Highlights
-
•
A wide range of studies have demonstrated that GPCRs sense membrane voltage.
-
•
MD simulations help illuminate the structure and dynamics of the GPCR voltage-sensor.
-
•
We discuss the implications of GPCR voltage-regulation for drug design.
Abstract
G-protein coupled receptors (GPCRs) form the largest class of membrane proteins in humans and the targets of most present drugs. Membrane potential is one of the defining characteristics of living cells. Recent work has shown that the membrane voltage, and changes thereof, modulates signal transduction and ligand binding in GPCRs. As it may allow differential signalling patterns depending on tissue, cell type, and the excitation status of excitable cells, GPCR voltage sensitivity could have important implications for their pharmacology. This review summarises recent experimental insights on GPCR voltage regulation and the role of molecular dynamics simulations in identifying the structural basis of GPCR voltage-sensing. We discuss the potential significance for drug design on GPCR targets from excitable and non-excitable cells.
Current Opinion in Pharmacology 2016, 30:44–50
This review comes from a themed issue on New technologies
Edited by Christofer S Tautermann and David E Gloriam
For a complete overview see the Issue and the Editorial
Available online 27th July 2016
http://dx.doi.org/10.1016/j.coph.2016.06.011
1471-4892/© 2016 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Introduction
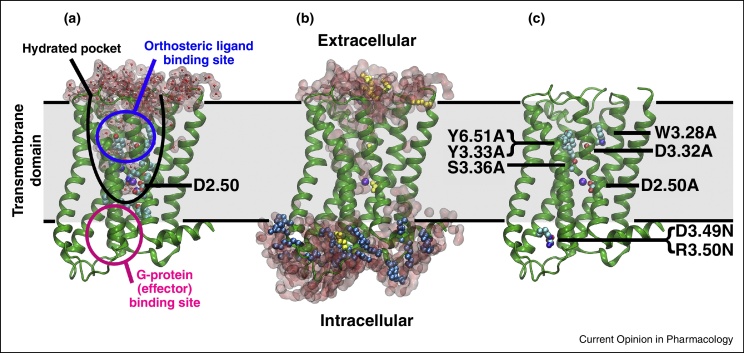
Membrane proteins form important interfaces mediating the exchange of matter and information between the cell and the external world. They are encoded by about 26% of the human genome [1] and represent a majority both of present as well as potential future drug targets [2]. G-protein coupled receptors (GPCRs) constitute the largest superfamily of membrane proteins in humans with more than 800 members [3]. They transmit binding information of a broad spectrum of extracellular ligands into a range of signalling pathways in the cell [4]. As a consequence, they play a paramount role in therapeutic intervention and are targeted by ∼30% of all presently marketed drugs [5]. Structurally, GPCRs form a bundle of seven transmembrane (TM) helices, which shape a ligand binding site on the extracellular face and an effector binding site on the intracellular side. Within the transmembrane domain, a conserved pocket, which is lined by polar residues and filled with water molecules [6] and a Na+ ion [7, 8••, 9••] extends from the ligand binding site towards the effector binding site and almost completely bridges these regions (Figure 1a).
Figure 1.
The structural features of class A GPCRs as exemplified by the M2 muscarinic receptor. (a) The major structural characteristics of class A GPCRs comprise seven transmembrane helices (green), an extracellular ligand binding site (blue circle), an internal hydrated pocket (black), and the intracellular effector protein interaction site (magenta). As both the polar hydrated pocket (water shown in red) and a Na+ (purple) ion binding to the charged residue D2.50 are conserved amongst class A GPCRs [9••], these features are highlighted. The locations of Na+ and water in the M2 receptor were inferred from MD simulations [10••], however the Na+ binding site is identical to that observed in crystal structures of other receptors [7, 8••]. (b) Distribution of charged residues within the M2 receptor (blue: positive; yellow: negative). Most residues, with the exception of three aspartates (D2.50, D3.32, and D3.26) are located outside of the direct influence of the membrane voltage. (c) All M2 receptor residues that were mutated in Ref [11] to probe the origin of voltage-sensing are shown in cyan. Mutation of these residues was demonstrated to have little or no effect upon gating charges with the exception of D2.50A [11].
Although the complete mechanism of signal transduction linking ligand binding to activation of the intracellular effector proteins is not yet fully understood, essential elements of this mechanism have been established. There is, for example, ample evidence for conformational changes in the TM domain of the receptors induced by extracellular ligand binding [12]. The changes propagate towards the intracellular side and facilitate the binding of effector proteins, which include a variety of G-proteins and β-arrestins [13]. In the G-protein-dependent signal transduction pathways, ligand binding on the extracellular side leads to the exchange of the nucleotide GDP by GTP in the bound effector G-protein complex. Nucleotide exchange triggers complex dissociation, and the activated G-protein components then transmit the signal to targets residing on the intracellular side [13].
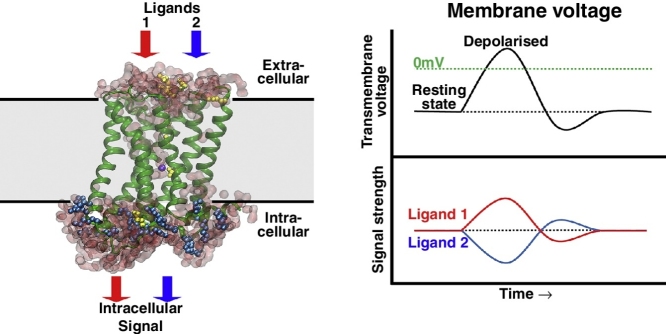
All plasma membranes exhibit a transmembrane potential difference or voltage (Vm), generated by electrochemical ion gradients across the bilayer [14]. Like all membrane proteins, GPCRs are therefore located in an environment in which strong electric fields of up to 107–108 V/m exist, as the physiologically relevant voltage gradients drop across the thin hydrophobic core of the membrane, which does not exceed dimensions of ∼3 nm along the membrane normal [15]. Electrically non-excitable cells maintain a resting voltage, which is negative on the intracellular side and undergoes slow oscillations during the cell cycle [14]. In electrically excitable cells — for example, neurons and muscle cells — the coordinated function of voltage-gated ion channels generates action potentials, in which the negative resting voltage displays rapid excursions towards positive values (termed depolarisation). Thus Vm typically adopts values between −90 and +50 mV; however, Vm can reach physiological levels of up to 150 mV, as demonstrated by hair cells in the inner ear [16].
The rapid Vm oscillations typical for action potentials are known to influence the conformation and function of some membrane proteins, an effect that is best understood for voltage-gated ion channels. These channel proteins contain specialised voltage-sensing domains, which are capable of inducing large-scale conformational transitions that gate the channels open or closed, even under small changes of Vm [17]. By contrast, voltage-related effects on other membrane proteins such as GPCRs seem less intuitive, although a number of studies have reported compelling evidence for a broad range of Vm-induced phenomena in GPCRs [11, 18••, 19, 20, 21, 22•] (for review see Ref [23]). Many important class A GPCR drug targets are expressed in excitable tissue, for instance the aminergic, opioid, adrenergic and purinergic receptors. Other important excitable tissue GPCRs include the class C metabotropic glutamate receptors, for instance in brain, for which voltage-induced effects have also been reported [24]. Class C GPCRs also have an extended allosteric pocket inside their transmembrane domain, as shown by recent crystal structures [25]. Currently, GPCR voltage regulation has been best characterised for class A GPCRs however, and therefore the focus of this review will be placed on this group. Because of their expression in excitable cells, effects related to Vm, and thus the excitation state of the cell, could have an important impact on the function of GPCRs and affect drug action on the receptors. Similarly, slower changes of Vm which have been reported to occur during the cell cycle could play a role in receptor-based signal transduction [14]. The aim of this review is therefore to summarise recent insights on the regulation of GPCRs by Vm, discuss its relevance for drug discovery, and highlight the important role of molecular dynamics (MD) simulations in deciphering the dynamic mechanisms of GPCR voltage sensing and their link to GPCR function.
Experimental evidence for voltage-induced effects in GPCRs
In recent years, Vm has been experimentally demonstrated to affect the conformation, function and transmitted signals of a range of GPCRs [26, 23, 19, 27, 18••]. Voltage-related effects have, for instance, been reported for the muscarinic, adrenergic, and purinergic receptor families [20, 18••, 19]. In most of the earlier work, evidence for voltage regulation was obtained indirectly, and measurements often relied on ionic current through downstream G-protein coupled inward rectifying potassium channels (GIRK) [28] or the use of intracellular calcium-sensitive dyes [26]. Voltage-induced conformational changes in GPCRs have recently also been confirmed directly by FRET-based reporters [18••]. Through both GIRK and FRET measurements, it has been shown that voltage can have opposite effects on the transmitted signal induced by agonist action on the receptors [11, 18••, 22•]. For example, the GIRK current elicited by acetylcholine binding to M2 receptors in rabbit or feline atrial myocytes is reduced by depolarisation, while that caused by the agonist pilocarpine is strongly enhanced [11, 22•].
The most quantitative measure of voltage-induced rearrangements in GPCRs are electrophysiological recordings, through which gating currents have been determined for several receptor types (Table 1). These transient currents reveal movements of charged regions in membrane proteins, which occur in response to voltage changes. Their name stems from their first observation, caused by the motion of Na+ channel voltage sensing domains during the process of channel gating [29]. The electric charge that resides on these voltage sensing domains, usually carried by charged amino acid side-chains, is multiplied by the fraction of the electric field they traverse upon channel gating to give the so-called gating charge. The gating charge can be derived from the gating currents and is expressed in terms of the elementary charge unit [30]. For instance, a singly charged particle moving across 50% of the voltage drop across the membrane would give rise to a gating charge of 0.5e.
Table 1.
Measured and calculated gating charges of class A GPCRs.
| Receptor | Gating charge (e) | Reporter method | Refs |
|---|---|---|---|
| m1 muscarinic: | |||
| wt | 0.72, 0.76a | FRET | [18••] |
| m2 muscarinic: | |||
| wt | 0.55 | Electrophysiology | [11] |
| wt | 0.53 (Na+) | MD simulation | [10••] |
| wt | 0.52 (proton) | MD simulation | [10••] |
| wt | 0.7, 0.85 | Electrophysiology | [32] |
| D692.50A | NR | Electrophysiology | [11] |
| W993.28A | 0.8 | Electrophysiology | [11] |
| D1033.32A | 0.5 | Electrophysiology | [11] |
| Y1043.33A | 0.54 | Electrophysiology | [11] |
| S1073.36A | 0.49 | Electrophysiology | [11] |
| D1203.49N | 0.66 | Electrophysiology | [32] |
| D1203.49N-R1203.50N | 0.52 | Electrophysiology | [11] |
| D1203.49N-R1203.50N | NR | Electrophysiology | [32] |
| Y4036.51A | 0.57 | Electrophysiology | [11] |
| α2A-adrenergic | |||
| wt | 0.5 | FRET | [19] |
| δ-opioid: | |||
| wt | 0.42 (Na+) | MD simulation | [10••] |
| N1313.35V | 0.63 (Na+) | MD simulation | [10••] |
NR, not resolved.
Precise value depends on methodology used.
Gating currents in GPCRs were first recorded for the wild-type (wt) M2 muscarinic receptor (M2 receptor) and the M2 receptor single-mutant (D1203.49N)a by cut-open oocyte electrophysiology. In these experiments, gating charges between 0.66 and 0.85e were inferred from the observed voltage dependence of the measured gating current (Table 1) [32]. In a more recent study, a gating charge of 0.55e on the wt M2 receptor was obtained by using the same technique (Table 1 and Figure 1) [11]. Interestingly, a wide range of mutants in which residues of particular interest were modified, including putative ligand binding contacts and conserved charged groups, did neither abolish the recorded gating currents nor markedly alter the observed gating charges [11]. The most prominent exception was the fully conserved residue D692.50 (Figure 1c), which has been identified as the main Na+ interacting residue in class A GPCRs [9••]. However, it was not clear if this finding, which was obtained before high-resolution crystal structures revealed ion binding in the TM section of GPCRs, resulted from lower surface expression of the mutant or was caused by the mutation itself [11].
Recently, it has been demonstrated by a combination of voltage-clamp and FRET experiments that both G-protein and β-arrestin signalling is strongly modulated by Vm in the muscarinic receptor family [18••]. The authors also studied the interplay between ligand action and voltage-induced effects. For instance, they showed that the effect of depolarisation on the transduced signal caused by the agonist carbachol in M3 receptors was inverted by a single mutation (N6.52Q) within the orthosteric ligand binding site, thereby demonstrating an interaction between the voltage sensor and the ligand binding site. The authors propose that the inversion in voltage sensitivity is due to a changed binding pose of the ligand [18••]. Notably, the magnitude of the voltage effect on the signal can be similar to the size of the ligand-induced signal such as in the case of acetylcholine acting upon the M1 receptor, as determined by FRET assays probing the arrestin3 signal under depolarisation [18••].
Role of MD simulations in deciphering the structural basis of GPCR voltage-sensing
Most GPCR structures so far have been resolved by X-ray crystallography (for review, see [33•, 34•]). To date, however, it has not yet been possible to experimentally determine membrane protein structures in the presence of a realistic transmembrane voltage. This also currently precludes the direct structural investigation of conformational changes triggered by altered Vm.
Present atomistic simulation techniques are commonly capable of modelling membrane proteins in model lipid bilayers over microsecond time spans, allowing MD studies to address many aspects of GPCR function in mechanistic detail. MD simulations have, for instance, been successfully used to shed light on the conformational transition towards the activated receptor state, the role of so-called micro-switches such as the DRY motif (ionic lock), receptor G-protein coupling specificity, nucleotide exchange in the effector complex, internal hydration of the polar pocket, and the processes of ligand attraction and binding [35, 12, 36, 37••, 13]. Voltages across the membrane can be readily included in the MD simulations, either by applying an external electric field 38, 39 or, similar to cells, by imposing TM electrochemical ion gradients 40, 41, as for instance implemented in the Computational Electrophysiology (CompEL) protocol [42•].
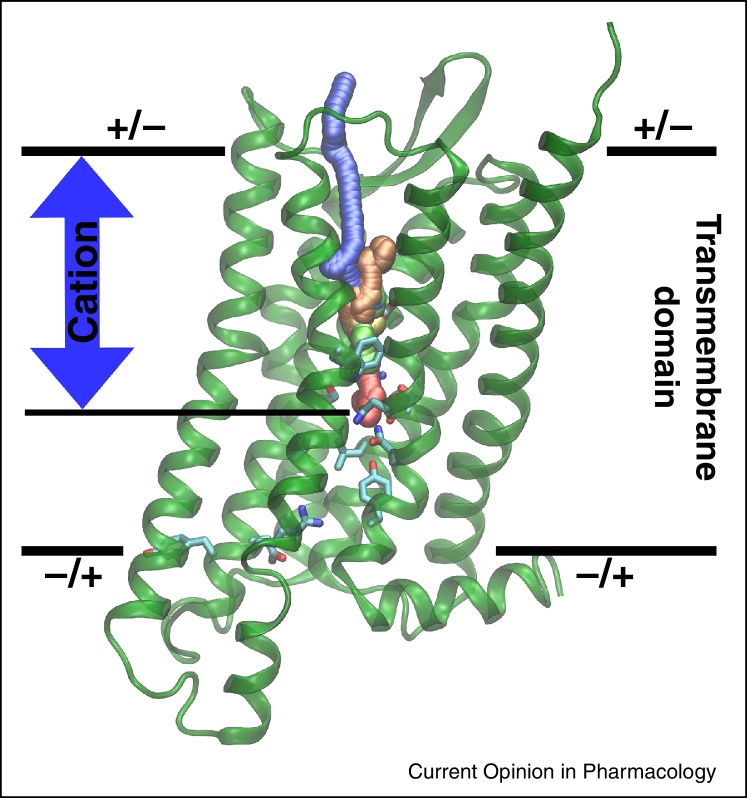
Recently, voltage-induced conformational changes and the observation of GPCR gating currents have been addressed by using MD simulations. First, a range of supra-physiological Vm were probed by CompEL simulations, followed by a further characterisation of the effects of physiological Vm by free energy calculations. The simulations showed that, in accordance with mutation experiments (Figure 1b,c) [11], none of the charged groups within or near the transmembrane region display substantial voltage-induced motions on the simulation timescales [10••]. By contrast, extensive voltage-induced movements of the Na+ ion, which binds internally in class A GPCRs to the highly conserved residue D2.50 [9••], along the water-filled pocket were observed (Figure 2). The movement of this single charge is triggered by depolarised voltages, facilitated by the hydration level of the pocket, and occurs directly within the transmembrane section of the receptor.
Figure 2.
Structural and mechanistic basis of a potential GPCR voltage sensor as derived by MD simulations. Depolarised Vm drives outward migration of an internal cation bound near D2.50 towards the extracellular space, crossing the ligand binding pocket. The observed gating charges for this transition are in excellent agreement with experimental values. Upon repolarisation or hyperpolarisation, the cation is attracted back into the allosteric binding pocket. The trajectory of a cation under depolarisation is colour-coded according to the simulation time, proceeding from red to blue.
Na+ has been detected in a range of high-resolution crystal structures of GPCRs [8••, 43, 44, 45•]. Because of the conservation level of D2.50 and the polar pocket in general, it is assumed that Na+ binding to D2.50 is a general feature of class A GPCRs [9••]. In addition, Na+ is known to have an allosteric effect on the function of most GPCRs [9••].
The expected gating charge for the observed movement of a cation from D2.50 towards the extracellular entrance of the receptor ligand binding pocket was determined from these MD simulations, and lies in the region of ∼0.53–0.63e for Na+ in M2 receptor and δ-OR variants [10••] (see Table 1). Both the observation that ion movement is triggered by depolarisation of Vm and the magnitudes of the gating charges are thus in excellent agreement with the experiments. Moreover, previous MD studies on the δ-OR without applied voltage have demonstrated the internal Na+ ion to be mobile, and able to leave the receptor under the influence of an applied force [46]. Importantly, it has also been shown that small organic cations such as amiloride can replace Na+ in the pocket under low Na+ concentration, exerting an allosteric effect similar to Na+ [47••]. It is therefore possible that other cations can undergo analogous movements within the pocket upon depolarisation, depending on experimental conditions, and give rise to comparable gating charges [32]. This includes potential protonation changes of the side chain of D2.50, during which a proton could be exchanged with the external solution [10••] (Table 1).
Implications for GPCR physiology and pharmacology
Na+ plays a central role in GPCR function, shifting the equilibrium between active and inactive receptors, regulating agonist binding, and biasing downstream signals [9••, 8••]. Any voltage-dependence of the occupancy of the GPCR allosteric pocket with Na+ or its position within the receptors could therefore have major functional implications for signal transduction and signal bias. Alongside its potential structural basis, voltage-dependence of GPCR conformation or signalling has now been established for a range of GPCRs and deserves the attention of drug designers and pharmacologists alike. As shown by Rinne et al. [18••], voltage can either enhance or attenuate the transmitted agonist signal depending on the ligand and precise environment of the binding site. In addition, ligand binding affinity has been shown to be voltage-dependent [32].
Electrophysiological properties such as resting Vm vary substantially between different cell types. For example, neurons display a markedly shifted Vm in various brain regions and developmental stages [48]. The action of GPCR ligands is therefore likely to depend on the cellular context. In electrically excitable cells, the transduced GPCR signal could also be altered by the excitation state of the cell. It has for instance been demonstrated that GPCR voltage sensing modulates synaptic neurotransmission by reshaping the kinetics of voltage-dependent transmitter release on the millisecond timescale [49]. Therefore, it is conceivable that GPCRs can establish dynamic feedback routes, by which voltage information is transmitted back into a range of intracellular signals on both fast and slow timescales. Notably, recent cancer research has revealed that a range of malignant cell types possess a more depolarised resting voltage than quiescent cells [50•, 14, 51]. Although GPCRs have traditionally received less attention than other proteins as cancer drug targets, GPCRs are known to be involved in cancer initiation and progression [52•]. The role of GPCR voltage regulation has, to our best knowledge, however not yet been investigated in this context.
Similarly, it has recently been demonstrated that oncogenic signalling pathways are influenced by Vm through the redistribution of charged lipids in the inner leaflet of the plasma membrane [50•]. Because membrane lipids allosterically modulate GPCR activity [53•], Vm could thus also have an indirect impact on receptor signal transduction via an effect on lipid distribution. As we only begin to appreciate the importance of Vm in regulating membrane proteins either directly or indirectly, much further work is needed to fully understand the role of Vm in GPCR signalling and its implications for the drug design process, which could be wide-ranging.
Conclusions
Recent experimental and computational insights suggest that the membrane voltage has an important impact on GPCR pharmacology. In particular, MD simulations under voltage are able to characterise functionally important movements in GPCRs driven by potential differences. Further simulations would be useful to investigate the interplay of voltage-induced changes with ligand binding and signal transduction. The fact that GPCRs are voltage-sensitive, together with its possible structural underpinning, should be taken into consideration during drug development on GPCR targets, as especially in excitable cell GPCRs, voltage-sensing could be an important mechanism of feeding back voltage information into intracellular signal transduction pathways. Thereby, the signal that is actually induced by a ligand might depend on the excitation state of the cell, which would have important consequences for drug discovery on excitable tissue GPCRs. It should also be investigated if agonists can show variations in their effect on different cell types, including non-excitable cells, owing to a difference in resting Vm.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
This work was supported by the BBSRC [Training Grant BB/J013072/1] and the Scottish Universities’ Physics Alliance. We thank Vsevolod Katritch and Daniel Seeliger for fruitful discussions.
Footnotes
Superscripts refer to the Ballesteros–Weinstein generic residue numbering nomenclature [31].
References
- 1.Fagerberg L., Jonasson K., von Heijne G., Uhlén M., Berglund L. Prediction of the human membrane proteome. Proteomics. 2010;10:1141–1149. doi: 10.1002/pmic.200900258. [DOI] [PubMed] [Google Scholar]
- 2.Lagerström M.C., Schiöth H.B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 3.Fredriksson R., Lagerström M.C., Lundin L.-G., Schiöth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 4.Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nat Rev Mol Cell Biol. 2002;3:639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 5.Hopkins A.L., Groom C.R. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. arXiv:Hopkins2002. [DOI] [PubMed] [Google Scholar]
- 6.Pardo L., Deupi X., Dölker N., López-Rodríguez M.L., Campillo M. The role of internal water molecules in the structure and function of the rhodopsin family of G protein-coupled receptors. ChemBioChem. 2007 doi: 10.1002/cbic.200600429. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Chun E., Thompson A.A., Chubukov P., Xu F., Katritch V., Han G.W., Roth C.B., Heitman L.H., IJzerman A.P. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8••.Fenalti G., Giguere P.M., Katritch V., Huang X.-P., Thompson A.a., Cherezov V., Roth B.L., Stevens R.C. Molecular control of δ-opioid receptor signalling. Nature. 2014;506:191–196. doi: 10.1038/nature12944. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the presence of an allosteric sodium ion in the transmembrane domain of a 1.8 Å resolution crystal structure of the δ-OR.
- 9••.Katritch V., Fenalti G., Abola E.E., Roth B.L., Cherezov V., Stevens R.C. Allosteric sodium in class A GPCR signaling. Trends Biochem Sci. 2014 doi: 10.1016/j.tibs.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; This excellent review provides a comprehensive analysis on sodium binding to GPCRs and its effects.
- 10••.Vickery O.N., Machtens J.-P., Tamburrino G., Seeliger D., Zachariae U. Structural mechanisms of voltage sensing in G protein-coupled receptors. Structure. 2016;24:997–1007. doi: 10.1016/j.str.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; This molecular dynamics study reveals the role of a mobile cation as the potential voltage sensor in class A GPCRs.
- 11.Navarro-Polanco R.a., Moreno Galindo E.G., Ferrer-Villada T., Arias M., Rigby J.R., Sánchez-Chapula J.a., Tristani-Firouzi M. Conformational changes in the M2 muscarinic receptor induced by membrane voltage and agonist binding. J Physiol. 2011;589:1741–1753. doi: 10.1113/jphysiol.2010.204107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nygaard R., Zou Y., Dror R.O., Mildorf T.J., Arlow D.H., Manglik A., Pan A.C., Liu C.W., Fung J.J., Bokoch M.P., Thian F.S., Kobilka T.S., Shaw D.E., Mueller L., Prosser R.S., Kobilka B.K. The dynamic process of β2-adrenergic receptor activation. Cell. 2013 doi: 10.1016/j.cell.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Audet M., Bouvier M. Restructuring G-protein-coupled receptor activation. Cell. 2012;151:14–23. doi: 10.1016/j.cell.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Yang M., Brackenbury W.J. Membrane potential and cancer progression. Front Physiol. 2013 doi: 10.3389/fphys.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ridgway N., McLeod R. Elsevier; 2015. Biochemistry of Lipids, Lipoproteins and Membranes. [Google Scholar]
- 16.Kandel E.R., Schwartz J.H., Jessell T.M. vol 4. McGraw-Hill; New York: 2000. (Principles of Neural Science). [Google Scholar]
- 17.Catterall W.A. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Rinne A., Mobarec J.C., Mahaut-Smith M., Kolb P., Bunemann M. The mode of agonist binding to a G protein-coupled receptor switches the effect that voltage changes have on signaling. Science Signal. 2015;8:ra110. doi: 10.1126/scisignal.aac7419. [DOI] [PubMed] [Google Scholar]; This paper uses FRET to analyse the effects of Vm on GPCRs, highlighting the differential effects of voltage on the action of a range of agonists on GPCR signalling.
- 19.Rinne A., Birk A., Bünemann M. Voltage regulates adrenergic receptor function. Proc Natl Acad Sci U S A. 2013;110:1536–1541. doi: 10.1073/pnas.1212656110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurung I.S., Martinez-Pinna J., Mahaut-Smith M.P. Novel consequences of voltage-dependence to G-protein-coupled P2Y1 receptors. Br J Pharmacol. 2008;154:882–889. doi: 10.1038/bjp.2008.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parnas H., Parnas I. The chemical synapse goes electric: Ca2+- and voltage-sensitive GPCRs control neurotransmitter release. Trends Neurosci. 2007;30:54–61. doi: 10.1016/j.tins.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 22•.Moreno-Galindo E.G., Alamilla J., Sanchez-Chapula J.A., Tristani-Firouzi M., Navarro-Polanco R.A. The agonist-specific voltage dependence of m2 muscarinic receptors modulates the deactivation of the acetylcholine-gated K+ current (IKACh) Pflügers Archiv Eur J Physiol. 2016:1–8. doi: 10.1007/s00424-016-1812-y. [DOI] [PubMed] [Google Scholar]; A recent paper showing the effects of voltage on the M2 receptor-induced GIRK current.
- 23.Mahaut-Smith M.P., Martinez-Pinna J., Gurung I.S. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008 doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Ohana L., Barchad O., Parnas I., Parnas H. The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J Biol Chem. 2006;281:24204–24215. doi: 10.1074/jbc.M513447200. [DOI] [PubMed] [Google Scholar]
- 25.Doré A.S., Okrasa K., Patel J.C., Serrano-Vega M., Bennett K., Cooke R.M., Errey J.C., Jazayeri A., Khan S., Tehan B. Structure of class C GPCR metabotropic glutamate receptor 5 transmembrane domain. Nature. 2014;511:557–562. doi: 10.1038/nature13396. [DOI] [PubMed] [Google Scholar]
- 26.Martinez-Pinna J., Tolhurst G., Gurung I.S., Vandenberg J.I., Mahaut-Smith M.P. Sensitivity limits for voltage control of P2Y receptor-evoked Ca2+ mobilization in the rat megakaryocyte. J Physiol. 2004;555:61–70. doi: 10.1113/jphysiol.2003.056846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ben Chaim Y., Bochnik S., Parnas I., Parnas H. Voltage affects the dissociation rate constant of the m2 muscarinic receptor. PLoS ONE. 2013;8:e74354. doi: 10.1371/journal.pone.0074354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ben-Chaim Y., Tour O., Dascal N., Parnas I., Parnas H. The M2 muscarinic G-protein-coupled receptor is voltage-sensitive. J Biol Chem. 2003;278:22482–22491. doi: 10.1074/jbc.M301146200. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong C.M., Bezanilla F. Currents related to movement of the gating particles of the sodium channels. Nature. 1973;242:459–461. doi: 10.1038/242459a0. [DOI] [PubMed] [Google Scholar]
- 30.Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- 31.Ballesteros J.A., Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25:366–428. [Google Scholar]
- 32.Ben-Chaim Y., Chanda B., Dascal N., Bezanilla F., Parnas I., Parnas H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. doi: 10.1038/nature05259. [DOI] [PubMed] [Google Scholar]
- 33•.Shonberg J., Kling R.C., Gmeiner P., Löber S. GPCR crystal structures: Medicinal chemistry in the pocket. Bioorg Med Chem. 2015;23:3880–3906. doi: 10.1016/j.bmc.2014.12.034. [DOI] [PubMed] [Google Scholar]; An excellent review describing the structure of ligands and their binding to GPCRs.
- 34•.Ghosh E., Kumari P., Jaiman D., Shukla A.K. Methodological advances: the unsung heroes of the GPCR structural revolution. Nat Rev Mol Cell Biol. 2015;16:69–81. doi: 10.1038/nrm3933. [DOI] [PubMed] [Google Scholar]; This review summarises the advances made in GPCR crystallization in recent years.
- 35.Rose A.S., Elgeti M., Zachariae U., Grubmüller H., Hofmann K.P., Scheerer P., Hildebrand P.W. Position of transmembrane helix 6 determines receptor G protein coupling specificity. J Am Chem Soc. 2014;136:11244–11247. doi: 10.1021/ja5055109. [DOI] [PubMed] [Google Scholar]
- 36.Dror R.O., Arlow D.H., Borhani D.W., Jensen M.Ø., Piana S., Shaw D.E. Identification of two distinct inactive conformations of the β2-adrenergic receptor reconciles structural and biochemical observations. Proc Natl Acad Sci U S A. 2009;106:4689–4694. doi: 10.1073/pnas.0811065106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Yuan S., Filipek S., Palczewski K., Vogel H. Activation of G-protein-coupled receptors correlates with the formation of a continuous internal water pathway. Nat Commun. 2014;5:4733. doi: 10.1038/ncomms5733. [DOI] [PubMed] [Google Scholar]; This paper highlights the use of molecular dynamics to elucidate the increased internal hydration of GPCRs upon activation and proposes the opening of a continuous pore through the GPCR.
- 38.Aksimentiev A., Schulten K. Imaging α-hemolysin with molecular dynamics: ionic conductance, osmotic permeability, and the electrostatic potential map. Biophys J. 2005;88:3745–3761. doi: 10.1529/biophysj.104.058727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roux B. The membrane potential and its representation by a constant electric field in computer simulations. Biophys J. 2008;95:4205–4216. doi: 10.1529/biophysj.108.136499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sachs J.N., Crozier P.S., Woolf T.B. Atomistic simulations of biologically realistic transmembrane potential gradients. J Chem Phys. 2004;121:10847–10851. doi: 10.1063/1.1826056. [DOI] [PubMed] [Google Scholar]
- 41.Delemotte L., Dehez F., Treptow W., Tarek M. Modeling membranes under a transmembrane potential. J Phys Chem B. 2008;112:5547–5550. doi: 10.1021/jp710846y. [DOI] [PubMed] [Google Scholar]
- 42•.Kutzner C., Köpfer D., Machtens J.-P., de Groot B.L., Song C., Zachariae U. Insights into the function of ion channels by computational electrophysiology simulations. Biochim Biophys Acta Biomembr. 2016;1858:1741–1752. doi: 10.1016/j.bbamem.2016.02.006. [DOI] [PubMed] [Google Scholar]; This paper describes the uses of computational electrophysiology implemented in GROMACS to study membrane proteins under Vm.
- 43.Liu W., Chun E., Thompson A.a., Chubukov P., Xu F., Katritch V., Han G.W., Roth C.B., Heitman L.H., IJzerman A.P., Cherezov V., Stevens R.C. Structural basis for allosteric regulation of GPCRs by sodium ions. Science. 2012;337:232–236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C., Srinivasan Y., Arlow D.H., Fung J.J., Palmer D., Zheng Y., Green H.F., Pandey A., Dror R.O., Shaw D.E., Weis W.I., Coughlin S.R., Kobilka B.K. High-resolution crystal structure of human protease-activated receptor 1. Nature. 2012;492:387–392. doi: 10.1038/nature11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45•.Miller-Gallacher J.L., Nehme R., Warne T., Edwards P.C., Schertler G.F., Leslie A.G., Tate C.G. The 2.1 Å resolution structure of cyanopindolol-bound β1-adrenoceptor identifies an intramembrane Na+ ion that stabilises the ligand-free receptor. PLoS ONE. 2014;9:e92727. doi: 10.1371/journal.pone.0092727. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reports the presence of an allosteric sodium ion in a high-resolution crystal structure of the β-adrenoceptor.
- 46.Shang Y., LeRouzic V., Schneider S., Bisignano P., Pasternak G.W., Filizola M. Mechanistic insights into the allosteric modulation of opioid receptors by sodium ions. Biochemistry. 2014;53:5140–5149. doi: 10.1021/bi5006915. [DOI] [PMC free article] [PubMed] [Google Scholar]; A molecular dynamics paper characterising the high mobility of the allosteric sodium ion within opioid receptors.
- 47••.Massink A., Gutierrez-de Teran H., Lenselink E.B., Ortiz Zacarias N.V., Xia L., Heitman L.H., Katritch V., Stevens R.C., IJzerman A.P. Sodium ion binding pocket mutations and adenosine A2A receptor function. Mol Pharmacol. 2015;87:305–313. doi: 10.1124/mol.114.095737. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper combines experimental and molecular dynamics approaches to investigate the effects of mutations within the allosteric sodium binding pocket and shows that organic cations can bind at the same site.
- 48.Ramoa A.S., McCormick D.A. Developmental changes in electrophysiological properties of LGNd neurons during reorganization of retinogeniculate connections. J Neurosci. 1994;14:2089–2097. doi: 10.1523/JNEUROSCI.14-04-02089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kupchik Y.M., Barchad-Avitzur O., Wess J., Ben-Chaim Y., Parnas I., Parnas H. A novel fast mechanism for GPCR-mediated signal transduction-control of neurotransmitter release. J Cell Biol. 2011;192:137–151. doi: 10.1083/jcb.201007053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Zhou Y., Wong C.-O., Cho K.-j., van der Hoeven D., Liang H., Thakur D.P., Luo J., Babic M., Zinsmaier K.E., Zhu M.X., Hu H., Venkatachalam K., Hancock J.F. Membrane potential modulates plasma membrane phospholipid dynamics and K-Ras signaling. Science. 2015;349:873–876. doi: 10.1126/science.aaa5619. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports a link between membrane voltage, membrane lipid distribution and signal transduction on oncogenic pathways.
- 51.Arcangeli A., Bianchi L., Becchetti A., Faravelli L., Coronnello M., Mini E., Olivotto M., Wanke E. A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol. 1995;489(Pt 2):455. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Liu Y., An S., Ward R., Yang Y., Guo X.-X., Li W., Xu T.-R. G protein-coupled receptors as promising cancer targets. Cancer Lett. 2016;376:226–239. doi: 10.1016/j.canlet.2016.03.031. [DOI] [PubMed] [Google Scholar]; This letter highlights the roles of GPCRs in cancer, suggesting GPCRs are promising cancer targets.
- 53•.Dawaliby R., Trubbia C., Delporte C., Masureel M., Van Antwerpen P., Kobilka B.K., Govaerts C. Allosteric regulation of G protein-coupled receptor activity by phospholipids. Nat Chem Biol. 2015;12:35–39. doi: 10.1038/nchembio.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper demonstrates that GPCRs can be allosterically modulated by various phospholipids.