Abstract
Background: Moderate-to-vigorous–intensity physical activity is recommended to maintain and improve health, but the mortality benefits of light activity and risk for sedentary time remain uncertain.
Objectives: Using accelerometer-based measures, we 1) described the mortality dose-response for sedentary time and light- and moderate-to-vigorous–intensity activity using restricted cubic splines, and 2) estimated the mortality benefits associated with replacing sedentary time with physical activity, accounting for total activity.
Design: US adults (n = 4840) from NHANES (2003–2006) wore an accelerometer for ≤7 d and were followed prospectively for mortality. Proportional hazards models were used to estimate adjusted HRs and 95% CIs for mortality associations with time spent sedentary and in light- and moderate-to-vigorous–intensity physical activity. Splines were used to graphically present behavior-mortality relation. Isotemporal models estimated replacement associations for sedentary time, and separate models were fit for low- (<5.8 h total activity/d) and high-active participants to account for nonlinear associations.
Results: Over a mean of 6.6 y, 700 deaths occurred. Compared with less-sedentary adults (6 sedentary h/d), those who spent 10 sedentary h/d had 29% greater risk (HR: 1.29; 95% CI: 1.1, 1.5). Compared with those who did less light activity (3 h/d), those who did 5 h of light activity/d had 23% lower risk (HR: 0.77; 95% CI: 0.6, 1.0). There was no association with mortality for sedentary time or light or moderate-to-vigorous activity in highly active adults. In less-active adults, replacing 1 h of sedentary time with either light- or moderate-to-vigorous–intensity activity was associated with 18% and 42% lower mortality, respectively.
Conclusions: Health promotion efforts for physical activity have mostly focused on moderate-to-vigorous activity. However, our findings derived from accelerometer-based measurements suggest that increasing light-intensity activity and reducing sedentary time are also important, particularly for inactive adults.
Keywords: sedentary behavior, physical activity, mortality, accelerometer, light-intensity activity, moderate-to-vigorous intensity activity
INTRODUCTION
During the past century, obligatory physical activity has been progressively engineered out of daily life, and the amount of sedentary time has increased, especially in more-developed countries (1–4). To prevent the adverse health effects of our increasingly sedentary ways of life, regular participation in physical activity of at least moderately vigorous intensity is recommended [i.e., energy cost ≥3 metabolic equivalents (METs) (5, 6)]. Recently, excessive sedentary time, or too much sitting, has emerged as a putative mortality risk factor independent from moderate-to-vigorous activity (7). Notably, the behavioral mechanism proposed to explain this association has been a loss of light-intensity physical activity (<3 METs) because of increased sedentary time (8, 9), suggesting that light activity may have greater health-enhancing impact than previously thought. This is important because interventions that seek to increase light-intensity physical activity could be a powerful additional strategy to increase physical activity and improve health.
Studies using accelerometers have now linked more light-intensity activity with lower mortality, but the dose-response relation remains uncertain. Early reports focused on older adults (10, 11) and had short follow-up periods (12–14)—raising concern about reverse causality. Two new reports with longer follow-up found light activity to be associated with lower mortality after adjusting for moderately vigorous activity (15, 16). Loprinzi (15) reported a 1-h increase in light activity to be associated with 16% lower mortality, whereas Fishman et al. (16) estimated that replacing 1 h of sedentary time with light activity was associated with 39% lower mortality. These studies provide important new evidence, but both used modeling methods that assume a linear relation between physical activity and mortality, yet the shape of this relation is generally believed to be nonlinear (17–19). To our knowledge, the mortality dose-response curves for accelerometer-measured physical activity and sedentary time have not been described in detail. Furthermore, a recent study found the mortality benefit associated with replacing sedentary time with physical activity to be dependent on one’s level of total activity (20). Accordingly, using accelerometer-based measures, we examined US adults (≥40 y old) 1) to describe the mortality dose response for sedentary time and light- and moderate-to-vigorous–intensity activity using restricted cubic splines, and 2) to estimate the mortality benefits associated with replacing sedentary time with physical activity, accounting for total activity levels. Completion of these objectives extends previous results from this publicly available data source (12–16) and provides new insights into the combined influence of sedentary time and different activity intensities on mortality that should inform future public health recommendations.
METHODS
Study population
We used the 2003–2006 accelerometer data from NHANES, a representative sample of US adults derived from a stratified, multistage sampling design. The National Death Index was used to ascertain mortality, and we analyzed data from the examination date through 31 December 2011. Of 6355 respondents aged ≥40 y, 4917 had valid accelerometer data. We excluded 6 participants who lacked follow-up time and 71 with missing covariates, resulting in 4840 adults for analysis (Supplemental Figure 1).
Measures and covariates
Our primary exposures, sedentary time and time spent in light and moderately vigorous physical activity, were measured by using an accelerometer (AM-7164; ActiGraph) (21). Participants were instructed to wear the monitor on their waist for 7 d, removing it to sleep and bathe. The monitors were set to record minute-by-minute observations of bodily movement, saving this information as an activity count (AC) (22). AC values ranged from 0 to >10,000 counts/min, reflecting the intensity of movement of the individual. ACs were used to identify monitor nonwear periods and to classify time spent in broad categories of activity intensity (i.e., sedentary, light, and moderate-to-vigorous activity). Nonwear time was defined per protocol (21), and those with ≥1 d of valid wear (i.e., ≥10 h/d) were included in the analysis. To estimate time spent in different activity intensities, we used standard cutoff methods (23–27). Sedentary time was defined as wear time with AC <100 (18), and physically active time (AC ≥100) was divided into light- and moderate-to-vigorous–intensity activity by using 2 moderate-intensity AC thresholds. We used the ≥760 cutoff because it was calibrated to differentiate between a broad range of light (<3 METs) and moderate- to vigorous-intensity (≥3 METs) lifestyle and ambulatory activities (AC ≥760), and it has been cross-validated (23–27). The AC ≥2020 cutoff, derived from studies that largely examined ambulatory walking and running activities (≥3 METs), has been widely used (21). We conducted analyses using both sets of cutoffs and obtained qualitatively similar results. For clarity of presentation, we present the AC ≥760 results and provide results for the 2020 cutoff in Supplemental materials. These methods combine both moderate- and vigorous-activity time because the accumulation of accelerometer-measured vigorous activity is a relatively rare occurrence. For each participant we calculated mean values for each activity intensity category from the valid days of accelerometer wear.
Confounders were included as covariates based on our previous investigation in these data (12); including, age (y), sex, race-ethnicity (white, black, Mexican American, or other), education (less than high school, high school diploma, or high school or more), alcohol consumption (never, former, or current), smoking status (never, former, or current), BMI (in kg/m2: <25, 25–29.9, or ≥30), self-reported diabetes, coronary artery disease, stroke, cancer, and mobility limitation (difficulty walking a quarter mile or up 10 stairs).
Statistical analysis
We first evaluated the descriptive characteristics of our study population and then examined the correlations between our primary exposures using Spearman correlations. Cox proportional hazard models with the use of follow-up time as the underlying time metric and adjusting for covariates listed above were used to estimate adjusted HRs and 95% CIs. We also tested for interaction between sex and our main exposures on mortality.
To describe the underlying dose-response mortality relation for sedentary and light- and moderate-to-vigorous–intensity activity, we used continuous measures of these exposures (28, 29) using the approach described by Desquilbet and Mariotti (30). This approach uses restricted cubic spline functions (29) to describe the shape of the dose-response curves and to test whether the association is nonlinear. Because the number of the knots specified to fit the splines might influence the shape of the associations (30), we initially evaluated models with 5, 4, and 3 knots placed at recommended percentiles. On visual inspection of the dose-response curves, we found minimal differences in results depending on the number of knots and elected to present our final models using 3 knots (at the 5th, 50th, and 95th percentiles), a choice that should enhance statistical power for testing for nonlinear associations (30). For nonlinear associations, the spline models were used to describe the associations, and when the association was determined to be linear, a simpler linear model was used. Our preliminary evaluation of spline results did reveal a strong influence of sparse data (few deaths) in the tails of the exposure distributions, so we trimmed the exposures to minimize this influence as described in figure footnotes. To enhance interpretability of these graphical results, we set the reference level at the ∼10th percentile of each exposure and then reported relevant risk estimates (HRs; 95% CIs) on the figures and in the text.
We did a second spline analysis for sedentary and light-intensity activity that further adjusted for moderate-to-vigorous physical activity. Because our preliminary analyses indicated nonlinear mortality associations for moderate-to-vigorous activity, we classified this variable into quintiles (<0.74, 0.75–1.27, 1.28–1.74, 1.75–2.40, and ≥2.41 h/d) to better account for the shape of the association. Spline results for moderate-to-vigorous activity were also adjusted for sedentary time.
We also investigated the interrelation between sedentary time and physical activity on mortality using the isotemporal substitution regression approach (31). To better understand results from this analysis, we initially fit 3 kinds of linear models: 1-factor, 2-factor, and 3-factor models (i.e., partition models) (31). To fit the isotemporal models, we included covariates, as well the continuous variables for light (hours per day) and moderate-to-vigorous activity (hours per day), and a variable for total time observed in both sedentary and physically active pursuits in the model [i.e., wear time (31, 32)]. A description of each type of model is provided in our Supplemental Materials. Results from this model, with the use of substitution of the association values in the overall model system of sedentary, light, and moderate-to-vigorous activity, provide an estimate of the mortality associations for replacing 1 h of sedentary time with an equal amount of time in physical activity of a specific intensity category, while holding total time constant. However, the isotemporal models assume a linear dose-response for physical activity and mortality, and we had observed nonlinearity in some of the physical activity mortality relation. To account for these nonlinear associations, we split our sample into “low active” (<5.8 h total activity/d) and “high active” (≥5.8 h/d) based on median total active time (AC ≥100) in the sample. These categories were determined by visual inspection of the restricted cubic spline for total physical activity to identify 2 groups in which the association was approximately linear (Supplemental Figure 2). Interactions between total activity level (low compared with high) and our main exposures on mortality were examined. For completeness and comparability to other studies, we also evaluated the associations in the overall study sample and then stratified by less- and more-active adults for all models.
We tested the proportional hazards assumption, including results for sedentary time and light- and moderate-to-vigorous–intensity activity, and isotemporal substitution by examining the interaction between follow-up time and each of these exposures. The proportional hazards assumption was not violated for sedentary time, moderate-to-vigorous activity, or the isotemporal models, but it was violated for light-intensity activity. To better understand the impact of different hazards over time, we estimated the shape of the light-activity curve, as well as curves for sedentary and moderate-to-vigorous time, for those with <2 y of follow-up, ≥2 y of follow-up, and in the overall sample (Supplemental Figure 3). These graphical sensitivity analyses indicated little effect of differential hazards over time on the overall association between light-intensity activity and mortality; therefore, we present the overall results in our main tables and figures.
We evaluated our key results for reverse causality in sensitivity analyses by excluding participants who accumulated <2 y of follow-up. We further examined the relation between mortality and activity stratified by major chronic diseases and key demographic and behavioral covariates. In addition, because the fixed amount of time in 1 d (24 h) works as de facto total time observed variable in all of our analysis, it is possible that substitution effects from exposures occurring during monitor nonwear periods (e.g., sleep) could affect our results. We therefore examined the mortality association for nonwear time and compared the results for individuals with low and high amounts of monitor wear time to better understand the potential for these effects to influence our results.
SAS-callable SUDAAN 10.0 (RTI International) was used to account for the complex survey design, address differential sample selection, sample nonresponse, and poststratification adjustments. Sample weights were adjusted per National Center for Health Statistics recommendations (33) to account for use of combined adjacent survey cycles (1/2 × WTMEC2YR), and stratum (SDMVSTRA) and primary sampling units (SDMVPSU) variables were included in our evaluation of exposure mortality associations. All statistical tests were 2-sided.
RESULTS
During 6.6 y of follow-up, 700 deaths occurred. Descriptive characteristics of our participants at baseline are presented in Table 1. Participants wore the monitors for a mean of 14 h/d and 5.6 d. On average, they spent 8.2, 4.2, 1.7 h/d in sedentary and light- and moderate-to-vigorous–intensity activity, respectively. Sedentary time was negatively correlated with light (r = −0.43) and moderate-to-vigorous activity (r = −0.52). Light- and moderate-to-vigorous–intensity time were positively correlated (r = 0.41; Supplemental Table 1). We found no statistical interaction between sex and sedentary time on mortality (P-interaction = 0.46) or for overall physical activity (P-interaction = 0.86); therefore, we report combined results. There was no association for nonwear time (P = 0.25; Supplemental Figure 4).
TABLE 1.
Characteristics of the study population and summary accelerometer values weighted to account for the survey design1
| Female (n = 2435) | Male (n = 2405) | Total (n = 4840) | |
| Weighted frequency | 53.5 (0.9)2 | 46.5 (0.9) | — |
| Age, y | 57.2 [0.4]3 | 56.3 [0.4] | 56.8 [0.4] |
| BMI, kg/m2 | 29.1 [0.2] | 28.8 [0.2] | 29.0 [0.1] |
| Ethnicity | |||
| Non-Hispanic white | 76.5 (2.3) | 78.5 (2.1) | 77.4 (2.2) |
| Non-Hispanic black | 10.8 (1.4) | 9.5 (1.2) | 10.2 (1.3) |
| Hispanic | 4.8 (1.0) | 5.8 (1.0) | 5.3 (1.0) |
| Other | 7.9 (0.8) | 6.3 (0.8) | 7.1 (0.7) |
| Education | |||
| Less than high school | 17.8 (1.3) | 17.1 (1.2) | 17.4 (1.2) |
| High school | 27.6 (1.1) | 25.0 (0.9) | 26.4 (0.8) |
| More than high school | 54.6 (1.7) | 58.0 (1.6) | 56.2 (1.4) |
| Smoking | |||
| Never | 54.9 (1.3) | 37.5 (1.3) | 46.8 (1.0) |
| Former | 26.5 (0.9) | 38.5 (1.3) | 32.1 (0.8) |
| Current | 18.6 (1.1) | 24.0 (1.1) | 21.1 (0.9) |
| Alcohol | |||
| Never | 15.7 (1.2) | 5.2 (0.5) | 10.8 (0.8) |
| Former | 21.1 (1.3) | 22.6 (1.7) | 21.8 (1.3) |
| Current | 57.6 (2.1) | 67.1 (1.7) | 62.0 (1.8) |
| Missing | 5.6 (0.5) | 5.1 (0.5) | 5.4 (0.4) |
| Diabetes | 13.0 (0.9) | 12.8 (0.8) | 12.9 (0.7) |
| Coronary artery disease | 9.2 (0.8) | 3.3 (1.0) | 11.1 (0.7) |
| Stroke | 4.3 (0.5) | 3.5 (0.4) | 3.9 (0.4) |
| Cancer | 14.3 (0.8) | 11.4 (0.7) | 12.9 (0.6) |
| Mobility limitation | 14.4 (0.8) | 10.9 (0.8) | 12.7 (0.6) |
| ActiGraph accelerometer | |||
| Sedentary (AC <100), h/d | 8.1 (0.05) | 8.3 (0.05) | 8.2 (0.04) |
| Light (100 ≥ AC < 760), h/d | 4.4 (0.04) | 4.1 (0.03) | 4.2 (0.02) |
| Moderate (760 ≥ AC < 2020), h/d | 1.5 (0.03) | 1.9 (0.02) | 1.7 (0.02) |
| Vigorous (AC ≥2020), h/d | 0.24 (0.01) | 0.40 (0.01) | 0.32 (0.01) |
| Valid wear days4 | 5.6 (0.1) | 5.7 (0.1) | 5.6 (0.1) |
| Valid wear time,4 h/d | 14.0 (0.06) | 14.3 (0.06) | 14.1 (0.06) |
All estimates are adjusted to account for the complex survey design. AC, activity count.
Percentage; SE in parentheses (all such values).
Mean; SE in brackets (all such values).
Valid wear days are days with ≥10 h of valid wear time. Valid wear time is 24 h minus nonwear time, with nonwear time defined as an interval of ≥60 min with allowance for 1–2 min of AC between 0 and 100 on days with ≥10 h of wear.
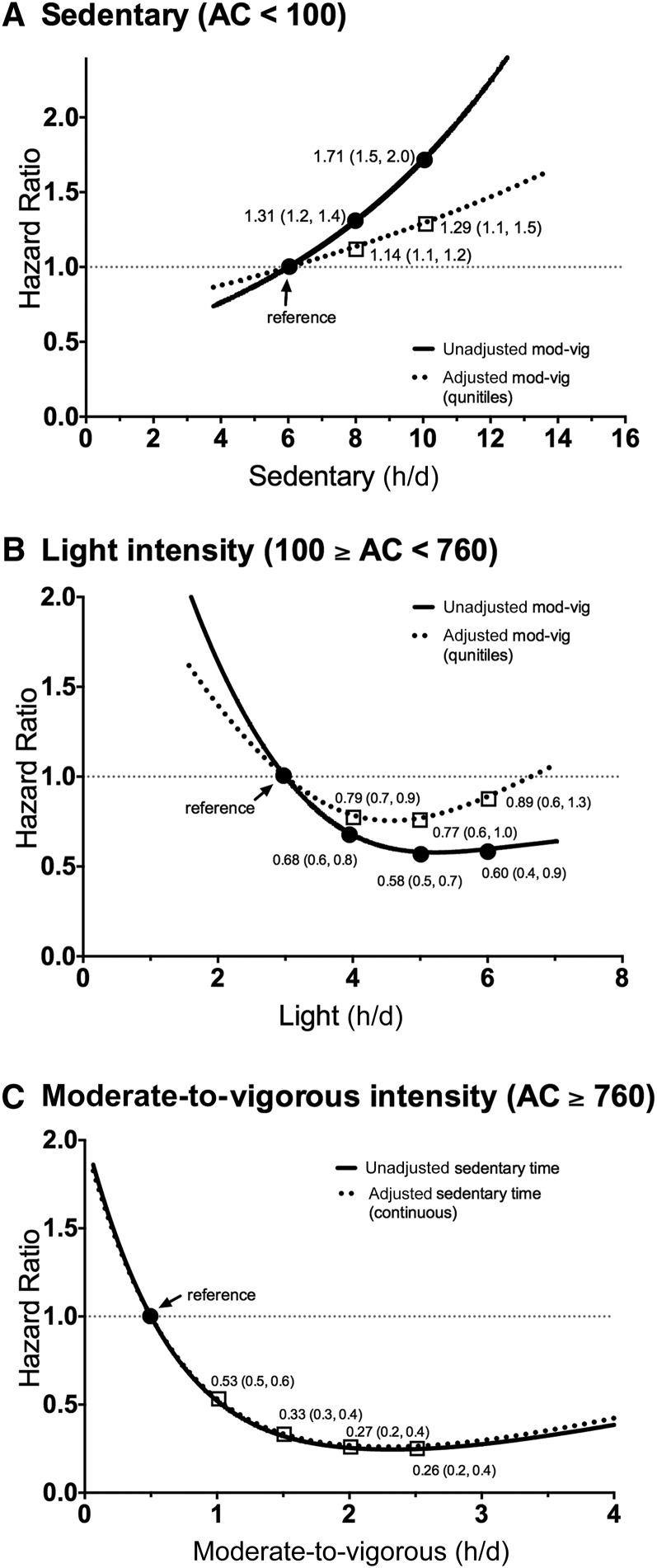
By using restricted cubic splines, sedentary time was associated with mortality in a linear manner in covariate adjusted models (P-linear ≤ 0.001, P-nonlinear = 0.17; Figure 1A). A 1-h increase in sedentary time was associated with a 12% greater mortality, but further adjustment for moderate-to-vigorous activity attenuated this association to a 5% greater risk per sedentary hour. Compared with adults who spent 6 sedentary h/d (reference), those who were sedentary for 8 h/d had a 14% greater risk (HR: 1.14; 95% CI: 1.1, 1.2), and those who spent 10 sedentary h/d had a 29% greater risk (HR: 1.29; 95% CI 1.1, 1.5).
FIGURE 1.
Dose-response relation for mortality and sedentary and light- and moderate-to-vigorous–intensity physical activity with and without adjustment for other behaviors. Numerical values reported are HRs (95% CIs). (A) Sedentary (AC <100) is a linear model, and results in (B) light (100 ≥ AC < 760) and (C) moderate-to-vigorous (AC ≥760) are from nonlinear models by using restricted cubic splines. Sedentary and light results are trimmed at 1st and 99th percentiles; moderate-to-vigorous results are trimmed at the 1st and 95th percentiles. The referent group is the ∼10th percentile. Associations are adjusted for age, race, education, smoking, alcohol, diabetes, coronary artery disease, cancer, stroke, mobility limitations, and BMI. Adjustment for moderate-to-vigorous physical activity was in quintiles (<0.74, 0.75–1.27, 1.28–1.74, 1.75–2.40, ≥2.41 h/d), and sedentary time was continuous. AC, activity count; mod-vig, moderate-to-vigorous.
In contrast, both light- and moderate-to-vigorous–intensity activity were associated in an inverse nonlinear manner in covariate-adjusted models (P-nonlinear = 0.02 and < 0.001, respectively; Figure 1B, C). Adjustment for moderate-to-vigorous–intensity activity attenuated the light-intensity association somewhat, such that compared with those engaging in 3 h light activity/d (reference), those who did 4 h light activity/d had a 21% lower risk (HR: 0.79; 95% CI: 0.7, 0.9), and those who did 5 h/d had only a small additional benefit of 23% lower risk (HR: 0.77; 95% CI: 0.6, 1.0). The HR associated with doing ≥6 h light activity, which is the ∼95th percentile of the distribution, are uncertain because of wide confidence intervals (HR: 0.89; 95% CI: 0.6, 1.3). For moderate-to-vigorous–intensity activity, there was minimal influence on risk after adjusting for either light-intensity activity or sedentary time. We elected to report results for sedentary adjustment. Compared with those engaging in 0.5 h/d of moderate-to-vigorous activity, those who did 1 h/d had a 47% lower risk (HR: 0.53; 95% CI: 0.5, 0.6), whereas those who did 1.5 h/d had a 67% lower risk (HR: 0.33; 95% CI: 0.3, 0.4). There were only modest additional benefits for additional moderate-to-vigorous activity >1.5 h/d.
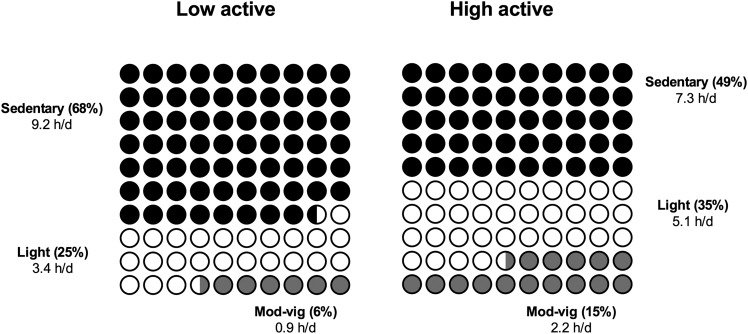
To understand and account for nonlinear associations between mortality and physical activity in our isotemporal substitution models, we examined results in our overall sample and among low- (<5.8 h/d of total activity) and high-active adults. We provide a description of the sedentary and activity profiles in each group in Figure 2. Adults in the low-active group spent 68% of their time sedentary, whereas those in the high-active group spent 49% of their time sedentary.
FIGURE 2.
Distribution of time spent in sedentary, light and moderate-to-vigorous time in low-active (n = 2618) and high-active (n = 2222) participants. Percent values are the proportion of total wear time spent in a given type of activity. Hours-per-day values are the group means. Mod-vig, moderate-to-vigorous.
Results for linear models in these data are presented in Table 2 for the overall sample and for low- and high-active adults. We found statistically significant (P < 0.01) interactions between total activity level and sedentary time on mortality and for total activity and both intensities of physical activity. Compared with single-factor models, the attenuation after adjustment for moderate-to-vigorous activity was similar to that observed in the spline results. Notably, we observed significant associations for sedentary time and light and moderate-to-vigorous activity in low-active adults but not high-active adults. Analyses with the use of alternate AC cutoffs for light (100 > AC < 2020) and moderate-to-vigorous activity (AC ≥2020) revealed a similar pattern of association for sedentary time and light activity (Supplemental Table 2).
TABLE 2.
Cox proportional hazards results for the relation between sedentary, light, and moderate-to-vigorous time and mortality in the overall study sample, and in low- and high-active groups1
| Models | Sedentary | Light | Moderate-to-vigorous |
| Overall (n = 4840, 700 deaths) | |||
| Single-factor models2 | 1.13 (1.09, 1.18) | 0.50 (0.38, 0.65) | 0.73 (0.65, 0.82) |
| 2-factor model3 | 1.05 (1.00, 1.10) | 0.53 (0.40, 0.71) | — |
| 2-factor model3 | — | 0.83 (0.74, 0.93) | 0.59 (0.44, 0.77) |
| Partition model4 | 1.03 (0.98, 1.08) | 0.84 (0.75, 0.95) | 0.60 (0.45, 0.81) |
| Low active (<5.8 h/d, n = 2618, 576 deaths)4 | |||
| Single-factor models2 | 1.14 (1.08, 1.20) | 0.71 (0.61, 0.83) | 0.31 (0.21, 0.46) |
| 2-factor model3 | 1.07 (1.02, 1.13) | — | 0.41 (0.28, 0.59) |
| 2-factor model3 | — | 0.82 (0.72, 0.94) | 0.37 (0.26, 0.53) |
| Partition model4 | 1.06 (1.00, 1.11) | 0.84 (0.74, 0.96) | 0.39 (0.27, 0.56) |
| High active (≥5.8 h/d, n = 2222, 124 deaths)4 | |||
| Single-factor models2 | 0.92 (0.82, 1.04) | 0.87 (0.56, 1.34) | 1.21 (0.88, 1.66) |
| 2-factor model3 | 0.89 (0.79, 1.00) | 0.80 (0.52, 1.24) | — |
| 2-factor model3 | — | 1.19 (0.85, 1.68) | 0.89 (0.58, 1.39) |
| Partition model4 | 0.90 (0.79, 1.03) | 0.83 (0.52, 1.32) | 1.16 (0.80, 1.66) |
Values are HRs (95% CIs) and adjusted for age, race, education, sex, smoking status, alcohol use, BMI, mobility limitation, and a history of the following conditions: diabetes, coronary artery disease, stroke, and cancer. Activity intensities are defined as sedentary time (AC <100) and light- (100 ≥ AC < 760) and moderate-to-vigorous–intensity physical activity (AC ≥760). Low- and high-active designations were based on sample-weighted medians of total active time (AC >100 min/d). There were statistically significant (P < 0.01) interactions between total activity level and sedentary time on mortality, and for total activity and both intensities of physical activity. AC, activity count.
Single-factor models are results from separate models for each type of behavior, adjusted only for covariates.
Two-factor model results are from separate models that included either sedentary time and light activity or sedentary time and moderate-to-vigorous activity and covariates, and “—“ indicates the variable was not included in the model.
Partition model results are from a single model that included sedentary time, light and moderate-to-vigorous activity, and covariates.
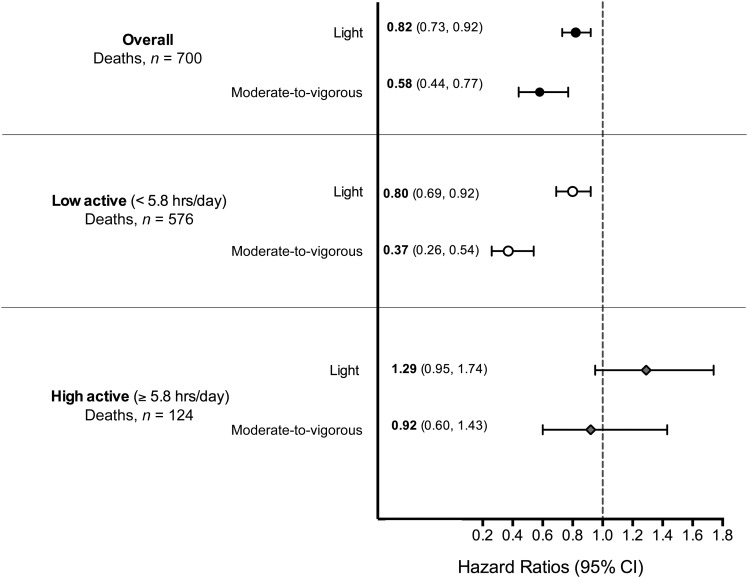
Next, we fit 3-factor (partition) models that mutually adjusted for sedentary and light and moderate-to-vigorous physical activity (Table 2) and explored the mortality benefits associated with replacing sedentary time with physical activity using isotemporal models (Figure 3). Overall, replacing 1 h of sedentary time with 1 h of light-intensity activity was associated with 18% lower mortality (HR: 0.82; 95% CI: 0.73, 92), and replacement with 1 h of moderate-to-vigorous–intensity activity was associated with 42% lower mortality (HR; 0.58; 95% CI: 0.44, 0.77; Figure 3). In low-active adults, replacing 1 h of sedentary time with physical activity was associated with a 20% lower risk for light-intensity (HR: 0.80; 95% CI: 0.69, 0.92) and a 63% lower risk for moderate-to-vigorous–intensity activity (HR: 0.37; 95% CI: 0.26, 0.54). In contrast, among high-active adults who were at 50% lower risk than less-active adults, replacement of sedentary time with physical activity showed no mortality benefit. Using alternate cutoffs, we found slightly stronger replacement associations. In the overall sample, replacing sedentary time with light-intensity activity (100 ≥ AC < 2020) was associated with 24% lower mortality and 63% lower mortality for moderate-to-vigorous intensity (AC ≥2020; Supplemental Table 3 and Supplemental Figure 5).
FIGURE 3.
Mortality associations for replacing 1 h of sedentary time with light- and moderate-to-vigorous–intensity activity in all participants and in low- and high-active groups. Numerical values reported are HRs (95% CIs). Associations are adjusted for age, race, education, sex, smoking status, alcohol use, BMI, and a history of the following conditions: diabetes, coronary artery disease, stroke, mobility limitation, and cancer. Activity intensities are defined as sedentary time (AC <100), light (100 ≥ AC < 760), and moderate-to-vigorous physical activity (AC ≥760). Low- and high-active designations were based on sample-weighted medians of total active time (AC ≥100 min/d). AC, activity count.
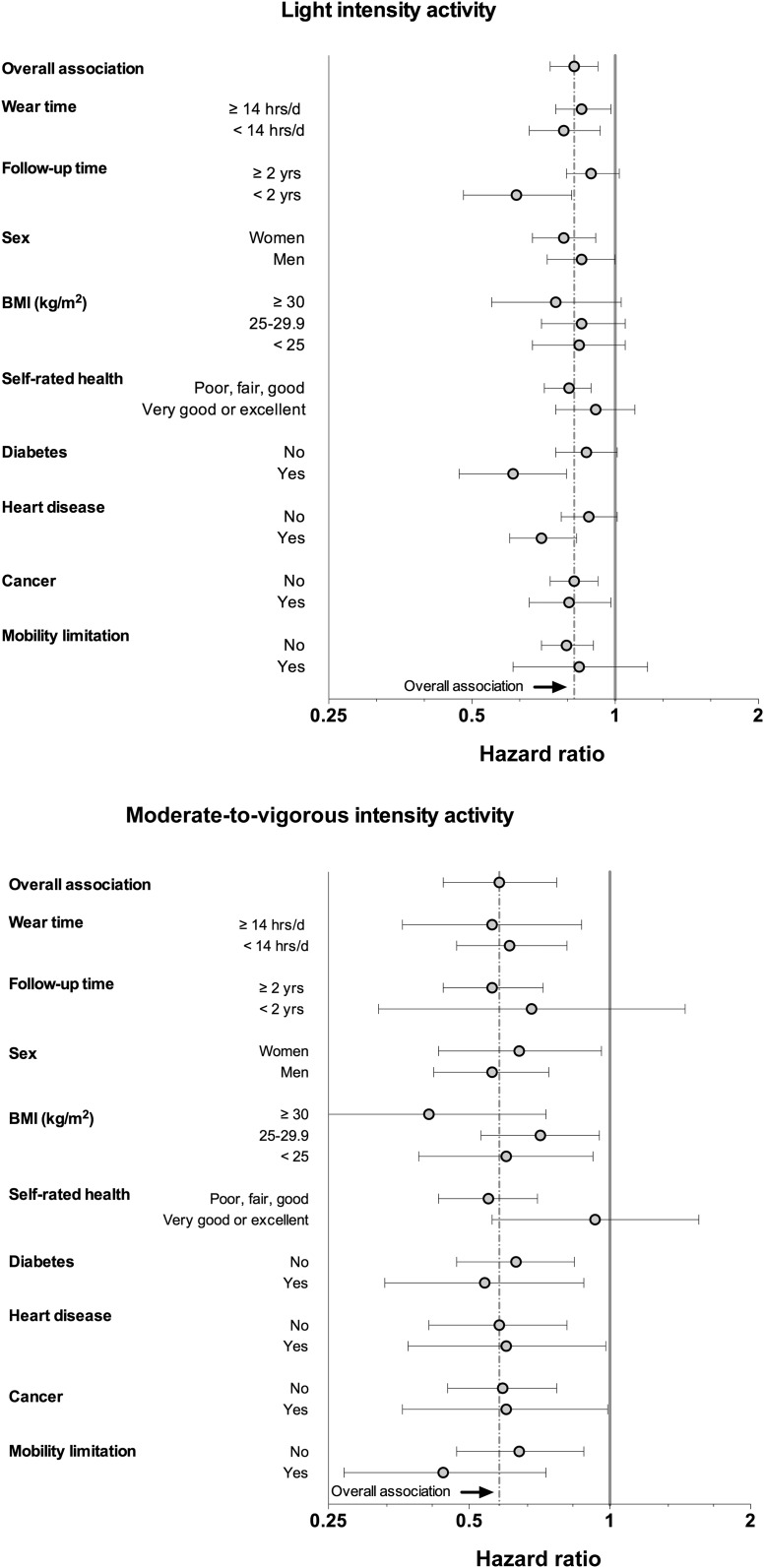
In sensitivity analysis we found little consistent evidence of variation from our original replacement associations for light- and moderate-to-vigorous–intensity activity by monitor-wear time, sex, and presence of chronic conditions, such as diabetes, heart disease, or cancer (Figure 4). However, there was evidence that the associations for replacement of sedentary time with light-intensity activity were stronger in the first 2 y of follow-up, whereas replacement associations for moderate-to-vigorous–intensity activity did not differ by follow-up time.
FIGURE 4.
Mortality associations for replacing 1 h of sedentary time with light- and moderate-to-vigorous–intensity activity by selected study characteristics. Numerical values reported are HRs (95% CIs). Associations are adjusted for age, race, education, sex, smoking status, alcohol use, BMI, and a history of the following conditions: diabetes, coronary artery disease, stroke, mobility limitation, and cancer (as appropriate). Activity intensities are defined as light (100 ≥ AC <760) and moderate-to-vigorous physical activity (AC ≥760). AC, activity count.
DISCUSSION
In this prospective study of US adults we found light- and moderate-to-vigorous–intensity physical activity to be associated with lower mortality in an inverse nonlinear manner. Benefits of light activity appeared to plateau between 4 and 5 h/d and for moderate-to-vigorous activity at ∼1.5 h/d. We found a linear mortality relation for sedentary time, and a 1-h increase in this prevalent behavior was associated with 7% greater mortality after adjustment for moderate-to-vigorous activity in less-active individuals. Isotemporal substitution models suggested that replacing sedentary time with either intensity of physical activity had important benefits for adults in the low-active group, whereas little additional benefit was noted for adults who were highly active.
An important finding is that there are significant differences in the relation between sedentary time and mortality depending on the amount of accelerometer-measured total physical activity accumulated in the day. The high-active group (>5.8 h/d of total activity) had half the mortality risk of the low-active group, and they spent ∼50% of their time sedentary and 50% physically active. This group was protected from health risks associated with increased sedentary time and did not gain additional benefits from further increases in physical activity. In contrast, the low-active group spent 68% of their time sedentary and 32% in physically active pursuits, and sedentary time was positively associated with increased risk. Collectively, we interpret our results to suggest that total physical activity, including light and moderate-to-vigorous physical activity, is an important determinant of mortality. Although our study begins to outline the shape of the mortality dose-response for duration through the use of an accelerometer, future studies are needed to better understand the most informative indicator of total physical activity (e.g., duration, total activity counts, MET h/d) and the amount of total activity that maximizes benefit and to disentangle the relative benefits of light- and moderate-to-vigorous–intensity activity.
Whether sedentary time has health effects that are biologically independent from physical activity has been a controversial question (33, 34). However, from a public health and time-use perspective, focusing on the risks and benefits associated with the trade-offs between sedentary and physically behaviors may be most relevant. Recent studies using that used self-reported measures found replacing 1 h of sitting with a variety of types and intensities of physical activity to be associated with lower mortality (20, 35) and that these benefits may be most pronounced in less-active adults (20). We also found that replacing sedentary time was most effective in less-active individuals and that little additional benefit was gained by increasing activity further in highly active adults. Using accelerometer-based measures in NHANES, Fishman et al. (16) found replacement of 1 h of sedentary time to be associated with 39% lower mortality, a slightly stronger effect than the 20% lower risk we observed. This difference could be due to their focus on adults >50 y of age, exclusion of accidental deaths, or differences in the methods used to define light activity in their study. Collectively, these studies provide consistent evidence that reducing sedentary behavior in favor of physical activity may have important mortality benefits. Notably, analysis of the NHANES accelerometer-based data by using distinct analytic approaches and inclusion criteria provides convergent and compelling evidence that greater amounts of light intensity are associated with important mortality benefits [e.g. (13–16)].
There are 2 important methodologic issues that merit comment. First, the isotemporal modeling approach only estimates mortality benefits for time trade-offs between activities by using results from statistical models, not from actual changes in behavior. Additionally, a challenge when using such models with accelerometer data is that the 24-h day effectively fixes total observation time, potentially introducing substitution effects from behaviors occurring during nonwear time, such as short sleep (36). To investigate this possible source of bias, we examined the nonwear time and mortality relation (no association) and evaluated those with long- and short-wear time (no major differences). We did not have information about sleep duration in our full sample.
Recent studies have begun to investigate the complex relation between sleep, sedentary behavior, physical activity, and health (32, 35, 37) and the analysis of health consequences of each of these behaviors over the 24-h day is an emerging research challenge. In this article we used the isotemporal substitution approach, but further work with branched equations (39), methods designed to find unknown dose-response breakpoints (40), or novel methods capable of integrating the effects of all of these behaviors within the full 24-h day could be informative (38). Second, we are acutely aware of the challenges associated with translating activity counts from 1-min epochs into categories of activity intensity (23, 40) and that newer methods are emerging (41, 42). Although a variety of activity count cutoffs have been proposed to classify sedentary time (18, 42, 43), we elected to use AC <100 because it adequately ranks adults in free-living studies (18, 27, 43). Several moderate-to-vigorous cutoffs have also been proposed (21, 22, 44). We elected to use the 760 threshold primarily because it was calibrated to differentiate between light and moderate-to-vigorous activity across a broad range of activities (23, 27) and provides useful estimates of each activity intensity in free-living studies compared with a variety of criterion measures (23–26, 45). We also examined results for the AC 2020 cutoff and found strong consistency in our results for sedentary time and light activity, supporting the idea that light-intensity activity has important health benefits. Stronger associations with using AC 2020 highlight the benefits of more intense moderate-to-vigorous activity.
Our study has a number of strengths. We used data from the first large-scale population-based cohort with accelerometer measurements with a large number of mortality endpoints to conduct a prospective analysis. In comparison with earlier reports from this cohort (12–14), we evaluated ∼4 more years of follow-up and 550 additional deaths. Unlike past studies, we explored the mortality dose-response in depth with restricted cubic splines and considered the role of total physical activity when estimating the mortality benefits associated with replacing sedentary time with physical activity. In addition, our estimate of the mortality benefits for a 1-h reduction in sedentary time is consistent with a reduction that was feasible in intervention trials (46), and there is considerable biologic plausibility for our results owing to the metabolic impact of light-intensity activity (32, 47, 48).
In conclusion, our results support the notion that light-intensity activities of everyday living, or “baseline activities” (49), may be associated with considerable mortality-sparing benefits for less-active adults in the population. Intervention efforts to increase light-intensity activity, perhaps by taking advantage of their time-inverse relation with sedentary time, may be an important adjunct to current health promotion efforts for physical activity.
Acknowledgments
We thank Doug Midthune, Victor Kipnis, James McClain, and Matthew Buman for very helpful discussion concerning this modeling approach and Penny Randall-Levy for help with the bibliography. In addition, we thank Barry Graubard for providing code to implement the restricted cubic spline analysis within this study with a complex survey design.
The authors’ responsibilities were as follows—CEM, SKK, RPT, AK, TBH, and DB: designed the research (project conception, development of overall research plan, and study oversight); RPT: conducted the research (hands-on conduct of the experiments and data collection); SKK, LK, RB, and DVD: provided essential reagents or provided essential materials (applies to authors who contributed by providing animals, constructs, databases, etc., necessary for research); CEM, SKK, LK, and DB: analyzed the data and performed the statistical analysis; CEM, SKK, RPT, AK, RB, DVD, PC, KYC, TBH, and DB: wrote the manuscript; and CEM, SKK, RPT, and DB: had primary responsibility for the final content. None of the authors reported a conflict of interest related to this study.
REFERENCES
- 1.Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev 2012;13:659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One 2011;6:e19657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownson RC, Boehmer TK, Luke DA. Declining rates of physical activity in the United States: what are the contributors? Annu Rev Public Health 2005;26:421–43. [DOI] [PubMed] [Google Scholar]
- 4.Archer E, Shook RP, Thomas DM, Church TS, Katzmarzyk PT, Hébert JR, McIver KL, Hand GA, Lavie CJ, Blair SN. 45-year trends in women’s use of time and household management energy expenditure. PLoS One 2013;8:e56620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee report. Washington (DC): Office of Disease Prevention and Health Promotion; 2008. p. A1–10. [Google Scholar]
- 6.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, Alter DA. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med 2015;162:123–32. [DOI] [PubMed] [Google Scholar]
- 8.Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, Troiano RP, Hollenbeck A, Schatzkin A. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr 2012;95:437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunstan DW, Barr ELM, Healy GN, Salmon J, Shaw JE, Balkau B, Magliano DJ, Cameron AJ, Zimmet PZ, Owen N. Television viewing time and mortality: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Circulation 2010;121:384–91. [DOI] [PubMed] [Google Scholar]
- 10.Ensrud KE, Blackwell TL, Cauley JA, Dam T-TL, Cawthon PM, Schousboe JT, Barrett-Connor E, Stone KL, Bauer DC, Shikany JM, et al. Objective measures of activity level and mortality in older men. J Am Geriatr Soc 2014;62:2079–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chipperfield JG. Everyday physical activity as a predictor of late-life mortality. Gerontologist 2008;48:349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koster A, Caserotti P, Patel KV, Matthews CE, Berrigan D, Van Domelen DR, Brychta RJ, Chen KY, Harris TB. Association of sedentary time with mortality independent of moderate to vigorous physical activity. PLoS One 2012;7:e37696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid D, Ricci C, Leitzmann MF. Associations of objectively assessed physical activity and sedentary time with all-cause mortality in US adults: the NHANES study. PLoS One 2015;10:e0119591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beddhu S, Wei G, Marcus RL, Chonchol M, Greene T. Light-intensity physical activities and mortality in the United States general population and CKD subpopulation. Clin J Am Soc Nephrol 2015;10:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loprinzi PD. Light-intensity physical activity and all-cause mortality. Am J Health Promot 2016Jan 5 (Epub ahead of print; DOI: 10.4278/ajhp.150515-ARB-882). [DOI] [PubMed] [Google Scholar]
- 16.Fishman EI, Steeves JA, Zipunnikov V, Koster A, Berrigan D, Harris TA, Murphy R. Association between objectively measured physical activity and mortality in NHANES. Med Sci Sports Exerc 2016;48:1303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paffenbarger RS, Hyde RT, Wing AL, Hsieh CC. Physical activity, all-cause mortality, and longevity of college alumni. N Engl J Med 1986;314:605–13. [DOI] [PubMed] [Google Scholar]
- 18.Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, et al. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee MC, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet 2011;378:1244–53. [DOI] [PubMed] [Google Scholar]
- 20.Matthews CE, Moore SC, Sampson J, Blair A, Xiao Q, Keadle SK, Hollenbeck A, Park Y. Mortality benefits for replacing sitting time with different physical activities. Med Sci Sports Exerc 2015;47:1833–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc 2008;40:181–8. [DOI] [PubMed] [Google Scholar]
- 22.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 1998;30:777–81. [DOI] [PubMed] [Google Scholar]
- 23.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc 2005;37(11 Suppl):S512–22. [DOI] [PubMed] [Google Scholar]
- 24.Crouter SE, DellaValle DM, Haas JD, Frongillo EA, Bassett DR. Validity of ActiGraph 2-regression model, Matthews cut-points, and NHANES cut-points for assessing free-living physical activity. J Phys Act Health 2013;10:504–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welk GJ, McClain JJ, Eisenmann JC, Wickel EE. Field validation of the MTI Actigraph and BodyMedia Armband Monitor using the IDEEA Monitor. Obesity (Silver Spring) 2007;15:918–28. [DOI] [PubMed] [Google Scholar]
- 26.van der Ploeg HP, Merom D, Chau JY, Bittman M, Trost SG, Bauman AE. Advances in population surveillance for physical activity and sedentary behavior: reliability and validity of time use surveys. Am J Epidemiol 2010;172:1199–206. [DOI] [PubMed] [Google Scholar]
- 27.Matthews CE, Keadle SK, Sampson J, Lyden K, Bowles HR, Moore SC, Libertine A, Freedson PS, Fowke JH. Validation of a previous-day recall measure of active and sedentary behaviors. Med Sci Sports Exerc 2013;45:1629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrell FE, Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst 1988;80:1198–202. [DOI] [PubMed] [Google Scholar]
- 29.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med 1989;8:551–61. [DOI] [PubMed] [Google Scholar]
- 30.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010;29:1037–57. [DOI] [PubMed] [Google Scholar]
- 31.Mekary RA, Willett WC, Hu FB, Ding EL. Isotemporal substitution paradigm for physical activity epidemiology and weight change. Am J Epidemiol 2009;170:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buman MP, Winkler EAH, Kurka JM, Hekler EB, Baldwin CM, Owen N, Ainsworth BE, Healy GN, Gardiner PA. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am J Epidemiol 2014;179:323–34. [DOI] [PubMed] [Google Scholar]
- 33.Hamilton MT, Hamilton DG, Zderic TW, Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 2007;56:2655–67. [DOI] [PubMed] [Google Scholar]
- 34.Maher C, Olds T, Mire E, Katzmarzyk PT. Reconsidering the sedentary behaviour paradigm. PLoS One 2014;9:e86403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamatakis E, Rogers K, Ding D, Berrigan D, Chau J, Hamer M, Bauman A. All-cause mortality effects of replacing sedentary time with physical activity and sleeping using an isotemporal substitution model: a prospective study of 201,129 mid-aged and older adults. Int J Behav Nutr Phys Act 2015;12:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao Q, Keadle SK, Hollenbeck AR, Matthews CE. Sleep duration and total and cause-specific mortality in a large US cohort: interrelationships with physical activity, sedentary behavior, and body mass index. Am J Epidemiol 2014;180:997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chastin SF, Palarea-Albaladejo J, Dontje ML, Skelton DA. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS One 2015;10:e0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thompson D, Batterham AM. Towards integrated physical activity profiling. PLoS One 2013;8:e56427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muggeo VM. Estimating regression models with unknown break-points. Stat Med 2003;22:3055–71. [DOI] [PubMed] [Google Scholar]
- 40.Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med 2014;48:1019–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kozey Keadle S, Lyden K, Staudenmayer J, Hickey A, Viskochil R, Braun B, Freedson P. The independent and combined effects of exercise training and reducing sedentary behavior on cardiometabolic risk factors. Appl Physiol Nutr Metab 2014;39:770–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crouter SE, Kuffel E, Haas JD, Frongillo EA, Bassett DRJ. Refined two-regression model for the ActiGraph Accelerometer. Med Sci Sports Exerc 2010;42:1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson P. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc 2011;43:1561–7. [DOI] [PubMed] [Google Scholar]
- 44.Matthews CE, Ainsworth BE, Hanby C, Pate RR, Addy C, Freedson PS, Jones DA, Macera CA. Development and testing of a short physical activity recall questionnaire. Med Sci Sports Exerc 2005;37:986–94. [PubMed] [Google Scholar]
- 45.Arem H, Moore SC, Park Y, Ballard-Barbash R, Hollenbeck A, Leitzmann M, Matthews CE. Physical activity and cancer-specific mortality in the NIH-AARP Diet and Health Study cohort. Int J Cancer 2013. Dec 18 (Epub ahead of print; DOI: 10.1002/ijc.28659). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prince SA, Saunders TJ, Gresty K, Reid RD. A comparison of the effectiveness of physical activity and sedentary behaviour interventions in reducing sedentary time in adults: a systematic review and meta-analysis of controlled trials. Obes Rev 2014;15:905–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, et al. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 2012;35:976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xiao Q, Moore SC, Keadle SK, Zheng W, Peters TM, Leitzmann MF, Ji BT, Sampson JN, Shu XO, Matthews CE. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int J Epidemiol 2016. Apr 12 (Epub ahead of print; DOI: 10.1002/ijc.28659). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell KE, Paluch AE, Blair SN. Physical activity for health: what kind? how much? how intense? on top of what? Annu Rev Public Health 2011;32:349–65. [DOI] [PubMed] [Google Scholar]