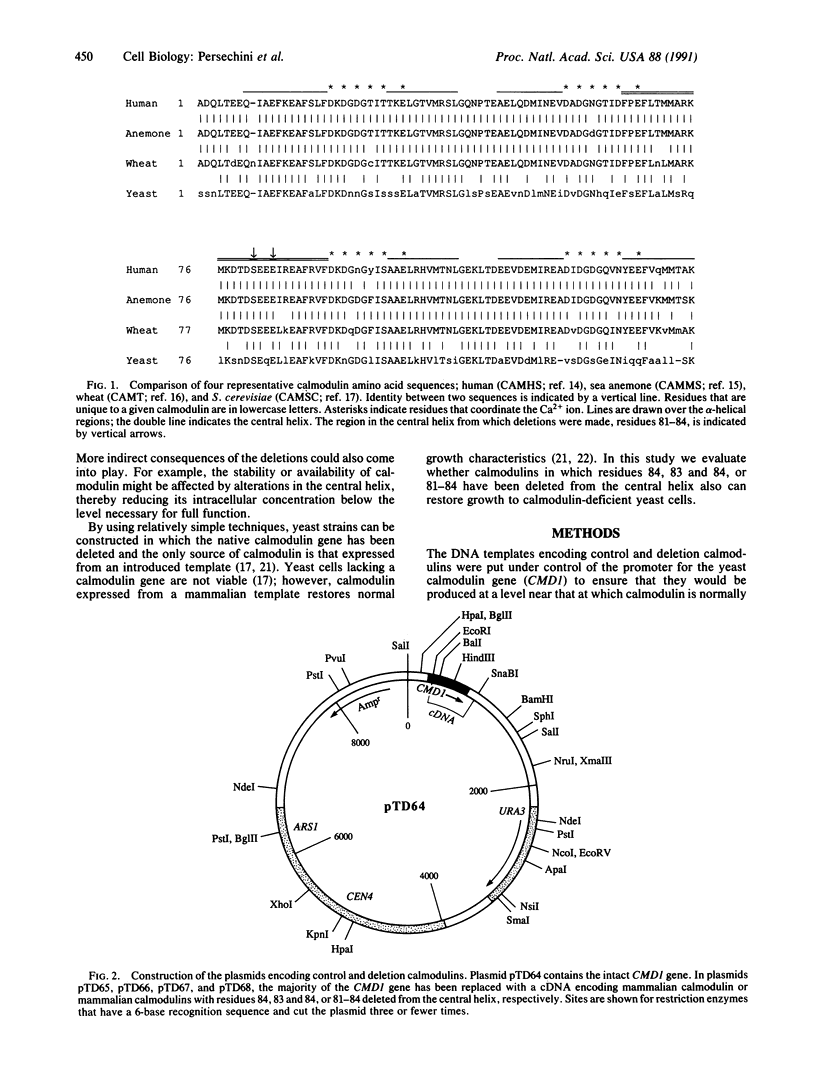
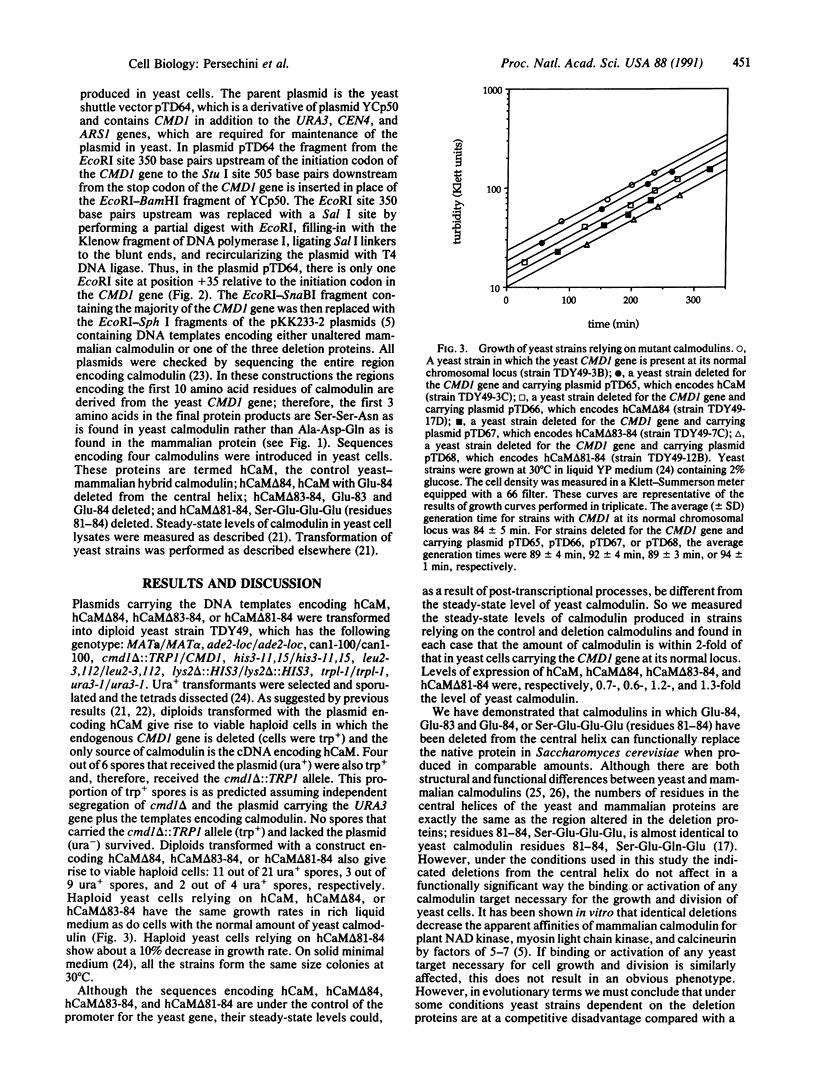
Abstract
Deletion of Glu-84, Glu-83 and Glu-84, or Ser-Glu-Glu-Glu (residues 81-84) from the central helix of mammalian calmodulin is known to result in a 5-7 times decrease in its apparent in vitro affinities for three calmodulin-dependent enzymes. However, based on in vitro experiments, it is difficult to estimate how these deletions might affect in vivo cellular function. The yeast Saccharomyces cerevisiae, which requires calmodulin for growth, provides an excellent system to evaluate these deletion proteins in vivo. Based on its ability to restore normal growth characteristics to yeast cells, mammalian calmodulin is functionally identical to the yeast protein; herein we evaluate the effect of deleting residues 84, 83 and 84, or 81-84 from the central helix. Sequences encoding the deletion proteins and an unaltered control sequence were introduced by means of a yeast shuttle vector and were expressed under control of the yeast calmodulin promoter. The deletion and control calmodulins are produced at levels similar to that observed for the yeast protein, and they completely restore normal growth characteristics. This result suggests that the regions deleted from the central helix are not critical for activation of any yeast calmodulin target normally required for cell growth or division. It is likely that there are twisting and shortening motions associated with the deletions from the central helix that alter significantly the spatial relationship between the two lobes of calmodulin. The abilities of the deletion calmodulins to restore completely normal growth characteristics to yeast cells suggest that the lobes of all the deletion proteins can still be appropriately positioned in calmodulin-target complexes. This is consistent with the hypothesis that the central helix of calmodulin is analogous to a flexible tether rather than to a rigid connector between the two lobes of the molecule.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu Y. S., Bugg C. E., Cook W. J. Structure of calmodulin refined at 2.2 A resolution. J Mol Biol. 1988 Nov 5;204(1):191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig T. A., Watterson D. M., Prendergast F. G., Haiech J., Roberts D. M. Site-specific mutagenesis of the alpha-helices of calmodulin. Effects of altering a charge cluster in the helix that links the two halves of calmodulin. J Biol Chem. 1987 Mar 5;262(7):3278–3284. [PubMed] [Google Scholar]
- Dalgarno D. C., Klevit R. E., Levine B. A., Williams R. J., Dobrowolski Z., Drabikowski W. 1H NMR studies of calmodulin. Resonance assignments by use of tryptic fragments. Eur J Biochem. 1984 Jan 16;138(2):281–289. doi: 10.1111/j.1432-1033.1984.tb07913.x. [DOI] [PubMed] [Google Scholar]
- Davis T. N., Thorner J. Vertebrate and yeast calmodulin, despite significant sequence divergence, are functionally interchangeable. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7909–7913. doi: 10.1073/pnas.86.20.7909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. N., Urdea M. S., Masiarz F. R., Thorner J. Isolation of the yeast calmodulin gene: calmodulin is an essential protein. Cell. 1986 Nov 7;47(3):423–431. doi: 10.1016/0092-8674(86)90599-4. [DOI] [PubMed] [Google Scholar]
- Heidorn D. B., Seeger P. A., Rokop S. E., Blumenthal D. K., Means A. R., Crespi H., Trewhella J. Changes in the structure of calmodulin induced by a peptide based on the calmodulin-binding domain of myosin light chain kinase. Biochemistry. 1989 Aug 8;28(16):6757–6764. doi: 10.1021/bi00442a032. [DOI] [PubMed] [Google Scholar]
- Heidorn D. B., Trewhella J. Comparison of the crystal and solution structures of calmodulin and troponin C. Biochemistry. 1988 Feb 9;27(3):909–915. doi: 10.1021/bi00403a011. [DOI] [PubMed] [Google Scholar]
- Klevit R. E., Blumenthal D. K., Wemmer D. E., Krebs E. G. Interaction of calmodulin and a calmodulin-binding peptide from myosin light chain kinase: major spectral changes in both occur as the result of complex formation. Biochemistry. 1985 Dec 31;24(27):8152–8157. doi: 10.1021/bi00348a047. [DOI] [PubMed] [Google Scholar]
- Luan Y., Matsuura I., Yazawa M., Nakamura T., Yagi K. Yeast calmodulin: structural and functional differences compared with vertebrate calmodulin. J Biochem. 1987 Dec;102(6):1531–1537. doi: 10.1093/oxfordjournals.jbchem.a122201. [DOI] [PubMed] [Google Scholar]
- Moncrief N. D., Kretsinger R. H., Goodman M. Evolution of EF-hand calcium-modulated proteins. I. Relationships based on amino acid sequences. J Mol Evol. 1990 Jun;30(6):522–562. doi: 10.1007/BF02101108. [DOI] [PubMed] [Google Scholar]
- Newton D. L., Oldewurtel M. D., Krinks M. H., Shiloach J., Klee C. B. Agonist and antagonist properties of calmodulin fragments. J Biol Chem. 1984 Apr 10;259(7):4419–4426. [PubMed] [Google Scholar]
- Newton D., Klee C., Woodgett J., Cohen P. Selective effects of CAPP1-calmodulin on its target proteins. Biochim Biophys Acta. 1985 Jun 30;845(3):533–539. doi: 10.1016/0167-4889(85)90222-8. [DOI] [PubMed] [Google Scholar]
- O'Neil K. T., DeGrado W. F. The interaction of calmodulin with fluorescent and photoreactive model peptides: evidence for a short interdomain separation. Proteins. 1989;6(3):284–293. doi: 10.1002/prot.340060311. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Anraku Y. Functional expression of chicken calmodulin in yeast. Biochem Biophys Res Commun. 1989 Jan 31;158(2):541–547. doi: 10.1016/s0006-291x(89)80083-x. [DOI] [PubMed] [Google Scholar]
- Ohya Y., Uno I., Ishikawa T., Anraku Y. Purification and biochemical properties of calmodulin from Saccharomyces cerevisiae. Eur J Biochem. 1987 Oct 1;168(1):13–19. doi: 10.1111/j.1432-1033.1987.tb13380.x. [DOI] [PubMed] [Google Scholar]
- Persechini A., Blumenthal D. K., Jarrett H. W., Klee C. B., Hardy D. O., Kretsinger R. H. The effects of deletions in the central helix of calmodulin on enzyme activation and peptide binding. J Biol Chem. 1989 May 15;264(14):8052–8058. [PubMed] [Google Scholar]
- Persechini A., Kretsinger R. H. The central helix of calmodulin functions as a flexible tether. J Biol Chem. 1988 Sep 5;263(25):12175–12178. [PubMed] [Google Scholar]
- Persechini A., Kretsinger R. H. Toward a model of the calmodulin-myosin light-chain kinase complex: implications for calmodulin function. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S1–12. [PubMed] [Google Scholar]
- Putkey J. A., Ono T., VanBerkum M. F., Means A. R. Functional significance of the central helix in calmodulin. J Biol Chem. 1988 Aug 15;263(23):11242–11249. [PubMed] [Google Scholar]
- Putkey J. A., Ono T., VanBerkum M. F., Means A. R. Functional significance of the central helix in calmodulin. J Biol Chem. 1988 Aug 15;263(23):11242–11249. [PubMed] [Google Scholar]
- Sasagawa T., Ericsson L. H., Walsh K. A., Schreiber W. E., Fischer E. H., Titani K. Complete amino acid sequence of human brain calmodulin. Biochemistry. 1982 May 11;21(10):2565–2569. doi: 10.1021/bi00539a041. [DOI] [PubMed] [Google Scholar]
- Toda H., Yazawa M., Sakiyama F., Yagi K. Amino acid sequence of calmodulin from wheat germ. J Biochem. 1985 Sep;98(3):833–842. doi: 10.1093/oxfordjournals.jbchem.a135342. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Yagi K. The amino acid sequence of the calmodulin obtained from sea anemone (metridium senile) muscle. Biochem Biophys Res Commun. 1980 Sep 16;96(1):377–381. doi: 10.1016/0006-291x(80)91225-5. [DOI] [PubMed] [Google Scholar]
- Yoshino H., Minari O., Matsushima N., Ueki T., Miyake Y., Matsuo T., Izumi Y. Calcium-induced shape change of calmodulin with mastoparan studied by solution X-ray scattering. J Biol Chem. 1989 Nov 25;264(33):19706–19709. [PubMed] [Google Scholar]


