Abstract
Background
We have previously shown increased resting-state functional connectivity (rsFC) in the frontoparietal network (FPN) and the default mode network (DMN) in patients with acute anorexia nervosa. Based on these findings we investigated within-network rsFC in patients recovered from anorexia nervosa to examine whether these abnormalities are a state or trait marker of the disease. To extend the understanding of functional connectivity in patients with anorexia nervosa, we also estimated rsFC between large-scale networks.
Methods
Girls and women recovered from anorexia nervosa and pair-wise, age- and sex-matched healthy controls underwent a resting-state fMRI scan. Using independent component analyses (ICA), we isolated the FPN, DMN and salience network. We used standard comparisons as well as a hypothesis-based approach to test the findings of our previous rsFC study in this recovered cohort. Temporal correlations between network time-course pairs were computed to investigate functional network connectivity (FNC).
Results
Thirty-one patients recovered from anorexia nervosa and 31 controls participated in our study. Standard group comparisons revealed reduced rsFC between the dorsolateral prefrontal cortex (dlPFC) and the FPN in the recovered group. Using a hypothesis-based approach we extended the previous finding of increased rsFC between the angular gyrus and the FPN in patients recovered from anorexia nervosa. No group differences in FNC were revealed.
Limitations
The study design did not allow us to conclude that the difference found in rsFC constitutes a scar effect of the disease.
Conclusion
This study suggests that some abnormal rsFC patterns found in patients recovered from anorexia nervosa normalize after long-term weight restoration, while distorted rsFC in the FPN, a network that has been associated with cognitive control, may constitute a trait marker of the disorder.
Introduction
Anorexia nervosa is a severe psychiatric disorder hallmarked by extreme weight loss (or failure to gain weight during growth) due to relentless control of food intake and has been associated with structural1 as well as functional brain alterations.2 Structural neuroimaging studies have reported grey matter atrophy in acutely ill patients to be largely reversible in weight-restored patients. On the level of brain functioning, abnormal neural responses in the lateral–frontal brain circuitry have been associated with excessive cognitive control in acutely ill as well as recovered patients.5–7
Despite these findings, we still lack understanding of the neural mechanisms underlying anorexia nervosa. One reason might be that the mainstay of studies using fMRI has been the identification of associations between specific brain regions with disorder-related dysfunction. However, this approach of mapping dysfunctions to individual brain areas has recently been challenged by a network perspective. It has been argued that aberrant functional connectivity (FC) of widespread brain regions seems more appropriate in explaining heterogeneous phenomenona, such as psychiatric disorders.8–10
Investigation of functional connectivity usually involves identifying temporal correlations in blood-oxygen level– dependent (BOLD) signal between spatially distinct brain areas.11 This can be applied to fMRI data acquired during tasks or during rest. The latter method, referred to as resting-state functional connectivity (rsFC), is particularly suitable for clinical application, as it requires little compliance from the participant12 and scanning times are relatively short (5–10 min). During a typical resting-state scan, participants are asked to lie still with closed eyes or to fixate on a crosshair.
Seed-based analysis is a widely used approach that involves selecting a specific brain region and evaluating its correlation with time course in other voxels. Beside this, independent component analysis (ICA) is one of the most common techniques used to analyze rsFC data.13 In contrast to the seed-based approach, it does not require strict a priori hypotheses regarding regions of interest and allows the detection and separation of multiple resting-state networks (RSN) at once. Independent component analysis uses a mathematical algorithm to separate the fMRI data set into maximally spatially independent components. However, the identified components do exhibit temporal correlation, which offers another valuable perspective.16 The identified RSNs have been found to be highly reliable across individuals and cover functional properties (e.g., motoric, salience detection) despite the absence of a task.17
Previous studies investigating rsFC in patients with anorexia nervosa have identified abnormalities in several large-scale RSNs. Our own work, in which we used the ICA approach,18 provided evidence for increased rsFC between the angular gyrus and the frontoparietal network (FPN) in acutely ill patients that was also associated with self-reported persistence, a personality dimension strongly pronounced in this group and associated with cognitive control. This finding is in line with a neurobiological framework proposed by Kaye and colleagues2 suggesting that enhanced executive functions mediated by pre-frontal brain areas play an important role in the etiology of anorexia nervosa. Furthermore we reported increased rsFC strength between the default mode network (DMN) and the anterior insula associated with self-reported problems of interoceptive awareness,18 a typical symptom of anorexia nervosa that is closely related to body image distortion. These findings are in line with those of Lee and colleagues,19 who also showed increased rsFC in the FPN and the DMN in acutely ill patients using a seed-based approach, whereas no difference in rsFC of the FPN was reported by Favaro and colleagues.20
Even though only large longitudinal studies can provide an ultimate answer, studying patients recovered from anorexia nervosa may help to disentangle whether aberrant rsFC in individuals with this disorder is a state marker caused by severe undernutrition or whether it constitutes a biological vulnerability (trait marker) to the disease. Previous work has suggested that alterations of rsFC are linked to reduced grey matter thickness in patients with depression.21 Therefore, we expected abnormal rsFC to recover after normalization of grey matter structure. To date, only 4 studies with limited sample sizes have investigated rsFC in patients recovered from anorexia nervosa, and results have been heterogeneous. Cowdrey and colleagues22 reported increased rsFC in the DMN but no differences in the FPN, the visual or somatosensory networks. In contrast, reduced rsFC in the visual network was reported by Favaro and colleagues,23 while McFadden and colleagues24 showed reduced rsFC strength in the salience network and sensorimotor network (the latter data were obtained during a task that may impact RSNs25). Using a seed-based approach, Favaro and colleagues26 reported that the observed alterations in rsFC of the striatal network in acutely ill patients vanished with recovery. To gain insight into whether aberrant rsFC is a state or trait marker of anorexia nervosa, further studies with larger samples of patients recovered from the disorder are needed.
Another neglected aspect in previous studies focusing on rsFC in patients with anorexia nervosa is that abnormal FC can occur on different hierarchical scales.27 One approach to investigate FC on a superordinate scale is functional network connectivity (FNC). This approach identifies interconnections between large-scale networks and captures weaker, but existing temporal correlations between the components identified by ICA.16 This method elucidates abnormal functional integration on a macroscopic level, as described by the triple network model of psychopathology.8 This model suggests that the insula as a central hub of the salience network mediates the switch between the DMN as a network involved in internally oriented mental states to the FPN, which is responsible for externally oriented attention as a response to salient stimuli.28 Alterations in this process are thought to contribute to dysfunction underlying psychiatric disorders.8 To date, evidence for changes in FNC in patients with psychiatric disorders is sparse. Jafri and colleagues16 could show increased FNC among all dominant RSNs in patients with schizophrenia, while von dem Hagen and colleagues29 demonstrated reduced FNC in patients with autism-spectrum disorders. Yet, to our knowledge, no studies have investigated FNC in patients with anorexia nervosa. However, our previous study investigating within-network rsFC in acutely ill patients with anorexia nervosa suggested a strong coupling between the DMN and the salience network manifested in an assignment of the anterior insula to the DMN in patients with the disorder. This coupling may indicate an altered interaction among the salience network, DMN and FPN in patients with anorexia nervosa, as proposed by the triple network model.
The aims of the present study were 2-fold. First, we compared within-network rsFC between patients recovered from anorexia nervosa and healthy controls to test whether abnormal FPN and DMN rsFC, as found in acutely ill patients, is detectable after recovery and can thus be considered a trait marker of the disorder. To probe effects of recovery, we used a targeted follow-up approach regarding the brain regions that showed group differences in our previous study. We also included the salience network in our analysis, as the anterior insula, a brain region that is often assigned to the salience network,30 showed group differences in our previous study. Second, we sought to examine FNC among the 3 networks of the triple network model of psychopathology.
Methods
Participants
We recruited girls and women recovered from anorexia nervosa and pairwise, age- and sex-matched controls for participation in this study. To be considered recovered, they had to have a diagnosis of anorexia nervosa and to have maintained a body mass index (BMI) above 18.5 (if age > 18 yr) or a BMI above the tenth age percentile (if age < 18 yr) for at least 6 months before the study; have regular menses; and have not binged, purged, or engaged in substantial restrictive eating patterns. Control participants had to have a healthy weight, be eumenorrhoeic and and have no history of psychiatric illness. Additional exclusion criteria for each group are outlined in Appendix 1, available at jpn.ca. Most importantly, we excluded individuals who were taking psychotropic medication within 6 weeks before the study and who had a diagnosis of bulimia nervosa, substance abuse, neurologic, or medical conditions.
This study was approved by our local institutional review board, and all participants or the guardians of underage participants gave written informed consent.
Clinical measures
To ascertain the absence of a current diagnosis of eating disorders the expert form SIAB-EX31 was conducted with all participants. Eating disorder–specific psychopathology, including interoceptive awareness, was assessed using the short version of the Eating Disorders Inventory-2 (EDI-232). We assessed the persistence personality dimension using the German version of the Junior Temperament and Character Inventory (JTCI33). Anxiety and depression were measured using the Symptom Check-List-90R (SCL-90-R34), and IQ was measured with a short version of the German adaption of the Wechsler Adult Intelligence Scale (WIE35) or with a short version of the German adaption of the Wechsler Intelligence Scale for Children (HAWIK36; Appendix 1).
Instrumental target reaction task
Participants performed a simple instrumental target reaction task as a measure of goal-directed behaviour to receive monetary rewards depending on their performance. Instrumental responding was defined by reaction time and by number of button presses in response to a target (Appendix 1).
Data acquisition
Data were acquired with a 3 T Siemens Trio scanner. The T1-weighted structural brain scans were acquired using a rapid acquisition gradient echo (MP-RAGE) sequence with the parameters described in Appendix 1.
An 8-min resting fMRI scan was acquired using a gradient-echo T2*-weighted echo planar imaging (EPI) sequence using standard parameters (Appendix 1). During fMRI, participants were instructed to lie still with their eyes closed and without falling asleep.
Image data preprocessing
Functional and structural images were processed using SPM8 (www.fil.ion.ucl.ac.uk/spm/) within the Nipype framework (http://nipy.sourceforge.net/nipype)37 following standard procedures (Appendix 1).
We evaluated the quality of the fMRI data by manual inspection and using artifact detection tools (ART38).
Cortical thickness measurement
The T1-weighted images were registered, motion-corrected, realigned, averaged and analyzed using the FreeSurfer software suite (http://surfer.nmr.mgh.harvard.edu) version 5.1.0. Cortical thickness was measured using standard Free-Surfer procedures (Appendix 1). We also extracted averaged thickness measurements for each participant from each of the respective labels of the Destrieux cortical atlas39 in each hemisphere according to the regions identified to show aberrant rsFC.
Independent component analysis
To identify temporally coherent RSNs, we conducted a spatial group ICA40 for all participants using the Group ICA fMRI Toolbox (GIFT) implemented in MATLAB (http://mialab.mrn.org/software/gift). The fMRI data were decomposed into maximally independent components according to the following steps. The number of components were estimated using modified minimum description length criteria,41 then we applied the infomax algorithm to the data.42 For each participant, we back-reconstructed component spatial maps using group independent component analysis (GICA) and converted them to z values.43
Component selection
To identify components of interest for further analysis, we applied a systematic 2-step process by correlating components with white matter and cerebral spinal fluid templates as well as RSN templates created by Yeo and colleagues44 The evaluation was confirmed by investigating spectral metrics (Appendix 1).
Analyses of independent components
For group analyses, spatial maps of the back-reconstructed components representing the networks of interest for each participant were entered into SPM8. The z values of these maps represent the concordance of the voxel-specific time-course to the averaged components time-course. The analysis of rsFC comprised 2 steps. First, we performed a 2-sample t test for each preselected component, masked with the aforementioned RSN templates.44 Between-group differences had to exceed p < 0.05, family-wise error (FWE)–corrected, to guard against type I errors. Second, to follow up the previous finding of increased rsFC in acutely ill patients in comparison to healthy controls in the FPN and DMN,18 we examined the contrast “recovered patients > healthy controls” by applying a mask (Appendix 1) that corresponds to the results of our previous study in acutely ill patients (angular gyrus, anterior insula).
Additional analyses
To further assess the association of the magnitude of group differences in rsFC with psychometric parameters, we extracted the b values of the respective clusters at a threshold of p < 0.001, uncorrected, using MarsBaR45 and computed the Pearson r correlation using SPSS statistical software version 21.0 (SPSS) for each diagnostic group separately and for both groups combined. Based on our previous work,18 we used a hypothesis-driven approach and planned to test the association between interoceptive awareness (EDI-2) and possible clusters showing group differences within the DMN as well as between persistence (JTCI) and the clusters of the FPN. To demonstrate external validity of the hypothesized function of the FPN, we investigated the association of instrumental responding and the magnitude of rsFC of the FPN. To address possible developmental effects, we tested for associations between age and rsFC using the same approach.
Additionally, we investigated the association between the cortical thickness measures and rsFC using partial correlation, with age as a covariate.
Functional network connectivity
The assessment of the FNC, defined by the temporal correlation between the spatially independent components, was conducted with the FNC toolbox (http://mialab.mrn.org/software/fnc). This toolbox computes the maximum lagged correlation, as described by Jafri and colleagues.16 First, the time courses of the respective components are filtered with cut-off frequencies below 0.017Hz and above 0.32Hz and interpolated to allow the detection of hemodynamic latencies below scanner repetition time.46 Next, the maximum lagged correlation is computed for all possible pairwise combinations of component time-courses according to the formula reported in Appendix 1.
Correlation combinations that exceeded a significance level of p < 0.05, false discovery rate (FDR)–corrected, and their corresponding lag values were separately extracted for both groups. Differences in FNC between recovered patients and healthy controls were tested using a 2-sample t test (p < 0.05, FDR-corrected).
Results
Participants
The study sample consisted of 62 female volunteers: 31 patients recovered from anorexia nervosa and 31 pairwise age- and sex-matched controls. There were no differences in age, body mass index standard deviation score (BMI SDS) and IQ between recovered patients and healthy controls. Recovered patients still had some residual eating disorder symptoms. The demographic and clinical characteristics of participants are described in Table 1.
Table 1.
Demographic and clinical characteristics of study participants
| Group; mean ± SD | ||||
|---|---|---|---|---|
|
|
||||
| Characteristic | Recovered* n = 31 |
Control n = 31 |
t | p value |
| Age, yr | 22.27 ± 3.08 | 21.73 ± 2.99 | −0.70 | 0.49 |
| BMI† | 20.69 ± 1.62 | 21.37 ± 2.10 | 1.42 | 0.16 |
| BMI SDS | −0.54 ± 0.55 | −0.29 ± 0.64 | 1.60 | 0.11 |
| IQ‡ | 108.52 ± 9.74 | 110.67 ± 8.80 | 0.91 | 0.37 |
| Age at onset, yr | 14.38 ± 1.93 | NA | — | — |
| Duration of recovery, mo | 53.24 ± 33.27 | NA | — | — |
| Illness duration, mo§ | 44.63 ± 31.93 | NA | — | — |
| EDI–2 total score | 162.05 ± 44.22 | 131.92 ± 25.89 | −3.26 | 0.002 |
| SCL–90–R, depression | 6.93 ± 7.41 | 5.17 ± 8.15 | −0.88 | 0.38 |
| SCL–90–R, anxiety | 3.97 ± 4.50 | 2.9 ± 6.32 | −0.75 | 0.45 |
BMI = body mass index; BMI SDS = body mass index standard deviation score; EDI-2 = Eating Disorders Inventory-2; NA = not applicable; SCL-90-R = Symptom Check-List-90R; SD = standard deviation.
One participant in the sample had been recovered for 9 mo, all other participants were recovered for at least 2 mo.
BMI is displayed but statistical comparisons are based on BMI-SDS values to ensure comparability across age.
IQ was assessed with a short version of the German adaption of the Wechsler Adult Intelligence Scale35 or a short version of the German adaption of the Wechsler Intelligence Scale for Children36 for participants aged 15 yr or younger.
Reported illness duration comprises the total time period from onset of the first episode until recovery following the last episode.
Component identification
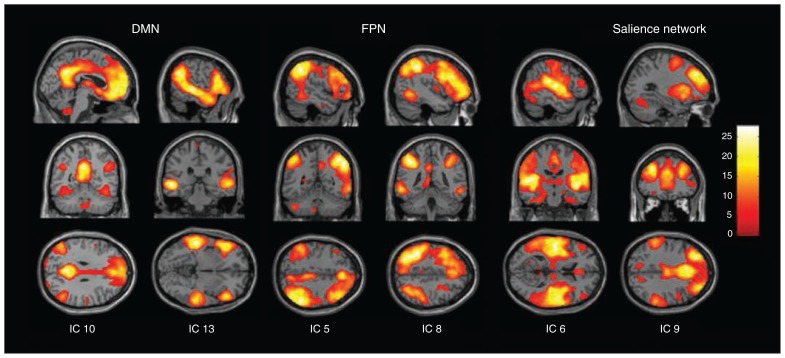
The dimension estimation revealed 23.76 ± 5.32 dimensions on average. The initial data reduction using principal components analysis (PCA) yielded 36 dimensions that were again reduced to 24 independent components using ICA. Six of these components were identified as RSNs of interest (Fig. 1).
Fig. 1.
Spatial maps of 6 independent components (IC) of interest grouped by network: frontoparietal network (FPN), default mode network (DMN) and salience network. Spatial maps are plotted as t statistics thresholded at p < 0.05, family-wise error–corrected.
Group comparison of independent components
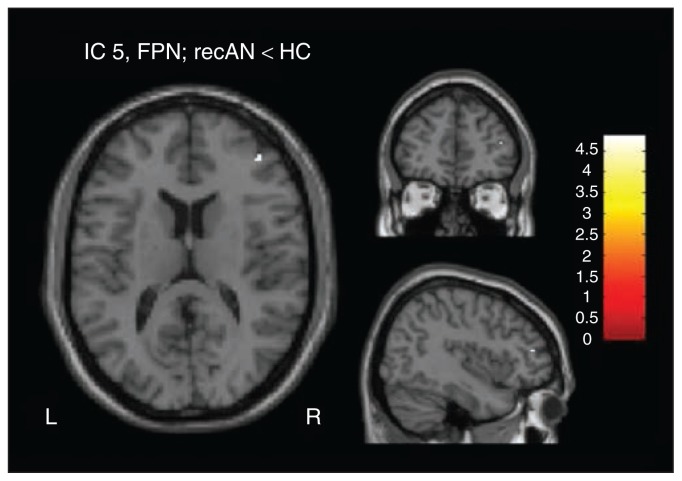
We found reduced functional connectivity between the right dorsolateral prefrontal cortex (dlPFC) and component 5, associated with FPN (tpeak = 4.87, p = 0.023, FWE-corrected) in recovered patients compared with healthy controls (Fig. 2). We did not observe any further group differences (below the threshold of p < 0.05, FWE-corrected) in the remaining 5 components.
Fig. 2.
Group difference at a threshold of p < 0.05, family-wise error–corrected, between patients recovered from anorexia nervosa (recAN) and healthy controls (HC) in component 5, representing the frontoparietal network (FPN; peak coordinates in Montreal Neurological Institute space: x, y, z = 42, 46, 14).
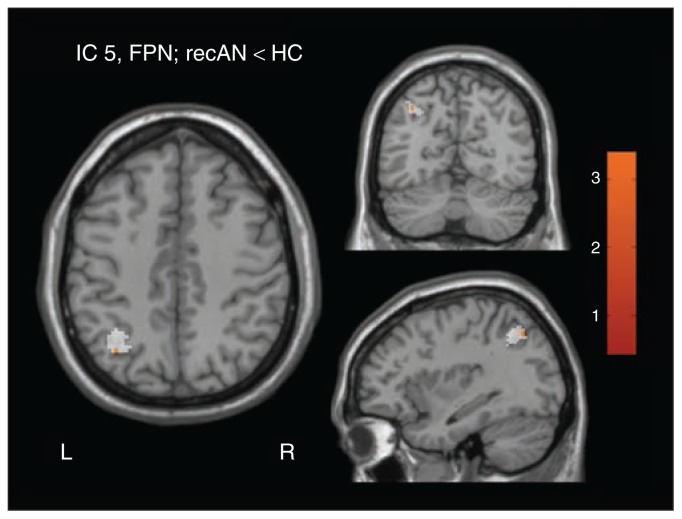
Using our targeted follow-up approach, we found increased rsFC of the left angular gyrus with component 5 associated with the FPN (tpeak = 3.16, p = 0.05, FWE-corrected, in recovered patients > healthy controls; Appendix 1 and Fig. 3). No increased rsFC was found between the left angular gyrus and component 8. Regarding the DMN, no increased rsFC between the anterior insula and component 10 or 13 was observed.
Fig. 3.
Targeted follow-up approach: group difference at a threshold of p = 0.05, familiy-wise error–corrected, between patients recovered from anorexia nervosa (recAN) and healthy controls (HC) in component 5, representing the frontoparietal network (FPN; peak coordinates in Montreal Neurological Institute space: x, y, z = 36, −64, 44). Applied mask covering the previous finding at angular gyrus (AG) is displayed in white.
Additional analyses
Since acutely ill patients are believed to exert excessive cognitive control that may be reflected in the persistence personality dimension, we tested this association in the FPN clusters, as described in our previous study.18 We found no association between persistence and reduced rsFC in the right dlPFC or increased rsFC in the angular gyrus in either of the groups or in both groups combined. To validate the function of the FPN, we tested the association between rsFC of the FPN and performance on an instrumental responding task. There was an association between the instrumental responding parameters, number of button presses and reaction time and increased rsFC between the angular gyrus and the rest of the FPN (component 5) in both groups combined (reaction time was also associated with rsFC in the angular gyrus within recovered patients; Table 2).
Table 2.
Correlations between the magnitude of the cluster-specific rsFC and psychometric parameters, cortical thickness, age, duration of illness, anxiety and depression for both groups and each group separately
| rsFC at dlPFC | rsFC at AG | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Analysis | Recovered | Control | Both | Recovered | Control | Both |
| Confirmatory analyses | ||||||
| Persistence | r = 0.35 | r = −0.03 | r = −0.05 | r = −0.07 | r = 0.27 | r = 0.20 |
| p = 0.06 | p = 0.86 | p = 0.72 | p = 0.68 | p = 0.14 | p = 0.12 | |
| Instrumental responding: #bp | r = 0.41 | r = 0.04 | r = 0.11 | r = 0.41 | r = 0.40 | r = 0.40 |
| p = 0.03 | p = 0.81 | p = 0.41 | p = 0.03 | p = 0.03 | p = 0.002† | |
| Instrumental responding: RT | r = −0.27 | r = −0.17 | r = −0.17 | r = −0.56 | r = −0.32 | r = −0.38 |
| p = 0.14 | p = 0.37 | p = 0.20 | p = 0.002† | p = 0.08 | p = 0.003† | |
| Exploratory analyses | ||||||
| Age | r = 0.05 | r = −0.12 | r = −0.09 | r = −0.04 | r = −0.05 | r = −0.001 |
| p = 0.78 | p = 0.54 | p = 0.51 | p = 0.85 | p = 0.79 | p = 0.99 | |
| Depression | r = 0.09 | r = −0.10 | r = −0.08 | r = −0.01 | r = 0.20 | r = 0.13 |
| p = 0.63 | p = 0.60 | p = 0.52 | p = 0.95 | p = 0.29 | p = 0.316 | |
| Anxiety | r = 0.03 | r = −0.05 | r = −0.07 | r = −0.09 | r = 0.24 | r = 0.12 |
| p = 0.89 | p = 0.80 | p = 0.58 | p = 0.63 | p = 0.21 | p = 0.35 | |
| Illness duration | r = −0.13 | — | — | r = 0.21 | — | — |
| p = 0.55 | p = 0.31 | |||||
| Cortical thickness* | r = 0.04 | r = 0.11 | r = 0.01 | r = −0.25 | r = 0.06 | r = −0.04 |
| p = 0.82 | p = 0.56 | p = 0.94 | p = 0.18 | p = 0.76 | p = 0.76 | |
AG = angular gyrus; dlPFC = dorsolateral prefrontal cortex; rsFC = resting-state functional connectivity; RT = reaction time.
A partial correlation with age as covariate was calculated for associations between rsFC and cortical thickness.
Significant when adjusting for multiple testing using the Bonferroni method.
Age was not associated with any finding of aberrant rsFC reported above. The magnitude of rsFC in brain regions showing group differences for FPN connectivity (dlPFC, angular gyrus) was neither associated with cortical thickness of the right dlPFC (middle frontal gyrus, Destrieux atlas) nor the left angular gyrus (superior parietal gyrus, Destrieux atlas). For means with standard deviations and statistics of group differences of persistence, instrumental responding parameters and cortical thickness measures, see Appendix 1.
Functional network connectivity
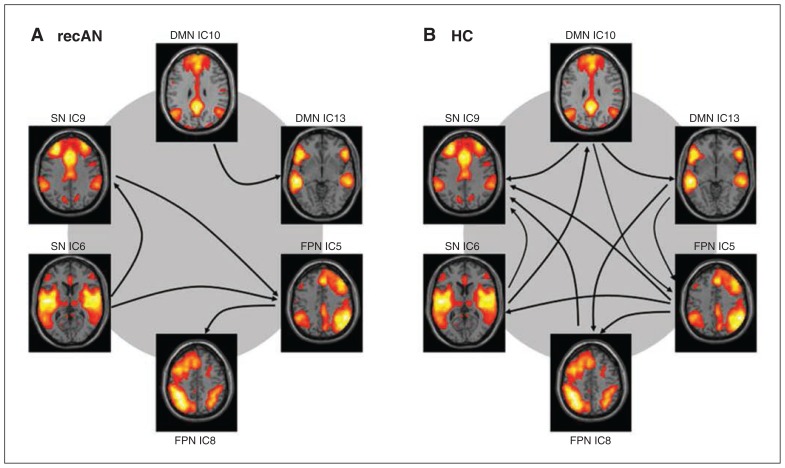
As expected, components identified to cover the same network were found to correlate significantly in both groups (all p < 0.048, FDR-corrected). In healthy controls we found significant FNC among the components of the DMN, the FPN and salience network. In recovered patients, only components of the FPN and the salience network significantly correlated; we found no correlation with the components of the DMN (Fig. 4). However, the group comparison of FNC revealed no difference between recovered patients and healthy controls (all p > 0.08, FDR-corrected).
Fig. 4.
Functional network connectivity in (A) patients recovered from anorexia nervosa (recAN) and (B) healthy controls (HC). Arrows show the significant correlation between resting-state networks. The direction of the arrow corresponds with the direction of the delay between 2 components. DMN = default mode network; FPN = frontoparietal network; IC = independent component; SN = salience network.
Discussion
The aim of this study was to investigate within- and between-network rsFC (or FNC) in patients recovered from anorexia nervosa compared with healthy controls. We found differences in within-network rsFC in the FPN. Specifically, our exploratory approach revealed reduced rsFC between the dlPFC and the FPN in recovered patients, while the targeted follow-up approach is in line with our previous finding (in acutely ill patients) of increased rsFC between the angular gyrus and the remaining parts of the FPN. Aberrant coupling between the anterior insula and the DMN, as found in acutely ill patients, was not detected. Regarding FNC, we found no group differences in interactions among the DMN, FPN and salience network.
Studying within- and between-network rsFC in patients recovered from anorexia nervosa enabled us to probe whether rsFC characteristics are trait or state markers of the disease. In line with the results of McFadden and colleagues,24 we found that aberrant rsFC in the DMN seems to normalize with recovery and therefore constitutes a state marker of the disease. In contrast, Cowdrey and colleagues22 reported increased rsFC in the DMN for recovered patients, but no difference in the FPN. Reasons for these discrepant findings may include the fact that ICA was performed independently in each group, which results in group-specific components, as well as heterogeneity in the population of patients with anorexia nervosa.
Likewise, task-based studies in patients with anorexia nervosa also indicate that some neural signatures persist into recovery, although discrepancies exist. Some studies investigating food cue reactivity in acutely ill and recovered patients found that increased neural activity in regions involved in the food motivation brain circuit (e.g., amygdala, hypothalamus, orbitofrontal cortex [OFC], insula) reflect trait vulnerability of the illness, while other studies found reduced neural activity in the insula in recovered patients. We in turn found no altered rsFC in the anterior insula (part of the DMN) or salience network in recovered patients. However, in line with our finding of altered rsFC of the FPN in recovered patients, task-related studies, such as the one by Sanders and colleagues,51 reported increased activity in the middle frontal gyrus in response to food cues during the acute as well as the recovered state of anorexia nervosa. Abnormal responses of frontoparietal brain regions in recovered patients, potentially indicative of trait-like characteristics, were also reported in studies using monetary reward tasks. An earlier study by our group5 found elevated activation of the dlPFC during anticipation of monetary rewards, failure to deactivate this region when feedback about the reward was presented and increased task-related connectivity between the dlPFC and OFC. Similarly, Wagner and colleagues7 reported that recovered patients showed an increased involvement of prefrontal and parietal cortices in response to monetary rewards.
A large body of research has shown that the FPN, including the lateral prefrontal cortex and the intraparietal lobule,44 are involved in executive functions, including attention allocation, working memory, performance monitoring and planning.52–55 Robust associations between rsFC in the FPN component identified here and instrumental responding underscore the importance of this network for cognitive control processes. Based on a cross-studies analysis of mixed blocked/event-related fMRI data as well as rsFC data, Dosenbach and colleagues56 argued that the FPN combines brain regions that initiate attentional control in response to performance feedback to adjust control settings. It has been suggested that parietal FPN regions, based on a detected conflict related to the stimulus, may signal the need to adjust processing priorities to the dlPFC, which in turn implements adaptive mechanisms.56 In this regard our results of increased rsFC in the angular gyrus and reduced rsFC in the dlPFC may reflect inefficient information transfer between these 2 major FPN hubs, which could lead to altered adaptive cognitive control processes.
In recent years, the notion of increased cognitive control underlying anorexia nervosa has gained a lot of interest. It was initially based on clinical observation of perseverative, obsessive and perfectionistic thinking styles in patients with this disorder.57 Scientific evidence stems from neuropsychological studies showing reduced cognitive flexibility and excessively detailed information processing and from task-based studies indicating alterations in frontal and parietal brain regions associated with executive functions in patients with anorexia nervosa. As reported previously, studies investigating neural response to monetary rewards have reported an increased involvement of prefrontal and parietal areas indicating a “strategic” response to rewards. The interpretation of our results in light of the aforementioned findings by Dosenbach and colleagues56 is in line with a study by Sato and colleagues60 reporting lower activation in lateral frontal brain regions in patients with anorexia nervosa when feedback about set shifting performance is presented in a Wisconsin Card Sorting Test. However, Zastrow and colleagues6 found evidence for greater neural response in the dlPFC and less temporoparietal activation in acutely ill patients during set shifting.
Taken together, the results of some task-based studies and the present study are in line with the hypothesis of anorexia nervosa as a disorder characterized by altered cognitive control processes underpinned by dysconnectivity within the FPN, which seems to persist into recovery, supporting the recently highlighted role of the FPN in psychiatric disorders.10 Nonetheless, conclusions from rsFC data with respect to task-related interpretations remain somewhat speculative. Alterations observed in the FPN could be interpreted as heightened cognitive control in patients with anorexia nervosa, as inefficient cognitive control mechanisms, or even as independent from any control processes.61 However, previous work has been able to demonstrate a direct link between rsFC and task-related neural activity.62
Regarding the interaction among the RSNs, we found no alterations among the DMN, FPN and salience network in patients recovered from anorexia nervosa. Based on these findings, we suggest that altered integration of information across these networks, as suggested by the triple network theory, may not be considered a trait marker of the disorder. Given that the most important evidence of abnormal FNC originates from research in patients with schizophrenia,16 one might speculate whether only a few very marked psychiatric symptoms, such as hallucinations, are associated with changes in rsFC between networks.
Limitations
Our study has to be evaluated in the light of the following limitations. First, owing to age differences we compared results obtained in acutely ill and recovered patients only across studies, but we did not include them into a single statistical model. Second, studying recovered patients allowed us to exclude the effects of acute undernutrition, but the cross- sectional nature of the study does not allow us to determine whether the observed differences in rsFC constitute a true trait marker or a possible scar effect of the disease. It is also possible that patients who eventually recover belong to a different subgroup than chronic or relapsing-remitting patients. Future research applying a longitudinal study design would help to clarify whether those neural substrates are a consequence or a potential antecedent of pathologic eating behaviour. Strengths of our study include the large, homogeneous sample consisting of young unmedicated patients recovered from anorexia nervosa, who are consistently of the restrictive subtype, as well as the fact that all participants were scanned in the morning after an overnight fast.
Conclusion
Our study demonstrates that some abnormal rsFC patterns found in patients with anorexia nervosa persist even after long-term weight restoration. While abnormal rsFC within the DMN seems to normalize, the increased rsFC between the angular gyrus and the FPN, which might indicate alterations in cognitive control functions, are still present after recovery, and abnormalities regarding rsFC between the dlPFC and the FPN re-emerge. Moreover, we found no altered FNC between networks in patients recovered from anorexia nervosa. Studies evaluating the predictive potential of differences in rsFC for treatment outcome may help to pave the way for potential clinical applications of resting-state fMRI.
Acknowledgments
This work was supported by Deutsche Forsc-hungsgemeinschaft (367/5-1 & SFB 940) and Swiss Anorexia Nervosa Foundation grants to S. Ehrlich. The authors thank all members of the research team for their assistance and thank all participants for their cooperation.
Footnotes
Competing interests: V. Roessner has received payment for consulting and writing activities from Lilly, Novartis and Shire Pharmaceuticals; lecture honoraria from Lilly, Novartis, Shire Pharmaceuticals and Medice Pharma; and support for research from Shire and Novartis. He has carried out (and is currently carrying out) clinical trials in cooperation with Novartis, Shire and Otsuka. No other competing interests declared.
Contributors: S. Ehrlich designed the study. I. Boehm, D. Geisler, F. Tam, J. King, F. Ritschel. M. Seidel. F. Bernardoni and J. Murr acquired the data, which I. Boehm, D. Geisler, T. Goschke, V. Calhoun, V. Roessner and S. Ehrlich analyzed. I. Boehm, J. King and S. Ehrlich wrote the article, which all authors reviewed and approved for publication.
References
- 1.Seitz J, Bühren K, von Polier GG, et al. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. Z Kinder Jugendpsychiatr Psychother. 2014;42:7–17. doi: 10.1024/1422-4917/a000265. [DOI] [PubMed] [Google Scholar]
- 2.Kaye WH, Fudge J, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 3.Wagner A, Greer P, Bailer UF, et al. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biol Psychiatry. 2006;59:291–3. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 4.King JA, Geisler D, Ritschel F, et al. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biol Psychiatry. 2015;77:624. doi: 10.1016/j.biopsych.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Ehrlich S, Geisler D, Ritschel F, et al. Elevated cognitive control over reward processing in recovered patients with anorexia nervosa. J Psychiatry Neurosci. 2015;40:307–15. doi: 10.1503/jpn.140249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zastrow A, Kaiser S, Stippich C, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry. 2009;166:608–16. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- 7.Wagner A, Aizenstein H, Venkatraman V, et al. Altered reward processing in women recovered from anorexia nervosa. Am J Psychiatry. 2007;164:1842–9. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 8.Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Goschke T. Dysfunctions of decision-making and cognitive control as transdiagnostic mechanisms of mental disorders: advances, gaps, and needs in current research. Int J Methods Psychiatr Res. 2014;23(suppl):41–57. doi: 10.1002/mpr.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole MW, Repovš G, Anticevic A. The frontoparietal control system a central role in mental health. Neuroscientist. 2014;20:652–64. doi: 10.1177/1073858414525995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friston KJ, Frith C, Liddle P, et al. Functional connectivity: the principal-component analysis of large (PET) data sets. J Cereb Blood Flow Metab. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. [DOI] [PubMed] [Google Scholar]
- 12.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun VD, Adali T. Multisubject independent component analysis of fMRI: a decade of intrinsic networks, default mode, and neuro-diagnostic discovery. IEEE Rev Biomed Eng. 2012;5:60–73. doi: 10.1109/RBME.2012.2211076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beckmann CF, DeLuca M, Devlin JT, et al. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci. 2005;360:1001–13. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calhoun VD, Adali T, Hansen LK, et al. ICA of functional MRI data: an overview. 2003 [Google Scholar]
- 16.Jafri MJ, Pearlson GD, Stevens M, et al. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–34. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Boehm I, Geisler D, King JA, et al. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front Behav Neurosci. 2014;8:346. doi: 10.3389/fnbeh.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Ran Kim K, Ku J, et al. Resting-state synchrony between anterior cingulate cortex and precuneus relates to body shape concern in anorexia nervosa and bulimia nervosa. Psychiatry Res Neuroimaging. 2014;221:43–8. doi: 10.1016/j.pscychresns.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Favaro A, Clementi M, Manara R, et al. Catechol-O-methyltransferase genotype modifies executive functioning and prefrontal functional connectivity in women with anorexia nervosa. J Psychiatry Neurosci. 2013;38:241–8. doi: 10.1503/jpn.120068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Tol MJ, Li M, Metzger C, et al. Local cortical thinning links to resting-state disconnectivity in major depressive disorder. Psychol Med. 2014;44:2053–65. doi: 10.1017/S0033291713002742. [DOI] [PubMed] [Google Scholar]
- 22.Cowdrey FA, Filippini N, Park RJ, et al. Increased resting state functional connectivity in the default mode network in recovered anorexia nervosa. Hum Brain Mapp. 2014;35:483–91. doi: 10.1002/hbm.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favaro A, Santonastaso P, Manara R, et al. Disruption of visuospatial and somatosensory functional connectivity in anorexia nervosa. Biol Psychiatry. 2012;72:864–70. doi: 10.1016/j.biopsych.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 24.McFadden KL, Tregellas JR, Shott ME, et al. Reduced salience and default mode network activity in women with anorexia nervosa. Journal of psychiatry & neuroscience JPN. 2014;39:178–88. doi: 10.1503/jpn.130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang T, He Y, Zang Y, et al. Modulation of functional connectivity during the resting state and the motor task. Hum Brain Mapp. 2004;22:63–71. doi: 10.1002/hbm.20012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Favaro A, Tenconi E, Degortes D, et al. Effects of obstetric complications on volume and functional connectivity of striatum in anorexia nervosa patients. Int J Eat Disord. 2014;47:686–95. doi: 10.1002/eat.22320. [DOI] [PubMed] [Google Scholar]
- 27.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6:15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 28.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von dem Hagen EA, Stoyanova RS, Baron-Cohen S, et al. Reduced functional connectivity within and between ‘social’ resting state networks in autism spectrum conditions. Soc Cogn Affect Neurosci. 2013;8:694–701. doi: 10.1093/scan/nss053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fichter M, Quadfiieg N. SIAB. Struckturiertes Inventar fuer anorektische und Bulimische Essstoerungen nach DSM-IV und ICD-10. Bern: Huber; 1999. [Google Scholar]
- 32.Paul T, Thiel A. Eating Disorder Inventory-2 (EDI-2): deutsche Version. Hogrefe: 2005. [Google Scholar]
- 33.Goth K, Schmeck K, editors. Das Junior Temprament und Character Inventar (JTCI) Goettingen: Hogrefe; 2009. [Google Scholar]
- 34.Franke GH. SCL-90-R: Symptom-Checkliste von LR Derogatis: Beltz Test. Göttingen, Germany: 2002. [Google Scholar]
- 35.Von Aster M, Neubauer A, Horn R. Wechsler Intelligenztest für Erwachsene (WIE) Deutschsprachige Bearbeitung und Adaptation des WAIS-III von David Wechsler. Frankfurt/Main, Germany: Harcourt Test Services; 2006. [Google Scholar]
- 36.Daseking M, Petermann U, Petermann F. Intelligenzdiagnostik mit dem HAWIK-IV. Kindh Entwickl. 2007;16:250–9. [Google Scholar]
- 37.Gorgolewski K, Burns CD, Madison C, et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in python. Front Neuroinform. 2011;22:13. doi: 10.3389/fninf.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci U S A. 2009;106:1279–84. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Destrieux C, Halgren E, Dale A, et al. Variability of the human brain studied on the flattened cortical surface. Abstr-Soc Neurosci. 1998 [Google Scholar]
- 40.Calhoun VD, Adali T, Pearlson G, et al. A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp. 2001;14:140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li YO, Adalı T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Hum Brain Mapp. 2007;28:1251–66. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Comput. 1995;7:1129–59. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 43.Erhardt EB, Rachakonda S, Bedrick EJ, et al. Comparison of multi-subject ICA methods for analysis of fMRI data. Hum Brain Mapp. 2011;32:2075–95. doi: 10.1002/hbm.21170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yeo BT, Krienen FM, Sepulcre J, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brett M, Anton J, Valabregue R, et al. Region of interest analysis using an SPM toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- 46.Calhoun V, Adalı T, Kraut M, et al. A weighted least-squares algorithm for estimation and visualization of relative latencies in event-related functional MRI. Magn Reson Med. 2000;44:947–54. doi: 10.1002/1522-2594(200012)44:6<947::aid-mrm17>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 47.Holsen LM, Lawson EA, Blum J, et al. Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci. 2012;37:322–32. doi: 10.1503/jpn.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lawson EA, Holsen LM, DeSanti R, et al. Increased hypothalamic–pituitary–adrenal drive is associated with decreased appetite and hypoactivation of food-motivation neurocircuitry in anorexia nervosa. Eur J Endocrinol. 2013;169:639–47. doi: 10.1530/EJE-13-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wagner A, Aizenstein H, Mazurkewicz L, et al. Altered insula response to taste stimuli in individuals recovered from restricting-type anorexia nervosa. Neuropsychopharmacology. 2008;33:513–23. doi: 10.1038/sj.npp.1301443. [DOI] [PubMed] [Google Scholar]
- 50.Oberndorfer T, Simmons A, McCurdy D, et al. Greater anterior insula activation during anticipation of food images in women recovered from anorexia nervosa versus controls. Psychiatry Res Neuroimaging. 2013;214:132–41. doi: 10.1016/j.pscychresns.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanders N, Smeets PA, van Elburg AA, et al. Altered food-cue processing in chronically ill and recovered women with anorexia nervosa. Front Behav Neurosci. 2015;27:46. doi: 10.3389/fnbeh.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanto TP, Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci. 2013;17:602–3. doi: 10.1016/j.tics.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 54.Ridderinkhof KR, van den Wildenberg WP, Segalowitz SJ, et al. Neurocognitive mechanisms of cognitive control: the role of pre-frontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain Cogn. 2004;56:129–40. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 55.Botvinick MM, Cohen JD. The computational and neural basis of cognitive control: charted territory and new frontiers. Cogn Sci. 2014;38:1249–85. doi: 10.1111/cogs.12126. [DOI] [PubMed] [Google Scholar]
- 56.Dosenbach NU, Fair DA, Cohen AL, et al. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schneider N, Salbach-Andrae H, Merle J, et al. Psychopathology in underweight and weight-recovered females with anorexia nervosa. Eat Weight Disord. 2009;14:e205–11. doi: 10.1007/BF03325118. [DOI] [PubMed] [Google Scholar]
- 58.Anderluh MB, Tchanturia K, Rabe-Hesketh S, et al. Childhood obsessive-compulsive personality traits in adult women with eating disorders: defining a broader eating disorder phenotype. Am J Psychiatry. 2003;160:242–7. doi: 10.1176/appi.ajp.160.2.242. [DOI] [PubMed] [Google Scholar]
- 59.Lilenfeld LR, Wonderlich S, Riso LP, et al. Eating disorders and personality: a methodological and empirical review. Clin Psychol Rev. 2006;26:299–320. doi: 10.1016/j.cpr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Sato Y, Saito N, Utsumi A, et al. Neural basis of impaired cognitive flexibility in patients with anorexia nervosa. PLoS ONE. 2013;8:e61108. doi: 10.1371/journal.pone.0061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hon N, Epstein RA, Owen AM, et al. Frontoparietal activity with minimal decision and control. J Neurosci. 2006;26:9805–9. doi: 10.1523/JNEUROSCI.3165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mennes M, Kelly C, Zuo X-N, et al. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]