Abstract
Background: Flavonoids are dietary polyphenolic compounds with a variety of proposed beneficial cardiovascular effects, but rigorous prospective studies that examine the association between flavonoid intake and incident coronary heart disease (CHD) in geographically and racially diverse US samples are limited.
Objective: With the use of the new, expanded USDA flavonoid database, we assessed the association between total flavonoid and flavonoid subclass intakes with incident CHD in a biracial and geographically diverse cohort, as well as effect modification by age, sex, race, and region of residence.
Design: Participants were 16,678 black and white men and women enrolled in the REGARDS (REasons for Geographic and Racial Differences in Stroke) study, a national prospective cohort study. All participants were without CHD at baseline, and all completed a Block98 food-frequency questionnaire. Flavonoid intakes were estimated from USDA flavonoid databases, which were recently improved to address missing values for cooked foods and to adjust for flavonoid losses due to processing. Incident CHD events were participant reported and adjudicated by experts. Quintiles of flavonoid intake were examined as predictors of incident CHD by using Cox proportional hazards regression to obtain HRs. Tests for trend used the quintile medians.
Results: Over a mean ± SD follow-up of 6.0 ± 1.9 y, 589 CHD events occurred. High flavonoid intake was associated with self-identified white race, exercise, not smoking, more education, and higher income. In models that adjusted for sociodemographic, health behavior, and dietary factors, there was an inverse association between anthocyanidin and proanthocyanidin intakes and incident CHD (HRs for quintile 5 compared with quintile 1—anthocyanidins: 0.71; 95% CI: 0.52, 0.98; P-trend = 0.04; proanthocyanidins: 0.63; 95% CI: 0.47, 0.84; P-trend = 0.02). There was no association between total flavonoid or other flavonoid subclass intakes and incident CHD.
Conclusions: Reported anthocyanidin and proanthocyanidin intakes were inversely associated with incident CHD. There was no significant effect modification by age, sex, race, or region of residence.
Keywords: flavonoids, coronary heart disease, epidemiology, diet, prospective studies, cardiovascular disease
INTRODUCTION
Black Americans and residents of the southeastern United States, a region also known as the Stroke Belt due to elevated stroke mortality, are at greater risk of coronary heart disease (CHD)9 mortality and fatal incident CHD than are whites and those living elsewhere (1–3). High adherence to a Southern dietary pattern, characterized by fried foods, organ meats, and sugar-sweetened beverages, is more common among men, non-Hispanic blacks, and residents of the southeastern United States and has been associated with a 66% increased risk of incident CHD (4). In contrast, diets high in plant-based foods have been associated with a decreased risk of CHD (5, 6). Although Americans generally consume inadequate amounts of fruit and vegetables, consumption among black Americans is even less likely to be adequate (7, 8), which may be related to socioeconomic or cultural dietary factors that differ between white and black Americans.
Flavonoids are bioactive, polyphenolic compounds found in a wide variety of plant-based foods, including fruit and vegetables, tea, wine, nuts, herbs, and spices. Subclasses of flavonoids commonly consumed in the United States include anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols, and proanthocyanidins. Cardioprotective mechanisms for flavonoids have been proposed, including antioxidant and anti-inflammatory actions, modulation of lipid metabolism and platelet function, and attenuation of hypertension (9). Consistent with these biological effects, epidemiologic studies to date suggest a protective effect of flavonoids against CHD mortality (10, 11), incident CHD (12, 13), and other outcomes relevant to CHD such as arterial stiffness, incident hypertension, and type 2 diabetes, as well as improved long-term weight maintenance (14–17). However, to our knowledge, no published studies that examined flavonoids and incident CHD enrolled a racially diverse participant population and only one study in the United States was geographically diverse (10, 11). Furthermore, this field of research has been hampered by a lack of comprehensive food flavonoid composition tables. To address this issue, in 2014 the USDA released the Provisional Flavonoid Addendum (18). With the use of this new, expanded flavonoid database, we evaluated the association of each of 7 flavonoid subclasses and the summed total of flavonoid subclass intakes with incident CHD in a large biracial cohort study. Furthermore, we assessed whether the association between flavonoid intake and CHD differed by age, sex, race, and residence in the southeastern United States.
METHODS
Study participants
The REGARDS (REasons for Geographic and Racial Differences in Stroke) Study is a prospective cohort study in 30,239 participants designed to examine regional and racial influences on stroke mortality. English-speaking, community-dwelling, non-Hispanic white and black adults living in the continental United States and >45 y old were recruited between 2003 and 2007 by using a combination of mail and telephone contacts. Self-reported race and sex were balanced by design, with oversampling from the southeastern United States, often referred to as the Stroke Belt, including Alabama, Arkansas, Georgia, Louisiana, Mississippi, Tennessee, North Carolina, and South Carolina. Within the Stroke Belt, the coastal plains regions of Georgia, North Carolina, and South Carolina, often referred to as the Stroke Buckle, experience an even higher rate of stroke mortality than the rest of the Stroke Belt. The final cohort included 56% residents from the southeastern United States and 44% residents of the contiguous lower 48 states, 42% blacks, and 55% women (19). Baseline data collection included computer-assisted telephone interviews to assess health status and medical history. Trained health care professionals conducted in-home examinations by using standardized, quality-controlled protocols to obtain fasting blood and urine samples, electrocardiograms, blood pressure, and height and weight measurements and to complete a medication inventory. The institutional review boards of all participating universities approved this study, and written informed consent was obtained from all participants.
Dietary assessment
A Block98 food-frequency questionnaire (FFQ) was left with all of the study participants at the conclusion of the in-home visit and was returned by mail. This self-administered FFQ is a 107-item questionnaire developed by NutritionQuest and has been validated in populations relevant to the REGARDS study (20–22). The Block98 FFQ enquires about plant-based foods with high flavonoid content, including fruit, vegetables, tea, nuts, and wine. NutritionQuest provided mean daily intake estimates, in grams, for FFQ items. Those who completed <85% of the FFQ and with reported caloric intakes of <800 kcal or >4200 kcal/d for men and <500 kcal or >3500 kcal/d for women were excluded from analyses.
Estimation of flavonoid intake
Specific flavonoid intakes of interest were anthocyanidins, flavan-3-ols, flavanones, flavone, flavonols, and proanthocyanidins, which are oligomers and polymers of flavan-3-ols, as well as total flavonoid intake. We did not consider isoflavones, which are flavonoids obtained primarily from soy-based foods, because intakes in the United States are generally low. The USDA Database for the Proanthocyanidin Content of Selected Foods and the USDA Provisional Flavonoid Addendum to the USDA Food and Nutrient Database for Dietary Studies (FNDDS), version 4.1, provide flavonoid content information for complementary sets of flavonoids, which are presented as aglycone equivalents (18, 23). The flavonoids included in these databases and used to estimate flavonoid intakes in this study are summarized in Supplemental Table 1. The Provisional Flavonoid Addendum is the most recent comprehensive food flavonoid database, providing data for 29 flavonoids in 6 subclasses for 7147 foods and beverages in the FNDDS, version 4.1 (2007–2008), and accounts for the effects of processing and cooking on flavonoids better than do previous releases of the USDA flavonoid databases (18). Foods and beverages, portions, and nutrient descriptions are updated in the FNDDS every 2 y on the basis of 24-h recalls in the dietary intake component of the nationally representative NHANES. In contrast, the USDA Database for the Proanthocyanidin Content of Selected Foods was released >10 y ago and has not been updated (23). The Provisional Flavonoid Addendum and Proanthocyanidin Database are largely complementary, although they contain overlapping information about flavan-3-ols, which are identified as monomers in the Proanthocyanidin Database. Given the methodologic differences between the databases, when a food was included in both databases, and if a flavan-3-ol value (Addendum), and monomer value (Proanthocyanidin Database) were both available, only the current flavan-3-ol value from the Provisional Flavonoid Addendum was used. Furthermore, 2 different measures of total flavonoid intake were used. One included only flavonoid values obtained from the Provisional Flavonoid Addendum (total I) and a second measure added proanthocyanidin intake (total II).
Food items comprising the Block98 FFQ were matched to corresponding food items in the Provisional Flavonoid Addendum by using the unique 8-digit FNDDS food code and the 5-digit USDA Standard Reference food codes in the Proanthocyanidin Database. When necessary, the USDA FNDDS–Standard Reference links file was used as a cross-walk between data sets. For food items that included only 1 food (e.g., bananas), the flavonoid values for the matching food in the USDA databases were used. Calculations for combined items on the FFQ, such as “apples or pears,” were weighted averages that used NHANES-based per capita consumption, consistent with weights used in the Block98 FFQ. Items on the FFQ with multiple ingredients (e.g., pizza) were assigned flavonoid values consistent with the USDA standard recipes. USDA recipes did not differentiate between foods eaten at home or outside of the home. Flavonoid values provided in the Provisional Flavonoid Addendum already account for processing factors. Estimated daily flavonoid intake for each participant was calculated by multiplying the reported amount (in grams) of food consumed by the flavonoid content of the corresponding food (expressed as mg flavonoid/100 g food) and summed across foods.
Acute CHD events
Participants were contacted every 6 mo with active telephone surveillance. Hospitalizations reported by living participants or proxies triggered medical record retrieval. Deaths were detected by report of next of kin, through online sources (e.g., Social Security Death Index), or by the National Death Index. Proxies or next of kin were interviewed about the circumstances surrounding the death including the presence of chest pain. Cause of death and cardiovascular outcomes were adjudicated by using medical records, death certificates, and autopsy reports following published guidelines and have been described previously (3). Only definite or probable myocardial infarctions (MIs) and CHD deaths that occurred through 31 December 2011 were included in this analysis.
Statistical analyses
We created quintiles of reported total and subclass flavonoid intakes defined from the total study population because sex-specific intakes were not meaningfully different. Median intakes and ranges of intakes for total flavonoids and each flavonoid subclass were calculated. Flavonoid values were energy-adjusted by using the residual method (24). Baseline characteristics were summarized by quintiles of total flavonoid I intake. The association between quintiles of flavonoid intake and the risk of incident CHD events was examined by using Cox proportional hazards models. Participants were censored at the date of CHD event, loss to follow-up, or last CHD adjudication, whichever occurred first. After verifying the proportional hazards assumption, models were built by sequential adjustment for potential confounders by first adding age, energy intake, and sex (model 1), followed by other demographic factors (race and region of residence), socioeconomic factors (household income and educational attainment), and health behaviors (smoking status, pack-years smoking, and physical activity) in model 2. These variables have been previously described (25). Next, in model 3, we adjusted for dietary factors that were associated with both flavonoid intake and acute CHD events, including percentage of energy consumed from sweetened foods and beverages (sweetened cereals and beverages, jelly, and sugar and/or honey added to coffee or tea), reported beer and liquor intake, and trans fat intake and the ratio of MUFAs to SFAs consumed. Wine intake was not included in models to avoid overadjustment for certain sources of flavonoids. Given evidence that flavonoid intake is associated with multiple CHD risk factors, we considered them to be potential intermediate variables along the causal pathway between flavonoid intake and incident CHD. We conducted secondary analyses, in model 4, adding terms to the model for BMI, history or use of medications for hyperlipidemia, diabetes, and hypertension, as well as aspirin use. We conducted stratified analyses on the basis of a priori (age at baseline, race, sex, and region of residence) and post hoc–identified (education, physical activity, and smoking status) potential effect modifiers. Interaction terms between quintiles of flavonoid intake and these variables were also added to models and likelihood ratio chi-square tests were used to formally test for statistical interaction. Trend tests were conducted by assigning each quintile its median value and modeling the exposure as a continuous variable. Sensitivity analysis that used multiply imputed missing covariates was conducted to assess possible bias due to the exclusion of participants with missing information on 3 key covariates, including smoking status (n = 66), education (n = 8), or physical activity (n = 233).
We also examined the association between selected FFQ food items and CHD endpoints with the use of multivariable models similar to those used for flavonoid intake. We included foods if they comprised ≥1% of reported total flavonoid intake. These foods included tea (63%), apples or pears (8%), cakes (6%), citrus fruit or juices (5%), canned fruit (4%), wine (4%), 100% fruit juice excluding orange (3%), peanuts or nuts (2%), legumes (2%), and berries (1%). Foods were categorized into quintiles, with the exception of wine and tea. Wine was a composite of primarily red wine, white wine, and wine cocktails weighted by NHANES-based population intake. Wine consumption was categorized as nondrinkers or consumption of ≤1 drink/wk, 2–4 drinks/wk, and ≥5 drinks/wk. Because neither the FFQ nor the Provisional Flavonoid Addendum differentiates the type of tea consumed, values are a composite of 84% black tea and 16% green tea based on market-share data, and intake was categorized as nondrinkers or consumption of 1–3 cups/mo, 1–6 cups/wk, or ≥1 cup/d. Final models in food analyses were adjusted for age, caloric intake, sex, race, region of residence, income, education, physical activity, smoking status, percentage of energy from sweets, reported beer and liquor intake, as well as trans fat intake and the ratio of MUFAs to SFAs consumed.
To investigate the effect of energy “misreporting,” flavonoid analyses were repeated after energy “underreporters” and “overreporters” were excluded (n = 5647 and 15, respectively) by using the Goldberg cutoffs (26, 27). Basal metabolic rate was estimated by using the Schofield equations for height, weight, and age (28); and physical activity level was assumed to be 1.55. Analyses were conducted by using SAS version 9.4 (SAS Institute).
RESULTS
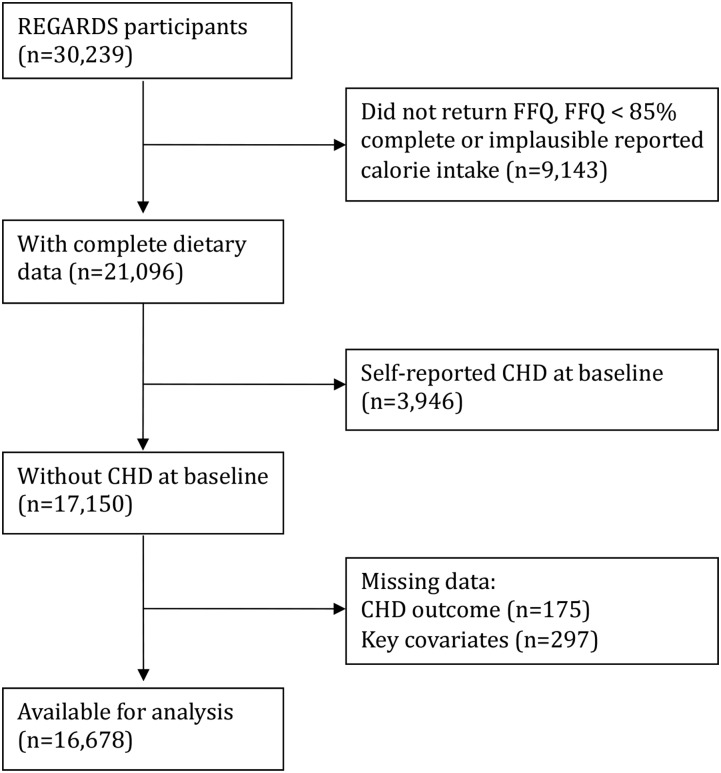
There were 21,096 participants (70%) who returned an FFQ with complete dietary data, defined by completing ≥85% of the FFQ items and plausible reported caloric intakes. After the exclusion of participants with a history of CHD at baseline (n = 3946), missing information on outcome (n = 175) or key covariates (n = 297), 16,678 participants were available for this analysis. During a mean ± SD follow-up of 6.0 ± 1.9 y, 589 incident CHD events were observed, 221 in women and 368 in men. The participant flow diagram is shown in Figure 1. Those excluded from the dietary subsample were more often black (61% compared with 39%), less educated (20% compared with 10% with less than a complete high school education), and had a lower reported annual household income (24% compared with 16% <$20,000/y) compared with those in the dietary subsample. There was no difference in the proportion of incident CHD events experienced in the dietary subsample than in those in the full sample.
FIGURE 1.
Participant flow diagram to determine the analytic cohort of 16,678 REGARDS participants without self-reported CHD at baseline. CHD, coronary heart disease; FFQ, food-frequency questionnaire; REGARDS, REasons for Geographic and Racial Differences in Stroke.
Mean and median total flavonoid intakes for women were 234 and 131 mg, respectively; for men, these values were 227 and 131 mg, respectively. Mean and median reported daily caloric intakes for women were 1561 and 1470 kcal, respectively; for men, these values were 1871 and 1758 kcal, respectively. The top food sources of flavonoids are listed in Table 1. Baseline characteristics by quintiles of flavonoid I intake are summarized in Table 2. The distribution of baseline characteristics did not differ when total flavonoid intake II was used instead (flavonoid intake I plus proanthocyanidins). Individuals with higher total flavonoid intakes were more likely to be white, more educated, and less sedentary; to have higher reported household income; and never to have smoked.
TABLE 1.
Proportional contribution of Block98 FFQ items to total flavonoid and flavonoid subclass intakes for 16,678 participants without CHD at baseline in the REGARDS study, 2003–20071
| Food source | % |
| Total flavonoids I2 | |
| Tea3 | 79 |
| Orange juice | 9 |
| Orange, raw | 3 |
| Wine4 | 2 |
| 100% real fruit juice, not orange | 2 |
| Apples or pears, raw | 2 |
| Other vegetables5 | 2 |
| Salad greens | <1 |
| Canned fruit6 | <1 |
| Grapefruit, raw | <1 |
| Anthocyanidins | |
| Wine4 | 27 |
| 100% real fruit juice, not orange | 16 |
| Berries | 14 |
| Coleslaw | 8 |
| Vegetable stews7 | 8 |
| Yogurt with fruit | 7 |
| Other fruit8 | 6 |
| Canned fruit6 | 4 |
| Other vegetables5 | 4 |
| Apples or pears | 3 |
| Flavanone | |
| Orange juice | 67 |
| Orange | 21 |
| Grapefruit | 7 |
| 100% real fruit juice, not orange | 2 |
| Liquor or cocktails | 1 |
| Tomato | <1 |
| Flavonol | |
| Tea3 | 44 |
| Salad greens | 18 |
| Other vegetables5 | 12 |
| Dark leafy greens | 8 |
| Apples or pears | 6 |
| Broccoli | 4 |
| 100% real fruit juice, not orange | 3 |
| Wine4 | 3 |
| Total flavonoids II9 | |
| Tea3 | 63 |
| Apples or pears | 8 |
| Cake10 | 6 |
| Orange juice | 4 |
| Pies and cobbler8 | 4 |
| Canned fruit6 | 4 |
| Wine4 | 4 |
| 100% real fruit juice, not orange | 2 |
| Peanuts and nuts | 1 |
| Beans | 1 |
| Flavan-3-ols | |
| Tea3 | 94 |
| Apples or pears | 1 |
| 100% real fruit juice, not orange | 1 |
| Bananas | 1 |
| Wine4 | 1 |
| Canned fruit6 | 1 |
| Flavone | |
| Other vegetables5 | 53 |
| Salad greens | 14 |
| Tea3 | 7 |
| Spinach | 5 |
| Cantaloupe | 4 |
| Orange | 3 |
| Vegetable stews7 | 3 |
| Apples or pears | 2 |
| Broccoli | 2 |
| Grapefruit | 1 |
| Proanthocyanidins | |
| Apples or pears | 18 |
| Cake10 | 16 |
| Pies and cobbler8 | 10 |
| Tea3 | 9 |
| Canned fruit6 | 8 |
| Beans | 6 |
| Peanuts and nuts | 6 |
| Wine4 | 6 |
| 100% real fruit juice, not orange | 4 |
| Berries | 3 |
CHD, coronary heart disease; FFQ, food-frequency questionnaire; REGARDS, REasons for Geographic and Racial Differences in Stroke.
Sum of flavonoid subclasses in the USDA Provisional Flavonoid Addendum (anthocyanidins, flavan-3-ols, flavanones, flavones, flavonols).
In the Provisional Flavonoid Addendum, flavonoid values for tea are a composite consisting of 84% black tea and 16% green tea, based on published market-share data.
A composite of red wine, white wine, cooking wine, and sangria, weighted by NHANES population-based reported intakes.
Includes pumpkin, squash, peppers, onions, artichokes, asparagus, lima beans, bean sprouts, beets, Brussels sprouts, cauliflower, celery, eggplant, mushrooms, okra, and parsnips that are cooked from fresh or frozen vegetables. Anthocyanidins are primarily from eggplant; flavones are from pumpkins, squash, peppers, celery, and okra; flavonols are from asparagus, peppers, Brussels sprouts, okra, and onions.
Canned fruit includes apricots, cherries, plums, peaches, pears, fruit cocktail, and applesauce.
Mix of vegetables, with or without meat, prepared as a stew, excluding soups. Anthocyanidins are primarily from eggplant; flavones are primarily from squashes, peppers, and herbs.
Includes fruit cobblers, chocolate pies, and turnovers; important sources of flavonoids include fruit, chocolate, and cinnamon.
Sum of total flavonoid I and proanthocyanidins.
Includes fruit, chocolate, and spice cakes; important sources of flavonoids include fruit, chocolate, and cinnamon.
TABLE 2.
Baseline characteristics for 16,678 participants without CHD at baseline in the REGARDS study 2003–2007, by quintile of total flavonoid intake (total I)1
| Quintile of total flavonoid I intake |
||||||
| 1 | 2 | 3 | 4 | 5 | P2 | |
| n | 3305 | 3341 | 3362 | 3362 | 3321 | |
| Age, y | 63.4 ± 9.03 | 64.4 ± 9.0 | 64.7 ± 9.2 | 64.6 ± 9.2 | 64.2 ± 8.9 | 0.002 |
| Energy intake, kcal | 2039 ± 548 | 1473 ± 530 | 1511 ± 588 | 1653 ± 611 | 1774 ± 779 | <0.001 |
| BMI, kg/m2 | 29.4 ± 6.3 | 29.0 ± 6.2 | 28.8 ± 5.9 | 29.1 ± 6.0 | 28.7 ± 6.0 | 0.003 |
| Male, n (%) | 1761 (53.3) | 1297 (38.8) | 1233 (36.8) | 1317 (39.2) | 1272 (38.3) | <0.001 |
| White, n (%) | 2070 (62.6) | 2011 (60.2) | 2034 (60.7) | 2241 (66.7) | 2643 (79.6) | <0.001 |
| Region,4 n (%) | <0.001 | |||||
| Stroke Belt | 1146 (34.7) | 1080 (32.3) | 1099 (32.8) | 1174 (34.9) | 1227 (37.0) | |
| Stroke Buckle | 575 (19.2) | 659 (20.0) | 681 (19.8) | 818 (23.2) | 909 (27.3) | |
| Not Stroke Belt | 1584 (47.9) | 1602 (47.8) | 1569 (46.9) | 1370 (40.8) | 1185 (35.7) | |
| Physical activity,5 n (%) | <0.001 | |||||
| None | 1174 (35.5) | 1113 (33.3) | 954 (28.5) | 1019 (30.3) | 1028 (30.9) | |
| 1–3 times/wk | 1174 (35.5) | 1254 (37.5) | 1359 (40.6) | 1294 (38.5) | 1238 (37.3) | |
| ≥4 times/wk | 957 (25.9) | 974 (29.2) | 1036 (30.9) | 1049 (31.2) | 1055 (31.8) | |
| Smoking status, n (%) | <0.001 | |||||
| Never | 1297 (39.2) | 1593 (46.1) | 1654 (49.4) | 1667 (49.6) | 1702 (51.3) | |
| Past | 1356 (41.0) | 1365 (40.9) | 1334 (39.8) | 1331 (39.6) | 1229 (37.0) | |
| Current | 364 (11.0) | 286 (8.6) | 269 (8.0) | 267 (7.9) | 233 (7.0) | |
| Education, n (%) | <0.001 | |||||
| Less than high school | 364 (11.0) | 286 (8.6) | 269 (8.0) | 267 (7.9) | 233 (7.0) | |
| High school graduate | 950 (28.4) | 806 (24.1) | 735 (22.0) | 794 (23.6) | 862 (25.9) | |
| Some college | 901 (27.6) | 900 (26.9) | 942 (28.1) | 920 (27.4) | 949 (28.6) | |
| College graduate | 1090 (32.8) | 1349 (40.4) | 1403 (41.9) | 1381 (41.0) | 1277 (38.5) | |
| Annual income, n (%) | <0.001 | |||||
| Did not respond | 367 (11.1) | 406 (12.2) | 381 (11.4) | 382 (11.4) | 386 (11.6) | |
| <$20,000 | 568 (17.2) | 487 (14.5) | 450 (13.4) | 497 (14.8) | 441 (13.3) | |
| $20,000–$34,000 | 788 (23.4) | 765 (22.9) | 763 (22.8) | 831 (24.7) | 786 (23.7) | |
| $35,000–$74,000 | 1036 (31.4) | 1067 (31.9) | 1102 (32.9) | 1047 (31.1) | 1070 (32.2) | |
| ≥$75,000 | 546 (16.5) | 616 (18.4) | 653 (19.5) | 605 (18.0) | 638 (19.2) | |
| Alcoholic drinks/wk | 2.9 ± 8.9 | 2.6 ± 7.3 | 2.4 ± 5.4 | 2.2 ± 5.4 | 1.9 ± 6.0 | <0.001 |
| Dietary fiber, g/d | 16.5 ± 8.4 | 14.3 ± 7.5 | 15.5 ± 7.9 | 16.2 ± 8.2 | 16.9 ± 8.4 | <0.001 |
| Saturated fat, g/d | 29.1 ± 7.5 | 20.5 ± 4.1 | 19.5 ± 4.3 | 19.6 ± 4.6 | 19.9 ± 5.3 | <0.001 |
| Monounsaturated fat, g/d | 29.1 ± 7.5 | 26.3 ± 5.8 | 26.3 ± 6.4 | 26.3 ± 6.4 | 26.4 ± 6.9 | <0.001 |
| trans Fats, g/d | 7.7 ± 4.0 | 5.9 ± 3.6 | 4.8 ± 2.7 | 4.6 ± 2.9 | 5.4 ± 3.3 | <0.001 |
| Vitamin C without supplements, mg/d | 84.6 ± 55.0 | 92.2 ± 55.1 | 118.9 ± 64.3 | 121.0 ± 78.8 | 115.6 ± 77.3 | <0.001 |
| Vitamin E, a-TEs/d | 11.0 ± 5.2 | 8.5 ± 4.3 | 8.7 ± 4.5 | 9.5 ± 4.8 | 10.0 ± 4.9 | <0.001 |
| β-Carotene, μg/d | 3323 ± 2797 | 3152 ± 2456 | 3576 ± 2892 | 3687 ± 3119 | 3777 ± 3177 | <0.001 |
| Folate without supplements, μg/d | 352 ± 153 | 297 ± 135 | 322 ± 143 | 345 ± 146 | 373 ± 153 | <0.001 |
| Energy from sweets, % | 17.6 ± 11.2 | 14.3 ± 9.6 | 13.0 ± 8.7 | 14.5 ± 9.2 | 16.1 ± 10.0 | <0.001 |
| Whole grains, servings/d | 1.4 ± 1.3 | 1.5 ± 1.3 | 1.5 ± 1.2 | 1.6 ± 1.3 | 1.6 ± 1.4 | <0.001 |
Sum of anthocyanidin, flavan3-ol, flavanone, flavone, and flavonol intakes. a-TE, α-tocopherol equivalent; CHD, coronary heart disease; REGARDS, REasons for Geographic and Racial Differences in Stroke.
Data were analyzed by using ANOVA and chi-square tests for continuous and categorical variables, respectively.
Mean ± SD (all such values).
The Stroke Belt includes Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee; the Stroke Buckle includes coastal plains of Georgia, and North and South Carolina; Not Stroke Belt includes the remaining area in the lower 48 contiguous states. Regions are mutually exclusive.
Answer to “How many times per week do you engage in intense physical activity, enough to work up a sweat?”
The associations of quintiles of total flavonoid and flavonoid subclasses with incident CHD in the total cohort are shown in Table 3. There was a significant inverse association, with a linear trend, between anthocyanidin intake and proanthocyanidin intake and incident CHD in models 1–3 and a 29% and 37% RR reduction after multivariable adjustment including dietary variables, respectively. Model 4, which included potential mediating CHD risk factors, yielded minimally attenuated effect estimates. The RR reductions associated with anthocyanidins and proanthocyanidins in risk factor–adjusted models were 27% and 34%, respectively. The significant association, with a significant linear trend, between flavones and incident CHD seen in model 1 was attenuated after adjustment for additional confounders in models 2 and 3 and mediators in model 4. Similarly, the significant linear trend for the association between flavonols and incident CHD in model 1 was no longer significant after covariate adjustment. There was no significant association between flavan-3-ol, flavanone, or total flavonoid intake and CHD. When energy “misreporters” were excluded in sensitivity analyses, estimates adjusted for sociodemographic, behavioral, and dietary factors were not meaningfully different for anthocyanidins [HR for quintile 5 compared with quintile 1 (HRQ5vQ1): 0.71; 95% CI: 0.50, 0.99; P-trend = 0.04] or for proanthocyanidins (HRQ5vQ1: 0.59; 95% CI: 0.43, 0.81; P-trend < 0.01). Additional adjustment for the mediators, cardiovascular disease risk factors, resulted in no meaningful change in the HRs for anthocyanidins (HRQ5vQ1: 0.72; 95% CI: 0.52, 1.00; P-trend = 0.05) or for proanthocyanidins (HRQ5vQ1: 0.61; 95% CI: 0.45, 0.82; P-trend < 0.01) (Supplemental Table 2).
TABLE 3.
HRs (95% CIs) for incident CHD by quintile of energy-adjusted flavonoid intake for 16,678 participants in the REGARDS study1
| Quintile |
||||||
| 1 | 2 | 3 | 4 | 5 | P-trend2 | |
| Total flavonoids I,3 mg/d | 46 (≤72)4 | 91 (73–113) | 141 (114–181) | 251 (182–352) | 599 (≥353) | |
| Model 1 | 1.00 | 0.94 (0.72, 1.21) | 0.77 (0.59, 1.00) | 0.80 (0.62, 1.04) | 1.02 (0.80, 1.30) | 0.27 |
| Model 2 | 1.00 | 1.00 (0.77, 1.29) | 0.88 (0.67, 1.15) | 0.87 (0.67, 1.13) | 1.12 (0.87, 1.43) | 0.25 |
| Model 3 | 1.00 | 1.03 (0.79, 1.34) | 0.92 (0.70, 1.21) | 0.91 (0.70, 1.19) | 1.13 (0.88, 1.45) | 0.09 |
| Model 4 | 1.00 | 0.94 (0.73, 1.21) | 0.85 (0.66, 1.10) | 0.82 (0.63, 1.35) | 1.06 (0.81, 1.38) | 0.40 |
| Anthocyanidins, mg/d | 4.0 (≤5.8) | 7.3 (5.9–8.7) | 10.3 (8.8–12.2) | 14.8 (12.3–18.5) | 24.7 (≥18.6) | |
| Model 1 | 1.00 | 0.78 (0.62, 0.99) | 0.74 (0.62, 0.95) | 0.62 (0.48, 0.80) | 0.53 (0.41, 0.69) | <0.001 |
| Model 2 | 1.00 | 0.83 (0.65, 1.06) | 0.84 (0.68, 1.11) | 0.74 (0.57, 0.96) | 0.70 (0.54, 0.92) | 0.01 |
| Model 3 | 1.00 | 0.82 (0.64, 1.05) | 0.86 (0.66, 1.11) | 0.74 (0.56, 0.99) | 0.71 (0.52, 0.98) | 0.04 |
| Model 4 | 1.00 | 0.83 (0.66, 1.06) | 0.85 (0.66, 1.09) | 0.81 (0.63, 1.05) | 0.73 (0.55, 0.96) | 0.05 |
| Flavan-3-ols, mg/d | 8 (≤25) | 38 (26–50) | 68 (51–112) | 187 (113–278) | 529 (≥279) | |
| Model 1 | 1.00 | 0.87 (0.66, 1.15) | 1.01 (0.77, 1.32) | 0.88 (0.68, 1.15) | 1.09 (0.86, 1.41) | 0.22 |
| Model 2 | 1.00 | 0.90 (0.68, 1.19) | 1.03 (0.78, 1.35) | 0.92 (0.71, 1.20) | 1.12 (0.87, 1.44) | 0.26 |
| Model 3 | 1.00 | 0.91 (0.69, 1.20) | 1.04 (0.79, 1.37) | 0.93 (0.72, 1.21) | 1.13 (0.87, 1.45) | 0.11 |
| Model 4 | 1.00 | 0.85 (0.65, 1.11) | 0.97 (0.74, 1.26) | 0.85 (0.66, 1.10) | 1.05 (0.82, 1.34) | 0.39 |
| Flavanones, mg/d | 2 (≤10) | 9 (10–14) | 17 (15–19) | 34 (20–46) | 58 (≥46) | |
| Model 1 | 1.00 | 0.94 (0.72, 1.23) | 0.77 (0.59, 1.02) | 0.96 (0.75, 1.24) | 0.81 (0.63, 1.04) | 0.22 |
| Model 2 | 1.00 | 1.02 (0.78, 1.33) | 0.86 (0.65, 1.13) | 1.11 (0.86, 1.43) | 0.95 (0.73, 1.23) | 0.97 |
| Model 3 | 1.00 | 1.03 (0.79, 1.35) | 0.88 (0.67, 1.16) | 1.15 (0.88, 1.49) | 0.97 (0.74, 1.26) | 0.52 |
| Model 4 | 1.00 | 1.06 (0.81, 1.37) | 0.92 (0.70, 1.20) | 1.17 (0.91, 1.50) | 1.03 (0.80, 1.33) | 0.62 |
| Flavones, mg/d | 0.4 (≤ 0.51) | 0.60 (0.52–0.69) | 0.80 (0.70–0.94) | 1.10 (0.95–1.45) | 2.09 (≥ 1.46) | |
| Model 1 | 1.00 | 0.88 (0.68, 1.12) | 1.04 (0.81, 1.32) | 0.89 (0.69, 1.14) | 0.75 (0.58, 0.99) | 0.01 |
| Model 2 | 1.00 | 0.98 (0.76, 1.26) | 1.22 (0.95, 1.26) | 1.12 (0.87, 1.45) | 0.97 (0.73, 1.27) | 0.82 |
| Model 3 | 1.00 | 1.02 (0.78, 1.31) | 1.31 (0.99, 1.70) | 1.27 (0.95, 1.69) | 0.95 (0.71, 1.26) | 0.18 |
| Model 4 | 1.00 | 0.97 (0.76, 1.24) | 1.22 (0.96, 1.56) | 1.11 (0.86, 1.43) | 0.97 (0.73, 1.28) | 0.78 |
| Flavonols, mg/d | 7 (≤9) | 11 (9–13) | 16 (14–18) | 23 (19–28) | 36 (≥28) | |
| Model 1 | 1.00 | 0.84 (0.66, 1.07) | 0.77 (0.59, 0.99) | 0.79 (0.61, 1.01) | 0.83 (0.64, 1.06) | 0.03 |
| Model 2 | 1.00 | 0.91 (0.72, 1.17) | 0.90 (0.69, 1.17) | 0.93 (0.72, 1.19) | 1.00 (0.77, 1.29) | 0.64 |
| Model 3 | 1.00 | 0.93 (0.72, 1.19) | 0.94 (0.72, 1.23) | 1.00 (0.76, 1.31) | 1.01 (0.77, 1.32) | 0.10 |
| Model 4 | 1.00 | 0.96 (0.76, 1.22) | 0.93 (0.72, 1.20) | 1.00 (0.78, 1.28) | 0.98 (0.75, 1.27) | 0.98 |
| Proanthocyanidins, mg/d | 42 (≤59) | 70 (60–81) | 91 (82–103) | 117 (104–136) | 168 (≥137) | |
| Model 1 | 1.00 | 0.68 (0.53, 0.87) | 0.58 (0.53, 0.76) | 0.68 (0.53, 0.86) | 0.51 (0.40, 0.67) | <0.001 |
| Model 2 | 1.00 | 0.73 (0.57, 0.93) | 0.68 (0.52, 0.88) | 0.79 (0.62, 1.01) | 0.63 (0.49, 0.82) | 0.003 |
| Model 3 | 1.00 | 0.72 (0.57, 0.92) | 0.68 (0.52, 0.88) | 0.79 (0.61, 1.02) | 0.63 (0.47, 0.84) | 0.02 |
| Model 4 | 1.00 | 0.69 (0.55, 0.89) | 0.76 (0.59, 0.97) | 0.87 (0.69, 1.11) | 0.66 (0.51, 0.85) | 0.03 |
| Total flavonoids II,5 mg/d | 108 (≤149) | 179 (150–211) | 250 (212–298) | 366 (299–477) | 717 (≥478) | |
| Model 1 | 1.00 | 0.87 (0.68, 1.11) | 0.71 (0.54, 0.92) | 0.77 (0.60, 1.00) | 0.87 (0.68, 1.11) | 0.47 |
| Model 2 | 1.00 | 0.95 (0.74, 1.22) | 0.80 (0.62, 1.04) | 0.90 (0.70, 1.16) | 0.97 (0.75, 1.24) | 0.31 |
| Model 3 | 1.00 | 0.97 (0.75, 1.25) | 0.83 (0.63, 1.09) | 0.94 (0.73, 1.23) | 1.05 (0.80, 1.33) | 0.46 |
| Model 4 | 1.00 | 0.92 (0.73, 1.18) | 0.79 (0.61, 1.02) | 0.91 (0.71, 1.17) | 0.93 (0.71, 1.19) | 0.86 |
HRs (95% CIs) were calculated with the use of Cox proportional hazards models. Model 1 adjusted for age, energy and sex; model 2 adjusted as for model 1, as well as for race, region of residence, educational attainment, household income, exercise, and smoking status; model 3 adjusted as for model 2, as well as for percentage of calories from sweets, fiber, trans fat, and n–3 fatty acids; model 4 adjusted as for model 3, as well as for BMI, aspirin use, and history of or use of medications for hypertension, diabetes, or hyperlipidemia. CHD, coronary heart disease; REGARDS, REasons for Geographic and Racial Disparities in Stroke.
P-trend values were calculated with the use of the Wald test.
Sum of anthocyanidin, flavan3-ol, flavanone, flavone, and flavonol intakes.
Median (range) (all such values).
Sum of anthocyanidin, flavan3-ol, flavanone, flavone, flavonol, and proanthocyanidin intakes.
Analyses stratified by the a priori factors age at baseline, sex, race, and region of residence or the post hoc factors education, physical activity, and smoking status did not show differences in the association between flavonoid intake and incident CHD, nor were there significant interactions (all P > 0.10). Analyses that used multiple imputation for missing covariates yielded similar results. Stratified results by sex and race are shown in Supplemental Tables 3and 4.
In food-based analyses, there was a significant association between the consumption of several food sources of flavonoids, including apples or pears, berries, and wine, and incident CHD. There was an inverse association between consumption of ∼≥3 small apples or pears/wk or ≥2 servings of berries/wk and incident CHD (apples—HRQ5vQ1: 0.74; 95% CI: 0.56, 0.99; P-trend = 0.02; berries—HRQ5vQ1: 0.70; 95% CI: 0.53, 0.93; P-trend = 0.01). There was a graded inverse association between wine consumption and incident CHD compared with nondrinkers (≤1 drink/wk—HR: 0.75; 95% CI: 0.61, 0.92; 2–4 drinks/wk—HR: 0.70; 95% CI: 0.50, 0.99; ≥5 drinks/wk—HR: 0.56; 95% CI: 0.40, 0.78). There was no significant association between incident CHD and any other main foods contributing to total flavonoid intake.
DISCUSSION
To our knowledge, this is the first prospective cohort study to evaluate the association of total flavonoid and flavonoid subclass intakes with incident CHD in a biracial cohort by using the newly expanded USDA Provisional Flavonoid Addendum. We were able to show that subclasses of flavonoids are more relevant to incident CHD than total flavonoid intake. Greater intakes of anthocyanidins and proanthocyanidins were associated with 29% and 37% reduced RR of incident CHD, respectively, after adjustment for potential confounders, and 27% and 34% reduced RR after additional CHD risk factor adjustment. The significant inverse association between proanthocyanidin intake and incident CHD was a novel finding. Our findings differ from recent results from a study of flavonoid intake and incident CVD in the Framingham Offspring Cohort, which found no association between total flavonoid or flavonoid subclass intakes and incident CHD (13). However, participants in the Framingham Offspring Cohort may be particularly attuned to modifiable cardiovascular disease risk factors. The only other previous study that evaluated proanthocyanidin or anthocyanidin intakes and incident CHD was conducted in the Nurses’ Health Study II (NHS II) (12). In the NHS II, anthocyanidin intake was associated with a 32% reduction of RR of incident CHD, which was similar to the reduction in RR seen in this study. Anthocyanidin intake was comparable between this study and the NHS II. Conversely, participants in our study who reported the highest quintile of proanthocyanidin intake experienced a 37% reduction in RR compared with only a 17% risk reduction in incident CHD in the NHS II. In addition to using a different version of the USDA flavonoid databases and a different FFQ, the NHS II enrolled female health care providers between 25 and 42 y old, which is a younger and more health-conscious cohort than in the REGARDS study, which may explain differences in the effect estimates.
There was no significant effect modification by a variety of factors, including age, sex, race, region of residence, educational attainment, smoking, or physical activity. Black participants reported lower flavonoid intakes than their white counterparts. This may be due to lower consumption of plant-based foods in this group, which is consistent with previous studies that showed that black participants in the REGARDS study more often consumed a Southern-style dietary pattern, which is associated with incident CHD (4). In secondary analyses, the addition of CHD risk factors or medications used to treat such conditions did not meaningfully change point estimates.
Observational and experimental evidence supports the inverse association of dietary anthocyanidins with incident CHD and CHD risk factors. In addition to a lower risk of incident CHD, high anthocyanidin intake may prevent the onset of type 2 diabetes (29). Most recently, physiologic concentrations of anthocyanidin metabolites were found to significantly reduce IL-6 and vascular cell adhesion molecule 1 (VCAM-1) in human vascular endothelial cells stimulated with oxidized LDL cholesterol or cluster of differentiation 40 (CD40) ligand, suggesting that anthocyanidin metabolites may modulate the expression of important inflammatory mediators (30). The association we found for dietary proanthocyanidins is particularly intriguing, because compounds found in the proanthocyanidin subclass are not well understood. Proanthocyanidins were not associated with CHD mortality in previous epidemiologic studies, and human intervention studies are scarce (10, 31). In these previous studies, outcomes were defined by International Classification of Diseases’ codes for ischemic heart disease (410–414), including subacute MI, old MI, angina pectoris, and other chronic ischemic heart disease, which is less specific than expert-adjudicated incident acute MI and CHD death. Animal and in vitro studies suggest that proanthocyanidins may have a role in reducing adiposity, a risk factor for CHD, through the inhibition of digestive enzymes in the small intestine, modulation of neuropeptides involved in satiety, and influence on lipid metabolism (32). Proanthocyanidins also appear to have antithrombotic and antihypertensive effects in animal models (32, 33).
Food-based analyses support the inverse associations between anthocyanidins, proanthocyanidins, and incident CHD. Apples and pears with peels are rich sources of proanthocyanidins and other flavonoids in smaller amounts. Berries are rich sources of anthocyanidins, and intakes ≥3 servings/wk are associated with a 30% reduction in RR of incident CHD (12). Wines are notable sources of polyphenols, although red wines are particularly rich in flavonoids, including anthocyanidins, proanthocyanidins, and other subclasses in smaller quantities. Although wine, apples, and berries are characteristically high in specific flavonoid subclasses, the effect of each subclass cannot be disentangled in food-based analyses. These foods may represent naturally potent mixtures of flavonoids.
Strengths of this study include the large, biracial, geographically diverse sample; prospective design; and excellent ascertainment of CHD outcomes. The flavonoid database used is the most comprehensive database available to assess flavonoid intake in the US population. The racial and geographic diversity of the study population contribute to the generalizability of the results.
Some limitations should be mentioned. The assessment of dietary flavonoids is susceptible to measurement error, because FFQs cannot capture all potential dietary sources of flavonoids. The Block98 FFQ was not specifically designed to measure flavonoid intake, leading to potential measurement error. However, the use of a detailed, comprehensive food-composition table and detailed FFQ should mitigate measurement error by capturing most flavonoid intake for Americans, especially because the exposure was rank ordered, rather than by absolute intake. Diet was assessed only at baseline; therefore, changes in dietary composition could not be assessed. Although there was a 10-y interval between the release of the Block98 FFQ and the end of enrollment in the REGARDS study in 2007, analysis of NHANES data over a similar time period indicate that flavonoid intake and the distribution of dietary flavonoid sources remained stable (34); therefore, the Block98 was likely able to capture flavonoid intake accurately. Although the flavonoid content of plants varies depending on cultivars, growing conditions, and storage, values used in this study were mean flavonoid values for foods, which are the most appropriate measure to use in a large epidemiologic study in which participants likely consumed various foods from various sources. In addition, although not all REGARDS study participants returned the dietary assessment, and some covariates were missing, there was no material difference in results when analyses were repeated after imputation of missing covariates. Energy misreporting may lead to bias in food survey research, including that utilizing FFQs. In this study, sensitivity analyses that excluded energy “misreporters” yielded point estimates that were not meaningfully different from the main analysis. Wider CIs, which in the case of anthocyanidin analysis included the null, were likely due to the reduced sample size after the exclusion of energy “misreporters.” As in all observational studies, confounding by unmeasured factors is possible. Residual confounding by smoking status is possible because features beyond smoking status influence cardiovascular disease risk prediction. Although time since quitting and age at quitting were not measured in this study, the inclusion of pack-years in models did not change estimates. Residual confounding by other dietary factors is unlikely, because many potential dietary confounders were evaluated and were not independently associated with incident CHD except for caloric intake from sweetened foods. Finally, significance should be interpreted cautiously, because some significant findings may be due to chance, given the number of comparisons in this study.
In conclusion, we found that higher reported intakes of anthocyanidins and proanthocyanidins were associated with a lower risk of incident CHD after adjustment for demographic, socioeconomic, health behavior, and dietary factors. Similarly, higher reported intakes of apples and pears, berries, and wine were also inversely associated with incident CHD. There was no effect modification by age, sex, race, or region of residence, although non-Hispanic blacks reported lower flavonoid intake, which may contribute to a greater CHD burden. Higher consumption of foods rich in anthocyanidins and proanthocyanidins may explain some of the cardioprotective effects of plant-rich diets, and their ability to prevent CHD should be evaluated in clinical trials.
Acknowledgments
We thank the other investigators and staff of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
The authors’ responsibilities were as follows—MEG, SEJ, and MMS: conducted the research; MEG: conducted the statistical analyses and had responsibility for the final content; and MEG and VV: wrote the manuscript; and all authors: designed the research and read and approved the final manuscript. The authors reported no conflicts of interest.
Footnotes
Abbreviations used: CD40, cluster of differentiation 40; CHD, coronary heart disease; FFQ, food-frequency questionnaire; FNDDS, Food and Nutrient Database for Dietary Studies; HRQ5vQ1, HR for quintile 5 compared with quintile 1; MI, myocardial infarction; NHS II, Nurses’ Health Study II; REGARDS, REasons for Geographic and Racial Differences in Stroke; VCAM-1, vascular cell adhesion molecule 1.
REFERENCES
- 1.Shuaib FM, Durant RW, Parmar G, Brown TM, Roth DL, Hovater M, Halanych JH, Shikany JM, Howard G, Safford MM. Awareness, treatment and control of hypertension, diabetes and hyperlipidemia and area-level mortality regions in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. J Health Care Poor Underserved 2012;23:903–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosamond WD, Chambless LE, Heiss G, Mosley TH, Coresh J, Whitsel E, Wagenknecht L, Ni H, Folsom AR. Twenty-two-year trends in incidence of myocardial infarction, coronary heart disease mortality, and case fatality in 4 US communities, 1987-2008. Circulation 2012;125:1848–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, et al. Association of race and sex with risk of incident acute coronary heart disease events. JAMA 2012;308:1768–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE. Southern dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circulation 2015;132:804–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr 2013;98:1514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, Gomez-Gracia E, Ruiz-Gutierrez V, Fiol M, Lapetra J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. [DOI] [PubMed] [Google Scholar]
- 7.Casagrande SS, Wang Y, Anderson C, Gary TL. Have Americans increased their fruit and vegetable intake? The trends between 1988 and 2002. Am J Prev Med 2007;32:257–63. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. State-specific trends in fruit and vegetable consumption among adults—United States, 2000-2009. MMWR Morb Mortal Wkly Rep 2010;59:1125–30. [PubMed] [Google Scholar]
- 9.Weseler AR, Bast A. Pleiotropic-acting nutrients require integrative investigational approaches: the example of flavonoids. J Agric Food Chem 2012;60:8941–6. [DOI] [PubMed] [Google Scholar]
- 10.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr 2012;95:454–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peterson JJ, Dwyer JT, Jacques PF, McCullough ML. Associations between flavonoids and cardiovascular disease incidence or mortality in European and US populations. Nutr Rev 2012;70:491–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassidy A, Mukamal KJ, Liu L, Franz M, Eliassen AH, Rimm EB. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013;127:188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacques PF, Cassidy A, Rogers G, Peterson JJ, Dwyer JT. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr 2015;114:1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings A, Welch AA, Fairweather-Tait SJ, Kay C, Minihane AM, Chowienczyk P, Jiang B, Cecelja M, Spector T, Macgregor A, et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am J Clin Nutr 2012;96:781–8. [DOI] [PubMed] [Google Scholar]
- 15.Cassidy A, O’Reilly EJ, Kay C, Sampson L, Franz M, Forman JP, Curhan G, Rimm EB. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am J Clin Nutr 2011;93:338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher dietary flavonol intake is associated with lower incidence of type 2 diabetes. J Nutr 2013;143:1474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertoia ML, Rimm EB, Mukamal KJ, Hu FB, Willett WC, Cassidy A. Dietary flavonoid intake and weight maintenance: three prospective cohorts of 124 086 US men and women followed for up to 24 years. BMJ 2016;352:i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebastian RS, Goldman JD, Martin CL, Steinfeldt LC, Enns CW, Moshfegh AJ. Flavonoid values for USDA survey foods and beverages 2007-2008: Provisional Flavonoid Addendum to the USDA Food and Nutrient Database for Dietary Studies, 4.1, and flavonoid intake files from What We Eat in America (WWEIA), National Health and Nutrition Examination Survey (NHANES) 2007-2008. Beltsville (MD): USDA–Agricultural Research Service, Food Surveys Research Group; 2014. [Google Scholar]
- 19.Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, Cushman M, Moy CS, Soliman EZ, Kissela BM, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol 2011;69:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 1990;43:1327–35. [DOI] [PubMed] [Google Scholar]
- 21.Caan BJ, Slattery ML, Potter J, Quesenberry CP Jr, Coates AO, Schaffer DM. Comparison of the Block and the Willett self-administered semiquantitative food frequency questionnaires with an interviewer-administered dietary history. Am J Epidemiol 1998;148:1137–47. [DOI] [PubMed] [Google Scholar]
- 22.Boucher B, Cotterchio M, Kreiger N, Nadalin V, Block T, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutr 2006;9:84–93. [DOI] [PubMed] [Google Scholar]
- 23.USDA, Agricultural Research Service. USDA database for the proanthocyanidin content of selected foods. Beltsville (MD): Agricultural Research Service Nutrient Data Laboratory; 2004. [Google Scholar]
- 24.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 25.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 26.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr 1991;45:569–81. [PubMed] [Google Scholar]
- 27.Black AE. Critical evaluation of energy intake using the Goldberg cut-off for energy intake:basal metabolic rate: a practical guide to its calculation, use and limitations. Int J Obes Relat Metab Disord 2000;24:1119–30. [DOI] [PubMed] [Google Scholar]
- 28.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 29.Wedick NM, Pan A, Cassidy A, Rimm EB, Sampson L, Rosner B, Willett WC, Hu FB, Sun Q, van Dam RM. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012;95:925–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin HP, Czank C, Raheem S, Zhang Q, Botting NP, Cassidy A, Kay CD. Anthocyanins and their physiologically relevant metabolites alter the expression of IL-6 and VCAM-1 in CD40L and oxidized LDL challenged vascular endothelial cells. Mol Nutr Food Res 2015;59:1095–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR Jr. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007;85:895–909. [DOI] [PubMed] [Google Scholar]
- 32.Salvadó MJ, Casanova E, Fernandez-Iglesias A, Arola L, Blade C. Roles of proanthocyanidin rich extracts in obesity. Food Funct 2015;6:1053–71. [DOI] [PubMed] [Google Scholar]
- 33.Liu X, Qiu J, Zhao S, You B, Ji X, Wang Y, Cui X, Wang Q, Gao H. Grape seed proanthocyanidin extract alleviates ouabain-induced vascular remodeling through regulation of endothelial function. Mol Med Rep 2012;6:949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Vance TM, Chun OK. Estimated intake and major food sources of flavonoids among US adults: changes between 1999-2002 and 2007-2010 in NHANES. Eur J Nutr 2016;55:833–43. [DOI] [PubMed] [Google Scholar]