Abstract
The objective of this review is to summarize and integrate specific clinical observations from the field of gastric bypass surgery and recent findings in beta cell biology. When considered together, these data sets suggest a previously unrecognized physiological mechanism which may explain how Roux-en-Y gastric bypass (RYGB) surgery mediates the early rapid reversal of hyperglycemia, observed before weight loss, in certain Type 2 Diabetes Mellitus (T2DM) patients. The novel mechanism is based on a recently recognized inhibitory circuit of glucose stimulated insulin secretion driven by dopamine (DA) stored in β-cell vesicles and the gut. We propose that dopamine (DA) and Glucagon like peptide 1 (GLP-1) represent two opposing arms of a glucose stimulated insulin secretion (GSIS) regulatory system and hypothesize that DA represents the “anti incretin” hypothesized to explain the beneficial effects of bariatric surgery on T2DM. These new hypotheses and the research driven by them may directly impact our understanding of: 1) the mechanisms underlying improved glucose homeostasis seen before weight loss following bariatric surgery, and 2) the regulation of glucose stimulated insulin secretion within islets. On a practical level, these studies may result in the development of novels drugs to modulate insulin secretion and/or methods to quantitatively asses in real time beta cell function and mass.
Keywords: Anti-incretin, Bariatric surgery, Dopamine, Glucose stimulated insulin secretion
Introduction
Bariatric surgery is an effective treatment for obesity and its associated comorbidities, such as T2DM [1]. Although improvement in insulin resistance secondary to weight loss and decrease in fat mass is a significant contributor to the improvement and reversal of T2DM, it alone does not fully account for the efficacy of specific types of metabolic surgery. Improved β-cell function, as well as rapid reversal of hyperglycemia in the absence of significant weight loss has been observed in operations where a portion of the gastrointestinal tract is bypassed [2–5]. In this review we summarize observations made in clinical studies of the efficacy of bariatric surgery, specifically Roux-en-Y-Gastric bypass (RYGB), and in studies of the regulation of β-cell insulin secretion which support the hypothesis of a previously unrecognized physiological mechanism of glucose homeostasis originating in the upper gut and stomach.
β-cells have a lot in common with CNS neurons
D-Glucose, with the synergistic effects of certain amino acids and fatty acids, is the major physiological stimulus for insulin secretion (reviewed in [6]). Net insulin production and glucose homeostasis, however, is regulated by a number of other molecules, including several gut derived peptide hormones and a number of classical neurotransmitters [7, 8], that act on β-cells, not only directly, but also indirectly through other target tissues such as liver and skeletal muscle. Many of these molecules function as amplifying agents that, although have little or no effect alone, enhance the signals generated by the β-cell glucose sensing apparatus. For example, rodent insulin secretion can be modulated by the monoamine neurotransmitter norepinephrine [9] elaborated by the adrenal medulla which reach islets via the islet capillary system, or alternatively, norepinephrine can be released locally from sympathetic innervation of the pancreas. Neuronal modulation (via innervation of the pancreas) of β-cell insulin secretion, however, may differ in rodents and humans. Relative to the structure of mouse islets, human islets are sparsely innervated with few contacts to autonomic and cholinergic axons. Moreover, in human islets, sympathetic axons are associated with blood vessel smooth muscle cells located around the islet rather than in direct contact with β-cells [10, 11]. To reconcile the apparent autonomy of human islets with the known effects of autonomic stimulation on islet hormone secretion, it has been suggested that neurotransmitter “spill-over” from innervation might be responsible for downstream effects on hormone secretion. Recent studies in our group point to an alternate possibility: the existence an autocrine circuit of neurotransmitter regulation of insulin secretion involving dopamine.
Dopamine's effects on insulin secretion have been considered for the last 40 years; however, neither the mechanism of action nor the origin of dopamine was understood until a few years ago. Injection of L-3, 4-dihydroxyphenylalanine (L-DOPA, the immediate biosynthetic precursor of dopamine) or dopamine itself produced a hyperglycemic response in mice. In these studies, both L-DOPA and DA were detected in isolated mouse islets; however, the contribution of adrenergic nerves secreting epinephrine and norepinephrine could not be excluded [12, 13]. Later, the mechanisms of monoamine uptake and action in the islets of the golden hamster were expanded [14, 15]. Dopamine was also detected in mice β-cell secretory granules by injection of radio-labeled L-DOPA. Following injection, partial inhibition of GSIS was observed; however, the authors of this study suggested that the dopamine might have originated in other tissues [16, 17].
There is ample evidence that all the components needed for dopamine synthesis, storage and secretion are expressed in β-cells. For example, aromatic L-amino acid decarboxylase and monoamine oxidases activities have been found in mouse islet homogenates [18–20]. Another key element in β-cell dopamine metabolism is the vesicular monoamine transporter type 2 (VMAT2). It sequesters dopamine within vesicles in β-cells, providing protection from DA catabolic enzymes such as monoamine oxidases. Although VMAT2 expression in human β-cells is widely accepted, [21] there is conflicting evidence whether or not it is expressed in rodent cells. While some have found no evidence using immunohistochemistry or RT-PCR, [22] others have found ample proof that VMAT2 is expressed in rodent pancreas. The convincing evidence supporting VMAT2 expression in rodent pancreatic β-cells is based on the binding or the inhibition of binding of dihydrotetrabenazine, a specific VMAT2 inhibitor [23, 24], the presence of serotonin and dopamine in islets [25–27] and that in in vitro cultures of purified rodent islets treated with dihydrotetrabenazine (DTBZ), enhanced insulin secretion can be observed [28].
VMAT2 appears to be an important nexus of dopaminergic control of glucose stimulated insulin secretion in rodents. This statement is based on a series of studies demonstrating that 1) in vivo glucose tolerance is improved by the specific VMAT2 inhibitor tetrabenazine (TBZ) administered during intraperitoneal glucose tolerance testing (IPGTT) and that L-DOPA reverses the effects of TBZ, 2) TBZ enhanced in vivo insulin secretion during IPGTT, 3) TBZ enhanced in vitro glucose dependent insulin secretion by purified rat islets, 4) The pancreatic beta cells are the major depot of total pancreas dopamine and 5) Western-blotting and RT-PCR experiments showing that VMAT2 is expressed by rodent β-cells. Together, these data suggested that VMAT2 regulates in vivo glucose homeostasis and insulin production via its role in vesicular transport and storage of DA in β-cells [28].
Other studies have shown that exogenous dopamine inhibits GSIS from isolated human and rodent islets. Rubi and colleagues demonstrated that D2-like receptors, the most likely mediator of the observed inhibition of insulin secretion by dopamine, are also expressed by islet β-cells [29]. D2-like receptors' role in regulating insulin secretion was further elucidated in two subsequent studies. In one, D2-like receptors (D2R) were knocked out in the rodent β-cell line INS-1 832/12 with a small interfering RNA, resulting in increased insulin secretion [30]. However a second study, using global D2R knockout mice, came to a different conclusion; that the lack of D2-like receptors impairs insulin secretion [31]. This apparent discrepancy resolves when the dose-response curve of beta cells to exogenous DA is considered [32]. In vitro, DA significantly stimulates insulin secretion at very low concentrations (10−8 M). Higher concentrations of dopamine (10−7 – 10−4 M) inhibits glucose-induced insulin secretion in the presence of both 4 mM and 20 mM glucose.
More recently, evidence has accumulated suggesting that VMAT2 and D2-Like receptors expressed by β-cells might be elements of a broader regulatory circuit [33]. The principle findings of these studies were that 1) within the human pancreas, D2R is expressed almost exclusively by β-cells and D2R co-localizes with insulin within vesicles as seen by dual color immunofluorescent histochemistry, 2) the main isoform expressed in islets is the D2R long variant, although D2R short transcripts can be found (in the CNS, there are functional differences between isoforms involving their ability to modulate potassium channels [34]), 3) pancreatic islets selectively express the LAT1/MDU1 branched chain and aromatic amino acid carrier system responsible for transport of L-DOPA, 4) islet β-cells express the dopamine active transporter/dopamine (reuptake) transporter (DAT), responsible for the transport of dopamine from the extracellular space into the cytosol, 5) inhibition of VMAT2 (by TBZ), antagonism of D2R (e.g. by Haloperidol or Sulpiride), or inhibition of DAT (by Vanoxerine, a.k.a GBR 12909), all enhance glucose-stimulated insulin release in vitro by human islets, 6) enhancement of insulin secretion by VMAT2 inhibition occurs by increasing the amplitude but not the frequency of pulsatile insulin secretion by human islets, 7) both TBZ and GBR 12909 deplete islet tissue of their DA content, 8) human islets secrete DA in a glucose-concentration dependent manner and DA release was coincident with insulin release, and 9) β-cells, via DAT, transport exogenous radiolabeled DA intracellularly. A similar report in the rodent system was published shortly thereafter [35].
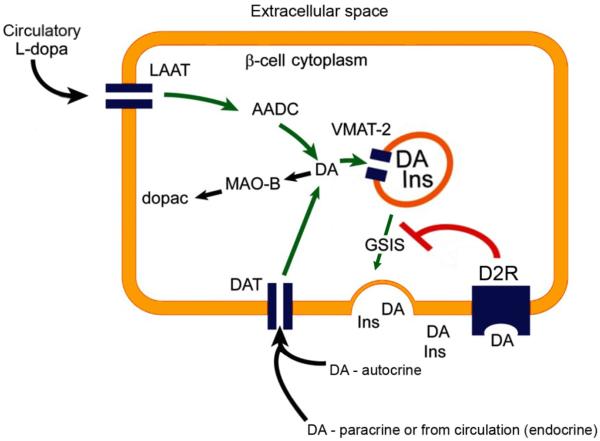
In this putative circuit, DA, synthesized de novo or imported by DAT, is stored in β-cell insulin granules by the action of VMAT2. The insulin granules also contain D2R. During GSIS, DA and insulin are released and D2R is delivered to the cell surface where it binds DA. DA signaling through D2R is a powerful inhibitor of glucose dependent insulin secretion [33]. While DA may be produced de novo by β-cells, as they express the full complement of transporters and enzymes needed to synthesize DA from tyrosine, it is also possible that β-cell DA is imported from other sources. Such sources might include, for example, “spillover” from the nerve terminals that innervate the pancreas (vide supra) and/or, since the pancreas is fed by three interlocking arterial circles and islets receive about 15% of the total circulation to the pancreas, uptake from blood might also be a source of L-DOPA and/or DA for β-cells. At the distal end of this circuit, β-cells selectively express (relative to the rest of the pancreas) the neutral amino acid carrier, LAT1/MDU1, as well as DAT (Figure 1). In support of our hypothesis regarding the relationship between dopamine and insulin secretion is anecdotal evidence that 1) aromatic L-amino acid decarboxylase (AADC) deficient individuals suffer from hypoglycemia [36] possibly due to increased insulin secretion as predicted by our hypothesis, and 2) Parkinson's patients receiving oral dopamine precursor L-DOPA have reduced insulin secretion during oral glucose tolerance testing [37].
Figure 1.
Dopaminergic Negative Feedback Regulating Insulin Secretion Schematic diagram of a β-cell expressing the molecular machinery for dopamine synthesis, storage, and secretion. The dopamine receptor D2R mediates the inhibition of glucose-stimulated insulin secretion. AADC, l-amino acid decarboxylase; DA, dopamine; GSIS, glucose-stimulated insulin secretion; INS, insulin; MAO, monoamine oxidase; LAAT, l-type amino acid transporter; D2R, dopamine 2-like receptors; dopac, 3,4-dihydroxyphenylacetic acid.
Dopamine in Peripheral Circulation Comes From The Gut
At this point it is important to understand that the foregut (including the stomach) appears to be the major source of peripheral circulating DA and L-DOPA [38]. Tyrosine hydroxylase (TH), the rate limiting DA biosynthetic enzyme, is expressed in parietal cells, Lieberkühn crypts, ileal epithelials and throughout the lamina propria of the small intestines [38, 39] and VMAT1, responsible for transport of serotonin (5-HT) or DA into storage vesicles, is expressed by enterochromaffin cells [38, 40] and the parietal cells of the oxyntic stomach [38]. Following ingestion of a standard mixed meal, healthy volunteers show significantly increased plasma levels of L-DOPA and DA [41, 42]. Similar results have been observed in the rat mixed meal testing model [43].
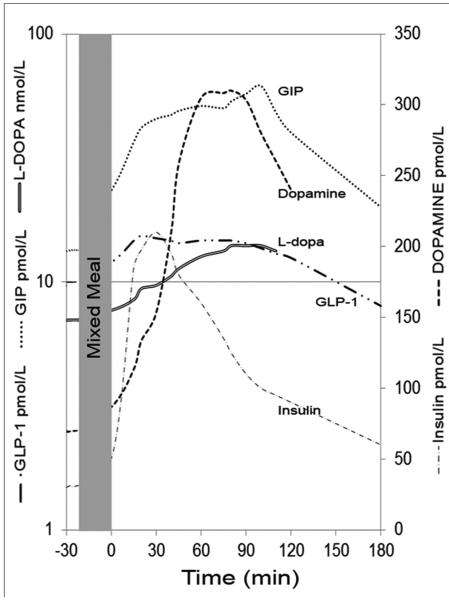
These postprandial arterial DA and L-DOPA excursions (up to 15 nmol/L) probably cannot directly act to significantly dampen insulin secretion. However, within human islets β-cells are in close contact to each other [10] analogous to synapses in the central nervous system. Since β-cells are able to take-up DA (and L-DOPA), concentrate DA by the action of VMAT2 for vesicular release, and release DA in high concentration near self or adjacent β-cell dopamine receptors, these excursions probably represent significant amounts with regard to regulating β-cell insulin secretion. If the dynamics of β-cell dopamine release are similar to that reported for the synapse, local concentrations of dopamine may rise up to 100 μM within a 5-μm radius from the release site [44], a finding that correlates with dose-response curve of human islet insulin secretion treated with exogenous dopamine [32]. Lastly, the kinetics of DA and L-DOPA appearance in the serum coincide with the postprandial rise and fall of immunoreactive incretin plasma levels [43] (Figure 2). Given that the foregut (including the stomach) is the major source of peripheral circulating DA and L-DOPA, it is reasonable to postulate that the foregut is also the origin of postprandial elevations of circulating DA and L-DOPA [45]. Supporting this idea is experimental evidence showing that the rate-limiting enzyme in the biosynthesis of dopamine, tyrosine hydroxylase, is expressed in cells throughout the proximal GI tract, including parietal cells, Lieberkuhn crypts, ileal epithelial cells, and throughout the lamina propria [39]. Furthermore, enterochromaffin cells and parietal cells express VMAT1, which is responsible for transport of monoamines into storage vesicles [38–40].
Figure 2.
Overlapping excursions of serum dopamine and GLP-1 and GIP in healthy volunteers during a mixed–meal tolerance test (MMTT). Excursions adapted from separate publications, L-dopa and dopamine in ref [41] and Insulin and Incretins in ref [43, 107].
Glucose-dependent insulinotropic peptide (GIP) and GLP-1 are well-characterized incretin insulin secretagogues originating from the foregut [46], the latter of which is believed to mediate many of the beneficial effects of bariatric surgery on glucose homeostasis [47]. Substantial evidence outlined above shows that DA and indirectly L-DOPA, via dopamine receptors are powerful inhibitors of insulin secretion [16, 17, 28, 29, 32, 33, 35, 43].
Dopamine Counter-regulates the effects of Incretins on Beta Cell Insulin Secretion
Since serum elevations of the incretins GLP-1 and GIP appear at roughly the same time as dopamine and L-DOPA, we performed a series of in vitro experiments examining what might be the net effects on insulin secretion and the signaling events which drive insulin secretion in β-cells [43]. We found that both DA and L-DOPA, abrogated incretin enhanced GSIS from human islets and the rodent beta cell line INS-1E. Underlying this effect, we found that DA suppressed many of the incretin enhanced glucose dependent phosphoprotein signaling events (e.g. phospho AKT), incretin enhanced glucose dependent intracellular cAMP elevation, and intracellular calcium fluxes. Lastly, DA suppressed GLP-1 stimulated rodent islet and beta-cell proliferation in vitro and had significant pro-apoptotic activity countering the effects of incretins.
The coincidence of the kinetics of release of GLP-1 and DA, and the counter regulatory-effects of DA on incretin-enhanced GSIS suggest the existence of a second, gut-based layer of regulation of glucose homeostasis. The idea of a second layer of regulation of glucose homeostasis was derived from the foregut and hindgut hypotheses (as reviewed by Rubino [48]) posited to explain the effects of bariatric surgery on T2DM. According to the hindgut hypothesis, the rapid delivery of nutrients to the distal intestine results in the secretion of incretins which enhances insulin release and/or action, resulting in a subsequent decrease in blood glucose levels. The major incretin responsible for this effect is now recognized as GLP-1 derived from L-cells (found in the ileum and colon) [49] (Figure 3). According to the foregut hypothesis, gastrointestinal bypass reduces the secretion of upper gastrointestinal factors that decrease insulin secretion and/or promote insulin resistance. DA, as described above, fulfills many of the requirements of an “anti-incretin” posited by the foregut hypothesis [50, 51] and, overall, it is plausible that bypass of organs (e.g. in RYGB), known to store and produce significant amounts of an insulin secretion inhibitor (i.e. DA) from nutritional sources [38] would move the organism to a more glucose-tolerant state. Surgical manipulation of total body DA and stimulation of GLP-1 secretion might be responsible for the rapid improvement in glucose tolerance and enhanced insulin secretion observed prior to weight loss in following RYGB surgery.
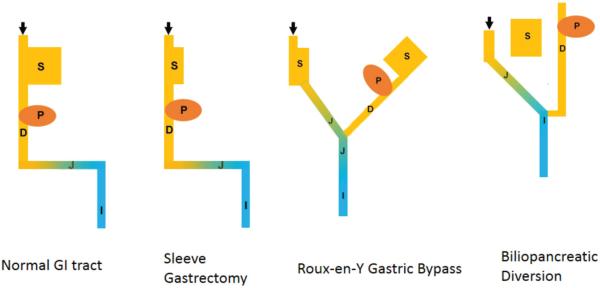
Figure 3. Schematic Diagram of Relationship between Different Bypass Surgeries, Dopamine and GLP-1 Producing Tissues.
Orange: Tissue elaborating Dopamine and/or L-DOPA. Blue: Tissue elaborating GLP-1. S: stomach, P: pancreas, D: duodenum, J: jejunum, I:Ileum, Arrow: Nutrient entry.
DA may not be the only anti-incretin. Ghrelin is a stomach and proximal intestine derived 28-amino acid peptide that may also play a role in T2DM reversal following RYGB surgery. Ghrelin has recently been reported to directly suppress the insulin secretion enhancing effects of GLP-1 [52] on β-cells. Several in vitro and in vivo (controversial) studies show that ghrelin induces hyperglycemia and reduces insulin secretion. A recent human in vivo study [53] shows that synthetic exogenous ghrelin has an inhibitory effect on glucose-stimulated insulin release and glucose disappearance. The ghrelin hypothesis [51] posits that ghrelin regulation might be disturbed following RYGB. During oral glucose tolerance testing, however, ghrelin does not show the same positive excursions as does DA. Nevertheless, ghrelin and DA share similar tissue distributions of origin and directly modulate β-cell insulin secretion and perhaps work together in maintaining glucose homeostasis.
Together these data suggest that 1) DA is an autocrine negative regulator of GSIS in human islets and 2) islets can “sense”, via DAT and LAT1/MDU1, circulating blood levels of DA and L-DOPA, respectively. Given that the GI tract (including the stomach) is the major source of DA (and L-DOPA) and GLP-1 in the body, GLP-1 and DA have opposing effects on β-cell insulin secretion, and finally certain types of bariatric surgery which separate out the DA producing arm of GI, while simultaneously enhancing the function of the GLP-1 producing arm of the GI to improve hyperglycemia, it is reasonable to propose that signaling through D2R within the β cell is the regulatory counterpart of GLP-1R signaling, mediating a second gut-based layer of postprandial blood glucose control that is integrated into the β-cell glucose sensing apparatus.
How Bariatric Surgery Might Alter Gastrointestinal DA and L-DOPA production
Currently there are five different bariatric procedures that are performed: adjustable gastric banding, Roux-en-Y gastric bypass, sleeve gastrectomy, biliopancreatic diversion, and biliopancreatic diversion with duodenal switch [54]. Adjustable gastric banding, Roux-en-Y gastric bypass, and sleeve gastrectomy are the most commonly utilized bariatric procedures utilized worldwide. Biliopancreatic diversion with or without duodenal switch is now only rarely used in select cases. Adjustable gastric banding involves the placement of an inflatable band over the upper portion of the stomach 1-2 cm below the gastroesophageal junction. The band's constriction can be adjusted by the injection or removal of saline through a subcutaneous port that is linked to a balloon in the band. Adjustable gastric banding thus creates a constricted upper gastric pouch that is readily adjustable. Routinely, this is performed by a laparoscopic approach but an open approach can be used if necessary. Gastric bypass surgery for weight loss originally consisted of a partitioning of the upper portion of the stomach to create a small gastric pouch, and restoring the continuity of the GI tract with an anastomosis between the stomach and a portion of the jejunum (i.e. gastrojejunostomy). Since its inception, this procedure has undergone many modifications. The construction of the pouch no longer consists of partitioning, but rather division of the stomach to avoid the creation of a gastrogastric fistula. Additionally, the most common method of re-establishing gastrointestinal continuity is the Roux-en-Y reconstruction. This involves the creation of a biliopancreatic limb that is not in continuity with the food stream, and an alimentary (or Roux) limb that does carry food which are then joined together as a jejunojejunostomy to form a common channel. The biliopancreatic limb is constructed from the duodenum and a length of proximal jejunum that is typically 30–60 cm in length. It carries bile and pancreatic secretions, but no food, to the jejunojejunostomy. The Roux limb re-establishes gastrointestinal tract continuity of the food stream by construction of a gastrojejunostomy of the remaining portion of jejunum and a jejunojejunostomy with the biliopancreatic limb. Usually the length of the Roux limb is 75–150 cm. Roux-en-Y Gastric Bypass not only reduces the size of the stomach pouch, but also bypasses a significant portion of the proximal small intestine. The other major mal-absorptive procedure is biliopancreatic diversion. In contrast to RYGB, a subtotal gastrectomy is performed rather than division of the stomach. A biliopancreatic limb is then created and reattached to the common channel 50–125 cm proximal to the ileocecal valve. A modification of this procedure, known as the duodenal switch, involves a sleeve gastrectomy instead of a subtotal gastrectomy. A significant portion of the stomach is removed along the greater curvature to create a tubular structure with a preserved pylorus. For the severely obese and other high-risk patients, this was initially performed as a two-stage procedure, where the sleeve gastrectomy was performed prior to the reconstruction of the GI tract. However, weight loss and improvement in co-morbidities of obesity were found to be clinically significant after sleeve gastrectomy, and it has since been used as a definitive bariatric procedure.
It has long been recognized that obesity and Type 2 Diabetes Mellitus are linked. In one series, 31% of morbidly obese subjects were also diabetic [55]. First-line management of diabetes in obese patients is life-style modifications that include increased exercise and weight loss prior to the initiation of anti-diabetic agents. However, conventional medical treatment of type 2 diabetes often achieves inadequate glycemic control, and concomitant obesity makes management particularly challenging. Although originally developed for weight loss, bariatric surgery has also been especially effective in managing diabetes. Particularly, procedures where a segment of the foregut is bypassed induce rapid and large improvement even before significant weight loss begins. Pories et al showed that 91% of obese diabetic patients can achieve normal fasting blood glucose after RYGB [4]. Schauer et al. observed that while 47–80% of gastric banding patients can discontinue anti-diabetic medication, the proportion increases to 80–98% with RYGB and 96–100% after biliopancreatic diversion procedures [5]. A meta-analysis also showed better remission rates in bypassing procedures such as biliopancreatic diversion and RYGB than restrictive procedures like gastric banding and sleeve gastrectomy [56]. This is in contrast to lifestyle modifications and medical management, both of which were shown not to be able to achieve remission or improvement in glycemic control for obese diabetic patients [57, 58]. In fact, in patients who failed to improve lifestyle and medical therapy for their diabetes, bariatric surgery was shown to normalize glucose levels, reduce HbA1c <6%, and reduce the need for anti-diabetic medication [59].
Surgeries where segments of the foregut are bypassed show particular effectiveness in improving Type 2 Diabetes Mellitus when compared to gastric banding (Figure 3). In fact, the improvements in glycemic control are seen before any significant weight loss occurs and is more robust. As many as 74% of patients who were on anti-diabetic medication pre-op no longer need it by 6 days post-op. After RYGB, 69.8% had regression of T2DM and another 19.1% had stable glycemia within 12 weeks. The level of improvement was positively correlated to the length of the biliopancreatic limb [60]. In the first month after surgery, there is a much greater improvement in insulin resistance as measured by the homeostatic model assessment index in patients who undergo RYGB than in those who undergo gastric banding. However, in the long-term the two procedures have equivalent rates of improved insulin resistance [61]. In contrast, another series showed RYGB improvement in HbA1c and insulin resistance is more robust when compared to gastric banding 1-2 years after surgery [62]. Where the improvement in T2DM after gastric banding relies solely on weight loss, other mechanisms in addition to weight loss are at play in patients who undergo RYGB.
Sleeve gastrectomy is another type of bariatric surgery where improvement in glucose control occurs independent of weight loss. Although considered a restrictive procedure, a large portion of the stomach along the greater curvature is eliminated from the food stream. Originally the first of a two-stage procedure for super-obese and high risk, as a stand-alone bariatric procedure it has achieved significant weight loss and improvement in glycemic control in up to 85% of patients [63]. In one series, all patients who underwent sleeve gastrectomy had improvement or remission of their diabetes within 6 months [64]. When compared to other bariatric procedures, sleeve gastrectomy's ability to achieve weight loss is generally better than gastric banding and less than or equivalent to that of RYGB; however, the rate of complications is greater than gastric banding and less than RYGB making it an attractive alternative [56, 64–68].
When compared to gastric banding, sleeve gastrectomy had superior rates of diabetes resolution and weight loss [69]. The superior rates of diabetes remission was also observed in severely obese patients with BMI>50 after one year [66]. However, compared to RYGB, T2DM remission rates of sleeve gastrectomy are inferior when measured one year post-op [65, 70]. Interestingly, the different rates of diabetes remission between sleeve gastrectomy and RYGB was shown not to be related to level of weight loss, as one series showed that RYGB had greater remission of diabetes despite similar reductions in BMI [71]. These observations of sleeve gastrectomy's efficacy in improving T2DM are consistent with the foregut hypothesis. In contrast to RYGB, where a significant portion of the stomach and proximal small intestine are eliminated from the food stream, sleeve gastrectomy eliminates a smaller portion of the foregut with the resection of the greater curvature of the stomach. This would account for sleeve gastrectomy's intermediate position between RYGB and gastric banding in regards to improvements in glycemic control.
In 1992, the National Institute of Health set forth the following criteria as indications for bariatric surgery. Individuals either must have a BMI ≥ 40 or a BMI ≥ 35 with high risk co-morbidity. Conditions that allow for patients with lower BMIs to meet the selection criteria included severe cardiopulmonary problems and type 2 diabetes [72]. As a result, there are only limited studies on the effects of bariatric surgery in non-obese individuals. However, the few reports that are in the literature of bypass operations for other indications show similar effects on insulin resistance and glycemic control in both less severely obese (BMI<35) and non-obese individuals. Patients who have gastrectomy for cancer often undergo reconstruction that bypasses duodenum and significant portion of the proximal jejunum. One retrospective review observed that a third non-obese diabetic patients who have either Roux-en-Y gastrojejunostomy or Billroth II gastrojejunostomy achieved remission of diabetes, while the remaining two-thirds had improvement in glycemic control [73]. Although average BMI reduction was only 9.8%, 15.1% of diabetic patients who undergo gastrectomy for cancer had resolution of diabetes and an additional 30.4% had improvement. Patients that had total gastrectomy with Roux-en-Y reconstruction showed the greatest improvement in glycemic control [74]. By three weeks post-gastrectomy, non-obese diabetic gastric cancer patients show improvement or remission of diabetes [75]. Even though weight was not a factor in the diabetes of these patients, bypassing segment of the foregut still resulted in improvement in Type 2 Diabetes Mellitus. These observations in non-obese patients support a weight-independent mechanism in the improvement of T2DM in obese patients who undergo bypass procedures.
When bariatric surgery is performed in obese individuals with BMI<35, similar patterns of improvement in diabetes is seen. In one series, 100% of obese diabetics with BMI from 30–35 had improvement of diabetes after RYGB. Within 20 months after surgery, 74% were able to discontinue all anti-diabetic medication and half had even achieved complete resolution [76]. In patients with a BMI<30, average HbA1c was able to be reduced from 8.3 to 6.6 in diabetics, with reduced requirements for insulin and oral diabetic agents [77]. Although patients with lower BMIs see great benefit from bariatric surgery, the greatest improvement in diabetes occurs in patients with BMI>32 [78]. The rapid improvement in glycemic control prior to significant weight loss that RYGB confers to diabetic obese patients is also seen in people with BMI<35. However, where normalization of HbA1c and fasting blood glucose was seen within 3 months for BMI>35 patients, it was not seen until 6 months after surgery in BMI<35. After one year, these differences disappeared; the authors of this study concluded that a longer biliopancreatic limb (or greater bypassed segment), which the higher BMI patients received, accounted for the differing dynamics in diabetes improvement [79].
The hormonal mechanisms involved in the improvement of diabetes in patients who undergo a bypass procedure have been investigated. After RYGB, patients were found to have increased insulin secretion and β-cell responsiveness via IV glucose tolerance test (IVGTT) 1 month after surgery prior to significant weight loss [80]. RYGB patient were also found to lack the compensatory increase in ghrelin that usually occurs with diet-induced weight loss and an exaggerated postprandial increase in peptide YY but no changes in leptin when compared with lean controls [81]. When examining insulin levels in RYGB patients, they were found to be lower at baseline when compared to gastric banding patients, but the same as lean individuals. However, postprandial levels exhibited an exaggerated acute phase increase in RYGB patients that were not seen in gastric banding patients [82]. The type of bariatric surgery a person undergoes also alters postprandial GLP-1 and GIP levels in different ways. RYGB showed much higher GLP-1 and lower GIP levels after a meal when compared to gastric banding and overweight controls [82]. The increase of GLP-1 secretion by RYGB was confirmed by another study which also showed it occurs early after RYGB [83]. However, gene expression of GIP has been found to increase over four times as much in patients who had improvement of diabetes after RYGB [84]. The changes in GLP-1 and GIP secretion after bypass do not fully account for the improvement in glycemic control. One randomized, prospective study compared post-prandial insulin levels, glucose and GLP-1 levels in patients after RYGB with sleeve gastrectomy. Sleeve gastrectomy also led to increase in GLP-1 and insulin levels long before any apparent weight loss occurs [85]. These data suggest that other mechanisms are at play in the improvement of glycemic control before the onset of weight loss when a portion of the proximal foregut is excluded from the food stream.
The clinical observations that bypass of proximal small intestine in patients, regardless of weight, improves glycemic control independent of weight loss were confirmed in a rat model [86]. RYGB in rats also induced upregulation of insulin receptors in skeletal muscle and liver, increased insulin receptor-β activity, and total GLUT4 levels in skeletal muscle, all contributing to reduction of insulin resistance [87]. The exclusion of proximal foregut was confirmed to be essential to bypass surgery's ability to improve glycemic control in diabetic rats when those that had undergone bypass were re-operated to restore the food stream in previously excluded portion of proximal intestine which in turn re-established impairment in glucose tolerance [88]. These observations in the rat do not fully account for RYGB surgery's ability to improve T2DM independent of weight loss.
The improvement in glucose tolerance following gastric banding appears to be related to weight loss alone and the pursuant reduction in peripheral insulin resistance [89, 90]. As described earlier, procedures such as RYGB and to a lesser extent sleeve gastrectomy have a marked improvement in glycemia seen almost immediately after surgery. If these surgeries decrease circulating levels of L-DOPA and dopamine, then β-cells would lack the substrate to produce this anti-incretin, and consequently glucose stimulated insulin secretion would increase. It is also relevant to note that L-DOPA and dopamine have effects on other tissues that affect peripheral insulin resistance. L-DOPA regulates glycogen concentration, glycogen synthase activity, and insulin stimulated glucose transport in rat muscle [91]. Additionally skeletal muscle responds to dopamine with increases in endogenous cAMP [92, 93] which negatively regulates the actions of insulin [93, 94]. Glycogen synthase (GS), the rate-limiting enzyme for the synthesis of glycogen from glucose in skeletal muscle, is activated by insulin and inhibited by agents that increase intracellular cAMP [95]. Insulin resistance and hyperglycemia in T2DM has been associated with impaired activation of GS [96–98]. The resulting down-regulation of glucose accumulation and GS in skeletal muscle is thought to be an important defect in the pathogenesis of T2DM [99]. Nevertheless, some tonic negative regulation of GS in skeletal muscle by dopamine (via β-adrenergic nerves) might cause rapid reduction of insulin resistance following surgical removal of organs contributing to circulating dopamine levels. Additionally, the reduction of dopamine levels, particularly the postprandial elevations observed after a mixed-meal tolerance test, might also lead to rapid improvement in β-cell function [100, 101].
An association between post-RYGB and hypoglycemia with nesidioblastosis has been recognized [102], with the first case being reported in 2005 [103]. Adult nesidioblastosis is characterized by beta-cell hypertrophy, islet hyperplasia, and complexes of insulin positive cells in close association with small pancreatic ducts and a resultant increase in beta cell mass. Given that RYGB increases plasma levels of gut incretin hormones, both GIP and GLP-1 protect human islets from apoptosis [104] or drive rodent β-cell mass proliferation [105, 106] and the proapoptotic / antiproliferative activity of DA on islets, it may not be surprising that decreasing peripheral dopaminergic tone favors increased beta cell mass and hyperinsulinemia.
Summary
Recent advancement in the knowledge of pancreatic β-cell biology and dopaminergic regulation of glucose stimulated insulin secretion has revealed a previously unrecognized mechanism in how certain types of bariatric surgery might be able to improve hyperglycemia independent of weight loss. According the foregut hypothesis, bypass of the proximal portion of the GI tract reduces the secretion of a factor that decreases insulin secretion/action; dopamine may very well be this “anti-incretin” factor. Multiple in vitro and in vivo models have shown that dopamine serves as an autocrine, negative regulator of glucose stimulated insulin secretion. Additionally, the proximal GI tract is the major source of peripheral circulating dopamine and L-DOPA secreting large amounts of both after a meal. Dopamine's role as the anti-incretin put forth by the foregut hypothesis is consistent with the clinical observations that bypass surgery improves hyperglycemia rapidly in a weight-loss independent manner. However, further investigation is required to properly delineate, dopamine's role in the regulation of glucose-stimulated insulin secretion after bariatric surgery.
References
- [1].Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric-metabolic surgery versus conventional medical treatment in obese patients with type 2 diabetes: 5 year follow-up of an open-label, single-centre, randomised controlled trial. Lancet. 2015;386:964–973. doi: 10.1016/S0140-6736(15)00075-6. [DOI] [PubMed] [Google Scholar]
- [2].Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes. 2006;55:2025–2031. doi: 10.2337/db06-0068. [DOI] [PubMed] [Google Scholar]
- [3].Pournaras DJ, Osborne A, Hawkins SC, et al. Remission of type 2 diabetes after gastric bypass and banding: mechanisms and 2 year outcomes. Ann Surg. 2010;252:966–971. doi: 10.1097/SLA.0b013e3181efc49a. [DOI] [PubMed] [Google Scholar]
- [4].Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. discussion 350–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Schauer PR, Burguera B, Ikramuddin S, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 2003;238:467–484. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 484–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Henquin JC. Triggering and amplifying pathways of regulation of insulin secretion by glucose. Diabetes. 2000;49:1751–1760. doi: 10.2337/diabetes.49.11.1751. [DOI] [PubMed] [Google Scholar]
- [7].Brunicardi FC, Shavelle DM, Andersen DK. Neural regulation of the endocrine pancreas. Int J Pancreatol. 1995;18:177–195. doi: 10.1007/BF02784941. [DOI] [PubMed] [Google Scholar]
- [8].Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- [9].McDermott AM, Sharp GW. Inhibition of insulin secretion: a fail-safe system. Cell Signal. 1993;5:229–234. doi: 10.1016/0898-6568(93)90014-d. [DOI] [PubMed] [Google Scholar]
- [10].Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci U S A. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rodriguez-Diaz R, Abdulreda MH, Formoso AL, et al. Innervation patterns of autonomic axons in the human endocrine pancreas. Cell Metab. 2011;14:45–54. doi: 10.1016/j.cmet.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hakanson R, Lundquist I, Rerup C. On the hyperglycaemic effect of DOPA and dopamine. Eur J Pharmacol. 1967;1:114–119. doi: 10.1016/0014-2999(67)90047-7. [DOI] [PubMed] [Google Scholar]
- [13].Hansen SE, Hedeskov CJ. Simultaneous determination of the content of serotonin, dopamine, noradrenaline and adrenaline in pancreatic islets isolated from fed and starved mice. Acta Endocrinol (Copenh) 1977;86:820–832. doi: 10.1530/acta.0.0860820. [DOI] [PubMed] [Google Scholar]
- [14].Mahony C, Feldman JM. Species variation in pancreatic islet monoamine uptake and action. Diabetes. 1977;26:257–261. doi: 10.2337/diab.26.4.257. [DOI] [PubMed] [Google Scholar]
- [15].Zern RT, Bird JL, Feldman JM. Effect of increased pancreatic islet norepinephrine, dopamine and serotonin concentration on insulin secretion in the golden hamster. Diabetologia. 1980;18:341–346. doi: 10.1007/BF00251017. [DOI] [PubMed] [Google Scholar]
- [16].Ericson LE, Hakanson R, Lundquist I. Accumulation of dopamine in mouse pancreatic B-cells following injection of L-DOPA. Localization to secretory granules and inhibition of insulin secretion. Diabetologia. 1977;13:117–124. doi: 10.1007/BF00745138. [DOI] [PubMed] [Google Scholar]
- [17].Lundquist I, Ahren B, Hansson C, Hakanson R. Monoamines in pancreatic islets of guinea pig, hamster, rat, and mouse determined by high performance liquid chromatography. Pancreas. 1989;4:662–667. doi: 10.1097/00006676-198912000-00002. [DOI] [PubMed] [Google Scholar]
- [18].Lundquist I, Panagiotidis G, Stenstrom A. Effect of L-dopa administration on islet monoamine oxidase activity and glucose-induced insulin release in the mouse. Pancreas. 1991;6:522–527. doi: 10.1097/00006676-199109000-00004. [DOI] [PubMed] [Google Scholar]
- [19].Lindstrom P. Aromatic-L-amino-acid decarboxylase activity in mouse pancreatic islets. Biochim Biophys Acta. 1986;884:276–281. doi: 10.1016/0304-4165(86)90174-1. [DOI] [PubMed] [Google Scholar]
- [20].Teitelman G, Joh TH, Reis DJ. Transformation of catecholaminergic precursors into glucagon (A) cells in mouse embryonic pancreas. Proc Natl Acad Sci U S A. 1981;78:5225–5229. doi: 10.1073/pnas.78.8.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Saisho Y, Harris PE, Butler AE, et al. Relationship between pancreatic vesicular monoamine transporter 2 (VMAT2) and insulin expression in human pancreas. J Mol Histol. 2008;39:543–551. doi: 10.1007/s10735-008-9195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schafer MK, Hartwig NR, Kalmbach N, et al. Species-specific vesicular monoamine transporter 2 (VMAT2) expression in mammalian pancreatic beta cells: implications for optimising radioligand-based human beta cell mass (BCM) imaging in animal models. Diabetologia. 2013;56:1047–1056. doi: 10.1007/s00125-013-2847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Scherman D, Jaudon P, Henry JP. Characterization of the monoamine carrier of chromaffin granule membrane by binding of [2–3H]dihydrotetrabenazine. Proc Natl Acad Sci U S A. 1983;80:584–588. doi: 10.1073/pnas.80.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singhal T, Ding YS, Weinzimmer D, et al. Pancreatic beta cell mass PET imaging and quantification with [11C]DTBZ and [18F]FP-(+)-DTBZ in rodent models of diabetes. Mol Imaging Biol. 2011;13:973–984. doi: 10.1007/s11307-010-0406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rosario LM, Barbosa RM, Antunes CM, et al. Regulation by glucose of oscillatory electrical activity and 5-HT/insulin release from single mouse pancreatic islets in absence of functional K(ATP) channels. Endocr J. 2008;55:639–650. doi: 10.1507/endocrj.k07e-131. [DOI] [PubMed] [Google Scholar]
- [26].Deeney JT, Branstrom R, Corkey BE, Larsson O, Berggren PO. 3H-serotonin as a marker of oscillatory insulin secretion in clonal beta-cells (INS-1) FEBS Lett. 2007;581:4080–4084. doi: 10.1016/j.febslet.2007.07.052. [DOI] [PubMed] [Google Scholar]
- [27].Braun M, Wendt A, Karanauskaite J, et al. Corelease and differential exit via the fusion pore of GABA, serotonin, and ATP from LDCV in rat pancreatic beta cells. J Gen Physiol. 2007;129:221–231. doi: 10.1085/jgp.200609658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Raffo A, Hancock K, Polito T, et al. Role of vesicular monoamine transporter type 2 in rodent insulin secretion and glucose metabolism revealed by its specific antagonist tetrabenazine. J Endocrinol. 2008;198:41–49. doi: 10.1677/JOE-07-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rubi B, Ljubicic S, Pournourmohammadi S, et al. Dopamine D2-like receptors are expressed in pancreatic beta cells and mediate inhibition of insulin secretion. J Biol Chem. 2005;280:36824–36832. doi: 10.1074/jbc.M505560200. [DOI] [PubMed] [Google Scholar]
- [30].Wu W, Shang J, Feng Y, et al. Identification of glucose-dependant insulin secretion targets in pancreatic beta cells by combining defined-mechanism compound library screening and siRNA gene silencing. J Biomol Screen. 2008;13:128–134. doi: 10.1177/1087057107313763. [DOI] [PubMed] [Google Scholar]
- [31].Garcia-Tornadu I, Ornstein AM, Chamson-Reig A, et al. Disruption of the dopamine d2 receptor impairs insulin secretion and causes glucose intolerance. Endocrinology. 2010;151:1441–1450. doi: 10.1210/en.2009-0996. [DOI] [PubMed] [Google Scholar]
- [32].Shankar E, Santhosh KT, Paulose CS. Dopaminergic regulation of glucose-induced insulin secretion through dopamine D2 receptors in the pancreatic islets in vitro. IUBMB Life. 2006;58:157–163. doi: 10.1080/15216540600687993. [DOI] [PubMed] [Google Scholar]
- [33].Simpson N, Maffei A, Freeby M, et al. Dopamine-mediated autocrine inhibitory circuit regulating human insulin secretion in vitro. Mol Endocrinol. 2012;26:1757–1772. doi: 10.1210/me.2012-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gantz SC, Robinson BG, Buck DC, et al. Distinct regulation of dopamine D2S and D2L autoreceptor signaling by calcium. Elife. 2015;4 doi: 10.7554/eLife.09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ustione A, Piston DW. Dopamine synthesis and D3 receptor activation in pancreatic beta-cells regulates insulin secretion and intracellular [Ca(2+)] oscillations. Mol Endocrinol. 2012;26:1928–1940. doi: 10.1210/me.2012-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arnoux JB, Damaj L, Napuri S, et al. Aromatic L-amino acid decarboxylase deficiency is a cause of long-fasting hypoglycemia. J Clin Endocrinol Metab. 2013;98:4279–4284. doi: 10.1210/jc.2013-2740. [DOI] [PubMed] [Google Scholar]
- [37].Rosati G, Maioli M, Aiello I, Farris A, Agnetti V. Effects of long-term L-dopa therapy on carbohydrate metabolism in patients with Parkinson's disease. Eur Neurol. 1976;14:229–239. doi: 10.1159/000114744. [DOI] [PubMed] [Google Scholar]
- [38].Eisenhofer G, Aneman A, Friberg P, et al. Substantial production of dopamine in the human gastrointestinal tract. J Clin Endocrinol Metab. 1997;82:3864–3871. doi: 10.1210/jcem.82.11.4339. [DOI] [PubMed] [Google Scholar]
- [39].Kozicz T, Arimura A. Distribution of urocortin in the rat's gastrointestinal tract and its colocalization with tyrosine hydroxylase. Peptides. 2002;23:515–521. doi: 10.1016/s0196-9781(01)00639-8. [DOI] [PubMed] [Google Scholar]
- [40].Mezey E, Eisenhofer G, Harta G, et al. A novel nonneuronal catecholaminergic system: exocrine pancreas synthesizes and releases dopamine. Proc Natl Acad Sci U S A. 1996;93:10377–10382. doi: 10.1073/pnas.93.19.10377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goldstein DS, Swoboda KJ, Miles JM, et al. Sources and physiological significance of plasma dopamine sulfate. J Clin Endocrinol Metab. 1999;84:2523–2531. doi: 10.1210/jcem.84.7.5864. [DOI] [PubMed] [Google Scholar]
- [42].Blum I, Vered Y, Graff E, et al. The influence of meal composition on plasma serotonin and norepinephrine concentrations. Metabolism. 1992;41:137–140. doi: 10.1016/0026-0495(92)90141-v. [DOI] [PubMed] [Google Scholar]
- [43].Maffei A, Segal AM, Alvarez-Perez JC, Garcia-Ocana A, Harris PE. Anti-incretin, Anti-proliferative Action of Dopamine on beta-Cells. Mol Endocrinol. 2015;29:542–557. doi: 10.1210/me.2014-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends Neurosci. 2004;27:270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- [45].Vieira-Coelho MA, Soares-da-Silva P. Dopamine formation, from its immediate precursor 3,4-dihydroxyphenylalanine, along the rat digestive tract. Fundam Clin Pharmacol. 1993;7:235–243. doi: 10.1111/j.1472-8206.1993.tb00237.x. [DOI] [PubMed] [Google Scholar]
- [46].Lovshin JA, Drucker DJ. Incretin-based therapies for type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5:262–269. doi: 10.1038/nrendo.2009.48. [DOI] [PubMed] [Google Scholar]
- [47].Manning S, Pucci A, Batterham RL. GLP-1: a mediator of the beneficial metabolic effects of bariatric surgery? Physiology (Bethesda) 2015;30:50–62. doi: 10.1152/physiol.00027.2014. [DOI] [PubMed] [Google Scholar]
- [48].Rubino F, R'Bibo SL, del Genio F, Mazumdar M, McGraw TE. Metabolic surgery: the role of the gastrointestinal tract in diabetes mellitus. Nat Rev Endocrinol. 2010;6:102–109. doi: 10.1038/nrendo.2009.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Tolhurst G, Reimann F, Gribble FM. Nutritional regulation of glucagon-like peptide-1 secretion. J Physiol. 2009;587:27–32. doi: 10.1113/jphysiol.2008.164012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Pories WJ, Albrecht RJ. Etiology of type II diabetes mellitus: role of the foregut. World J Surg. 2001;25:527–531. doi: 10.1007/s002680020348. [DOI] [PubMed] [Google Scholar]
- [51].Cummings DE. Endocrine mechanisms mediating remission of diabetes after gastric bypass surgery. Int J Obes (Lond) 2009;33(Suppl 1):S33–40. doi: 10.1038/ijo.2009.15. [DOI] [PubMed] [Google Scholar]
- [52].Damdindorj B, Dezaki K, Kurashina T, et al. Exogenous and endogenous ghrelin counteracts GLP-1 action to stimulate cAMP signaling and insulin secretion in islet beta-cells. FEBS Lett. 2012;586:2555–2562. doi: 10.1016/j.febslet.2012.06.034. [DOI] [PubMed] [Google Scholar]
- [53].Tong J, Prigeon RL, Davis HW, et al. Ghrelin suppresses glucose-stimulated insulin secretion and deteriorates glucose tolerance in healthy humans. Diabetes. 2010;59:2145–2151. doi: 10.2337/db10-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- [55].Hofso D, Jenssen T, Hager H, Roislien J, Hjelmesaeth J. Fasting plasma glucose in the screening for type 2 diabetes in morbidly obese subjects. Obes Surg. 2010;20:302–307. doi: 10.1007/s11695-009-0022-5. [DOI] [PubMed] [Google Scholar]
- [56].Panunzi S, De Gaetano A, Carnicelli A, Mingrone G. Predictors of remission of diabetes mellitus in severely obese individuals undergoing bariatric surgery: do BMI or procedure choice matter? A meta-analysis. Ann Surg. 2015;261:459–467. doi: 10.1097/SLA.0000000000000863. [DOI] [PubMed] [Google Scholar]
- [57].Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. doi: 10.1056/NEJMoa1200111. [DOI] [PubMed] [Google Scholar]
- [58].Courcoulas AP, Belle SH, Neiberg RH, et al. Three-Year Outcomes of Bariatric Surgery vs Lifestyle Intervention for Type 2 Diabetes Mellitus Treatment: A Randomized Clinical Trial. JAMA Surg. 2015 doi: 10.1001/jamasurg.2015.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Cohen R, Pinheiro JS, Correa JL, Schiavon CA. Laparoscopic Roux-en-Y gastric bypass for BMI < 35 kg/m(2): a tailored approach. Surg Obes Relat Dis. 2006;2:401–404. doi: 10.1016/j.soard.2006.02.011. discussion 404. [DOI] [PubMed] [Google Scholar]
- [60].Proczko-Markuszewska M, Stefaniak T, Kaska L, Kobiela J, Sledzinski Z. Impact of Roux-en-Y gastric bypass on regulation of diabetes type 2 in morbidly obese patients. Surg Endosc. 2012;26:2202–2207. doi: 10.1007/s00464-012-2160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lee WJ, Lee YC, Ser KH, Chen JC, Chen SC. Improvement of insulin resistance after obesity surgery: a comparison of gastric banding and bypass procedures. Obes Surg. 2008;18:1119–1125. doi: 10.1007/s11695-008-9457-3. [DOI] [PubMed] [Google Scholar]
- [62].Ballantyne GH, Wasielewski A, Saunders JK. The surgical treatment of type II diabetes mellitus: changes in HOMA Insulin resistance in the first year following laparoscopic Roux-en-Y gastric bypass (LRYGB) and laparoscopic adjustable gastric banding (LAGB) Obes Surg. 2009;19:1297–1303. doi: 10.1007/s11695-009-9870-2. [DOI] [PubMed] [Google Scholar]
- [63].Perathoner A, Weissenbacher A, Sucher R, Laimer E, Pratschke J, Mittermair R. Significant weight loss and rapid resolution of diabetes and dyslipidemia during short-term follow-up after laparoscopic sleeve gastrectomy. Obes Surg. 2013;23:1966–1972. doi: 10.1007/s11695-013-1038-4. [DOI] [PubMed] [Google Scholar]
- [64].Moon Han S, Kim WW, Oh JH. Results of laparoscopic sleeve gastrectomy (LSG) at 1 year in morbidly obese Korean patients. Obes Surg. 2005;15:1469–1475. doi: 10.1381/096089205774859227. [DOI] [PubMed] [Google Scholar]
- [65].Zhang Y, Wang J, Sun X, et al. Laparoscopic sleeve gastrectomy versus laparoscopic Roux-en-Y gastric bypass for morbid obesity and related comorbidities: a meta-analysis of 21 studies. Obes Surg. 2015;25:19–26. doi: 10.1007/s11695-014-1385-9. [DOI] [PubMed] [Google Scholar]
- [66].Thereaux J, Corigliano N, Poitou C, Oppert JM, Czernichow S, Bouillot JL. Comparison of results after one year between sleeve gastrectomy and gastric bypass in patients with BMI>/=50 kg/m(2) Surg Obes Relat Dis. 2014 doi: 10.1016/j.soard.2014.11.022. [DOI] [PubMed] [Google Scholar]
- [67].Vidal J, Ibarzabal A, Nicolau J, et al. Short-term effects of sleeve gastrectomy on type 2 diabetes mellitus in severely obese subjects. Obes Surg. 2007;17:1069–1074. doi: 10.1007/s11695-007-9180-5. [DOI] [PubMed] [Google Scholar]
- [68].Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254:410–420. doi: 10.1097/SLA.0b013e31822c9dac. discussion 420–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Pham S, Gancel A, Scotte M, et al. Comparison of the effectiveness of four bariatric surgery procedures in obese patients with type 2 diabetes: a retrospective study. J Obes. 2014;2014:638203. doi: 10.1155/2014/638203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Zhang N, Maffei A, Cerabona T, Pahuja A, Omana J, Kaul A. Reduction in obesity-related comorbidities: is gastric bypass better than sleeve gastrectomy? Surg Endosc. 2013;27:1273–1280. doi: 10.1007/s00464-012-2595-7. [DOI] [PubMed] [Google Scholar]
- [71].Milone M, Di Minno MN, Leongito M, et al. Bariatric surgery and diabetes remission: sleeve gastrectomy or mini-gastric bypass? World J Gastroenterol. 2013;19:6590–6597. doi: 10.3748/wjg.v19.i39.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr. 1992;55:615S–619S. doi: 10.1093/ajcn/55.2.615s. [DOI] [PubMed] [Google Scholar]
- [73].Zervos EE, Agle SC, Warren AJ, et al. Amelioration of insulin requirement in patients undergoing duodenal bypass for reasons other than obesity implicates foregut factors in the pathophysiology of type II diabetes. J Am Coll Surg. 2010;210:564–572. 572–564. doi: 10.1016/j.jamcollsurg.2009.12.025. [DOI] [PubMed] [Google Scholar]
- [74].Kim JW, Cheong JH, Hyung WJ, Choi SH, Noh SH. Outcome after gastrectomy in gastric cancer patients with type 2 diabetes. World J Gastroenterol. 2012;18:49–54. doi: 10.3748/wjg.v18.i1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Shen Z, Yu J, Lei S, et al. Glycemic changes after gastrectomy in non-morbidly obese patients with gastric cancer and diabetes. Hepatogastroenterology. 2015;62:245–250. [PubMed] [Google Scholar]
- [76].de Sa VC, Ferraz AA, Campos JM, Ramos AC, Araujo JG, Jr, Ferraz EM. Gastric bypass in the treatment of type 2 diabetes in patients with a BMI of 30 to 35 kg/m2. Obes Surg. 2011;21:283–287. doi: 10.1007/s11695-010-0318-5. [DOI] [PubMed] [Google Scholar]
- [77].Garcia-Caballero M, Valle M, Martinez-Moreno JM, et al. Resolution of diabetes mellitus and metabolic syndrome in normal weight 24–29 BMI patients with One Anastomosis Gastric Bypass. Nutr Hosp. 2012;27:623–631. doi: 10.1590/S0212-16112012000200041. [DOI] [PubMed] [Google Scholar]
- [78].Huang CK, Shabbir A, Lo CH, Tai CM, Chen YS, Houng JY. Laparoscopic Roux-en-Y gastric bypass for the treatment of type II diabetes mellitus in Chinese patients with body mass index of 25–35. Obes Surg. 2011;21:1344–1349. doi: 10.1007/s11695-011-0408-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kaska L, Proczko M, Kobiela J, Stefaniak TJ, Sledzinski Z. Dynamics of type 2 diabetes mellitus laboratory remission after Roux-en-Y gastric bypass in patients with body mass index lower than 35 kg/m(2) and higher than 35 kg/m(2) in a 3-year observation period. Wideochir Inne Tech Malo Inwazyjne. 2014;9:523–530. doi: 10.5114/wiitm.2014.44427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lin E, Davis SS, Srinivasan J, et al. Dual mechanism for type-2 diabetes resolution after Roux-en-Y gastric bypass. Am Surg. 2009;75:498–502. discussion 502–493. [PMC free article] [PubMed] [Google Scholar]
- [81].Korner J, Bessler M, Cirilo LJ, et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab. 2005;90:359–365. doi: 10.1210/jc.2004-1076. [DOI] [PubMed] [Google Scholar]
- [82].Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis. 2007;3:597–601. doi: 10.1016/j.soard.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Elahi D, Galiatsatos P, Rabiee A, et al. Mechanisms of type 2 diabetes resolution after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2014;10:1028–1039. doi: 10.1016/j.soard.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Moran-Atkin E, Brody F, Fu SW, Rojkind M. Changes in GIP gene expression following bariatric surgery. Surg Endosc. 2013;27:2492–2497. doi: 10.1007/s00464-012-2764-8. [DOI] [PubMed] [Google Scholar]
- [85].Peterli R, Wolnerhanssen B, Peters T, et al. Improvement in glucose metabolism after bariatric surgery: comparison of laparoscopic Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: a prospective randomized trial. Ann Surg. 2009;250:234–241. doi: 10.1097/SLA.0b013e3181ae32e3. [DOI] [PubMed] [Google Scholar]
- [86].Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg. 2004;239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bonhomme S, Guijarro A, Keslacy S, et al. Gastric bypass up-regulates insulin signaling pathway. Nutrition. 2011;27:73–80. doi: 10.1016/j.nut.2010.08.005. [DOI] [PubMed] [Google Scholar]
- [88].Rubino F, Forgione A, Cummings DE, et al. The mechanism of diabetes control after gastrointestinal bypass surgery reveals a role of the proximal small intestine in the pathophysiology of type 2 diabetes. Ann Surg. 2006;244:741–749. doi: 10.1097/01.sla.0000224726.61448.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- [90].Ponce J, Haynes B, Paynter S, et al. Effect of Lap-Band-induced weight loss on type 2 diabetes mellitus and hypertension. Obes Surg. 2004;14:1335–1342. doi: 10.1381/0960892042583932. [DOI] [PubMed] [Google Scholar]
- [91].Smith JL, Ju JS, Saha BM, Racette BA, Fisher JS. Levodopa with carbidopa diminishes glycogen concentration, glycogen synthase activity, and insulin-stimulated glucose transport in rat skeletal muscle. J Appl Physiol (1985) 2004;97:2339–2346. doi: 10.1152/japplphysiol.01219.2003. [DOI] [PubMed] [Google Scholar]
- [92].Schubert D, Tarikas H, LaCorbiere M. Neurotransmitter regulation of adenosine 3',5'-monophosphate in clonal nerve, glia, and muscle cell lines. Science. 1976;192:471–473. doi: 10.1126/science.176728. [DOI] [PubMed] [Google Scholar]
- [93].Hunt DG, Ding Z, Ivy JL. Clenbuterol prevents epinephrine from antagonizing insulin-stimulated muscle glucose uptake. J Appl Physiol (1985) 2002;92:1285–1292. doi: 10.1152/japplphysiol.01009.2001. [DOI] [PubMed] [Google Scholar]
- [94].Lee AD, Hansen PA, Schluter J, Gulve EA, Gao J, Holloszy JO. Effects of epinephrine on insulin-stimulated glucose uptake and GLUT-4 phosphorylation in muscle. Am J Physiol. 1997;273:C1082–1087. doi: 10.1152/ajpcell.1997.273.3.C1082. [DOI] [PubMed] [Google Scholar]
- [95].Lawrence JC, Jr, Roach PJ. New insights into the role and mechanism of glycogen synthase activation by insulin. Diabetes. 1997;46:541–547. doi: 10.2337/diab.46.4.541. [DOI] [PubMed] [Google Scholar]
- [96].Vind BF, Birk JB, Vienberg SG, et al. Hyperglycaemia normalises insulin action on glucose metabolism but not the impaired activation of AKT and glycogen synthase in the skeletal muscle of patients with type 2 diabetes. Diabetologia. 2012;55:1435–1445. doi: 10.1007/s00125-012-2482-8. [DOI] [PubMed] [Google Scholar]
- [97].Iozzo P, Pratipanawatr T, Pijl H, et al. Physiological hyperinsulinemia impairs insulin-stimulated glycogen synthase activity and glycogen synthesis. Am J Physiol Endocrinol Metab. 2001;280:E712–719. doi: 10.1152/ajpendo.2001.280.5.E712. [DOI] [PubMed] [Google Scholar]
- [98].Beck-Nielsen H. The role of glycogen synthase in the development of hyperglycemia in type 2 diabetes: `To store or not to store glucose, that's the question'. Diabetes Metab Res Rev. 2012;28:635–644. doi: 10.1002/dmrr.2337. [DOI] [PubMed] [Google Scholar]
- [99].Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990;322:223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- [100].Falken Y, Hellstrom PM, Holst JJ, Naslund E. Changes in glucose homeostasis after Roux-en-Y gastric bypass surgery for obesity at day three, two months, and one year after surgery: role of gut peptides. J Clin Endocrinol Metab. 2011;96:2227–2235. doi: 10.1210/jc.2010-2876. [DOI] [PubMed] [Google Scholar]
- [101].Korner J, Inabnet W, Febres G, et al. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond) 2009;33:786–795. doi: 10.1038/ijo.2009.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mala T. Postprandial hyperinsulinemic hypoglycemia after gastric bypass surgical treatment. Surg Obes Relat Dis. 2014;10:1220–1225. doi: 10.1016/j.soard.2014.01.010. [DOI] [PubMed] [Google Scholar]
- [103].Service GJ, Thompson GB, Service FJ, Andrews JC, Collazo-Clavell ML, Lloyd RV. Hyperinsulinemic hypoglycemia with nesidioblastosis after gastric-bypass surgery. N Engl J Med. 2005;353:249–254. doi: 10.1056/NEJMoa043690. [DOI] [PubMed] [Google Scholar]
- [104].Farilla L, Bulotta A, Hirshberg B, et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- [105].Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- [106].Perfetti R, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology. 2000;141:4600–4605. doi: 10.1210/endo.141.12.7806. [DOI] [PubMed] [Google Scholar]
- [107].Rask E, Olsson T, Soderberg S, et al. Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care. 2001;24:1640–1645. doi: 10.2337/diacare.24.9.1640. [DOI] [PubMed] [Google Scholar]