Abstract
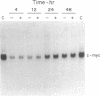
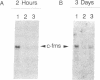
6-Thioguanine (6-TG)-induced differentiation of hypoxanthine phosphoribosyltransferase (IMP: pyrophosphate phosphoribosyltransferase, EC 2.4.2.8)-deficient HL-60 cells is characterized by 2 days of growth, after which morphological differentiation proceeds. Addition of the tRNA wobble base queuine, in the presence of 6-TG, maintains the proliferative capability of the cells. The ability of 6-TG to induce differentiation correlates with c-myc mRNA down-regulation, but queuine has no effect on this parameter. Treatment with 6-TG for 2-3 days commits HL-60 cells to granulocytic differentiation, and, once committed, these cells do not respond to the monocytic inducer phorbol 12-myristate 13-acetate. Nonetheless, when cells are treated with queuine and 6-TG, they maintain the promyelocytic morphology and are capable of being induced down the monocytic pathway by phorbol 12-myristate 13-acetate as indicated by stabilization of c-fms mRNA and cell adherence. In the absence of queuine, phorbol 12-myristate 13-acetate is incapable of inducing monocytic markers in the 6-TG-treated cells. The data presented indicate that 6-TG-induced differentiation of HL-60 cells is a tRNA-facilitated event and that the tRNA wobble base queuine is capable of maintaining both the proliferative and pluripotent potential of the cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodner A. J., Ting R. C., Gallo R. C. Induction of differentiation of human promyelocytic leukemia cells (HL-60) by nucleosides and methotrexate. J Natl Cancer Inst. 1981 Nov;67(5):1025–1030. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Bodner A., Ting R., Gallo R. C. Induction of morphological and functional differentiation of human promyelocytic leukemia cells (HL-60) by componuds which induce differentiation of murine leukemia cells. Int J Cancer. 1980 Feb 15;25(2):213–218. doi: 10.1002/ijc.2910250208. [DOI] [PubMed] [Google Scholar]
- Farkas W. R., Jacobson K. B., Katze J. R. Substrate and inhibitor specificity of tRNA-guanine ribosyltransferase. Biochim Biophys Acta. 1984 Feb 24;781(1-2):64–75. doi: 10.1016/0167-4781(84)90124-6. [DOI] [PubMed] [Google Scholar]
- Freytag S. O. Enforced expression of the c-myc oncogene inhibits cell differentiation by precluding entry into a distinct predifferentiation state in G0/G1. Mol Cell Biol. 1988 Apr;8(4):1614–1624. doi: 10.1128/mcb.8.4.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher R. E., Ferrari A. C., Zulich A. W., Yen R. W., Testa J. R. Cytotoxic and cytodifferentiative components of 6-thioguanine resistance in HL-60 cells containing acquired double minute chromosomes. Cancer Res. 1984 Jun;44(6):2642–2653. [PubMed] [Google Scholar]
- Gibboney D. S., French B. T., Patrick D. E., Trewyn R. W. 6-ethylmercaptopurine-mediated growth inhibition of HL-60 cells in vitro irrespective of purine salvage. Cancer Chemother Pharmacol. 1989;25(3):189–194. doi: 10.1007/BF00689581. [DOI] [PubMed] [Google Scholar]
- Gusella J. F., Housman D. Induction of erythroid differentiation in vitro by purines and purine analogues. Cell. 1976 Jun;8(2):263–269. doi: 10.1016/0092-8674(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro K., Schwartz E. L., Sartorelli A. C. Characterization of the metabolic forms of 6-thioguanine responsible for cytotoxicity and induction of differentiation of HL-60 acute promyelocytic leukemia cells. J Cell Physiol. 1984 Nov;121(2):383–390. doi: 10.1002/jcp.1041210216. [DOI] [PubMed] [Google Scholar]
- Katze J. R., Gündüz U., Smith D. L., Cheng C. S., McCloskey J. A. Evidence that the nucleic acid base queuine is incorporated intact into tRNA by animal cells. Biochemistry. 1984 Mar 13;23(6):1171–1176. doi: 10.1021/bi00301a022. [DOI] [PubMed] [Google Scholar]
- Kretz K. A., Katze J. R., Trewyn R. W. Guanine analog-induced differentiation of human promyelocytic leukemia cells and changes in queuine modification of tRNA. Mol Cell Biol. 1987 Oct;7(10):3613–3619. doi: 10.1128/mcb.7.10.3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralidhar G., Ochieng J., Trewyn R. W. Altered queuine modification of transfer RNA involved in the in vitro transformation of Chinese hamster embryo cells. Cancer Res. 1989 Dec 15;49(24 Pt 1):7110–7114. [PubMed] [Google Scholar]
- Muralidhar G., Utz E. D., Elliott M. S., Katze J. R., Trewyn R. W. Identifying inhibitors of queuine modification of tRNA in cultured cells. Anal Biochem. 1988 Jun;171(2):346–351. doi: 10.1016/0003-2697(88)90496-4. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Structure, biosynthesis, and function of queuosine in transfer RNA. Prog Nucleic Acid Res Mol Biol. 1983;28:49–73. doi: 10.1016/s0079-6603(08)60082-3. [DOI] [PubMed] [Google Scholar]
- Pegoraro L., Abrahm J., Cooper R. A., Levis A., Lange B., Meo P., Rovera G. Differentiation of human leukemias in response to 12-0-tetradecanoylphorbol-13-acetate in vitro. Blood. 1980 May;55(5):859–862. [PubMed] [Google Scholar]
- Schwartz E. L., Blair O. C., Sartorelli A. C. Cell cycle events associated with the termination of proliferation by cytotoxic and differentiation-inducing actions of 6-thioguanine on HL-60 cells. Cancer Res. 1984 Sep;44(9):3907–3910. [PubMed] [Google Scholar]
- Shindo-Okada N., Okada N., Ohgi T., Goto T., Nishimura S. Transfer ribonucleic acid guanine transglycosylase isolated from rat liver. Biochemistry. 1980 Jan 22;19(2):395–400. doi: 10.1021/bi00543a023. [DOI] [PubMed] [Google Scholar]
- Weber B., Horiguchi J., Luebbers R., Sherman M., Kufe D. Posttranscriptional stabilization of c-fms mRNA by a labile protein during human monocytic differentiation. Mol Cell Biol. 1989 Feb;9(2):769–775. doi: 10.1128/mcb.9.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]