Abstract
Cryopreservation of human umbilical vein endothelial cells (HUVECs) is important to tissue engineering applications and the study of the role of endothelial cells in cardiovascular and cerebrovascular diseases. The traditional methods for cryopreservation by vitrification (cooling samples to a cryogenic temperature without apparent freezing) using high concentration of cryoprotective agents (CPAs) and slow freezing are suboptimal due to the severe toxicity of high concentration of CPAs and ice formation-induced cryoinjuries, respectively. In this study, we developed a method to cryopreserve HUVECs by vitrification with low concentration of CPAs. This is achieved by optimizing the CPAs and using highly thermally conductive quartz capillary (QC) to contain samples for vitrification. The latter minimizes the thermal mass to create ultra-fast cooling/warming rates. Our data demonstrate that HUVECs can be vitrified in the QC using 1.4 mol/L ethylene glycol and 1.1 mol/L dimethyl sulfoxide with more than 90% viability. Moreover, this method significantly improves the attachment efficiency of the cryopreserved HUVECs. The attached cells post-cryopreservation proliferate similarly to fresh cells. Therefore, this study may provide an effective vitrification technique to bank HUVECs for vascular tissue engineering and other applications.
Introduction
Human umbilical vein endothelial cells (HUVECs) are in high demand for engineering blood vessels1,2 and vascularized tissues3 to treat diseases due to their low immunogenicity,4 and an important cell model for investigating the role of endothelial cells in cardiovascular and cerebrovascular diseases.5,6 Therefore, effective cryopreservation of HUVECs to ensure its convenient availability is of great significance. However, damage to endothelial cells due to the formation of ice crystals and toxicity of cryoprotective agents (CPAs) during cryopreservation could compromise the barrier function of the endothelium.7 Therefore, it is important to develop an effective method for cryopreservation of HUVECs with minimal damage.
Up to now, slow freezing is the most extensively used approach for the cryopreservation of HUVECs.8,9 Although a low and minimally toxic concentration of cell membrane-permeable CPAs is used for this approach, it is associated with cell damage due to severe cell dehydration along with prolonged exposure to CPAs and ice formation because of the requirement of slowly freezing to form ice for dehydrating cells.10,11
In contrast, the goal of vitrification is to cool cells to a cryogenic temperature without any lethal effects of ice formation, which is considered to be a promising alternative to the conventional slow-freezing approach for cell cryopreservation.12,13 Vitrification is defined as the solidification of a solution by an extreme increase in viscosity without crystallization. In an aqueous solution, vitrification means ice-free solidification. However, the high concentration of CPAs (usually more than 4 mol/L) commonly used for vitrification are toxic to many types of cells and tissues.14 Therefore, it is desired to decrease the CPA concentration to a low and relatively nontoxic level for cell vitrification, which could be achieved by creating a high cooling/warming rate.15
Various devices have been used to create a high cooling/warming rate for cell vitrification, including the conventional French-type straws (CS),16 cryotop,17 electron microscopy copper grid,18,19 and cryoloop.20,21 The cooling rate and warming rate for CS determined in our laboratory are around 1200°C/min and 700°C/min, respectively (unpublished data). Nevertheless, the improvement of cooling/warming rates with all these technologies is limited and a toxic concentration of CPAs is still needed.17,22 More recently, thin-walled (10 μm) quartz capillary (QC) has been explored to achieve low-CPA vitrification of stem cells and oocytes, taking advantage of the miniaturized size and highly conductive quartz wall of the QC for creating an ultrafast cooling/warming rate. It is reported that quartz capillaries combined with slush nitrogen raised the cooling rate to 250,000°C/min between 20°C and −150°C and warming rate to 75,000°C/min between −150°C and 20°C.23,24 However, such study has not been reported with endothelial cells.
The goal of this study was to develop an approach for cryopreserving HUVECs at a low and relatively nontoxic level of CPAs by using the QC to achieve vitrification by ultrafast freezing and by identifying the optimal CPA combination. This approach can combine the advantages of the conventional slow freezing and vitrification with a high concentration of CPAs while avoiding their shortcomings. Six different CPA solutions were compared and tested during this study. Cell viability, attachment efficiency, and proliferation rate of HUVECs were investigated with QC-assisted vitrification. The results indicate that vitreous cryopreservation of HUVECs at a high survival with a low concentration of CPAs can be achieved using the QC-assisted approach.
Materials and Methods
Cell culture
HUVECs were purchased from Jiangsu KeyGen Biotechnology Corporation, Ltd. (Nanjing, Jiangsu, China) and were grown in RPMI-1640 medium (Hyclone, USA) supplemented with 10% (v/v) fetal bovine serum (FBS) in a humidified 5% carbon dioxide incubator at 37°C. Cells were plated in 25-cm2 T-flasks and were trypsinized with 0.25 wt% trypsin (Hyclone) for 5 min at 37°C after washing three times with phosphate-buffered saline (1× PBS) when the cells reached 80% of confluence. After being centrifuged at 100 g for 5 min, the cells were resuspended in cold RPMI-1640 containing 10% (v/v) FBS (on ice, ∼4°C) for further experiments.
Conventional straws and quartz capillary
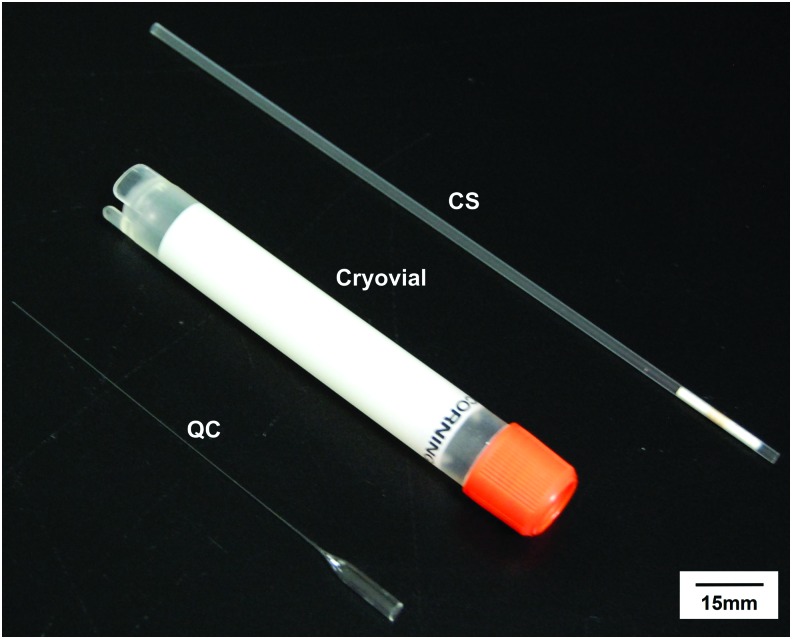
The straw used in this study was CS (FHK, Japan) as shown in Figure 1. The outer diameter is 2 mm and the thickness of its wall is 0.16 mm. The thin-walled QC (Wolfgang Muller Glass Technik, Germany) used has an outer diameter of 200 μm and a wall thickness of 10 μm, which is much smaller than CS as shown in Figure 1. Therefore, the CS has a sample volume (200 μL) of 100 times more than that of QC (∼2 μL). These geometrical minimizations (10 times smaller in outer diameter and 16 times thinner in wall thickness) facilitate a faster heat transfer. The probability of ice formation is strongly volume dependent. Therefore, QC is beneficial because of its small volume.23 Additionally, it can achieve an ultrafast cooling rate by direct immersion in liquid nitrogen (LN2) to minimize ice formation.25
FIG. 1.
A comparison of cryodevices used in this study, including CS, QC, and the 5 mL cryovial. The diameter of QC is 200 μm with a thin wall of 10 μm. Scale bar: 15 mm. CS, conventional straw; QC, quartz capillary. Color images available online at www.liebertpub.com/tec
CPA-laden cryopreservation solutions
In this study, we used vitrification solutions with three different cell membrane-penetrating CPAs, including dimethyl sulfoxide (DMSO; Sangon Biotech Co., Ltd., China), 1,2-propanediol (PROH; Sigma, St. Louis, MO), ethylene glycol (EG; Sigma, St. Louis, MO), together with trehalose (Sinozyme Biotechnology Co., Ltd., China) that does not penetrate the cell membrane. The combinations of two cell membrane-penetrating CPAs were used with a ratio of 1:1 in volume. CPA solutions of high concentration 15% (v/v) and low concentration 8% (v/v) were considered. Since carbohydrates are often used in cryopreservation by vitrification, further opacity parameters were investigated using the conventional straw for solutions with 0.5 or 1 mol/L trehalose and low-concentration CPAs in HUVEC medium.
Eventually, we designed the vitrification solutions for CS as follows: two kinds of 15% (v/v) penetrating CPAs and 0.5 mol/L trehalose in HUVEC medium with 20% (v/v) FBS (VSCSHC) and two kinds of 8% (v/v) penetrating CPAs and 1 mol/L trehalose in HUVEC medium with 20% (v/v) FBS (VSCSLC) that can be vitrified in the bulk solution by directly immersing the CS into LN2 for cooling. The CPA solutions were loaded into the CS with the aid of a rubber suction bulb.
The QC has small size and small sample volumes compared with CS. Therefore, we found the VSCSLC that allows apparent vitrification in CS also works for QC. The vitrification solution for QC (VSQC) was the same as VSCSLC (vitrification solution for CS at low concentration) and can be vitrified with no apparent ice formation by plunging the QC into LN2 for cooling. In addition, we also use this low concentration of CPA solution for slow freezing HUVECs by using CS (SFSCSLC-ED) to compare the results of different methods. The components of CPA solutions and cryopreservation conditions used in this study are summarized in Table 1.
Table 1.
List of the Abbreviations for Cryoprotective Agent Solutions and Cryopreservation Methods
| CPA solution | |||||||
|---|---|---|---|---|---|---|---|
| Permeating CPA (%, v/v) | |||||||
| Group | EG | DMSO | PROH | Trehalose (mol/L) | CPA concentration | Cryodevice | Method |
| VSQC-ED | 8% | 8% | 1 | Low | QC | Vitrification | |
| SFSCSLC-ED | 8% | 8% | 1 | Low | CS | Slow freezing | |
| VSCSLC-ED | 8% | 8% | 1 | Low | CS | Vitrification | |
| VSCSLC-EP | 8% | 8% | 1 | Low | CS | Vitrification | |
| VSCSLC-PD | 8% | 8% | 1 | Low | CS | Vitrification | |
| VSCSHC-ED | 15% | 15% | 0.5 | High | CS | Vitrification | |
| VSCSHC-EP | 15% | 15% | 0.5 | High | CS | Vitrification | |
| VSCSHC-PD | 15% | 15% | 0.5 | High | CS | Vitrification | |
CPA, cryoprotective agent; CS, conventional straw; DMSO, dimethyl sulfoxide; EG, ethylene glycol; PROH, 1,2-propanediol; QC, quartz capillary.
Vitrification of HUVECs using CS
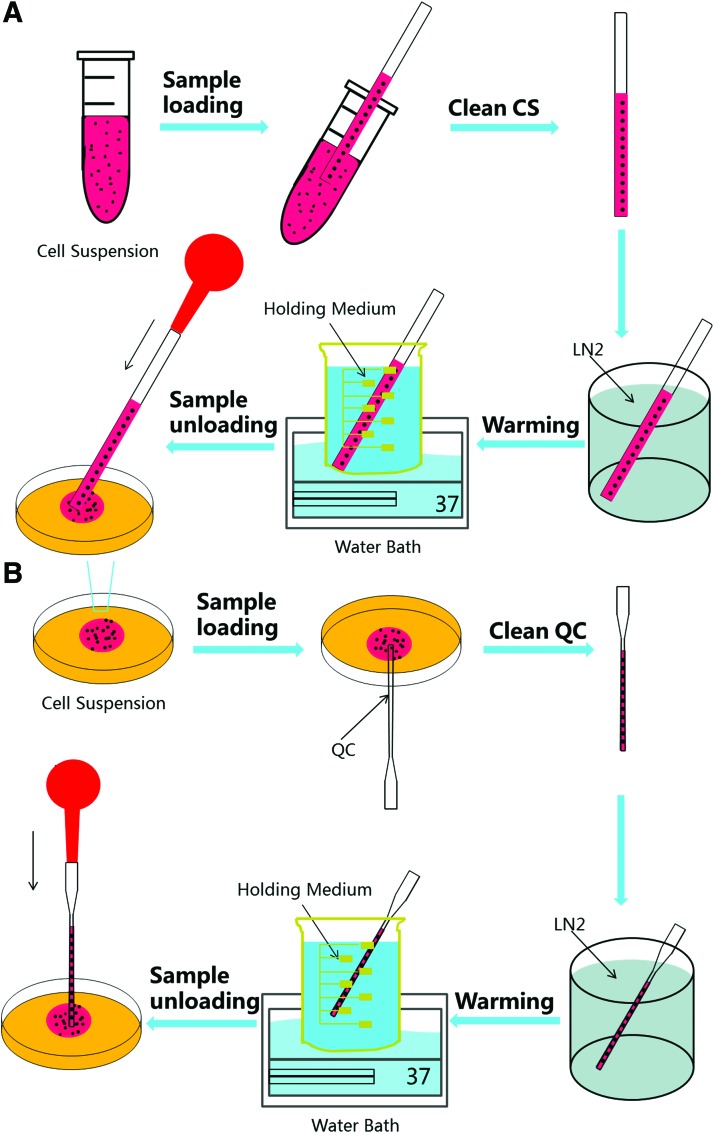
The procedure for cell cryopreservation by vitrification using CS is illustrated in Figure 2A. On the day of experiment, the attached HUVECs were harvested when they reached 85–90% confluency. Trypsin solution was added into a T-flask and incubated in an incubator for 5 min. The cells were divided into four groups, one of which is control and centrifuged at 100 g for 5 min, and then were resuspended in a cold culture medium (on ice, ∼4°C) for further use.
FIG. 2.
Schematic illustration of the procedures for vitrifying HUVECs with CS (A) and QC (B). HUVECs, human umbilical vein endothelial cells; LN2, liquid nitrogen. Color images available online at www.liebertpub.com/tec
For vitrification, high-CPA and low-CPA vitrification protocols were tested. In the high-CPA protocol, the isolated cells were spun down at 100 g for 5 min and incubated in three equilibrium solutions: 7.5% (v/v) (1.35 mol/L) EG + 7.5% (v/v) (1.05 mpl/L) DMSO, 7.5% (v/v) (1.35 mol/L) EG + 7.5% (v/v) (1.00 mol/L) PROH, and 7.5% (v/v) (1.00 mol/L) PROH + 7.5% (v/v) (1.05 mol/L) DMSO in HM20 (HUVEC medium containing 20% FBS) for 5 min on ice. After centrifugation, the cells were resuspended in three high concentration vitrification solutions made of 15% (v/v) (2.7 mol/L) EG + 15% (v/v) (2.1 mol/L) DMSO + 0.5 mol/L trehalose (VSCSHC-ED), 15% (v/v) (2.7 mol/L) EG + 15% (v/v) (2.0 mol/L) PROH + 0.5 mol/L trehalose (VSCSHC-EP), 15% (v/v) (2.0 mol/L) PROH + 15% (v/v) (2.1 mol/L) DMSO + 0.5 mol/L trehalose (VSCSHC-PD) in HM20 for 1 min at 4°C.
In the low-CPA protocol, cells were equilibrated in three equilibrium solutions: 4% (v/v) (0.72 mol/L) EG + 4% (v/v) (0.56 mol/L) DMSO, 4% (v/v) (0.72 mol/L) EG + 4% (v/v) (0.54 mol/L) PROH, and 4% (v/v) (0.56 mol/L) PROH +4% (v/v) (0.56 mol/L) DMSO in HM20 (RPMI medium containing 20% FBS) for 5 min at 4°C. After that, cells were equilibrated in three low-concentration vitrification solutions: 8% (v/v) (1.44 mol/L) EG +8% (v/v) (1.12 mol/L) DMSO + 1 mol/L trehalose (VSCSLC-ED), 8% (v/v) (1.44 mol/L) EG + 8% (v/v) (1.08 mol/L) PROH + 1 mol/L trehalose (VSCSLC-EP), and 8% (v/v) (1.08 mol/L) PROH+ 8% (v/v) (1.12 mol/L) DMSO + 1 mol/L trehalose (VSCSLC-PD) in HM20 for 1 min at 4°C.
After exposure to the vitrification solution, 200 μL of cell suspension was put at the bottom of a centrifugal tube and was loaded into CS with the aid of a rubber suction bulb. Then, the CS was plunged into LN2 at a high speed as shown in Supplementary Movie S1 (Supplementary Data are available online at www.liebertpub.com/tec). The CS was held in LN2 for 5 min, which is enough for cooling the sample to the temperature of LN2.24 For warming, the vitrified cells were plunged into a holding medium made of 1 mol/L trehalose (for high-CPA vitrification) or 0.5 mol/L trehalose (low-CPA vitrification) in 1× PBS at 37°C for 1 min. The cell suspension was then expelled from CS into 1 mol/L trehalose or 0.5 mol/L trehalose in HM10 (HUVEC medium containing 10% FBS) for 3 to 4 min at 37°C. Next, the cells were further processed for analyzing immediate viability and attachment efficiency. The control group was nonfrozen cells harvested from the same batch.
Vitrification of HUVECs using QC
The objective is to achieve low-CPA vitrification of HUVECs with high survival. The vitrification solution of QC (VSQC) is the same as the low-CPA vitrification solution used for CS. Therefore, vitrification protocol was the same as that aforementioned for low-CPA vitrification of HUVECs using CS. This process is illustrated in Figure 2B. After exposure to the vitrification solution, cell suspension drop was put on the bottom surface of a Petri dish, which was then inverted to form a hanging drop. The hanging drop was then loaded into the QC by touching the stem tip of the QC on the lowest surface of the hang drop vertically. After that, the cells were loaded into the QC by both gravity and capillary effects. The cell suspension in the QC was vitrified by immersing the QC into LN2, as shown in Supplementary Movie S2, and held in there for at least 5 min.
The vitrified cell suspension was melted by plunging the QC into a holding medium made of 0.5 mol/L trehalose in 1× PBS at 37°C for 1 min. The cell suspension was then unloaded from QC into 0.5 mol/L trehalose in HM10 (HUVEC medium containing 10% FBS) for 3 to 4 min at 37°C with the aid of a rubber suction bulb. After that, the cells were further processed for analyzing immediate viability, attachment efficiency, and proliferation.
Slow freezing of HUVECs using CS
To compare the effect of different cryopreservation methods, we cryopreserved HUVECs using the conventional slow freezing method at low-CPA concentration. The cells were washed with 1× PBS, detached using trypsin, and collected by centrifugation at 100 g for 5 min. The collected cells were then washed with 1× PBS and resuspended in equilibrium solutions: 4% (v/v) EG + 4% (v/v) DMSO in HM20 for 5 min at 4°C. After that, cells were equilibrated in a low-concentration solution: 8% (v/v) EG +8% (v/v) DMSO + 1 mol/L trehalose in HM20 (SFSCSLC-ED). After exposure to the solution, 200 μL of cell suspension was transferred into a CS and was held at 4°C for 30 min. Then, the CS was hermetically sealed before being placed into a freezer at −20°C overnight. On the second day, the CS was transferred into the LN2 tank. After 5 min, the CS was removed from the LN2 tank and thawed in 37°C water bath. The process of removing CPAs was the same as that mentioned above for vitrification using CS.
Analysis of cell viability, attachment efficiency, and proliferation postvitrification
The immediate viability of postcryopreservation HUVECs was evaluated with the Muse™ Cell Analyzer (EMD Millipore, the Life Science division of Merck KGaA of Darmstadt, Germany) using a Cell Count and Viability Kit (EMD Millipore).26,27 As aforementioned, the cell suspension in the CS or QC after warming was expelled into 2 mL of holding medium containing 1 mol/L trehalose or 20 μL of holding medium containing 0.5 mol/L trehalose in HM10 for 3 to 4 min at 37°C, respectively. The cells were then incubated in the Cell Count and Viability Kit for 5 min for dye uptake in the dark at room temperature. Data from the stained HUVECs were acquired using the Count & Viability Software Module.
The immediate cell viability postvitrification was also analyzed using a Standard Live/Dead Staining Kit (KeyGen Biotech Co., Ltd., China) of fluorescent probes: Acridine Orange and Ethidium Bromide to check the cell membrane integrity.24,28 The cells were incubated in dark at room temperature for 5 min, and fluorescence images of HUVECs were taken using a Nikon fluorescence inverted microscope (10× objective).
To further check the viability of cells postcryopreservation at a longer time, cell attachment was investigated at day 1 and proliferation was investigated over a 3-day observation period as described in previous studies.29,30 Briefly, after incubating in warm medium for 3 to 4 min at 37°C, the cell suspension was then transferred into warm fresh HM10 medium and incubated for another 10 min at 37°C. The cells were spun down at 100 g for 5 min and suspended in 2 mL of warm fresh HM10 medium at 37°C. Then, the cells were cultured in six-well plates for further study. Fresh cells without vitrification were seeded at the same total cell concentration as control.
At different time points (1, 2, and 3 days), cells were washed using 1× PBS twice and lightly trypsinized. The cells were then counted with the Muse™ Cell Analyzer using a Cell Count and Viability Kit. The attachment efficiency was calculated as the percentage of the total number of cells in post-vitrification sample relative to that in the control nonfrozen sample at day 1. The proliferation was calculated as the percentage of the cell number of days 2 and 3 to the cell number of day 1.
Statistical analysis
All results are reported as mean ± standard deviation. Independent experiments were performed at least six times. Statistical significance comparison of data between different groups was conducted using Microsoft Excel (2013) based on One-Way ANOVA. A p-value <0.05 was considered to be statistically significant.
Results and Discussion
Vitrification of HUVECs using CS
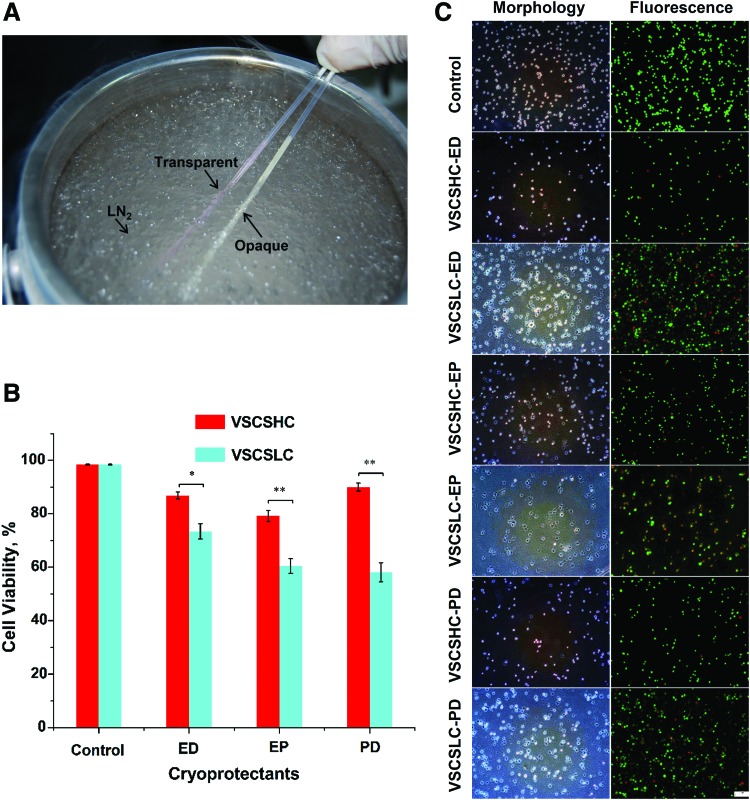
One convenient way for confirming nonvitrification is the appearance of opacity (or visible ice formation) when cooling solutions below their freezing point. If there is no observable opacity in an aqueous solution, it is called apparent vitrification. As shown in Figure 3A and Supplementary Movie S1, the vitrification solution for CS (VSCSLC) stays transparent, while the cell culture medium without any CPA appears opaque (whitish) after plunging into LN2. This indicates that VSCSLC was successfully vitrified while there was extensive ice formation in the cell culture medium without any CPA. The results of further study on the immediate viability of HUVECs postvitrification in CS are shown in Figure 3B. The quantitative data of cell viability were obtained by a Muse Cell Analyzer. Viability of fresh cells without vitrification was quantified using the same approach. Fresh cells without cryopreservation were studied as control and the viability of control cells was 98 ± 0.2%.
FIG. 3.
Vitrification of HUVECs with CS. (A) A typical picture showing the appearances of VSCSLC-ED (transparent due to vitrification with no apparent ice formation) and culture medium without any CPA (opaque due to freezing with apparent ice formation) after plunging them into liquid nitrogen with CS. (B) Viability of HUVECs after vitrification in various vitrification solutions using CS. (C) Typical phase and fluorescence micrographs showing the viability of HUVECs vitrified with the various vitrification solutions. *p < 0.05 and **p < 0.01. CPA, cryoprotective agent; ED, EG+DMSO; EP, EG+PROH; PD, PROH+DMSO; scale bar, 100 μm. Color images available online at www.liebertpub.com/tec
HUVECs in VSCSHC solution can survive the vitrification procedure, even though a large number of HUVECs are dead in VSCSLC solution after vitrification. The postcryopreservation viability for vitrified HUVECs in VSCSHC groups is all high, especially when the viability reaches up to 90 ± 3.6% in VSCSHC-PD group. By contrast, the viability for vitrified HUVECs in VSCSLC-EP and VSCSLC-PD groups are about 60%, while in VSCSLC-ED it is 73 ± 7%. The corresponding typical figures obtained from Muse Cell Analyzer are shown in Supplementary Figure S1. We also evaluated the immediate viability by cell membrane integrity (i.e., Live/Dead dye stain) postcryopreservation using various cryoprotectants. Typical phase and fluorescence micrographs of HUVECs are shown in Figure 3C and Supplementary Figure S2.
Although no opacity due to ice was observed when cooling all these samples, there was ice formation during warming for the VSCSLC groups. Probably, the large thermal mass of the samples and the low thermal conductivity of the wall material limit the melting rate in the CS, which leads to recrystallization or/and devitrification. Our experiments indicate that EG and DMSO are good candidates for the cell membrane-permeating component of the vitrification solution. Therefore, we applied the VSCSLC-ED solution to QC for exploring the possibilities of using the low/nontoxic concentrations of CPAs.
Vitrification of HUVECs using QC
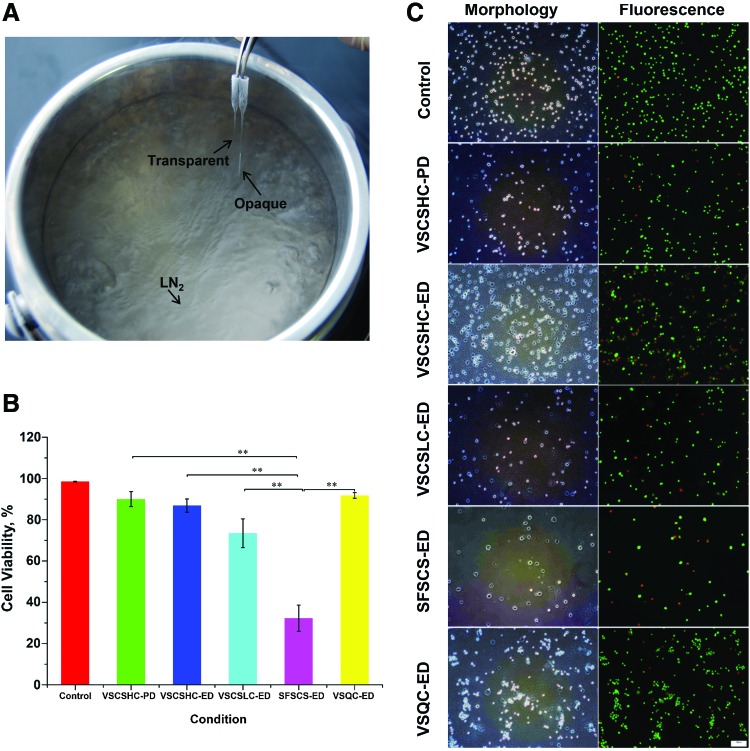
Due to the smaller sample volume and higher thermal conductivity of QC than CS, we found that the VSCSLC-ED solution is good for apparent vitrification in QC, while whitish opaque ice formation is observed in the medium without any CPA (Figure 4A and Supplementary Movie S2). In addition, the cytotoxicity of EG is low even at high concentrations.31 Moreover, cell permeability is lower for EG than PROH, and the cytotoxic effectors of EG inside cell are less.32 Therefore, VSCSLC-ED solution is chosen as the vitrification solution of QC (VSQC). All of the HUVECs that were loaded into the QC were recovered after vitrification.
FIG. 4.
Vitrification of HUVECs using QC. (A) A typical picture showing the appearances of VSCSLC-ED (transparent due to vitrification with no apparent ice formation) and culture medium without any CPA (opaque due to freezing with apparent ice formation) after plunging them into liquid nitrogen with QC. (B) Viability of HUVECs after vitrification in various vitrification solutions using QC. (C) Typical phase and fluorescence micrographs showing the viability of HUVECs vitrified with the various vitrification solutions. **p < 0.01. Color images available online at www.liebertpub.com/tec
As shown in Figure 4B, a significant improvement of cell viability is seen when comparing to that using CS. The cell viability was quantified by Muse Cell Analyzer. We can see from the results that a large number of HUVECs in VSCSLC-ED and SFSCSLC-ED groups with CS are dead after cryopreservation. Most importantly, the quantitative data of cell viability of HUVECs in VSCSLC-ED solution postvitrification using the QC are comparative with the results of VSCSHC-ED vitrification, up to 91 ± 1.3%. The typical figures obtained from Muse Cell Analyzer are shown in Supplementary Figure S3. The cell viability for the VSCSLC-ED group may be even lower because the morphology of many cells changed, while only a very small number of cells have the morphological change in the VSQC-ED group. The representative qualitative data of typical phase and fluorescence micrographs are shown in Figure 4C and Supplementary Figure S4. The viability of fresh cells without cryopreservation was high.
These results demonstrate that using the VSQC and QC for vitrification can effectively eliminate ice formation in the small volume of solution during cooling. With the usage of the quartz capillaries, significantly higher cooling and warming rates can be achieved, as a result of its reduced inner diameter along with the thin wall and large thermal conductivity. Nevertheless, the survived HUVECs must be able to attach to a substrate to proliferate normally, for confirming the effectiveness of the novel low-CPA (2.5 mol/L penetrating CPA) vitrification approach with QC developed in this study. Therefore, the attachment efficiency were compared between different conditions. In addition, proliferation of HUVECs was examined before and after the low-CPA vitrification with QC.
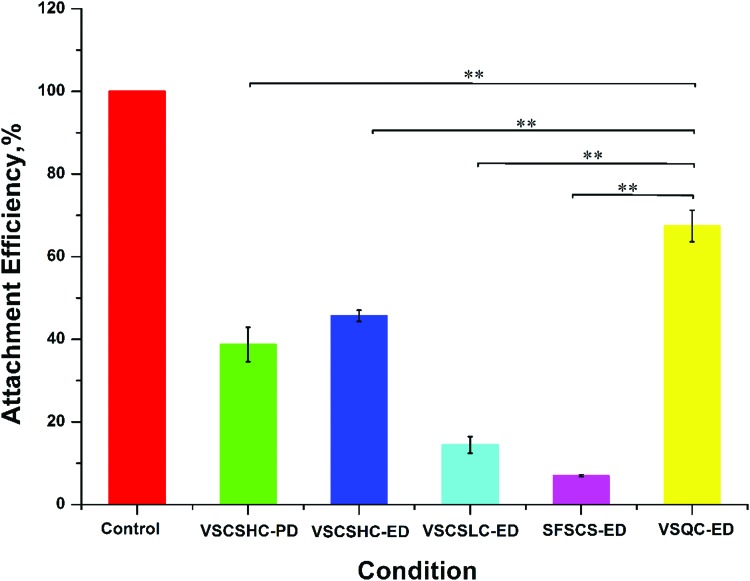
We examined the viability of the HUVECs 1 day postvitrification by quantifying the attachment efficiency, which was calculated as the number of attached live cells postcryopreservation, relative to that of fresh cells after 1 day of culture. As shown in Figure 5, only a minimal percentage of cells were able to attach when using VSCSLC-ED solution for vitrification in CS. Of note, a significant improvement of attachment efficiency was observed using VSQC-ED solution for vitrification in QC and the percentage is as high as 67 ± 6.5% after 1 day of culture. However, the attachment efficiency was found to be almost the same for the two experimental conditions with VSCSHC-PD and VSCSHC-ED solution vitrification in CS. While the immediate cell viability of VSCSHC-PD and VSCSHC-ED groups are not significantly different from that in VSQC-ED group, the attachment efficiency of VSCSHC-PD and VSCSHC-ED groups are much lower than that in VSQC-ED group (p < 0.01). This is probably because concentrations of PROH and DMSO in VSCSHC groups are high and toxic, which leads to the low attachment efficiency.
FIG. 5.
Attachment efficiency of HUVECs postvitrification using VSCSLC-ED and QC. The attachment efficiency was calculated as the percentage of the number of attached live cells after 1 day of culture postcryopreservation out of the number of attached live fresh cells seeded and cultured in the same way. **p < 0.01. Color images available online at www.liebertpub.com/tec
It is worth noting that the viability of cells determined by attachment efficiency is lower than the immediate viability because vitrified HUVECs experience a delay in growth postcryopreservation compared with fresh cells during the first day of culture. However, a comparison of the data between different groups shows that quartz capillaries are superior to the CS technique for low-CPA vitrification of HUVECs.
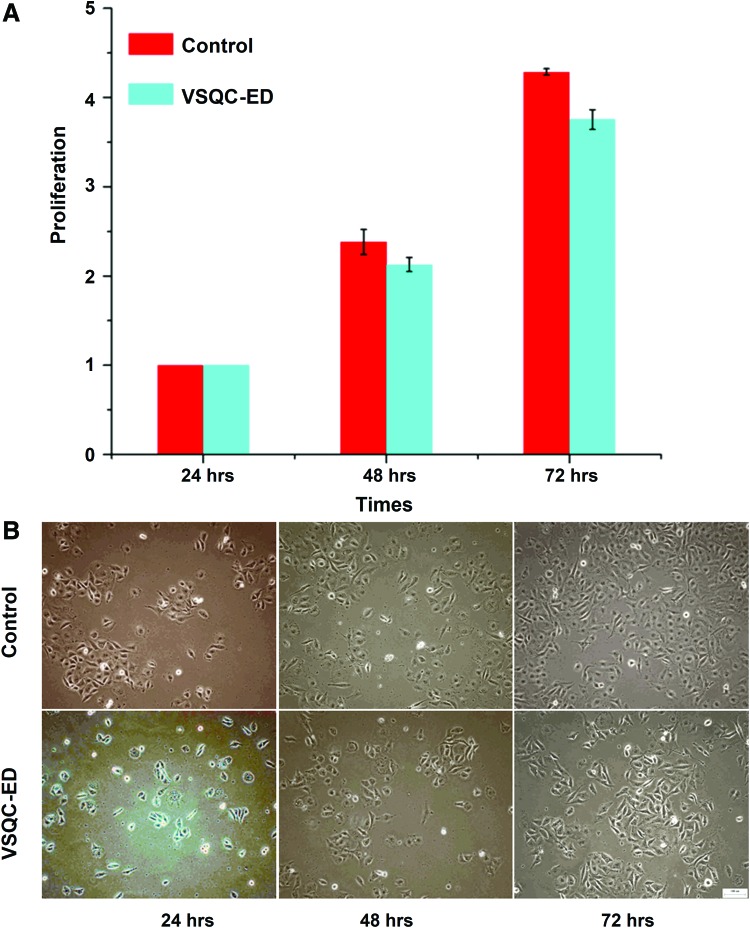
The growth of the attached HUVECs post-vitrification using QC and VSQC-ED solution for vitrification was very similar to the proliferation of the control nonfrozen samples. Figure 6A shows the normalized number of cells in a well calculated as the ratio of the cell number that was obtained from Muse Cell Analyzer at days 2 and 3 with respect to that on day 1 for each group. Evidently, the same growth pattern was observable for the vitrified and the control HUVECs. The qualitative number of cells per field of view that was observed had a significant increase over a 3-day period for both the vitrified HUVECs and the control samples, as shown in Figure 6B. In addition, the attached HUVECs postcryopreservation retained their cobblestone-like morphology similar to nonfrozen cells. Therefore, the vitrification process has no significant effects on the growth characteristics of HUVECs when they attached postvitrification. Collectively, the immediate and long-term cell survival data indicate that HUVECs can survive the QC vitrification procedure well.
FIG. 6.
Proliferation of HUVECs postvitrification using VSQC-ED and QC in 3 days. (A) Quantitative data showing similar proliferation of cryopreserved to fresh HUVECs. (B) Typical phase micrographs showing similar morphology of fresh to cryopreserved HUVECs. Color images available online at www.liebertpub.com/tec
Conclusions
In summary, we show that the 200 μm (outer diameter) QC is excellent for ultrafast vitrification of HUVECs compared with CS. By using the QC, we were able to significantly lower the concentration of CPAs required for vitrification of HUVECs. The intracellular concentration of CPAs used in this protocol is close to that for conventional slow freezing. With the decrease in CPA concentration, we can effectively reduce the steps and time for removing CPAs to minimize cell injury. Ultimately, the use of QC and an optimized combination of cell membrane-penetrating CPAs (1.4 mol/L EG and 1.1 mol/L DMSO) enables successful cryopreservation of HUVECs with high viability (>90%). Approximately 70% of the HUVECs post vitrification can attach. Moreover, the proliferation of the attached cells is similar to fresh cells. This study may provide a valuable vitrification approach to bank HUVECs for engineering blood vessels and vascularized tissues and studying endothelial cell biology.
Supplementary Material
Acknowledgments
This work was partially supported by grants from NSFC (Nos. 51276179, 51476160 and 51528601). X.H. was supported by the National Science Foundation (NSF CBET-1154965) and the National Institutes of Health (NIH R01EB012108).
Disclosure Statement
No competing financial interests exist.
References
- 1.Rouwkema J., Boer J.D., and Blitterswijk C.A.V. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng 12, 2685, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Koike N., Fukumura D., Gralla O., Au P., Schechner J.S., and Jain R.K. Tissue engineering: creation of long-lasting blood vessels. Nature 428, 138, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Inamori M., Mizumoto H., and Kajiwara T. An approach for formation of vascularized liver tissue by endothelial cell-covered hepatocyte spheroid integration. Tissue Eng Part A 15, 2029, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Mutin M., Dignat-George F., and Sampol J. Immunologic phenotype of cultured endothelial cells: quantitative analysis of cell surface molecules. Tissue Antigens 50, 449, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Riddle D.L., and Schappert S.M. Volume of ambulatory care visits and patterns of care for patients diagnosed with plantar fasciitis: a national study of medical doctors. Foot Ankle Int 25, 303, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Baudin B., Bruneel A., Bosselut N., and Vaubourdolle M. A protocol for isolation and culture of human umbilical vein endothelial cells. Nat Protoc 2, 481, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Carbognani P., Spaggiari L., Rusca M., Cattelani L., Solli P., Romani A., Alessandrini F., Dell'Abate P., Valente M., and Bobbio P. Pulmonary endothelial cell modifications after storage in solid-organ preservation solutions. J Int Med Res 23, 200, 1995 [DOI] [PubMed] [Google Scholar]
- 8.Lehle K., Hoenicka M., Jacobs V.R., Schmid F.X., and Birnbaum D.E. Identification and reduction of cryoinjury in endothelial cells: a first step toward establishing a cell bank for vascular tissue engineering. Tissue Eng 12, 3439, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Walcerz D.B., and Karow A.M., Jr Cryopreservation of cells for tissue engineering. Tissue Eng 2, 85, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Mazur P. Freezing of living cells—Mechanisms and implications. Am J Physiol 247, C125, 1984 [DOI] [PubMed] [Google Scholar]
- 11.Mazur P., Rall W.F., and Leibo S.P. Kinetics of water-loss and the likelihood of intracellular freezing in mouse ova—influence of the method of calculating the temperature-dependence of water permeability. Cell Biophys 6, 197, 1984 [DOI] [PubMed] [Google Scholar]
- 12.Fahy G.M., Macfarlane D.R., Angell C.A., and Meryman H.T. Vitrification as an approach to cryopreservation. Cryobiology 21, 407, 1984 [DOI] [PubMed] [Google Scholar]
- 13.Fowler A., and Toner M. Cryo-injury and biopreservation. Ann N Y Acad Sci 1066, 119, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Fahy G., Levy D., and Ali S. Some emerging principles underlying the physical properties, biological actions, and utility of vitrification solutions. Cryobiology 24, 196, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Katkov I.I., Isachenko V., Isachenko E., Kim M.S., Lulat A.G.M.I., Mackay A.M., and Levine F. Low- and high-temperature vitrification as a new approach to bio stabilization of reproductive and progenitor cells. Int J Refrig 29, 346, 2006 [Google Scholar]
- 16.Booth P.J., Vajta G., Hoj A., Holm P., Jacobsen H., Greve T., and Callesen H. Full-term development of nuclear transfer calves produced from open-pulled straw (OPS) vitrified cytoplasts: work in progress. Theriogenology 51, 999, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Kuwayama M., Vajta G., Kato O., and Leibo S.P. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online 11, 300, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Chen S.U., Lien Y.R., Cheng Y.Y., Chen H.F., Ho H.N., and Yang Y.S. Vitrification of mouse oocytes using closed pulled straws (CPS) achieves a high survival and preserves good patterns of meiotic spindles, compared with conventional straws, open pulled straws (OPS) and grids. Hum Reprod 16, 2350, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Martino A., Pollard J.W., and Leibo S.P. Effect of chilling bovine oocytes on their developmental competence. Mol Reprod Dev 45, 503, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Mukaida T., Nakamura S., Tomiyama T., Wada S., Kasai M., and Takahashi K. Successful birth after transfer of vitrified human blastocysts with use of a cryoloop containerless technique. Fertil Steril 76, 618, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Begin I., Bhatia B., Baldassarre H., Dinnyes A., and Keefer C.L. Cryopreservation of goat oocytes and in vivo derived 2-to 4-cell embryos using the cryoloop (CLV) and solid-surface vitrification (SSV) methods. Theriogenology 59, 1839, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Kuwayama M. Highly efficient vitrification for cryopreservation of human oocytes and embryos: the Cryotop method. Theriogenology 67, 73, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Risco R., Elmoazzen H., Doughty M., He X., and Toner M. Thermal performance of quartz capillaries for vitrification. Cryobiology 55, 222, 2007 [DOI] [PubMed] [Google Scholar]
- 24.He X., Park E.Y., Fowler A., Yarmush M.L., and Toner M. Vitrification by ultra-fast cooling at a low concentration of cryoprotectants in a quartz micro-capillary: a study using murine embryonic stem cells. Cryobiology 56, 223, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berejnov V., Husseini N.S., Alsaied O.A., and Thorne R.E. Effects of cryoprotectant concentration and cooling rate on vitrification of aqueous solutions. J Appl Crystallogr 39, 244, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millipore E. Results and discussions. BioTechniques 52, 200, 2012 [Google Scholar]
- 27.Byrne S.M., Ortiz L., Mali P., Aach J., and Church G.M. Multi-kilobase homozygous targeted gene replacement in human induced pluripotent stem cells. Nucl Acids Res 43, e21, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mo C., Zhao R., Vallejo J., Igwe O., Bonewald L., Wetmore L., and Brotto M. Prostaglandin E2 promotes proliferation of skeletal muscle myoblasts via EP4 receptor activation. Cell Cycle 14, 1507, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gong M., Yang H., Zhang S., Yang Y., Zhang D., Qi Y., and Zou L. Superparamagnetic core/shell GoldMag nanoparticles: size-, concentration-and time-dependent cellular nanotoxicity on human umbilical vein endothelial cells and the suitable conditions for magnetic resonance imaging. J Nanobiotechnology 13, 24, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao W., Huang H., Wang H., Zhao S., Dumbleton J., Zhao G., and He X. Nanoparticle-mediated intracellular delivery enables cryopreservation of human adipose-derived stem cells using trehalose as the sole cryoprotectant. ACS Appl Mater Interfaces 7, 5017, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukaida T., Wada S., Takahashi K., Pedro P.B., An T.Z., and Kasai M. Vitrification of human embryos based on the assessment of suitable conditions for 8-cell mouse embryos. Hum Reprod 13, 2874, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Eto T., Takahashi R., Kamisako T., Hioki K., and Sotomaru Y. A study on cryoprotectant solution suitable for vitrification of rat two-cell stage embryos. Cryobiology 68, 147, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.