Abstract
Background
Preclinical and clinical studies suggest that a fish oil-based diet may play a role in delaying the progression of prostate cancer through a number of different mechanisms involving inflammatory pathways. Given the importance of tumor-associated macrophages (TAMs) in carcinogenesis, we hypothesized that a fish oil-based diet will inhibit TAM infiltration and delay the growth of prostate cancer.
Methods
Androgen sensitive mouse prostate cancer (MycCaP) allograft tumors were grown in fully immunocompetent FVB mice fed a high- fat fish oil (omega-3) or corn oil (omega-6) diet. Gene expression of markers for immune cell populations, cytokines, chemokines and signaling pathways were determined by real-time PCR and western blot in tumor tissue. Cell proliferation and apoptosis in vitro were measured by MTS assay and flow cytometry.
Results
Tumor volumes were significantly smaller in mice in ω-3 vs the ω-6 group (P=0.048). Gene expression of markers for M1 and M2 macrophages (F4/80, iNOS, ARG1), associated cytokines (IL-6, TNF alpha, IL-10) and the chemokine CCL-2 were also lower in the omega-3 group. Correlative in vitro studies were performed in M1 and M2 polarized macrophages and mirrored the in vivo findings. Dietary fish oil and in vitro omega-3 fatty acid administration reduced protein expression of transcription factors in the nuclear factor kappa B pathway leading to a significant decrease in gene expression of downstream targets (Bcl-2, BCL-XL, XIAP, survivin) in MycCap cells.
Conclusions
These findings underscore the potential of fish oil in modulating the clinical course of human prostate cancer through the immune system. Further preclinical and clinical studies are warranted evaluating fish oil-based therapies for inhibiting the recruitment and function of M1 and M2 tumor infiltrating macrophages.
Keywords: Fish oil, omega-3 and omega-6 fatty acid, tumor-associated macrophages, allograft prostate tumor, FVB mouse
Introduction
In animal studies, fish oil derived omega-3 fatty acids (ω-3 FAs), primarily docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), delay the development and progression of prostate cancer [1-4]. Epidemiologic studies generally support the preventive role of dietary fish and omega-3 fatty acids [5-7]. This association, however, is not supported by other reports [8, 9]. Short-term prospective clinical trials suggest favorable effects of dietary fish oil [10, 11]. In a pre-prostatectomy study, a low-fat diet with fish oil supplementation resulted in a decrease in the Ki67 index and the Cell Cycle Progression Score in prostate cancer tissue compared with a Western diet [12]. Likewise, in an observational trial in men on active surveillance, prostate tissue EPA levels were inversely correlated with prostate cancer progression [13]. To date, there are no long-term prospective randomized trials evaluating dietary fish oil for prostate cancer prevention and treatment.
Multiple mechanisms have been proposed to explain the anticancer effects of omega-3 fatty acids on prostate cancer [14]. The anti-inflammatory effects of fish oil have been well described [15], and may play a role in the anticancer effects observed in tissue culture and animal models [16, 17]. Macrophages are the most abundant inflammatory cells in the tumor microenvironment and higher macrophage infiltration is associated with more aggressive prostate cancer [18]. Distinct states of polarized TAMs have been identified: the ‘classically’ activated (M1) macrophages produce pro-inflammatory cytokines and have tumoricidal activity while the ‘alternatively’ activated (M2) macrophages make up the majority of TAMs and are pro-angiogenic, have immunosuppressive effects, and promote tumor progression and metastasis [19-21]. In co-culture studies, Li et al. reported that in vitro administration of conditioned medium generated from EPA/DHA-treated M2-type macrophages decreased PC3 cell migration and invasion [22]. The clinical importance of M2 macrophages is highlighted by the finding that up to 40% of cells in prostate cancer bone metastases in warm autopsy studies are M2 macrophages [23]. Zarif et al. proposed that specific strategies targeting M2 macrophage recruitment and function may play an important role in prostate cancer therapeutics [23].
Based on the anti-inflammatory and potential immunomodulatory effects of fish oil-derived omega-3 fatty acids, we sought to determine if an omega-3 diet alters inflammatory and immune cell infiltration in prostate tumors. To accomplish this we used an allograft model system in which mouse prostate cancer cells (MycCap) were implanted subcutaneously into fully immunocompetent FVB mice [24]. We evaluated gene expression of marker genes for tumor associated immune cells and associated inflammatory chemokines and cytokines. We hypothesized that a fish oil-based diet would result in decreased expression of genes characteristic for TAMs and would decrease the pro-inflammatory milieu in the tumor microenvironment.
Materials and methods
Chemicals and reagents
DHA was obtained from Cayman Chemical (Ann Harbor, MI, USA). RPMI and DMEM media and fetal bovine serum, were purchased from Invitrogen (Carlsbad, CA, USA); p-IKKβ, total IκBα antibodies from Cell Signaling (Beverly, MA, USA) and LPS, human IL-4, IL-13 from Sigma Chemical (St Louis, MO, USA).
Diet
The diets were prepared by DYETS, Inc. (Bethlehem, PA). In the ω-6 diet 30% of energy (134g/kg) was provided by corn oil, and the ω-6 to ω-3 ratio was 18:1. In the ω-3 diet 30% of energy was provided by menhaden oil (134g/kg) and the ω-6 to ω-3 ratio was 1:8 (Supplemental Table 1).
Animal Husbandry, Feeding Protocol, and MycCaP Allograft Tumors
Thirty male FVB mice (8 weeks old) were obtained from Jackson Laboratory (Bar Harbor, ME, USA). The mice were housed individually to monitor and measure food intake. The experiments were approved by the UCLA Animal Research Committee, and animals were cared for in accordance with institutional guidelines. Mice were acclimated for 7-days on a standard AIN- 93 G diet (DYETS, Bethlehem, PA). 5×105 MycCap cells, derived from the FVB genetic background [24] , generously provided by Dr. L. Wu, UCLA, were injected subcutaneously into the flank. When tumor volume reached 30-50 mm3, the mice were randomly assigned to either the omega-3 or omega-6 diet (n=15). In a palatability study we found that caloric intake was the same for the omega-3 and omega-6 diets, therefore a pair-feeing design was not required to maintain equal caloric intake between the groups. Body weight, food intake, and tumor volume were measured three times per week. Mice were sacrificed 35 days after starting the omega-3 and omega-6 diets. Tumor tissue was weighed and rinsed with saline. Half of the tumor tissue was snap-frozen in liquid nitrogen, and the other half fixed for 24 h in 10% neutral buffered formalin and embedded in paraffin blocks for histological sectioning
Fatty Acid Analysis of Red Blood Cell Membranes and Mouse Diets
The fatty acid analysis of diets and red blood cells was performed as previously described (1). Fatty acids were converted to methyl esters (FAME) in a methanol/benzene mixture (7:3 vol:vol) and quantified using an Agilent Technologies (San Diego, CA) 7890A gas chromatograph. The ratio of omega-3 to omega-6 fatty acids was calculated using the sum of linolenic acid, eicosapentaenoic acid, docosapentaenoic acid, docosahexaenoic acid for omega-3 and linoleic acid, eicosadienoic acid and arachidonic acid for omega-6 fatty acids.
F4/80 and Ki67 Tissue Staining
Paraffin-embedded sections were cut at 4 μm thickness and paraffin removed with xylene and rehydrated through graded ethanol. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 10 min. Proteolytic induced epitope retrieval (PIER) was carried out with proteinase K (Dako, S3020, Carpinteria, CA) at 37°C for 10 min. Tissue was incubated with primary antibody F4/80 at 1:50 dilution, diluted with BSA at 4°C overnight, and Ki67 at 1:100 dilution for 1 hr followed by 30 min with secondary polyclonal rabbit anti-rat immunoglobulin/biotinylated (Dako, E0468, Carpinteria, CA) 1:20. The signal was detected using the mouse DAKO horseradish peroxidase EnVision kit (DAKO) and anti-rabbit HRP polymer and visualized with the diaminobenzidine reaction. The sections were counterstained with hematoxylin.
mRNA Isolation and Quantitative PCR
Total RNA was isolated from tumors using RNeasy Mini kit (Qiagen, Valencia, CA) according to the manufacturer's protocols. The reverse-transcriptional PCR and quantitative real-time PCR were performed as previously described [25]. Briefly, first-strand cDNA was synthesized using MLV-Reverse Transcriptase and random hexamers (Promega, Madison, MI, USA). Quantitative PCR was performed using a Universal SYBR Green mastermix (Applied Biosystems, Grand Island, NY, USA) on CFX96 Real time PCR system (Bio-Rad, Hercules, CA, USA). Gene expression was calculated after normalization to GAPDH using the ΔΔCT method and expressed as relative mRNA level compared to control. Primer sequences used in the experiments are listed in Supplemental Table 2 and 3.
M1 and M2 Macrophage Differentiation and MycCaP Cell Culture
Human THP-1 monocytic cells were obtained from American Type Culture Collection (Manassas,VA, USA). Mouse prostate cancer cell line MycCap was a gift from Dr. Lily Wu (UCLA, CA). MycCap cells were authenticated by measuring gene expression of human c-myc using qPCR and overexpression of c-myc was confirmed. MycCap cells were maintained in DMEM medium and other cell lines (PC-3 and DU145) were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin (10 ng/mL penicillin and 10 U/mL streptomycin), and 2.5mM glutamine at 37°C in a humidified 5% CO2 incubator. M1 and M2 macrophage differentiation was performed as previously described with some modification [26]. To generate M1-type macrophages, THP-1 cells were treated with 320 nM phorbol 12-myristate 13-acetate (PMA; Sigma). After 24 h, PMA was removed and cells were treated with 10ng/ml lipopolysaccharide (LPS) for 3 h. For THP-1 M2-type macrophage differentiation, cells were treated with 320 nM PMA (Sigma). After 24 h, PMA was removed and cells were treated with 20 ng/mL interleukin (IL)-4, and 20 ng/mL IL-13 (Sigma, USA) for an additional 24 h. M1 macrophage polarization was confirmed by increased gene expression of IL-6, TNF-α and MCP-1 but not IL-10 (Supplementary Figure 1A). M2-type macrophage characteristics were confirmed by increased gene expression of IL-10, IL-1Ra, and CCL17 but not IL-6 and TNF-α (Supplementary Figure 1B).
Cell Viability Assay
Cell viability was determined by CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega). Briefly, cells were seeded into 96-well plates and then treated with different doses of DHA (omega-3 fatty acid) and linoleic acid (omega-6 fatty acid). Ethanol was used as a vehicle control. After 24 h of treatment, 20 μL of MTS and PMS were added and incubated at 37°C for 4 h, when the colorimetric change was measured with a microplate reader at 490 nM.
Flow Cytometry and Apoptosis Assay
Vehicle control or DHA-treated cells were stained with annexin V-phycoerythrin and 7-amino-actinomycin D (7-AAD) according to the manufacturer's directions (Apoptosis Detection Kit; BD Biosciences). The percentage of apoptotic cells was determined by flow cytometry (BD Biosciences).
Western blotting
The western blots were performed as previously described [27, 28]. Immunoblots were probed with antibodies against phospho-inhibitor of nuclear factor kappa-B kinase beta (IKKβ), total IKKβ. Total IKappaB-alpha, GAPDH (Cell Signaling, Beverly, MA, USA).
Statistical Analysis
Quantitative measures were compared between the two groups (omega-3 and omega-6 diet) using two-tailed Student's t test calculated by GraphPadPrism6.0 software (GraphPad Software, La Jolla CA). The data are represented as mean ± standard deviation (SD) or standard error of the mean (SEM). In vitro experiments were performed in triplicate. Tumor growth was compared between groups by fitting a piece-wise linear GEE model [29]. This model fit tumor size over time with two separate linear components, first the early phase between day 10 and 42 and a later phase after day 42. Our primary comparison was the difference in growth rate after day 42. Data are represented by the mean ± SEM. A p-value <0.05 was considered statistically significant.
Results
Effect of Dietary ω-3 FAs on MycCap Tumor Growth
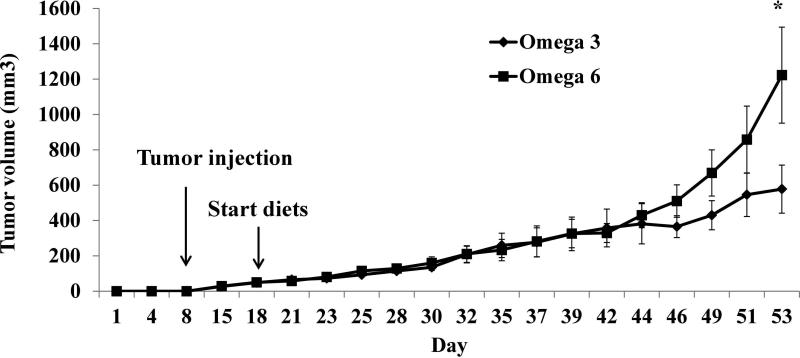
There was no significant difference in mean caloric intake or mouse body weight between mice on the ω-6 and ω-3 diets (Supplementary Figure 2A, B). Starting at day 43 tumors from mice fed the ω-3 diet had significantly slower growth rates (p=0.046) compared with tumors from mice fed the ω-6 diet. The tumor volume was significantly smaller at day 53 in mice fed the ω-3 vs the ω-6 diet (P=0.048) (Figure 1A). There was no difference in the mean Ki-67 index of the tumors between the ω-6 and ω-3 groups.
Figure 1.
Effect of ω-3 diet on tumor volume. Thirty eight-week-old male immunocompetent FVB mice were fed AIN93G diet for 1 week prior to injection of 5 × 105 MycCap in the flank. When tumor volumes reached 30-50 mm3, the mice were randomly assigned to either the ω-3 or ω-6 diet (n=15 for ω-6 diet group; n=14 for ω-3 diet group). Data are presented as the mean ±SEM; Student's t-test was used to compared ω-3 diet with the ω-6 diet (*, p<0.05).
Effect of ω-3 FAs on Gene expression of Markers of Tumor-Associated Macrophages In Vivo and In Vitro
MycCap tumor tissue had significant infiltration of macrophages as demonstrated by F4/80 immunohistochemistry (Supplementary Figure 3A). Gene expression of markers for total macrophages (F4/80) and M1 (iNOS) and M2 (ARG1, CD206, CD204) macrophages were significantly lower in tumor tissue from the ω-3 group as compared to the ω-6 group (Figure 2A). To test whether these markers were specific for macrophages or expressed in MycCap cells, gene expression of the same markers were measured in MycCap cells in vitro. With the exception of Arg 1, none of the markers were expressed in MycCap cells (Supplementary Figure 3B). Gene expression of the chemokine CCL2 and cytokines IL-6, IL-10, and TNF-α, was significantly lower in the ω-3 group tumor tissue vs the ω-6 group tumors (Figure 2B). In addition the protein concentration of phospho-IKKβ was lower and total IκBα was higher in the ω-3 vs the ω-6 diet group (Figure 3 A-C).
Figure 2.
Effect of ω-3 diet on gene expression of immune cell markers and cytokine expression. A. mRNA expression level of markers of general macrophages (F4/80), M1 (iNOS) and M2 (ARG1, CD206, CD204) macrophages were determined using real-time qPCR relative to GAPDH mRNA. B. mRNA expression level of cytokines associated with general macrophages (CCL2), M1 (IL-6, TNF-α) and M2 (IL-10) was determined using real-time qPCR relative to GAPDH mRNA. n=15 for ω-6 diet group and n=14 for ω-3 diet group. Data are presented as the mean ±SEM; Student's t-test was used to compared ω-3 diet with the ω-6 diet (*, p<0.05).
Figure 3.
Feeding the ω-3 diet down regulated NF-κB in mouse tumor. A. Western blot analysis of p-IKKβ and total IκBα protein level, Lane 1 to 7 are protein samples from seven different tumors from the ω-6 diet group and lane 8 to 14 contain individual protein samples from seven tumors from the ω-3 diet group. The GAPDH band was used for protein loading control. B and C. The optimized bands were quantified by intensity and shown as an average of each diet group for protein levels normalized by GAPDH band. n=15 for ω-6 diet group and n=14 for ω-3 diet group. Data are presented as the mean ±SD; Student's t-test was used to compared ω-3 diet with the ω-6 diet (*, p<0.05).
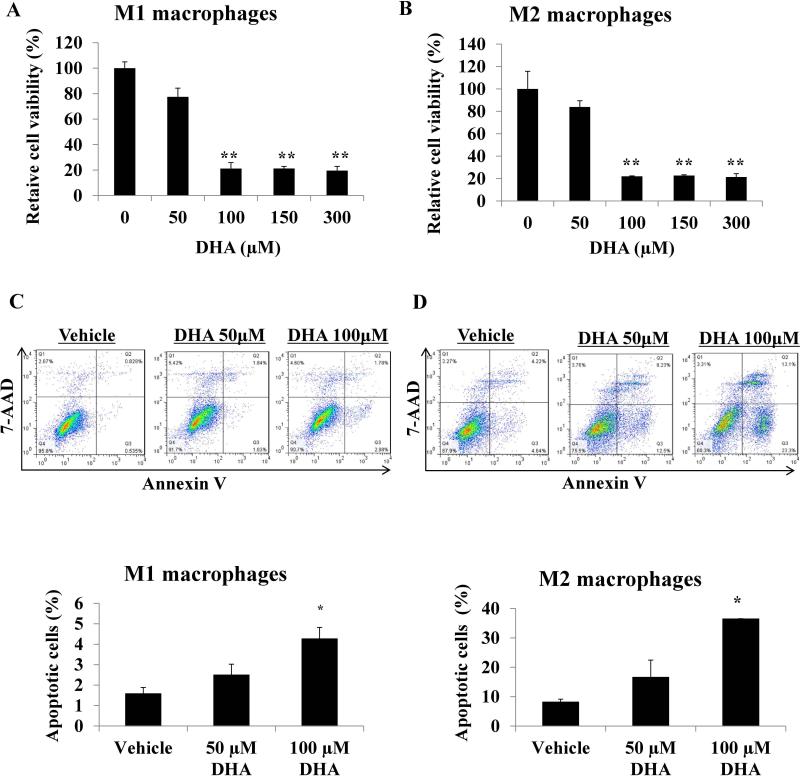
The in vitro effects of DHA on M1 and M2 polarized macrophages mirrored the in vivo findings. DHA treatment decreased viability and induced apoptosis in both macrophage subtypes (Figure 4 A-D), and decreased expression of M1 macrophage derived cytokines IL-6 and TNF-α, and the M2 derived cytokine IL-10 (Supplementary Figure 4 A and B). DHA also inhibited the NF-κB pathway (Supplementary Figure 4A-C). Treatment with the ω-6 FA linoleic acid did not affect the viability of M1 and M2 macrophages. (Supplementary Figure 5A and B).
Figure 4.
Effects of different doses of docosahexaenoic acid (DHA) on M1 and M2 macrophage cell viability and apoptosis. THP-1 cells were treated with 320 nM phorbol 12-myristate 13-acetate (PMA; Sigma). After 24 h, PMA was removed and cells were treated with 10ng/ml lipopolysaccharide (LPS) for 3 h to get M1 macrophages. For THP-1 M2 macrophages differentiation, cells were treated with 320 nM PMA (Sigma). After 24 h, PMA was removed and cells were treated with 20 ng/mL interleukin (IL)-4, and 20 ng/mL IL-13 (Sigma, USA) for an additional 24 h. A. M1 and B. M2 macrophages were incubated with DHA overnight, cell viability was analyzed by MTS assay. C and D. M1 and M2 macrophages were incubated with DHA overnight, cells were collected and stained with Annexin V and 7-AAD, apoptosis was analyzed by flow cytometry. Data are presented as the mean ± SD (*, p<0.05; **, p<0.01).
Effect of ω-3 FAs on Gene Expression of Markers of Other Tumor Infiltrating Immune Cells in MycCaP Tumors
Gene expression of the B cell surface marker B220 was higher in tumor tissue from mice fed the ω-3 diet compared to the ω-6 diet (Table 1). There was a trend for higher gene expression of the T cell marker CD4+ (p=0.10) in ω-3 fed mice compared to ω-6 fed mice (Table 1). There was no significant difference between the two groups in gene expression of markers for CD8+ T cells, MDSC's, NK cells and neutrophils (Table 1). There was a trend for lower expression of a B cell chemokine (CXCL13) and T cell chemokines (CXCL9, CXCR3) (p=0.103, 0.096 and 0.09 respectively) in the tumor tissue from mice fed the ω-3 vs ω-6 diet.
Table 1.
Effects of ω-3 diet on mRNA levels of immune cell markers.
| Immune cell type | Neutrophil | MDSC | CD4+ T-cell | CD8+ T-cell | B-cell | NK cell |
|---|---|---|---|---|---|---|
| Immune marker | CXCR1 | CSF-1R | CD4 | CD8 | B220 | NK1.1 |
| ω-6 | 1±0.11 | 1±0.11 | 1±0.06 | 1±0.4 | 1±0.27 | 1±0.21 |
| ω-3 | 0.4±0.04 | 0.48±0.04 | 1.7±0.10 | 1.4±0.49 | 2.2±0.44 | 1.1±0.49 |
| T test (p-value) | 0.47 | 0.38 | 0.10 | 0.27 | 0.04 | 0.85 |
Gene expression is evaluated relative to data from mice fed the ω-6 diet.
Fatty Acid Content of the Diets and Mouse Red Blood Cells
Fatty acid analysis of the diets confirmed that they contained appropriate levels of ω-6 and ω-3 fatty acids (Supplementary Figure 6A). Likewise, consumption of the diets resulted in higher levels of EPA, DPA and DHA (ω-3 fatty acids) in red blood cells in the ω-3 diet group, and higher levels of linoleic acid (ω-6 fatty acid) in the ω-6 group (Supplementary Figure 6B).
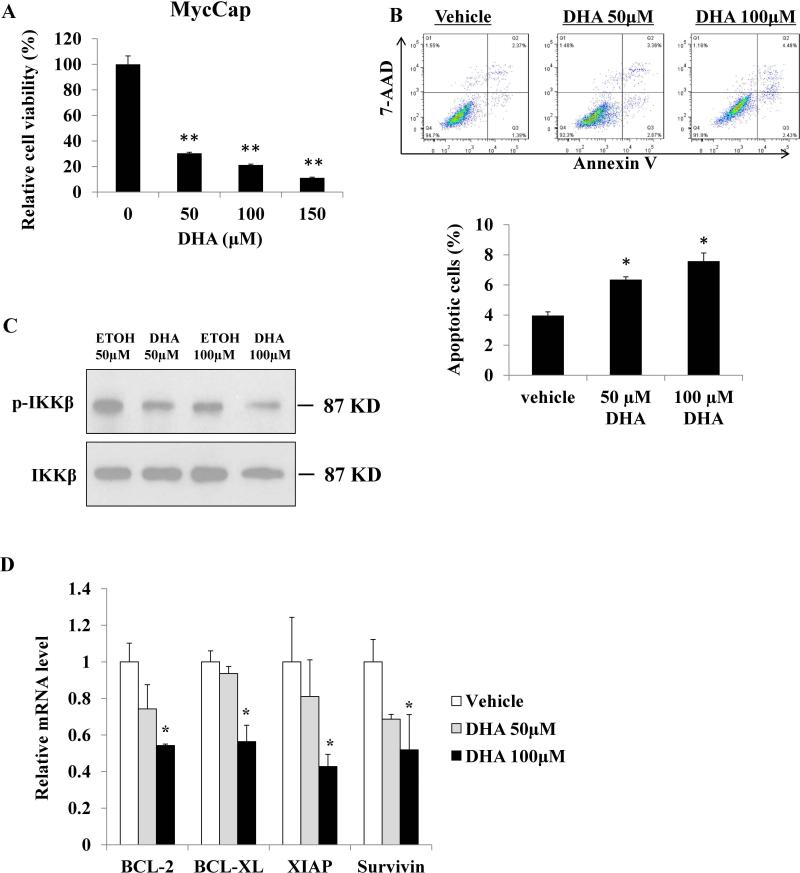
In vitro Effects of DHA and Linoleic acid on MycCaP Cells
DHA inhibited viability and induced apoptosis in MycCaP cells and human prostate cancer cells PC-3 and DU145 in vitro (Figure 5A, B and Supplementary Figure 7A). In addition, DHA inhibited protein expression of members of the NF-κB pathway in MycCaP cells and decreased gene expression of NF-κB pathway target genes BCL-2, BCL-XL, XIAP and survivin (Figure 5C and D). Treatment with the ω-6 FA linoleic acid did not affect the viability of MycCap cells (Supplementary Figure 7B).
Figure 5.
Effects of DHA on MycCap cell viability, apoptosis and NF-κB pathway. A. MycCap cells were incubated with DHA overnight, cell viability was analyzed by MTS assay. B. MycCap cells were incubated with DHA overnight, cells were collected and stained with Annexin V and 7-AAD and apoptotic cells were analyzed by flow cytometry. C. MycCap cells were incubated with DHA or ethanol as vehicle control overnight, protein level of p-IKKβ and total IKKβ was determined by Western blot. D. MycCap cells were incubated with DHA or ethanol as vehicle control overnight, mRNA level of NF-KB apoptosis-associated target genes were determined by real-time qPCR. Data are presented as the mean ± SD (*, p<0.05; **, p<0.01).
Discussion
Previous studies reported that fish oil-based diets decreased tumor growth in various mouse models of prostate cancer using immunocompromised mouse strains [1, 2], and immunocompetent transgenic models [2]. The allograft mouse model used in the present study offers the advantage of evaluating the effect of dietary fish oil on host immune cell infiltration and function. MycCap tumor tissue showed significant infiltration of macrophages as demonstrated by F4/80 immunohistochemistry, making this an ideal model to study the effect of dietary fish oil on TAMs. The most intriguing finding in the present trial is that tumor tissue from the fish oil group had decreased gene expression for markers of total macrophages and M1 and M2 macrophages, and decreased gene expression of CCL-2, a chemokine recruiting monocytes and macrophages to the site of inflammation. DHA also inhibited M1 and M2 macrophage function in vitro. Given the critical role of TAMs in prostate cancer development, progression and metastasis, these findings lay the foundation for future investigations on the potential role of dietary fish oil as a form of “nutritional immunotherapy” for prostate cancer patients.
Cancer cells in the prostate coexist in the stromal microenvironment with a variety of cells including fibroblasts, endothelial cells, immune cells and other stromal cells. Tumor and stromal cells actively promote a pro-inflammatory environment by secreting cytokines, chemokines and growth factors attracting tumor infiltrating immune cells. Tumor-associated macrophages make up a major portion of the immune cells in the stroma. Interferon gamma and LPS induce polarization to M1 macrophages, which produce nitric oxide and pro-inflammatory cytokines such as IL-6 and TNF alpha. M2 macrophages (induced by IL-4 and IL-13) make up the majority of TAMs and promote tumor growth, angiogenesis, epithelial to mesenchymal transition, and metastasis [30]. Preclinical and clinical studies are investigating therapies targeting M2 macrophage recruitment, survival, and function for a variety of malignancies including breast, lung, and prostate cancer [31]. For example, anti CCL-2 therapy appears to have significant activity against breast cancer [32]. In the present study, a fish oil-based diet was found to delay tumor progression (as compared to a corn oil-based diet), decrease gene expression of CCL2, decrease levels of M2 macrophage markers (ARG1, CD206, CD204), and decrease IL-10 gene expression in the tumor tissue, an immunosuppressive cytokine produced by M2 macrophages. M1 macrophages have been described as tumoricidal, but also as supporting tumor growth [30]. Since in the present study the fish oil intervention led to a decreased tumor volume and gene expression of markers for M1 macrophages were significantly lower compared to the corn oil group, we hypothesize that M1 macrophages in this mouse model support tumor growth. Supporting this hypothesis is the observation that gene expression of IL-6 and TNF alpha, which are secreted in part by M1 macrophages and may promote prostate cancer progression, were significantly decreased in the tumor tissue.
As part of the adaptive immune response macrophages communicate with T and B cells by displaying antigens or secreting chemokines. In the present study there was no significant difference between the diet groups in cell surface markers for MDSCs, CD8+ T cells, and NK cells in the tumor tissue. The cell surface marker for B cells (B220) was significantly higher in the fish oil vs corn oil group and there was a trend for increase in the marker for CD4+ T cells. There was also a trend for higher gene expression of T cell cytokines CXCL9 and CXCL3 and the B cell cytokine CXCL13. Noy R. et al recently reviewed multiple potential mechanisms whereby TAMS inhibit the host immune response including expression of ligands of the inhibitory receptors programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4), secretion of IL-10 and TGF-beta, and depletion of L-arginine [30]. Possibly the fish oil-induced reduction in M2 macrophage activity, through altering levels of IL-10 (immunosuppressive cytokine), resulted in higher number of B cells and a trend for higher CD4+ cells. This hypothesis, however, would not explain the lack of an effect on CD8+ T cells. Possibly a longer term dietary intervention would result in more significant changes in the host immune response. It has previously been reported that androgen ablation caused infiltration with leukocytes, including B cells, leading to IKK-β activation and androgen-free survival of prostate cancer [33]. However, in the setting of androgen sensitive prostate cancer, as was the case in the prese nt experiment, the role of increased B cell markers is uncertain. Future experiments are warranted evaluating the effect of fish oil on the microenvironment and the host immune response in immunocompetent mouse models.
In prior studies, ω-3 FAs were found to inhibit prostate cancer progression through a number of mechanisms including inhibition of cyclooxygenase 2 mediated prostaglandin E2 formation, lipoxygenase activity, toll-like receptors, formation of pro-resolvin metabolites, activation of PPARγ and inhibition of NF-κB [34]. In the present study, dietary ω-3 FAs decreased IKKβ phosphorylation, increased total IκBα and inhibited gene expression of NF-κB target genes in MycCap cells in vitro and in the tumor tissue. The NF-κB inhibitory effect of ω-3 FAs was also reported by Li et al. in PC-3 cells treated with conditioned medium from DHA-treated macrophages (22). Activation of NF-κB signaling provides a critical link between inflammation and cancer progression Through up-regulation of tumor promoting inflammatory cytokines (IL-6, TNF-a), and survival genes (Bcl-XL) NF-κB inhibits apoptosis and enhances tumor growth, and metastases [35]. The mechanism through which ω-3 FAs inhibit NF-κB signaling pathway is under investigation. Several receptors of DHA including GPR120 and PPARγ have been reported to play a role in the regulation of the NF-kB pathway [36, 37]. Further studies are required to elucidate the mechanisms whereby ω-3 FAs regulate the NF-kB pathway.
The fact that fish oil appears to inhibit prostate cancer progression through multiple mechanisms complicates our ability to define specific mechanisms in animal models. That being said, affecting multiple pathways directly against cancer cells and indirectly through effects on the host immune response represents a potential strength with regards to the clinical utility of fish oil-based interventions. Whereas malignancies are known to develop resistance mechanisms against treatments targeting specific pathways, fish-oil based therapies may offer the benefit of targeting multiple pathways and potentially offsetting host resistance mechanisms. Prospective trials with appropriate tissue studies are required in men with varying stages of prostate cancer to elucidate the biological effects of ω-3 FAs on prostate cancer tissue and the tumor microenvironment. A randomized prospective trial is underway evaluating a fish oil-based diet in men on active surveillance with tissue markers being evaluated at baseline and after a 1-year intervention (NCT02176902). Three other prospective trials on fish oil are described in ClinicalTrials.Gov. Fish oil-based diets may potentially be effective as monotherapy, or combined with targeted therapies to improve efficacy and potentially offset side effects. Future preclinical and clinical studies are required to determine the potential of fish oil-based diets to be used as nutritional immunotherapy in patients with prostate cancer.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by National Institute of Health [P50CA92131 to W.J.A.] and Department of Defense Prostate Cancer Research Program [PC141593 to P.L.].
Footnotes
Disclosure Statement: None of the authors has any conflict of interest.
Supplementary material
Supplementary Figures 1-7 and Tables 1-3 can be found in the online version of this article at the publisher's web-site.
REFERENCES
- 1.Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, Leung P, Hong-Gonzalez J, Freedland SJ, Said J, Gui D, Seeram NP, Popoviciu LM, Bagga D, Heber D, Glaspy JA, Aronson WJ. Effect of altering dietary omega-6/omega-3 fatty acid ratios on prostate cancer membrane composition, cyclooxygenase-2, and prostaglandin E2. Clin Cancer Res. 2006;12:4662–4670. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, Edwards IJ, D'Agostino R, Zhang H, Wu H, Kang JX, Chen YQ. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saw CL, Wu TY, Paredes-Gonzalez X, Khor TO, Pung D, Kong AN. Pharmacodynamics of fish oil: protective effects against prostate cancer in TRAMP mice fed with a high fat western diet. Asian Pac J Cancer Prev. 2011;12:3331–3334. [PubMed] [Google Scholar]
- 4.Lloyd JC, Masko EM, Wu C, Keenan MM, Pilla DM, Aronson WJ, Chi JT, Freedland SJ. Fish oil slows prostate cancer xenograft growth relative to other dietary fats and is associated with decreased mitochondrial and insulin pathway gene expression. Prostate Cancer Prostatic Dis. 2013;16:285–291. doi: 10.1038/pcan.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CG, Willett WC. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 6.Leitzmann MF, Stampfer MJ, Michaud DS, Augustsson K, Colditz GC, Willett WC, Giovannucci EL. Dietary intake of n-3 and n-6 fatty acids and the risk of prostate cancer. Am J Clin Nutr. 2004;80:204–216. doi: 10.1093/ajcn/80.1.204. [DOI] [PubMed] [Google Scholar]
- 7.Terry P, Lichtenstein P, Feychting M, Ahlbom A, Wolk A. Fatty fish consumption and risk of prostate cancer. Lancet. 2001;357:1764–1766. doi: 10.1016/S0140-6736(00)04889-3. [DOI] [PubMed] [Google Scholar]
- 8.Brasky TM, Till C, White E, Neuhouser ML, Song X, Goodman P, Thompson IM, King IB, Albanes D, Kristal AR. Serum phospholipid fatty acids and prostate cancer risk: results from the prostate cancer prevention trial. Am J Epidemiol. 2011;173:1429–1439. doi: 10.1093/aje/kwr027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SY, Wilkens LR, Henning SM, Le ML, Gao K, Goodman MT, Murphy SP, Henderson BE, Kolonel LN. Circulating fatty acids and prostate cancer risk in a nested case-control study: the Multiethnic Cohort. Cancer Causes Control. 2009;20:211–223. doi: 10.1007/s10552-008-9236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronson WJ, Kobayashi N, Barnard RJ, Henning S, Huang M, Jardack PM, Liu B, Gray A, Wan J, Konijeti R, Freedland SJ, Castor B, Heber D, Elashoff D, Said J, Cohen P, Galet C. Phase II prospective randomized trial of a low-fat diet with fish oil supplementation in men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2011;4:2062–2071. doi: 10.1158/1940-6207.CAPR-11-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JM, Weinberg V, Magbanua MJ, Sosa E, Simko J, Shinohara K, Federman S, Mattie M, Hughes-Fulford M, Haqq C, Carroll PR. Nutritional supplements, COX-2 and IGF-1 expression in men on active surveillance for prostate cancer. Cancer Causes Control. 2011;22:141–150. doi: 10.1007/s10552-010-9684-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galet C, Gollapudi K, Stepanian S, Byrd JB, Henning SM, Grogan T, Elashoff D, Heber D, Said J, Cohen P, Aronson WJ. Effect of a low-fat fish oil diet on proinflammatory eicosanoids and cell-cycle progression score in men undergoing radical prostatectomy. Cancer Prev Res (Phila) 2014;7:97–104. doi: 10.1158/1940-6207.CAPR-13-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreel X, Allaire J, Leger C, Caron A, Labonte ME, Lamarche B, Julien P, Desmeules P, Tetu B, Fradet V. Prostatic and dietary omega-3 fatty acids and prostate cancer progression during active surveillance. Cancer Prev Res (Phila) 2014;7:766–776. doi: 10.1158/1940-6207.CAPR-13-0349. [DOI] [PubMed] [Google Scholar]
- 14.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calder PC. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim Biophys Acta. 2015;1851:469–484. doi: 10.1016/j.bbalip.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Chung H, Lee YS, Mayoral R, Oh DY, Siu JT, Webster NJ, Sears DD, Olefsky JM, Ellies LG. Omega-3 fatty acids reduce obesity-induced tumor progression independent of GPR120 in a mouse model of postmenopausal breast cancer. Oncogene. 2015;34:3504–3513. doi: 10.1038/onc.2014.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng H, Shen Y, Shen J, Zhou F, Shen S, Das UN. Effect of n-3 and n-6 unsaturated fatty acids on prostate cancer (PC-3) and prostate epithelial (RWPE-1) cells in vitro. Lipids Health Dis. 2013;12:160. doi: 10.1186/1476-511X-12-160. doi: 10.1186/1476-511X-12-160.:160-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanciotti M, Masieri L, Raspollini MR, Minervini A, Mari A, Comito G, Giannoni E, Carini M, Chiarugi P, Serni S. The role of M1 and M2 macrophages in prostate cancer in relation to extracapsular tumor extension and biochemical recurrence after radical prostatectomy. Biomed Res Int. 2014;2014:486798. doi: 10.1155/2014/486798. doi: 10.1155/2014/486798. Epub;%2014 Mar 11.:486798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33:2423–2431. doi: 10.1038/onc.2013.191. [DOI] [PubMed] [Google Scholar]
- 20.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–46. doi: 10.1146/annurev-physiol-021909-135846. doi: 10.1146/annurev-physiol-021909-135846.:219-246. [DOI] [PubMed] [Google Scholar]
- 21.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 22.Li CC, Hou YC, Yeh CL, Yeh SL. Effects of eicosapentaenoic acid and docosahexaenoic acid on prostate cancer cell migration and invasion induced by tumor-associated macrophages. PLoS One. 2014;9:e99630. doi: 10.1371/journal.pone.0099630. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Zarif JC, Taichman RS, Pienta KJ. TAM macrophages promote growth and metastasis within the cancer ecosystem. Oncoimmunology. 2014;3:e941734. doi: 10.4161/21624011.2014.941734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 25.Liang P, Cheng SH, Cheng CK, Lau KM, Lin SY, Chow EY, Chan NP, Ip RK, Wong RS, Ng MH. Platelet factor 4 induces cell apoptosis by inhibition of STAT3 via up-regulation of SOCS3 expression in multiple myeloma. Haematologica. 2013;98:288–295. doi: 10.3324/haematol.2012.065607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tjiu JW, Chen JS, Shun CT, Lin SJ, Liao YH, Chu CY, Tsai TF, Chiu HC, Dai YS, Inoue H, Yang PC, Kuo ML, Jee SH. Tumor-associated macrophage-induced invasion and angiogenesis of human basal cell carcinoma cells by cyclooxygenase-2 induction. J Invest Dermatol. 2009;129:1016–1025. doi: 10.1038/jid.2008.310. [DOI] [PubMed] [Google Scholar]
- 27.Cheng Y, Geng H, Cheng SH, Liang P, Bai Y, Li J, Srivastava G, Ng MH, Fukagawa T, Wu X, Chan AT, Tao Q. KRAB zinc finger protein ZNF382 is a proapoptotic tumor suppressor that represses multiple oncogenes and is commonly silenced in multiple carcinomas. Cancer Res. 2010;70:6516–6526. doi: 10.1158/0008-5472.CAN-09-4566. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Liang P, Geng H, Wang Z, Li L, Cheng SH, Ying J, Su X, Ng KM, Ng MH, Mok TS, Chan AT, Tao Q. A novel 19q13 nucleolar zinc finger protein suppresses tumor cell growth through inhibiting ribosome biogenesis and inducing apoptosis but is frequently silenced in multiple carcinomas. Mol Cancer Res. 2012;10:925–936. doi: 10.1158/1541-7786.MCR-11-0594. [DOI] [PubMed] [Google Scholar]
- 29.Diggle Peter, Heagerty Patrick, Liang Kung-Yee, Zeger Scott, Diggle Peter J., Zeger Scott L. Analysis of Longitudinal Data. 2nd ed. Oxford University Press; 2002. [Google Scholar]
- 30.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jinushi M, Komohara Y. Tumor-associated macrophages as an emerging target against tumors: Creating a new path from bench to bedside. Biochim Biophys Acta. 2015;1855:123–130. doi: 10.1016/j.bbcan.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Knight DA, Snyder A, Smyth MJ, Stewart TJ. A role for CCL2 in both tumor progression and immunosurveillance. Oncoimmunology. 2013;2:e25474. doi: 10.4161/onci.25474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Im DS. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol. 2015:10. doi: 10.1016/j.ejphar.2015.03.094. [DOI] [PubMed] [Google Scholar]
- 35.Karin M. NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141. doi: 10.1101/cshperspect.a000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calder PC. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142:592S–599S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.