Abstract
Background
Phosphate binders, such as lanthanum carbonate, control elevated serum-phosphate levels in patients with end-stage renal disease (ESRD). Lanthanum carbonate is available in oral powder and tablet form. The aim of this survey was to investigate satisfaction with, preference for, and adherence to lanthanum carbonate oral powder in patients with ESRD.
Scope
Patients from France and Spain who had been taking lanthanum carbonate powder for at least 4 weeks, and who had experience of other phosphate binders of any formulation, were asked to complete an online or telephone survey. Treatment satisfaction was measured using the Treatment Satisfaction Questionnaire for Medication-9; preference was measured using 5-point Likert scale agreement ratings; and adherence was measured using the Morisky Medication Adherence Scale-4. Data were evaluated using bivariate analyses.
Findings
Overall, 160 patients participated (80 per country). Lanthanum carbonate powder was reported to have a higher effectiveness rating (p<0.05), be more convenient (p<0.05), and provide a higher level of satisfaction (p<0.01) than previous binders. There was an overall preference for lanthanum carbonate powder over previous binders of any formulation (p<0.001). Adherence to medication was similar for all binders analysed: 66.3% of French patients adhered to lanthanum carbonate powder, and 65.0% adhered to previous binder treatment (p=not significant); 52.5% of Spanish patients adhered to lanthanum carbonate powder, and 56.3% adhered to previous binder treatment (p=not significant).
Limitations
The survey enrolled patients who had already experienced phosphate binders before the study began. Information on patient preferences for and adherence to previous phosphate binders was therefore based on the patients’ memories of these experiences, which may have been subject to change over time. Although most participants completed the online survey in this study, a telephone survey was used for individuals who could not access the online version; if only one method of data recording had been used, there may have been reduced variation in responses.
Conclusion
Patients with ESRD report increased satisfaction with and preference for lanthanum carbonate powder over other formulations, suggesting that lanthanum carbonate powder is more convenient and easier to use than other formulations.
Keywords: chronic kidney failure, chronic renal insufficiency, lanthanum carbonate, medication adherence, patient preference, personal satisfaction, powders, phosphates
Introduction
Patients with end-stage renal disease (ESRD) have severely diminished kidney function; this may be associated with elevated serum-phosphate levels [1,2]. The Dialysis Outcomes and Practice Patterns Study, a 12-country observational study of patients receiving haemodialysis, reported that between half and two-thirds of these patients have hyperphosphataemia [3]. Elevated serum-phosphate levels may in turn be associated with hyperparathyroidism [4], vascular calcification [5,6], and mineral bone disorder [6,7]. Furthermore, hyperphosphataemia is an independent predictor of mortality [8–10]. The increased risk of cardiac death from elevated phosphate levels is particularly noteworthy: cardiovascular events are the primary cause of death in patients receiving dialysis, accounting for nearly half of all such deaths [11,12]. This highlights the need for proper management of elevated serum-phosphate levels in patients with ESRD [13,14].
Recommendations in clinical-practice guidelines for chronic kidney disease suggest that patients with ESRD should aim for a target serum-phosphate range of 3.5–5.5 mg/dL [15,16]. Dietary changes and dialysis are usually insufficient to maintain phosphate within the recommended range. Oral phosphate-binding agents are therefore also recommended to manage serum-phosphate levels [16]. Phosphate binders include calcium-based (calcium acetate, calcium acetate/magnesium carbonate combination, and calcium carbonate) as well as calcium-free (lanthanum carbonate, sevelamer carbonate, sevelamer hydrochloride, and the iron-based binders ferric citrate and sucroferric oxyhydroxide) binders [17].
Lanthanum carbonate (FOSRENOL® [lanthanum carbonate], Shire Pharmaceutical Contracts Ltd, Basingstoke, UK) in tablet form has been shown to be effective in treating hyperphosphataemia in patients with ESRD [18,19]. An oral powder formulation of lanthanum carbonate has also been approved in Europe. The tablet and powder formulations of lanthanum carbonate have been shown to be pharmacodynamically equivalent [20]; therefore, it is expected that the level of phosphate control would be the same regardless of formulation.
Non-adherence to phosphate binders is a recognized issue [17]. High tablet burden has been shown to correlate with low levels of adherence [12]. Furthermore, there may be no noticeable impact on symptoms in patients adhering to the complex treatment regimens, which may make them less inclined to adhere to treatment. Therefore, a better understanding of patient preferences is needed. It is possible that powder formulations may be preferred by patients to other formulations (e.g. tablets), and could potentially facilitate greater adherence to medication and better patient outcomes. No study to date has examined patient preferences and treatment satisfaction with lanthanum carbonate powder, either alone or in comparison with other tablet or powder phosphate binders. The aim of the present study was to investigate the levels of satisfaction with, preference for, and adherence to lanthanum carbonate powder compared with other phosphate binders of any formulation, in French and Spanish patients with ESRD.
Methods
Study design
This study was a questionnaire-based survey completed in France and Spain from 1 January to 31 March 2014. Patients with ESRD who met the study-inclusion criteria (see below) were identified and contacted by local physicians. All patients provided informed consent. All procedures followed were in accordance with the Helsinki Declaration of 1964, as revised in 2013. The study was granted approval by the relevant ethics committee in Spain (Comité Ético de Investigación Clínica del Área de Salud de Valladolid Este, Facultad de Medicina, C/Ramón y Cajal 7, 47005 Valladolid, Spain). In France, a local review was not required because the French institutional review board (Comité Consultatif sur le Traitement de l’Information en Matière de Recherche dans le Domaine de la Santé) considered this study to be market research, which does not require approval. Nominative patient data were not collected in this study, eliminating the need for approval from the French data-protection agency (Commission Nationale de l’Informatique et des Libertés). A mixed online and telephone approach was used because the number of patients taking lanthanum carbonate powder was potentially small, owing to the short time period since its launch on the market (France, June 2013; Spain, January 2013), and because of the desire to maximize the number of qualifying patients participating in the survey. For patients who could not complete the survey online, a computer-assisted telephone-interviewing system was used in which the respondent completed the survey over the telephone with the help of an interviewer.
Patients
Shire Pharmaceutical Contracts Ltd, Basingstoke, UK, provided lists of physicians to be contacted to obtain their assistance in recruiting patients with ESRD who met the study-inclusion criteria. Some of these physicians were recruited via the contacts of the Kantar Health network of interviewers. After giving oral consent to participate, physicians were briefed about the study details over the telephone. A total of 160 patients were planned to be recruited, based on the number of physicians expected to participate and the number of patients expected to be recruited per physician. Patients were recruited with the assistance of the participating physicians as soon as the physicians had been briefed.
The inclusion criteria were: ability to read and write the native-country language; diagnosed with ESRD; currently receiving haemodialysis; currently taking lanthanum carbonate powder for at least 4 weeks; and experience of taking another phosphate-binder medication. These inclusion criteria were provided to physicians during their briefing, and each patient confirmed that they met these criteria upon participation. All information was self-reported by the patients. Physicians provided patients with the appropriate interviewer and agency contact information. Patients then telephoned or emailed the professional interviewer through the agency Kantar Health (France) or a subcontractor (Spain) to complete the survey. In France, patients who completed the survey received a low-value gift via their physician. The value of the gift was based on the amount of time required to participate in the study (a 15–25-min survey). In Spain, patients could not be rewarded in order to comply with national ethical requirements. Payments to physicians in France and Spain were adjusted according to physicians’ incomes in the two countries, and were based on the amount of time required to recruit each patient (estimated at 30–45 min).
Questionnaire
The online questionnaire was designed to take approximately 15 min to complete, while telephone-based interviews lasted 5–10 min longer. The questionnaire covered patient demographics, medical history, patient satisfaction with medication, reasons for switching from a previous phosphate binder to lanthanum carbonate powder, preference for lanthanum carbonate powder compared with previous phosphate-binder medication, and adherence to medication. The key outcomes of interest were satisfaction, preference, and adherence.
Treatment satisfaction was measured using the validated Treatment Satisfaction Questionnaire for Medication (TSQM)-9 for both current and previous medication [21,22]. The TSQM-9 uses a self-reported scale assessing patients’ satisfaction with medication, providing scores on three scales: patient-reported effectiveness, convenience, and global satisfaction. These are self-reported measures; patient-reported effectiveness may, therefore, not reflect an actual increase in the effectiveness of lanthanum carbonate to treat hyperphosphataemia. The TSQM-9 comprises nine items, each scored on a 7-point Likert scale (1=most negative; 7=most positive). Overall TSQM-9 scores range from 0 (lowest satisfaction) to 100 (highest satisfaction). A patient’s level of satisfaction with their treatment can impact the patient’s health-related decisions and treatment-related behaviours, which will affect the success of treatment outcomes [21]. The nine items outlined in the TSQM-9 provide a breakdown of the overall definition of satisfaction in this context.
Questions on treatment preference used 5-point Likert scale agreement ratings (1=strong preference for previous phosphate binder; 5=strong preference for lanthanum carbonate powder). Preference was defined by 11 items addressing a number of factors, including the ease of taking the treatment with meals, the impact of the treatment on the taste, flavour, or enjoyment of meals, and practicalities associated with taking the treatment.
Adherence to medication was measured using the validated Morisky Medication Adherence Scale (MMAS)-4, which is a generic self-reported, medication-taking behaviour questionnaire [23,24]. It consists of four items (questions labelled 1–4) with a scoring of yes=1 and no=0. The scores for the four items are summed to give a score of 0–4, ranging from high adherence to low adherence. This score can be recoded in three adherence levels (high adherence=score of 0; medium adherence=score of 1 or 2; low adherence=score of 3 or 4). Alternatively, the score can be recoded in a binary adherence variable (adherent=score of 0; non-adherent=score of 1–4), to determine the percentages of adherent and non-adherent patients.
The complete questionnaire was produced in English and then translated by a bilingual native speaker into French or Spanish, depending on the language in question. Subsequently, a review and approval of the initial translation was undertaken by a second bilingual native speaker.
Statistical analyses
The analyses were conducted using the statistical software Stata®, version 13.1 (StataCorp, College Station, TX, USA).
Descriptive statistics (means and standard deviations [SDs] for continuous variables; frequencies and percentages for categorical variables) were performed on the overall sample (France and Spain together) and within each country.
Bivariate analyses were performed on the overall sample and within each country. Patient satisfaction with, preference for, and adherence to lanthanum carbonate powder at the time of the survey versus the phosphate binder that the patient was using prior to the survey (all classes of phosphate binder apart from lanthanum carbonate powder) were compared. Differences between categorical variables were examined using McNemar’s χ2 tests, and differences between continuous variables were examined using paired-sample t-tests. Single-sample t-tests of chewing, swallowing, and meal-enjoyment measures tested whether patients’ opinions of lanthanum carbonate powder and a previous phosphate binder were significantly different from the midpoint (a score of 3, which represents indifference between past and current treatment).
Results
Patient demographics
A total of 160 patients were recruited for the study, 80 from each country (France and Spain). None of the patients who were contacted refused to take part in the study or asked for their data to be removed once they had completed the questionnaire. Patient demographics are presented in Table 1 and were similar in both countries. The most notable difference was that 63.8% of French patients but only 45.0% of Spanish patients never ate ready-to-use food (Table 1).
Table 1.
Patient demographics.
| France (n=80) | Spain (n=80) | Total (N=160) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| n | Value | n | Value | n | Value | |
| Median age, years (range) | 80 | 63.0 (19–90) | 80 | 66.0 (29–87) | 160 | 63.5 (19–90) |
|
| ||||||
| Sex | ||||||
| Male | 54 | 67.5% | 48 | 60.0% | 102 | 63.8% |
| Female | 26 | 32.5% | 32 | 40.0% | 58 | 36.3% |
|
| ||||||
| Living | ||||||
| With others | 63 | 78.8% | 69 | 86.3% | 132 | 82.5% |
| Alone | 17 | 21.3% | 11 | 13.8% | 28 | 17.5% |
|
| ||||||
| Eat ready-to-use food | ||||||
| Never | 51 | 63.8% | 36 | 45.0% | 87 | 54.4% |
| 1–2 times per week | 24 | 30.0% | 33 | 41.3% | 57 | 35.6% |
| 3–6 times per week | 4 | 5.0% | 9 | 11.3% | 13 | 8.1% |
| ≥7 times per week | 1 | 1.3% | 2 | 2.5% | 3 | 1.9% |
Note: values have been rounded up or down to the nearest decimal point.
Patient history of taking phosphate-binder medication and reasons for switching are presented in Table 2. Overall, at the time of survey completion, patients in France and Spain had been receiving haemodialysis for an average of 43.3 months (SD, 45.6; Table 2), and had been taking a phosphate binder (including lanthanum carbonate powder) for an average of 35.4 months (SD, 48.9; Table 2). French patients had been taking lanthanum carbonate powder for an average of 3.2 months (SD, 2.6), while Spanish patients had been receiving this medication for 7.9 months (SD, 5.9; Table 2). Overall, patients reported taking an average of 2.4 (SD, 0.8) sachets per day of lanthanum carbonate powder. The majority of patients (60.0%) reported taking sachets at a dose of 1000 mg per sachet, compared with only 40.0% of patients using 750 mg sachet doses. In France and Spain, patients reported taking on average a similar number of sachets of lanthanum carbonate powder per day (France, 2.3; Spain, 2.5); however, the majority of French patients were taking these at the higher dose of 1000 mg (76.3%), while only 43.8% of Spanish patients were taking this dose (Table 2). Overall, 33.8% of patients combined lanthanum carbonate powder with another phosphate binder of any formulation (other phosphate binders taken at the time of the survey are described in Table 2). There was some variation between the two countries in this (France, 16.3%; Spain, 51.3%; Table 2). Patients took an average of 9.7 tablets, pills, capsules, and/or sachets per day when combining all phosphate binders (France, 10.3; Spain, 9.1; Table 2). The most common phosphate binder taken prior to the time of the survey was lanthanum carbonate in tablet form (53.8% in both France and Spain). Overall, 46.7% of these patients reported having crushed the tablets; however, there was some variation between the countries as only 22.7% of French patients but 69.6% of Spanish patients crushed their tablets (Table 2). Medical reasons were most commonly cited to explain why patients switched from a previous phosphate binder to lanthanum carbonate (reported by 45.0% of French patients; 75.0% of Spanish patients; 60.0% of all patients) (Table 2). Problems with chewing previous binders (reported by 16.3% of French patients; 23.8% of Spanish patients; 20.0% of all patients), swallowing previous binders (reported by 15.0% of French patients; 11.3% of Spanish patients; 13.1% of all patients), or wanting to take fewer tablets, pills, or capsules (reported by 7.5% of French patients; 13.8% of Spanish patients; 10.6% of all patients) were also frequently reported as reasons for switching medications.
Table 2.
Patient history of taking phosphate-binder medication.
| France (n=80) | Spain (n=80) | Total (N=160) | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | |
| Time (months) receivinga | ||||||
| Haemodialysis | 40.52 | (45.43) | 46.13 | (45.83) | 43.33 | (45.58) |
| Phosphate binder | 34.05 | (51.33) | 36.80 | (46.67) | 35.42 | (48.92) |
| Lanthanum carbonate powder | 3.15 | (2.62) | 7.88 | (5.87) | 5.52 | (5.11) |
|
| ||||||
| Lanthanum carbonate powdera | ||||||
| Number of sachets per day | 2.25 | (0.86) | 2.50 | (0.78) | 2.38 | (0.83) |
|
| ||||||
| All phosphate binders combineda | ||||||
| Number of tablets, pills, capsules, and/or sachets per day | 10.26 | (4.66) | 9.14 | (6.79) | 9.70 | (5.83) |
|
| ||||||
| n | (%) | n | (%) | n | (%) | |
|
| ||||||
| Lanthanum carbonate powder dose strengtha | ||||||
| 750 mg | 19 | (23.8) | 45 | (56.3) | 64 | (40.0) |
| 1000 mg | 61 | (76.3) | 35 | (43.8) | 96 | (60.0) |
|
| ||||||
| Take another phosphate binder?a | ||||||
| Yes | 13 | (16.3) | 41 | (51.3) | 54 | (33.8) |
| No | 67 | (83.8) | 39 | (48.8) | 106 | (66.3) |
|
| ||||||
| If previously taking lanthanum carbonate tablets, did you frequently crush the tablets?b | ||||||
| Yes | 10 | (22.7) | 32 | (69.6) | 42 | (46.7) |
| No | 34 | (77.3) | 14 | (30.4) | 48 | (53.3) |
|
| ||||||
| Other phosphate binder(s) takena | ||||||
| Calcium acetate/magnesium carbonate tablets | – | – | 10 | (12.5) | – | – |
| Sevelamer hydrochloride tablets | 3 | (3.8) | 2 | (2.5) | 5 | (3.1) |
| Sevelamer carbonate oral suspension | 2 | (2.5) | 7 | (8.8) | 9 | (5.6) |
| Sevelamer carbonate tablets | 1 | (1.3) | 8 | (10.0) | 9 | (5.6) |
| Calcium acetate | – | – | 12 | (15.0) | – | – |
| Calcium carbonate | 3 | (3.8) | – | – | – | – |
| Lanthanum carbonate tablets | 2 | (2.5) | – | – | – | – |
| Other | 3 | (3.8) | 2 | (2.5) | 5 | (3.1) |
|
| ||||||
| Other phosphate binder(s) taken prior to the survey | ||||||
| Calcium acetate/magnesium carbonate tablets | – | – | 4 | (5.0) | – | – |
| Sevelamer hydrochloride tablets | 17 | (21.3) | 9 | (11.3) | 26 | (16.3) |
| Sevelamer carbonate oral suspension | 6 | (7.5) | 4 | (5.0) | 10 | (6.3) |
| Sevelamer carbonate tablets | 6 | (7.5) | 7 | (8.8) | 13 | (8.1) |
| Calcium acetate | 3 | (3.8) | 17 | (21.3) | 20 | (25.1) |
| Calcium carbonate | 9 | (11.3) | 1 | (1.3) | 10 | (12.6) |
| Lanthanum carbonate tablets | 43 | (53.8) | 43 | (53.8) | 86 | (53.8) |
|
| ||||||
| Reasons for switching to lanthanum carbonate powderc | ||||||
| Medical reasons | 36 | (45.0) | 60 | (75.0) | 96 | (60.0) |
| The patient had problems with chewing previous binders | 13 | (16.3) | 19 | (23.8) | 32 | (20.0) |
| The patient had problems with swallowing previous binders | 12 | (15.0) | 9 | (11.3) | 21 | (13.1) |
| The patient wanted to take fewer tablets, pills, or capsules | 6 | (7.5) | 11 | (13.8) | 17 | (10.6) |
| The patient did not like the taste of previous binders | 7 | (8.8) | 7 | (8.8) | 14 | (8.8) |
| The patient wanted to try lanthanum carbonate powder | 6 | (7.5) | 0 | (0.0) | 6 | (3.8) |
| The patient’s doctor proposed a switch in medication | 5 | (6.3) | 0 | (0.0) | 5 | (3.1) |
| Adverse events (itching, diarrhoea) | 2 | (2.5) | 3 | (3.8) | 5 | (3.1) |
| Other difficulties with using previous medication (patient reported having to drink lots of water with previous medication) | 3 | (3.8) | 1 | (1.3) | 4 | (2.5) |
| The patient hoped that this treatment would be more effective than previous phosphate binders | 1 | (1.3) | 0 | (0.0) | 1 | (0.6) |
| Other reasons (cost of different binders) | 1 | 1.3 | 0 | 0.0 | 1 | 0.6 |
At time of survey.
Not all patients who were taking lanthanum carbonate tablets answered this question.
Patients could choose more than one answer.
Note: values have been rounded up or down to the nearest decimal point.
SD, standard deviation.
Satisfaction
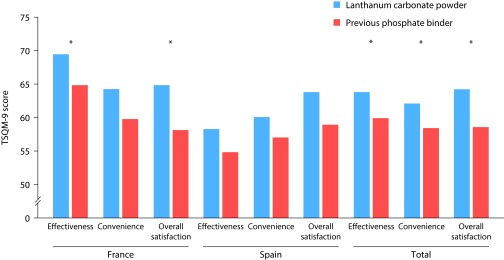
Based on the TSQM-9 scores, French patients reported lanthanum carbonate powder as having a statistically significantly higher effectiveness rating than previous phosphate binders (lanthanum carbonate powder was rated as having a mean effectiveness of 69.5/100; previous phosphate binders were rated at 64.8/100; p<0.05; Table 3 and Figure 1). French patients were also significantly more satisfied with lanthanum carbonate powder than with previous phosphate binders (lanthanum carbonate powder was rated to have a mean overall satisfaction score of 64.8/100; previous phosphate binders were rated at 58.0/100; p<0.05; Table 3). French patients did not report any significant differences in convenience between lanthanum carbonate powder and previous phosphate binders, while Spanish patients did not report any statistically significant difference in effectiveness, convenience, or overall satisfaction between the different medication types (Table 3). Compared with previous phosphate binders, French patients were statistically significantly more satisfied with the way lanthanum carbonate powder relieved their symptoms (on the 7-point Likert scale, where 1=most negative and 7=most positive, lanthanum carbonate powder: 5.26; previous phosphate binder: 4.93; p<0.05 [type of symptom was not specified in the questionnaire]), with the time it took to start working (lanthanum carbonate powder: 5.20; previous phosphate binder: 4.88; p<0.05), with its ease of use (lanthanum carbonate powder: 4.79; previous phosphate binder: 4.33; p<0.05), and with the medication overall (lanthanum carbonate powder: 5.15; previous phosphate binder: 4.70; p<0.01) (Table 3). They were also more confident that the medication was a good thing for them when compared with previous phosphate binders (lanthanum carbonate powder: 3.53; previous phosphate binder: 3.26; p<0.05). There were no statistically significant differences for Spanish patients between lanthanum carbonate powder and any other phosphate binders of any formulation in these measures (Table 3). The combined data for both countries showed that patients reported lanthanum carbonate powder as having a significantly higher effectiveness rating (lanthanum carbonate powder: 63.9/100; previous phosphate binders: 59.8/100; p<0.05) and being more convenient (lanthanum carbonate powder: 62.2/100; previous phosphate binders: 58.3/100; p<0.05) than previous phosphate binders, and showed an overall increased satisfaction for lanthanum carbonate powder compared with previous phosphate binders (lanthanum carbonate powder: 64.3/100; previous phosphate binders: 58.5/100; p<0.01).
Table 3.
Patient satisfaction with phosphate-binder medication.
| France | Spain | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Lanthanum carbonate powder (n=80) | Previous phosphate binder (n=80) | Standard error | t-test | Lanthanum carbonate powder (n=80) | Previous phosphate binder (n=80) | Standard error | t-test | Lanthanum carbonate powder (N=160) | Previous phosphate binder (N=160) | Standard error | t-test | |
| Mean | Mean | p value | Mean | Mean | p value | Mean | Mean | p value | ||||
| TSQM-9 scorea | ||||||||||||
| Effectiveness | 69.51 | 64.79 | 2.31 | 0.043 | 58.26 | 54.86 | 2.10 | NS | 63.89 | 59.83 | 1.67 | 0.015 |
| Convenience | 64.38 | 59.65 | 3.04 | NS | 60.00 | 57.01 | 2.39 | NS | 62.19 | 58.33 | 1.94 | 0.047 |
| Overall satisfaction | 64.82 | 58.04 | 2.60 | 0.01 | 63.75 | 58.93 | 2.52 | NS | 64.29 | 58.48 | 1.80 | 0.001 |
|
| ||||||||||||
| TSQM-9 itemb | ||||||||||||
| 1. Satisfaction with the ability of the medication to prevent or treat the condition | 5.05 | 4.86 | 0.17 | NS | 4.53 | 4.33 | 0.14 | NS | 4.79 | 4.59 | 0.11 | NS |
| 2. Satisfaction with the way the medication relieves symptoms | 5.26 | 4.93 | 0.14 | 0.02 | 4.51 | 4.35 | 0.14 | NS | 4.89 | 4.64 | 0.11 | 0.02 |
| 3. Satisfaction with the time taken for the medication to start working | 5.20 | 4.88 | 0.14 | 0.022 | 4.45 | 4.20 | 0.13 | NS | 4.83 | 4.54 | 0.11 | 0.006 |
| 4. Ease of using the medication in its current form | 4.79 | 4.33 | 0.22 | 0.038 | 4.59 | 4.31 | 0.17 | NS | 4.69 | 4.32 | 0.14 | 0.008 |
| 5. Ease of planning when to use the medication each time | 5.13 | 4.90 | 0.21 | NS | 4.79 | 4.76 | 0.16 | NS | 4.96 | 4.83 | 0.13 | NS |
| 6. Convenience of taking the medication as instructed | 4.68 | 4.51 | 0.22 | NS | 4.43 | 4.19 | 0.16 | NS | 4.55 | 4.35 | 0.13 | NS |
| 7. Overall confidence that taking the medication is a good thing | 3.53 | 3.26 | 0.12 | 0.029 | 3.73 | 3.50 | 0.14 | NS | 3.63 | 3.38 | 0.09 | 0.008 |
| 8. Certainty that the good things about the medication outweigh the bad things | 3.40 | 3.16 | 0.14 | NS | 3.55 | 3.36 | 0.12 | NS | 3.48 | 3.26 | 0.09 | 0.021 |
| 9. Overall satisfaction with this medication | 5.15 | 4.70 | 0.15 | 0.002 | 4.65 | 4.39 | 0.14 | NS | 4.90 | 4.54 | 0.11 | 0.001 |
Scores range from 0 (lowest satisfaction) to 100 (highest satisfaction).
Individual items: 7-point Likert scales (1=most negative; 7=most positive).
NS, not significant; TSQM, Treatment Satisfaction Questionnaire for Medication.
Figure 1. Patient satisfaction with lanthanum carbonate powder compared with previous phosphate binders.
Satisfaction was measured using the TSQM-9 and rated effectiveness, convenience, and overall satisfaction with medication on a scale of 0–100. Lanthanum carbonate powder, N=160. Previous phosphate binder, N=160. *p<0.05. TSQM, Treatment Satisfaction Questionnaire for Medication.
Preference
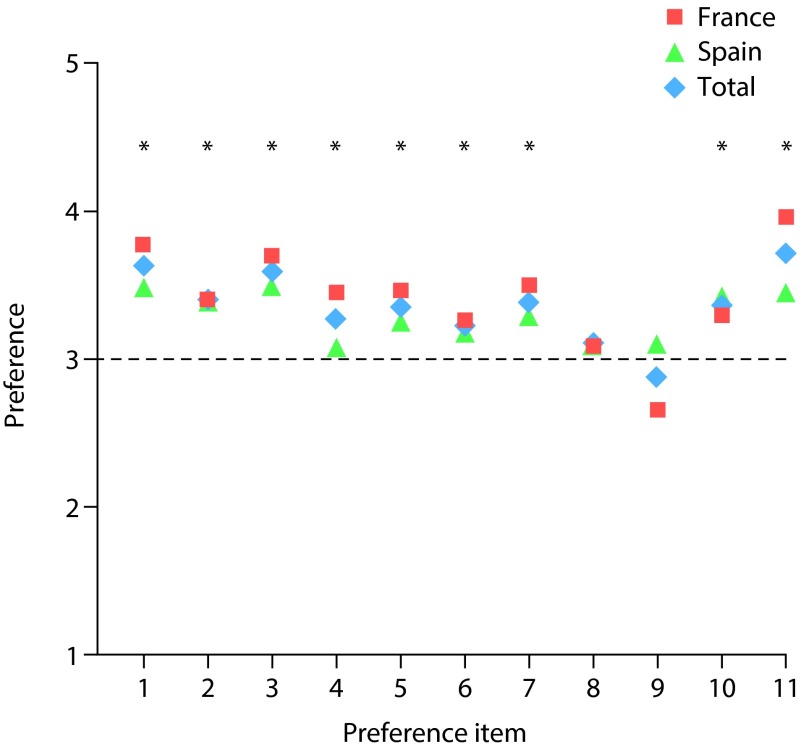
According to item 1 described in Table 4, there was a preference for continuing lanthanum carbonate powder treatment over previous phosphate binders. On the Likert scale, where 1=strong preference for previous phosphate binder, 3=indifference, and 5=strong preference for lanthanum carbonate powder, French patients scored 3.8 while Spanish patients scored 3.5 (total score for both countries combined was 3.6; Table 4). For each individual country as well as for the combined data, there was a statistically significant preference for lanthanum carbonate powder over previous phosphate binders (p<0.001; Table 4). Figure 2 illustrates that, for the majority of the 11 items measured, patients preferred lanthanum carbonate powder over previous phosphate binders. Compared with previous phosphate binders, patients found lanthanum carbonate powder significantly easier to take with food (France, p<0.05; Spain, p<0.01) and easier to swallow (France, p<0.001; Spain, p<0.001; Table 4). Lanthanum carbonate powder was shown to have a significantly better taste than other phosphate binders of any formulation (France, p<0.001; Spain, p<0.05), and did not require the patient to drink additional fluid (France, p<0.001; Spain, p<0.05; Table 4). Overall, lanthanum carbonate powder was significantly easier to take than other medication (France, p<0.05; Spain, p<0.01) and easier to mix with food (France, p<0.001; Spain, p<0.001; Table 4).
Table 4.
Preference of patients for lanthanum carbonate powder.
| France (n=80) | Spain (n=80) | Total (N=160) | ||||
|---|---|---|---|---|---|---|
| Preference | t-test | Preference | t-test | Preference | t-test | |
| Meana (SD) | p valueb | Meana (SD) | p valueb | Meana (SD) | p valueb | |
| 1. Preference for continuing lanthanum carbonate powder treatment | 3.77 (1.28) | <0.001 | 3.49 (1.04) | <0.001 | 3.63 (1.17) | <0.001 |
| 2. Ease of taking with food | 3.40 (1.38) | 0.012 | 3.40 (1.12) | 0.002 | 3.40 (1.25) | <0.001 |
| 3. Ease of swallowing | 3.70 (1.24) | <0.001 | 3.50 (1.19) | <0.001 | 3.60 (1.21) | <0.001 |
| 4. Enjoyment of meals | 3.45 (1.11) | 0.001 | 3.09 (0.98) | NS | 3.27 (1.06) | 0.002 |
| 5. The taste or flavour of the medicine | 3.46 (1.01) | <0.001 | 3.26 (0.94) | 0.014 | 3.36 (0.97) | <0.001 |
| 6. Not impacting the taste of my food | 3.26 (0.92) | 0.013 | 3.19 (1.02) | NS | 3.23 (0.97) | 0.004 |
| 7. The need to drink additional fluid or water | 3.50 (0.95) | <0.001 | 3.29 (1.20) | 0.036 | 3.39 (1.09) | <0.001 |
| 8. Having the ability to modify dosage | 3.09 (0.51) | NS | 3.11 (0.90) | NS | 3.10 (0.73) | NS |
| 9. Ease of opening the medication packet | 2.66 (0.86) | 0.001 | 3.09 (0.89) | NS | 2.88 (0.90) | NS |
| 10. Overall ease of taking the medication | 3.30 (1.19) | 0.028 | 3.41 (1.14) | 0.002 | 3.36 (1.17) | <0.001 |
| 11. Overall ease of mixing with food | 3.96 (1.08) | <0.001 | 3.45 (0.94) | <0.001 | 3.71 (1.04) | <0.001 |
5-point Likert scale (1=strong preference for previous phosphate binder; 3=indifference; 5=strong preference for lanthanum carbonate powder).
One-sample t-test against indifference (score=3).
NS, not significant; SD, standard deviation.
Figure 2. Patient preference for lanthanum carbonate powder compared with previous phosphate binders.
Preference was measured using 5-point Likert scale agreement ratings. Preference items: (1) Preference for continuing lanthanum carbonate powder treatment; (2) ease of taking with food; (3) ease of swallowing; (4) enjoyment of meals; (5) the taste or flavour of the medicine; (6) not impacting the taste of my food; (7) the need to drink additional fluid or water; (8) having the ability to modify dosage; (9) ease of opening the medication packet; (10) overall ease of taking the medication; (11) overall ease of mixing with food. Lanthanum carbonate powder, N=160. Previous phosphate binder, N=160. *p<0.05.
Adherence
Overall, 66.3% of French patients were considered to adhere to lanthanum carbonate powder treatment (scoring 0 on the MMAS-4; 33.7% reported non-adherence, scoring 1–4; Table 5). Similarly, 65.0% of French patients reported adherence to previous phosphate binders (difference in adherence between lanthanum carbonate powder and previous phosphate binders, p=not significant). Likewise, there was no significant difference among Spanish patients between adherence to lanthanum carbonate powder (52.5%) and adherence to previous phosphate binders (56.3%; p=not significant; Table 5). This trend of non-significance was observed for all four items of the MMAS-4 (Table 5). The actual MMAS-4 scores reflected these results as well. Mean adherence was slightly higher in France than Spain (0.5 vs. 0.9, respectively, while total score for both countries combined was 0.7); these scores represent medium–high adherence (0=high adherence; 3 and 4=low adherence).
Table 5.
Adherence of patients to phosphate-binder medication.
| France | Spain | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Lanthanum carbonate powder (n=80) | Previous phosphate binder (n=80) | Lanthanum carbonate powder (n=80) | Previous phosphate binder (n=80) | Lanthanum carbonate powder (N=160) | Previous phosphate binder (N=160) | |||||||
|
|
||||||||||||
| n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| Adherence score (MMAS-4) | ||||||||||||
| Non-adherent (score of 1–4) | 27 | (33.7) | 28 | (35.0) | 38 | (47.5) | 35 | (43.7) | 65 | (40.6) | 63 | (39.4) |
| Adherent (score of 0) | 53 | (66.3) | 52 | (65.0) | 42 | (52.5) | 45 | (56.3) | 95 | (59.4) | 97 | (60.6) |
| χ2(1)=0.02; p=NS | χ2(1)=0.22; p=NS | χ2(1)=0.05; p=NS | ||||||||||
|
| ||||||||||||
| 1. MMAS-4: Do you ever forget to take your medicine? | ||||||||||||
| No | 54 | (67.5) | 53 | (66.3) | 44 | (55.0) | 46 | (57.5) | 98 | (61.3) | 99 | (61.9) |
| Yes | 26 | (32.5) | 27 | (33.7) | 36 | (45.0) | 34 | (42.5) | 62 | (38.7) | 61 | (38.1) |
| χ2(1)=0.02; p=NS | χ2(1)=0.10; p=NS | χ2(1)=0.01; p=NS | ||||||||||
|
| ||||||||||||
| 2. MMAS-4: Are you careless at times about taking your medicine? | ||||||||||||
| No | 75 | (93.7) | 71 | (88.7) | 64 | (80.0) | 64 | (80.0) | 139 | (86.9) | 135 | (84.4) |
| Yes | 5 | (6.3) | 9 | (11.3) | 16 | (20.0) | 16 | (20.0) | 21 | (13.1) | 25 | (15.6) |
| χ2(1)=1.25; p=NS | χ2(1)=0.00; p=NS | χ2(1)=0.41; p=NS | ||||||||||
|
| ||||||||||||
| 3. MMAS-4: When you feel better do you sometimes stop taking your medicine? | ||||||||||||
| No | 76 | (95.0) | 76 | (95.0) | 72 | (90.0) | 72 | (90.0) | 148 | (92.5) | 148 | (92.5) |
| Yes | 4 | (5.0) | 4 | (5.0) | 8 | (10.0) | 8 | (10.0) | 12 | (7.5) | 12 | (7.5) |
| χ2(1)=0.00; p=NS | χ2(1)=0.00; p=NS | χ2(1)=0.00; p=NS | ||||||||||
|
| ||||||||||||
| 4. MMAS-4: Sometimes if you feel worse when you take the medicine, do you stop taking it? | ||||||||||||
| No | 75 | (93.7) | 74 | (92.5) | 69 | (86.3) | 68 | (85.0) | 144 | (90) | 142 | (88.7) |
| Yes | 5 | (6.3) | 6 | (7.5) | 11 | (13.7) | 12 | (15.0) | 16 | (10) | 18 | (11.3) |
| χ2(1)=0.09; p=NS | χ2(1)=0.05; p=NS | χ2(1)=0.13; p=NS | ||||||||||
MMAS, Morisky Medication Adherence Scale; NS, not significant.
Discussion
The objectives of the current study, which was the first of its kind, were to document the level of satisfaction with lanthanum carbonate powder among French and Spanish patients with ESRD, to establish their preferences for the powder formulation of lanthanum carbonate compared with other phosphate binders of any formulation, and to determine adherence to medication. The results indicate that French but not Spanish patients report being more satisfied with lanthanum carbonate powder than other phosphate binders (in tablet or oral suspension form) and that both French and Spanish patients prefer lanthanum carbonate powder to their previous phosphate-binder medication. However, switching from a previous phosphate binder to lanthanum carbonate powder did not improve patient adherence to medication in either country. It is interesting to note that the most common phosphate binder taken prior to the time of the survey was lanthanum carbonate in tablet form. It has been previously reported that patients have to take a mean daily dose of 9.6 sevelamer hydrochloride tablets to achieve similar phosphate levels to those taking 2.8 lanthanum carbonate tablets per day [25]. Thus, lanthanum carbonate tablets may be preferred over other phosphate binders of the same formulation because fewer lanthanum carbonate tablets are required to achieve the same level of phosphate control as with other phosphate binders. Alternatively, physicians may have been more likely to recommend that those patients already taking lanthanum carbonate (in tablet form) rather than those taking calcium-based tablets should try the powder formulation. In this way, further variations in treatment (and possible related side effects) would be reduced. Finally, it is not possible to determine from the data which other phosphate binder patients were taking prior to the one used immediately before switching to lanthanum carbonate powder. It is possible that many of the patients who changed from lanthanum carbonate tablets to powder had previously taken calcium-based binders.
The majority of French patients were using the higher-dose formulation of lanthanum carbonate powder (1000 mg), while a much smaller proportion of Spanish patients were taking this dose. This may have had an impact on the number of patients taking another phosphate binder concurrently, with a higher dose potentially reducing the desire or need to take further medication. This may have also contributed to the low number of French patients taking another phosphate binder at the time of the survey.
Overall, both French and Spanish patients took a similar number of phosphate binders per day and had a similar history in terms of time spent receiving haemodialysis and other phosphate binders. There were, however, some country-specific differences that may reflect variations in the time taken for the product to come to market. Spanish patients had been taking lanthanum carbonate powder for a longer period at the time of the survey than French patients, possibly because the medicine was available to patients in Spain a few months before being available in France. This may have influenced their opinion of lanthanum carbonate powder because Spanish patients would have had more time to adjust to using the new formulation than French patients. Additionally, Spanish patients were much more likely than French patients to be combining another phosphate binder of any formulation with lanthanum carbonate powder. It is possible that French patients, who had more recently switched to lanthanum carbonate powder than Spanish patients, would want to test out the new powder formulation as a sole treatment before incorporating additional (previously used) phosphate-binder treatments. This may have further influenced patients’ opinions on the advantages or disadvantages of lanthanum carbonate powder.
French but not Spanish patients were significantly more satisfied with lanthanum carbonate powder than with other phosphate binders of any formulation. Compared with oral medications (in any formulation) for other chronic diseases (asthma, arthritis, cancer, cardiovascular disease, depression/anxiety, diabetes, infectious disease, migraine, or psoriasis), patients with ESRD assessed using the TSQM-9 in the present study were generally less satisfied with their medications [21]. The result is, however, consistent with another study on patient treatment satisfaction in ESRD [26]. This study examined 977 participants (84% were patients with ESRD, 16% were caregivers to such patients) who completed a survey on satisfaction with renal replacement therapy and treatment education. In line with the results in the current study, the authors showed that treatment satisfaction among patients with ESRD lay between 58% and 66% [26]. Taking into account that patients with ESRD are often not aware of their symptoms, this relatively low level of satisfaction is not surprising. French patients also reported lanthanum carbonate powder as having a statistically significantly higher effectiveness rating than previous phosphate binders. French patients were also statistically significantly more satisfied with the way lanthanum carbonate powder relieved their symptoms. However, the clinical relevance of these statistical differences is unknown.
Patients satisfied with lanthanum carbonate powder would be more likely to continue with this treatment than patients who were initially not as satisfied. Thus, it is interesting to examine the reasons for switching medications to determine what may have produced patient satisfaction with lanthanum carbonate powder. The main reasons reported for switching from a previous phosphate binder to lanthanum carbonate powder (medical reasons, problems with chewing and swallowing previous binders, or a desire to reduce the large quantity of tablets, pills, or capsules the patient was taking) indicate that a powder formulation is a preferable alternative to tablets for patients taking phosphate-binder medication. Lanthanum carbonate in tablet form was the most common phosphate binder taken prior to the study, suggesting that medical and other reasons given for switching from this treatment were related to issues with the tablet formulation but not with the efficacy of lanthanum carbonate; in fact, the tablet and powder formulations have been shown to be pharmacodynamically equivalent [20]. The powder formulation of lanthanum carbonate may therefore have contributed somewhat to increased patient satisfaction; however, this cannot be defined clearly in the current study owing to the lack of other phosphate binders in powder form. Future studies should determine whether patient satisfaction increases in general for powder formulations of any phosphate binders compared with other formulations, or whether this effect is specific to lanthanum carbonate.
Two French patients were reported to take lanthanum carbonate both in tablet and in powder formulation at the time of the survey. It may be that the different formulations suited the patients’ needs at different times, whereby a tablet or powder formulation may have been easier to take, depending on the particular meal or other daily changing conditions under which the patients were receiving treatment. Alternatively, the patients may have wanted to change gradually from tablet to powder formulation, rather than switching immediately from one to the other, resulting in an overlap of the two formulations.
Patients in France and Spain showed a clear preference for lanthanum carbonate powder over other phosphate binders of any formulation, as they found it easy to swallow and to mix with food. It has been reported that elderly patients with ESRD have difficulties with chewing lanthanum carbonate tablets [27]. Understanding patient preference is important in maximizing medication adherence, and preference for lanthanum carbonate powder is thus a valuable measure of the usefulness of this formulation, which may encourage patient compliance and adherence. Nevertheless, despite the higher satisfaction with lanthanum carbonate powder than with other phosphate binders, no significant difference was observed in adherence to medication. The large number of phosphate binders taken by patients each day (9–10 tablets, pills, capsules, and/or sachets per day for both French and Spanish patients), which can impact on their lifestyle [28], may have had an effect on adherence. The possibility of increased adherence with a powder over tablet formulations has been previously suggested [28]. The powder form of lanthanum carbonate was proposed to increase adherence by aiding patients with reduced ability to chew, whose cognitive functions or medication compliance are poor, and by decreasing the daily required number of tablets. In a survey of 79 patients on maintenance haemodialysis, the preference for lanthanum carbonate in chewable tablet versus granule form was investigated [29]. In this study, 46.8% of patients found granules easier to take, while 27.8% reported that tablets were easier to take and 25.3% reported no differences between the formulations. This supports the idea that a physically smaller-sized formulation of lanthanum carbonate may be preferred and could therefore increase adherence. Nevertheless, lanthanum carbonate powder, like other phosphate binders, must be taken together with meals, which may affect adherence if it is not compatible with the patient’s lifestyle [28]. A relatively low level of adherence to phosphate binders has been previously reported in a review of online medical databases searching for predictors of non-adherence to phosphate-binding medication in patients with ESRD [12]. This review of 34 studies demonstrated a low level of adherence in 51% of patients [12]. While there was some variation between the studies, the overall result suggests that non-adherence to phosphate binders in treatment for ESRD is prevalent. Alongside high tablet burdens, it is reported that a lack of understanding regarding treatment and patients questioning the need for phosphate binders may decrease adherence [17], which would apply for formulations such as lanthanum carbonate powder as well as tablets. Steps should therefore be taken in patient care to improve adherence, potentially through a better understanding of patients’ beliefs and concerns [30,31]. This may lead to optimal adherence to prescribed treatment. An explanation for the lack of increased adherence observed in this study is that the MMAS-4, used to measure adherence, has a relatively low sensitivity and does not have good psychometric properties [32–34]. In this study, the MMAS-4 may therefore not have recorded adequately any differences in adherence that are associated with different phosphate-binder formulations.
An important issue to address when considering lanthanum carbonate powder as an alternative treatment for patients with ESRD is the associated treatment cost versus other available phosphate binders. There are no cost-effectiveness studies comparing lanthanum carbonate powder to other binders of different formulations; however, studies have reported that lanthanum carbonate tablets are cost-effective compared with sevelamer hydrochloride [35] as well as calcium-containing binders in patients not responding to this treatment [36,37]. Further studies should be completed to understand the cost-effectiveness of lanthanum carbonate powder versus other binders.
Certain limitations should be considered in the current study. A key limitation was that the survey enrolled patients who had already experienced phosphate binders before the study began. Information on patient preferences for and adherence to previous phosphate binders was therefore based on the patients’ memories of these experiences, which may have been subject to change over time. Also, because the data were self-reported by the survey participants, there was potential for self-selection bias when comparing treatments.
The use of an online versus telephone survey (which included the help of an interviewer) may have influenced the responses given by patients. Although most participants completed the online survey in this study, a telephone survey was used for individuals who could not access the online version; if only one method of data recording had been used, there may have been reduced variation in responses.
The study analysed patient satisfaction with, preference for, and adherence to phosphate binders over a 4-week recollection period, although overall, patients were taking lanthanum carbonate for an average of 5.52 months. Longer studies may be helpful to determine changes in patient satisfaction over greater periods of time.
Future studies should focus on determining benefits of lanthanum carbonate powder over other powder phosphate binders on the market. Increased levels of satisfaction were observed in French but not Spanish patients; thus, it would be interesting to determine the level of satisfaction with lanthanum carbonate oral powder in other European patients. Indeed, there may be regional variation among cultural preferences for powder formulations of phosphate binders and other medications, which could affect satisfaction with, as well as adherence to such medications. Furthermore, developing a comprehensive understanding of patients’ expectations and the low level of patient adherence to phosphate-binder medication in ESRD will provide valuable insight for future treatments.
Conclusions
The data suggest that the more convenient formulation of lanthanum carbonate powder compared with other phosphate binders of any formulation results in higher levels of satisfaction among French patients with ESRD. Satisfaction was not accompanied by increased adherence to lanthanum carbonate powder over other formulations; nevertheless, both French and Spanish patients demonstrated a clear preference for lanthanum carbonate powder over other phosphate binders of any formulation. The results suggest that a powder may be more convenient and easy to use than other formulations, although further studies should be performed to determine whether this effect is observed only in French patients or also in patients from other countries.
Acknowledgements
Medical writing support was provided by Noëlle L O’Regan PhD of PharmaGenesis London, London, UK, and was funded by Shire. The results were presented previously as a poster at the 52nd Congress of the European Renal Association – European Dialysis and Transplant Association (ERA-EDTA) in May 2015.
Abbreviations
- ESRD
end-stage renal disease
- MMAS
Morisky Medication Adherence Scale
- NS
not significant
- SD
standard deviation
- TSQM
Treatment Satisfaction Questionnaire for Medication
Footnotes
Disclosure and potential conflicts of interest: Michael Keith was an employee and a stock holder of Shire at the time of the study. Patricia de Sequera has no conflicts of interest to declare. François Clair has received funding from Shire. Riccardo Pedersini was an employee of Kantar Health at the time of the study and is currently an employee of RTI Health Solutions.
The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests forms for the authors are available for download at: http://www.drugsincontext.com/wp-content/uploads/2016/10/dic.212300-COI.pdf.
Contributions: All named authors contributed to the conception of the work; the acquisition, analysis, and interpretation of the data; and drafting and revision of the manuscript. All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval of the version to be published.
Funding declaration: This study was conducted by Kantar Health and funded by Shire Development LLC.
Compliance with ethics guidelines: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964, as revised in 2013. Informed consent was obtained from all patients for being included in the study.
Correct attribution: Copyright © 2016 Keith M, de Sequera P, Clair F, Pedersini R. http://dx.doi.org/10.7573/dic.212300. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 3.0.
Provenance: Submitted, externally peer reviewed
Peer review comments to author: 30 August 2016
Drugs in Context is published by Just Medical Media Ltd. Registered office: Gatelands, Patterdale Road, Windermere, Cumbria, LA23 1NH, UK
Just Medical Media Limited is registered in England Number 6891187. VAT GB 945 1713 22
For all manuscript and submissions enquiries, contact Julia Savory, Head of Digital Publishing and Submissions Management julia@justmedicalmedia.com
For all permissions, rights, and reprints, contact Stephen I’Anson, Commercial Director steve@justmedicalmedia.com
References
- 1.Albaaj F, Hutchison A. Hyperphosphataemia in renal failure: causes, consequences and current management. Drugs. 2003;63:577–96. doi: 10.2165/00003495-200363060-00005. http://dx.doi.org/10.2165/00003495-200363060-00005. [DOI] [PubMed] [Google Scholar]
- 2.Hruska KA, Mathew S, Lund R, Qiu P, Pratt R. Hyperphosphatemia of chronic kidney disease. Kidney Int. 2008;74:148–57. doi: 10.1038/ki.2008.130. http://dx.doi.org/10.1038/ki.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodkin DA, Bragg-Gresham JL, Koenig KG, Wolfe RA, Akiba T, Andreucci VE, Saito A, Rayner HC, Kurokawa K, Port FK, Held PJ, Young EW. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–7. doi: 10.1097/01.asn.0000100127.54107.57. http://dx.doi.org/10.1097/01.ASN.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 4.Goodman WG, Quarles LD. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int. 2008;74:276–88. doi: 10.1038/sj.ki.5002287. http://dx.doi.org/10.1038/sj.ki.5002287. [DOI] [PubMed] [Google Scholar]
- 5.Goodman WG. Vascular calcification in chronic renal failure. Lancet. 2001;358:1115–16. doi: 10.1016/S0140-6736(01)06299-7. http://dx.doi.org/10.1016/S0140-6736(01)06299-7. [DOI] [PubMed] [Google Scholar]
- 6.Roman-Garcia P, Carrillo-Lopez N, Fernandez-Martin JL, Naves-Diaz M, Ruiz-Torres MP, Cannata-Andia JB. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone. 2010;46:121–8. doi: 10.1016/j.bone.2009.09.006. http://dx.doi.org/10.1016/j.bone.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Pelletier S, Roth H, Bouchet JL, Drueke T, London G, Fouque D, French P French Phosphorus and Calcium Observatory investigators. Mineral and bone disease pattern in elderly haemodialysis patients. Nephrol Dial Transplant. 2010;25:3062–70. doi: 10.1093/ndt/gfq128. http://dx.doi.org/10.1093/ndt/gfq128. [DOI] [PubMed] [Google Scholar]
- 8.Haider DG, Lindner G, Wolzt M, Ahmad SS, Sauter T, Leichtle AB, Fiedler GM, Fuhrmann V, Exadaktylos AK. Hyperphosphatemia is an independent risk factor for mortality in critically ill patients: results from a cross-sectional study. PLoS One. 2015;10:e0133426. doi: 10.1371/journal.pone.0133426. http://dx.doi.org/10.1371/journal.pone.0133426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hruska K, Mathew S, Lund R, Fang Y, Sugatani T. Cardiovascular risk factors in chronic kidney disease: does phosphate qualify? Kidney Int Suppl. 2011;121:S9–13. doi: 10.1038/ki.2011.24. http://dx.doi.org/10.1038/ki.2011.24. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez OM. The connection between dietary phosphorus, cardiovascular disease, and mortality: where we stand and what we need to know. Adv Nutr. 2013;4:723–9. doi: 10.3945/an.113.004812. http://dx.doi.org/10.3945/an.113.004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(Suppl 1):A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. http://dx.doi.org/10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Karamanidou C, Clatworthy J, Weinman J, Horne R. A systematic review of the prevalence and determinants of nonadherence to phosphate binding medication in patients with end-stage renal disease. BMC Nephrol. 2008;9:2. doi: 10.1186/1471-2369-9-2. http://dx.doi.org/10.1186/1471-2369-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31:607–17. doi: 10.1053/ajkd.1998.v31.pm9531176. http://dx.doi.org/10.1053/ajkd.1998.v31.pm9531176. [DOI] [PubMed] [Google Scholar]
- 14.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–18. doi: 10.1097/01.ASN.0000133041.27682.A2. http://dx.doi.org/10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 15.KDIGO. Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 16.National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–201. [PubMed] [Google Scholar]
- 17.Floege J. Phosphate binders in chronic kidney disease: a systematic review of recent data. J Nephrol. 2016;29:329–40. doi: 10.1007/s40620-016-0266-9. http://dx.doi.org/10.1007/s40620-016-0266-9. [DOI] [PubMed] [Google Scholar]
- 18.Hutchison AJ, Maes B, Vanwalleghem J, Asmus G, Mohamed E, Schmieder R, Backs W, Jamar R, Vosskuhler A. Long-term efficacy and tolerability of lanthanum carbonate: results from a 3-year study. Nephron Clin Pract. 2006;102:c61–71. doi: 10.1159/000088932. http://dx.doi.org/10.1159/000088932. [DOI] [PubMed] [Google Scholar]
- 19.Joy MS, Finn WF. Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis. 2003;42:96–107. doi: 10.1016/s0272-6386(03)00554-7. http://dx.doi.org/10.1016/S0272-6386(03)00554-7. [DOI] [PubMed] [Google Scholar]
- 20.Pierce D, Hossack S, Robinson A, Zhang P, Martin P. Assessment of pharmacodynamic equivalence and tolerability of lanthanum carbonate oral powder and tablet formulations: a single-center, randomized, open-label, 2-period crossover study in healthy subjects. Clin Ther. 2012;34:1290–300 e2. doi: 10.1016/j.clinthera.2012.05.003. http://dx.doi.org/10.1016/j.clinthera.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson MJ, Sinha A, Hass SL, Colman SS, Kumar RN, Brod M, Rowland CR. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12. http://dx.doi.org/10.1186/1477-7525-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bharmal M, Payne K, Atkinson MJ, Desrosiers MP, Morisky DE, Gemmen E. Validation of an abbreviated Treatment Satisfaction Questionnaire for Medication (TSQM-9) among patients on antihypertensive medications. Health Qual Life Outcomes. 2009;7:36. doi: 10.1186/1477-7525-7-36. http://dx.doi.org/10.1186/1477-7525-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. http://dx.doi.org/10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Morisky DE, Malotte CK, Choi P, Davidson P, Rigler S, Sugland B, Langer M. A patient education program to improve adherence rates with antituberculosis drug regimens. Health Educ Behav. 1990;17:253–66. doi: 10.1177/109019819001700303. http://dx.doi.org/10.1177/109019819001700303. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RJ, Keith MS, Preston P, Copley JB. The real-world dose-relativity of sevelamer hydrochloride and lanthanum carbonate monotherapy in patients with end-stage renal disease. Adv Ther. 2013;30:1100–10. doi: 10.1007/s12325-013-0077-5. http://dx.doi.org/10.1007/s12325-013-0077-5. [DOI] [PubMed] [Google Scholar]
- 26.Fadem SZ, Walker DR, Abbott G, Friedman AL, Goldman R, Sexton S, Buettner K, Robinson K, Peters TG. Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol. 2011;6:605–12. doi: 10.2215/CJN.06970810. http://dx.doi.org/10.2215/CJN.06970810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arenas MD, Malek T, Alvarez-Ude F, Gil MT, Moledous A, Reig-Ferrer A. [Phosphorus binders: preferences of patients on haemodialysis and its impact on treatment compliance and phosphorus control]. Nefrologia. 2010;30:522–30. doi: 10.3265/Nefrologia.pre2010.may.10275. [Spanish] http://dx.doi.org/10.3265/Nefrologia.pre2010.may.10275. [DOI] [PubMed] [Google Scholar]
- 28.Lloret MJ, Ruiz-Garcia C, Dasilva I, Furlano M, Barreiro Y, Ballarin J, Bover J. Lanthanum carbonate for the control of hyperphosphatemia in chronic renal failure patients: a new oral powder formulation – safety, efficacy, and patient adherence. Patient Prefer Adher. 2013;7:1147–56. doi: 10.2147/PPA.S31694. http://dx.doi.org/10.2147/PPA.S31694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mukai I, Yoshizawa T, Kumagai J, Takahashi N, Tsuchiya S. Questionnaire survey and serum phosphorus levels in maintenance hemodialysis patients switching lanthanum carbonate formulation from chewable tablets to granules. Ther Apher Dial. 2014;(18 Suppl 1):28–33. doi: 10.1111/1744-9987.12205. http://dx.doi.org/10.1111/1744-9987.12205. [DOI] [PubMed] [Google Scholar]
- 30.Horne R, Chapman SC, Parham R, Freemantle N, Forbes A, Cooper V. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One. 2013;8:e80633. doi: 10.1371/journal.pone.0080633. http://dx.doi.org/10.1371/journal.pone.0080633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riley S, Bradley R, Craig K, Wells L. Examining patient perceptions of phosphate binders. Br J Renal Med. 2007;12:19–21. [Google Scholar]
- 32.Lam WY, Fresco P. Medication adherence measures: an overview. Biomed Res Int. 2015;2015:217047. doi: 10.1155/2015/217047. http://dx.doi.org/10.1155/2015/217047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–54. doi: 10.1111/j.1751-7176.2008.07572.x. http://dx.doi.org/10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Tan X, Patel I, Chang J. Review of the four item Morisky Medication Adherence Scale (MMAS-4) and eight item Morisky Medication Adherence Scale (MMAS-8) Innov Pharm. 2014;5(3) Article 165. http://pubs.lib.umn.edu/innovations/vol5/iss3/5/ [Google Scholar]
- 35.Park H, Rascati KL, Keith MS, Hodgkins P, Smyth M, Goldsmith D, Akehurst R. Cost-effectiveness of lanthanum carbonate versus sevelamer hydrochloride for the treatment of hyperphosphatemia in patients with end-stage renal disease: a US payer perspective. Value Health. 2011;14:1002–9. doi: 10.1016/j.jval.2011.05.043. http://dx.doi.org/10.1016/j.jval.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 36.Brennan A, Akehurst R, Davis S, Sakai H, Abbott V. The cost-effectiveness of lanthanum carbonate in the treatment of hyperphosphatemia in patients with end-stage renal disease. Value Health. 2007;10:32–41. doi: 10.1111/j.1524-4733.2006.00142.x. http://dx.doi.org/10.1111/j.1524-4733.2006.00142.x. [DOI] [PubMed] [Google Scholar]
- 37.Gros B, Galan A, Gonzalez-Parra E, Herrero JA, Echave M, Vegter S, Tolley K, Oyaguez I. Cost effectiveness of lanthanum carbonate in chronic kidney disease patients in Spain before and during dialysis. Health Econ Rev. 2015;5:49. doi: 10.1186/s13561-015-0049-3. http://dx.doi.org/10.1186/s13561-015-0049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]