Abstract
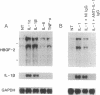
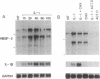
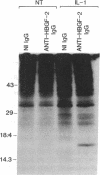
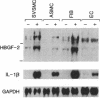
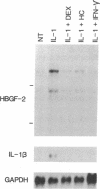
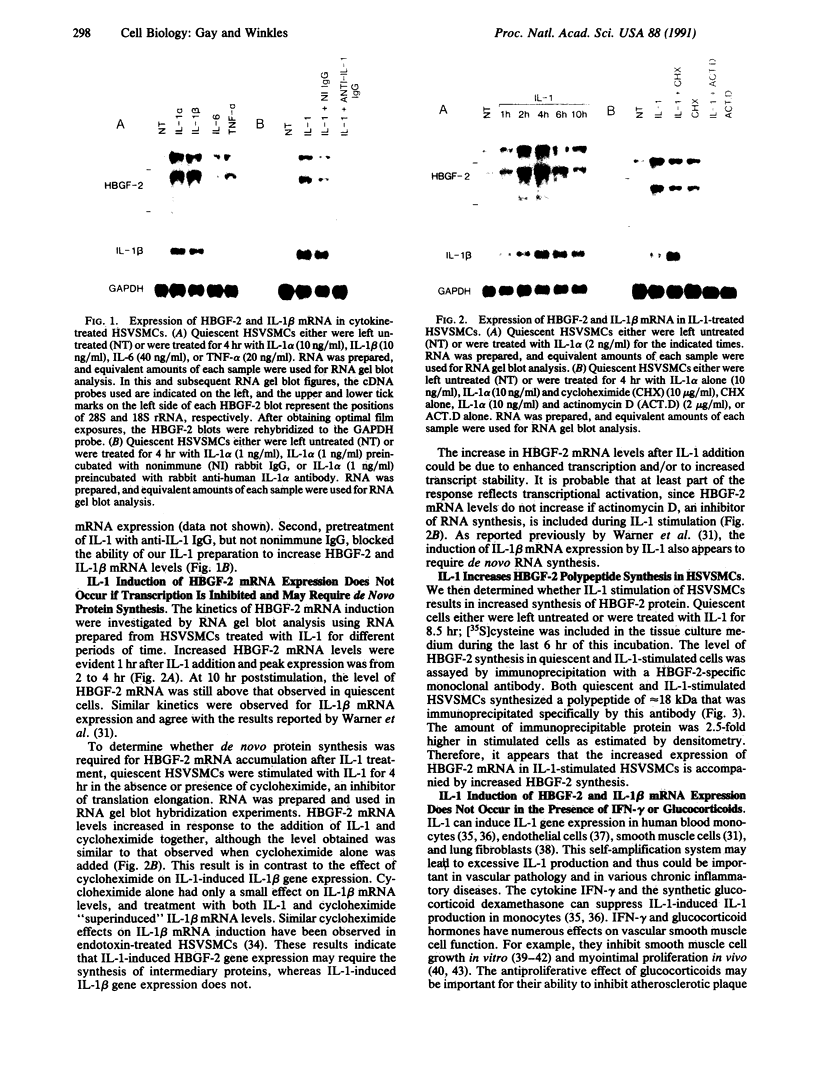
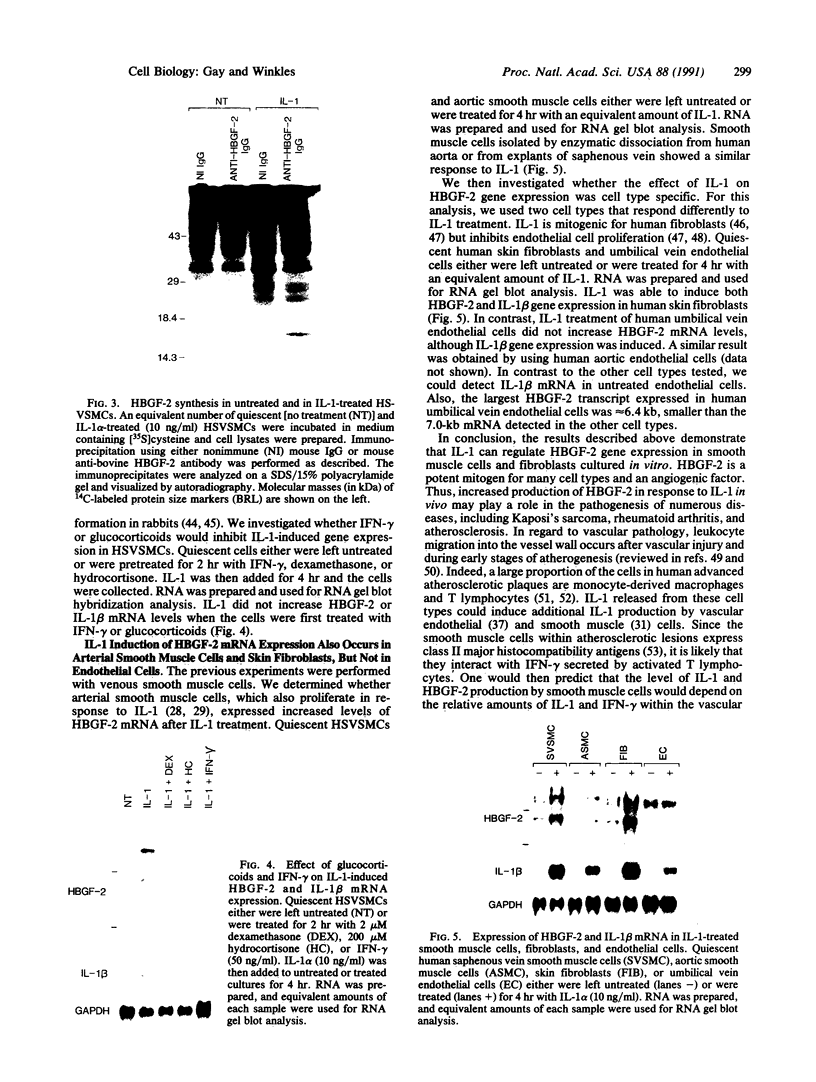
The angiogenic polypeptide heparin-binding growth factor 2 (HBGF-2), or basic fibroblast growth factor, is a mitogen for vascular smooth muscle cells in vitro and in vivo. Smooth muscle cells also synthesize HBGF-2; thus, it may stimulate their proliferation in vivo by both autocrine and paracrine mechanisms. We report here that HBGF-2 gene expression in human saphenous vein smooth muscle cells is induced by interleukin (IL)-1 alpha and IL-1 beta, inflammatory cytokines produced by many cell types in response to a variety of signals. Maximal HBGF-2 mRNA levels are detected 2-4 hr after IL-1 treatment; induction may require de novo protein synthesis and does not occur if transcription is inhibited. Immunoprecipitation analysis indicates that IL-1-stimulated cells also express an increased amount of HBGF-2 protein. Interferon gamma and glucocorticoids, inhibitors of smooth muscle cell proliferation in vitro and in vivo, suppress the induction of HBGF-2 expression by IL-1. These results imply that cytokines released at sites of vascular injury or inflammation may regulate HBGF-2 production by smooth muscle cells. Increased HBGF-2 levels within the vessel wall could play a role in both the smooth muscle cell proliferation and the neovascularization associated with the development of atherosclerotic lesions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albini A., Mitchell C. D., Thompson E. W., Seeman R., Martin G. R., Wittek A. E., Quinnan G. V. Invasive activity and chemotactic response to growth factors by Kaposi's sarcoma cells. J Cell Biochem. 1988 Apr;36(4):369–376. doi: 10.1002/jcb.240360406. [DOI] [PubMed] [Google Scholar]
- Barger A. C., Beeuwkes R., 3rd, Lainey L. L., Silverman K. J. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984 Jan 19;310(3):175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Vallega G., Griendling K. K., Gordon J. B., Cragoe E. J., Jr, Canessa M., Alexander R. W. Effects of glucocorticoids on Na+/H+ exchange and growth in cultured vascular smooth muscle cells. J Cell Physiol. 1988 Dec;137(3):391–401. doi: 10.1002/jcp.1041370302. [DOI] [PubMed] [Google Scholar]
- Bikfalvi A., Alterio J., Inyang A. L., Dupuy E., Laurent M., Hartmann M. P., Vigny L., Raulais D., Courtois Y., Tobelem G. Basic fibroblast growth factor expression in human omental microvascular endothelial cells and the effect of phorbol ester. J Cell Physiol. 1990 Jul;144(1):151–158. doi: 10.1002/jcp.1041440120. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Pukac L. A., Caleb B. L., Wright T. C., Jr, Karnovsky M. J. Heparin selectively inhibits a protein kinase C-dependent mechanism of cell cycle progression in calf aortic smooth muscle cells. J Cell Biol. 1989 Dec;109(6 Pt 1):3147–3155. doi: 10.1083/jcb.109.6.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervu A., Moore W. S., Quiñones-Baldrich W. J., Henderson T. Efficacy of corticosteroids in suppression of intimal hyperplasia. J Vasc Surg. 1989 Aug;10(2):129–134. doi: 10.1067/mva.1989.0100129. [DOI] [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias J. A., Reynolds M. M., Kotloff R. M., Kern J. A. Fibroblast interleukin 1 beta: synergistic stimulation by recombinant interleukin 1 and tumor necrosis factor and posttranscriptional regulation. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6171–6175. doi: 10.1073/pnas.86.16.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch F., Baird A., Ling N., Ueno N., Hill F., Denoroy L., Klepper R., Gospodarowicz D., Böhlen P., Guillemin R. Primary structure of bovine pituitary basic fibroblast growth factor (FGF) and comparison with the amino-terminal sequence of bovine brain acidic FGF. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6507–6511. doi: 10.1073/pnas.82.19.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. Heparin-binding growth factor-1 stimulation of human endothelial cells induces platelet-derived growth factor A-chain gene expression. J Biol Chem. 1990 Feb 25;265(6):3284–3292. [PubMed] [Google Scholar]
- Ghezzi P., Dinarello C. A. IL-1 induces IL-1. III. Specific inhibition of IL-1 production by IFN-gamma. J Immunol. 1988 Jun 15;140(12):4238–4244. [PubMed] [Google Scholar]
- Gonzalez A. M., Buscaglia M., Ong M., Baird A. Distribution of basic fibroblast growth factor in the 18-day rat fetus: localization in the basement membranes of diverse tissues. J Cell Biol. 1990 Mar;110(3):753–765. doi: 10.1083/jcb.110.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Ferrara N., Haaparanta T., Neufeld G. Basic fibroblast growth factor: expression in cultured bovine vascular smooth muscle cells. Eur J Cell Biol. 1988 Apr;46(1):144–151. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hannan R. L., Kourembanas S., Flanders K. C., Rogelj S. J., Roberts A. B., Faller D. V., Klagsbrun M. Endothelial cells synthesize basic fibroblast growth factor and transforming growth factor beta. Growth Factors. 1988;1(1):7–17. doi: 10.3109/08977198809000242. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Claesson-Welsh L. Class II MHC antigen expression in the atherosclerotic plaque: smooth muscle cells express HLA-DR, HLA-DQ and the invariant gamma chain. Clin Exp Immunol. 1986 May;64(2):261–268. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Jonasson L., Holm J., Clowes M. M., Clowes A. W. Gamma-interferon regulates vascular smooth muscle proliferation and Ia antigen expression in vivo and in vitro. Circ Res. 1988 Oct;63(4):712–719. doi: 10.1161/01.res.63.4.712. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Edelman E. R. Biological and biochemical properties of fibroblast growth factors. Implications for the pathogenesis of atherosclerosis. Arteriosclerosis. 1989 May-Jun;9(3):269–278. doi: 10.1161/01.atv.9.3.269. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1989;1(4):207–235. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker J. P., Kilty L. A., Johnson L. K. Glucocorticoid inhibition of vascular smooth muscle cell proliferation: influence of homologous extracellular matrix and serum mitogens. J Cell Biol. 1984 Feb;98(2):534–540. doi: 10.1083/jcb.98.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loppnow H., Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990 Mar;85(3):731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makheja A. N., Bloom S., Muesing R., Simon T., Bailey J. M. Anti-inflammatory drugs in experimental atherosclerosis. 7. Spontaneous atherosclerosis in WHHL rabbits and inhibition by cortisone acetate. Atherosclerosis. 1989 Apr;76(2-3):155–161. doi: 10.1016/0021-9150(89)90099-3. [DOI] [PubMed] [Google Scholar]
- Mansson P. E., Malark M., Sawada H., Kan M., McKeehan W. L. Heparin-binding (fibroblast) growth factors type one and two genes are co-expressed in proliferating normal human vascular endothelial and smooth muscle cells in culture. In Vitro Cell Dev Biol. 1990 Feb;26(2):209–212. doi: 10.1007/BF02624114. [DOI] [PubMed] [Google Scholar]
- Matsuzaki K., Yoshitake Y., Matuo Y., Sasaki H., Nishikawa K. Monoclonal antibodies against heparin-binding growth factor II/basic fibroblast growth factor that block its biological activity: invalidity of the antibodies for tumor angiogenesis. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9911–9915. doi: 10.1073/pnas.86.24.9911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Murphy P. R., Sato R., Sato Y., Friesen H. G. Fibroblast growth factor messenger ribonucleic acid expression in a human astrocytoma cell line: regulation by serum and cell density. Mol Endocrinol. 1988 Jul;2(7):591–598. doi: 10.1210/mend-2-7-591. [DOI] [PubMed] [Google Scholar]
- Murphy P. R., Sato Y., Sato R., Friesen H. G. Regulation of multiple basic fibroblast growth factor messenger ribonucleic acid transcripts by protein kinase C activators. Mol Endocrinol. 1988 Dec;2(12):1196–1201. doi: 10.1210/mend-2-12-1196. [DOI] [PubMed] [Google Scholar]
- Mäkelä T. P., Alitalo R., Paulsson Y., Westermark B., Heldin C. H., Alitalo K. Regulation of platelet-derived growth factor gene expression by transforming growth factor beta and phorbol ester in human leukemia cell lines. Mol Cell Biol. 1987 Oct;7(10):3656–3662. doi: 10.1128/mcb.7.10.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postlethwaite A. E., Raghow R., Stricklin G. P., Poppleton H., Seyer J. M., Kang A. H. Modulation of fibroblast functions by interleukin 1: increased steady-state accumulation of type I procollagen messenger RNAs and stimulation of other functions but not chemotaxis by human recombinant interleukin 1 alpha and beta. J Cell Biol. 1988 Feb;106(2):311–318. doi: 10.1083/jcb.106.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta M., Moscatelli D., Joseph-Silverstein J., Rifkin D. B. Purification from a human hepatoma cell line of a basic fibroblast growth factor-like molecule that stimulates capillary endothelial cell plasminogen activator production, DNA synthesis, and migration. Mol Cell Biol. 1986 Nov;6(11):4060–4066. doi: 10.1128/mcb.6.11.4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Masuda J., Raines E. W., Gown A. M., Katsuda S., Sasahara M., Malden L. T., Masuko H., Sato H. Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science. 1990 May 25;248(4958):1009–1012. doi: 10.1126/science.2343305. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Saegusa Y., Ziff M., Welkovich L., Cavender D. Effect of inflammatory cytokines on human endothelial cell proliferation. J Cell Physiol. 1990 Mar;142(3):488–495. doi: 10.1002/jcp.1041420307. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M., Kajio T., Kawahara K., Kato K. Antibodies against basic fibroblast growth factor inhibit the autocrine growth of pulmonary artery endothelial cells. FEBS Lett. 1988 Jun 6;233(1):163–166. doi: 10.1016/0014-5793(88)81376-0. [DOI] [PubMed] [Google Scholar]
- Sarzani R., Brecher P., Chobanian A. V. Growth factor expression in aorta of normotensive and hypertensive rats. J Clin Invest. 1989 Apr;83(4):1404–1408. doi: 10.1172/JCI114029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y., Rifkin D. B. Autocrine activities of basic fibroblast growth factor: regulation of endothelial cell movement, plasminogen activator synthesis, and DNA synthesis. J Cell Biol. 1988 Sep;107(3):1199–1205. doi: 10.1083/jcb.107.3.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R., Ghezzi P., Dinarello C. A. IL-1 induces IL-1. IV. IFN-gamma suppresses IL-1 but not lipopolysaccharide-induced transcription of IL-1. J Immunol. 1990 Mar 15;144(6):2216–2222. [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Schweigerer L., Neufeld G., Friedman J., Abraham J. A., Fiddes J. C., Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987 Jan 15;325(6101):257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Stemme S., Jonasson L., Holm J., Hansson G. K. Immunologic control of vascular cell growth in arterial response to injury and atherosclerosis. Transplant Proc. 1989 Aug;21(4):3697–3699. [PubMed] [Google Scholar]
- Sternfeld M. D., Hendrickson J. E., Keeble W. W., Rosenbaum J. T., Robertson J. E., Pittelkow M. R., Shipley G. D. Differential expression of mRNA coding for heparin-binding growth factor type 2 in human cells. J Cell Physiol. 1988 Aug;136(2):297–304. doi: 10.1002/jcp.1041360212. [DOI] [PubMed] [Google Scholar]
- Terranova V. P., DiFlorio R., Lyall R. M., Hic S., Friesel R., Maciag T. Human endothelial cells are chemotactic to endothelial cell growth factor and heparin. J Cell Biol. 1985 Dec;101(6):2330–2334. doi: 10.1083/jcb.101.6.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. A., Rios-Candelore M., Giménez-Gallego G., DiSalvo J., Bennett C., Rodkey J., Fitzpatrick S. Pure brain-derived acidic fibroblast growth factor is a potent angiogenic vascular endothelial cell mitogen with sequence homology to interleukin 1. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6409–6413. doi: 10.1073/pnas.82.19.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- Warner S. J., Friedman G. B., Libby P. Immune interferon inhibits proliferation and induces 2'-5'-oligoadenylate synthetase gene expression in human vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1174–1182. doi: 10.1172/JCI113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkles J. A., Friesel R., Burgess W. H., Howk R., Mehlman T., Weinstein R., Maciag T. Human vascular smooth muscle cells both express and respond to heparin-binding growth factor I (endothelial cell growth factor). Proc Natl Acad Sci U S A. 1987 Oct;84(20):7124–7128. doi: 10.1073/pnas.84.20.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]