Abstract
Atherosclerosis is a maladaptive, nonresolving chronic inflammatory disease that occurs at sites of blood flow disturbance. The disease usually remains silent until a breakdown of integrity at the arterial surface triggers the formation of a thrombus. By occluding the lumen, the thrombus or emboli detaching from it elicits ischaemic symptoms that may be life-threatening. Two types of surface damage can cause atherothrombosis: plaque rupture and endothelial erosion. Plaque rupture is thought to be caused by loss of mechanical stability, often due to reduced tensile strength of the collagen cap surrounding the plaque. Therefore, plaques with reduced collagen content are thought to be more vulnerable than those with a thick collagen cap. Endothelial erosion, on the other hand, may occur after injurious insults to the endothelium instigated by metabolic disturbance or immune insults. This review discusses the molecular mechanisms involved in plaque vulnerability and the development of atherothrombosis.
Keywords: atherosclerosis, atherothrombosis, endothelial erosion, inflammation, plaque rupture
Atherosclerosis: chronic inflammation in the artery wall
Atherosclerosis is a maladaptive, nonresolving chronic inflammatory disease that occurs at sites of blood flow disturbance. The atherogenic process is thought to be triggered by the subendothelial retention of cholesterol-containing plasma lipoproteins at these sites and by flow-mediated inflammatory changes in endothelial cells [1, 2]. The lesions contain monocyte-derived macrophages and T cells interspersed with acellular regions containing lipids and debris from dead cells, embedded in an extracellular matrix composed of collagen fibres and other constituents produced primarily by vascular smooth muscle cells [3, 4]. The collagenous matrix typically forms a fibrous cap that overlies the lipid-rich region in the plaque core. Lesions generally remain covered by an intact endothelium until the late stages of the disease. The eventual breakdown of endothelial continuity can promote lesion progression and complication.
Cells of the atherosclerotic lesion display features of ongoing inflammation, with macrophages and T cells producing a host of mediators including proinflammatory cytokines, costimulatory factors for immune activation, eicosanoids and reactive oxygen and nitrogen species [5, 6]. In addition, many of the macrophages internalize cholesterol through their scavenger receptors and some also produce anti-inflammatory cytokines. Furthermore, certain T cells of the regulatory phenotype display anti-inflammatory and immunosuppressive features. This delicate balance between pro- and anti-inflammatory signals results in a slowly progressive, nonresolving, chronic inflammation [7].
Innate and adaptive immune reactions in the artery
Several reactions link lipid accumulation to inflammation. In the macrophage, pattern recognition receptors selected in evolution for handling components of microbial pathogens also mediate internalization of modified lipoproteins [5, 6]. These scavenger receptors evade suppression due to increases in intracellular cholesterol concentrations and can therefore mediate continued lipoprotein uptake that permits overloading the cell with lipids. At a certain point, intracellular cholesterol precipitates as microcrystals. Analogously with urate crystals, these cholesterol microcrystals can activate an inflammasome, that is a cytosolic molecular machine that cleaves a proforma of interleukin (IL)-1 β, converting it into bioactive IL1 β that can be secreted by the cell [8]. When released in the arterial intima, IL-1 β induces production of a set of other pro-inflammatory molecules, including the cytokine IL-6 and the pro-inflammatory eicosanoid, PGE2 [9, 10]. IL-1 β also promotes expression of leucocyte adhesion molecules and matrix-degrading metalloproteinases. Thus, cholesterol accumulation begets inflammation and tissue remodelling.
Another set of pattern recognition receptors, the Toll-like receptors, may bind modified lipoprotein particles in the arterial intima [11–14], triggering phosphorylation cascades that elicit expression of a set of pro-inflammatory genes similar but not identical to that elicited by IL-1 β. For instance, TNF induces the expression of matrix metalloproteinases that degrade collagen and promotes tissue remodelling [15]. TNF has crucial pathogenetic importance in rheumatoid arthritis and other inflammatory diseases and also impacts atherosclerosis substantially [16–18].
Presentation by dendritic cells of fragments of LDL particles to T cells in lymph nodes draining the atherosclerotic lesion calls adaptive immunity into action [19, 20]. Clones of T cells that recognize peptide fragments of the main LDL apoprotein (apoprotein B) that can act as autoantigens. This encounter tends to differentiate the T cells into pro-inflammatory Th1 effector cells under the influence of pro-inflammatory mediators such as IL-12 found in plaque [20, 21]. Effector T cells patrol the body, enter at sites such as the plaque, where endothelial cells express leucocyte adhesion molecules. These T cells may undergo reactivation by LDL fragments. Such renewed activation prompts the Th1 cell to produce large amounts of TNF and also another pro-inflammatory cytokine, interferon-gamma [21, 22]. This interferon strongly stimulates macrophages and also profoundly effects vascular endothelial and smooth muscle cells, causing them to express leucocyte adhesion molecules, modulate their fibrinolytic properties, reduce proliferation and, in the case of the smooth muscle cell, inhibit fibrillar collagen formation [23, 24]. Interestingly, in keeping with the counterbalancing forces mentioned above, lesional dendritic cells can also promote the development of proresolving regulatory T cells in early atherosclerosis [25, 26], but ultimately the effector: regulatory T-cell balance promotes progressive inflammation.
In the advanced atherosclerotic plaque, infiltrating mast cells contribute to the pro-inflammatory milieu [27]. Upon activation, these cells release a host of mediators and enzymes, including histamine, serotonin, thromboxane and other eicosanoids, cytokines and a set of serine proteases, all of which may profoundly affect the atherosclerotic lesion.
The concerted action of all pro-inflammatory signals operating in the plaque not only enhances inflammation but also hampers renewal of the structural elements that support the mechanical stability of the inflamed tissue.
Clinical and histopathological features of culprit lesions
The atherosclerotic process typically lies silent for months, years and even decades and may never result in clinical manifestations [2]. Yet, if the plaque’s surface is damaged, thrombotic occlusion of the artery may ensue. Surface continuity may be damaged by fissuring (so-called plaque rupture, observed in 60% to 80% of cases of acute coronary syndrome) or surface erosion (present in 20% to 40% of cases with coronary thrombosis, especially in women and young victims of sudden coronary death) [28, 29]. Figures 1 and 2 depict these two different types of discontinuity of the plaque surface. Recent studies suggest that the proportion of infarctions caused by rupture versus erosion is changing, with more cases due to erosion and fewer to overt plaque rupture [30].
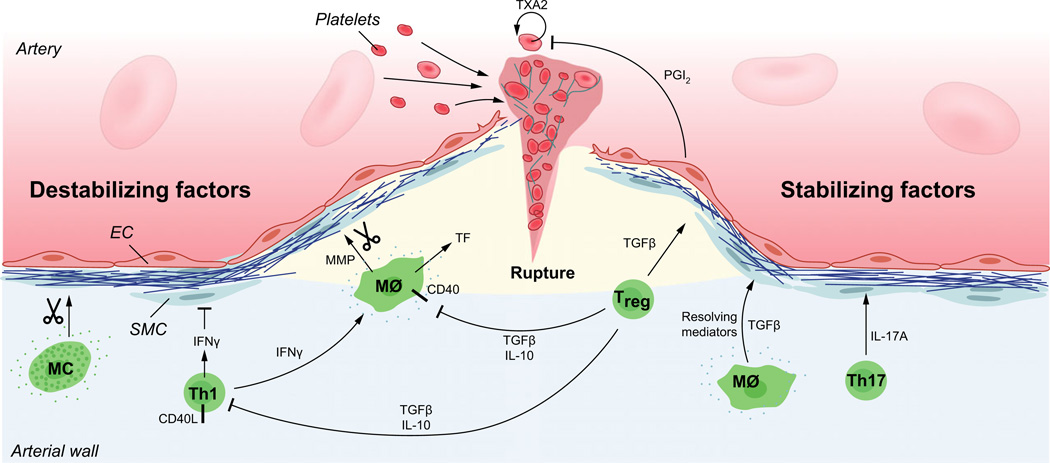
Fig. 1.
Mechanisms of plaque rupture. Activated macrophages and Th1 cells produce metalloproteinases and cytokines that hamper the tensile strength of the collagen cap. Several pro-inflammatory cytokines including interferon-Γ (IFNΓ) and tumour necrosis factor (TNF), as well as CD40/CD40L cell surface receptors of the TNF superfamily promote an inflammatory state that enhance cell death and prothrombotic activity in the plaque. When the cap no longer can withstand the mechanical force of the blood pressure, superficial fissures are formed in the plaque. Exposure of the plaque’s inner core with its thrombogenic material rapidly triggers platelet activation, humoral coagulation and the formation of a thrombus that may either occlude the artery at the site of plaque rupture or dissociate as an embolus and occlude the arterial lumen at a site downstream of the ruptured plaque. Counteracting all these pro-inflammatory and tissue-destructive signals, subsets of macrophages and T cells produce anti-inflammatory molecules that counteract vascular inflammation and reduce the risk for plaque rupture and atherothrombosis. Amongst them, transforming growth factor-β (TGF-β) and interleukin-10 (IL-10) inhibit inflammation and immune cell activation. In addition, TGF-β has fibrogenic properties that it shares with IL-17A produced by Th17 cells. The resolution of plaque inflammation depends not only on anti-inflammatory signals but also on resolving mediators such as eicosanoids of the resolvin type and Annexin I, both of which ligate the FPR/ALX receptor. EC, endothelial cell; SMC, smooth muscle cell; MΦ, macrophage; MMP, metalloproteinase; TXA2, thromboxane A2; PGI2, prostaglandin I2 (prostacyclin).
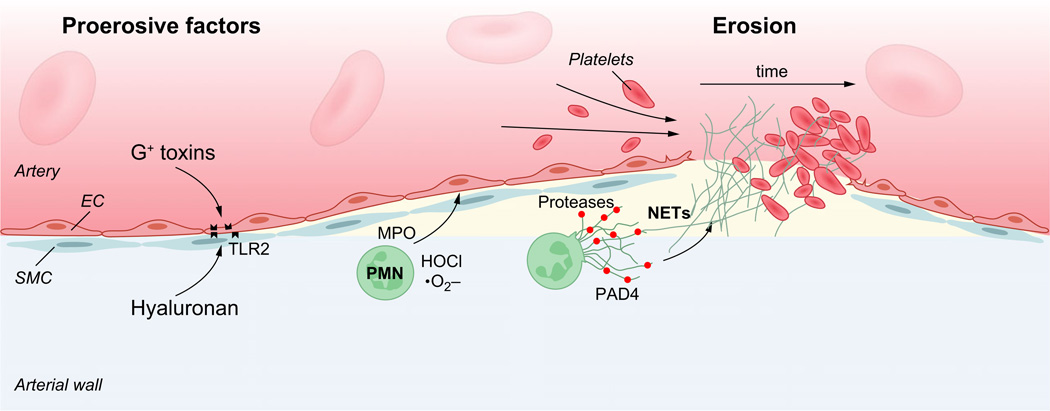
Fig. 2.
Mechanisms of plaque erosion. Endothelial cells of atherosclerotic plaques commonly express Toll-like receptor −2 (TLR2) that can ligate both Gram-positive toxins (G+ toxins) of bacterial pathogens and hyaluronan released from the extracellular matrix. TLR2 ligation can trigger endothelial dysfunction with endoplasmic reticulum stress and apoptosis. Such reactions are further enhanced by neutrophil attack on the endothelium. As a result, endothelial cells may detach, exposing the subendothelial matrix with its thrombogenic components. Activated neutrophils contribute to a prothrombotic state by releasing a set of proteases including neutrophil elastase and by forming neutrophil extracellular traps (NETs) that can damage endothelial cells, trap leucocytes and enhance thrombosis. PAD4, Peptide arginine deaminase-4, a component of NETs.
Fissures and erosions trigger atherothrombosis by exposing thrombogenic material inside the plaque, such as phospholipids, tissue factor and matrix molecules, to platelets and coagulation factors [2]. Platelet aggregates precipitating on these exposed surfaces are stabilized by fibrin networks. Tissue factor, expressed by macrophages and by vascular smooth muscle cells in the atherosclerotic plaque, can initiate the blood coagulation cascade that leads to fibrin formation [31]. Atherothrombi expand rapidly and can fill the lumen within minutes, thereby leading to ischaemia and infarction.
A range of factors may contribute to atherothrombosis. Disturbance of the balance between prothrombotic and fibrinolytic activity on the plaque surface probably plays an important role for precipitating the thrombotic event [32], but the precise sequence of events that operate in vivo is not yet known.
The ‘vulnerable plaque’
Thrombi precipitate on damaged vascular surfaces, as recognized by Rudolf Virchow in 1856 [33]. The cause of the damage leading to plaque rupture or erosion remains incompletely understood, despite considerable progress in this regard. Constantinides, Davies, Falk and their colleagues observed that ruptured plaques display thin fibrous caps and large lipid core regions [34–36]. These findings highlighted structural abnormalities in the vessel wall as a cause of atherothrombosis. Subsequent investigations have revealed that culprit lesions of fatal thrombi in coronary arteries contain reduced amounts of mature, cross-linked collagen and increased levels of collagen-degrading enzymes.
In vivo imaging technology now offers approaches to the analysis of major plaque components. For example, optical coherence tomography (OCT) and magnetic resonance imaging can identify thin-cap plaques. Computerized tomographic angiography can identify outward arterial remodelling, radiolucency and spotty calcification associated with coronary events. Such approaches, albeit incompletely validated, are currently used to obtain surrogate end-point data on effects of putative plaque-stabilizing therapies [37–39].
Histopathological analysis of lesions that have provoked fatal myocardial infarction (MI) shows stimata of inflammation including accumulation of macrophages, activated T cells, dendritic cells and mast cells as well as reduced thickness of the fibrous cap and increased neovascularization at sites of plaque rupture and thrombosis [40] (Fig. 1). Matrix metalloproteinases and cysteine proteinases, products of activated macrophages, localize at sites of plaque rupture [41]. Several of these enzymes digest fibrillar collagen, thus reducing the mechanical stability of the plaque [41, 42]. These proteinases likely render plaques susceptible to rupture, but have complex effects on the composition and size of lesions in mouse experiments.
Lesional cell death
Cell death may also predispose to plaque rupture [7, 43]. Smooth muscle cells (SMC) synthesize the bulk of the arterial extracellular matrix. Site of fatal plaque rupture display depletion of SMC needed to repair and maintain the collagen that comprises the plaque’s fibrous cap. Apoptosis of SMC documented in atheromata may thus lead to their relative lack at sites of plaque rupture. Rapid phagocytosis usually clears the remnants of cells that have undergone apoptosis, a process known as efferocytosis [44]. If this process fails, secondary necrosis ensues, contributing to the formation of the plaques lipid core, also known as the ‘necrotic core’. Computational analyses indicate that lipid core accumulation can reduced the mechanical integrity of the plaque.
Plaque necrosis results from death of lesional cells, mostly macrophages. Cell death can lead to necrosis by at least two mechanisms: apoptosis followed by defective phagocytic clearance (‘efferocytosis’) of the apoptotic cells and a process called primary necrosis [7]. Macrophage apoptosis occurs in lesions of all stages. A number of plaque factors are likely to trigger lesional macrophage apoptosis, including excessive inflammation, oxidized lipids and cholesterol, often in combination through a ‘multihit’ process. Observational data in human atheromata and molecular-genetic causation data in mouse models of advanced atherosclerosis indicate that one of the hits caused by these factors in chronic endoplasmic reticulum (ER) stress [45]. In particular, the ER stress effector CHOP is tightly associated with cell death and plaque necrosis in human coronary artery lesions, and genetic deletion of CHOP in mice protects against advanced lesional macrophage apoptosis and plaque necrosis [45].
In early atherosclerosis, the apoptotic cells are properly cleared by neighbouring phagocytes, which prevents postapoptotic necrosis and triggers proresolving processes that are linked to efferocytosis [46]. In advanced plaques, however, efferocytosis is defective, leading to cell necrosis, release of pro-inflammatory damage-associated molecular patterns (DAMPs) and lack of efferocytosis-mediated proresolving signalling [47–49]. Collectively, these processes promote the type of inflammatory, necrotic lesions that are characteristics of vulnerable plaques (see below). The mechanisms of defective efferocytosis in advanced atherosclerotic lesions are not known and are likely to be multifactorial. A recent study provided correlative evidence in human atheromata suggesting a role for ADAM17-mediated cleavage of MerTK, a macrophage efferocytosis receptor shown to be important in the progression of murine atherosclerosis [49–51]. It is also interesting to note that defective efferocytosis is a cardinal sign of defective inflammation resolution [52] and that a therapeutic strategy that enhanced resolution in advanced murine plaques markedly suppressed plaque necrosis [53].
Whereas defective efferocytosis leads to plaque necrosis through secondary necrosis of uncleared apoptotic cells, cells can undergo another process in which necrosis develops as a primary event. In this case, a signalling cascade involving RIP1 and RIP3 kinases is involved, and when RIP3 kinase was genetically targeted in fat fed LDL receptor null mice, plaque necrosis was partially suppressed [54]. These data suggest that, at least in advanced murine atheroma, both secondary and primary apoptosis contribute to plaque necrosis.
Plaque erosion
Plaques that have disrupted due to fibrous cap fracture tend to have a large lipid core [30], and the potent procoagulant tissue factor localizes in these cores [7] (Fig. 1). Those disrupted by erosion, another substrate for thrombus formation, do not have a large lipid core and show less inflammatory cell accumulation than fissured plaques (Fig. 2). Plaques frequently rupture without clinical manifestations, possibly reflecting variation in the thrombotic response depending on the thrombogenicity of exposed plaque constituents, local hemorheology, shear-induced platelet activation systemic clotting activity, fibrinolytic function and sensitivity of the end organ to ischaemia.
Plaques displaying endothelial erosion seem to differ from rupturing ones in some important aspects [29]. They appear to be less inflamed but contain proliferating smooth muscle cells, abundant proteoglycans and hyaluronan, and substantial neovascularization. Therefore, pathogenetic mechanisms may differ between these two conditions, and we will consider them separately.
Why do plaques rupture?
Most of our knowledge about plaque rupture comes from studies of human autopsy specimens and surgical material. Key histopathological findings associated with regions of fatal disruption include a thin fibrous cap (<50–60 micrometers), increased signs of inflammatory activity and heightened amounts of proteolytic enzymes [35, 55–58]. Therefore, inflammatory stimuli such as local immune reactions might activate macrophages, mast cells and T cells to release cytokines that inhibit cap formation and proteases that digest fibrous components of the cap (Fig. 1).
Much interest has focused on the collagenolytic action of matrix metalloproteinases and cysteine proteases in the plaque. A set of such enzymes is present in the human atherosclerotic plaque and has shown proteolytic activity in culprit lesions [56, 59]. These findings have encouraged attempts at developing plaque-stabilizing therapies by targeting proteases. Several excellent reviews cover this interesting development in detail [60, 61].
A set of immune cytokines impacts powerfully on the fibrous cap (Fig. 1). Interferon-Γ, a pro-inflammatory, macrophage-activating cytokine produced by Th1-type T cells and NK cells, inhibits collagen fibre formation, causing plaques to adopt a vulnerable phenotype with reduced collagen content. This is due to a triple action of interferon-gamma, as it both inhibits smooth muscle differentiation [24], procollagen- I gene expression [23] and the collagen cross-linking enzyme, lysyl oxidase [62].
The action of Th1 cells is counterbalanced by Treg cells producing TGF-β [63] (Fig. 1). This cytokine has a direct, fibrogenic action on smooth muscle cells and fibroblasts. In addition, it inhibits Th1 and macrophage activity, leading to reduced plaque inflammation. Treg also enhance the catabolism of very low-density lipoproteins, resulting in reduced plasma lipid levels.
A third type of T cells, the Th17 cell type, is involved in wound healing and exerts powerful fibrogenic activity [64]. Th17 cells activated in the context of atherosclerosis promote the formation of thick collagen fibres that can withstand the mechanical assault on the plaque exerted by hemodynamic forces [65]. This is due to the capacity of the signature Th17 cytokine, IL-17A, to promote procollagen expression (Fig. 1).
In addition to reducing the capacity of the tissue to withstand mechanical strain, immune signals may also promote atherothrombosis by increasing the tendency to form platelet aggregates and clots (Fig. 1). The TNF/TNF receptor superfamily members, CD40 ligand (CD40L, CD154) and CD40, may have particular importance in this context. CD40L, typically expressed on activated T cells, ligates CD40 on cells of the macrophage lineage. This stimulation triggers expression of tissue factor as well as matrix metalloproteinase secretion [66]. In addition, activated platelets also express CD40L [67] and endothelial cells exhibit its receptor CD40 [68], allowing for multiple heterophilic interactions that may promote atherothrombosis [69, 70].
Lipid mediators are at least as important as cytokines in the sequence of events leading to atherothrombosis (Fig. 1). The prothrombotic effect of thromboxane A2 released from platelets and the counterbalancing, antithrombotic effect of endothelium-derived prostaglandin I2 (PGI2, a.k.a. prostacyclin) is well known, crucial for vascular homoeostasis, and the target of aspirin used in cardiovascular prevention [71, 72]. Other prostaglandins play different roles in the atherosclerotic artery wall. Thus, PGE2 produced by several cell types promotes vasodilation and macrophage activation but also increases expression of the anti-inflammatory cytokine, IL-10 [73].
The leukotriene pathway of lipid mediators also exerts powerful effects on atherosclerosis. Leukotriene (LT) B4 is a pro-inflammatory leukotriene expressed in plaques [74, 75]. Through its BLT1 receptor, it promotes plaque growth and enhances its inflammatory properties [76]. It also increases vascular restenosis after endothelial injury [74]. 5-lipoxygenase-activating protein (FLAP), a cofactor for the enzyme that converts arachidonic acid into the leukotriene pathway, is upregulated in plaques and promotes LTB4 production [77]. Genetic polymorphism in the FLAP encoding gene, ALOX5AP, was associated with cardiovascular disease in several genetic studies [78], although it did not turn out to be a major genetic risk factor in genomewide association studies. However, this does not rule out a possible role for leukotriene signalling in cardiovascular disease. As many patients with asthma are treated with leukotriene receptor blockers, long-term follow-up of these individuals permits an assessment of the importance of leukotriene signalling in cardiovascular disease [79]. A population-based Swedish study of 7 million cases revealed that those treated with the leukotriene receptor blocker, montelukast had a 35% reduced risk of recurrent stroke and myocardial infarction [79].
Lipoxins and resolvins produced in the 12/15-lipoxygenase pathway counterbalance the pro-inflammatory effects of leukotrienes and may inhibit atherosclerosis and its clinical complications [80]. In line with this notion, targeting the lipoxin receptor FPR2/ALX by genetic abrogation leads to features of reduced plaque stability [81]. Further studies will be required to clarify the role of pro- and anti-inflammatory lipoxygenase products in atherosclerosis.
Clinical studies have associated ischaemic atherothrombotic events such as MI and stroke with infections. Acute infections, via elicitation of systemic cytokines, may elicit an ‘echo’ of inflammatory activation in the plaque, leading to bursts of pro-inflammatory, proteolytic and prothrombotic activity, although we currently lack definitive evidence to confirm such a chain of events [82].
The lack of suitable animal models has hampered research on plaque disruption. Although under circumstances that should promote thrombosis on plaques in rodents, such experiments yielded a low incidence of thrombosis and lack of linear relationship between events and histopathological findings such as ‘buried caps’ [83–85]. Such studies have not generally dealt with coronary arteries, rather the aorta or its large calibre branches. Yet, more recent work has described promising experimental preparations that may be more suitable for addressing mechanisms of plaque rupture [86]. In genetically hypercholesterolemic mutant mice, several interventions can precipitate rupture of existing atherosclerotic plaques, for example virally directed local overexpression of an active form of the MMP stromelysin, the long-term infusion of angiotensin II [87], placement of a cuff around the carotid artery [88], partial ligation of this artery [89] or increasing elastin fragmentation through a ‘knock-in’ mutation in the fibrillin-1 gene [90]. Yet, none of these preparations induce standardized plaque ruptures at a given time in a controlled manner. Instead, they increase the tendency for the plaque to rupture, and heal, spontaneously.
Signs of plaque rupture include intraplaque haemorrhage, fractured cap fibres and multilayered ‘buried’ caps [91]. Enumeration of these signs by microscopy permits quantification of the phenomenon. Such methods have obvious limitations but may permit investigators to assess the effects of various treatments on the tendency for plaques to rupture. The contrived nature of these manipulations, however, limits the generalizability of such experiments. For example, a blocker of angiotensin II should limit disruptions produced by infusions of this mediator, and MMP inhibitors will reduce the consequences of stromelysin overexpression with no predictive value for the effects of these interventions on plaque rupture in humans.
Why does the endothelium erode?
Mechanisms instigating endothelial erosion have been unclear. However, recent studies point to a role for innate immunity in this process (Fig. 2). Endothelial cells overlying atherosclerotic lesions abundantly express the pattern recognition receptor, Toll-like receptor-2 (TLR2) [11]. Ligation of this receptor results in endothelial apoptosis in a process accelerated by polymorphonuclear leucocytes, a cell type found at sites of fatal plaque erosion [92]. TLR2 ligands include the extracellular molecule hyaluronan as well as components of Gram-positive bacteria [93]; therefore, endogenous as well as infectious factors may operate to promote atherothrombosis through this mechanism [92]. Stressful events also associate with acute ischaemic events. For example, the incidence of myocardial infarction often rises shortly after major sports events (particularly in males) and peaked after stressful events such as a major earthquake [94, 95]. This association may result from acute changes in local hemodynamics of the atherosclerotic artery. Exposure of atherosclerotic mice to stressful stimuli led to endothelin-dependent vasoconstriction that preceded thrombosis and myocardial ischaemia, possibly because the vasoconstrictive episode had caused endothelial erosion [35]. Likewise, infusion of spasmogenic stimuli in MI-prone rabbits elicited occasional coronary artery thrombi resembling human superficial erosion [96].
How can plaques be stabilized?
Abundant experimental and some clinical data using MRI or intravascular imaging suggest that lipid lowering, and statin therapy in particular may alter plaque properties implicated in susceptibility to rupture. Several other approaches may stabilize plaques (Fig. 3). None of them have entered clinical trials on the indication to stabilize plaques, in part due to the difficulties in identifying vulnerable, ruptured, eroded and thrombosed lesions in the living patient. Current progress in in vivo imaging techniques might enable such trials in the future.
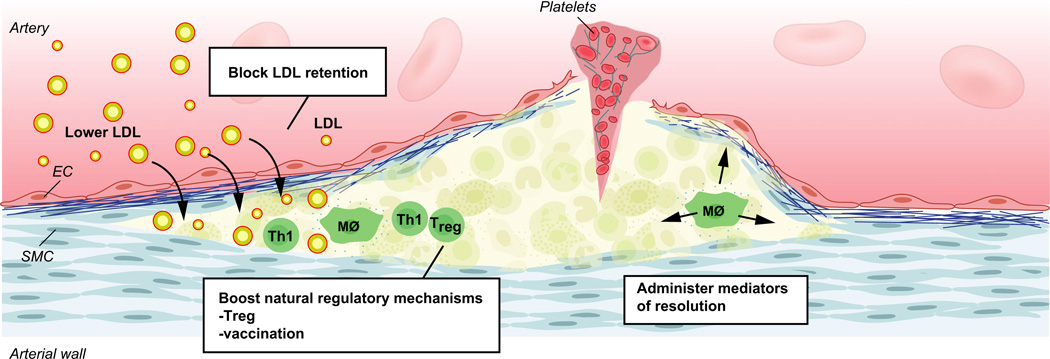
Fig. 3.
Therapy targets for prevention of atherothrombosis. Reduction of LDL (and other large lipoproteins) by lipid-lowering therapy and prevention of LDL retention in the artery wall, both act to reduce cholesterol accumulation, an initiator of atherosclerosis. Stimulation of immunoregulatory mechanisms reduces vascular inflammation; they include administration of anti-inflammatory cytokines, enhancing Treg cells and vaccination to elicit atheroprotective immunity. Mediators of resolution include resolvin-type eicosanoids, peptide mimetics of Annexin I and other substances.
Conclusion
Atherosclerosis associates strongly with systemic risk factors (e.g. high LDL, hypertension, diabetes), yet the lesions distribute multifocally. Most plaques remain silent throughout life but certain individual lesions may provoke thrombotic complication and ischaemia, resulting in life-threatening complications. The discovery of plaque rupture and endothelial erosion as two main causes of atherothrombosis helps us to understand why this very chronic condition manifests clinically in an episodic and unpredictable fashion. Further studies have clarified that inflammation, proteolysis and reduced collagen fibre content predispose to plaque rupture, whereas endothelial erosion followed by neutrophil infiltration typically complicates lesions of a distinct morphology.
Lack of animal preparations that develop disruption of atherosclerotic plaques has, however, hampered progress in mechanistic research on atherothrombosis. Similarly, limitations of noninvasive in vivo imaging of so-called vulnerable plaques in humans have hampered clinical work in this domain. Recent progress in both these areas may address these issues and aid the development and evaluation of plaque-stabilizing therapies beyond lipid lowering in the forthcoming years. The many unanswered questions in this field provide ample opportunity for future research and may yield avenues to improve patient outcomes.
Acknowledgments
Grant Support
Dr Hansson is supported by the Swedish Research Council (project grant 6816 and Linnaeus Support 8703), the Swedish Heart-Lung Foundation, Stockholm County Council, the Foundation for Strategic Research, and the European Commission (FP7 Projects AtheroFlux and VIA). Dr Libby is supported by the US National Institutes of Health (R01 HL080472). Dr Tabas receives funding from the National Institutes of Health (NIH grants HL107497 and HL075662 and Program of Excellence in Nanotechnology (PEN) Award, Contract #HHSN268201000045C).
Conflict of interest statement
Dr. Tabas has a patent targeted polymeric inflammation-resolving nanoparticles pending. Dr. Hansson has patents on the treatment of atherosclerosis issued and under consideration. Dr. Libby reports nonfinancial support from Amgen, AstraZeneca, Boehringer Ingleheim, Bristol-Myers Squibb, Eli Lilly and Company, Esperion Therapeutics, Genzyme, GlaxoSmithKline, Isis Pharmaceuticals, Kowa Pharmaceuticals, Merck, Novartis, Pfizer, Sanofi-Regeneron and Takeda Pharmaceuticals; other from Athera biotechnologies and Interleukin Genetics; and grants from General Electric, GlaxoSmithKline and Novartis, outside the submitted work.
References
- 1.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 3.Jonasson L, Holm J, Skalli O, Bondjers G, Hansson GK. Regional accumulations of t cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986;6:131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- 4.Hansson GK, Holm J, Jonasson L. Detection of activated t lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–175. [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Lichtman AH, Hansson GK. Immune effector mechanisms implicated in atherosclerosis: From mice to humans. Immunity. 2013;38:1092–1104. doi: 10.1016/j.immuni.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–1879. doi: 10.1161/CIRCRESAHA.114.302699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duewell P, Kono H, Rayner KJ, et al. Nlrp3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Libby P, Warner S, Friedman GB. Interleukin-1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988;81:487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loppnow H, Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990;85:731–738. doi: 10.1172/JCI114498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 12.Miller YI, Viriyakosol S, Worrall DS, Boullier A, Butler S, Witztum JL. Toll-like receptor 4-dependent and -independent cytokine secretion induced by minimally oxidized low-density lipoprotein in macrophages. Arterioscler Thromb Vasc Biol. 2005;25:1213–1219. doi: 10.1161/01.ATV.0000159891.73193.31. [DOI] [PubMed] [Google Scholar]
- 13.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 15.Saren P, Welgus HG, Kovanen PT. TNF-alpha and IL-1beta selectively induce expression of 92-kda gelatinase by human macrophages. J Immunol. 1996;157:4159–4165. [PubMed] [Google Scholar]
- 16.Branen L, Hovgaard L, Nitulescu M, Bengtsson E, Nilsson J, Jovinge S. Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein e knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:2137–2142. doi: 10.1161/01.ATV.0000143933.20616.1b. [DOI] [PubMed] [Google Scholar]
- 17.Bjorkbacka H, Kunjathoor VV, Moore KJ, et al. Reduced atherosclerosis in myd88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–421. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 18.Michelsen KS, Wong MH, Shah PK, et al. Lack of toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein e. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein b-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–1091. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]
- 20.Hermansson A, Ketelhuth DF, Strodthoff D, et al. Inhibition of t cell response to native low-density lipoprotein reduces atherosclerosis. J Exp Med. 2010;207:1081–1093. doi: 10.1084/jem.20092243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frostegard J, Ulfgren AK, Nyberg P, et al. Cytokine expression in advanced human atherosclerotic plaques: dominance of pro-inflammatory (th1) and macrophage-stimulating cytokines. Atherosclerosis. 1999;145:33–43. doi: 10.1016/s0021-9150(99)00011-8. [DOI] [PubMed] [Google Scholar]
- 23.Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11:1223–1230. doi: 10.1161/01.atv.11.5.1223. [DOI] [PubMed] [Google Scholar]
- 24.Hansson GK, Hellstrand M, Rymo L, Rubbia L, Gabbiani G. Interferon gamma inhibits both proliferation and expression of differentiation-specific alpha-smooth muscle actin in arterial smooth muscle cells. J Exp Med. 1989;170:1595–1608. doi: 10.1084/jem.170.5.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi JH, Cheong C, Dandamudi DB, et al. Flt3 signaling-dependent dendritic cells protect against atherosclerosis. Immunity. 2011;35:819–831. doi: 10.1016/j.immuni.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian M, Thorp E, Hansson GK, Tabas I. Treg-mediated suppression of atherosclerosis requires myd88 signaling in dcs. J Clin Invest. 2013;123:179–188. doi: 10.1172/JCI64617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaartinen M, Penttilä A, Kovanen PT. Mast cells of two types differing in neutral protease composition in the human aortic intima. Demonstration of tryptase- and tryptase/chymase-containing mast cells in normal intimas, fatty streaks, and the shoulder region of atheromas. Arterioscler Thromb Vasc Biol. 1994;14:966–972. doi: 10.1161/01.atv.14.6.966. [DOI] [PubMed] [Google Scholar]
- 28.Davies MJ, Thomas A. Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984;310:1137–1140. doi: 10.1056/NEJM198405033101801. [DOI] [PubMed] [Google Scholar]
- 29.Farb A, Burke AP, Tang AL, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. doi: 10.1161/01.cir.93.7.1354. [DOI] [PubMed] [Google Scholar]
- 30.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J. 2013;34:719–728. doi: 10.1093/eurheartj/ehs411. [DOI] [PubMed] [Google Scholar]
- 31.Wilcox JN, Smith KM, Schwartz SM, Gordon D. Localization of tissue factor in the normal vessel wall and in the atherosclerotic plaque. Proc Natl Acad Sci U S A. 1989;86:2839–2843. doi: 10.1073/pnas.86.8.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamsten A. Hemostatic function and coronary artery disease. N Engl J Med. 1995;332:677–678. doi: 10.1056/NEJM199503093321011. [DOI] [PubMed] [Google Scholar]
- 33.Virchow R. Der atheromatose prozess der arterien. Wien Med Wochenschr. 1856;6:825–828. [Google Scholar]
- 34.Davies MJ, Thomas A. Plaque fissuring – the cause of acute myocardial infarction, sudden ischaemic death and crescendo angina. Br Heart J. 1985;53:363–373. doi: 10.1136/hrt.53.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Falk E, Shah P, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–671. doi: 10.1161/01.cir.92.3.657. [DOI] [PubMed] [Google Scholar]
- 36.Constantinides P. The role of arterial wall injury in atherogenesis and arterial thrombogenesis. Zentralbl Allg Pathol. 1989;135:517–530. [PubMed] [Google Scholar]
- 37.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 38.Cheng JM, Garcia-Garcia HM, de Boer SP, et al. In vivo detection of high-risk coronary plaques by radiofrequency intravascular ultrasound and cardiovascular outcome: results of the atheroremo-ivus study. Eur Heart J. 2014;35:639–647. doi: 10.1093/eurheartj/eht484. [DOI] [PubMed] [Google Scholar]
- 39.Yonetsu T, Kakuta T, Lee T, et al. In vivo critical fibrous cap thickness for rupture-prone coronary plaques assessed by optical coherence tomography. Eur Heart J. 2011;32:1251–1259. doi: 10.1093/eurheartj/ehq518. [DOI] [PubMed] [Google Scholar]
- 40.Shah PK, Falk E, Badimon JJ, et al. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. [PubMed] [Google Scholar]
- 41.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnarable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newby AC. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc Med. 2007;17:253–258. doi: 10.1016/j.tcm.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michel JB. Anoikis in the cardiovascular system: known and unknown extracellular mediators. Arterioscler Thromb Vasc Biol. 2003;23:2146–2154. doi: 10.1161/01.ATV.0000099882.52647.E4. [DOI] [PubMed] [Google Scholar]
- 44.Liao X, Sluimer JC, Wang Y, et al. Macrophage autophagy plays a protective role in advanced atherosclerosis. Cell Metab. 2012;15:545–553. doi: 10.1016/j.cmet.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Myoishi M, Hao H, Minamino T, et al. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 46.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I. Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of apoe−/− and ldlr−/− mice lacking chop. Cell Metab. 2009;9:474–481. doi: 10.1016/j.cmet.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geng YJ, Libby P. Evidence for apoptosis in advanced human atheroma. Colocalization with interleukin-1 beta-converting enzyme. Am J Pathol. 1995;147:251–266. [PMC free article] [PubMed] [Google Scholar]
- 48.Schrijvers DM, De Meyer GR, Kockx MM, Herman AG, Martinet W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1256–1261. doi: 10.1161/01.ATV.0000166517.18801.a7. [DOI] [PubMed] [Google Scholar]
- 49.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 50.Thorp E, Cui D, Schrijvers DM, Kuriakose G, Tabas I. Mertk receptor mutation reduces efferocytosis efficiency and promotes apoptotic cell accumulation and plaque necrosis in atherosclerotic lesions of apoe−/− mice. Arterioscler Thromb Vasc Biol. 2008;28:1421–1428. doi: 10.1161/ATVBAHA.108.167197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ait-Oufella H, Pouresmail V, Simon T, et al. Defective mer receptor tyrosine kinase signaling in bone marrow cells promotes apoptotic cell accumulation and accelerates atherosclerosis. Arterioscler Thromb Vasc Biol. 2008;28:1429–1431. doi: 10.1161/ATVBAHA.108.169078. [DOI] [PubMed] [Google Scholar]
- 52.Serhan CN, Fredman G, Yang R, et al. Novel proresolving aspirin-triggered dha pathway. Chem Biol. 2011;18:976–987. doi: 10.1016/j.chembiol.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fredman G, Kamaly N, Spolitu S, et al. Targeted nanoparticles containing the proresolving peptide ac2–26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci Transl Med. 2015;7:275ra220. doi: 10.1126/scitranslmed.aaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin J, Li H, Yang M, et al. A role of rip3-mediated macrophage necrosis in atherosclerosis development. Cell Rep. 2013;3:200–210. doi: 10.1016/j.celrep.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 55.van der Wal AC, Becker AE, van der Loos CM, Das PK. Site of intimal rupture or erosion of thrombosed coronary atherosclerotic plaques is characterized by an inflammatory process irrespective of the dominant plaque morphology. Circulation. 1994;89:36–44. doi: 10.1161/01.cir.89.1.36. [DOI] [PubMed] [Google Scholar]
- 56.Henney AM, Wakeley PR, Davies MJ, et al. Localization of stromelysin gene expression in atherosclerotic plaques by in situ hybridization. Proc Natl Acad Sci U S A. 1991;88:8154–8158. doi: 10.1073/pnas.88.18.8154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circ Res. 2014;114:1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 58.Kaartinen M, Penttila A, Kovanen PT. Mast cells in rupture-prone areas of human coronary atheromas produce and store tnf-alpha. Circulation. 1996;11:2787–2792. doi: 10.1161/01.cir.94.11.2787. [DOI] [PubMed] [Google Scholar]
- 59.Sukhova G, Shi G-P, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins s and k in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Newby AC. Proteinases and plaque rupture: unblocking the road to translation. Curr Opin Lipidol. 2014;25:358–366. doi: 10.1097/MOL.0000000000000111. [DOI] [PubMed] [Google Scholar]
- 61.Ketelhuth DF, Back M. The role of matrix metalloproteinases in atherothrombosis. Curr Atheroscler Rep. 2011;13:162–169. doi: 10.1007/s11883-010-0159-7. [DOI] [PubMed] [Google Scholar]
- 62.Ovchinnikova O, Robertson AK, Wagsater D, et al. T-cell activation leads to reduced collagen maturation in atherosclerotic plaques of apoe(−/−) mice. Am J Pathol. 2009;174:693–700. doi: 10.2353/ajpath.2009.080561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klingenberg R, Gerdes N, Badeau RM, et al. Depletion of foxp3+ regulatory t cells promotes hypercholesterolemia and atherosclerosis. J Clin Invest. 2013;123:1323–1334. doi: 10.1172/JCI63891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.MacLeod AS, Hemmers S, Garijo O, et al. Dendritic epidermal t cells regulate skin antimicrobial barrier function. J Clin Invest. 2013;123:4364–4374. doi: 10.1172/JCI70064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gistera A, Robertson AK, Andersson J, et al. Transforming growth factor-beta signaling in t cells promotes stabilization of atherosclerotic plaques through an interleukin-17-dependent pathway. Sci Transl Med. 2013;5:196ra100. doi: 10.1126/scitranslmed.3006133. [DOI] [PubMed] [Google Scholar]
- 66.Mach F, Schonbeck U, Bonnefoy JY, Pober JS, Libby P. Activation of monocyte/macrophage functions related to acute atheroma complication by ligation of cd40: induction of collagenase, stromelysin, and tissue factor. Circulation. 1997;96:396–399. doi: 10.1161/01.cir.96.2.396. [DOI] [PubMed] [Google Scholar]
- 67.Henn V, Slupsky JR, Gräfe M, et al. Cd40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 68.Mach F, Schonbeck U, Sukhova GK, et al. Functional cd40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages – implications for cd40-cd40 ligand signaling in atherosclerosis. Proc Natl Acad Sci U S A. 1997;94:1931–1936. doi: 10.1073/pnas.94.5.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lievens D, Zernecke A, Seijkens T, et al. Platelet cd40 l mediates thrombotic and inflammatory processes in atherosclerosis. Blood. 2010;116:4317–4327. doi: 10.1182/blood-2010-01-261206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf D, Hohmann JD, Wiedemann A, et al. Binding of cd40 l to mac-1’s i-domain involves the eqlkksktl motif and mediates leukocyte recruitment and atherosclerosis–but does not affect immunity and thrombosis in mice. Circ Res. 2011;109:1269–1279. doi: 10.1161/CIRCRESAHA.111.247684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samuelsson B. Role of basic science in the development of new medicines: examples from the eicosanoid field. J Biol Chem. 2012;287:10070–10080. doi: 10.1074/jbc.X112.351437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gabrielsen A, Qiu H, Back M, et al. Thromboxane synthase expression and thromboxane a2 production in the atherosclerotic lesion. J Mol Med. 2010;88:795–806. doi: 10.1007/s00109-010-0621-6. [DOI] [PubMed] [Google Scholar]
- 73.MacKenzie KF, Clark K, Naqvi S, et al. Pge(2) induces macrophage il-10 production and a regulatory-like phenotype via a protein kinase a-sik-crtc3 pathway. J Immunol. 2013;190:565–77. doi: 10.4049/jimmunol.1202462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Back M, Bu DX, Branstrom R, Sheikine Y, Yan ZQ, Hansson GK. Leukotriene b4 signaling through nf-kappab-dependent blt1 receptors on vascular smooth muscle cells in atherosclerosis and intimal hyperplasia. Proc Natl Acad Sci U S A. 2005;102:17501–17506. doi: 10.1073/pnas.0505845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qiu H, Gabrielsen A, Agardh HE, et al. Expression of 5-lipoxygenase and leukotriene a4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci U S A. 2006;103:8161–8166. doi: 10.1073/pnas.0602414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heller EA, Liu E, Tager AM, et al. Inhibition of atherogenesis in blt1-deficient mice reveals a role for ltb4 and blt1 in smooth muscle cell recruitment. Circulation. 2005;112:578–586. doi: 10.1161/CIRCULATIONAHA.105.545616. [DOI] [PubMed] [Google Scholar]
- 77.Back M, Sultan A, Ovchinnikova O, Hansson GK. 5-lipoxygenase-activating protein: a potential link between innate and adaptive immunity in atherosclerosis and adipose tissue inflammation. Circ Res. 2007;100:946–949. doi: 10.1161/01.RES.0000264498.60702.0d. [DOI] [PubMed] [Google Scholar]
- 78.Helgadottir A, Manolescu A, Thorleifsson G, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 79.Ingelsson E, Yin L, Back M. Nationwide cohort study of the leukotriene receptor antagonist montelukast and incident or recurrent cardiovascular disease. J Allergy Clin Immunol. 2012;129:702–707. e702. doi: 10.1016/j.jaci.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 80.Merched AJ, Ko K, Gotlinger KH, Serhan CN, Chan L. Atherosclerosis: evidence for impairment of resolution of vascular inflammation governed by specific lipid mediators. FASEB J. 2008;22:3595–3606. doi: 10.1096/fj.08-112201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petri MH, Laguna-Fernandez A, Gonzalez-Diez M, Paulsson-Berne G, Hansson GK, Back M. The role of the fpr2/alx receptor in atherosclerosis development and plaque stability. Cardiovasc Res. 2015;105:65–74. doi: 10.1093/cvr/cvu224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Libby P, Egan D, Skarlatos S. Roles of infectious agents in atherosclerosis and restenosis. An assessment of the evidence and need for future research. Circulation. 1997;96:4095–4103. doi: 10.1161/01.cir.96.11.4095. [DOI] [PubMed] [Google Scholar]
- 83.Caligiuri G, Lévy B, Pernow J, Thorén P, Hansson GK. Myocardial infarction mediated by endothelin receptor signaling in hypercholesterolemic mice. Proc Natl Acad Sci U S A. 1999;96:6920–6924. doi: 10.1073/pnas.96.12.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Johnson JL, Jackson CL. Atherosclerotic plaque rupture in the apolipoprotein e knockout mouse. Atherosclerosis. 2001;154:399–406. doi: 10.1016/s0021-9150(00)00515-3. [DOI] [PubMed] [Google Scholar]
- 85.Schwartz SM, Galis ZS, Rosenfeld ME, Falk E. Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol. 2007;27:705–713. doi: 10.1161/01.ATV.0000261709.34878.20. [DOI] [PubMed] [Google Scholar]
- 86.Matoba T, Sato K, Egashira K. Mouse models of plaque rupture. Curr Opin Lipidol. 2013;24:419–425. doi: 10.1097/MOL.0b013e3283646e4d. [DOI] [PubMed] [Google Scholar]
- 87.Katsuki S, Matoba T, Nakashiro S, et al. Nanoparticle-mediated delivery of pitavastatin inhibits atherosclerotic plaque destabilization/rupture in mice by regulating the recruitment of inflammatory monocytes. Circulation. 2014;129:896–906. doi: 10.1161/CIRCULATIONAHA.113.002870. [DOI] [PubMed] [Google Scholar]
- 88.Sasaki T, Kuzuya M, Nakamura K, et al. A simple method of plaque rupture induction in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1304–1309. doi: 10.1161/01.ATV.0000219687.71607.f7. [DOI] [PubMed] [Google Scholar]
- 89.Chen YC, Bui AV, Diesch J, et al. A novel mouse model of atherosclerotic plaque instability for drug testing and mechanistic/therapeutic discoveries using gene and microrna expression profiling. Circ Res. 2013;113:252–265. doi: 10.1161/CIRCRESAHA.113.301562. [DOI] [PubMed] [Google Scholar]
- 90.Van der Donckt C, Van Herck JL, Schrijvers DM, et al. Elastin fragmentation in atherosclerotic mice leads to intraplaque neovascularization, plaque rupture, myocardial infarction, stroke, and sudden death. Eur Heart J. 2015;36:1049–1058. doi: 10.1093/eurheartj/ehu041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Williams H, Johnson JL, Carson KG, Jackson CL. Characteristics of intact and ruptured atherosclerotic plaques in brachiocephalic arteries of apolipoprotein e knockout mice. Arterioscler Thromb Vasc Biol. 2002;22:788–792. doi: 10.1161/01.atv.0000014587.66321.b4. [DOI] [PubMed] [Google Scholar]
- 92.Quillard T, Araujo HA, Franck G, Shvartz E, Sukhova G, Libby P. Tlr2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 94.Carroll D, Ebrahim S, Tilling K, Macleod J, Smith GD. Admissions for myocardial infarction and world cup football: database survey. BMJ. 2002;325:1439–1442. doi: 10.1136/bmj.325.7378.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leeka J, Schwartz BG, Kloner RA. Sporting events affect spectators’ cardiovascular mortality: it is not just a game. Am J Med. 2010;123:972–977. doi: 10.1016/j.amjmed.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 96.Shiomi M, Ishida T, Kobayashi T, et al. Vasospasm of atherosclerotic coronary arteries precipitates acute ischemic myocardial damage in myocardial infarction-prone strain of the Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb Vasc Biol. 2013;33:2518–2523. doi: 10.1161/ATVBAHA.113.301303. [DOI] [PMC free article] [PubMed] [Google Scholar]