Abstract
Background. After decades of obscurity, Zika virus (ZIKV) has spread through the Americas since 2015 accompanied by congenital microcephaly and Guillain-Barré syndrome. Although these epidemics presumably involve transmission by Aedes aegypti, no direct evidence of vector involvement has been reported, prompting speculation that other mosquitoes such as Culex quinquefasciatus could be involved.
Methods. We detected an outbreak of ZIKV infection in southern Mexico in late 2015. Sera from suspected ZIKV-infected patients were analyzed for viral RNA and antibodies. Mosquitoes were collected in and around patient homes and tested for ZIKV.
Results. Of 119 suspected ZIKV-infected patients, 25 (21%) were confirmed by RT-PCR of serum collected 1–8 days after the onset of signs and symptoms including rash, arthralgia, headache, pruritus, myalgia, and fever. Of 796 mosquitoes collected, A. aegypti yielded ZIKV detection by RT-PCR in 15 of 55 pools (27.3%). No ZIKV was detected in C. quinquefasciatus. ZIKV sequences derived from sera and mosquitoes showed a monophyletic relationship suggestive of a point source introduction from Guatemala.
Conclusions. These results demonstrate the continued, rapid northward progression of ZIKV into North America with typically mild disease manifestations, and implicate A. aegypti for the first time as a principal vector in North America.
Keywords: Zika, flavivirus, arbovirus, mosquito, transmission
Zika virus (ZIKV) is a mosquito-borne flavivirus in the family Flaviviridae, which includes human-pathogenic arthropod-borne viruses (arboviruses) such as dengue virus (DENV), yellow fever virus, and West Nile virus. It was first discovered in the Zika forest of Uganda, in 1947, during investigations of enzootic yellow fever, and the first human cases of ZIKV infection were described in 1954, in Nigeria. However, until 2007, only 14 sporadic human cases were reported, although serological studies and virus isolation from mosquitoes suggested widespread ZIKV circulation in Africa and Asia [1, 2].
The first major outbreak of ZIKV infection was detected in 2007 in Yap Island, where up to 73% of the population was estimated to have been infected [3]. Another outbreak occurred the same year in Gabon [4]. These were followed by a large epidemic in French Polynesia in 2013–2014, where up to 66% of the population was infected [5], and spread of ZIKV to New Caledonia, the Cook Islands, Easter Island, Vanuatu, and the Solomon Islands [6]. In the Americas, phylogenetic analyses suggest ZIKV importation into Brazil from the Pacific in 2013 [7]. In early 2015, the first ZIKV infections were described in Brazil [7], harbingers of an explosive hemispheric epidemic.
As of August 2016, local ZIKV infections had been reported in 45 countries and territories in the Americas, with the epidemic spreading throughout most of northern South America and nearly all of Central America and the Caribbean [8], presumably through travelers who initiated transmission by the urban mosquito vector, Aedes aegypti. Many countries in North America and Europe have also reported hundreds of imported cases. The lack of direct evidence of A. aegypti infection has led to speculation that other common tropical urban mosquitoes, such as Culex quinquefasciatus, could be involved [9]. Culex species have not been associated with ZIKV transmission during outbreaks in Yap [3], Gabon [4], or French Polynesia [2]. However, other Aedes species have been implicated in urban transmission in Gabon [4] and Oceania [2, 3, 10].
Serosurveys suggest that most ZIKV infections are asymptomatic [11]. In symptomatic cases of Zika fever (ZIKF), signs and symptoms are similar to those described for infections by DENV and chikungunya virus (CHIKV), 2 other arboviruses present in most regions affected by ZIKV, making diagnosis challenging. ZIKF typically includes fever, arthralgia, maculopapular rash, conjunctivitis, and retro-orbital pain. However, ZIKV can also lead to more-serious neurological complications, such as Guillain-Barré syndrome and, in newborns, congenital microcephaly syndrome [12–16].
In southern Mexico, near the Guatemala border, an increasing number of patients exhibiting conjunctivitis, fever, and rash was observed in Tapachula and surrounding rural localities in November 2015. Initially, medical personnel were confused because 12 months earlier, a major chikungunya fever (CHIKF) outbreak affected the same region [17]. However, most cases did not present with arthralgia of the hands and feet, which is typical of CHIKF, and the retroocular pain seen in most cases, which is not typical of CHIKF, was suggestive of dengue. Blood samples were obtained from patients showing signs and symptoms consistent with ZIKF, including fever, conjunctivitis, myalgias, retroocular pain, and rash. Mosquitoes were also collected in and around the homes of patients with suspected ZIKF to identify the vector(s) transmitting.
METHODS
Clinical Presentations and Sample Collection
In Mexico-Guatemala border communities, physicians from Suchiate, Frontera Hidalgo, and Metapa municipalities reported to the Centro Regional de Investigación en Salud Pública (CRISP), in Tapachula, an increase of febrile patients with signs and symptoms perceived to be slightly different from those of CHIKF or dengue. Therefore, a house-to-house survey was implemented to identify patients who met the World Health Organization case definition for ZIKF [18]: presentation with rash and/or fever and at least 1 of the following signs or symptoms: arthralgia, arthritis, or conjunctivitis (nonpurulent/hyperemic). Also, patients reporting directly to the CRISP for diagnostic assays and meeting this case definition were included. Blood samples were collected by venipuncture from acute and convalescent patients who volunteered and provided written informed consent.
Detection of Viral RNA
RNA was extracted from serum samples collected 1–61 days after fever onset, using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, California) following the manufacturer's protocol. All RNA samples were tested using a 1-step reverse transcription–polymerase chain reaction (RT-PCR) with the Qiagen OneStep RT-PCR kit (Qiagen, Valencia, California) and previously described ZIKV- or DENV-specific primers [19]. Quantitative RT-PCR was also performed, using the Taqman RNA-to-C 1-step kit (Applied Biosystems, San Francisco, California) and CHIKV-specific primers [17]. All ZIKV-PCR-positive samples were confirmed using end point real-time quantitative RT-PCR and previously described primers 1086/1162c/1107-FAM [20] and were also inoculated onto Vero cells for virus isolation attempts and plaque assays [21].
Serologic Testing
Because dengue fever is hyperendemic in the Tapachula region and results in cross-reactions in ZIKV enzyme-linked immunosorbent assays (ELISAs), sera were analyzed using the more-specific plaque-reduction neutralization test (PRNT) [21] for ZIKV and the most recently circulating DENV-2 and DENV-3 serotypes. All samples negative for ZIKV and CHIKV RNA by RT-PCR and negative for ZIKV antibodies by PRNT were also screened by ELISA for CHIKV immunoglobulin M (IgM) [22] (CHIKjj Detect IgM ELISA Kit; InBiOS, Seattle, Washington) and DENV IgM (Detect IgM Capture ELISA; InBiOS).
ZIKV infection was confirmed if RNA was detected in a patient's serum or if neutralizing antibodies were present in the absence of DENV neutralization.
Mosquito Collections
From 9 to 17 December, adult mosquitoes were collected between 9:00 am and 3:00 pm, using backpack aspirators, in and around homes of patients with suspected ZIKF (Figure 1). Unengorged mosquitoes (ie, those with no visible blood in the abdomen) were pooled by species, sex, and date and location of collection and then triturated in cell culture medium supplemented with 5% fetal bovine serum, using the Tissue Lyzer II (Qiagen). Homogenates were centrifuged, and viral RNA was extracted from supernatants, using the QIAamp Viral RNA Mini Kit (Qiagen), and screened for the presence of ZIKV RNA by RT-PCR as described above.
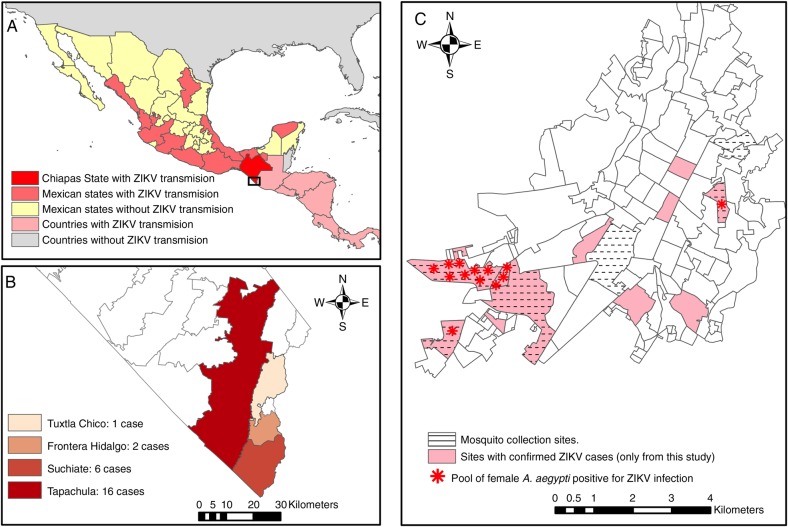
Figure 1.
Study areas of Zika fever (ZIKF) outbreak in Chiapas State, southern Mexico. A, Map showing the current epidemiological situation of Zika virus (ZIKV) transmission in Mexico [23]. B, Distribution of confirmed ZIKF cases among Mexico-Guatemala border municipalities. C, Map of Tapachula city, showing the sites with at least 1 confirmed ZIKF case and where ZIKV-infected female Aedes aegypti pools were collected.
Virus isolation was performed by inoculation of Vero cells (ATCC, Bethesda, Maryland) with 100 µL of mosquito homogenates followed by incubation for up to 7 days for observation of cytopathic effects (CPE). The supernatant from CPE-positive cultures was harvested and reinoculated onto Vero cells for viral amplification. RNA was extracted from CPE-positive supernatants and sequenced using the Illumina 1500 HiSeq next-generation sequencer (Illumina, San Diego, California).
Illumina Sequencing
Viral RNA (approximately 0.9 µg) was fragmented by incubation at 94°C for 8 minutes in 19.5 µL of buffer (Illumina 15016648). Sequencing libraries were prepared using an Illumina TruSeq RNA v2 kit and then sequenced using the Rapid-Run 2 × 50 paired-end protocol. Reads were quality filtered, and adapter sequences were removed using Trimmomatic software [24]. The de novo assembly program ABySS [25] was used to assemble contigs covering nearly the full length of the ZIKV genome, using k values from 20–40 and 500 000 (MEX_1–7 and MEX_2–81) or 250 000 (MEX_1–44) reads. Reads were mapped back to the contigs by using bowtie2 [26] and were visualized with the Integrated Genomics Viewer [27] to verify correct assembly. There were 15.2, 14.1, and 13.5 million read pairs in samples MEX_1–7, MEX_1–44, and MEX_2–81, respectively, with approximately 32 300 (0.24%), 18 600 000 (13.3%), and 12 100 (0.09%), respectively, mapping to the ZIKV genome.
Sequencing of PCR-Positive Serum Samples
Amplicons were generated from ZIKV-positive RNA extractions by RT-PCR (Superscript III RT System, Invitrogen, and Phusion DNA polymerase; New England BioLabs, Ipswich, Massachusetts) targeting the partial nonstructural protein 5 (NS5) gene. Direct Sanger sequencing of amplicons was performed using the BigDye terminator v1.3 kit and ABI 3500 Genetic Analyzer (Applied Biosystems, Carlsbad, California). Alignments and analysis were performed using Sequencher 4.9 (Ann Arbor, Michigan).
The ZIKV evolutionary history was inferred by using the maximum likelihood method based on the general time reversible model. The tree with the highest log likelihood (−31 884.9247) is shown in Figure 2. Initial tree(s) for the heuristic search were obtained using neighbor-joining and BioNJ algorithms, with pair-wise distances estimated by using the maximum composite likelihood (MCL) and then selecting the topology with the superior log likelihood value. A discrete gamma distribution was used to model evolutionary rate differences among sites (5 categories [+G, parameter, 0.2579]). The rate variation model allowed for some sites to be evolutionarily invariable ([+I], 0.0010% sites). All positions containing gaps and missing data were eliminated, leaving 10 250 positions in the final data set. Evolutionary analyses were conducted in MEGA7 [28].
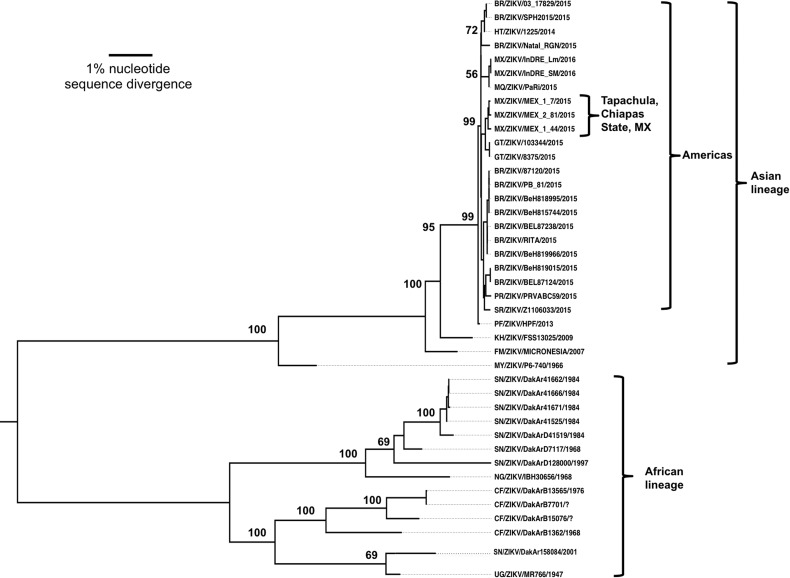
Figure 2.
Maximum likelihood phylogenetic tree of Zika virus (ZIKV) strain genomic open reading frame sequences determined as part of this study, as well as homologous sequences from the GenBank library. Strains are listed by abbreviated country or territory, followed by strain and year of collection. Numbers indicate bootstrap values. Abbreviations: BR, Brazil; CF, Ivory Coast; FM, Yap, Micronesia; GT, Guatemala; HT, Haiti; KH, Cambodia; MQ, Martinique; MX, Mexico; MY, Malaysia; NG, Nigeria; PF, French Polynesia; PR, Puerto Rico; SR, Suriname; SN, Senegal; UG, Uganda.
RESULTS
Case Descriptions and RT-PCR Results
A total of 119 serum samples was collected in several locations near Tapachula, Chiapas State, Mexico, between 30 November and 18 December 2015 from patients with signs/symptoms consistent with ZIKF (Tables 1 and 2); 70 (58.8%) were females, and 49 (41.2%) were males, with ages ranging from 4 to 80 years and a mean age of 34.7 years. Blood samples were also obtained from convalescent patients in March 2016. Patient data were deidentified, and laboratory testing was approved by the University of Texas Medical Branch Institutional Review Board.
Table 1.
Localities Where Blood Specimens Were Collected During a Zika Fever Outbreak in Chiapas State, Southern Mexico, 2015
| Collection Site | Samples Collected, No. | RT-PCR–Positive for ZIKVa |
|---|---|---|
| Tapachula | 66 | 16 |
| Suchiate | 39 | 6 |
| Frontera Hidalgo | 3 | 2 |
| Tuxtla Chico | 1 | 1 |
| Metapa | 7 | 0 |
| Tuxtla Chico | 1 | 0 |
| Cacahoatan | 1 | 0 |
| Huixta | 1 | 0 |
| Total | 119 | 25 (21) |
Abbreviations: RT-PCR reverse transcription–polymerase chain reaction; ZIKV, Zika virus.
a Data are no. or no. (%).
Table 2.
Clinical Signs and Symptoms of Zika Fever in 25 Patients With Polymerase Chain Reaction–Confirmed Zika Virus Infection Who Were Studied in Chiapas State, Southern Mexico, 2015
| Sign/Symptom | Patients, No. (%) |
|---|---|
| Rash | 21 (88) |
| Arthralgia | 21 (88) |
| Headache | 21 (88) |
| Pruritus | 18 (758) |
| Myalgia | 17 (71) |
| Fever | 17 (71) |
| Chills | 15 (62) |
| Somnolence | 15 (62) |
| Asthenia | 12 (50) |
| Vertigo | 12 (50) |
| Conjunctivitis | 9 (38) |
| Retro-orbital pain | 9 (38) |
| Anorexia | 9 (38) |
| Edema | 8 (33) |
| Nausea | 8 (33) |
| Dysgeusia | 8 (33) |
| Diarrhea | 6 (25) |
| Abdominal pain | 6 (25) |
| Adenopathy | 6 (25) |
| Mind alteration | 4 (17) |
| Mental confusion | 3 (12) |
| Vomit | 2 (8) |
| Gingivorrhagia | 2 (8) |
| Bleeding | 1 (4) |
| Hematuria | 1 (4) |
| Petechiae | 1 (4) |
All samples were analyzed for ZIKV RNA by RT-PCR, and serological analyses were performed using PRNT and ELISA. ZIKV RNA was detected in 25 sera (21%) collected 1–10 days after signs/symptoms onset; 15 of these confirmed cases (60%) were female (age range, 11–58 years; mean age, 26.3 years), and 10 (40%) were male (age, 25–80 years; mean age, 42.6 years), with an overall mean age of 33.1 years. A high proportion (88%) of patients with PCR-confirmed ZIKV RNA presented with a maculopapular rash, headache, and arthralgia (Table 2). Fever and myalgia were also common (71%), and ocular signs and symptoms (conjunctivitis and retroocular pain) were observed in 38% of confirmed cases. Virus isolation on Vero cells was negative for all sera. All samples negative for ZIKV by RT-PCR also tested negative for DENV and CHIKV RNA. Extrapolation of the cycle threshold values of PCR-positive sera, using a standard curve generated from a closely related ZIKV stock, yielded estimated viremia titers ranging from 0.32 to 2454 focus-forming units/mL from days 1 to 10 after the onset of signs or symptoms (Figure 3).
Figure 3.
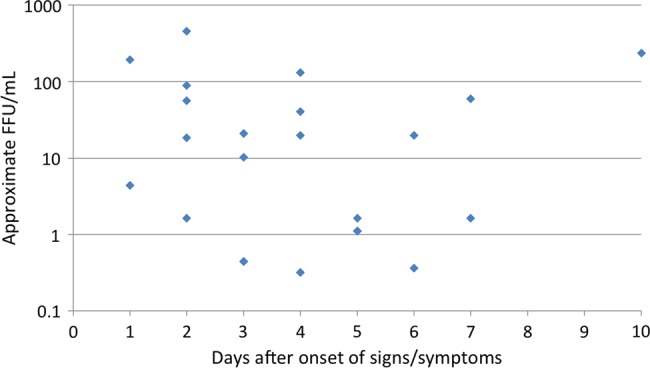
Estimated infectious viremia titers expressed as focus-forming units(FFU) per milliliter of serum, extrapolated from cycle threshold values found in Supplementary Table 1.
Serology Results
As expected in a DENV-hyperendemic region, many sera were positive in multiple flavivirus assays (Table 3 and Supplemental Table 1). Of 74 patients (62.1%) with ZIKV-neutralizing antibodies, all but 2 had some level of DENV neutralization, rendering results inconclusive. Only 2 patients had ZIKV PRNT titers at least 4-fold higher than DENV titers. Some patients showing high ZIKV PRNT titers were also positive for DENV IgM, although no DENV RNA was detected. Of the 25 samples positive for ZIKV by PCR, only 8 had neutralizing activity against ZIKV, consistent with clearance of ZIKV viremia by the antibody response. Sera from convalescent patients were obtained for 19 of 25 ZIKF-confirmed (RT-PCR–positive) cases. A minimum 4-fold increase in the ZIKV PRNT titer was observed in all of these convalescent sera, consistent with ZIKV seroconversion (Table 3). However, only 2 of these 19 convalescent patients showed a 4-fold higher titer for ZIKV than for DENV, probably reflecting prior DENV infection in the remaining 17 and an anamnestic increase in DENV PRNT titers due to original antigenic sin [29]. Although no samples tested positive for CHIKV RNA, CHIKV IgM was detected in 12 sera negative for ZIKV by PCR. One ZIKV-negative patient tested positive for IgM against both DENV and CHIKV.
Table 3.
Acute and Convalescent Neutralization Test Results from 25 ZIKV-RNA-positive Patients, Chiapas, Mexico, 2015
| Sample ID | Sex | Age, y | PRNT80 Titer |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acute Phase |
Convalescent Phase |
|||||||||
| Day After Onset | ZIKV | DENV-2 | DENV-3 | Day After Onset | ZIKV | DENV-2 | DENV-3 | |||
| ZK-LI-0011Pa | F | 58 | 6 | 320 | 640 | ≥1280 | 124 | ≥1280 | 320 | 160 |
| ZK-LI-0012P | F | 13 | NA | <20 | NT | NT | NA | NA | NA | NA |
| ZK-TA-0018-P | F | 36 | 6 | <20 | 80 | 80 | 120 | 80 | 160 | ≥1280 |
| ZK-FH-0026-P | M | 49 | 4 | <20 | 160 | 80 | 120 | 320 | 640 | 320 |
| ZK-TC-0027-P | M | 53 | 1 | <20 | NT | NT | NA | NA | NA | NA |
| ZK-FH-0028-P | M | 19 | 2 | 20 | <40 | <40 | 115 | 640 | 80 | 640 |
| ZK-TA-0031-P | F | 24 | 4 | <20 | 40 | 40 | 131 | 320 | 160 | 160 |
| ZK-TA-0032-P | F | NA | NA | 20 | 160 | <40 | 100 | 320 | 640 | ≥1280 |
| ZK-TA-0034-P | F | 21 | 2 | <20 | 20 | 160 | 107 | 640 | 20 | ≥1280 |
| ZK-TA-0035-P | M | 65 | 4 | <20 | 20 | 80 | 109 | 40 | 160 | 320 |
| ZK-TA-0037-P | F | 14 | 7 | <20 | NT | NT | NA | NA | NA | NA |
| ZK-TA-0038-P | M | 25 | 7 | <20 | 40 | 40 | 111 | 160 | 80 | 320 |
| ZK-TA-0041-P | M | 80 | 5 | 40 | 160 | 160 | NA | NA | NA | NA |
| ZK-CH-0044-P | M | 31 | 2 | <20 | ≥1280 | 80 | NA | NA | NA | NA |
| ZK-CH-0049-P | F | 11 | 2 | <20 | 160 | <40 | 97 | 80 | ≥1280 | ≥1280 |
| ZK-CH-0060-P | F | 40 | 5 | 20 | 40 | 40 | NA | NA | NA | NA |
| ZK-CH-0072-P | F | 19 | 5 | <20 | NT | NT | 111 | 320 | 320 | 320 |
| ZK-TA-0078-P | M | 53 | 4 | 20 | 160 | 80 | 99 | 160 | 320 | 640 |
| ZK-TA-0081-P | F | 26 | 3 | 20 | 40 | 80 | 96 | 640 | 80 | 320 |
| ZK-TA-0082-P | F | 22 | 3 | 40 | <40 | 80 | 95 | 160 | ≥1280 | 80 |
| ZK-TA-0089-Pa | F | 14 | 3 | <20 | <40 | <40 | 96 | 640 | <20 | 40 |
| ZK-TA-0090-P | M | 25 | 3 | <20 | 20 | ≥1280 | 95 | 40 | 640 | ≥1280 |
| ZK-TA-0096-P | F | 28 | 10 | <20 | 80 | 20 | 110 | 320 | 40 | 320 |
| ZK-TA-0107-P | M | 26 | 2 | <20 | NT | NT | 107 | 160 | <20 | 80 |
| ZK-TA-0108-P | F | 42 | 1 | <20 | NT | NT | 105 | 80 | ≥1280 | ≥1280 |
Abbreviations: DENV, dengue virus; ID, identifier; NA, not available; NT, not tested; PRNT80, 80% plaque-reduction neutralizing test.
a PRNT80 convalescent ZIKV titer showed a ≥4-fold rise over the convalescent titer and was ≥4-fold greater than DENV-2 and DENV-3 titers.
Mosquito Screening
Mosquito collections were performed in 69 households, of which 58 were from Tapachula and 11 were from Suchiate. A total of 796 mosquitoes were collected, with A. aegypti being the most abundant (59.3%; 59% female), followed by C. quinquefasciatus (40.5%; 47% female). Only 2 A. albopictus mosquitoes were captured (0.2%). At least 1 A. aegypti mosquito was collected from most households (81.2%), but the densities of females per household were low (median 2.0; Table 4).
Table 4.
Aedes aegypti Mosquitoes Collected in 69 Households from Tapachula and Suchiate Municipalities, Chiapas State, Southern Mexico, 2015
| Variable | Tapachula | Suchiate (Hidalgo City) | Total |
|---|---|---|---|
| Households surveyed. no. | 58 | 11 | 69 |
| Households with A. aegypti, % (no.) | 89.3 (50) | 54.5 (6) | 81.2 (56) |
| Female mosquitoes collected, no. | 274 | 5 | 279 |
| Male mosquitoes collected, no. | 186 | 7 | 193 |
| Total mosquitoes collected, no. | 460 | 12 | 472 |
| Female mosquitoes collected per household, no., median (95% CI) | 2.0 (1.0–2.0) | … | … |
| Female mosquito pools assayed for ZIKV, total no. | 50 | 5 | 55 |
| ZIKV-positive mosquito pools, total no. | 13 | 2 | 15 |
| Maximum likelihood estimation of infected mosquitoes per 1000 (95% CI) | 52.49–172.66 | … | … |
Abbreviations: CI, confidence interval; ZIKV, Zika virus.
Of the 472 female A. aegypti tested in 55 pools, ZIKV RNA was detected by RT-PCR in 15 pools. Three of these produced CPE in Vero cells after 3 days of incubation, and virus isolates were obtained. ZIKV was not detected in male mosquitoes, including 52 pools of A. aegypti. The lack of virus isolation from the RT-PCR–positive sera and most of the mosquito pools may reflect low ZIKV titers, further reduced by repeated freezing and thawing. ZIKV was not detected in any A. albopictus or C. quinquefasciatus mosquitoes. Despite the small numbers collected in most households, the minimum field infection rate for A. aegypti was estimated at 52.49–172.66 infections per 1000 mosquitoes (Table 3).
Sequencing and Phylogenetic Results
Three serum samples were selected from different locations for direct Illumina and Sanger sequencing. Because of low genome coverage, presumably reflecting low viral titers, no useful sequences were obtained using Illumina, but Sanger sequencing generated partial ZIKV NS5 sequences. Full-genome sequences were obtained using Illumina from RNA extracted directly from the 3 mosquito pools from which ZIKV was isolated.
A phylogenetic analysis based on 44 complete genome sequences supported a ZIKV origin in Africa and divergence in the distant past into 2 major lineages: African and Asian/American (Figure 2). African strains fell into 2 distinct groups: (1) the Uganda cluster, anchored by the prototype 1947 MR766 strain and including isolates from western Africa to 2001, and (2) the Nigeria group, with strains isolated in Nigeria and Senegal from 1968–1997. The Asian lineage was anchored by the prototype 1966 P6-740 Malaysian strain and included all isolates from Oceania. Within this Asian clade, the new American lineage emerged, including strains from Latin America and the Caribbean. A major characteristic of the American lineage was its rapid expansion to cover a widespread geographic area of transmission, consistent with a pattern of intense diversification with expansion into new territories inhabited by immunologically naive human populations.
Based on the occurrence of Mexican ZIKV strains in 2 independent clades, the introduction of ZIKV into Chiapas probably occurred twice; our strains from the Tapachula area probably resulted from a point introduction from neighboring Guatemala, likely following established migration routes (Figure 2). The other 2 Mexican strains are from an unknown location in Chiapas State (GenBank accession nos. KU922960.1 and KU922923.1), and their grouping with a Martinique strain suggests an introduction from that Caribbean Island. Our NS5 sequences derived from sera had no more than one nucleotide difference from the mosquito strains, confirming a transmission link.
DISCUSSION
We detected an outbreak of ZIKF in southern Mexico, near the Guatemalan border, from 30 November to 18 December 2015. The 25 PCR-confirmed cases, collected 1–10 days after the onset of signs and/or symptoms, suggested that viremia can last for >10 days, assuming that it can precede clinical disease. The majority of patients with ZIKF presented with rash, arthralgia, headache, pruritus, myalgia, fever, chills, and somnolescence. Other signs and symptoms reported previously [3], including conjunctivitis and retro-orbital pain, occurred at lower frequencies. No signs of neurologic disease, such as Guillain-Barré syndrome, were detected, and no pregnant female patients were studied.
Serological results were inconclusive because nearly all patients had DENV-neutralizing antibodies that presumably reflected earlier DENV infections. This underscores the challenge of serodiagnostics in DENV-hyperendemic areas and emphasizes the need for improved flavivirus antigens lacking cross-reactivity.
Although limited in scope, our study indicates that A. aegypti was the principal vector of ZIKV in the Tapachula area of Chiapas State, based on the detection of ZIKV in several mosquito pools and the previous demonstrated transmission competence of this species [30, 31]. Our estimated field infection rates were very high, as expected for viruses like DENV and CHIKV when sampling is focused on the homes of suspected cases with active transmission [32, 33]. An important finding was that ZIKV was not detected in C. quinquefasciatus, another common urban tropical mosquito discussed as a potential ZIKV vector [9]. Only 2 A. albopictus mosquitoes, another proven ZIKV vector [4, 31, 34], were collected, so the lack of infection of this species is not conclusive regarding its potential role in other regions where it is more abundant.
Our implication of A. aegypti in ZIKV transmission in southern Mexico is important because no natural mosquito infections have been reported in the peer-reviewed literature since ZIKV reached the Americas, and some experimental studies suggest that this species develops disseminated infections at a low frequency even after high-titer blood meals [31]. However, based on its nearly ideal anthropophilic behavior, this species can transmit human viruses to epidemic proportions even when it is relatively insusceptible [35]. Our first conclusive implication of this species in ZIKV transmission in the Americas, based on the detection of virus identical or nearly so to strains from humans living in the same households, supports the need for additional efforts to control A. aegypti as one of the few interventions currently available for the ongoing outbreak.
Our phylogenetic results suggest that the Tapachula outbreak occurred following a point source ZIKV introduction from Guatemala. However, our Chiapas isolates were phylogenetically distinct from 2 other strains isolated from human infections in the same state, which were probably introduced directly from the Caribbean island of Martinique. The ability of infected people to transport ZIKV, perhaps even when asymptomatically infected or during incubation, to initiate mosquito-borne transmission is undoubtedly a major factor in its explosive spread from French Polynesia since 2013. This ability, combined by transmission by A. aegypti, as indicated by our results, suggests a poor prognosis for controlling the current ZIKF outbreak in the Americas. Considering the ongoing CHIKF outbreak, which followed a similar pathway to the Americas in 2013, followed by an epidemic of hemispheric proportions [36], as well as our inability to control DENV in the Americas for many decades, the prospects for controlling or slowing the spread of ZIKV are poor. Nevertheless, source reduction and insecticide-based control inside and around homes, schools, and places of work should be redoubled and supplemented by more-recently developed methods, such as lethal traps, until transgenic and Wolbachia-based methods can be scaled-up for use in large populations [37]. Fortunately, the majority of ZIKF cases are mild or unapparent, and the overall global disease burden is smaller than that of CHIKV and DENV. However, the high risk of severe ZIKV outcomes such as microcephaly following infection during pregnancy [5, 38] indicate a need for extraordinary measures to protect pregnant women and their sex partners from exposure to A. aegypti and perhaps other vectors during the peak of ZIKV transmission occurring now in many regions of the Americas.
Supplementary Data
Supplementary materials are available at http://jid.oxfordjournals.org. Consisting of data provided by the author to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the author, so questions or comments should be addressed to the author.
Notes
Financial support. This work was supported by the National Institutes of Health (grantAI120942).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Weaver SC, Costa F, Garcia-Blanco MA et al. . Zika Virus: History, Emergence, Biology, and Prospects for Control. Antiviral Res 2016; 130:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musso D, Gubler DJ. Zika Virus. Clin Microbiol Rev 2016; 29:487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duffy MR, Chen TH, Hancock WT et al. . Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med 2009; 360:2536–43. [DOI] [PubMed] [Google Scholar]
- 4.Grard G, Caron M, Mombo IM et al. . Zika virus in Gabon (Central Africa)--2007: a new threat from Aedes albopictus? PLoS Negl Trop Dis 2014; 8:e2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cauchemez S, Besnard M, Bompard P et al. . Association between Zika virus and microcephaly in French Polynesia, 2013–15: a retrospective study. Lancet 2016; 387:2125–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Musso D, Cao-Lormeau VM, Gubler DJ. Zika virus: following the path of dengue and chikungunya? Lancet 2015; 386:243–4. [DOI] [PubMed] [Google Scholar]
- 7.Zanluca C, de Melo VC, Mosimann AL, Dos Santos GI, Dos Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz 2015; 110:569–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.http://ais.paho.org/phip/viz/ed_zika_countrymap.asp. Accessed 20 August 2016. [Google Scholar]
- 9.Ayres CF. Identification of Zika virus vectors and implications for control. Lancet Infect Dis 2016; 16:278–9. [DOI] [PubMed] [Google Scholar]
- 10.Ledermann JP, Guillaumot L, Yug L et al. . Aedes hensilli as a potential vector of Chikungunya and Zika viruses. PLoS Negl Trop Dis 2014; 8:e3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musso D, Nhan T, Robin E et al. . Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19:1–3. [DOI] [PubMed] [Google Scholar]
- 12.Besnard M, Eyrolle-Guignot D, Guillemette-Artur P et al. . Congenital cerebral malformations and dysfunction in fetuses and newborns following the 2013 to 2014 Zika virus epidemic in French Polynesia. Euro Surveill 2016; 21:1–9. [DOI] [PubMed] [Google Scholar]
- 13.Mlakar J, Korva M, Tul N et al. . Zika Virus Associated with Microcephaly. N Engl J Med 2016; 374:951–8. [DOI] [PubMed] [Google Scholar]
- 14.Cao-Lormeau VM, Blake A, Mons S et al. . Guillain-Barre Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet 2016; 22:913–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oehler E, Watrin L, Larre P et al. . Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill 2014; 19:1–3. [DOI] [PubMed] [Google Scholar]
- 16.Calvet G, Aguiar RS, Melo AS et al. . Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 2016; 16:653–60. [DOI] [PubMed] [Google Scholar]
- 17.Kautz TF, Diaz-Gonzalez EE, Erasmus JH et al. . Chikungunya Virus as Cause of Febrile Illness Outbreak, Chiapas, Mexico, 2014. Emerg Infect Dis 2015; 21:2070–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Zika virus disease, interim case definitions. WHO/ZIKV/SUR/16.1. 2016. http://apps.who.int/iris/bitstream/10665/204381/1/WHO_ZIKV_SUR_16.1_eng.pdf?ua=1. Accessed 22 August 2016. [Google Scholar]
- 19.Balm MN, Lee CK, Lee HK, Chiu L, Koay ES, Tang JW. A diagnostic polymerase chain reaction assay for Zika virus. J Med Virol 2012; 84:1501–5. [DOI] [PubMed] [Google Scholar]
- 20.Lanciotti RS, Kosoy OL, Laven JJ et al. . Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis 2008; 14:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Lennete ET, Lennete DA, eds. Diagnostic procedures for viral, rickettsial and chlamydial infections, 7th ed Washington, D. C: American Public Health Association, 1995: 189–212. [Google Scholar]
- 22.Erasmus JH, Needham J, Raychaudhuri S et al. . Utilization of an Eilat Virus-Based Chimera for Serological Detection of Chikungunya Infection. PLoS Negl Trop Dis 2015; 9:e0004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sistema Nacional de Vigilancia Epidemiológica. Boletín epidemiológico semana 14. Secretaría de Salud 2016; 33:14. http://www.gob.mx/cms/uploads/attachment/file/83566/sem14.pdf. Accessed 22 August 2016. [Google Scholar]
- 24.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 2014; 30:2114–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simpson JT, Wong K, Jackman SD, Schein JE, Jones SJ, Birol I. ABySS: a parallel assembler for short read sequence data. Genome Res 2009; 19:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods 2012; 9:357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson JT, Thorvaldsdottir H, Winckler W et al. . Integrative genomics viewer. Nat Biotechnol 2011; 29:24–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol 2016; 33:1870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halstead SB, Rojanasuphot S, Sangkawibha N. Original antigenic sin in dengue. Am J Trop Med Hyg 1983; 32:154–6. [DOI] [PubMed] [Google Scholar]
- 30.Li MI, Wong PS, Ng LC, Tan CH. Oral susceptibility of Singapore Aedes (Stegomyia) aegypti (Linnaeus) to Zika virus. PLoS Negl Trop Dis 2012; 6:e1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chouin-Carneiro T, Vega-Rua A, Vazeille M et al. . Differential Susceptibilities of Aedes aegypti and Aedes albopictus from the Americas to Zika Virus. PLoS Negl Trop Dis 2016; 10:e0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz-Gonzalez EE, Kautz TF, Dorantes-Delgado A et al. . First Report of Aedes aegypti Transmission of Chikungunya Virus in the Americas. Am J Trop Med Hyg 2015; 93:1325–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Rejon J, Lorono-Pino MA, Farfan-Ale JA et al. . Dengue virus-infected Aedes aegypti in the home environment. Am J Trop Med Hyg 2008; 79:940–50. [PubMed] [Google Scholar]
- 34.Wong PS, Li MZ, Chong CS, Ng LC, Tan CH. Aedes (Stegomyia) albopictus (Skuse): a potential vector of Zika virus in Singapore. PLoS Negl Trop Dis 2013; 7:e2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller BR, Monath TP, Tabachnick WJ, Ezike VI. Epidemic yellow fever caused by an incompetent mosquito vector. Trop Med Parasitol 1989; 40:396–9. [PubMed] [Google Scholar]
- 36.Weaver SC, Lecuit M. Chikungunya virus and the global spread of a mosquito-borne disease. N Engl J Med 2015; 372:1231–9. [DOI] [PubMed] [Google Scholar]
- 37.Fernandez-Salas I, Danis-Lozano R, Casas-Martinez M et al. . Historical inability to control Aedes aegypti as a main contributor of fast dispersal of chikungunya outbreaks in Latin America. Antiviral Res 2015; 124:30–42. [DOI] [PubMed] [Google Scholar]
- 38.Brasil P, Pereira JP Jr, Raja Gabaglia C et al. . Zika Virus Infection in Pregnant Women in Rio de Janeiro - Preliminary Report. N Engl J Med 2016; doi:10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.