Abstract
Classical insulin and insulin-like growth factor I (IGF-I) receptors exist as well defined alpha 2 beta 2 heterotetrameric complexes that are assembled from two identical alpha beta heterodimeric half-receptor precursors. Recent evidence suggests that insulin and IGF-I half-receptors can heterologously assemble to form alpha 2 beta 2 insulin/IGF-I hybrid receptor complexes in vivo and in vitro. We have utilized hybrid receptor complexes to examine ligand-stimulated transmembrane signaling of wild-type insulin (alpha beta INS.WT) or IGF-I (alpha beta IGF.WT) half-receptors assembled with a kinase-defective insulin half-receptor mutant (alpha beta INS.A/K). In vitro assembly of either (alpha beta)IGF.WT/(alpha beta)INS.A/K or (alpha beta)INS.WT/(alpha beta)INS.A/K hybrid receptors resulted in decreased substrate protein kinase activity. The degree of protein kinase inactivation directly correlated with the amount of immunologically cross-reactive hybrid receptors formed. In contrast to substrate kinase activity, insulin-stimulated autophosphorylation of the (alpha beta)INS.WT/(alpha beta)INS.A/K hybrid receptor complex was completely unaffected in comparison to the wild-type (alpha beta)INS.WT/(alpha beta)INS.WT receptor. To assess a molecular basis for this difference, autophosphorylation of a hybrid receptor composed of a truncated beta-subunit insulin half-receptor with the kinase-defective half-receptor, (alpha beta)INS. delta CT/(alpha beta)INS.A/K, demonstrated the exclusive autophosphorylation of the (alpha beta)INS.A/K half-receptor beta subunit. These results demonstrate that ligand-dependent substrate phosphorylation by insulin and IGF-I holoreceptors requires interactions between two functional beta subunits within the alpha 2 beta 2 heterotetrameric complex and occurs through an intramolecular trans-phosphorylation reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
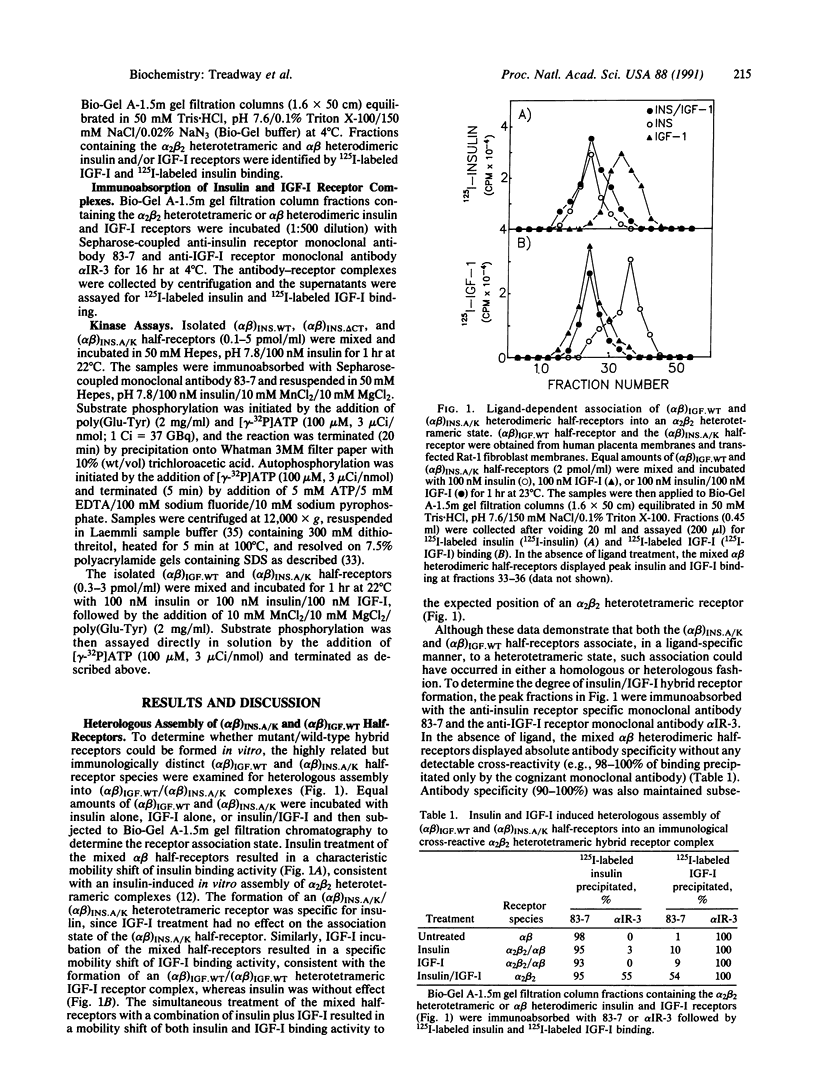
- Böni-Schnetzler M., Kaligian A., DelVecchio R., Pilch P. F. Ligand-dependent intersubunit association within the insulin receptor complex activates its intrinsic kinase activity. J Biol Chem. 1988 May 15;263(14):6822–6828. [PubMed] [Google Scholar]
- Böni-Schnetzler M., Rubin J. B., Pilch P. F. Structural requirements for the transmembrane activation of the insulin receptor kinase. J Biol Chem. 1986 Nov 15;261(32):15281–15287. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Deutsch P. J., Wan C. F., Rosen O. M., Rubin C. S. Latent insulin receptors and possible receptor precursors in 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1983 Jan;80(1):133–136. doi: 10.1073/pnas.80.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltz S. M., Swanson M. L., Wemmie J. A., Pessin J. E. Functional properties of an isolated alpha beta heterodimeric human placenta insulin-like growth factor 1 receptor complex. Biochemistry. 1988 May 3;27(9):3234–3242. doi: 10.1021/bi00409a017. [DOI] [PubMed] [Google Scholar]
- Forsayeth J., Maddux B., Goldfine I. D. Biosynthesis and processing of the human insulin receptor. Diabetes. 1986 Jul;35(7):837–846. doi: 10.2337/diab.35.7.837. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D. The insulin receptor: molecular biology and transmembrane signaling. Endocr Rev. 1987 Aug;8(3):235–255. doi: 10.1210/edrv-8-3-235. [DOI] [PubMed] [Google Scholar]
- Grunberger G., Zick Y., Gorden P. Defect in phosphorylation of insulin receptors in cells from an insulin-resistant patient with normal insulin binding. Science. 1984 Mar 2;223(4639):932–934. doi: 10.1126/science.6141638. [DOI] [PubMed] [Google Scholar]
- Hedo J. A., Collier E., Watkinson A. Myristyl and palmityl acylation of the insulin receptor. J Biol Chem. 1987 Jan 25;262(3):954–957. [PubMed] [Google Scholar]
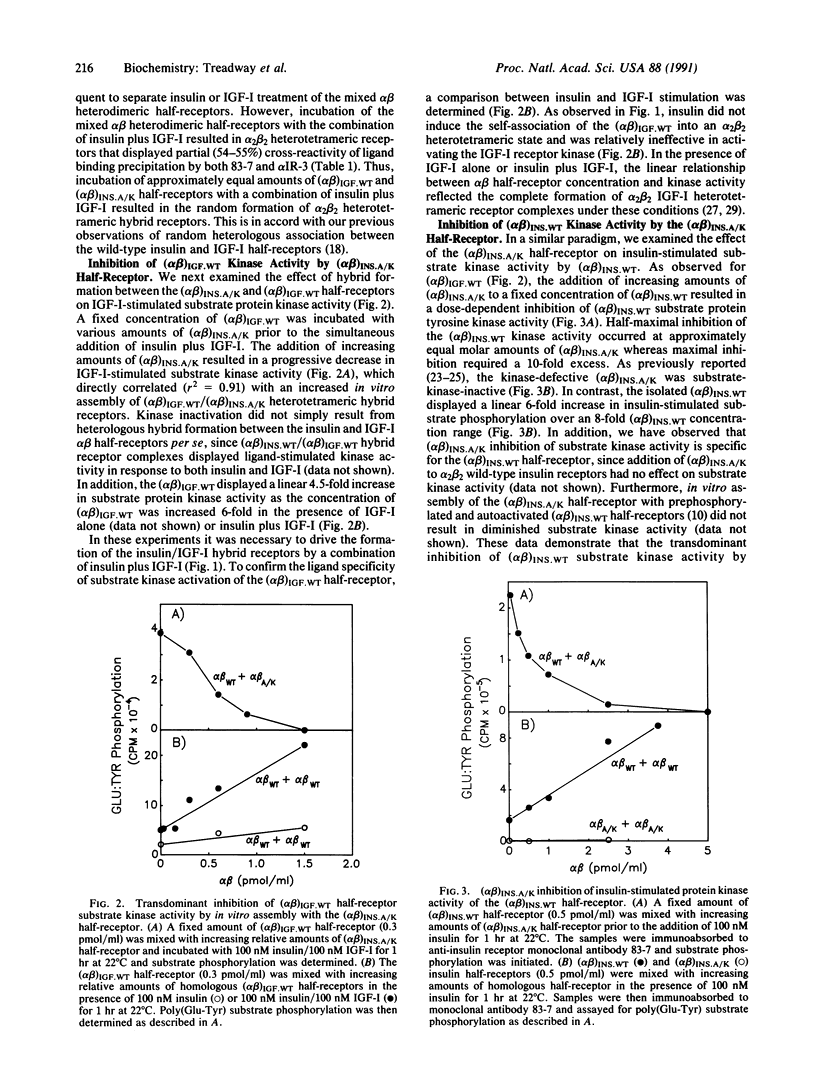
- Hedo J. A., Kasuga M., Van Obberghen E., Roth J., Kahn C. R. Direct demonstration of glycosylation of insulin receptor subunits by biosynthetic and external labeling: evidence for heterogeneity. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4791–4795. doi: 10.1073/pnas.78.8.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Cuatrecasas P. Monensin blocks the maturation of receptors for insulin and somatomedin C: identification of receptor precursors. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1228–1231. doi: 10.1073/pnas.80.5.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H. H., Freidenberg G. R., Kladde M., Olefsky J. M. Insulin activation of insulin receptor tyrosine kinase in intact rat adipocytes. An in vitro system to measure histone kinase activity of insulin receptors activated in vivo. J Biol Chem. 1986 Apr 5;261(10):4691–4697. [PubMed] [Google Scholar]
- Kull F. C., Jr, Jacobs S., Su Y. F., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Monoclonal antibodies to receptors for insulin and somatomedin-C. J Biol Chem. 1983 May 25;258(10):6561–6566. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Levy J., Huecksteadt T., Dull T. J., Lee J., Ullrich A., Olefsky J. M. Properties of a human insulin receptor with a COOH-terminal truncation. I. Insulin binding, autophosphorylation, and endocytosis. J Biol Chem. 1988 Jun 25;263(18):8904–8911. [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Thies R. S., Olefsky J. M. Dissection of the growth versus metabolic effects of insulin and insulin-like growth factor-I in transfected cells expressing kinase-defective human insulin receptors. J Biol Chem. 1990 Jan 25;265(3):1678–1682. [PubMed] [Google Scholar]
- Moller D. E., Flier J. S. Detection of an alteration in the insulin-receptor gene in a patient with insulin resistance, acanthosis nigricans, and the polycystic ovary syndrome (type A insulin resistance). N Engl J Med. 1988 Dec 8;319(23):1526–1529. doi: 10.1056/NEJM198812083192306. [DOI] [PubMed] [Google Scholar]
- Morrison B. D., Feltz S. M., Pessin J. E. Polylysine specifically activates the insulin-dependent insulin receptor protein kinase. J Biol Chem. 1989 Jun 15;264(17):9994–10001. [PubMed] [Google Scholar]
- Morrison B. D., Swanson M. L., Sweet L. J., Pessin J. E. Insulin-dependent covalent reassociation of isolated alpha beta heterodimeric insulin receptors into an alpha 2 beta 2 heterotetrameric disulfide-linked complex. J Biol Chem. 1988 Jun 5;263(16):7806–7813. [PubMed] [Google Scholar]
- Moxham C. P., Duronio V., Jacobs S. Insulin-like growth factor I receptor beta-subunit heterogeneity. Evidence for hybrid tetramers composed of insulin-like growth factor I and insulin receptor heterodimers. J Biol Chem. 1989 Aug 5;264(22):13238–13244. [PubMed] [Google Scholar]
- O'Hare T., Pilch P. F. Separation and characterization of three insulin receptor species that differ in subunit composition. Biochemistry. 1988 Jul 26;27(15):5693–5700. doi: 10.1021/bi00415a045. [DOI] [PubMed] [Google Scholar]
- Odawara M., Kadowaki T., Yamamoto R., Shibasaki Y., Tobe K., Accili D., Bevins C., Mikami Y., Matsuura N., Akanuma Y. Human diabetes associated with a mutation in the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):66–68. doi: 10.1126/science.2544998. [DOI] [PubMed] [Google Scholar]
- Rechler M. M., Nissley S. P. The nature and regulation of the receptors for insulin-like growth factors. Annu Rev Physiol. 1985;47:425–442. doi: 10.1146/annurev.ph.47.030185.002233. [DOI] [PubMed] [Google Scholar]
- Ronnett G. V., Knutson V. P., Kohanski R. A., Simpson T. L., Lane M. D. Role of glycosylation in the processing of newly translated insulin proreceptor in 3T3-L1 adipocytes. J Biol Chem. 1984 Apr 10;259(7):4566–4575. [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. S., Gherzi R., Johnson E. L., Chou C. K., Rosen O. M. The protein-tyrosine kinase activity of the insulin receptor is necessary for insulin-mediated receptor down-regulation. J Biol Chem. 1987 Aug 25;262(24):11833–11840. [PubMed] [Google Scholar]
- Soos M. A., O'Brien R. M., Brindle N. P., Stigter J. M., Okamoto A. K., Whittaker J., Siddle K. Monoclonal antibodies to the insulin receptor mimic metabolic effects of insulin but do not stimulate receptor autophosphorylation in transfected NIH 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5217–5221. doi: 10.1073/pnas.86.14.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos M. A., Siddle K., Baron M. D., Heward J. M., Luzio J. P., Bellatin J., Lennox E. S. Monoclonal antibodies reacting with multiple epitopes on the human insulin receptor. Biochem J. 1986 Apr 1;235(1):199–208. doi: 10.1042/bj2350199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soos M. A., Siddle K. Immunological relationships between receptors for insulin and insulin-like growth factor I. Evidence for structural heterogeneity of insulin-like growth factor I receptors involving hybrids with insulin receptors. Biochem J. 1989 Oct 15;263(2):553–563. doi: 10.1042/bj2630553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet L. J., Morrison B. D., Pessin J. E. Isolation of functional alpha beta heterodimers from the purified human placental alpha 2 beta 2 heterotetrameric insulin receptor complex. A structural basis for insulin binding heterogeneity. J Biol Chem. 1987 May 25;262(15):6939–6942. [PubMed] [Google Scholar]
- Taira M., Taira M., Hashimoto N., Shimada F., Suzuki Y., Kanatsuka A., Nakamura F., Ebina Y., Tatibana M., Makino H. Human diabetes associated with a deletion of the tyrosine kinase domain of the insulin receptor. Science. 1989 Jul 7;245(4913):63–66. doi: 10.1126/science.2544997. [DOI] [PubMed] [Google Scholar]
- Treadway J. L., Morrison B. D., Goldfine I. D., Pessin J. E. Assembly of insulin/insulin-like growth factor-1 hybrid receptors in vitro. J Biol Chem. 1989 Dec 25;264(36):21450–21453. [PubMed] [Google Scholar]
- Van Obberghen E., Ksauga M., Le Cam A., Hedo J. A., Itin A., Harrison L. C. Biosynthetic labeling of insulin receptor: studies of subunits in cultured human IM-9 lymphocytes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1052–1056. doi: 10.1073/pnas.78.2.1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilden P. A., Morrison B. D., Pessin J. E. Relationship between insulin receptor subunit association and protein kinase activation: insulin-dependent covalent and Mn/MgATP-dependent noncovalent association of alpha beta heterodimeric insulin receptors into an alpha 2 beta 2 heterotetrameric state. Biochemistry. 1989 Jan 24;28(2):785–792. doi: 10.1021/bi00428a056. [DOI] [PubMed] [Google Scholar]
- Wilden P. A., Morrison B. D., Pessin J. E. Relationship between insulin receptor subunit association and protein kinase activation: insulin-dependent covalent and Mn/MgATP-dependent noncovalent association of alpha beta heterodimeric insulin receptors into an alpha 2 beta 2 heterotetrameric state. Biochemistry. 1989 Jan 24;28(2):785–792. doi: 10.1021/bi00428a056. [DOI] [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of insulin receptor beta subunit activates the receptor tyrosine kinase in intact H-35 hepatoma cells. J Biol Chem. 1986 Apr 5;261(10):4715–4722. [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]