doi: 10.1124/jpet.114.216028. [DOI] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B, Mohammadi E, Tyler K, Van Gordon S, Parker A, Towner R, Hurst R. Mechanisms of Visceral Organ Crosstalk: Importance of Alterations in Permeability in Rodent Models. J Urol. 2015;194:804–811. doi: 10.1016/j.juro.2015.02.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Bjorling DE. Nerve growth factor mediates peripheral mechanical hypersensitivity that accompanies experimental cystitis in mice. Neurosci Lett. 2006;392:193–197. doi: 10.1016/j.neulet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Guerios SD, Wang ZY, Boldon K, Bushman W, Bjorling DE. Blockade of NGF and trk receptors inhibits increased peripheral mechanical sensitivity accompanying cystitis in rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R111–122. doi: 10.1152/ajpregu.00728.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haab F, Braticevici B, Krivoborodov G, Palmas M, Zufferli Russo M, Pietra C. Efficacy and safety of repeated dosing of netupitant, a neurokinin-1 receptor antagonist, in treating overactive bladder. Neurourol Urodyn. 2014;33:335–340. doi: 10.1002/nau.22406. [DOI] [PubMed] [Google Scholar]
- Heng YJ, Saunders CI, Kunde DA, Geraghty DP. TRPV1, NK1 receptor and substance P immunoreactivity and gene expression in the rat lumbosacral spinal cord and urinary bladder after systemic, low dose vanilloid administration. Regul Pept. 2011;167:250–258. doi: 10.1016/j.regpep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Hu VY, Zvara P, Dattilio A, Redman TL, Allen SJ, Dawbarn D, Stroemer RP, Vizzard MA. Decrease in bladder overactivity with REN1820 in rats with cyclophosphamide induced cystitis. J Urol. 2005;173:1016–1021. doi: 10.1097/01.ju.0000155170.15023.e5. [DOI] [PubMed] [Google Scholar]
- Ishizuka O, Igawa Y, Mattiasson A, Andersson KE. Capsaicin-induced bladder hyperactivity in normal conscious rats. J Urol. 1994;152:525–530. doi: 10.1016/s0022-5347(17)32787-8. [DOI] [PubMed] [Google Scholar]
- Ishizuka O, Mattiasson A, Andersson KE. Tachykinin effects on bladder activity in conscious normal rats. J Urol. 1995;154:257–261. [PubMed] [Google Scholar]
- Iuchi H, Satoh Y, Ono K. Postnatal development of neuropeptide Y- and calcitonin gene-related peptide-immunoreactive nerves in the rat urinary bladder. Anat Embryol (Berl) 1994;189:361–373. doi: 10.1007/BF00190591. [DOI] [PubMed] [Google Scholar]
- Jaggar SI, Scott HC, Rice AS. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. Br J Anaesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Skau KA, Gerald MC, Wallace LJ. Regional noradrenergic and cholinergic neurochemistry in the rat urinary bladder: effects of age. J Urol. 1988;139:611–615. doi: 10.1016/s0022-5347(17)42543-2. [DOI] [PubMed] [Google Scholar]
- Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol. 2007;292:F1065–1072. doi: 10.1152/ajprenal.00229.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keast JR, De Groat WC. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992;319:615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Keast JR, Smith-Anttila CJ, Osborne PB. Developing a functional urinary bladder: a neuronal context. Front Cell Dev Biol. 2015;3:53. doi: 10.3389/fcell.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger MB, Girard B, Vizzard MA. p75NTR expression in rat urinary bladder sensory neurons and spinal cord with cyclophosphamide-induced cystitis. J Comp Neurol. 2008;507:1379–1392. doi: 10.1002/cne.21627. [DOI] [PubMed] [Google Scholar]
- Klinger MB, Vizzard MA. Role of p75NTR in female rat urinary bladder with cyclophosphamide-induced cystitis. Am J Physiol Renal Physiol. 2008;295:F1778–1789. doi: 10.1152/ajprenal.90501.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBerge J, Malley SE, Zvarova K, Vizzard MA. Expression of corticotropin-releasing factor and CRF receptors in micturition pathways after cyclophosphamide-induced cystitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R692–703. doi: 10.1152/ajpregu.00086.2006. [DOI] [PubMed] [Google Scholar]
- Lamb K, Gebhart GF, Bielefeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Lecci A, Maggi CA. Spinal cord tachykinins in the micturition reflex. Prog Brain Res. 1995;104:145–159. doi: 10.1016/s0079-6123(08)61789-6. [DOI] [PubMed] [Google Scholar]
- Lecci A, Maggi CA. Tachykinins as modulators of the micturition reflex in the central and peripheral nervous system. Regul Pept. 2001;101:1–18. doi: 10.1016/s0167-0115(01)00285-3. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Geppetti P, Santicioli P, Frilli S, Giuliani S, Furio M, Theodorsson E, Fusco B, Meli A. Tachykinin-like immunoreactivity in the mammalian urinary bladder: correlation with the functions of the capsaicin-sensitive sensory nerves. Neuroscience. 1988;26:233–242. doi: 10.1016/0306-4522(88)90140-6. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Santicioli F, Meli A. Evidence for the involvement of a capsaicin-sensitive innervation in the afferent branch of micturition reflex in rats. Acta Physiol Hung. 1987;69:425–435. [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A. Postnatal development of micturition reflex in rats. Am J Physiol. 1986;250:R926–931. doi: 10.1152/ajpregu.1986.250.5.R926. [DOI] [PubMed] [Google Scholar]
- Marti E, Gibson SJ, Polak JM, Facer P, Springall DR, Van Aswegen G, Aitchison M, Koltzenburg M. Ontogeny of peptide- and amine-containing neurones in motor, sensory, and autonomic regions of rat and human spinal cord, dorsal root ganglia, and rat skin. J Comp Neurol. 1987;266:332–359. doi: 10.1002/cne.902660304. [DOI] [PubMed] [Google Scholar]
- McMahon SB. NGF as a mediator of inflammatory pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:431–440. doi: 10.1098/rstb.1996.0039. [DOI] [PubMed] [Google Scholar]
- Meini S, Patacchini R, Maggi CA. Tachykinin NK1 receptor subtypes in the rat urinary bladder. Br J Pharmacol. 1994;111:739–746. doi: 10.1111/j.1476-5381.1994.tb14800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Girard B, Arms L, Guertin P, Vizzard MA. Neuropeptide/Receptor expression and plasticity in micturition pathways. Curr Pharm Des. 2013;19:4411–4422. doi: 10.2174/1381612811319240008. [DOI] [PubMed] [Google Scholar]
- Merrill L, Girard BM, May V, Vizzard MA. Transcriptional and translational plasticity in rodent urinary bladder TRP channels with urinary bladder inflammation, bladder dysfunction, or postnatal maturation. J Mol Neurosci. 2012;48:744–756. doi: 10.1007/s12031-012-9867-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill L, Vizzard MA. Intravesical TRPV4 blockade reduces repeated variate stress-induced bladder dysfunction by increasing bladder capacity and decreasing voiding frequency in male rats. Am J Physiol Regul Integr Comp Physiol. 2014;307:R471–480. doi: 10.1152/ajpregu.00008.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YK, de Groat WC, Wu HY. Smooth muscle and neural mechanisms contributing to the downregulation of neonatal rat spontaneous bladder contractions during postnatal development. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2100–2112. doi: 10.1152/ajpregu.00779.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nsa Allogho S, Nguyen-Le XK, Gobeil F, Pheng LH, Regoli D. Neurokinin receptors (NK1, NK2) in the mouse: a pharmacological study. Can J Physiol Pharmacol. 1997;75:552–557. [PubMed] [Google Scholar]
- Puder BA, Papka RE. Distribution and origin of corticotropin-releasing factor-immunoreactive axons in the female rat lumbosacral spinal cord. J Neurosci Res. 2001;66:1217–1225. doi: 10.1002/jnr.10033. [DOI] [PubMed] [Google Scholar]
- Regoli D, Nguyen QT, Jukic D. Neurokinin receptor subtypes characterized by biological assays. Life Sci. 1994;54:2035–2047. doi: 10.1016/0024-3205(94)00712-8. [DOI] [PubMed] [Google Scholar]
- Ribeiro-da-Silva A, Cuello AC, Henry JL. NGF over-expression during development leads to permanent alterations in innervation in the spinal cord and in behavioural responses to sensory stimuli. Neuropeptides. 2000;34:281–291. doi: 10.1054/npep.2000.0822. [DOI] [PubMed] [Google Scholar]
- Saban R, Saban MR, Nguyen NB, Lu B, Gerard C, Gerard NP, Hammond TG. Neurokinin-1 (NK-1) receptor is required in antigen-induced cystitis. Am J Pathol. 2000;156:775–780. doi: 10.1016/S0002-9440(10)64944-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sann H, Walb G, Pierau FK. Postnatal development of the autonomic and sensory innervation of the musculature in the rat urinary bladder. Neurosci Lett. 1997;236:29–32. doi: 10.1016/s0304-3940(97)00752-0. [DOI] [PubMed] [Google Scholar]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2009 doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed]
- Schnegelsberg B, Sun TT, Cain G, Bhattacharya A, Nunn PA, Ford AP, Vizzard MA, Cockayne DA. Overexpression of NGF in mouse urothelium leads to neuronal hyperinnervation, pelvic sensitivity, and changes in urinary bladder function. Am J Physiol Regul Integr Comp Physiol. 2010;298:R534–547. doi: 10.1152/ajpregu.00367.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, Artim DE, de Groat WC. Neurokinins inhibit low threshold inactivating K+ currents in capsaicin responsive DRG neurons. Exp Neurol. 2009;219:562–573. doi: 10.1016/j.expneurol.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, Aura Kullmann F, de Groat WC. Neurokinin 2 receptor-mediated activation of protein kinase C modulates capsaicin responses in DRG neurons from adult rats. Eur J Neurosci. 2008;27:3171–3181. doi: 10.1111/j.1460-9568.2008.06267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, de Groat WC. Neurokinins enhance excitability in capsaicin-responsive DRG neurons. Exp Neurol. 2007;205:92–100. doi: 10.1016/j.expneurol.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki S, Erickson KA, Seki M, Nishizawa O, Igawa Y, Ogawa T, de Groat WC, Chancellor MB, Yoshimura N. Elimination of rat spinal neurons expressing neurokinin 1 receptors reduces bladder overactivity and spinal c-fos expression induced by bladder irritation. Am J Physiol Renal Physiol. 2005;288:F466–473. doi: 10.1152/ajprenal.00274.2004. [DOI] [PubMed] [Google Scholar]
- Sillen U. Bladder function in healthy neonates and its development during infancy. J Urol. 2001;166:2376–2381. doi: 10.1016/s0022-5347(05)65594-2. [DOI] [PubMed] [Google Scholar]
- Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny S, Cheppudira BP, Meyers S, Balestreire EM, Apodaca G, Birder LA, Braas KM, Waschek JA, May V, Vizzard MA. Urinary bladder function and somatic sensitivity in vasoactive intestinal polypeptide (VIP)−/− mice. J Mol Neurosci. 2008;36:175–187. doi: 10.1007/s12031-008-9100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studeny S, Vizzard MA. Corticotropin-releasing factor (CRF) expression in postnatal and adult rat sacral parasympathetic nucleus (SPN) Cell Tissue Res. 2005;322:339–352. doi: 10.1007/s00441-005-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, Roppolo JR, Yoshimura N, Card JP, de Groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neurosci Lett. 1997;223:197–200. doi: 10.1016/s0304-3940(97)13433-4. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Alterations in spinal cord Fos protein expression induced by bladder stimulation following cystitis. Am J Physiol Regul Integr Comp Physiol. 2000a;278:R1027–1039. doi: 10.1152/ajpregu.2000.278.4.R1027. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Changes in urinary bladder neurotrophic factor mRNA and NGF protein following urinary bladder dysfunction. Exp Neurol. 2000b;161:273–284. doi: 10.1006/exnr.1999.7254. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Increased expression of spinal cord Fos protein induced by bladder stimulation after spinal cord injury. Am J Physiol Regul Integr Comp Physiol. 2000c;279:R295–305. doi: 10.1152/ajpregu.2000.279.1.R295. [DOI] [PubMed] [Google Scholar]
- Vizzard MA. Up-regulation of pituitary adenylate cyclase-activating polypeptide in urinary bladder pathways after chronic cystitis. J Comp Neurol. 2000d;420:335–348. [PubMed] [Google Scholar]
- Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erdman SL, Forstermann U, de Groat WC. Ontogeny of nitric oxide synthase in the lumbosacral spinal cord of the neonatal rat. Brain Res Dev Brain Res. 1994;81:201–217. doi: 10.1016/0165-3806(94)90307-7. [DOI] [PubMed] [Google Scholar]
- Zhang X, Pietra C, Lovati E, de Groat WC. Activation of neurokinin-1 receptors increases the excitability of guinea pig dorsal root ganglion cells. J Pharmacol Exp Ther. 2012;343:44–52. doi: 10.1124/jpet.112.196113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvara P, Vizzard MA. Exogenous overexpression of nerve growth factor in the urinary bladder produces bladder overactivity and altered micturition circuitry in the lumbosacral spinal cord. BMC Physiol. 2007;7:9. doi: 10.1186/1472-6793-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvarova K, Vizzard MA. Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: a developmental study. J Comp Neurol. 2005;489:501–517. doi: 10.1002/cne.20657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvarova K, Zvara P. Urinary bladder function in conscious rat pups: a developmental study. Am J Physiol Renal Physiol. 2012;302:F1563–1568. doi: 10.1152/ajprenal.00567.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]