Abstract
Background. The global regulator sarA modulates virulence of methicillin-resistant Staphylococcus aureus (MRSA) via regulation of principal virulence factors (eg, adhesins and toxins) and biofilm formation. Resistance of S. aureus strains to β-lactam antibiotics (eg, oxacillin) depends on the production of penicillin-binding protein 2a (PBP2a), encoded by mecA.
Methods. In the present study, we investigated the impact of sarA on the phenotypic and genotypic characteristics of oxacillin resistance both in vitro and in an experimental endocarditis model, using prototypic healthcare- and community-associated MRSA parental and their respective sarA mutant strain sets.
Results. All sarA mutants (vs respective MRSA parental controls) displayed significant reductions in oxacillin resistance and biofilm formation in vitro and oxacillin persistence in an experimental endocarditis model in vivo. These phenotypes corresponded to reduced mecA expression and PBP2a production and an interdependency of sarA and sigB regulators. Moreover, RNA sequencing analyses showed that sarA mutants exhibited significantly increased levels of primary extracellular proteases and suppressed pyrimidine biosynthetic pathway, argininosuccinate lyase–encoding, and ABC transporter–related genes as compared to the parental strain.
Conclusions. These results suggested that sarA regulates oxacillin resistance in mecA-positive MRSA. Thus, abrogation of this regulator represents an attractive and novel drug target to potentiate efficacy of existing antibiotic for MRSA therapy.
Keywords: sarA, β-lactam antibiotic resistance, MRSA endocarditis, treatment
Staphylococcus aureus is a leading cause of endovascular infections, including infective endocarditis [1]. Despite the use of modern antibiotics, morbidity and mortality associated with these infections remain unacceptably high [2–4]. Therefore, there is a critical need to understand the regulation of resistance and virulence determinants driving these treatment failure outcomes to enable development of novel treatment strategies.
Resistance of methicillin-resistant S. aureus (MRSA) to β-lactam antibiotics, including nafcillin and oxacillin, depends on elaboration of penicillin-binding protein 2a (PBP2a). PBP2a, encoded by mecA, is an alternative cell wall cross-linking enzyme with reduced affinity for virtually all β-lactam antibiotics. Previous studies showed that the expression of mecA closely correlated with the level of oxacillin resistance [5]. mecA is part of the staphylococcal chromosome cassette (SCCmec), which is present in MRSA but absent in methicillin (oxacillin)–susceptible S. aureus (MSSA) [6]. Importantly, the SCCmec element is transferrable among staphylococci by transduction and conjugation in the laboratory, which contributes to dissemination of resistance [7]. Furthermore, excision of the SCCmec element in MRSA strains results in oxacillin susceptibility [8]. Likewise, inactivation of mecA in MRSA strains reverses the MRSA phenotype to that of MSSA [5].
The staphylococcal accessory regulator (SarA) protein is a global regulator that governs many of the virulence factors produced by S. aureus [9–11]. SarA is also an important positive regulator of biofilm formation, in part because of its reciprocal repressive activity on protease and nuclease production [12]. Importantly, our previous investigation demonstrated that sarA mutant strains had significantly slower growth rates in the presence of oxacillin as compared to their respective parental MRSA strains [13]. Thus, we hypothesized that a combination of the impacts of sarA on methicillin/oxacillin resistance and biofilm formation may provide a novel strategy to treat MRSA biofilm-related endovascular infections. It is well known that the alternative sigma factor protein, SigB, can influence other regulons, including sarA, affording adaptive responses to a variety of environmental stresses [14, 15]. However, the relationship between sigB and oxacillin resistance is not well understood.
The present study is the first to our knowledge to demonstrate that sarA mutants exhibit significantly reduced β-lactam (eg, oxacillin) resistance as compared to respective MRSA parental strains in vitro and in experimental infective endocarditis. In addition, we delineate that these effects are based on the impact of sarA on mecA and also involve regulatory impact on sigB. These findings provide potentially unique approaches to overcome intrinsic resistance to existing β-lactam antibiotics (eg, oxacillin) in MRSA strains.
MATERIALS AND METHODS
Bacterial Strains, Plasmids, and Growth Medium
The bacterial strains and plasmids used in this study are listed in Table 1. The 2 hospital-associated MRSA (HA-MRSA) strains were from patients with persistent bacteremia derived from a multinational clinical trial collection [16, 18, 19]. The 2 community-associated MRSA (CA-MRSA) strains were MW2 (USA400, reported to cause fatal infection in children [13]) and JE2 (USA300 strain LAC, obtained from the National Institutes of Health Network on Antimicrobial Resistance in Staphylococcus aureus [NARSA] [17]). These MRSA strains belong to SCCmec type IV, in which strains are mecI and mecR2 negative and only have a truncated mecR1 [20]. In the current studies, the JE2 sarA mutant was complemented with sarA by transforming it with the plasmid pALC1215, which is pSPT181 carrying the entire sarA locus under its native promoter [22]. In addition, we introduced pALC6185, in which mecA was expressed from a xylose-inducible promoter into the JE2 sarA mutant [21]. These 2 mutant strains were confirmed with polymerase chain reaction (PCR) and sequencing. The other transposon mutants were obtained from NARSA. The S. aureus strains were routinely grown in tryptic soy broth (TSB) or TSB agar plates.
Table 1.
Staphylococcus aureus Strains and Plasmids Used in This Study
| Strain or Plasmid | Relevant Characteristic(s) | Source or Reference(s) |
|---|---|---|
| Strain | ||
| 300-169 | MRSA (agr-I, SCCmec IV, and CC45) | [12, 16] |
| 300-169 ΔsarA | 300-169 sarA::kan | [12] |
| 324-136 | MRSA (agr-I, SCCmec IV, and CC45) | [12, 16] |
| 324-136 ΔsarA | 324-136 sarA::Tn917LTV1 | [12] |
| MW2 | MRSA (SCCmec IV), USA400 | [12, 13] |
| ALC5415 | MW2 sarA::kan | [12] |
| JE2 | MRSA (SCCmec IV), LAC, USA300 | [17] |
| JE2 ΔsarA | JE2 sarA::kan with ALC6185 | [12] |
| JE2 ΔsarA/psarA | JE2 ΔsarA complemented with pALC1215 | This study |
| JE2 ΔsarA/pmecA | JE2 ΔsarA complemented with pALC6185 | This study |
| JE2 ΔmecA | Transposon mutant with insertion in S. aureus USA300_0032 | NTML |
| JE2 ΔsigB | Transposon mutant with insertion in S. aureus USA300_2022 | NTML |
| S. aureus ATCC43300 | MRSA | ATCC |
| S. aureus ATCC25923 | MSSA | ATCC |
| Plasmid | ||
| pALC6185 | pEPSA5::mecA Cmr | [21] |
| pALC1215 | pSPT181::sarA Tetr | [22] |
Abbreviations: ATCC, American Type Culture Collection; MRSA, methicillin-resistant S. aureus; MSSA, methicillin-susceptible S. aureus; NTML, Nebraska Transposon Mutant Library; SCCmec, staphylococcal chromosome cassette mec.
Determination of Oxacillin Minimum Inhibitory Concentrations (MICs)
The oxacillin MICs of study MRSA strain sets were determined by using both the standard Etest method (BioMerieux, La Balme-les-Grottes, France), according to the manufacturer's recommended protocols, and the standard broth microdilution method, as recommended by the Clinical and Laboratory Standards Institute [23].
Population Analyses
Oxacillin population analyses were performed to determine whether the MRSA strains exhibited heterogeneous or homogeneous resistance to oxacillin, using standard protocols [24, 25]. The classification of resistance as heterogeneous versus homogeneous was based on plating findings, defined as the number of colony-forming units (CFU) on oxacillin-containing (50 µg/mL) agar plates divided by the number on drug-free agar plates, multiplied by 100%. Heterogeneous and homogeneous were defined as <1% and ≥1% of CFU growth, respectively [26].
Production of PBP2a
A semiquantitative, rapid and reliable latex agglutination method was used to measure the PBP2a production according to a modified manufacture's instruction (Denka Seiken, Tokyo, Japan) [27]. A positive reaction is indicated by the development of an overt agglutination pattern within 3 minutes, while a negative reaction is indicated by a homogenous suspension appearance. The intensity of agglutination was scored blindly by 2 of us (L. L. and W. A.) as high (+++), moderate (++), or low (+), whereas the negative control, which showed no activity, was scored as negative (−). S. aureus ATCC 43300 (MRSA) and ATCC 25923 (MSSA) were used as positive and negative controls, respectively.
Biofilm Formation
Biofilm formation was performed as previously described [12, 16, 25]. Adhering dye (0.1% safranin) was dissolved in 30% acetic acid, and absorption was measured as OD490nm to quantify biofilm formation [12, 16, 25].
Isolation of RNA
Total RNA was isolated from study strain cells after 24 hours of culture at 37°C, using an RNeasy Kit (Qiagen, Valencia, CA) [12]. For the mecA transcription assays, total RNA was isolated from all parental MRSA and their respective sarA mutant strains in the presence and absence of half the MICs of oxacillin after exposure for 24 hours at 37°C, using the RNeasy kit as described above.
Transcription Analyses by Real-Time Quantitative PCR (qPCR)
Real-time qPCR was performed as described previously [12, 28]. The amplification of mecA, asp23 (surrogate for sigB expression), sarA, and gyrB was performed using primers as described previously [12, 29, 30]. gyrB was used to normalize for transcript quantification. Relative quantification was calculated by the ΔΔCT method.
Whole-Genome Sequencing (WGS)
The MRSA JE2 and its sarA mutant strain-pair underwent WGS. Genomic DNA isolation and library preparation were performed as described previously [31, 32]. The DNA library was mapped against published NC_007793 (S. aureus USA300_FPR3757) genomes, and the single-nucleotide polymorphism (SNP) and InDels were examined [34, 35].
RNA Sequencing (RNA-seq)
The RNA samples of JE2 and its sarA mutant strain-pair described above were used for RNA-Seq. Sequencing was conducted on an Illumina NextSeq platform. The RNA-Seq data were analyzed by CLC Genomics Workbench (V8.5), and the number of reads per kilobase of transcript per million mapped reads for each gene was compared between the parental and sarA mutant strains.
Experimental Endocarditis Model in Rabbits
To better define the role of sarA in vivo in regard to oxacillin responsiveness in MRSA, a well-characterized rabbit model of catheter-induced aortic valve infective endocarditis was used [12, 28]. After catheterization, animals were infected intravenously (with 105 CFU/animal, a 95% infective dose, as established in previous studies [12]) of either JE2 parental, its sarA mutant, or complemented strains. Twenty-four hours after infection, animals were randomized to receive no therapy (controls) or oxacillin at 50 mg/kg intramuscularly 3 times daily for 3 days. This oxacillin dose achieves a serum maximum concentration (Cmax) of approximately 25 µg/mL (unpublished data), which was significantly lower than the Cmax (43 µg/mL) of the recommended human clinical dose for mild-to-moderate MSSA infections (500 mg intravenously [33]). Twenty-four hours after the last oxacillin treatment, animals were euthanized. The cardiac vegetation, kidney, and spleen were then removed and quantitatively cultured. The limitation of organism density detection in the target tissues is approximately 1 log10 CFU per gram of tissue. This value was assigned to all culture-negative (sterile) tissues as a baseline for the purposes of relative calculation of the mean log10 CFU per gram of tissue (±SD) for statistical comparisons.
The Institutional Animal Care and Use Committee of the Los Angeles Biomedical Research Institute at Harbor–UCLA Medical Center approved all animal study protocols.
Statistics
To compare MRSA counts in target tissues, univariate analyses were performed using the Student t test [16]. For other experiments described, unpaired 2-tailed Student t tests were performed. P values of <.05 were considered statistically significant.
RESULTS
Effect of sarA on Oxacillin MICs
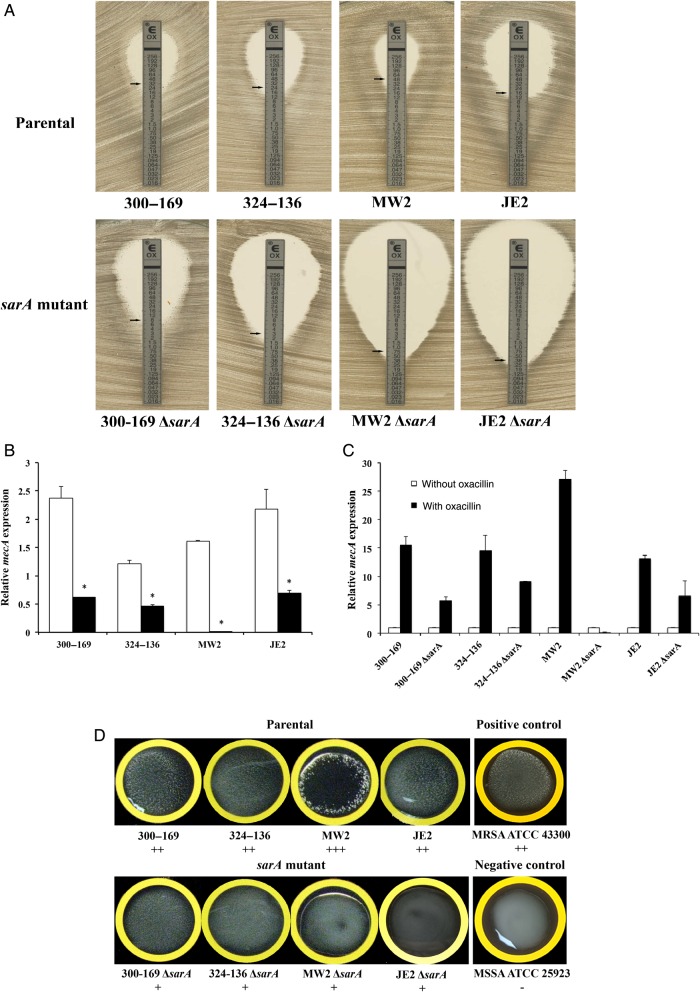
As expected, all parental MRSA strains were oxacillin resistant, with MICs ranging from 16 to 48 µg/mL (Figure 1A). Interestingly, the sarA mutants exhibited a 4–64-fold reduction in oxacillin MICs versus their respective parental strains. For instance, the 2 CA-MRSA sarA mutants were hypersusceptible to oxacillin (MICs ≤ 1.0 µg/mL). In addition, we found an agreement of overall MICs (within ±2-fold dilutions) between the results of the Etest and broth microdilution in all study strains.
Figure 1.
Oxacillin minimum inhibitory concentrations (MICs) determined by the Etest (A), relative expression of mecA in the absence (B), and the presence of half MICs of oxacillin (C) and penicillin-binding protein 2a (PBP2a) agglutination (D) of study methicillin-resistant Staphylococcus aureus parental and sarA mutant strain sets. B, Relative transcription levels of mecA represent the mean (+SD) of 3 biological replicates in vitro. The fold changes represent the normalized mecA expression (2−ΔCt) to the housekeeping gene gyrB. *P < .05, compared with their respective parental strains. Open bars, parental strain; shaded bars, sarA mutant. D, mecA-positive (ATCC 43300) and mecA-negative (ATCC 25923) controls. Interpretation of agglutination intensity: +++, high; ++, moderate; +, low; and −, none.
Population Analyses
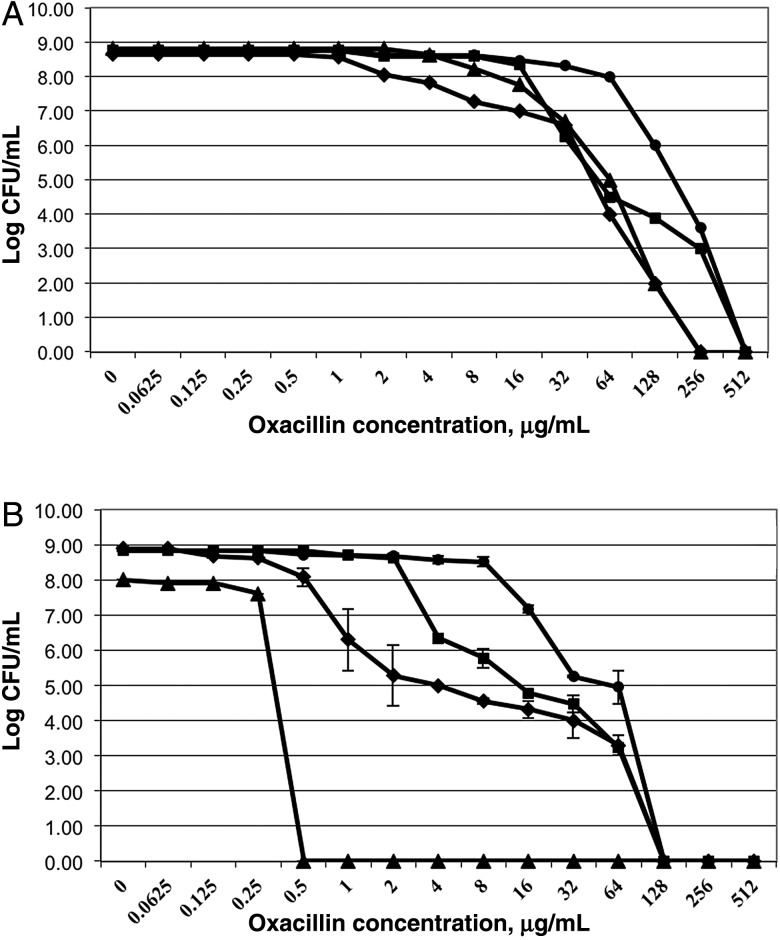
We demonstrated that only 1 MRSA parental strain (300-169) exhibited oxacillin homogeneous resistance, while the other 3 parental strains showed heterogeneous resistance to oxacillin (Figure 2). In addition, 3 of 4 sarA mutants, except sarA mutant in the MW2 background, had hetero-oxacillin–resistant subpopulations (Figure 2).
Figure 2.
Population analyses of methicillin-resistant Staphylococcus aureus parental (A) and their respective sarA mutant (B) strains upon exposure to a range of oxacillin concentrations. These data represent the means (±SD) for 2 separate assays. Strain 300-169, circles; strain 324-136, squares; strain MW2, triangles; and strain JE2, diamonds. CFU, colony-forming units.
Impact of sarA on mecA Transcription and PBP2a Production
The effect of sarA on oxacillin susceptibility suggested that this outcome might be due to its influence on mecA expression and PBP2a production. Consistent with the oxacillin MICs, all parental strains had significantly higher mecA expression as compared to their respective sarA mutants (P < .005; Figure 1B). Additionally, induced mecA expression was observed in the presence of half the MICs of oxacillin versus the absence of oxacillin in all study strains (Figure 1C). However, the increased degree of mecA expression was substantially lower in the sarA mutants as compared to their respective parental strain (Figure 1C).
For PBP2a production, as expected, the positive MRSA control strain had strong agglutination, while PBP2a production was not detected in the negative control MSSA strain (Figure 1D). In accordance with the mecA expression results, a higher intensity of agglutination, indicating higher PBP2a production, was observed in the MRSA parental strains vs their respective sarA mutants (Figure 1D).
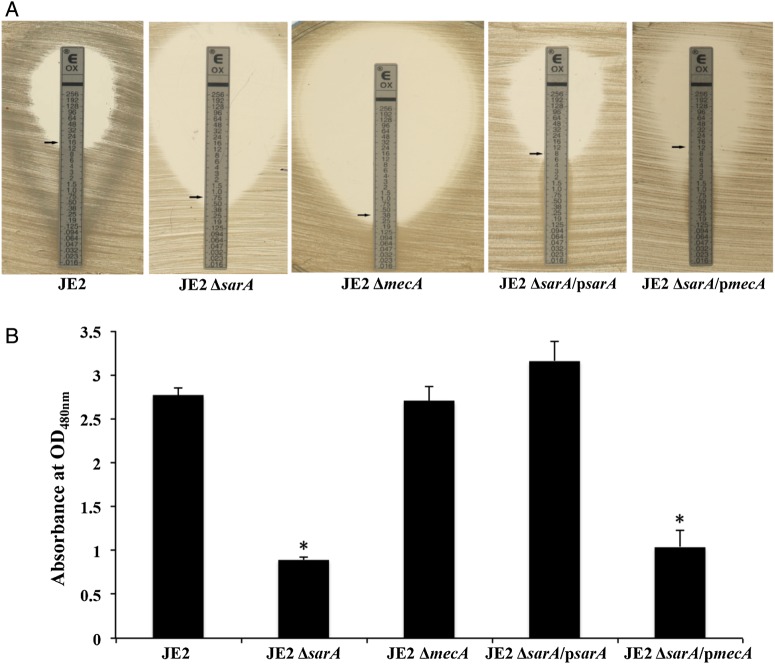
To define the relationship between sarA and mecA on abrogation of MRSA phenotype, we examined oxacillin MICs using sarA and mecA mutants, and their complemented strains in the sarA mutant strain in the JE2 background. We found that oxacillin MICs were significantly reduced in the sarA and mecA mutants, thus changing the MRSA phenotype to methicillin susceptible (Figure 3A). These reduced oxacillin MICs were restored to MRSA parental levels with complementation of sarA or mecA in the sarA mutant strain (Figure 3A). In addition, a similar intensity of agglutination was observed from the PBP2a production assays between the JE2 parental and ΔsarA/psarA strains (data not shown).
Figure 3.
Oxacillin minimum inhibitory concentrations determined by the Etest (A) and biofilm formation (B) of the methicillin-resistant Staphylococcus aureus JE2 parental strain, its sarA mutant, mecA mutant, ΔsarA/psarA (sarA complemented in the sarA mutant strain), and ΔsarA/pmecA (mecA complemented in the sarA mutant strain). Results of biofilm formation are shown as the mean of the OD490nm (+SD) from 3 biological replicates, each of which was done in triplicate. *P < .05, for the strains, compared with the JE2 parental strain.
Biofilm Formation
The ΔsarA strain formed significantly less biofilm as compared to its parental JE2 strain and the complemented sarA mutant restored formation of biofilm to the level of the parent JE2 strain (Figure 3B). Based on the observations of sarA on biofilm formation and oxacillin susceptibility, we hypothesized that oxacillin treatment may have good efficacy against biofilm-related MRSA infections. However, there were no significant differences in biofilm formation between the mecA mutant and its parental strain, as well as between the JE2 ΔsarA and ΔsarA/pmecA strains, which collectively suggests that mecA itself has no effect on biofilm formation (Figure 3B).
Influence of sarA on sigB
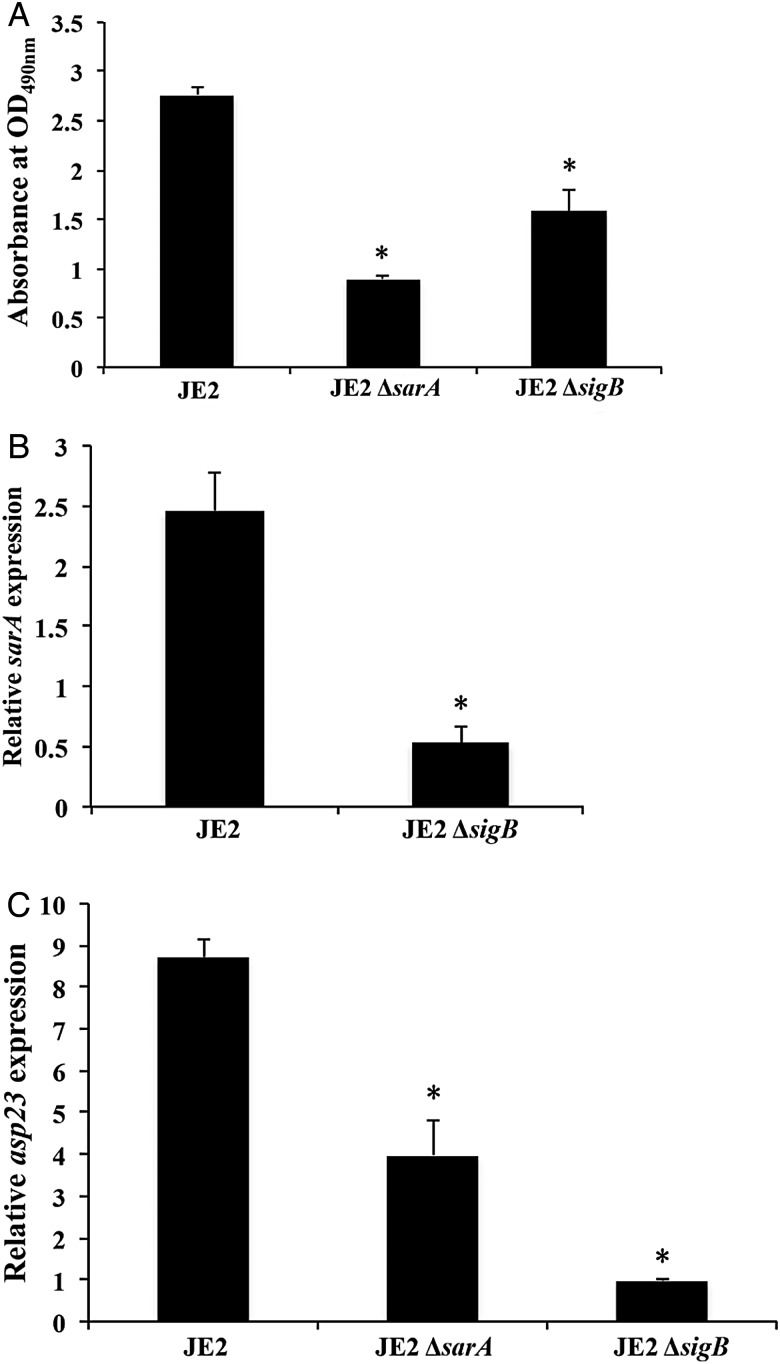
It has been demonstrated that modulation of the sarA locus is dependent on the stress-induced transcription factor SigB. Thus, the interaction between sarA and sigB and their impact on oxacillin MICs, PBP2a production, biofilm formation, and sarA expression were studied by using sarA and sigB mutant strains in the JE2 background. As shown in Figure 4A, the sigB knockout of strain JE2 also had significantly reduced oxacillin MICs (4 µg/mL) versus its parental strain, in part due to the lower expression of mecA and lower production of PBP2a (Figure 4B and 4C). Furthermore, the sigB mutant formed significantly less biofilm (P < .01; Figure 5A). Given that sigB is a regulator of sarA [15], we found that the sigB mutant had significantly less sarA expression when compared to the JE2 parental strain (P < .01; Figure 5B). Interestingly, the sarA mutant strain exhibited approximately 2-fold lower asp23 expression (a marker for sigB activity) as compared to the parental strain (P < .01; Figure 5C). As a control, the sigB mutant had very low level of asp23 expression as compared to JE2 and the sarA mutant. Our data suggest interdependency between the sarA and sigB regulators in terms of the MRSA phenotype and biofilm formation, as well as a positive regulatory effect of sarA on sigB activity.
Figure 4.
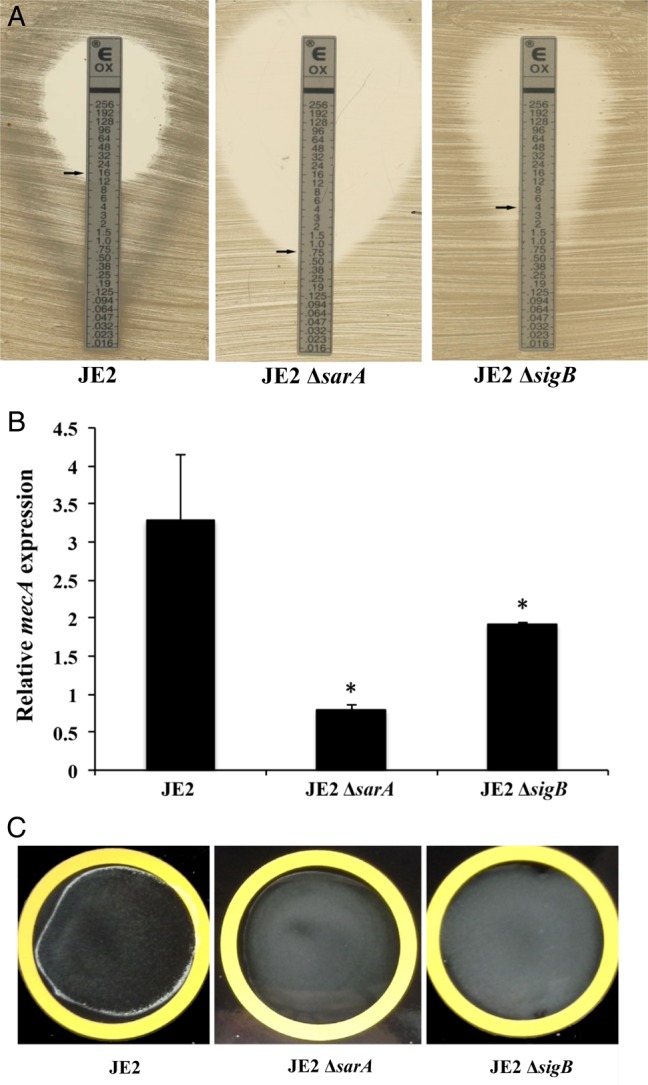
Oxacillin minimum inhibitory concentrations determined by the Etest (A), relative expression of mecA (B), and PBP2a agglutination (C) of the JE2 parental strain and its sarA and sigB mutants. Relative transcript levels of mecA represent the mean (+SD) of 3 biological replicates in vitro. The fold changes represent normalized target gene expression (2−ΔCt) to the housekeeping gene gyrB. *P < .05, compared with the JE2 parental strain.
Figure 5.
Biofilm formation (A) and relative expression of sarA (B) and asp23 (C) in the methicillin-resistant Staphylococcus aureus JE2 parental strains and its sarA and/or sigB mutant. Relative transcript levels of sarA and asp23 represent the mean (+SD) of 3 biological replicates in vitro. The fold changes represent normalized target gene expression (2−ΔCt) to the housekeeping gene gyrB. *P < .05, compared with the JE2 parental strain.
RNA-seq Findings
We found that 9 and 15 genes were expressed significantly higher and lower, respectively, in the sarA mutant versus the JE2 parental strain (P < .01; Supplementary Table 1). For instance, 4 major extracellular proteases (sspB, sspA, scp, and aur) and proton antiporter genes were expressed higher in the sarA mutant versus the parental strain. On the other hand, inactivation of sarA decreased the expression of pyrimidine biosynthetic pathway genes (pyrF, pyrE, carB, pyrB, pyrC, and pyrP) and argininosuccinate lyase–encoding genes. In addition, similar RNA-seq results were obtained for MW2 and its sarA mutant strain (data not shown). Moreover, 2 sarA upregulated (argH and SAUSA300-1477) and 2 sarA downregulated (ear and sspB) genes with the most substantial changes were selected for validation of their expression in JE2 and its sarA mutant strains by real-time qPCR. Our data demonstrated that the expression of these genes yielded outcomes similar to those observed by RNA-seq (data not shown).
WGS Findings
Interestingly, our data indicated that JE2 and the JE2 sarA mutant had identical WGS findings, with the exception of the insertion of aph(3′)-III (which confers resistance to kanamycin) within the JE2 sarA mutant. There were also approximately 45 SNPs demonstrated between the USA300_FPR3757 and JE2 strains.
Impact of sarA on the Efficacy of Oxacillin in a Rabbit Infective Endocarditis Model
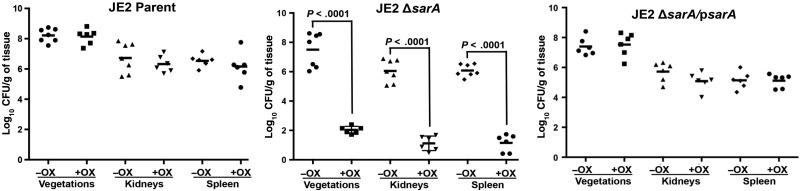
Of interest, in the absence of oxacillin therapy, the sarA mutant was associated with reduced MRSA densities in vegetations (P < .05) but not in other hematogenously seeded target organs (kidneys and spleen), compared with its respective JE2 parental strain (Figure 6). As expected, oxacillin treatment had no efficacy in the model of infective endocarditis caused by the JE2 parental strain. However, of great significance, animals infected with the sarA mutant of JE2 were hypersusceptible to oxacillin treatment, with 100% sterile tissue cultures (Figure 6). In addition, animals infected by the sarA-complemented variant did not respond to oxacillin treatment; this confirmed the important roles of sarA in maintaining the MRSA phenotype and, consequently, the resistance to oxacillin therapeutic outcomes in this model.
Figure 6.
Densities of methicillin-resistant Staphylococcus aureus in target tissues in the infective endocarditis model due to challenge with 105 colony-forming units (CFU) of the JE2 parental strain, its sarA mutant, or the sarA complementary strain, with (+) or without (−) oxacillin (OX) therapy (50 mg/kg intramuscularly 3 times daily for 3 days). Each dot represents 1 rabbit. Horizontal black bars indicate mean values for observations.
DISCUSSION
In the current study, we demonstrated that sarA mutants exhibited significantly reduced oxacillin MICs relative to respective parental strains. This finding was attributable in part to decreased mecA expression and corresponding reduced PBP2a production, which was consistent with previous reports [36, 37]. For example, Arede et al reported that the inactivation of MecR2, a robust activator of mecA transcription that disrupts binding of the repressor MecI to the mecA promoter, converted a MRSA strain to methicillin-susceptible phenotype [37]. Previous studies also showed that blaI and blaR1, 2 genes located upstream of β-lactamase gene (blaZ), regulate β-lactamase and PBP2a production in S. aureus [38]. Published findings of WGS analysis demonstrated that the β-lactamase genes (blaI-blaR1-blaZ) were not found in our 2 study CA-MRSA strains. In addition, our unpublished WGS data showed that, although one of the HA-MRSA strains does have these genes, there are a number of SNPs identified (as compared to a reference strain; data not shown). Therefore, the current study was not designed to address the potential impact of sarA on these genes. In addition to mecA and blaZ, other genes have been reported to influence the MRSA phenotype. For example, inactivation of llm, agr, and fmtA each impart reductions in methicillin resistance without converting the MRSA strain to a mecA-negative MSSA strain and with no apparent affect on PBP2a production [39, 40]. In addition, factors essential for methicillin resistance, such as FemA, FemB, and FemC, are also important to the essential resistance phenotype by affecting peptidoglycan precursor synthesis [41, 42]. It has been reported that inactivation of femAB renders MRSA hypersusceptible to β-lactam. However, inactivation of femC has less effects on methicillin resistance since highly resistant subpopulations are still present [41]. Moreover, Boyle-Vavra et al reported that VraSR, a 2-component regulatory system, regulates downstream genes that presumably facilitate resistance to cell wall–inhibitory antibiotics, and inactivation of vraSR decreases oxacillin resistance without decreasing mecA expression [43, 44]. Taken together, these results indicate that, in addition to mecA and blaZ, the methicillin resistance phenotype is also influenced by other chromosomal factors and that the mechanism(s) of these effects are mecA independent.
Our WGS data showed that JE2 and its sarA mutant are identical except for the insertion of aph(3′)-III, which was used to disrupt sarA in the sarA mutant. These data underscore the independent role of sarA in the MRSA phenotypes observed in our study. Our RNA-Seq data showed that the pyrimidine biosynthetic pathway genes significantly increased in parent versus sarA mutants. These findings were consistent with reports by Cordwell et al [45], who demonstrated that the pyrimidine biosynthetic pathway genes had higher expression in MRSA (COL) than MSSA (8325). However, the impact of the perturbation of the pyrimidine biosynthetic pathways on the MRSA phenotype via sarA-mecA interactions remains to be elucidated.
Biofilm formation is a key virulence strategy of staphylococci [8, 46]. In the current study, as expected, we found that the sarA mutant formed significantly less biofilm than the parental strain and had increased expression of major extracellular proteases [46, 47]. Therefore, it is conceivable that sarA-mediated reduction in biofilm formation influences methicillin/oxacillin susceptibility beyond its negative impact on protease genes. For example, a logical hypothesis might posit that the sarA mutant derepresses protease expression, leading to the increased proteolytic activity and degradation of PBP2a necessary for oxacillin resistance. Of special interest, we also observed that the mecA mutant displayed significantly reduced oxacillin resistance but without a discernible effect on biofilm formation. Like most clinical MRSA strains [48], 3 of the 4 MRSA strains in this study exhibited a heterogeneous pattern of resistance to oxacillin. Pozzi et al recently reported that biofilm formation by S. aureus 8325-4 (oxacillin susceptible) and its isogenic strain carrying pmecA, which exhibits a heterogeneous oxacillin resistance phenotype, were similar [8]. These findings corroborated our current results, indicating that the heterogeneous oxacillin susceptibility may have no direct impact on biofilm formation.
In parallel to sarA, we also investigated another key global regulon, sigB, because of its impact on expression of multiple virulence genes and global regulators, including sarA [14]. It is instructive to note that the influence of sigB on sarA is somewhat controversial. Bischoff et al reported a positive regulatory effect of sigB on sarA [15]. In contrast, Cheung et al [49] demonstrated a negative impact of sigB on sarA transcription. These contrasting findings may be due to differences in genetic backgrounds of study strains used in the 2 studies. In the present work, we discovered that not only did the sigB mutant exhibit decreased sarA expression, but also that the sarA mutant had reduced asp23 transcription (a surrogate marker of sigB activation). This outcome indicated a positive regulatory effect of sigB on sarA, as well as a positive, reciprocal feedback influence of sarA on sigB. The mechanism(s) of this internally amplifying autocrine feedback loop between sarA and sigB is not well understood. Several observations have suggested that the regulatory effects of SarA might be more complex than initially appreciated [11]. Besides transcriptional regulation, the present results also revealed that the ΔsigB null mutation significantly reduced oxacillin resistance and biofilm formation as compared to its JE2 parental strain. This finding was in accordance with previous findings in which inactivation of sigB resulted in reduced methicillin resistance [50]. These results support our hypothesis that the sigB mutant exhibited reduced sarA expression, subsequently resulting in downregulated mecA. Consequently, this effect would lead to the reduction of oxacillin resistance, a phenotype potentially amplified by the positive feedback impact imposed by sarA on sigB activation.
Perhaps most importantly, the genotypic and phenotypic findings described above translated into a significant outcome of S. aureus infection in vivo. These data underscored the significance of sarA in differential oxacillin resistance in the relevant setting of infective endocarditis. As expected, oxacillin had no therapeutic effect in the model of infective endocarditis caused by MRSA strains. However, animals infected with the sarA mutant exhibited a striking degree of hypersusceptibility to oxacillin treatment. The mechanism(s) of this therapeutic effect are thought to occur by inactivation of sarA, which subsequently reduces oxacillin resistance and biofilm formation. From a broader perspective, these results raise the exciting possibility that novel compounds targeting sarA expression and/or function may add to the future anti-MRSA armamentarium. In addition, these data provide an impetus into further studies to investigate the mechanism(s) of how sarA regulates mecA, including defining whether sarA binds to the mecA promoter and/or its messenger RNA and whether increased oxacillin susceptibility in the sarA mutants involves other regulatory factors downstream of or beyond the sarA network that regulates mecA.
Supplementary Material
Notes
Financial support. This work was supported by the National Institutes of Health (grants AI-097657 [to Y. Q. X.], AI-091801 [to A. L. C.], AI-039108-19 [to A. S. B.], and AI-124319 and AI-111661 [to M. R. Y.]) and the Chinese National Natural Science Foundation (grant 31272609 to L. L.).
Potential conflicts of interest. Y. Q. X. received research grants from ContraFect, Meck and Theravance. A. S. B. is a member of scientific advisory board of ContraFect and received research grants from ContraFect and Theravance. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Fowler VG Jr, Justice A, Moore C et al. Risk factors for hematogenous complications of intravascular catheter-associated Staphylococcus aureus bacteremia. Clin Infect Dis 2005; 40:695–703. [DOI] [PubMed] [Google Scholar]
- 2.Boucher H, Miller LG, Razonable RR. Serious infections caused by methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2010; 51(suppl 2):S183–97. [DOI] [PubMed] [Google Scholar]
- 3.Boucher HW, Sakoulas G. Perspectives on Daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin Infect Dis 2007; 45:601–8. [DOI] [PubMed] [Google Scholar]
- 4.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2008; 46(suppl 5):S344–9. [DOI] [PubMed] [Google Scholar]
- 5.Chen FJ, Wang CH, Chen CY, Hsu YC, Wang KT. Role of the mecA gene in oxacillin resistance in a Staphylococcus aureus clinical strain with a pvl-positive ST59 genetic background. Antimicrob Agents Chemother 2014; 58:1047–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katayama Y, Ito T, Hiramatsu K. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 2000; 44:1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray MD, Boundy S, Archer GL. Transfer of the methicillin resistance genomic island among staphylococci by conjugation. Mol Microbiol 2016; 100:675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pozzi C, Waters EM, Rudkin JK et al. Methicillin resistance alters the biofilm phenotype and attenuates virulence in Staphylococcus aureus device-associated infections. PLoS Pathog 2012; 8:e1002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunman PM, Murphy E, Haney S et al. Transcription profiling-based identification of Staphylococcus aureus genes regulated by the agr and/or sarA loci. J Bacteriol 2001; 183:7341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien Y, Manna AC, Cheung AL. SarA level is a determinant of agr activation in Staphylococcus aureus. Mol Microbiol 1998; 30:991–1001. [DOI] [PubMed] [Google Scholar]
- 11.Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus. Int J Biochem Cell Biol 2008; 40:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdelhady W, Bayer AS, Seidl K et al. Impact of vancomycin on sarA-mediated biofilm formation: role in persistent endovascular infections due to methicillin-resistant Staphylococcus aureus. J Infect Dis 2014; 209:1231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trotonda MP, Xiong YQ, Memmi G, Bayer AS, Cheung AL. Role of mgrA and sarA in methicillin-resistant Staphylococcus aureus autolysis and resistance to cell wall-active antibiotics. J Infect Dis 2009; 199:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bischoff M, Dunman P, Kormanec J et al. Microarray-based analysis of the Staphylococcus aureus sigmaB regulon. J Bacteriol 2004; 186:4085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bischoff M, Entenza JM, Giachino P. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J Bacteriol 2001; 183:5171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seidl K, Chen L, Bayer AS, Hady WA, Kreiswirth BN, Xiong YQ. Relationship of agr expression and function with virulence and vancomycin treatment outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2011; 55:5631–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spentzas T, Kudumula R, Acuna C et al. Role of bacterial components in macrophage activation by the LAC and MW2 strains of community-associated, methicillin-resistant Staphylococcus aureus. Cell Immunol 2011; 269:46–53. [DOI] [PubMed] [Google Scholar]
- 18.Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis 2009; 199:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fowler VG Jr, Sakoulas G, McIntyre LM et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis 2004; 190:1140–9. [DOI] [PubMed] [Google Scholar]
- 20.Milheirico C, Oliveira DC, de Lencastre H. Multiplex PCR strategy for subtyping the staphylococcal cassette chromosome mec type IV in methicillin-resistant Staphylococcus aureus: ‘SCCmec IV multiplex’. J Antimicrob Chemother 2007; 60:42–8. [DOI] [PubMed] [Google Scholar]
- 21.Memmi G, Filipe SR, Pinho MG, Fu Z, Cheung A. Staphylococcus aureus PBP4 is essential for beta-lactam resistance in community-acquired methicillin-resistant strains. Antimicrob Agents Chemother 2008; 52:3955–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manna AC, Bayer MG, Cheung AL. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol 1998; 180:3828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 8th ed Approved standard M07-A8 Wayne, PA: Clinical and Laboratory Standards Institute, 2009. [Google Scholar]
- 24.Moore MR, Perdreau-Remington F, Chambers HF. Vancomycin treatment failure associated with heterogeneous vancomycin-intermediate Staphylococcus aureus in a patient with endocarditis and in the rabbit model of endocarditis. Antimicrob Agents Chemother 2003; 47:1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdelhady W, Bayer AS, Seidl K et al. Reduced vancomycin susceptibility in an in vitro catheter-related biofilm model correlates with poor therapeutic outcomes in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2013; 57:1447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartman BJ, Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother 1986; 29:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jafri AK, Reisner BS, Woods GL. Evaluation of a latex agglutination assay for rapid detection of oxacillin resistant Staphylococcus aureus. Diagn Microbiol Infect Dis 2000; 36:57–9. [DOI] [PubMed] [Google Scholar]
- 28.Abdelhady W, Chen L, Bayer AS et al. Early agr activation correlates with vancomycin treatment failure in multi-clonotype MRSA endovascular infections. J Antimicrob Chemother 2015; 70:1443–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oliveira DC, de Lencastre H. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 2002; 46:2155–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ster C, Gilbert FB, Cochard T, Poutrel B. Transcriptional profiles of regulatory and virulence factors of Staphylococcus aureus of bovine origin: oxygen impact and strain-to-strain variations. Mol Cell Probes 2005; 19:227–35. [DOI] [PubMed] [Google Scholar]
- 31.Mwangi MM, Wu SW, Zhou Y et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A 2007; 104:9451–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda M, Ohta T, Uchiyama I et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001; 357:1225–40. [DOI] [PubMed] [Google Scholar]
- 33.PDR Network. Oxacillin (oxacillin)—drug summary http://www.pdr.net/drug-summary/Oxacillin-oxacillin-3066 Accessed 29 August 2016. [Google Scholar]
- 34.Diep BA, Gill SR, Chang RF et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006; 367:731–9. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Durbin R. Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 2010; 26:589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brakstad OG, Maeland JA. Mechanisms of methicillin resistance in staphylococci. APMIS 1997; 105:264–76. [DOI] [PubMed] [Google Scholar]
- 37.Arede P, Milheirico C, de Lencastre H, Oliveira DC. The anti-repressor MecR2 promotes the proteolysis of the mecA repressor and enables optimal expression of beta-lactam resistance in MRSA. PLoS Pathog 2012; 8:e1002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hackbarth CJ, Chambers HF. blaI and blaR1 regulate beta-lactamase and PBP 2a production in methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 1993; 37:1144–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maki H, Yamaguchi T, Murakami K. Cloning and characterization of a gene affecting the methicillin resistance level and the autolysis rate in Staphylococcus aureus. J Bacteriol 1994; 176:4993–5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Komatsuzawa H, Ohta K, Labischinski H, Sugai M, Suginaka H. Characterization of fmtA, a gene that modulates the expression of methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 1999; 43:2121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berger-Bachi B, Tschierske M. Role of fem factors in methicillin resistance. Drug Resist Updat 1998; 1:325–35. [DOI] [PubMed] [Google Scholar]
- 42.de Lencastre H, Tomasz A. Reassessment of the number of auxiliary genes essential for expression of high-level methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 1994; 38:2590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boyle-Vavra S, Yin S, Jo DS, Montgomery CP, Daum RS. VraT/YvqF is required for methicillin resistance and activation of the VraSR regulon in Staphylococcus aureus. Antimicrob Agents Chemother 2013; 57:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin S, Daum RS, Boyle-Vavra S. VraSR two-component regulatory system and its role in induction of pbp2 and vraSR expression by cell wall antimicrobials in Staphylococcus aureus. Antimicrob Agents Chemother 2006; 50:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cordwell SJ, Larsen MR, Cole RT, Walsh BJ. Comparative proteomics of Staphylococcus aureus and the response of methicillin-resistant and methicillin-sensitive strains to Triton X-100. Microbiol 2002; 148:2765–81. [DOI] [PubMed] [Google Scholar]
- 46.Otto M. Staphylococcal biofilms. Curr Top Microbiol Immunol 2008; 322:207–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zielinska AK, Beenken KE, Mrak LN et al. sarA-mediated repression of protease production plays a key role in the pathogenesis of Staphylococcus aureus USA300 isolates. Mol Microbiol 2012; 86:1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chambers HF. Methicillin-resistant staphylococci. Clin Microbiol Reviews 1988; 1:173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung AL, Chien YT, Bayer AS. Hyperproduction of alpha-hemolysin in a sigB mutant is associated with elevated SarA expression in Staphylococcus aureus. Infect Immun 1999; 67:1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh VK, Schmidt JL, Jayaswal RK, Wilkinson BJ. Impact of sigB mutation on Staphylococcus aureus oxacillin and vancomycin resistance varies with parental background and method of assessment. Int J Antimicrob Agents 2003; 21:256–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.