Abstract
Background and Purpose
Ventricular assist devices (VADs) have advanced the management of end-stage heart failure. However, these devices are associated with hemorrhagic and thrombotic complications, including stroke. We assessed the incidence, risk factors, and outcomes of ischemic and hemorrhagic stroke after VAD placement.
Methods
Using administrative claims data from acute care hospitals in California, Florida, and New York from 2005–2013, we identified patients who underwent VAD placement, defined by International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 37.66. Ischemic and hemorrhagic strokes were identified by previously validated coding algorithms. We used survival statistics to determine incidence rates and Cox proportional hazard analyses to examine associations.
Results
Among 1,813 patients, we identified 201 ischemic strokes and 116 hemorrhagic strokes during 3.4 (±2.0) years of follow-up after implantation of a VAD. The incidence of stroke was 8.7% per year (95% confidence interval [CI], 7.7–9.7%). The annual incidence of ischemic stroke (5.5%; 95% CI, 4.8–6.4%) was nearly double that of hemorrhagic stroke (3.1%; 95% CI, 2.6–3.8%). Women faced a higher hazard of stroke than men (hazard ratio [HR], 1.6; 95% CI, 1.2–2.1), particularly hemorrhagic stroke (HR, 2.2; 95% CI, 1.4–3.4). Stroke was strongly associated with subsequent in-hospital mortality (HR, 6.1; 95% CI, 4.6–7.9).
Conclusions
The incidence of stroke after VAD implantation was 8.7% per year, and incident stroke was strongly associated with subsequent in-hospital mortality. Notably, ischemic stroke occurred at nearly twice the rate of hemorrhagic stroke. Women appeared to face a higher risk for hemorrhagic stroke than men.
Indexing terms: Ventricular Assist Device, Stroke, Hemorrhagic Stroke, Ischemic Stroke, Heart Failure
Ventricular assist devices (VADs) play an important role in the management of advanced heart failure. VAD therapy is intended for patients with severe heart failure that causes disabling symptoms such as dyspnea at rest or with minimal activity or such heart failure that requires inotrope therapy.1 VADs provide mechanical circulatory support to patients primarily as a bridge to heart transplantation.2 More recently, an increasing proportion of VADs are being implanted as destination therapy, particularly in older patients with multiple comorbidities who are not candidates for heart transplantation.2–4
VADs are associated with several thrombotic and hemorrhagic complications, such as pump thrombosis, systemic bleeding, thromboembolic stroke, and intracranial hemorrhage.2, 5 It is customary to maintain VAD patients on therapeutic anti-coagulation with warfarin and an anti-platelet agent, typically aspirin.1, 6, 7 Estimates of stroke risk in patients with VADs vary widely,8–11 but registry data show a cumulative overall stroke rate of 19% by 3 years.4 Risk factors and the characteristics and outcomes of stroke in patients with VADs have not been well defined. We therefore used comprehensive administrative claims data from three states to assess the relationship between VADs and stroke.
Methods
Design
We used administrative claims data on all admissions to nonfederal acute care hospitals in California, Florida, and New York. These heterogeneous states comprise approximately 25% of the total U.S. population and were chosen because they are the three largest states with publicly available deidentified data that allow tracking of individual patients from visit to visit across years. Data were available for 2005–2011 in California, 2005–2013 in Florida, and 2006–2013 in New York. The Agency for Healthcare Research and Quality12 collects these data from the California Office of Statewide Health Planning and Development, the New York State Department of Health Statewide Planning and Research Cooperative System, and the Florida Agency for Health Care Administration, and subsequently de-identifies the data by assigning each patient an anonymous record number. The Weill Cornell Medicine institutional review board approved our analyses.
Patient Population
We identified all patients who underwent VAD placement, defined as International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) code 37.66 (insertion of implantable heart assist system). The vast majority (95%) of VADs placed in the United States are durable continuous flow left ventricular assist devices, and this code has been used to study the epidemiology of these devices.2, 13 Patients were included regardless of whether they underwent VAD placement as destination therapy or as a bridge to transplantation. We excluded patients with a documented stroke prior to the index visit for VAD placement. Non-residents of California, Florida and New York were excluded to maximize follow-up.
Measurements
Stroke was defined as a composite of ischemic or hemorrhagic stroke, as identified by previously validated ICD-9-CM discharge diagnosis code algorithms.14 Ischemic stroke was defined by ICD-9-CM codes 433.x1, 434.x1, or 436 in any discharge diagnosis position in the absence of any concomitant code for trauma or intracranial hemorrhage or a primary code for rehabilitation.
This algorithm was found to be 86% sensitive and 95% specific for ischemic stroke.14 Hemorrhagic stroke was defined as any diagnosis code for intracerebral hemorrhage (ICD-9-CM code 431) or subarachnoid hemorrhage (430) in the absence of any concomitant code for trauma or a primary code for rehabilitation. This has been shown to be 82% sensitive and 93% specific for intracerebral hemorrhage and 98% sensitive and 92% specific for subarachnoid hemorrhage.11 Additionally, we included nontraumatic subdural hematoma (in the absence of any trauma codes or a primary code for rehabilitation) in our definition of hemorrhagic stroke given evidence that nontraumatic subdural hematomas comprise a substantial proportion of intracranial hemorrhages.15 The ICD-9-CM code for subdural hematoma (432.1) has been validated to be 96% sensitive and 89% specific.16
Additional covariates were demographic characteristics such as age, sex, race, insurance status, household income; vascular risk factors such as hypertension, diabetes, coronary heart disease, peripheral vascular disease, chronic obstructive pulmonary disease, chronic kidney disease, and atrial fibrillation; and the overall burden of medical illness as measured by the Elixhauser comorbidity index.17
Statistical Analysis
Baseline characteristics were reported with standard descriptive statistics and compared using the chi-square test or t-test, as appropriate. Survival statistics were used to calculate incidence rates, and Kaplan-Meier analysis was used to calculate cumulative rates. Patients were censored at the time of in-hospital death, or date of the last available follow-up data. For secondary analyses of ischemic stroke and hemorrhagic stroke, patients were censored at the time of their first recorded stroke, whether ischemic or hemorrhagic. After confirming that the proportional hazard assumption was met, Cox proportional hazards modeling was used to identify factors associated with stroke. Additionally, we used separate Cox models to assess the association between stroke—modeled as a time-varying covariate—and subsequent in-hospital mortality. Finally, because pulsatile VADs were largely discontinued in favor of continuous flow devices after 2008, subgroup analyses and tests of interaction were performed to examine the effect of the time period of VAD placement (2005–2008 versus 2009–2013) on our findings.2 All analyses were performed using Stata/MP version 13 (StataCorp, TX).
Results
We identified 1,813 patients with implantation of a VAD. The mean age of the patients was 56.1 (±13.1) years, and patients were predominantly male (79.8%). They had a high proportion of baseline vascular risk factors, including coronary artery disease, hypertension, diabetes, and atrial fibrillation. Patients who experienced a stroke after VAD implantation were younger and more likely to be female (Table 1).
Table 1.
Characteristics of Patients, Stratified by Stroke
| Characteristic* | Stroke (N = 301) | No Stroke (N = 1,512) | P value |
|---|---|---|---|
| Age, mean (SD), y | 54.0 (12.1) | 56.5 (13.3) | 0.002 |
| Female | 74 (24.6) | 292 (19.3) | 0.04 |
| Race† | 0.70 | ||
| White | 170 (57.6) | 901 (60.9) | |
| Black | 48 (16.3) | 205 (13.9) | |
| Hispanic | 37 (12.5) | 189 (12.8) | |
| Asian | 14 (4.8) | 54 (3.7) | |
| Other | 26 (8.8) | 130 (8.8) | |
| Payment source | 0.02 | ||
| Medicare | 106 (35.2) | 667 (44.1) | |
| Medicaid | 53 (17.6) | 214 (14.2) | |
| Private or Other | 142 (47.2) | 631 (41.7) | |
| Income quartile | 0.01 | ||
| 1st | 84 (28.7) | 315 (21.1) | |
| 2nd | 60 (20.5) | 367 (24.6) | |
| 3rd | 82 (28.0) | 390 (26.1) | |
| 4th | 67 (22.9) | 420 (28.2) | |
| Hypertension | 133 (44.2) | 669 (44.3) | 0.99 |
| Diabetes | 112 (37.2) | 519 (34.3) | 0.34 |
| Coronary heart disease | 174 (57.8) | 859 (56.8) | 0.75 |
| Peripheral vascular disease | 28 (9.3) | 151 (10.0) | 0.72 |
| Chronic obstructive pulmonary disease | 45 (15.0) | 206 (13.6) | 0.54 |
| Chronic kidney disease | 111 (36.9) | 527 (34.9) | 0.50 |
| Atrial fibrillation | 127 (42.2) | 678 (44.8) | 0.40 |
| Obesity | 35 (11.6) | 156 (10.3) | 0.50 |
| Elixhauser comorbidities‡, mean (SD) | 3.5 (1.9) | 3.3 (1.8) | 0.04 |
Abbreviations: SD, standard deviation
Data are presented as number (%) unless otherwise specified.
Self-reported by patients or their surrogates. Numbers do not sum to group totals because of missing race/ethnicity data in 2.2% of patients.
Numbers represent the number of Elixhauser comorbid conditions, which comprise a comprehensive set of 28 comorbidity measures for use with large administrative datasets.
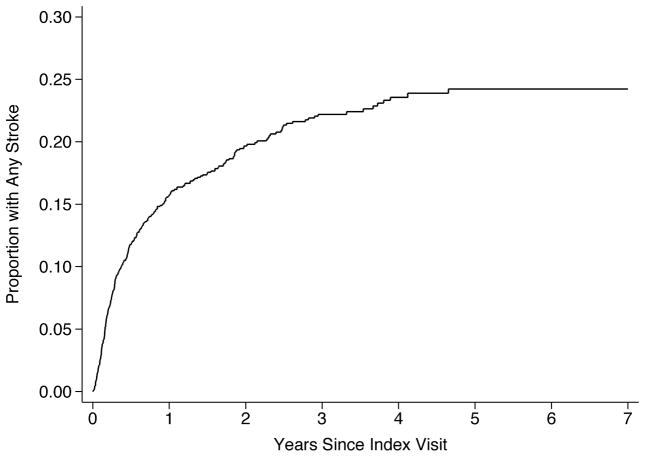
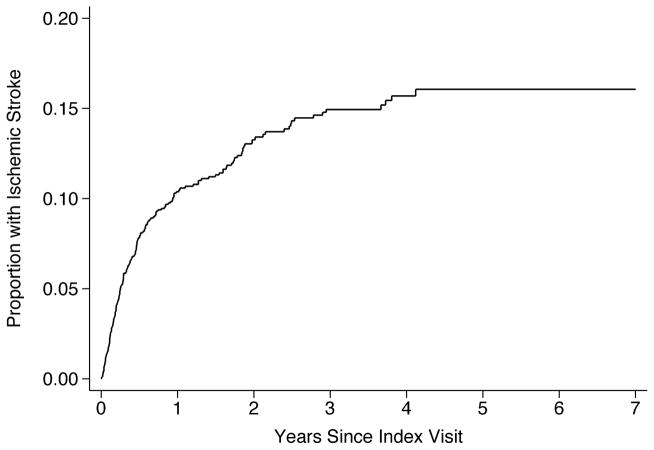
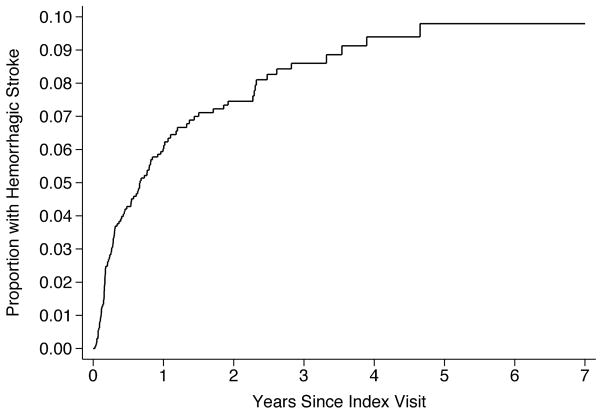
Over 3.4 (±2.0) years of follow-up, we identified 301 patients with a stroke (16.6%). Of these, 201 patients had ischemic stroke, 116 had hemorrhagic stroke, and 16 had both. The overall incidence of any stroke was 8.7% (95% CI, 7.7–9.7%) per year. The annual incidence was 5.5% (95% CI, 4.8–6.4%) for ischemic stroke and 3.1% (95% CI, 2.6–3.8%) for hemorrhagic stroke. By 3 years after VAD placement, this resulted in a cumulative overall stroke rate of 22.2% (95% CI, 19.9–24.7%) (Figure 1), a cumulative ischemic stroke rate of 14.9% (95% CI, 13.0–17.2%; Figure 2), and a cumulative hemorrhagic stroke rate of 8.6% (95% CI, 7.1–10.4%; Figure 3).
Figure 1.
Cumulative Rate of Stroke after Ventricular Assist Device Placement.
Figure 2.
Cumulative Rate of Ischemic Stroke after Ventricular Assist Device Placement.
Figure 3.
Cumulative Rate of Hemorrhagic Stroke after Ventricular Assist Device Placement.
In multivariable analysis adjusting for demographics, vascular risk factors, and comorbidities, female sex was independently associated with stroke (hazard ratio [HR], 1.6; 95% CI, 1.2–2.1). This association was not present for ischemic stroke (HR 1.3, 95% CI, 0.9–1.9) but was particularly pronounced for hemorrhagic stroke (HR, 2.2; 95% CI, 1.4–3.4). In the overall sample, the cumulative hemorrhagic stroke rate in women (14.0%; 95% CI, 10.0%–19.4%) was substantially greater than in men (8.8%; 95% CI, 6.9–11.3%; P < 0.001).
The higher risk of hemorrhagic stroke in women compared to men was not changed when the time period of VAD placement (2005–2008 versus 2009–2013) was included as a covariate, nor was the period of VAD placement itself independently associated with hemorrhagic stroke (HR, 0.9; 95% CI, 0.6–1.4). However, the relative hazard of hemorrhagic stroke in women compared to men was greater in 2005–2008 (HR, 4.1; 95% CI, 2.0–8.4) than in 2009–2013 (HR, 1.8; 95% CI, 0.9–3.4) (P = 0.02 for interaction), indicating that the degree of disparity between men and women in hemorrhagic stroke risk after VAD placement has declined significantly over time.
After discharge from index hospitalization for VAD placement, 16.3% (95% CI, 14.6–18.0%) of patients faced in-hospital death. After adjusting for demographics, vascular risk factors, and comorbidities, stroke was strongly associated with subsequent in-hospital mortality (HR, 6.1; 95% CI, 4.6–7.9). The association between stroke and in-hospital mortality was similar irrespective of whether the stroke was ischemic (HR, 8.5; 95% CI, 5.9–12.2) or hemorrhagic (HR, 6.7; 95% CI, 4.7–9.5).
Discussion
In a large, population-based sample of patients, approximately one in every 10 patients experienced a stroke each year after VAD implantation. This is comparable to the contemporary incidence of gastrointestinal hemorrhage of approximately 10% per year18 and of pump thrombosis of 9% at 12 months in 2013.19 The stroke rate in our cohort significantly exceeded the highest observed rates in multiple historical cohorts of medically managed mild to moderate heart failure and in a recent analysis of patients with dramatically reduced ejection fraction (<15%),20–23 which supports the conclusion that the association is with the VAD implant itself, not just with the patient population requiring the implant. Additionally, we found that ischemic stroke was nearly twice as common as hemorrhagic stroke, despite it being customary for VAD patients to be treated with warfarin and an anti-platelet agent.1 Although VAD recipients were predominantly male, women had an increased risk of stroke, especially hemorrhagic stroke. Stroke was strongly associated with subsequent in-hospital mortality.
Our findings are generally consistent with and build upon the existing VAD registry results, which have not reported granular data on stroke subtypes (e.g., ischemic versus hemorrhagic). The 22.2% 3-year cumulative stroke rate in our cohort is consistent with the 19% cumulative stroke rate in registry data.4 Our finding of an 8.7% annual incidence of stroke is also similar to the findings of a recent single-center analysis of stroke in LVAD recipients, which found a 0.094 events per patient-year rate of stroke.6 However, this study found an approximately 3:1 ratio of ischemic stroke to ICH, in contrast to the 2:1 ratio seen in our results. This may be due to the small sample size of their study and their exclusion of subdural hematoma from the definition of ICH.6 Additionally, their study found a higher rate of in-hospital mortality after ICH than after ischemic stroke; however, their ICH sample size was small (n=8), and they did not assess the effect of stroke on mortality in this population.6 Our study found that the risk of in-hospital mortality after stroke did not vary with stroke subtype, which is consistent with a recent single-center study of outcome after stroke in the LVAD population.7 However, in contrast to the twofold risk of mortality after stroke seen in their cohort, we found a six-fold risk of in-hospital mortality.7 The discrepancy may be because we considered only in-hospital mortality, the rate of which may be greater in stroke patients. Our finding of an elevated risk of in-hospital mortality after stroke in VAD patients is also consistent with findings of registry data2 and a large study demonstrating reduced survival in patients with LVAD-related thromboembolic events.24 Our data reveal a strong association between stroke and in-hospital mortality and also suggest an association with mortality overall. Generally, other studies of stroke in patients with LVADs vary widely in their findings, which is likely a result of small sample sizes, single-center design, and inclusion of transient ischemic attack in the definition of stroke.8
A key finding in our study was the strong association between female sex and hemorrhagic stroke, and the decrease in the disparity between men and women in hemorrhagic stroke risk over the past decade. Female sex is not associated with higher rates of hemorrhagic stroke in the general population.25 Two prior studies also found female sex to be a risk factor for hemorrhagic stroke after VAD placement,26, 27 but these studies were small, included only clinical trial patients, and could not assess temporal trends. Other studies have not identified this association perhaps due to their limited sample sizes. 6, 7 Potential explanations for the higher hemorrhagic stroke risk in women include lower body mass index,28 longer duration on heart transplant waiting lists,29 and more advanced heart failure at the time of presentation.30 The decrease in sex-based disparity in hemorrhagic stroke risk over time may be due to changes in pump technology and anti-coagulation management.1, 31 Our findings suggest that some risk factors are being better addressed over time, but even in the most recent epoch female VAD recipients remained at a nearly two-fold higher risk of hemorrhagic stroke than men. Efforts to identify other modifiable risk factors for hemorrhagic stroke in women after VAD placement may help to further mitigate sex-based disparities in cardiovascular health.
Limitations
A number of limitations should be considered. First, our use of administrative claims data means that clinical variables such as stroke severity, antithrombotic medication use, laboratory values, imaging findings, and other possible mediators of outcomes were not included in the analysis. As noted, however, the anti-thrombotic regimen in LVAD patients is standardized.1, 6, 7 Second, the use of in-patient ICD-9-CM codes for identification of vascular risk factors and comorbidities may result in incomplete ascertainment. Third, data regarding out-of-hospital death were not available, which could result in an over-estimation of the effect of stroke on mortality. However, the majority of VAD recipients during our study period would have died in a hospital,32–34 so though our findings may over-estimate the strength of the association between stroke and mortality, we do not expect this bias to fully attenuate the observed relationship. Additionally, the specific finding of increased risk of in-hospital death after stroke is germane to increasingly important end-of-life considerations in this population.35 Fourth, the ICD-9-CM code used to identify patients with VADs is all-inclusive, capturing primarily left ventricular devices of various models and types but also a small number of other type of VADs.
Future Directions
Given the growing public health burden of advanced heart failure and the tremendous resources invested in the care of these patients, understanding and preventing complications is crucial.35–37 Future investigations of modifiable stroke risk factors, sex-specific stroke risk factors, and optimal anti-thrombotic regimens for these patients are warranted.
Conclusions
Our population-level analysis demonstrates that patients, especially women, with VADs are at high risk for stroke, and stroke is associated with an increased risk of in-hospital mortality in VAD recipients.
Acknowledgments
Sources of Funding: This study was supported by grant K23NS082367 (Kamel) from the National Institute of Neurological Disorders and Stroke.
Footnotes
Disclosures: None.
References
- 1.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, et al. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 2.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. doi: 10.1016/j.healun.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, et al. Sixth INTERMACS annual report: a 10,000-patient database. J Heart Lung Transplant. 2014;33:555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, et al. Fifth INTERMACS annual report: risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Willey JZ, Demmer RT, Takayama H, Colombo PC, Lazar RM. Cerebrovascular disease in the era of left ventricular assist devices with continuous flow: risk factors, diagnosis, and treatment. J Heart Lung Transplant. 2014;33:878–887. doi: 10.1016/j.healun.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Willey JZ, Gavalas MV, Trinh PN, Yuzefpolskaya M, Reshad Garan A, Levin AP, et al. Outcomes after stroke complicating left ventricular assist device. [Accessed July 5, 2016];J Heart Lung Transplant. 2016 doi: 10.1016/j.healun.2016.03.014. published online ahead of print March 30, 2016. http://dx.doi.org/10.1016/j.healun.2016.03.014. [DOI] [PMC free article] [PubMed]
- 7.Harvey L, Holley C, Roy SS, Eckman P, Cogswell R, Liao K, et al. Stroke After Left Ventricular Assist Device Implantation: Outcomes in the Continuous-Flow Era. Ann Thorac Surg. 2015;100:535–541. doi: 10.1016/j.athoracsur.2015.02.094. [DOI] [PubMed] [Google Scholar]
- 8.Backes D, van den Bergh WM, van Duijn AL, Lahpor JR, van Dijk D, Slooter AJ. Cerebrovascular complications of left ventricular assist devices. Eur J Cardiothorac Surg. 2012;42:612–620. doi: 10.1093/ejcts/ezs320. [DOI] [PubMed] [Google Scholar]
- 9.Arnaoutakis GJ, George TJ, Kilic A, Beaty CA, Weiss ES, Conte JV, et al. Risk factors for early death in patients bridged to transplant with continuous-flow left ventricular assist devices. Ann Thorac Surg. 2012;93:1549–1554. doi: 10.1016/j.athoracsur.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitson BA, Eckman P, Kamdar F, Lacey A, Shumway SJ, Liao KK, et al. Hemolysis, pump thrombus, and neurologic events in continuous-flow left ventricular assist device recipients. Ann Thorac Surg. 2014;97:2097–2103. doi: 10.1016/j.athoracsur.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 11.Kato TS, Schulze PC, Yang J, Chan E, Shahzad K, Takayama H, et al. Pre-operative and post-operative risk factors associated with neurologic complications in patients with advanced heart failure supported by a left ventricular assist device. J Heart Lung Transplant. 2012;31:1–8. doi: 10.1016/j.healun.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett M, Steiner C, Andrews R, Kassed C, Nagamine M. Methodological Issues when Studying Readmissions and Revisits Using Hospital Adminstrative Data. [Accessed June 1, 2016];HCUP Methods Series Report # 2011-01. 2011 https://www.hcup-us.ahrq.gov/reports/methods/2011_01.pdf.
- 13.Lampropulos JF, Kim N, Wang Y, Desai MM, Barreto-Filho JA, Dodson JA, et al. Trends in left ventricular assist device use and outcomes among Medicare beneficiaries, 2004–2011. Open Heart. 2014;1:e000109. doi: 10.1136/openhrt-2014-000109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 15.Hart RG, Diener HC, Yang S, Connolly SJ, Wallentin L, Reilly PA, et al. Intracranial hemorrhage in atrial fibrillation patients during anticoagulation with warfarin or dabigatran: the RE-LY trial. Stroke. 2012;43:1511–1517. doi: 10.1161/STROKEAHA.112.650614. [DOI] [PubMed] [Google Scholar]
- 16.Morris NA, Merkler AE, Parker WE, Claassen J, Connolly ES, Sheth KN, et al. Adverse Outcomes After Initial Non-surgical Management of Subdural Hematoma: A Population-Based Study. Neurocrit Care. 2016;24:226–232. doi: 10.1007/s12028-015-0178-x. [DOI] [PubMed] [Google Scholar]
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Joy PS, Kumar G, Guddati AK, Bhama JK, Cadaret LM. Risk Factors and Outcomes of Gastrointestinal Bleeding in Left Ventricular Assist Device Recipients. Am J Cardiol. 2016;117:240–244. doi: 10.1016/j.amjcard.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 19.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, et al. Pump thrombosis in the Thoratec HeartMate II device: An update analysis of the INTERMACS Registry. J Heart Lung Transplant. 2015;34:1515–1526. doi: 10.1016/j.healun.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 20.Homma S, Thompson JL, Pullicino PM, Levin B, Freudenberger RS, Teerlink JR, et al. Warfarin and aspirin in patients with heart failure and sinus rhythm. N Engl J Med. 2012;366:1859–1869. doi: 10.1056/NEJMoa1202299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massie BM, Collins JF, Ammon SE, Armstrong PW, Cleland JG, Ezekowitz M, et al. Randomized trial of warfarin, aspirin, and clopidogrel in patients with chronic heart failure: the Warfarin and Antiplatelet Therapy in Chronic Heart Failure (WATCH) trial. Circulation. 2009;119:1616–1624. doi: 10.1161/CIRCULATIONAHA.108.801753. [DOI] [PubMed] [Google Scholar]
- 22.Abdul-Rahim AH, Perez AC, Fulton RL, Jhund PS, Latini R, Tognoni G, et al. Risk of Stroke in Chronic Heart Failure Patients Without Atrial Fibrillation: Analysis of the Controlled Rosuvastatin in Multinational Trial Heart Failure (CORONA) and the Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca-Heart Failure (GISSI-HF) Trials. Circulation. 2015;131:1486–1494. doi: 10.1161/CIRCULATIONAHA.114.013760. [DOI] [PubMed] [Google Scholar]
- 23.Di Tullio MR, Qian M, Thompson JL, Labovitz AJ, Mann DL, Sacco RL, et al. Left Ventricular Ejection Fraction and Risk of Stroke and Cardiac Events in Heart Failure: Data From the Warfarin Versus Aspirin in Reduced Ejection Fraction Trial. [Accessed July 5, 2016];Stroke. 2016 doi: 10.1161/STROKEAHA.116.013679. published online ahead of print June 28, 2016. http://dx.doi.org/10.1161/STROKEAHA.116.013679. [DOI] [PMC free article] [PubMed]
- 24.Wever-Pinzon O, Naka Y, Garan AR, Takeda K, Pan S, Takayama H, et al. National trends and outcomes in device-related thromboembolic complications and malfunction among heart transplant candidates supported with continuous-flow left ventricular assist devices in the United States. [Accessed July 5, 2016];J Heart Lung Transplant. 2016 doi: 10.1016/j.healun.2016.02.004. published online ahead of print March 11, 2016. http://dx.doi.org/10.1016/j.healun.2016.02.004. [DOI] [PubMed]
- 25.van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. doi: 10.1016/S1474-4422(09)70340-0. [DOI] [PubMed] [Google Scholar]
- 26.Bogaev RC, Pamboukian SV, Moore SA, Chen L, John R, Boyle AJ, et al. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–522. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, et al. Pre-operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014;63:880–888. doi: 10.1016/j.jacc.2013.08.1656. [DOI] [PubMed] [Google Scholar]
- 28.Butler J, Howser R, Portner PM, Pierson RN. Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg. 2005;79:66–73. doi: 10.1016/j.athoracsur.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Morris AA, Cole RT, Laskar SR, Kalogeropoulos A, Vega JD, Smith A, et al. Improved Outcomes for Women on the Heart Transplant Wait List in the Modern Era. J Card Fail. 2015;21:555–560. doi: 10.1016/j.cardfail.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 30.Morgan JA, Weinberg AD, Hollingsworth KW, Flannery MR, Oz MC, Naka Y. Effect of gender on bridging to transplantation and posttransplantation survival in patients with left ventricular assist devices. J Thorac Cardiovasc Surg. 2004;127:1193–1195. doi: 10.1016/s0022-5223(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 31.Boyle AJ, Russell SD, Teuteberg JJ, Slaughter MS, Moazami N, Pagani FD, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28:881–887. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 32.Brush S, Budge D, Alharethi R, McCormick AJ, MacPherson JE, Reid BB, et al. End-of-life decision making and implementation in recipients of a destination left ventricular assist device. J Heart Lung Transplant. 2010;29:1337–1341. doi: 10.1016/j.healun.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Whellan DJ, Cox M, Hernandez AF, Heidenreich PA, Curtis LH, Peterson ED, et al. Utilization of hospice and predicted mortality risk among older patients hospitalized with heart failure: findings from GWTG-HF. J Card Fail. 2012;18:471–477. doi: 10.1016/j.cardfail.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Olshansky B, Wood F, Hellkamp AS, Poole JE, Anderson J, Johnson GW, et al. Where patients with mild to moderate heart failure die: results from the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Am Heart J. 2007;153:1089–1094. doi: 10.1016/j.ahj.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 35.Whellan DJ, Goodlin SJ, Dickinson MG, Heidenreich PA, Jaenicke C, Stough WG, et al. End-of-life care in patients with heart failure. J Card Fail. 2014;20:121–134. doi: 10.1016/j.cardfail.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Chen-Scarabelli C, Saravolatz L, Hirsh B, Agrawal P, Scarabelli TM. Dilemmas in end-stage heart failure. J Geriatr Cardiol. 2015;12:57–65. doi: 10.11909/j.issn.1671-5411.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang JC, Ewald GA, Allen LA, Butler J, Westlake Canary CA, Colvin-Adams M, et al. Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee. J Card Fail. 2015;21:519–534. doi: 10.1016/j.cardfail.2015.04.013. [DOI] [PubMed] [Google Scholar]