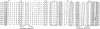Abstract
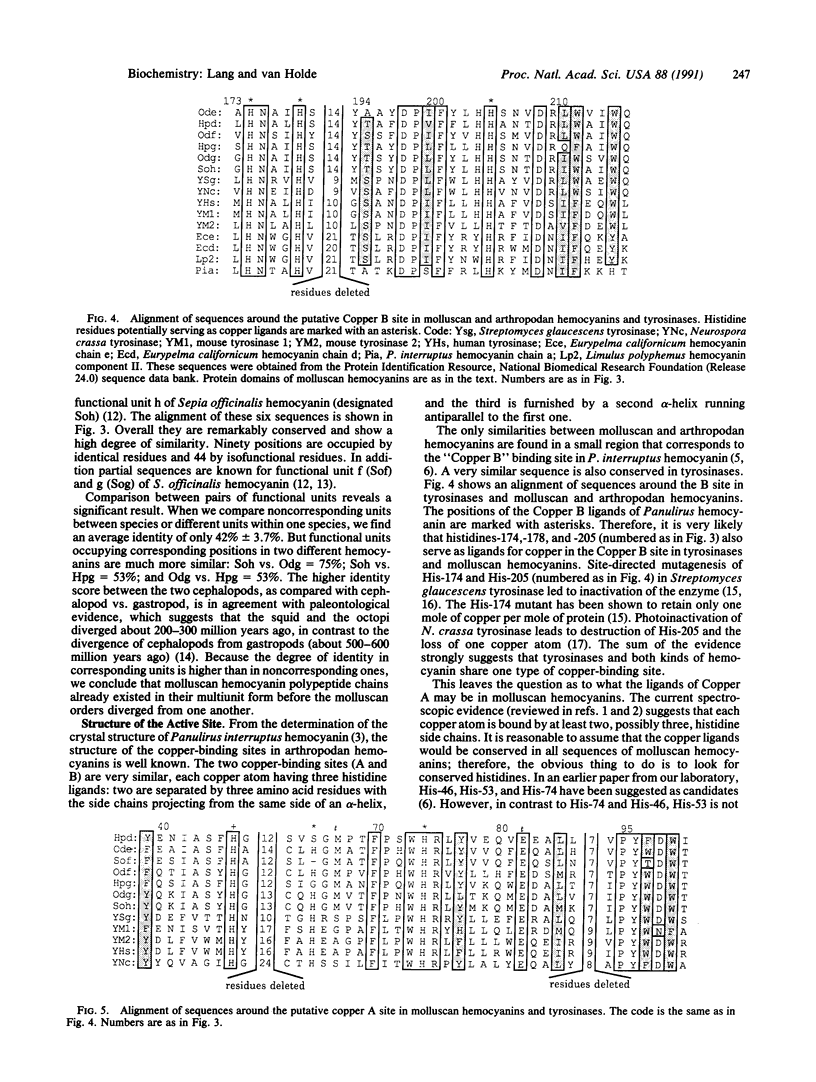
A number of additional cDNA clones coding for portions of the very large polypeptide chain of Octopus dofleini hemocyanin were isolated and sequenced. These data reveal two very similar coding sequences, which we have denoted "A-type" and "G-type." We have obtained complete A-type sequences coding for functional units Ode and Odf; consequently a total of three such unit sequences are now known from a single subunit of one molluscan hemocyanin. This presents the opportunity to make sequence comparisons within one hemocyanin subunit. Domains within one subunit show on the average 42% identity in amino acid residues; corresponding functional units from hemocyanins of different species show degrees of identity of 53-75%. Therefore, molluscan hemocyanins already existed before the individual molluscan classes diverged in the early Cambrian. Sequence comparisons of molluscan hemocyanins with arthropodan hemocyanins and tyrosinases allow us to identify the ligands of the "Copper B" site with high probability. Possible ligands for the "Copper A" site are proposed, based on sequence comparisons between molluscan hemocyanins and tyrosinases. Besides two histidine side chains, a methionine side chain might be involved in binding of Copper A, a result not in conflict with spectroscopic studies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuff M. E., Hendrickson W. A., Lamy J., Lamy J. N., Miller K. I., van Holde K. E. Crystals of the carboxyl-terminal functional unit from Octopus dofleini hemocyanin. J Mol Biol. 1990 May 5;213(1):11–15. doi: 10.1016/S0022-2836(05)80117-2. [DOI] [PubMed] [Google Scholar]
- Drexel R., Siegmund S., Schneider H. J., Linzen B., Gielens C., Préaux G., Lontie R., Kellermann J., Lottspeich F. Complete amino-acid sequence of a functional unit from a molluscan hemocyanin (Helix pomatia). Biol Chem Hoppe Seyler. 1987 Jun;368(6):617–635. doi: 10.1515/bchm3.1987.368.1.617. [DOI] [PubMed] [Google Scholar]
- Ellerton H. D., Ellerton N. F., Robinson H. A. Hemocyanin--a current perspective. Prog Biophys Mol Biol. 1983;41(3):143–248. doi: 10.1016/0079-6107(83)90028-7. [DOI] [PubMed] [Google Scholar]
- Himmelwright R. S., Eichman N. C., Solomon E. I. Preparation and characterization of met apo hemocyanin: a single copper (II) active site. Biochem Biophys Res Commun. 1978 Mar 15;81(1):243–247. doi: 10.1016/0006-291x(78)91656-x. [DOI] [PubMed] [Google Scholar]
- Huber M., Lerch K. Identification of two histidines as copper ligands in Streptomyces glaucescens tyrosinase. Biochemistry. 1988 Jul 26;27(15):5610–5615. doi: 10.1021/bi00415a032. [DOI] [PubMed] [Google Scholar]
- LONTIE R. The binding of copper in haemocyanins. Clin Chim Acta. 1958 Jan;3(1):68–71. doi: 10.1016/0009-8981(58)90058-5. [DOI] [PubMed] [Google Scholar]
- Lang W. H. cDNA cloning of the Octopus dofleini hemocyanin: sequence of the carboxyl-terminal domain. Biochemistry. 1988 Sep 20;27(19):7276–7282. doi: 10.1021/bi00419a015. [DOI] [PubMed] [Google Scholar]
- van Holde K. E., Miller K. I. Haemocyanins. Q Rev Biophys. 1982 Feb;15(1):1–129. doi: 10.1017/s0033583500002705. [DOI] [PubMed] [Google Scholar]