Abstract
Background
Obesity is a consequence of chronic energy imbalance. We need accurate and precise measurements of energy intake and expenditure, as well as the related behaviors, to fully understand how energy homeostasis is regulated in order to develop interventions and evaluate their effectiveness to combat the global obesity epidemic.
Scope of review
We provide an in-depth review of the methodologies currently used to measure energy intake and expenditure in humans, including their principles, advantages, and limitations in the clinical research setting. The aim is to provide researchers with a comprehensive guide to conduct obesity research of the highest possible quality.
Major conclusions
An array of methodologies is available to measure various aspects of energy metabolism and none is perfect under all circumstances. The choice of methods should be specific to particular research questions with practicality and quality of data the priorities for consideration. A combination of complementary measurements may be preferable. There is an imperative need to develop new methodologies to improve the accuracy and precision of energy intake assessments.
Keywords: Energy expenditure, Dietary assessment, Clinical study methodology
Highlights
-
•
Image-based technology is a significant step to improve energy intake measurement.
-
•
Physical activity informs patterns but not absolute energy expenditure.
-
•
Combining complementary measurements overcomes shortfalls of individual methods.
1. Introduction
Obesity is an escalating global epidemic with considerable personal and societal consequences. In 2014, the World Health Organization estimated that more than 1.9 billion adults (or 39% of the world population aged 18 years and over) are overweight and among those over 600 million are obese [1]. As a major risk factor for a range of chronic diseases including diabetes, cardiovascular diseases and certain cancers, overweight, and obesity have been attributed to cause 3.4 million deaths in 2010 [2]. Obesity also imposes substantial economic impacts. In addition to a ∼40% higher per capita medical spending than individuals with a healthy body weight [3], obesity also incurs indirect costs due to reduced workforce productivity, e.g. unable to work at full capacity, absent from work or premature mortality [4]. In the United States, it has been estimated that the national cost of obesity-related absenteeism ranges from $3 billion to $6 billion annually [5]. Despite the effort to create a supportive environment for healthy lifestyle and implementing aggressive interventions, there have been no indication for any declining trend to lessen the obesity epidemic [6].
Obesity, as a disease of excessive fat deposition, is essentially a consequence of chronic energy imbalance with energy intake consistently exceeding expenditure, which then leads to the storage of surplus energy in the white adipose tissue. While the solution to obesity appears to be as simple as reducing calorie intake (e.g. avoid energy-dense food) and increasing energy expenditure (e.g. increase physical activity), the failure of decades of public health initiatives to remove the “obesogenic” environmental factors clearly indicates that obesity is a problem far more complex than the prevailing wisdom of “low willpower”. Indeed, we are yet to fully understand the sophisticated interactions between genetics, physiology, and cognitive behavior that regulate energy homeostasis. One of the greatest challenges in obesity research is to accurately measure energy intake and expenditure, as well as the related behaviors. Such measurement tools would allow for identification of causal associations between energy homeostasis and health outcomes, inform on the mechanisms by which energy metabolism is regulated, and accurately define how energy balance changes in response to interventions. In this article, we provide an in-depth review of the methodologies used to measure energy intake and expenditure in humans, with the aim of providing researchers with a comprehensive guide to conduct obesity research of the highest possible quality.
2. A brief overview on energy homeostasis
2.1. Energy balance
Energy balance is the difference between energy intake and energy expenditure. Energy equilibrium, i.e., zero energy balance, is reached when metabolizable energy consumption matches perfectly with the amount of energy spent. A non-zero energy balance, on the other hand, must implicate an equal change in the energy content of the body in the form of changes in the weight of some body constituent [7] which subsequently alters energy expenditure. In the case of a positive energy balance, basal metabolic rate increases with weight gain because of the growth of lean mass to support the expended fat depots, and, conversely, a decrease in basal metabolic rate when a negative energy balance triggers a reduction in body mass. Assuming energy intake remains unchanged after the initial intervention, one might expect that energy expenditure will eventually match energy intake and body weight will be stabilized at a new set-point.
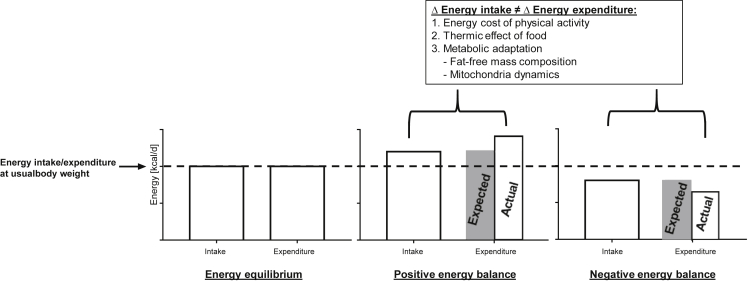
In reality, however, when there is persistent deviation from energy equilibrium, changes in energy expenditure is no longer predictable on the basis of metabolic mass. Our CALERIE study [8], among many others [9], [10], has shown that weight loss is associated with a reduction in 24-h energy expenditure up to 15% lower than what is predicted after adjustment for changes in body composition, whereas the opposite is observed in overfeeding [9], [11]. A recent study on morbidly obese individuals reported a lower resting metabolic rate immediately following intensive diet and exercise interventions, which continued to decline (from −275 to −499 kcal/d) at 6 years post-intervention despite significant regain of both lean and fat mass [12]. These data clearly suggest a disproportionate change in energy expenditure not only during, but also well beyond, the dynamic phase of weight change, a phenomenon known as metabolic adaptation or adaptive thermogenesis. The relationship between energy balance and metabolic adaptation is illustrated in Figure 1.
Figure 1.
Illustration of energy balance and metabolic adaptation. When energy intake equals to total energy expenditure, a state of energy equilibrium is reached, and the body weight stays at the usual set-point. When energy intake exceeds or falls below the level required to maintain the usual body weight, energy expenditure no longer matches the intake (indicated by the gray shaded area), with expenditure exceeding intake in positive energy balance and the reverse for negative energy balance. Most of this difference is explained by changes in energy cost of physical activity associated with a different body mass and thermic effect of food. Metabolic adaptation refers to the phenomenon in which energy expenditure is adjusted independent of metabolic mass, possibly via altered mitochondrial dynamics, as a potential mechanism to restore body weight to the usual set-point.
Besides the changes in energy expenditure related to the changes in fat-free and fat mass, another obvious factor for changes in 24-h energy expenditure is of course an altered energy cost of physical activity given a different body mass. Variations in food intake also modify the amount of energy spent on nutrient processing and assimilation (thermic effect of food; see Section 2.3 below). It is perhaps somewhat intriguing that basal metabolic rate also has been shown to be disproportionally regulated relative to metabolic mass [13], [14]. Some hypothesize a change in the fat-free mass composition. While this is supported by data from Bosy-Westphal and colleagues [15] that demonstrated a small and yet significant greater loss of organ mass with high metabolic activity (e.g. heart, liver and kidneys) as compared to the total loss of fat-free mass following weight reduction. In recent years, there is emerging evidence for a link between mitochondrial dynamics, nutrient availability, and energy expenditure, which implicates some form of cellular bioenergetics adaptation. Mitochondria in cells under starvation have been shown to increase the ratio of ATP produced per unit of nutrient consumption, whereas those in nutrient excess increase energy waste in the form of heat via mitochondrial proton leak (for details refer to a recent review by Liesa and Shirihai [16]). In other words, we adapt to changes in energy supply and demand by adjusting our capacity and/or efficiency of ATP synthesis. Prolonged changes in mitochondrial dynamics (e.g. chronic positive energy balance in obesity), however, may lead to mitochondrial dysfunction characteristic of metabolic diseases [17], [18].
2.2. Food intake
The regulation of food intake is primarily a feedback system that responds to both physiological (internal) and environmental (external) signals. These signals act directly on the brain or alter secretions from other organs, influencing ingestive behavior from meal size to diet selection, and impacting daily energy consumption. The majority of physiological signals come from organs that are involved in the acquisition and storage of nutrients (such as liver, adipose and skeletal muscle tissues), which inform on nutrient availability. Modulation of food intake occurs even before feeding begins. Sensory cues (taste, smell, texture and sight) and the thought or discussion of food are cephalic signals that trigger physiological response in preparation for a meal, which include increasing salivation and the secretion of gastric acid, orexigenic hormones, and insulin [19], [20]. Upon ingestion, gastric distension stimulates the vagal mechanosensitive fibers that contribute to postprandial satiety [21]. The direct contact of nutrients with the gastrointestinal tract also stimulates the secretion of satiation hormones, mainly cholecystokinin, peptide YY, glucose-dependent insulinotropic polypeptide (GIP), and glucagon-like peptide 1 (GLP-1). Collectively these hormones activate the proopiomelanocortin/cocaine-and amphetamine-related transcript (POMC/CART) neurons in the arcuate nucleus of the hypothalamus and the brainstem–vagus complex to suppress food intake (for details refer to reviews by Lancha et al. [22] and Scott et al. [23]). On the contrary, ghrelin is the only gastrointestinal hormone that has an orexigenic effect. Ghrelin is considered as one of the main hunger signals, as variations in its circulating level, which increases during fasting and peaks before meals, precedes the sensation of hunger, and parallels meal initiation patterns [24]. Once released, ghrelin stimulates the hypothalamus (via multiple pathways including neuropeptide Y (NPY)/agouti-related peptide (AgRP) secretion, AMP-activated protein kinase (AMPK) and mechanistic target of rapamycin (mTOR) signaling) and the vagus nerve to promote food intake [25]. In addition to direct communications with the brain, some gut hormones also modulate feeding behavior by interacting with other organs that regulate energy homeostasis. Known as the incretin effect, food ingestion increases the circulating levels of GLP-1 and GIP, which then bind to specific G protein-coupled receptors in the pancreatic β-cells and stimulate insulin biosynthesis and secretion. This is an integral mechanism to amplify the glucose signal and has been estimated to be responsible for up to 70% of postprandial insulin secretion [26].
While instant feedback from the gastrointestinal tract controls food intake during feeding, hormones that inform on the overall nutritional status act as signals to regulate longer-term ingestive behavior. Post-absorptive regulation of ingestive behavior is primarily driven by changes in circulating level of glucose. The pancreas acutely secretes insulin in response to the rise in blood glucose following a meal [27]. Activation of the insulin signaling cascade phosphorylates the transcription factor FOXO, which subsequently leads to POMC transcription in the hypothalamic arcuate nucleus and an anorectic effect [28]. The hypothalamus also contains glucose-sensing neurons that directly contribute to meal-related regulation of feeding [29].
The status of energy stores in the white adipose tissue provides signals for the longer-term level of food intake control to achieve long-term energy balance. Leptin, secreted primarily from the adipocytes, is a well-characterized “adiposity signal” to the brain, as its circulating concentration is positively correlated with fat mass [30]. Leptin functions via mechanisms similar to those of insulin; binding to the respective receptor activates downstream signaling pathways that converge at the level of phosphatidylinositol 3 kinase (PI3K) and triggers the POMC/CART neurons to stimulate the sensation of satiety [28]. In the case of chronic energy surplus, the expanded fat mass produces more leptin that is supposed to negatively impact on food intake to restore energy balance, although resistance to leptin appears to disrupt this defensive mechanism [31]. Starvation, on the other hand, triggers a fall in leptin level that precedes fat loss, and this temporary disassociation between adiposity and leptin production has been proposed as an important mechanism to efficiently compensate for the energy deficit to defend a set-point for adiposity [32].
Ingestive behavior is also shaped by external factors that are not directly involved in energy homeostasis but have more to do with preferences and eating patterns. Irrespective of physiological needs, the food per se determines whether or not and how much we decide to eat. Sweet and high-fat foods have a strong sensory appeal, which likely originates from our evolutionary preference for a concentrated source of energy [33]. Sweetness and fat content also provide aroma, flavor, and texture that are major contributors to food palatability. A review by Sorensen and colleagues [34] concludes that energy intake always increases with palatability, although whether palatability impacts subjective appetite sensations remains largely debatable. Another important factor that comes into play in the context of palatability is sensory perception, which is known to be altered by numerous factors, most notably age [35]. Other meal-associated factors, including portion size [36] and food variety [37], are also known to alter energy intake. Finally, psychological and socio-cultural events also interact with physiological processes to determine food choices and consumption [38], [39].
2.3. Energy expenditure
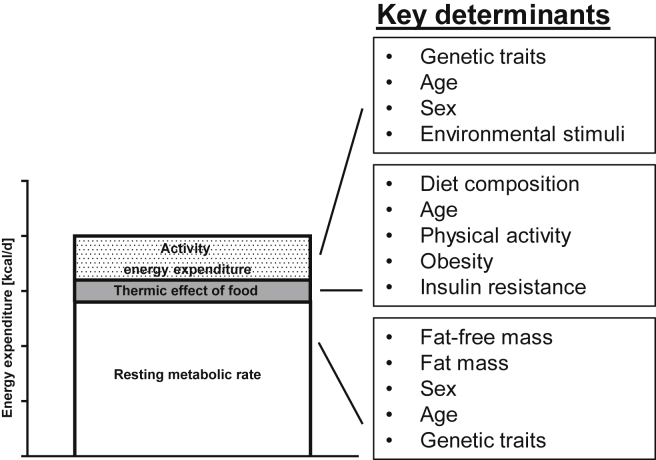
In humans, about 90% of energy ingested is metabolizable energy, with the rest being lost in the feces, urine, or leaving the body via the skin [40]. Total daily energy expenditure (TDEE) is comprised of three components: resting (or basal) metabolic rate (RMR), the thermic effect of food (TEF; also known as diet-induced thermogenesis), and activity energy expenditure (Figure 2). RMR refers to the energy required to sustain the biochemical systems of the body at complete rest and accounts for ∼70% of TDEE in sedentary individuals [41]. RMR can be further divided into energy expenditure during sleep (sleep metabolic rate; SMR) and that to maintain wakefulness without physical activity, with the latter contributing ∼5% of RMR with slight variations across race, sex, and obesity [42], [43], [44]. RMR or SMR is often used interchangeably to reflect energy expenditure independent of physical activity and TEF. Fat-free mass is by far the strongest determinant of RMR and accounts for ∼70% of its variance, with fat mass, sex, age, and familial traits being some of the remaining significant contributors to RMR [45], [46].
Figure 2.
Components of total daily energy expenditure.
Energy cost of physical activity is the most variable component of TDEE, which accounts for energy consumed in muscular work during spontaneous and voluntary exercise. It has been estimated that activity energy expenditure ranges from ∼15% in very sedentary individuals to up to 50% in highly active individuals [47]. Spontaneous physical activity (SPA) primarily refers to fidgeting, posture maintenance, non-specific ambulatory behavior (e.g. pacing), and activities of daily living [48]. In 24-h whole-room respiratory chamber studies, subjects spent 4–17% of their time on SPA, and this accounted for 100–700 kcal/d [49]. Physical activity level, both spontaneous and voluntary, is determined by genetic traits, age, sex, and complex interactions between biochemical, physiological, and brain reward pathways, as well as response to environmental stimuli (for details refer to a comprehensive review by Garland and colleagues [48]). The actual energy cost of physical activity is then be a function of the activity level and the energetic efficiency with which these activities are performed [47].
TEF refers to the energy expenditure that relates to food consumption, i.e., energy required to digest, absorb, assimilate, and store nutrients, and thus is dependent on the amount and the type of nutrients consumed. TEF has been reported as 5–10%, 0–3% and 20–30% of the energy content of carbohydrates, lipids, and proteins respectively [50] and in the case of energy balance on a Western diet accounts for ∼10% of TDEE [51]. Aging, physical activity, obesity, and insulin resistance have all been reported to impact on TEF independent of food consumption with the sympathetic nervous system as the possible mechanistic link, but it has also been suggested otherwise [51], [52], [53]. Some questioned the physiological relevance of TEF in positive energy balance as part of the etiology of obesity, as the reduction in TEF during weight gain, if any, would be compensated by the increase in RMR associated with the greater fat and fat-free mass [50], [54].
Temperature, both inside and outside the body, is a key determinant of energy expenditure. About two-thirds of RMR contributes to heat production to maintain a constant core body temperature of 37 °C in humans, and it has been estimated that every 1 °C increase in core temperature is associated with 10–13% increase in metabolic rate [55]. The tight regulation of core temperature within a range of ∼0.2 °C, however, makes it unlikely to play a major role in altering RMR and thus overall energy balance [56]. Along this notion, we reported a small and yet significant reduction in core temperature during sleep in Pima Indians but similar metabolic rate as compared to weight and body composition-matched Caucasians [56]. Ambient temperature, on the other hand, has been increasingly appreciated as a potential avenue to modulate energy expenditure. When mammals are exposed to temperatures outside their thermoneutral zone (a range of ambient temperatures within which basal metabolism is sufficient to maintain body temperature), thermoregulation mechanisms kick in to defend the body temperature set-point, and, subsequently, metabolic rate will increase [57].
In recent years non-shivering thermogenesis in brown adipose tissue (BAT) via mitochondrial uncoupling has been implicated as a key therapeutic target to increase energy expenditure. Transient cold challenges induce acute increase in endogenous heat production, but extended periods of cold exposure will lead to a sustained higher metabolic rate with reports of increased energy expenditure ranging from 2 to 17% including elevations in both SMR and TEF [57], [58], [59]. It has been proposed that the amount of BAT that one possesses is a genetically predisposed phenotype, but there is also evidence for cold acclimation to increase both the quantity and activity of BAT [60], [61], [62]. There is also the recent discovery of the beige (or brite) adipocytes in the subcutaneous white adipose tissue that express high levels of UCP-1 and have thermogenic capacity similar to that of the classic brown adipocytes [63], [64]. Importantly, in addition to cold exposure, exercise training and long-term peroxisome proliferator-activated receptor-γ agonist treatment have also been shown to induce the “browning” of white adipose tissue [65] that appear to be more feasible options to increase thermogenesis as a treatment for obesity.
3. Methodologies to assess energy intake and appetite-related parameters
3.1. Food recall and food diary
Energy intake measurement requires documenting food intake over a specified period of time. In 24-h food recall respondents are interviewed to recall what they consumed in the preceding day. The relative ease of administration makes it the choice of dietary assessment tool in nationwide nutrition surveys [66], [67]. The quality of data obtained depends considerably on the interviewers, who must be skillful in prompting respondents to recall their intake accurately [68]. Structured interviews with pre-determined questions and checklists of commonly forgotten foods improve the consistency of the recall process across respondents, but they are labor-intensive procedures. Limited representability of single 24-h recall (e.g. day-to-day and seasonal variations) is another major disadvantage, which may be overcome by using the average of multiple recalls across a specific timeframe.
An alternative is to use food diary that requires respondents to write down everything they eat and drink over a period typically of 3–7 days. When completing a food diary, respondents are instructed to record at the time of eating to minimize misreporting, but this leads to significant participant burden. It is not uncommon for respondents to alter their diet during the recording period, e.g. to avoid complicated foods like mixed dishes [69]. More problematic is under-reporting across all population groups, with energy intake derived from food diaries found to be 20–60% lower than that determined using doubly labeled water [70]. Usually food diary data are verified during an interview during which researchers clarify any missing details.
Mis-quantifying food intake and laborious data analyses are key inherent limitations of 24-h food recall and food diary. Food weighing is the gold standard, but, for obvious practical reasons, food recall and diary mainly rely on estimation of portion size using standard units, household measures, food models, or images of food on standard plate, cup, and bowl, etc. However, even with training, the use of these measurement aids only leads to modest improvements in the accuracy of estimating food intake [70], [71]. Data analysis is another major challenge when using food recall and food diary. Because food intake is reported in a non-uniform format, the data will need to be entered manually. Limitations in food database also make it difficult to code food items for nutritional analyses.
Computer-assisted or internet-based 24-h food recall or food diary makes these methods feasible for large-scale nutritional studies. In these programs, respondents either report food intake to interviewers who enter the intake electronically, or they directly record their own intake on the computer if the program is self-administered. The data are entered by selecting the food consumed from the list provided, and then program prompts for estimation of portion sizes usually with the aid of food photographs or food models [72]. The Automated Multiple-Pass Method (AMPM) developed by the US Department of Agriculture is the most well-established computer-assisted 24-h food recall available [68] and has been used to collect two days of intake data from each participant of the National Health and Nutrition Examination Survey since 2002. This system navigates the interviewers through the recall process who then enter the responses in the program that automates data formatting, coding, and analyses. AMPM was able to accurately estimate energy intake (underreported by <3%) in individuals in the healthy BMI range when validated against doubly labeled water [73], and the estimate was not significantly different from the observed intake in a smaller cohort under laboratory-controlled conditions irrespective of BMI [74]. Fully automated data collection, processing, and analyses in self-administered food record further simplify the process, improves accessibility, and lowers the cost. A web-based self-administered version of the interviewer-assisted AMPM captured similar number of food items (∼80% of the actual consumption), and the estimated energy intake was not significantly different between the two methods [75]. Intake data collected using web-based food record have also been shown to be comparable to those collected using handwritten food record analyzed by dieticians [76] and perform reasonably well during validation against biomarkers [77], [78], [79]. As such, web-based food record has gained popularity in recent years especially in epidemiological studies following improvements in user interface and food composition database [80], [81].
3.2. Image-assisted dietary assessment
Recent advances in image-assisted assessment of dietary intake, i.e., use image or video as the primary record of food intake, is a significant step in improving self-reporting accuracy. Respondents are asked to capture images of foods before and after each eating episode with a visual reference marker in the images (for portion size estimation) and provide a description of the food either by text or voice recordings [82], [83], [84]. Alternatively, respondents may use wearable cameras to capture point-of-view images during eating episodes [85], [86]. The major advantages here are that it allows objective estimation of portion size, minimizes systematic bias in self-reported intake, and thus provides more accurate estimation of energy consumption. The Remote Food Photography Method (RFPM) is arguably the most sophisticated image-assisted dietary assessment tool to date [87]. In a small pilot study, Martin and colleagues [88] showed that energy intake estimated using the RFPM was not significantly different from that using doubly labeled water in free-living conditions or weighed intake in a laboratory setting.
A key challenge in implementing image-assisted dietary assessment is data collection and analyses. Failure for respondents to capture images is not uncommon often due to forgetfulness, loss of imaging device, or equipment breakdown. In this case, a back-up method (e.g. food record) is used. To minimize missing data, prompts to smartphones to remind respondents to capture food images have been trialled. These prompts can be set at generic mealtimes or customized to individuals' timing and frequency of meal consumption, with the latter shown to improve the accuracy of food intake [88]. When it comes to data analyses, typically the food images are analyzed manually by researchers using a reference database, which is a labor-intensive process [82]. RFPM incorporates semi-automated procedures to simplify data analyses. These include a computer software that corrects and standardizes food images, a smartphone-based application that automatically identifies foods using bar code scanning and Price Look Up codes, and imaging algorithms that automatically identifies foods and estimates portion size [87], [89]. Although at this stage a human operator is still required to oversee data management and the analysis process, these are important steps towards fully automated data analysis.
3.3. Food frequency questionnaire
Food frequency questionnaire (FFQ) is a commonly used dietary assessment tool in epidemiological studies. It consists of a list of food items and respondents are required to report how frequently they consume these foods during a specific period of time. Some also ask for information on portion sizes, and these are known as semi-quantitative FFQs. FFQ is often the preferred tool to assess habitual dietary intake in large population-based cohorts for its consideration of seasonal intake variations, occasional food consumption, and its ease of administration and data analysis [68]. While FFQ is better suited for ranking individuals (i.e., distinguishing population subgroups who differ with respect to intake), it has also been used to estimate absolute intake but is generally considered as less accurate. When validated against doubly labeled water, FFQ has been shown to underestimate energy intake by up to 36% [90]. It has been argued that food frequency estimates over a long period of time are unlikely to be based on memory for dietary events but on people's mental image of their habitual diet, e.g. food likes and dislikes and concerns about dieting and health [91]. Reporting food frequency, therefore, is more a measure of attitude rather than actual dietary behavior and thus is likely to be influenced by variables including age and gender [91], [92].
How the FFQ is designed and administered influence the response rate, completeness, and accuracy of the information collected and thus is a key factor that determines the quality of research data [93]. FFQs may be adapted from existing questionnaires (e.g. the NCI/Block Health Habits and History Questionnaire [94] and the Harvard Semiquantitative Food Frequency Questionnaire [95]) or they can be developed from scratch using basic principles. Due to the enormous variability of the population's diet it is impossible to list all available food items on the FFQ. As such, it is common to design FFQs specifically for certain populations (e.g. Mediterranean) or population sub-groups (e.g. children) and are often further narrowed down to focus on certain nutrients or food groups depending on the purpose of the dietary assessment. According to a quantitative review by Molag and colleagues [90], the average number of items on FFQs is 134 (range 44–350) and those with >200 items perform better at ranking individuals than those with a short item list (≤100 items). Validation studies have shown that there is a rapid decrease in marginal gain in information as the complexity of FFQs increases [96]. The level of accuracy of dietary data required, therefore, should be the key criterion to determine the number of foods listed in the FFQ [96]. How the data is collected, e.g. via a postal questionnaire, phone interview, in-person interview, computer- or web-based, also influences data quality. Computer-readable forms eliminate data-entry errors; Prompts can be incorporated in computerized FFQs to correct incomplete or implausible data; correlation coefficients for repeatability between FFQs and reference measures are higher for interviewer-administered than self-administered FFQs [68], [96].
3.4. Observed intake in controlled environment
Having respondents eat in a controlled environment (e.g. laboratory, hospital, and childcare) allows researchers to measure food intake more objectively and more accurately. The most common way to do so is to quantify “plate waste” i.e., participants are provided with a known amount of food to consume ad libitum and the food uneaten is measured [97], [98]. Alternatively the amount of food consumed may be visually estimated and recorded by trained personnel if weighing food remains is not possible [99]. In addition to simply quantifying food intake, devices have also been developed to collect data on eating behavior. The “universal eating monitor” was one of the earliest devices of this kind ever documented in the literature. Invented in 1980 by Kissileff and colleagues [100], the “universal eating monitor” was an electronic platform scale connected to a computer that measured meal duration and rate of food consumption in real-time. Nowadays, a combination of food weighing and video recording is commonly used to record food intake and eating behavior in a laboratory setting [101], [102].
The quality of food intake data collected in a tightly controlled environment, however, has been subjected to criticism as this may create less realistic food consumption. The fact that respondents know that their food intake is being monitored or measured is sufficient to alter their eating behavior. A meta-analysis by Robinson and colleagues [103] showed that heightened awareness of being observed significantly reduced energy intake in a laboratory setting as compared to that in free-living conditions, which may be due to self-presentation motives (e.g. eat minimally to portray a positive image). Buffet-style meals used to measure spontaneous intake in the laboratory setting potentially create another source of deviation from the “real” intake, with food provided in excessive quantities promoting over-consumption [98], [101]. Buffet-style meals may initially produce a sense of novelty but repeated use on the same respondent over a short period of time can lead to boredom and lower energy intake [97]. There are reports of disguising the measurement of food intake and eating behavior by blinding respondents to study aims, using cover stories, or concealing the measurement procedures, which may minimize awareness-related confounding factors of food consumption [103].
3.5. Biomarkers of nutrient intake
Some consider quantifying biomarkers as the most objective and unbiased way to assess the intake of particular diet components. The idea is to measure biochemical markers in specimens (e.g. plasma, urine and tissue biopsy) that have strong and independent relationship with the nutrients to be assessed that can indicate intake or the status of these nutrients [104], [105]. Biomarkers are commonly used to assess micronutrient intake as distinct metabolites can inform on consumption with high specificity [105], [106]. Biomarkers for energy intake are yet to be identified, but some can be used to assess intake of individual macronutrient. Twenty four hours urinary nitrogen has been validated to assess protein intake in individuals who are in nitrogen balance. Multiple collections are preferable to account for day-to-day variations, with eight days of 24-h urine collection reported to improve the correlation between daily nitrogen intake and output to 0.95 [107]. Blood or tissue lipids are able to reflect dietary fat subtype intake [108], and a combination of selected fatty acid in tissue specimens has been shown to differentiate total fat consumption [109]. Biomarkers for carbohydrates are less common, but there are recent reports of using plasma alkylresorcinol and urinary sucrose and fructose as biomarkers for wholegrain [110] and sugar [111] consumption respectively. Stable isotope ratios have also been proposed as novel biomarkers for food intake as the stable isotope ratios of light elements (e.g. carbon, nitrogen and sulfur) vary naturally in food and these variations are captured in tissues minimally affected by endogenous processes [112].
A major limitation of using biomarkers is that the respondent characteristics, e.g. age, body weight stability, pregnancy, diseases, genetics, and lifestyle factors (e.g. smoking), may affect the biological pool of nutrients and/or their metabolites and thus its ability to detect changes over time and across diverse populations [104], [113]. The nature of the research also limits the choice of biomarkers. Blood or urine samples are easy to obtain, but the relatively quick turnover of nutrients and/or their metabolites (usually ranges from hours to days) means that these samples are only useful for indicating short-term intake. In contrast, biomarker abundance in tissues (e.g. membrane phospholipid in adipose biopsy) is applicable in assessing long-term consumption patterns, although invasive sampling may limit its use in large-scale epidemiological studies.
Recent advances in metabolomics are expected to significantly improve the application of biomarkers in dietary assessment. Food metabolome, i.e., the sum of all metabolites that are derived from the digestion and biotransformation of food components [114], enables the use of a combination of biomarkers to assess exposure to dietary components [115], food groups [116], [117], or even dietary patterns with high specificity [118], [119]. More importantly, the largely unexploited food metabolome will also certainly lead to the discovery of novel biomarkers for dietary exposure and/or diet-disease relationships with a high level of detail and precision [114]. While the complexity of metabolomics analyses makes it unlikely to replace more cost-effective dietary assessment tools, a more common use of biomarkers is to validate self-reported measures. For example, an increase in plasma carotenoids levels has often been used to validate high fruit and vegetables intake reported in FFQs and food diaries [120], [121]. Prentice and colleagues [122] demonstrated the use of biomarkers from sub-groups within study cohorts to develop algorithms to calibrate self-reported dietary measures as an alternative for more objective measures at an acceptable cost.
3.6. Mathematical modeling and intake-balance method
Based on the energy balance principle, energy intake equals to the sum of energy expenditure and changes in energy stores in the body. Theoretically, energy intake, therefore, can be retrospectively calculated if measures of energy expenditure and storage are available. While the latter two can both be objectively quantified, this is arguably the most unbiased way to assess energy intake. When participants maintained energy balance (i.e., energy intake = energy out), Seale and Rumpler [123] showed that the energy intake estimated by doubly labeled water differed from metabolizable energy intake by only 0.3%, whereas the difference between metabolizable energy consumption and intake from self-reported diet record was 22%.
Mathematical modeling to assess energy intake is particularly useful in obesity research when weight change (and thus energy store) usually occurs over extended periods of time and corresponding long-term tracking is impractical. Our laboratory successfully validated the intake-balance method in controlled overfeeding studies in which the calculated energy intake differed from the actual intake by 0.8% and 4% in institutionalized and free-living participants respectively with changes in body composition assessed using dual-energy X-ray absorptiometry and energy expenditure using either whole-room indirect calorimetry and doubly labeled water [124]. While the labor- and cost-intensive processes of measuring body composition and energy expenditure limit the practicality of the intake-balance method, similar mathematical models have been developed to calculate changes in energy intake based on demographics and repeated body weight measurements [125], [126]. This provides an inexpensive and simple alternative to obtain energy intake data during weight management interventions, although baseline indirect calorimetry or doubly labeled water would still be required to determine the absolute time-course energy intake.
It should be noted that assumptions are involved in various stages of mathematical modeling of energy intake, e.g. coefficients for energy content of tissues and the energy cost of tissue synthesis [124], body composition dynamics [126], individual biological variations, and physical activity energy expenditure [125], that limit the precision of model-calculated energy intake. While mathematically derived energy intake measures are unlikely to become mainstream in dietary assessment, developing user-friendly platforms to use these models [127] is an alternate way to benefit obesity research by providing ongoing feedback and thus improving adherence to dietary protocols.
3.7. Appetite measurement
Originally developed to assess pain, visual analogue scales (VAS) is the most widely used questionnaire to assess subjective ratings of appetitive sensations. VAS typically consists of a series of questions about motivation to eat and gastrointestinal repletion (e.g. how hungry do you feel?) and below each question there is a 100 mm straight line with the two extreme responses at the ends (e.g. not at all hungry/as hungry as I have ever felt) [128]. Respondents place a mark on the line to indicate their sensations, and, usually, they are required to complete the VAS at regular time intervals throughout the study. The area-under-curves of the time-course plots are then used to quantify appetite ratings [129]. Traditionally VAS is administered using pen and paper, but manual measurement and data recording are time-consuming and error-prone and thus are commonly replaced by electronic appetite rating systems that fully automate data collection and analyses [129], [130], [131].
A major shortfall of VAS is the validity and reproducibility of subjective appetite ratings. Appetite has a physiological basis but is also strongly influenced by cognitive and environmental factors including the surroundings, habitual meal times, sensory stimulation, familiarity, and availability of food [128], [132], [133]. Circulating levels of gut peptides, e.g. ghrelin, cholecystokinin and GLP-1, often are assessed concurrently as objective biomarkers of appetite although their secretions are also known to be modified by cognitive and sensory influences [128], albeit to a lesser extent. Further, the relationships between gut peptides and appetite regulation are not always straightforward, and, therefore, they are unlikely to replace subjective appetitive ratings, but they remain critical to inform on mechanisms by which nutrients or dietary factors impact on food intake [134].
4. Methodologies to assess energy expenditure
4.1. Self-reporting and direct observation
Interest in measuring energy expenditure (primarily physical activity level) arose in the early 20th century with the aim of improving productivity and worker efficiency [135]. Subjective reports, including diary records and questionnaires, were commonly used to seek information on the type, frequency, duration, and intensity of physical activity [136], [137]. Before measurements of physiological responses were possible, objective measures of energy expenditure were limited to direct observation of physical activity patterns or analysis of film records [135].
4.2. Direct calorimetry
Direct calorimetry is based on the first law of thermodynamics and the assumptions of thermal stability and low energy storage capacity, that energy spent in all physiological processes is ultimately dissipated as heat and thus total energy expenditure can be assessed by directly measuring heat production [138]. Direct calorimetry is technically challenging as it requires measurements of all heat transfers including radiation, convection, and conduction as well as heat loss due to evaporation [139]. Various isothermic and gradient-layer whole-room systems have been developed, with one of the most common systems consisting of a chamber surrounded by a shell space that is maintained at the same temperature as the inside of the chamber, and heat production is calculated by measuring the differences in air temperature and humidity between the inlet and outlet of the chamber [138]. Besides the complex design of the chamber, other limitations also preclude direct calorimetry from being the mainstream methodology to measure energy expenditure. For instance, due to the non-negligible heat capacity of the human body and slow heat exchange as compared to respiratory gas exchange, whole-room direct calorimetry is not able to detect acute changes in energy expenditure (e.g. TEF). The need to confine respondents in a small space also limits its applicability in study protocols.
Recent advances in wearable technology have led to renewal interest in direct calorimetry to measure energy expenditure with improved portability. Armband-like devices have been developed to detect skin temperature, heat flux, and evaporative heat loss from the skin's surface to estimate energy expenditure continuously over extended periods of time [140], [141]. The greatest advantage is that they overcome the problem of measuring inhale and exhale gas composition for indirect calorimetry so they are more user-friendly in free-living conditions. Initial trials showed that these devices accurately estimated energy expenditure (validated against indirect calorimetry), although ambient temperatures outside the thermoneutral zone and intense physical activity appeared to affect the accuracy of energy expenditure measures [140], [142], [143].
4.3. Indirect calorimetry
Rather than measuring the actual heat production, indirect calorimetry assesses energy expenditure by calculating the amount of energy released when energy substrates are oxidized. Weir [144] proposed that total heat output [kcal] = 3.9 × oxygen used [L] + 1.11 × carbon dioxide produced [L] and therefore energy expenditure can be estimated by relatively simple measurements of oxygen consumption and carbon dioxide production with the assumption of energy balance and negligible anaerobic respiration.
Indirect calorimetry is most commonly used to assess energy expenditure in clinical research settings primarily due to the advantages of continuous energy expenditure measurement with high accuracy and precision, as well as flexibility of equipment design to suit various experimental settings [145]. The ratio of oxygen consumption to carbon dioxide production in indirect calorimetry also informs on carbohydrate and fat oxidation. It is common to derive protein oxidation data from 24-h urine output in order to gain insight into the balance of macronutrient utilization.
4.3.1. Whole-room respiratory chamber
Using indirect calorimetry to measure TDEE in whole-room respiratory chamber was first pioneered by the laboratory of Jéquier in Lausanne [146] and in the Dunn Clinical Nutrition Centre in Cambridge [147] since the late 1970s. A modified design of the chamber was described by Ravussin and colleagues [148] and has been widely adopted in metabolic research facilities across the world, which typically involves inputs of air with constant or known gas composition into an air-tight chamber and continuous sampling of the outflowing air that is either dried or have water vapor pressure measured to allow accurate measurement of oxygen and carbon dioxide concentrations using mass spectrometer, paramagnetic, or infrared analyzers [145].
Whole-room respiratory chambers allow continuous measurement of TDEE up to several days [149]. These chambers are also used to assess TEF, although how this should be calculated remains debatable. Schutz and colleagues [150] proposed that in a thermoneutral environment, TEF can be derived by subtracting basal metabolic rate from the intercept of the linear regression between total energy expenditure and physical activity (i.e., energy expenditure at zero activity). Other attempts to assess TEF include using the difference in TDEE between fed and fasted states or the 24-h resting energy expenditure above SMR [151] or a mathematical modeling approach that estimates TEF using raw data from a 24-h indirect calorimetry measures in the respiratory chamber [152]. All of these, however, are shown to have very low reproducibility, with intra-individual coefficient of variations reported to be as high as 43% [148]. Limited consensus in how TEF is mathematically defined, variability in measuring the components for calculations, as well as day-to-day biological variation in postprandial processing of nutrients, all contribute to TEF being the most difficult component of daily energy expenditure to measure [52], [151].
4.3.2. Metabolic carts
Measuring RMR using metabolic carts [153] and in combination with an estimated physical activity level [154] provide some estimate of TDEE that is feasible in most clinical research settings. In contrast to whole-room respiratory chambers that confer considerable limitations in study protocols, metabolic carts use a facemask, mouthpiece, hood, or canopy to capture exhaled gas, which is connected to analyzers mounted on mobile carts. The smaller “dead space” in the ventilation system improves response time in the measurement (from ∼5–30 min for a chamber system to ∼1–2 min for a hood or canopy to <30 s for a mask or mouthpiece) [145]. The set up and operation of these metabolic carts are substantially more user-friendly compared to chambers, although respondent mobility is largely limited and thus these systems are only applicable in studies that last up to a few hours. A portable calorimeter that has the gas analyzer and power supply in a backpack is the indirect calorimetry system most applicable in free-living conditions, but the device remains cumbersome and the power supply limits recording time [155].
4.4. Doubly labeled water
Using doubly labeled water (DLW) to measure energy expenditure in humans was first described by Schoeller and van Santen [156] in 1982; since then, it has become the gold standard for measuring long-term free-living energy expenditure. The principles, assumptions, and calculations underlying the measurement of energy expenditure using DLW were detailed elsewhere [157], [158]. Briefly, when dosed with water containing known amounts of non-radioactive isotopes of hydrogen (2H; deuterium) and oxygen (18O), these isotopes equilibrate with hydrogen and oxygen in the body water, and, subsequently, 2H exits the body as water, and 18O exits the body as both water and carbon dioxide. Turnover rates of 2H and 18O are determined by quantifying isotope concentrations in body fluids (most commonly urine) using mass spectrometry. The differential disappearance of the two isotopes provides a measure of carbon dioxide production. When this is used in combination with the average respiratory quotient during the study period (measured by indirect calorimetry or approximated by food quotient), the ratio of carbon dioxide production and oxygen consumption allows the calculation of energy expenditure. Typically DLW can be used to measure TDEE over 4–21 days, with spot urine samples collected immediately prior to dosing and daily or weekly thereafter depending on the duration of the study period to estimate the rate of isotope elimination [159], [160].
The relative ease of administering DLW and the highly sensitive techniques of quantifying isotopes (albeit at a high cost) minimize measurement error and make it the gold standard for measuring energy expenditure in free-living conditions. Importantly, without the limitations of confined space or gaseous sampling attachment, DLW remains as the only tool for assessing energy expenditure in true free-living conditions and thus is ideal for a wide array of applications in particularly those that involve physical activity. The use of the DLW protocol also extends to validation of dietary or exercise assessment techniques, as well as the measurement of total body water, water turnover, and body composition given the assumption of the hydration of fat-free mass [161]. Using DLW to assess energy expenditure, of course, is not without its limitations. Numerous assumptions involved in the calculations may compromise validity of the measurements [162], [163]; time-course energy expenditure data are not possible, and there is no information on the nature and intensity of physical activity [48]. Finally, the cost of isotopes, access to the equipment and expertise to perform isotope-ratio mass spectrometry limit the availability of the DLW methodology in metabolic research facilities and clinical setting.
4.5. Non-calorimetric techniques
4.5.1. Physical activity log and kinematic measurements
Physical activity log and kinematic measurements are commonly used in clinical research due to their low costs, non-invasiveness, and relative ease of administration. An activity log requires respondents to log all physical activities over a period of time (e.g. 7 days). Activity energy expenditure is then calculated by multiplying the energy equivalent for the activities (usually estimated using predictive equations [164] but ideally measured using calorimeters) with the time spent in each activity. Kinematic measurements provide an objective record of physical activity. In confined space, such as a whole-room respiratory chamber, radar tracking reports the percentage of time during which the respondent is moving [165]. Movement of free-living individuals is typically measured using pedometers and accelerometers. Pedometers detect vertical acceleration of the hips during ambulation and report the data as an accumulated step count but are not able to indicate patterns and intensity of physical activity [166], whereas accelerometers detect body displacement, with uniaxial accelerometers in one axis and triaxial accelerometers in three axes. Time-course activity counts are then translated into activity duration in pre-specified intensity categories [167]. Extrapolating these movement data to estimation of energy expenditure, however, is not always practical and has been shown to introduce significant errors. There have been attempts to develop regression equations to predict activity energy expenditure from accelerometer activity counts, but the equations have to be device- and activity-specific, and validation in the laboratory setting limits generalizability to free-living conditions, with reports of underestimating 24-h activity energy expenditure up to 59% when compared to DLW [168], [169]. The complex relationship between movement and energy expenditure, which needs to take into account variables like gender, age, body mass, and efficiency of movement, may indeed implicate that there is no simple solution to accurately predicting energy expenditure from any kind of physical activity measurements [166].
4.5.2. Heart rate and ventilation monitoring
Heart rate has long been considered a surrogate marker of physical activity level and thus an attractive physiological variable to inform on energy expenditure. Except during very low and very high exercise intensities, there is a significant linear correlation between heart rate and the rate of oxygen consumption (VO2), which can then be extrapolated to predictions of energy expenditure or metabolic rate [170]. The slope of this heart rate-VO2 relationship, however, varies considerably across individuals at least partly due to age, sex, aerobic fitness, and movement efficiency. Thus, an individualized calibration procedure is required to use heart rate to predict energy expenditure. Typically, this involves measuring heart rate, VO2, and VCO2 (using a metabolic cart) during a series of workload with progressive intensity from light to strenuous levels, and a regression line of heart rate and energy expenditure is developed for each individual [171]. The known physiological differences between exercises (a good example here is running vs cycling) [172] have led to attempts to derive predictive equations specific to exercise regimens [171], [173], but there are questions about the feasibility of such a highly specific approach. A general consensus is that heart rate provides a reasonable estimate of energy expenditure at a group level but with limited accuracy at individual estimations [170]. Further, under circumstances like very low energy expenditure level (where slight movements increase heart rate but VO2 remain almost unchanged) or intermittent exercise (where there is a lag in heart rate response to changes in work rate), the assumption of a linear heart rate-VO2 relationship no longer applies [170]. Finally, heart rate measures are often affected by significant loss of data points.
Based on similar principles of the VO2-energy expenditure relationship, some argue for ventilation as a better surrogate marker of energy expenditure, with the advantage of ventilating variables (as compared to heart rate) are less sensitive to physical and mental stress that can significantly confound energy expenditure prediction [174]. As early as the 1950s, a linear relationship between ventilation and energy expenditure had been established that spans across the majority of everyday life activities [175]. Spirometry remains the gold standard but in clinical setting ventilation measurement usually involves placing sensors on the body surface to detect movements of the chest and abdominal walls to calculate variations of the volumes of thorax and abdomen. Technological advances in sensors to improve precision, accuracy in measuring ventilation, as well as wearability of the devices will be critical to expand the use of ventilation in metabolic research.
5. Conclusion: choice of methodologies is the key to success
Chronic positive energy balance is arguably the strongest predictor of metabolic diseases. Decades of research have been devoted to understand how the system of energy homeostasis is perturbed, its metabolic consequences, and possible solutions to restore homeostasis. While basic science research using animal models and cell cultures has made great advancements in elucidating the complex array of molecular mechanisms underlying energy metabolism, translation of this knowledge into clinical benefits lacks significant progress. A key challenge here is to properly evaluate the effectiveness of interventions, including lifestyle modifications, supplementations, or pharmacological treatments, to provide feedback for further improvement. Currently there is an array of methodologies designed for measurements of various aspects of energy metabolism, each with its pros and cons. It is imperative to understand the relative merits of each methodology in order to choose the most appropriate ones for the research (Table 1, Table 2). Further, it is critical to develop new methodology to measure energy intake with the accuracy and precision like DLW has been for energy expenditure.
Table 1.
Summary of methods to assess energy intake.
| Method | Duration of use | Accuracy & precision | Cost | Advantages | Limitations |
|---|---|---|---|---|---|
| Food recall | 1 day | Interviewer-dependenta | Low | Easy to administer, suitable for assessing short-term dietary interventions | Low representability, labor-intensive analysisa |
| Food diary | 3–7 days | Low due to under-reporting and mis-quantifying food intakea | Low | Easy to administer, suitable for assessing short-term dietary interventions | Participant burden, labor-intensive analysisa |
| Food frequency questionnaire | 3–12 months | Low due to “non-memory-based” response | Low (develop in-house) to moderate (use commercially available questionnaire) | Easy to administer, suitable for epidemiological studies and ranking individuals, can be tailored for specific populations, nutrients or food groups | Less accurate for absolute intake estimation |
| Observed intake | Flexible | High with food weighing | Low | Tightly controlled environmental factors | Creates less realistic eating behavior, repetitive testing alters “real” intake |
| Biomarkers | Hours to days for nutrient/metabolite turnover, months for biomarker abundance in tissues | High | High | Objective and unbiased, high specificity | Limited well-validated markers, often requires invasive sampling (e.g. blood draw), confounded by respondent characteristics |
| Mathematical modeling and intake-balance method | Flexible | Limited due to multiple assumptions in modeling | Low (based on demographics and anthropometry) to high (based on precise body composition and energy expenditure measurements) | Objective and unbiased, ongoing tracking allows real-time assessment of intake | Labor-intensive for body composition and energy expenditure measurements, no consumption data on specific nutrients |
Possible improvements with computer-, internet- or image-assisted technology.
Table 2.
Summary of methods to measure energy expenditure (EE).
| Method | Duration of use | Accuracy & precision | Cost | Advantages | Limitations |
|---|---|---|---|---|---|
| Direct calorimetry | Hours to several days | High except with intense physical activity or temperature outside the thermoneutal zone | High due to equipment set up and maintenance | Direct measure of heat production, complete control of environmental factors | Technically demanding, unable to detect acute changes, respondent restricted to confined space |
| Whole-room respiratory chamber | Hours to several days | High | High due to equipment set up and maintenance | Real-time minute-by-minute data, allow measurement of components of EE and substrate utilization | Technically demanding, respondent restricted to confined space |
| Metabolic cart | Hours | High for resting metabolic rate, moderate when estimating total daily EE | Moderate | Quick response time, easy to operate, feasible in clinical setting | Restricted respondent mobility |
| Doubly labeled water | 4–21 days | High | High due to isotope cost | Gold standard in free-living conditions, applicable to wide range of protocols | No time-course data, unable to differentiate components of EE |
| Physical activity log | 3–7 days | Low due to significant errors in extrapolating activity data to EE estimation | Low | Easy to administer | Participant burden may compromise data quality |
| Kinematic measurements | Flexible | Low due to significant errors in extrapolating movement data to EE estimation | Low to moderate | Easy to administer, objective and unbiased | Pedometers provide no data on patterns and intensity of physical activity |
| Heart rate monitoring | Flexible | Moderate at a group level, low at individual estimations | Low to moderate | Easy to administer, objective and unbiased | Requires individualized calibration, significant loss of data points |
| Ventilation monitoring | Hours | Low to moderate | Low to moderate | Less sensitive to physical and mental confounders | Low applicability in free-living conditions |
When choosing methodologies, one has to be aware that the “gold standards” are not necessarily the most appropriate for a particular research question. The following are a few important things to consider [176], [177], [178]: 1) required precision and accuracy – e.g. what is the expected coefficient of variation of the variables to be measured? Are the data used at the group or individual level? 2) Practicality – e.g. how much time and cost are involved for both researchers and respondents? What are the logistical constraints? 3) Quality of data – e.g. is the methodology going to impose a learning effect and/or participant burden that will increase the risk of an altered response? While no methodology is perfect under all circumstances, using a combination of complementary measurements may be useful to overcome some of the shortfalls. For example, at Pennington Biomedical we are among the first to routinely use both DLW and indirect calorimetry to assess energy expenditure, which allows simultaneous measurement of free-living energy homeostasis and insights into components of energy expenditure and substrate utilization [8], [179], [180]. When assessing dietary intake, it is common to use at least two assessment tools and cross-validate the response, e.g. generate correlation and calibration coefficients from 24-h food recall or weighed food records to validate FFQs [68], [96].
In this review article, we provide information on the utility and limitations of methodologies that are commonly used in obesity research, with a specific focus on those that quantify energy intake and expenditure. We encourage researchers to think critically when designing clinical trial protocols, selecting methodologies that provide the best possible answer to the specific research question(s) without compromising the overall quality of the work.
Acknowledgement
This work was partially supported by a NORC Center Grant #P30DK072476.
Contributor Information
Yan Y. Lam, Email: yan.lam@sydney.edu.au.
Eric Ravussin, Email: eric.ravussin@pbrc.edu.
Conflict of interest
None declared.
References
- 1.Global status report on noncommunicable diseases 2014. World Health Organization; Geneva: 2014. [Google Scholar]
- 2.Ng M., Fleming T., Robinson M., Thomson B., Graetz N., Margono C. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 Aug 30;384(9945):766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkelstein E.A., Trogdon J.G., Cohen J.W., Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Affairs (Millwood) 2009;28(5):w822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 4.Lehnert T., Sonntag D., Konnopka A., Riedel-Heller S., Konig H.H. Economic costs of overweight and obesity. Best Practice & Research. Clinical Endocrinology & Metabolism. 2013;27(2):105–115. doi: 10.1016/j.beem.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Trogdon J.G., Finkelstein E.A., Hylands T., Dellea P.S., Kamal-Bahl S.J. Indirect costs of obesity: a review of the current literature. Obesity Reviews. 2008;9(5):489–500. doi: 10.1111/j.1467-789X.2008.00472.x. [DOI] [PubMed] [Google Scholar]
- 6.Flegal K.M., Kruszon-Moran D., Carroll M.D., Fryar C.D., Ogden C.L. Trends in obesity among adults in the United States, 2005 to 2014. JAMA: the Journal of the American Medical Association. 2016;315(21):2284–2291. doi: 10.1001/jama.2016.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hervey G.R., Tobin G. The part played by variation of energy expenditure in the regulation of energy balance. Proceedings of the Nutrition Society. 1982;41(2):137–153. doi: 10.1079/pns19820024. [DOI] [PubMed] [Google Scholar]
- 8.Heilbronn L.K., de Jonge L., Frisard M.I., DeLany J.P., Larson-Meyer D.E., Rood J. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2006;295(13):1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leibel R.L., Rosenbaum M., Hirsch J. Changes in energy expenditure resulting from altered body weight. The New England Journal of Medicine. 1995;332(10):621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- 10.Weigle D.S., Sande K.J., Iverius P.H., Monsen E.R., Brunzell J.D. Weight loss leads to a marked decrease in nonresting energy expenditure in ambulatory human subjects. Metabolism. 1988;37(10):930–936. doi: 10.1016/0026-0495(88)90149-7. [DOI] [PubMed] [Google Scholar]
- 11.Bray G.A., Redman L.M., de Jonge L., Covington J., Rood J., Brock C. Effect of protein overfeeding on energy expenditure measured in a metabolic chamber. American Journal of Clinical Nutrition. 2015;101(3):496–505. doi: 10.3945/ajcn.114.091769. [DOI] [PubMed] [Google Scholar]
- 12.Fothergill E., Guo J., Howard L., Kerns J.C., Knuth N.D., Brychta R. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity (Silver Spring) 2016 Aug;24(8):1612–1619. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dulloo A.G., Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. American Journal of Clinical Nutrition. 1998;68(3):599–606. doi: 10.1093/ajcn/68.3.599. [DOI] [PubMed] [Google Scholar]
- 14.Luke A., Schoeller D.A. Basal metabolic rate, fat-free mass, and body cell mass during energy restriction. Metabolism. 1992;41(4):450–456. doi: 10.1016/0026-0495(92)90083-m. [DOI] [PubMed] [Google Scholar]
- 15.Bosy-Westphal A., Kossel E., Goele K., Later W., Hitze B., Settler U. Contribution of individual organ mass loss to weight loss-associated decline in resting energy expenditure. American Journal of Clinical Nutrition. 2009;90(4):993–1001. doi: 10.3945/ajcn.2008.27402. [DOI] [PubMed] [Google Scholar]
- 16.Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metabolism. 2013;17(4):491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jheng H.F., Huang S.H., Kuo H.M., Hughes M.W., Tsai Y.S. Molecular insight and pharmacological approaches targeting mitochondrial dynamics in skeletal muscle during obesity. Annals of the New York Academy of Sciences. 2015;1350:82–94. doi: 10.1111/nyas.12863. [DOI] [PubMed] [Google Scholar]
- 18.Paradies G., Paradies V., Ruggiero F.M., Petrosillo G. Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World Journal of Gastroenterology. 2014;20(39):14205–14218. doi: 10.3748/wjg.v20.i39.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katschinski M. Nutritional implications of cephalic phase gastrointestinal responses. Appetite. 2000;34(2):189–196. doi: 10.1006/appe.1999.0280. [DOI] [PubMed] [Google Scholar]
- 20.Smeets P.A., Erkner A., de Graaf C. Cephalic phase responses and appetite. Nutrition Reviews. 2010;68(11):643–655. doi: 10.1111/j.1753-4887.2010.00334.x. [DOI] [PubMed] [Google Scholar]
- 21.Chaudhri O.B., Salem V., Murphy K.G., Bloom S.R. Gastrointestinal satiety signals. Annual Review of Physiology. 2008;70:239–255. doi: 10.1146/annurev.physiol.70.113006.100506. [DOI] [PubMed] [Google Scholar]
- 22.Lancha A., Fruhbeck G., Gomez-Ambrosi J. Peripheral signalling involved in energy homeostasis control. Nutrition Research Reviews. 2012;25(2):223–248. doi: 10.1017/S0954422412000145. [DOI] [PubMed] [Google Scholar]
- 23.Scott R., Tan T., Bloom S. Gut hormones and obesity: physiology and therapies. Vitamins and Hormones. 2013;91:143–194. doi: 10.1016/B978-0-12-407766-9.00007-9. [DOI] [PubMed] [Google Scholar]
- 24.Patterson M., Bloom S.R., Gardiner J.V. Ghrelin and appetite control in humans–potential application in the treatment of obesity. Peptides. 2011;32(11):2290–2294. doi: 10.1016/j.peptides.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Delporte C. Structure and physiological actions of ghrelin. Scientifica. 2013;2013:518909. doi: 10.1155/2013/518909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girard J. The incretins: from the concept to their use in the treatment of type 2 diabetes. Part A: incretins: concept and physiological functions. Diabetes & Metabolism. 2008;34(6 Pt 1):550–559. doi: 10.1016/j.diabet.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Prentki M., Matschinsky F.M., Madiraju S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metabolism. 2013;18(2):162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 28.Konner A.C., Klockener T., Bruning J.C. Control of energy homeostasis by insulin and leptin: targeting the arcuate nucleus and beyond. Physiology & Behavior. 2009;97(5):632–638. doi: 10.1016/j.physbeh.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Williams G., Bing C., Cai X.J., Harrold J.A., King P.J., Liu X.H. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiology & Behavior. 2001;74(4–5):683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum M., Nicolson M., Hirsch J., Heymsfield S.B., Gallagher D., Chu F. Effects of gender, body composition, and menopause on plasma concentrations of leptin. Journal of Clinical Endocrinology & Metabolism. 1996;81(9):3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 31.Myers M.G., Jr., Leibel R.L., Seeley R.J., Schwartz M.W. Obesity and leptin resistance: distinguishing cause from effect. Trends in Endocrinology and Metabolism. 2010;21(11):643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gautron L., Elmquist J.K. Sixteen years and counting: an update on leptin in energy balance. Journal of Clinical Investigation. 2011;121(6):2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drewnowski A. Energy intake and sensory properties of food. American Journal of Clinical Nutrition. 1995;62(5 Suppl.):1081S–1085S. doi: 10.1093/ajcn/62.5.1081S. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen L.B., Moller P., Flint A., Martens M., Raben A. Effect of sensory perception of foods on appetite and food intake: a review of studies on humans. International Journal of Obesity and Related Metabolic Disorders. 2003;27(10):1152–1166. doi: 10.1038/sj.ijo.0802391. [DOI] [PubMed] [Google Scholar]
- 35.Drewnowski A. Sensory control of energy density at different life stages. Proceedings of the Nutrition Society. 2000;59(2):239–244. [PubMed] [Google Scholar]
- 36.Ello-Martin J.A., Ledikwe J.H., Rolls B.J. The influence of food portion size and energy density on energy intake: implications for weight management. American Journal of Clinical Nutrition. 2005;82(1 Suppl.):236S–241S. doi: 10.1093/ajcn/82.1.236S. [DOI] [PubMed] [Google Scholar]
- 37.McCrory M.A., Burke A., Roberts S.B. Dietary (sensory) variety and energy balance. Physiology & Behavior. 2012;107(4):576–583. doi: 10.1016/j.physbeh.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Hetherington M.M. The physiological-psychological dichotomy in the study of food intake. Proceedings of the Nutrition Society. 2002;61(4):497–507. doi: 10.1079/pns2002187. [DOI] [PubMed] [Google Scholar]
- 39.Gibson E.L. Emotional influences on food choice: sensory, physiological and psychological pathways. Physiology & Behavior. 2006;89(1):53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 40.Widdowson E.M. Assessment of the energy value of human foods. Proceedings of the Nutrition Society. 1955;14(2):142–154. doi: 10.1079/pns19550031. [DOI] [PubMed] [Google Scholar]
- 41.Ravussin E., Bogardus C. A brief overview of human energy metabolism and its relationship to essential obesity. American Journal of Clinical Nutrition. 1992;55(1 Suppl.):242S–245S. doi: 10.1093/ajcn/55.1.242s. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg G.R., Prentice A.M., Davies H.L., Murgatroyd P.R. Overnight and basal metabolic rates in men and women. European Journal of Clinical Nutrition. 1988;42(2):137–144. [PubMed] [Google Scholar]
- 43.Fontvieille A.M., Ferraro R.T., Rising R., Larson D.E., Ravussin E. Energy cost of arousal: effect of sex, race and obesity. International Journal of Obesity and Related Metabolic Disorders. 1993;17(12):705–709. [PubMed] [Google Scholar]
- 44.Kumahara H., Yoshioka M., Yoshitake Y., Shindo M., Schutz Y., Tanaka H. The difference between the basal metabolic rate and the sleeping metabolic rate in Japanese. Journal of Nutritional Science and Vitaminology (Tokyo) 2004;50(6):441–445. doi: 10.3177/jnsv.50.441. [DOI] [PubMed] [Google Scholar]
- 45.Weyer C., Snitker S., Rising R., Bogardus C., Ravussin E. Determinants of energy expenditure and fuel utilization in man: effects of body composition, age, sex, ethnicity and glucose tolerance in 916 subjects. International Journal of Obesity and Related Metabolic Disorders. 1999;23(7):715–722. doi: 10.1038/sj.ijo.0800910. [DOI] [PubMed] [Google Scholar]
- 46.Bogardus C., Lillioja S., Ravussin E., Abbott W., Zawadzki J.K., Young A. Familial dependence of the resting metabolic rate. The New England Journal of Medicine. 1986;315(2):96–100. doi: 10.1056/NEJM198607103150205. [DOI] [PubMed] [Google Scholar]
- 47.Levine J.A. Non-exercise activity thermogenesis (NEAT) Nutrition Reviews. 2004;62(7 Pt 2):S82–S97. doi: 10.1111/j.1753-4887.2004.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 48.Garland T., Jr., Schutz H., Chappell M.A., Keeney B.K., Meek T.H., Copes L.E. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. Journal of Experimental Biology. 2011;214(Pt 2):206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Johannsen D.L., Ravussin E. Spontaneous physical activity: relationship between fidgeting and body weight control. Current Opinion in Endocrinology, Diabetes, and Obesity. 2008;15(5):409–415. doi: 10.1097/MED.0b013e32830b10bb. [DOI] [PubMed] [Google Scholar]
- 50.Tappy L. Thermic effect of food and sympathetic nervous system activity in humans. Reproduction Nutrition Development. 1996;36(4):391–397. doi: 10.1051/rnd:19960405. [DOI] [PubMed] [Google Scholar]
- 51.de Jonge L., Bray G.A. The thermic effect of food and obesity: a critical review. Obesity Research. 1997;5(6):622–631. doi: 10.1002/j.1550-8528.1997.tb00584.x. [DOI] [PubMed] [Google Scholar]
- 52.Granata G.P., Brandon L.J. The thermic effect of food and obesity: discrepant results and methodological variations. Nutrition Reviews. 2002;60(8):223–233. doi: 10.1301/002966402320289359. [DOI] [PubMed] [Google Scholar]
- 53.Jones P.P., Van Pelt R.E., Johnson D.G., Seals D.R. Role of sympathetic neural activation in age- and habitual exercise-related differences in the thermic effect of food. Journal of Clinical Endocrinology & Metabolism. 2004;89(10):5138–5144. doi: 10.1210/jc.2004-0101. [DOI] [PubMed] [Google Scholar]
- 54.Weinsier R.L., Hunter G.R., Heini A.F., Goran M.I., Sell S.M. The etiology of obesity: relative contribution of metabolic factors, diet, and physical activity. American Journal of Medicine. 1998;105(2):145–150. doi: 10.1016/s0002-9343(98)00190-9. [DOI] [PubMed] [Google Scholar]
- 55.Landsberg L. Core temperature: a forgotten variable in energy expenditure and obesity? Obes Rev. 2012;13(Suppl. 2):97–104. doi: 10.1111/j.1467-789X.2012.01040.x. [DOI] [PubMed] [Google Scholar]
- 56.Rising R., Fontvieille A.M., Larson D.E., Spraul M., Bogardus C., Ravussin E. Racial difference in body core temperature between Pima Indian and Caucasian men. International Journal of Obesity and Related Metabolic Disorders. 1995;19(1):1–5. [PubMed] [Google Scholar]
- 57.Cannon B., Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. Journal of Experimental Biology. 2011;214(Pt 2):242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 58.Wijers S.L., Saris W.H., van Marken Lichtenbelt W.D. Recent advances in adaptive thermogenesis: potential implications for the treatment of obesity. Obes Rev. 2009;10(2):218–226. doi: 10.1111/j.1467-789X.2008.00538.x. [DOI] [PubMed] [Google Scholar]
- 59.Westerterp-Plantenga M.S., van Marken Lichtenbelt W.D., Strobbe H., Schrauwen P. Energy metabolism in humans at a lowered ambient temperature. European Journal of Clinical Nutrition. 2002;56(4):288–296. doi: 10.1038/sj.ejcn.1601308. [DOI] [PubMed] [Google Scholar]
- 60.van Marken Lichtenbelt W.D., Vanhommerig J.W., Smulders N.M., Drossaerts J.M., Kemerink G.J., Bouvy N.D. Cold-activated brown adipose tissue in healthy men. The New England Journal of Medicine. 2009;360(15):1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 61.Cypess A.M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A.B. Identification and importance of brown adipose tissue in adult humans. The New England Journal of Medicine. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orava J., Nuutila P., Lidell M.E., Oikonen V., Noponen T., Viljanen T. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metabolism. 2011;14(2):272–279. doi: 10.1016/j.cmet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Wu J., Bostrom P., Sparks L.M., Ye L., Choi J.H., Giang A.H. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petrovic N., Walden T.B., Shabalina I.G., Timmons J.A., Cannon B., Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. Journal of Biological Chemistry. 2010;285(10):7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sidossis L., Kajimura S. Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. Journal of Clinical Investigation. 2015;125(2):478–486. doi: 10.1172/JCI78362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Briefel R.R., Sempos C.T., McDowell M.A., Chien S., Alaimo K. Dietary methods research in the third National Health and Nutrition Examination Survey: underreporting of energy intake. American Journal of Clinical Nutrition. 1997;65(4 Suppl.):1203S–1209S. doi: 10.1093/ajcn/65.4.1203S. [DOI] [PubMed] [Google Scholar]
- 67.Gose M., Krems C., Heuer T., Hoffmann I. Trends in food consumption and nutrient intake in Germany between 2006 and 2012: results of the German National Nutrition Monitoring (NEMONIT) British Journal of Nutrition. 2016:1–10. doi: 10.1017/S0007114516000544. [DOI] [PubMed] [Google Scholar]
- 68.Falomir Z., Arregui M., Madueno F., Corella D., Coltell O. Automation of food questionnaires in medical studies: a state-of-the-art review and future prospects. Computers in Biology and Medicine. 2012;42(10):964–974. doi: 10.1016/j.compbiomed.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 69.Trabulsi J., Schoeller D.A. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. American Journal of Physiology. Endocrinology and Metabolism. 2001;281(5):E891–E899. doi: 10.1152/ajpendo.2001.281.5.E891. [DOI] [PubMed] [Google Scholar]
- 70.Hill R.J., Davies P.S. The validity of self-reported energy intake as determined using the doubly labeled water technique. British Journal of Nutrition. 2001;85(4):415–430. doi: 10.1079/bjn2000281. [DOI] [PubMed] [Google Scholar]
- 71.Martin C.K., Anton S.D., York-Crowe E., Heilbronn L.K., VanSkiver C., Redman L.M. Empirical evaluation of the ability to learn a calorie counting system and estimate portion size and food intake. British Journal of Nutrition. 2007;98(2):439–444. doi: 10.1017/S0007114507708802. [DOI] [PubMed] [Google Scholar]
- 72.Subar A.F., Thompson F.E., Potischman N., Forsyth B.H., Buday R., Richards D. Formative research of a quick list for an automated self-administered 24-hour dietary recall. Journal of the American Dietetic Association. 2007;107(6):1002–1007. doi: 10.1016/j.jada.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 73.Moshfegh A.J., Rhodes D.G., Baer D.J., Murayi T., Clemens J.C., Rumpler W.V. The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. American Journal of Clinical Nutrition. 2008;88(2):324–332. doi: 10.1093/ajcn/88.2.324. [DOI] [PubMed] [Google Scholar]
- 74.Conway J.M., Ingwersen L.A., Moshfegh A.J. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. Journal of the American Dietetic Association. 2004;104(4):595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 75.Kirkpatrick S.I., Subar A.F., Douglass D., Zimmerman T.P., Thompson F.E., Kahle L.L. Performance of the Automated Self-Administered 24-hour Recall relative to a measure of true intakes and to an interviewer-administered 24-h recall. American Journal of Clinical Nutrition. 2014;100(1):233–240. doi: 10.3945/ajcn.114.083238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raatz S.K., Scheett A.J., Johnson L.K., Jahns L. Validity of electronic diet recording nutrient estimates compared to dietitian analysis of diet records: randomized controlled trial. Journal of Medical Internet Research. 2015;17(1):e21. doi: 10.2196/jmir.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lassale C., Castetbon K., Laporte F., Deschamps V., Vernay M., Camilleri G.M. Correlations between fruit, vegetables, fish, vitamins, and fatty acids estimated by web-based nonconsecutive dietary records and respective biomarkers of nutritional status. Journal of the Academy of Nutrition. 2016;116(3) doi: 10.1016/j.jand.2015.09.017. 427–438 e425. [DOI] [PubMed] [Google Scholar]
- 78.Lassale C., Castetbon K., Laporte F., Camilleri G.M., Deschamps V., Vernay M. Validation of a Web-based, self-administered, non-consecutive-day dietary record tool against urinary biomarkers. British Journal of Nutrition. 2015;113(6):953–962. doi: 10.1017/S0007114515000057. [DOI] [PubMed] [Google Scholar]
- 79.Warensjo Lemming E., Nalsen C., Becker W., Ridefelt P., Mattisson I., Lindroos A.K. Relative validation of the dietary intake of fatty acids among adults in the Swedish National Dietary Survey using plasma phospholipid fatty acid composition. Journal of Nutritional Science. 2015;4:e25. doi: 10.1017/jns.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ax E., Warensjo Lemming E., Becker W., Andersson A., Lindroos A.K., Cederholm T. Dietary patterns in Swedish adults; results from a national dietary survey. British Journal of Nutrition. 2016;115(1):95–104. doi: 10.1017/S0007114515004110. [DOI] [PubMed] [Google Scholar]
- 81.Knudsen V.K., Matthiessen J., Biltoft-Jensen A., Sorensen M.R., Groth M.V., Trolle E. Identifying dietary patterns and associated health-related lifestyle factors in the adult Danish population. European Journal of Clinical Nutrition. 2014;68(6):736–740. doi: 10.1038/ejcn.2014.38. [DOI] [PubMed] [Google Scholar]
- 82.Gemming L., Utter J., Ni Mhurchu C. Image-assisted dietary assessment: a systematic review of the evidence. Journal of the Academy of Nutrition. 2015;115(1):64–77. doi: 10.1016/j.jand.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 83.Wang D.H., Kogashiwa M., Kira S. Development of a new instrument for evaluating individuals' dietary intakes. Journal of the American Dietetic Association. 2006;106(10):1588–1593. doi: 10.1016/j.jada.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 84.Rollo M.E., Ash S., Lyons-Wall P., Russell A. Trial of a mobile phone method for recording dietary intake in adults with type 2 diabetes: evaluation and implications for future applications. Journal of Telemedicine and Telecare. 2011;17(6):318–323. doi: 10.1258/jtt.2011.100906. [DOI] [PubMed] [Google Scholar]
- 85.Gemming L., Doherty A., Kelly P., Utter J., Ni Mhurchu C. Feasibility of a SenseCam-assisted 24-h recall to reduce under-reporting of energy intake. European Journal of Clinical Nutrition. 2013;67(10):1095–1099. doi: 10.1038/ejcn.2013.156. [DOI] [PubMed] [Google Scholar]
- 86.Pettitt C., Liu J., Kwasnicki R.M., Yang G.Z., Preston T., Frost G. A pilot study to determine whether using a lightweight, wearable micro-camera improves dietary assessment accuracy and offers information on macronutrients and eating rate. British Journal of Nutrition. 2016;115(1):160–167. doi: 10.1017/S0007114515004262. [DOI] [PubMed] [Google Scholar]
- 87.Martin C.K., Nicklas T., Gunturk B., Correa J.B., Allen H.R., Champagne C. Measuring food intake with digital photography. Journal of Human Nutrition and Dietetics. 2014;27(Suppl. 1):72–81. doi: 10.1111/jhn.12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin C.K., Correa J.B., Han H., Allen H.R., Rood J.C., Champagne C.M. Validity of the Remote Food Photography Method (RFPM) for estimating energy and nutrient intake in near real-time. Obesity (Silver Spring) 2012;20(4):891–899. doi: 10.1038/oby.2011.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martin C.K., Kaya S., Gunturk B.K. Quantification of food intake using food image analysis. Institute of Electrical and Electronics Engineers Engineering in Medicine and Biology Society. 2009;2009:6869–6872. doi: 10.1109/IEMBS.2009.5333123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Molag M.L., de Vries J.H., Ocke M.C., Dagnelie P.C., van den Brandt P.A., Jansen M.C. Design characteristics of food frequency questionnaires in relation to their validity. American Journal of Epidemiology. 2007;166(12):1468–1478. doi: 10.1093/aje/kwm236. [DOI] [PubMed] [Google Scholar]
- 91.Drewnowski A. Diet image: a new perspective on the food-frequency questionnaire. Nutrition Reviews. 2001;59(11):370–372. doi: 10.1111/j.1753-4887.2001.tb06964.x. [DOI] [PubMed] [Google Scholar]
- 92.Lee H., Kang M., Song W.O., Shim J.E., Paik H.Y. Gender analysis in the development and validation of FFQ: a systematic review. British Journal of Nutrition. 2016;115(4):666–671. doi: 10.1017/S0007114515004717. [DOI] [PubMed] [Google Scholar]
- 93.Kuskowska-Wolk A., Holte S., Ohlander E.M., Bruce A., Holmberg L., Adami H.O. Effects of different designs and extension of a food frequency questionnaire on response rate, completeness of data and food frequency responses. International Journal of Epidemiology. 1992;21(6):1144–1150. doi: 10.1093/ije/21.6.1144. [DOI] [PubMed] [Google Scholar]
- 94.Block G., Hartman A.M., Dresser C.M., Carroll M.D., Gannon J., Gardner L. A data-based approach to diet questionnaire design and testing. American Journal of Epidemiology. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 95.Willett W.C., Reynolds R.D., Cottrell-Hoehner S., Sampson L., Browne M.L. Validation of a semi-quantitative food frequency questionnaire: comparison with a 1-year diet record. Journal of the American Dietetic Association. 1987;87(1):43–47. [PubMed] [Google Scholar]
- 96.Cade J., Thompson R., Burley V., Warm D. Development, validation and utilisation of food-frequency questionnaires – a review. Public Health Nutrition. 2002;5(4):567–587. doi: 10.1079/PHN2001318. [DOI] [PubMed] [Google Scholar]
- 97.Nair N.S., Brennan I.M., Little T.J., Gentilcore D., Hausken T., Jones K.L. Reproducibility of energy intake, gastric emptying, blood glucose, plasma insulin and cholecystokinin responses in healthy young males. British Journal of Nutrition. 2009;101(7):1094–1102. doi: 10.1017/S0007114508042372. [DOI] [PubMed] [Google Scholar]
- 98.Rising R., Alger S., Boyce V., Seagle H., Ferraro R., Fontvieille A.M. Food intake measured by an automated food-selection system: relationship to energy expenditure. American Journal of Clinical Nutrition. 1992;55(2):343–349. doi: 10.1093/ajcn/55.2.343. [DOI] [PubMed] [Google Scholar]
- 99.Ball S.C., Benjamin S.E., Ward D.S. Development and reliability of an observation method to assess food intake of young children in child care. Journal of the American Dietetic Association. 2007;107(4):656–661. doi: 10.1016/j.jada.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Kissileff H.R., Klingsberg G., Van Itallie T.B. Universal eating monitor for continuous recording of solid or liquid consumption in man. American Journal of Physiology. 1980;238(1):R14–R22. doi: 10.1152/ajpregu.1980.238.1.R14. [DOI] [PubMed] [Google Scholar]
- 101.Allirot X., Saulais L., Disse E., Roth H., Cazal C., Laville M. Validation of a buffet meal design in an experimental restaurant. Appetite. 2012;58(3):889–897. doi: 10.1016/j.appet.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 102.Nornberg T.R., Houlby L., Jorgensen L.N., He C., Perez-Cueto F.J. Do we know how much we put on the plate? Assessment of the accuracy of self-estimated versus weighed vegetables and whole grain portions using an Intelligent Buffet at the FoodScape Lab. Appetite. 2014;81:162–167. doi: 10.1016/j.appet.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 103.Robinson E., Hardman C.A., Halford J.C., Jones A. Eating under observation: a systematic review and meta-analysis of the effect that heightened awareness of observation has on laboratory measured energy intake. American Journal of Clinical Nutrition. 2015;102(2):324–337. doi: 10.3945/ajcn.115.111195. [DOI] [PubMed] [Google Scholar]
- 104.Silva V., Barazzoni R., Singer P. Biomarkers of fish oil omega-3 polyunsaturated fatty acids intake in humans. Nutrition in Clinical Practice: Official Publication of the American Society for Parenteral and Enteral Nutrition. 2014;29(1):63–72. doi: 10.1177/0884533613516144. [DOI] [PubMed] [Google Scholar]
- 105.Elmadfa I., Meyer A.L. Developing suitable methods of nutritional status assessment: a continuous challenge. Advances in Nutrition. 2014;5(5):590S–598S. doi: 10.3945/an.113.005330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zamora-Ros R., Touillaud M., Rothwell J.A., Romieu I., Scalbert A. Measuring exposure to the polyphenol metabolome in observational epidemiologic studies: current tools and applications and their limits. American Journal of Clinical Nutrition. 2014;100(1):11–26. doi: 10.3945/ajcn.113.077743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bingham S.A. Urine nitrogen as a biomarker for the validation of dietary protein intake. Journal of Nutrition. 2003;133(Suppl. 3):921S–924S. doi: 10.1093/jn/133.3.921S. [DOI] [PubMed] [Google Scholar]
- 108.Hodson L., Skeaff C.M., Fielding B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Progress in Lipid Research. 2008;47(5):348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 109.King I.B., Lemaitre R.N., Kestin M. Effect of a low-fat diet on fatty acid composition in red cells, plasma phospholipids, and cholesterol esters: investigation of a biomarker of total fat intake. American Journal of Clinical Nutrition. 2006;83(2):227–236. doi: 10.1093/ajcn/83.2.227. [DOI] [PubMed] [Google Scholar]
- 110.Biltoft-Jensen A., Damsgaard C.T., Andersen E.W., Ygil K.H., Andersen R., Ege M. Validation of reported whole-grain intake from a web-based dietary record against plasma alkylresorcinol concentrations in 8- to 11-year-olds participating in a randomized controlled trial. Journal of Nutrition. 2016;146(2):377–383. doi: 10.3945/jn.115.222620. [DOI] [PubMed] [Google Scholar]
- 111.Tasevska N. Urinary sugars–a biomarker of total sugars intake. Nutrients. 2015;7(7):5816–5833. doi: 10.3390/nu7075255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.O'Brien D.M. Stable isotope ratios as biomarkers of diet for health research. Annual Review of Nutrition. 2015;35:565–594. doi: 10.1146/annurev-nutr-071714-034511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hedrick V.E., Dietrich A.M., Estabrooks P.A., Savla J., Serrano E., Davy B.M. Dietary biomarkers: advances, limitations and future directions. Nutrition Journal. 2012;11:109. doi: 10.1186/1475-2891-11-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Scalbert A., Brennan L., Manach C., Andres-Lacueva C., Dragsted L.O., Draper J. The food metabolome: a window over dietary exposure. American Journal of Clinical Nutrition. 2014;99(6):1286–1308. doi: 10.3945/ajcn.113.076133. [DOI] [PubMed] [Google Scholar]
- 115.Edmands W.M., Ferrari P., Rothwell J.A., Rinaldi S., Slimani N., Barupal D.K. Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. American Journal of Clinical Nutrition. 2015;102(4):905–913. doi: 10.3945/ajcn.114.101881. [DOI] [PubMed] [Google Scholar]
- 116.Tulipani S., Llorach R., Jauregui O., Lopez-Uriarte P., Garcia-Aloy M., Bullo M. Metabolomics unveils urinary changes in subjects with metabolic syndrome following 12-week nut consumption. Journal of Proteome Research. 2011;10(11):5047–5058. doi: 10.1021/pr200514h. [DOI] [PubMed] [Google Scholar]
- 117.Pujos-Guillot E., Hubert J., Martin J.F., Lyan B., Quintana M., Claude S. Mass spectrometry-based metabolomics for the discovery of biomarkers of fruit and vegetable intake: citrus fruit as a case study. Journal of Proteome Research. 2013;12(4):1645–1659. doi: 10.1021/pr300997c. [DOI] [PubMed] [Google Scholar]
- 118.Bouchard-Mercier A., Rudkowska I., Lemieux S., Couture P., Vohl M.C. The metabolic signature associated with the Western dietary pattern: a cross-sectional study. Nutrition Journal. 2013;12:158. doi: 10.1186/1475-2891-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vazquez-Fresno R., Llorach R., Urpi-Sarda M., Lupianez-Barbero A., Estruch R., Corella D. Metabolomic pattern analysis after mediterranean diet intervention in a nondiabetic population: a 1- and 3-year follow-up in the PREDIMED study. Journal of Proteome Research. 2015;14(1):531–540. doi: 10.1021/pr5007894. [DOI] [PubMed] [Google Scholar]
- 120.Burrows T.L., Hutchesson M.J., Rollo M.E., Boggess M.M., Guest M., Collins C.E. Fruit and vegetable intake assessed by food frequency questionnaire and plasma carotenoids: a validation study in adults. Nutrients. 2015;7(5):3240–3251. doi: 10.3390/nu7053240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Biltoft-Jensen A., Bysted A., Trolle E., Christensen T., Knuthsen P., Damsgaard C.T. Evaluation of web-based dietary assessment software for children: comparing reported fruit, juice and vegetable intakes with plasma carotenoid concentration and school lunch observations. British Journal of Nutrition. 2013;110(1):186–195. doi: 10.1017/S0007114512004746. [DOI] [PubMed] [Google Scholar]
- 122.Prentice R.L., Tinker L.F., Huang Y., Neuhouser M.L. Calibration of self-reported dietary measures using biomarkers: an approach to enhancing nutritional epidemiology reliability. Current Atherosclerosis Reports. 2013;15(9):353. doi: 10.1007/s11883-013-0353-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Seale J.L., Rumpler W.V. Comparison of energy expenditure measurements by diet records, energy intake balance, doubly labeled water and room calorimetry. European Journal of Clinical Nutrition. 1997;51(12):856–863. doi: 10.1038/sj.ejcn.1600498. [DOI] [PubMed] [Google Scholar]
- 124.Gilmore L.A., Ravussin E., Bray G.A., Han H., Redman L.M. An objective estimate of energy intake during weight gain using the intake-balance method. American Journal of Clinical Nutrition. 2014;100(3):806–812. doi: 10.3945/ajcn.114.087122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thomas D.M., Schoeller D.A., Redman L.A., Martin C.K., Levine J.A., Heymsfield S.B. A computational model to determine energy intake during weight loss. American Journal of Clinical Nutrition. 2010;92(6):1326–1331. doi: 10.3945/ajcn.2010.29687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sanghvi A., Redman L.M., Martin C.K., Ravussin E., Hall K.D. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. American Journal of Clinical Nutrition. 2015;102(2):353–358. doi: 10.3945/ajcn.115.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hall K.D., Sacks G., Chandramohan D., Chow C.C., Wang Y.C., Gortmaker S.L. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mattes R.D., Hollis J., Hayes D., Stunkard A.J. Appetite: measurement and manipulation misgivings. Journal of the American Dietetic Association. 2005;105(5 Suppl. 1):S87–S97. doi: 10.1016/j.jada.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 129.Rumbold P.L., Dodd-Reynolds C.J., Stevenson E. Agreement between paper and pen visual analogue scales and a wristwatch-based electronic appetite rating system (PRO-Diary(c)), for continuous monitoring of free-living subjective appetite sensations in 7-10 year old children. Appetite. 2013;69:180–185. doi: 10.1016/j.appet.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 130.Stratton R.J., Stubbs R.J., Hughes D., King N., Blundell J.E., Elia M. Comparison of the traditional paper visual analogue scale questionnaire with an Apple Newton electronic appetite rating system (EARS) in free living subjects feeding ad libitum. European Journal of Clinical Nutrition. 1998;52(10):737–741. doi: 10.1038/sj.ejcn.1600636. [DOI] [PubMed] [Google Scholar]
- 131.Gibbons C., Caudwell P., Finlayson G., King N., Blundell J. Validation of a new hand-held electronic data capture method for continuous monitoring of subjective appetite sensations. The International Journal of Behavioral Nutrition and Physical Activity. 2011;8:57. doi: 10.1186/1479-5868-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Flint A., Raben A., Blundell J.E., Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. International Journal of Obesity and Related Metabolic Disorders. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 133.Stubbs R.J., Hughes D.A., Johnstone A.M., Rowley E., Reid C., Elia M. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. British Journal of Nutrition. 2000;84(4):405–415. doi: 10.1017/s0007114500001719. [DOI] [PubMed] [Google Scholar]
- 134.Delzenne N., Blundell J., Brouns F., Cunningham K., De Graaf K., Erkner A. Gastrointestinal targets of appetite regulation in humans. Obes Rev. 2010;11(3):234–250. doi: 10.1111/j.1467-789X.2009.00707.x. [DOI] [PubMed] [Google Scholar]
- 135.Shephard R.J., Aoyagi Y. Measurement of human energy expenditure, with particular reference to field studies: an historical perspective. European Journal of Applied Physiology. 2012;112(8):2785–2815. doi: 10.1007/s00421-011-2268-6. [DOI] [PubMed] [Google Scholar]
- 136.Mundal R., Erikssen J., Rodahl K. Assessment of physical activity by questionnaire and personal interview with particular reference to fitness and coronary mortality. European Journal of Applied Physiology and Occupational Physiology. 1987;56(3):245–252. doi: 10.1007/BF00690888. [DOI] [PubMed] [Google Scholar]
- 137.Kannel W.B. Habitual level of physical activity and risk of coronary heart disease: the Framingham study. Canadian Medical Association Journal. 1967;96(12):811–812. [PMC free article] [PubMed] [Google Scholar]
- 138.Jequier E. Human whole body direct calorimetry. IEEE Engineering in Medicine and Biology Magazine. 1986;5(2):12–14. doi: 10.1109/MEMB.1986.5006277. [DOI] [PubMed] [Google Scholar]
- 139.Webb P. The measurement of energy exchange in man: an analysis. American Journal of Clinical Nutrition. 1980;33(6):1299–1310. doi: 10.1093/ajcn/33.6.1299. [DOI] [PubMed] [Google Scholar]
- 140.Lyden K., Swibas T., Catenacci V., Guo R., Szuminsky N., Melanson E.L. Estimating energy expenditure using heat flux measured at a single body site. Medicine and Science in Sports and Exercise. 2014;46(11):2159–2167. doi: 10.1249/MSS.0000000000000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Melanson E.L., Dykstra J.C., Szuminsky N. A novel approach for measuring energy expenditure in free-living humans. Institute of Electrical and Electronics Engineers Engineering in Medicine and Biology Society. 2009;2009:6873–6877. doi: 10.1109/IEMBS.2009.5333124. [DOI] [PubMed] [Google Scholar]
- 142.Benito P.J., Neiva C., Gonzalez-Quijano P.S., Cupeiro R., Morencos E., Peinado A.B. Validation of the SenseWear armband in circuit resistance training with different loads. European Journal of Applied Physiology. 2012;112(8):3155–3159. doi: 10.1007/s00421-011-2269-5. [DOI] [PubMed] [Google Scholar]
- 143.Drenowatz C., Eisenmann J.C. Validation of the SenseWear Armband at high intensity exercise. European Journal of Applied Physiology. 2011;111(5):883–887. doi: 10.1007/s00421-010-1695-0. [DOI] [PubMed] [Google Scholar]
- 144.Weir J.B. New methods for calculating metabolic rate with special reference to protein metabolism. Journal of Physiology. 1949;109(1–2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Levine J.A. Measurement of energy expenditure. Public Health Nutrition. 2005;8(7A):1123–1132. doi: 10.1079/phn2005800. [DOI] [PubMed] [Google Scholar]
- 146.Ravussin E., Burnand B., Schutz Y., Jequier E. Twenty-four-hour energy expenditure and resting metabolic rate in obese, moderately obese, and control subjects. American Journal of Clinical Nutrition. 1982;35(3):566–573. doi: 10.1093/ajcn/35.3.566. [DOI] [PubMed] [Google Scholar]
- 147.Murgatroyd P.R., Davies H.L., Prentice A.M. Intra-individual variability and measurement noise in estimates of energy expenditure by whole body indirect calorimetry. British Journal of Nutrition. 1987;58(3):347–356. doi: 10.1079/bjn19870104. [DOI] [PubMed] [Google Scholar]
- 148.Ravussin E., Lillioja S., Anderson T.E., Christin L., Bogardus C. Determinants of 24-hour energy expenditure in man. Methods and results using a respiratory chamber. Journal of Clinical Investigation. 1986;78(6):1568–1578. doi: 10.1172/JCI112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Smith S.R., de Jonge L., Zachwieja J.J., Roy H., Nguyen T., Rood J.C. Fat and carbohydrate balances during adaptation to a high-fat. American Journal of Clinical Nutrition. 2000;71(2):450–457. doi: 10.1093/ajcn/71.2.450. [DOI] [PubMed] [Google Scholar]
- 150.Schutz Y., Bessard T., Jequier E. Diet-induced thermogenesis measured over a whole day in obese and nonobese women. American Journal of Clinical Nutrition. 1984;40(3):542–552. doi: 10.1093/ajcn/40.3.542. [DOI] [PubMed] [Google Scholar]
- 151.Tataranni P.A., Larson D.E., Snitker S., Ravussin E. Thermic effect of food in humans: methods and results from use of a respiratory chamber. American Journal of Clinical Nutrition. 1995;61(5):1013–1019. doi: 10.1093/ajcn/61.4.1013. [DOI] [PubMed] [Google Scholar]
- 152.Marino S., De Gaetano A., Giancaterini A., Giordano D., Manco M., Greco A.V. Computing DIT from energy expenditure measures in a respiratory chamber: a direct modeling method. Computers in Biology and Medicine. 2002;32(4):297–309. doi: 10.1016/s0010-4825(02)00007-0. [DOI] [PubMed] [Google Scholar]
- 153.Psota T., Chen K.Y. Measuring energy expenditure in clinical populations: rewards and challenges. European Journal of Clinical Nutrition. 2013;67(5):436–442. doi: 10.1038/ejcn.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Human energy requirements: report of a joint FAO/WHO/UNU Expert Consultation. Food and Nutrition Bulletin. 2005;26(1):166. [PubMed] [Google Scholar]
- 155.Brychta R., Wohlers E., Moon J., Chen K. Energy expenditure: measurement of human metabolism. IEEE Engineering in Medicine and Biology Magazine. 2010;29(1):42–47. doi: 10.1109/MEMB.2009.935463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schoeller D.A., van Santen E. Measurement of energy expenditure in humans by doubly labeled water method. Journal of Applied Physiology Respiratory Environmental and Exercise Physiology. 1982;53(4):955–959. doi: 10.1152/jappl.1982.53.4.955. [DOI] [PubMed] [Google Scholar]
- 157.Coward W.A. Stable isotopic methods for measuring energy expenditure. The doubly-labeled-water (2H2(18)O) method: principles and practice. Proceedings of the Nutrition Society. 1988;47(3):209–218. doi: 10.1079/pns19880037. [DOI] [PubMed] [Google Scholar]
- 158.Schoeller D.A., Ravussin E., Schutz Y., Acheson K.J., Baertschi P., Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. American Journal of Physiology. 1986;250(5 Pt 2):R823–R830. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 159.Rochon J., Bales C.W., Ravussin E., Redman L.M., Holloszy J.O., Racette S.B. Design and conduct of the CALERIE study: comprehensive assessment of the long-term effects of reducing intake of energy. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2011;66(1):97–108. doi: 10.1093/gerona/glq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bratteby L.E., Sandhagen B., Fan H., Samuelson G.A. 7-day activity diary for assessment of daily energy expenditure validated by the doubly labeled water method in adolescents. European Journal of Clinical Nutrition. 1997;51(9):585–591. doi: 10.1038/sj.ejcn.1600449. [DOI] [PubMed] [Google Scholar]
- 161.Westerterp K.R. Body composition, water turnover and energy turnover assessment with labeled water. Proceedings of the Nutrition Society. 1999;58(4):945–951. doi: 10.1017/s0029665199001251. [DOI] [PubMed] [Google Scholar]
- 162.Coward W.A. Measurement of energy expenditure: the doubly-labeled-water method in clinical practice. Proceedings of the Nutrition Society. 1991;50(2):227–237. doi: 10.1079/pns19910032. [DOI] [PubMed] [Google Scholar]
- 163.Speakman J.R. Principles, problems and a paradox with the measurement of energy expenditure of free-living subjects using doubly-labeled water. Statistics in Medicine. 1990;9(11):1365–1380. doi: 10.1002/sim.4780091113. [DOI] [PubMed] [Google Scholar]
- 164.Hall C., Figueroa A., Fernhall B., Kanaley J.A. Energy expenditure of walking and running: comparison with prediction equations. Medicine and Science in Sports and Exercise. 2004;36(12):2128–2134. doi: 10.1249/01.mss.0000147584.87788.0e. [DOI] [PubMed] [Google Scholar]
- 165.Schutz Y., Ravussin E., Diethelm R., Jequier E. Spontaneous physical activity measured by radar in obese and control subject studied in a respiration chamber. International Journal of Obesity. 1982;6(1):23–28. [PubMed] [Google Scholar]
- 166.Tudor-Locke C., Williams J.E., Reis J.P., Pluto D. Utility of pedometers for assessing physical activity: convergent validity. Sports Medicine. 2002;32(12):795–808. doi: 10.2165/00007256-200232120-00004. [DOI] [PubMed] [Google Scholar]
- 167.Freedson P.S., Melanson E., Sirard J. Calibration of the computer science and applications, Inc. accelerometer. Medicine and Science in Sports and Exercise. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 168.Crouter S.E., Churilla J.R., Bassett D.R., Jr. Estimating energy expenditure using accelerometers. European Journal of Applied Physiology. 2006;98(6):601–612. doi: 10.1007/s00421-006-0307-5. [DOI] [PubMed] [Google Scholar]
- 169.Plasqui G., Westerterp K.R. Physical activity assessment with accelerometers: an evaluation against doubly labeled water. Obesity (Silver Spring) 2007;15(10):2371–2379. doi: 10.1038/oby.2007.281. [DOI] [PubMed] [Google Scholar]
- 170.Achten J., Jeukendrup A.E. Heart rate monitoring: applications and limitations. Sports Medicine. 2003;33(7):517–538. doi: 10.2165/00007256-200333070-00004. [DOI] [PubMed] [Google Scholar]
- 171.Hiilloskorpi H.K., Pasanen M.E., Fogelholm M.G., Laukkanen R.M., Manttari A.T. Use of heart rate to predict energy expenditure from low to high activity levels. International Journal of Sports Medicine. 2003;24(5):332–336. doi: 10.1055/s-2003-40701. [DOI] [PubMed] [Google Scholar]
- 172.Millet G.P., Vleck V.E., Bentley D.J. Physiological differences between cycling and running: lessons from triathletes. Sports Medicine. 2009;39(3):179–206. doi: 10.2165/00007256-200939030-00002. [DOI] [PubMed] [Google Scholar]
- 173.Charlot K., Cornolo J., Borne R., Brugniaux J.V., Richalet J.P., Chapelot D. Improvement of energy expenditure prediction from heart rate during running. Physiological Measurement. 2014;35(2):253–266. doi: 10.1088/0967-3334/35/2/253. [DOI] [PubMed] [Google Scholar]
- 174.Gastinger S., Donnelly A., Dumond R., Prioux J. A review of the evidence for the use of ventilation as a surrogate measure of energy expenditure. JPEN. Journal of Parenteral and Enteral Nutrition. 2014;38(8):926–938. doi: 10.1177/0148607114530432. [DOI] [PubMed] [Google Scholar]
- 175.Durnin J.V., Edwards R.G. Pulmonary ventilation as an index of energy expenditure. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1955;40(4):370–377. doi: 10.1113/expphysiol.1955.sp001137. [DOI] [PubMed] [Google Scholar]
- 176.Grandjean A.C. Dietary intake data collection: challenges and limitations. Nutrition Reviews. 2012;70(Suppl. 2):S101–S104. doi: 10.1111/j.1753-4887.2012.00545.x. [DOI] [PubMed] [Google Scholar]
- 177.Sternfeld B., Goldman-Rosas L. A systematic approach to selecting an appropriate measure of self-reported physical activity or sedentary behavior. Journal of Physical Activity & Health. 2012;9(Suppl. 1):S19–S28. doi: 10.1123/jpah.9.s1.s19. [DOI] [PubMed] [Google Scholar]
- 178.Lagerros Y.T., Lagiou P. Assessment of physical activity and energy expenditure in epidemiological research of chronic diseases. European Journal of Epidemiology. 2007;22(6):353–362. doi: 10.1007/s10654-007-9154-x. [DOI] [PubMed] [Google Scholar]
- 179.Bray G.A., Smith S.R., de Jonge L., Xie H., Rood J., Martin C.K. Effect of dietary protein content on weight gain, energy expenditure, and body composition during overeating: a randomized controlled trial. JAMA: the Journal of the American Medical Association. 2012;307(1):47–55. doi: 10.1001/jama.2011.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Harrington D.M., Martin C.K., Ravussin E., Katzmarzyk P.T. Activity related energy expenditure, appetite and energy intake: potential implications for weight management. Appetite. 2013;67:1–7. doi: 10.1016/j.appet.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]