Abstract
Elevated intraocular pressure is the primary cause of open angle glaucoma. Outflow resistance exists within the trabecular meshwork but also at the level of Schlemm's canal and further downstream within the outflow system. Viral vectors allow to take advantage of naturally evolved, highly efficient mechanisms of gene transfer, a process that is termed transduction. They can be produced at biosafety level 2 in the lab using protocols that have evolved considerably over the last 15 to 20 years. Applied by an intracameral bolus, vectors follow conventional as well as uveoscleral outflow pathways. They may affect other structures in the anterior chamber depending on their transduction kinetics which can vary among species when using the same vector. Not all vectors can express long-term, a desirable feature to address the chronicity of glaucoma. Vectors that integrate into the genome of the target cell can achieve transgene function for the life of the transduced cell but are mutagenic by definition. The most prominent long-term expressing vector systems are based on lentiviruses that are derived from HIV, FIV, or EIAV. Safety considerations make non-primate lentiviral vector systems easier to work with as they are not derived from human pathogens. Non-integrating vectors are subject to degradation and attritional dilution during cell division. Lentiviral vectors have to integrate in order to express while adeno-associated viral vectors (AAV) often persist as intracellular concatemers but may also integrate. Adeno- and herpes viral vectors do not integrate and earlier generation systems might be relatively immunogenic. Nonviral methods of gene transfer are termed transfection with few restrictions of transgene size and type but often a much less efficient gene transfer that is also short-lived. Traditional gene transfer delivers exons while some vectors (lentiviral, herpes and adenoviral) allow transfer of entire genes that include introns. Recent insights have highlighted the role of non-coding RNA, most prominently, siRNA, miRNA and lncRNA. SiRNA is highly specific, miRNA is less specific, while lncRNA uses highly complex mechanisms that involve secondary structures and intergenic, intronic, overlapping, antisense, and bidirectional location. Several promising preclinical studies have targeted the RhoA or the prostaglandin pathway or modified the extracellular matrix. TGF-β and glaucoma myocilin mutants have been transduced to elevate the intraocular pressure in glaucoma models. Cell based therapies have started to show first promise. Past approaches have focused on the trabecular meshwork and the inner wall of Schlemm's canal while new strategies are concerned with modification of outflow tract elements that are downstream of the trabecular meshwork.
Keywords: Glaucoma, outflow tract, gene therapy, intraocular pressure, trabecular meshwork
1. Introduction
Open angle glaucoma (OAG), the most common form of glaucoma, is characterized by a decreased outflow facility and elevated intraocular pressure (IOP) (Stamer and Acott, 2012). Progressive loss of retinal ganglion cells is the clinical consequence in all glaucomas and the leading cause of irreversible vision loss worldwide. IOP remains the major modifiable cause and risk factor (Caprioli and Varma, 2011). Compared to traditional interventions that consist of frequently used drops (Friedman et al., 2007), laser (Kaplowitz et al., 2015; Lin, 2008) or filtering surgery (Bussel et al., 2014), gene and cell based therapies have potential to address OAG in a more specific yet long lasting fashion that may also be less traumatic. Gene therapy changes expression in the target tissue by introducing exogenous nucleic acids such as DNA, mRNA, small interfering RNA (siRNA), microRNA (miRNA), or antisense oligonucleotides. Both coding and, more recently, non-coding nucleic acid (Husso et al., 2014) have been used. Cell based therapies often involve transplantation of cells that have been engineered with gene therapy tools. The eye makes for a good target because it is a relatively confined anatomic space that can be directly observed.
Gene transfer using viral vectors has shown promise for many eye diseases but compared to retinal disorders, there is a noticeable paucity of gene therapy trials for glaucoma. Of 2210 clinical gene therapy trials, only 31 (1.4%) were concerned with ocular diseases (“Gene Therapy Clinical Trials Worldwide,” n.d.). Twenty-two of those are based in the United States, two in France (FR-0059, FR-0060), two in Australia (AU-0025, AU-0029), one in the United Kingdom (UK0192), one in China (CN-0025), and one in Israel (IL-0008). There is presently only a single clinical glaucoma gene therapy trial, US-0589, which involves a subconjunctivally injected adenoviral vector prior to trabeculectomy. In this phase 1 trial, p21, a potent cyclin-dependent kinase inhibitor, is expressed to reduce scar formation.
This review will provide an overview of gene transfer to the primary site of glaucoma pathogenesis, the outflow tract, and discuss what has been accomplished and which approaches have not yet been attempted.
2. Established methods of ocular gene therapy
In gene therapy, genes can be added, altered, or knocked down by viral vectors, a process termed transduction, or by non-viral methods, termed transfection. Delivery may be directly in vivo or in vitro to cultured cells that are then transplanted. Although most cells in the eye are nondividing, not all vectors can transduce them. Predominantly used viral vectors are based on adenovirus (AdV), adeno-associated virus (AAV), herpes simplex virus (HSV), type C-retrovirus, and lentivirus (Fig. 1). The most important distinguishing features are 1) transgene persistence, 2) packaging capacity, 3) cell type preference, 4) immunogenicity, and 5) safety. Transgene persistence depends on whether or not genomic integration is achieved since episomes or concatemers that contain the transgene are subject to degradation or dilutional attrition by cell division. Transgene integration is by definition mutagenic and may result in unpredictable consequences (disruption of host genes, expression of downstream genes). The packaging capacity is sufficient in most vectors to deliver exons of no more than 2 kilo-basepairs (kb). More complex genes or entire introns, in contrast, can only be packaged by some vectors as reviewed below. Cell type preference depends on transduction kinetics (affected by contact time, transducing units per volume, media) and receptor tropism. Immunogenicity is influenced by viral proteins, prior exposure, transgene species, and cell lines and media used during production (Bessis et al., 2004). The biosafety level is especially concerned with tropism of the natural host (human versus nonhuman), creation of aerosol, and potential for a replication-competent vector that might start to propagate.
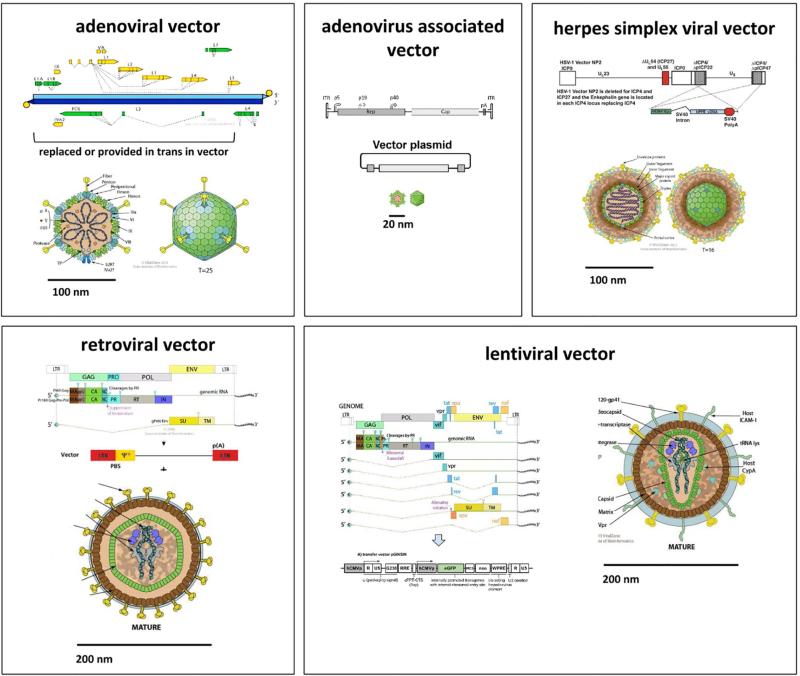
Fig. 1. Genome and structure models of vectors commonly used in ocular gene transfer.
Adenoviral vectors are non-integrating and can deliver up to 38 kilo-basepairs (kb). Adeno-associated viral vectors may integrate or persist as concatemers and have a small physical size with a packaging capacity of 1.6 kb. Herpes simplex viral vectors do not integrate and have a very large capacity of up to 150 kb. Newer adeno- and herpes viral vectors are less immunogenic than earlier generations. Retroviral vectors have a packaging capacity of 7 kb and only transduce dividing cells into which they permanently integrate. Lentiviruses belong to the family of retroviridae but have evolved a nuclear import mechanism for nondividing cells. Derived vectors have a capacity of 7 kb and permanently integrate into both dividing and nondividing cells. All vectors shown here, but not the adeno-associated and retroviral vector, are capable of delivering complex genes that include introns. All of them can deliver exons and small, non-coding RNA (siRNA).
Viruses evolved highly efficient mechanisms of gene transfer and immune evasion a long time ago. The simian immunodeficiency virus, for instance, can be traced back more than 10 million years (Compton et al., 2013) while retroviral orthologues predating the divergence of placental mammals exceed 100 million years (Lee et al., 2013). The downside is that host defense mechanisms are equally mature and can result in pronounced immune reactions in addition to innate restriction at the post-entry level (Kajaste-Rudnitski and Naldini, 2015). A prominent example is the shock syndrome, cytokine release, acute respiratory distress, and multiorgan failure that systemically applied adenoviral vectors can cause (Wilson, 2009). Non-viral delivery using physical or chemical methods to transfer exogenous genes into target cells have a more favorable safety profile than viral vectors, but with lower transduction efficiency (Zulliger et al., 2015).
2.1. Viral gene delivery
2.1.1. Adenoviral vectors (AdV)
Adenoviruses contain linear, double-stranded DNA. Of species A to F that are subdivided into 50 serotypes (Warnock et al., 2011), B and E are the most frequently used for vectors in clinical trials (Ginn et al., 2013). AdV can be produced with relative ease at high titers and high purity (Luo et al., 2007). Gutted AdV are more challenging to generate, however (Kreppel, 2014). They can deliver large transgenes of up to 38 kb that can contain full genes including introns (DelloRusso et al., 2002). The trabecular meshwork (TM) and the inner wall of Schlemm's canal have been successfully transduced without significantly influencing lens transparency and other tissue architecture nearby (Borrás et al., 1998; Lee et al., 2010). AdV vector preps are unlikely to be affected by pseudotransduction, where contaminating cell fragment proteins from producer cells co-pellet, as may happen with non-banded preps of pseudotyped retroviral vectors (Liu et al., 1996). AdV can transduce both dividing and nondividing tissues. Trabecular meshwork transduction can be achieved without a significant impact on IOP (Borrás et al., 1999).
One shortcoming is that most humans have been exposed to serotypes B and E during their lifetime and have preexisting immunity as a result. Expression in dividing cells is also limited to 5 to 50 days (Borrás et al., 1998; Budenz et al., 1995; Giovingo et al., 2013; Vittitow et al., 2002) because AdV does not integrate. In nondividing cells or in spaces with a relative immune privilege, expression can last for up to a year (Loewen et al., 2004b). AdV is often immunogenic due to coexpressed viral proteins (Borrás et al., 2001) which can be addressed by anti-CD44L treatment (Millar et al., 2008), adjusting the titer (Ethier et al., 2004), or vector gutting (DelloRusso et al., 2002). AdV requires handling at biosafety level (BSL) 2. The nonspecific transduction can be made more specific by transcriptional targeting (Gonzalez et al., 2004) or choosing select sub-serotypes (Ueyama et al., 2014).
2.1.2. Adenovirus-associated vectors (AAV)
AAV, an adenoviral helper-dependent, replication-defective parvovirus, can efficiently infect both dividing and nondividing cells with only a mild immune response and no known pathogenicity (Warnock et al., 2011). AAV-derived vectors have a packaging capacity of approximately 4.6 kb. They are relatively difficult to produce but high titers can be achieved (Grieger et al., 2006). There is an established track record for AAV vectors with inherited eye diseases (Bainbridge et al., 2008) but the transduction efficiency of conventional AAV in TM is very low due to the inability to convert single-stranded DNA into double-stranded DNA, which requires DNA polymerase complexes in host cells (Borrás et al., 2006). Transduction efficiency varies among serotypes of scAAV and host species (Bogner et al., 2015). Expression is slower in the outflow tract than other regions and takes approximately 1 week (Borrás et al., 2015). Gene expression is more persistent with self-complementary vectors and has been reported for at least 3.5 months in rat and 2.3 years in monkey TM (Buie et al., 2010). TM transduction is relatively IOP neutral but mild intraocular inflammation may limit expression (Buie et al., 2010). AAV vectors can be handled at BSL 1 but production occurs at BSL 2 due to the use of transformed HEK293 cells (“293 [HEK-293] ATCC ® CRL-1573™ Homo sapiens embryonic kidney,” n.d.) that contain adenoviral elements and are also transfected during production with adenoviral helper plasmids. Although AAV integrates preferentially into chromosome 19, this is not the case for AAV-derived vectors which typically persist as intracellular head to tail concatemers (Yan et al., 2006, 2005), integrate without specificity, or insert into active genes (Nakai et al., 2003).
2.1.3. HSV vectors
HSV is a 152 kb double-stranded DNA virus (Warnock et al., 2011). HSV-1 based vectors are more commonly used for CNS gene therapy targets than HSV-2 due to the tropism of the HSV-1 wild type (Goins et al., 2014). Wild type HSV-2 is less epileptogenic than HSV-1 yet can also be used for CNS gene therapy and can, for instance, use an intranasal delivery route to treat seizures in a model (Laing et al., 2006). The neurotropism and long latency of HSV may benefit HSV-based vectors, especially for gene transfer to neurons (Chattopadhyay et al., 2005). The packaging capacity of 150 kb is very large (Marconi et al., 2015). First generation HSV vectors can cause severe intraocular inflammation in primates (Liu et al., 1999), but less so in rodents (Spencer et al., 2000). Ninety percent of humans already have antibodies to HSV-1 or HSV-2 (Wald and Corey, 2011). In nonhuman primates, nearly 100% of the TM and non-pigmented ciliary epithelium cells were transduced (Liu et al., 1999), but achieved only 10%-20% transduction efficiency in rats and none in mice following an intracameral bolus (Spencer et al., 2000). While early generation vectors had short term expression measured in days (Liu et al., 1999), long-term expression has been achieved in the CNS with later generation vectors (Zhang et al., 2012). New HSV vectors that are devoid of all five immediate-early genes appear to be able to achieve longer-term expression also in non-neuronal cells (Miyagawa et al., 2015). HSV is handled as BSL 2.
2.1.4. Retroviral vectors (RV)
Retroviruses contain a single-stranded RNA genome of 7 to 12 kb length, which is converted into DNA by reverse transcriptase to linearly integrate into the host genome (Coffin et al., n.d.). The historical classification into type-A, -B, -C and -D is based on their morphology in electron microscopy and is of limited use today. All retroviruses depend on dissolution of the nuclear membrane during cell division to obtain access to the chromatin except lentiviruses, which have evolved an import mechanism. The most commonly used RV is based on a type-C oncovirus, the Moloney murine leukemia virus (MLV), with a simple genome and a packaging capacity of about 7 kb (Nayerossadat et al., 2012). The normal wild type genome length cannot be exceeded in a vector that consists of a minimized viral backbone and a transgene. Expression of transgenes is driven by long terminal repeats (LTRs) that contain splice donors susceptible to cryptic splicing (Armentano et al., 1987). Only dividing cells can be transduced (Lewis and Emerman, 1994) which can be exploited to target proliferative processes (Kimura et al., 1996). MLV appears to favor integration in close proximity to transcription start sites, which may be oncogenic if this occurs near a proto-oncogene or disrupts a tumor suppressor gene (Wu et al., 2003).
2.1.5. Lentiviral vectors (LV)
Lentiviruses belong to the family of retroviruses but have a more complex genome (Warnock et al., 2011) with a packaging capacity of about 7 kb. LV genomically integrate into nondividing cells such as neurons (Loewen et al., 2004b) or nondividing, terminally differentiated cells, that represent the vast majority of cells in an organism, and include the TM (Fig. 2 (Loewen et al., 2001)) or the retinal pigment epithelium (Loewen et al., 2004b, 2003). There is only one clinical trial that uses lentiviral vectors, a phase 1 dose escalation safety study of a subretinally injected vector that expresses endostatin and angiostatin for advanced neovascular age-related macular degeneration (US-1061). Rev restricts RNA splicing in lentiviruses and thereby allows transfer of nucleic acid with cryptic splice sites and complex genes containing introns (Puthenveetil et al., 2004). LVs have been derived from many species and include the human (HIV (Naldini et al., 1996)), simian (SIV (Mangeot et al., 2000)), bovine (BIV (R. Berkowitz et al., 2001)) and feline immunodeficiency virus (FIV (Poeschla et al., 1998)), as well as the equine infectious anemia virus (EIAV (Olsen, 1998)) and visna virus (R. D. Berkowitz et al., 2001). Because the LTRs of HIV vectors are active promoters in human cells, a self-inactivating modification (SIN) was developed (Miyoshi et al., 1998). While 3'-LTR promoter activity could lead to expression of open reading frames downstream of the insertion site, 5'-LTR promoter activity may generate antigenic peptides from Δgag. The U3 deletion of the 3'-LTR SIN design is copied to the 5'-LTR during reverse transcription and inactivates both LTRs of the integrated vector (Loewen and Poeschla, 2005). FIV LTR promoter function is minimal in non-feline cells and any residual promoter activity from either 5'- or 3'-LTR can be avoided by deleting the 172 bp of the U3 Region of the 3'LTR (including the TATA box and binding sites for transcription factors NFκB, NF-ATc and SP1). FIV vectors are typically produced by transient transfection of a three plasmid system (Saenz et al., 2006) while packaging cell lines as well as four plasmid systems are available for HIV vectors (Barde et al., 2010). Insulator elements can be used to prevent silencing (Romero et al., 2015). There may be low-level expression from non-integrating, circular episomal elements that occur after cell entry (Saenz et al., 2004; Yáñez-Muñoz et al., 2006). Non-primate lentiviral vectors are handled at BSL 2 but HIV based vectors require BSL 2+ at most institutions.
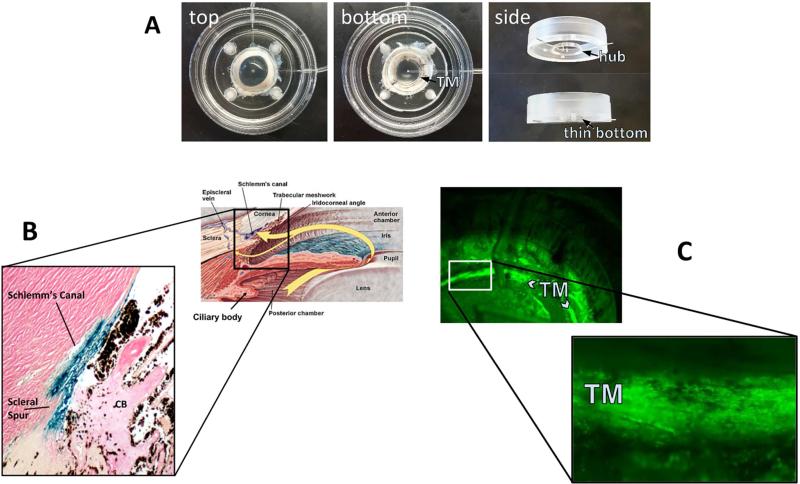
Fig. 2. Anterior segment perfusion culture system.
(Loewen et al., 2016) (A). FIV transduction of human (B) and porcine eyes (C). A redesigned culture bottom allows direct observation of vector function through the bottom of the dish (C).
FIV and HIV vectors have a high TM transduction efficiency in perfusion-cultured human eyes (Fig. 2 B, (Loewen et al., 2001)) and pig eyes (Fig. 2 C (Loewen et al., 2016)) when compared to transducing units-adjusted Ad vector and MLV with a low cytotoxic effect and immunogenicity (Barraza et al., 2009; Challa et al., 2005; Loewen et al., 2004b). Only transient (Barraza et al., 2009) or no (Barraza et al., 2010; Challa et al., 2005; Zhang et al., 2014) inflammation was recorded. Long-term expression of 455 days in monkeys (Barraza et al., 2009) and 840 days in domestic cats (Khare et al., 2008) has been serially followed by live gonioscopy (Fig. 3). A temporary decrease of outflow facility was seen in human anterior segment perfusion cultures (Loewen et al., 2002; Zhang et al., 2014) but not in cats (Khare et al., 2008; Loewen et al., 2004a).
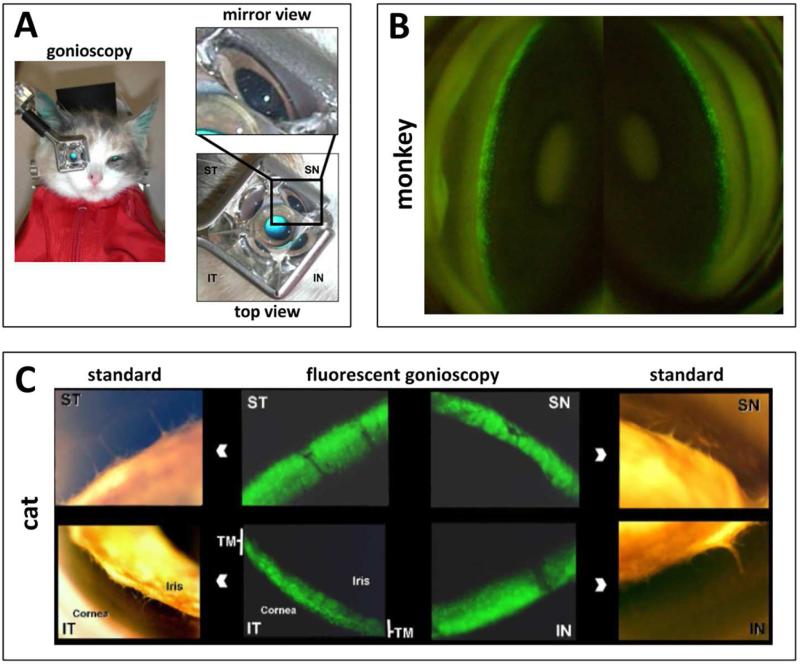
Fig. 3. Live observation of transgene expression with a standard goniolens.
Setup for observation of mid-sized animals (A). Serial observation of EGFP marker gene expression in a macaque eye following an intracameral FIV bolus (B (Barraza et al., 2009)). Relatively selective TM transduction and marker gene in the domestic cat (C (Loewen et al., 2004a)).
3. Non-viral gene delivery
Nucleic acid can also be delivered by injection or a combination of chemical and physical methods. Advantages are low immunogenicity, low mutagenicity, and large capacity. The limitations are that such transfection is transient and inefficient (Conley et al., 2008).
Naked siRNA can suppress targets in human TM cells. Function is limited to about 48 hours (Comes and Borrás, 2007; Gonzalez and Tan, 2014) (Comes and Borrás, 2007; Gonzalez and Tan, 2014) systemic exposure can be detected (Lawrence et al., 2011). Transfection of naked DNA to the outflow tract has not been described.
Nanoparticle can allow non-invasive gene delivery to ocular tissues at greater efficacy than naked DNA (Eljarrat-Binstock et al., 2008). Expression can be detected as early as 4 hours (Losa et al., 1991) and is longer lasting in inner than in outer ocular tissues (Eljarrat-Binstock et al., 2008). Intracameral injection allows transfection of TM (Farjo et al., 2006; Zou et al., 2011). Intravitreal injection of poly (llactic acid), polystyrene, and Poly N-isopropylacrylamide particles caused a substantial reduction of IOP and TM inflammation making hyaluronic acid a better candidate for TM gene delivery (Zou et al., 2011).
The sleeping beauty transposon system was first developed 20 years ago (Ivics et al., 1997). It consists of a transposon with the transgene expression cassette and the transposase which can bind to inverted and direct repeats in transposons to facilitate integration. Advantages are long-term expression, high transfection efficiency (Johnen et al., 2012), low pathogenicity, and integration independent of the cell cycle (Ivics and Izsvák, 2011). Electroporation (Johnen et al., 2012), transfection reagents (Belur et al., 2007), and hydrodynamic injection (Bell et al., 2007) do not appear to be effective methods of delivering transposon elements in the anterior segment. To address this problem, a hybrid vector combining a chimeric virus and transposons has been developed (de Silva et al., 2010). EGFP expression was seen in RPE, corneal endothelial, and iris cells after subretinal injection of such hybrid vector in mice (Turunen et al., 2014). However, the advantages provided by hybrid vector should be weighed against a low cost-efficient ratio and the complexity of vector production (Aronovich et al., 2011).
Replicating episomal vectors, which include small circular vectors and artificial chromosomes, can provide sustained gene expression (Zehnpfennig et al., 2010) and avoid unpredictable consequences of gene integration (Lipps et al., 2003). Another possibility is the use of bacteriophage ΦC31 integrase and the attB recognition sequence to enable genomic integration and prolonged expression (at least 4.5 months in mouse retina (Chalberg et al., 2005)). This system has to be electroporated which often causes cataracts, inflammation, and nanophthalmos (Chalberg et al., 2005).
4. Applications
4.1. Enhancing outflow
Choosing a transgene for gene therapy of glaucoma is difficult because in only less than 10% of glaucoma patients can a single gene be identified to be at fault (Fan et al., 2006): MYOC (Stone et al., 1997), CYP1B1 (Lim et al., 2013), CAV1/CAV2 (Thorleifsson et al., 2010), LTBP2 (Ali et al., 2009), GAS7,TMCO1 (van Koolwijk et al., 2012), and ARHGEF12 (Springelkamp et al., 2015) can all alter outflow. In addition, the conventional outflow tract is a complex structure that consists of many distinctly different components and cell types: the uveoscleral, corneoscleral and deeper juxtacanalicular TM, the inner and outer wall of Schlemm's canal, collector channels and aqueous veins. These structures have different transduction properties and kinetics: the TM is a phagocytotically active tissue that consists primarily of terminally differentiated, nondividing cells which facilitates transduction with VSV-G pseudotyped lentiviral vectors while the directly adjacent cornea is less transduced when exposed to the same vector bolus (Loewen et al., 2001). The ability of the TM and Schlemm's canal to present antigens and induce tolerance (anterior chamber associated immune deviation, ACAID, (Stein-Streilein and Streilein, 2002), may work in favor of gene transfer to the outflow tract by reducing immunity against vector and transgene. Schlemm's canal has both typical and lymphatic vascular properties (Kizhatil et al., 2014) from which collector channels start to sprout during embryogenesis (Ramírez et al., 2004). An application of gene therapeutic substances to the anterior chamber, Schlemm's canal, or the collector channels has potential to cause a systemic exposure since they drain into the venous circulation.
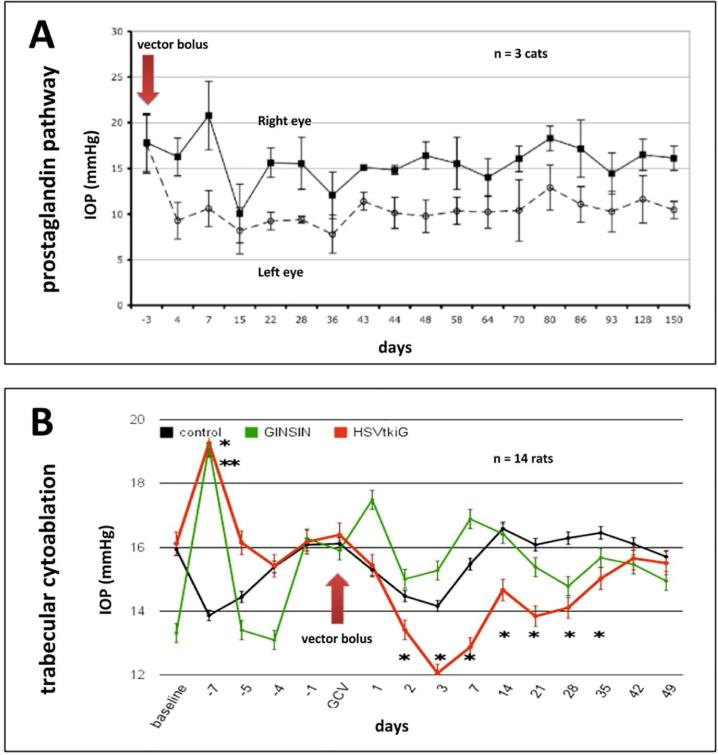
A well-studied therapeutic target to increase outflow facility is the RhoA and Rho-kinase pathway, which modulates the actin cytoskeleton, cell adhesive interactions, ECM formation, and TM contraction (Wang et al., 2013; Zhang et al., 2008). Increased outflow facility after dominant-negative RhoA or exoenzyme C3 (Rho GTPase inhibitor) by an adenoviral vector was observed in a primate anterior segment perfusion model (Liu et al., 2005; Vittitow et al., 2002). Borras et al used a scAAV-mediated RhoA transfer to prevented elevation of IOP for more than 4 weeks in rats (Borrás et al., 2015). Rho-kinase, a key downstream effector of activated RhoA, may present as a more effective therapeutic target since specific inhibition of this gene increased the outflow facility much greater than inhibiting RhoA (80% vs. 32.5%) (Rao et al., 2005; Vittitow et al., 2002). Barraza et al transduced components (receptor and enzymes) of the prostaglandin pathway using FIV to reduce IOP in domestic cats, an approach that involved codon optimization to avoid rapid RNA degradation of the expressed transgenes (Fig. 4A (Barraza et al., 2010)). Using a similar approach in primate model to study prostaglandin pathway gene therapy for future human use, Lee et al. transduced cynomolgus monkeys with bovine PGF synthase alone using FIV vectors and observed a significant IOP reduction for 5 months (Lee et al., 2014).
Fig. 4. Examples of gene therapeutic IOP lowering.
Prostaglandin pathway transduction shows a long-term IOP reduction after a single vector bolus in the cat (A (Barraza et al., 2010)). Inducible cytoablative vector causes a rapid TM cell loss followed by a recovery of IOP and TM cell numbers (Zhang et al., 2014) in this model of TM regeneration (B).
Kumar and colleagues found that transduction of tissue plasminogen activator by an adenoviral vector increased outflow facility by 60% in steroid induced glaucoma (Kumar et al., 2013). Herpes simplex thymidine kinase/ganciclovir based TM ablation using conditionally cytotoxic FIV vectors reduced IOP in a rodent model (Fig. 4B (Zhang et al., 2014)). Naked glucocorticoid receptor small interfering RNA (siRNA) in a human anterior segment perfusion model caused significant reduction of MYOC and CDT6 expression by 98% and 85%, respectively (Comes and Borrás, 2007). Similarly, a recent study by Gonzalez and Tan also found that a low dose of siRNA significantly inhibited targeted gene by 53.9% (Gonzalez and Tan, 2014). Silencing ChGn, an enzyme specific to chondroitin sulfate glycosaminoglycan biosynthesis, and caveolin-1 by shRNA caused increased outflow facility in the same model (Aga et al., 2014; Keller et al., 2011). Matrix metalloproteinases play an important role in degrading ECM and decrease the resistance of outflow (De Groef et al., 2013). Gerometta et al (Gerometta et al., 2010) and Spiga et al (Spiga and Borrás, 2010) developed a glucocorticoid-inducible metalloproteinase 1 Ad vector to reduce IOP in the presence of continued corticosteroid application.
4.2. Generating disease models
Gene transfer to the outflow tract can be used to develop OAG disease models. Members of the TGF-β superfamily (Buie et al., 2013; Robertson et al., 2010) and their downstream effectors (Junglas et al., 2012; Lee et al., 2010; Oh et al., 2013) can induce ECM remodeling in the TM (Fuchshofer and Tamm, 2012; Wordinger et al., 2014) and increase IOP. AdV-mediated overexpression of TGF-β1 and TGF-β2 resulted in reduced outflow facility and ocular hypertension in a dose-dependent manner (Robertson et al., 2010; Shepard et al., 2010). Transduction of BMP2 into TM also increased IOP in rats (Buie et al., 2013) as did transduction of downstream effectors COCH (Lee et al., 2010), CTGF (Junglas et al., 2012), and SPARC (Oh et al., 2013). IOP increased in a dose-dependent fashion following intravitreal injection of adenoviral vector encoding sFRP1 (Wang et al., 2008) and sCD44 (Giovingo et al., 2013) in mice. Lentiviral shRNA silencing was used to investigate the role of three different hyaluronan genes in outflow in human eyes (Keller et al., 2012). Similar methods have identified various roles of versican, a large aggregating CS proteoglycan (Keller et al., 2011), tenascin, a matricellular glycoprotein (Keller et al., 2013), and different caveolins on aqueous outflow resistance and ECM remodeling (Aga et al., 2014).
5. Current Challenges and Future Directions
5.1. New promoters, models, transgenes
Because cell type specific promoters are not always known, the highly expressing CMV or EF1-α promoters are most commonly used, which has led to expression not only in the TM but also occasionally in corneal endothelial cells (Challa et al., 2005; Zhang et al., 2014) and non-pigmented ciliary epithelial cells (Barraza et al., 2010; Bogner et al., 2015). A TM preferential promoter is the matrix Gla protein promoter (Gonzalez et al., 2004) or chitinase 3-like 1 promoter (Liton et al., 2005). Ueyama et al found that an unmodified Ad35 serotype preferentially transduces TM cells (Ueyama et al., 2014).
To establish a glaucoma animal model by tissue specific transgenesis using viral vectors, other transgenes should be considered. For instance, smad3 is such a candidate that amplifies the TGF-β signal and is essential for TGF-β2 induced ocular hypertension in mice (McDowell et al., 2013). Similarly, Gremlin could be tested as it blocks the negative effect of BMP-4 on TGF-β induced TM fibrosis (Wordinger et al., 2007). S1P2 receptor activation has also shown significant increases in the conventional outflow resistance of human and porcine eyes (Sumida and Stamer, 2011) and might allow to create a glaucoma model in those eyes.
Conversely, BMP-4 (Wordinger et al., 2007), Smad7 (Fuchshofer et al., 2009), and BMP7 (Fuchshofer et al., 2007) can antagonize TGF-β mediated TM changes and may be suitable for glaucoma gene therapy. Nitric oxide is an intracellular signaling molecule with IOP-lowering effects (Cavet et al., 2014). Overexpression of endogenous NO synthases (Stamer et al., 2011) and Ghrelin (upstream modulator of NO) (Azevedo-Pinto et al., 2013) could decrease IOP and increase pressure dependent drainage. Transduction of eNOS and Ghrelin into the outflow tract may be a viable strategy for glaucoma therapy. If outflow resistance is located downstream of the TM, overexpression in TM cells may not be sufficient.
5.2. New targets and delivery methods
5.2.1. Targets downstream of the trabecular meshwork
The majority of outflow resistance is produced by the extracellular matrix in the juxtacanalicular TM and possibly the inner wall endothelium of Schlemm's canal while the larger pores of the outer meshwork are not able to generate a significant resistance. In theory, a single pore of 100 micrometer length and a diameter of 20 micrometers may allow for a pressure drop of 5 mmHg (Johnson and Erickson, 2000). It has previously been thought that the aqueous veins would not be able to produce a significant outflow resistance (estimated to be 20 vessels of 50 micrometer diameter and an average length of 1 mm (Rosenquist et al., 1989)). However, this contradicts a mounting body of both experimental (Schuman et al., 1999; Van Buskirk, 1977) and clinical evidence of residual outflow resistance in that after trabecular ablation, IOP remains above 15 mmHg (Bussel et al., 2015, 2014; Kaplowitz et al., 2014). This often overlooked outflow resistance downstream of the TM may provide new treatment opportunities. Although the episcleral venous pressure in normal subjects is between 8 to 10 mmHg (Sultan and Blondeau, 2003), it is estimated to be near 12 mmHg in untreated POAG (Selbach et al., 2005). This matches IOP reduction after trabeculectomy ab interno by 30 to 40% or to 3 to 4 mmHg above that of episcleral venous pressure with an average near 15 to 16 mmHg as evidenced by a formal meta-analysis (Kaplowitz et al., 2016). It is possible that pressure dependent outflow vessel kinking (Francis et al., 2012) and valve-like suspended structures at the collector openings (Martin et al., 2014) have a pressure and flow regulatory function (Hann et al., 2011; Schieber and Toris, 2013) and represent novel structural treatment targets.
As a vessel-like endothelial layer, supplement of endogenous NO in Schlemm's canal decreased the volume of endothelial cells (Ellis et al., 2010) and increased outflow facility (Chang et al., 2015). A recently identified cytokine signaling pathway may provide additional gene therapy targets that are downstream of the TM (Alvarado et al., 2015). Initiating an expansion of the aqueous vascular bed by reinitiating vessel sprouting may also be feasible. This suggests that transduction of collector channels and Schlemm's canal might be a possible approach for glaucoma, but only if vectors can make it past the TM. Transduction of the downstream outflow tract is possible using FIV vectors (Fig. 5 C).
Fig. 5. New cell and gene delivery approaches.
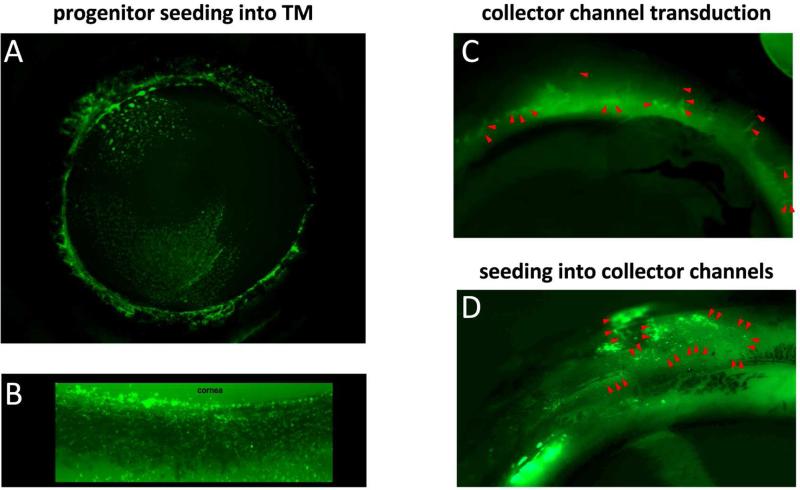
(A) Adipose derived tissue stem cells transduced with EGFP expressing FIV vector (Zhang et al., 2014) were seeded into the TM of anterior perfusion cultures of human eyes. View from inside into everted eye. (B) Magnified view of TM from experiment (A). (C) Collector channel (marked by red arrowheads) transduction with EGFP expressing FIV vector. External, frontal view of perfused porcine eyes (Loewen et al., 2016). An ab interno trabeculectomy (Kaplowitz et al., 2014) was performed followed by an intracameral vector bolus. (D) Cell seeding model with EGFP expressing fibroblasts (Oatts et al., 2013). External, frontal view of perfused porcine eyes. An ab interno trabeculectomy (Kaplowitz et al., 2014) was performed followed by an intracameral cell bolus. Collect channels were marked by red arrowheads.
5.2.2. Treatment with engineered cells
As an alternative approach, progenitor or other suitable cells could be engineered in vitro and then seeded into the outflow tract (Fig. 5 A and B) as done in other compartments of the eye (Flachsbarth et al., 2014). Human TM stem cells can be seeded into mouse TM (Du et al., 2013). Cells that are genetically engineered to overexpress therapeutic genes could similarly be transplanted into the TM or into the outflow tract (Fig. 5 D).
5.2.2. Delivery of non-coding RNA
In addition to the long established ribosomal and transfer RNA (tRNA), and siRNA discussed above, several other non-coding RNA (ncRNA) species exist, including micro RNA (miRNA), small nuclear RNA (sn), P-element induced wimpy testis interacting RNA (piwi- or piRNA), as well as long non-coding RNAs (lncRNAs). The major difference between siRNAs and miRNAs is that miRNAs have multiple targets whereas siRNAs are highly specific. MiRNAs can inhibit the contraction of TM cells and ECM short term and up to 10 miRNAs can be delivered by one viral vector (Gonzalez et al., 2014). LncRNAs exceed 200 nucleotides in length, and regulate expression at the transcriptional and post-transcriptional level and make up the majority (about 98%) of the transcriptome (Mercer et al., 2009). LncRNAs may be transcribed as partial or whole natural antisense transcripts (NAT) of coding genes, or they might be located between genes or within introns. Some lncRNAs originate from so-called pseudogenes (Milligan and Lipovich, 2014). They can be described as intergenic, intronic, overlapping, antisense, bidirectional or processed (Mattick and Rinn, 2015; Peschansky and Wahlestedt, 2014). Other characteristics are tissue specific expression, formation of secondary structures, post-transcriptional processing, and mostly nuclear location.
LncRNA MALAT1, implicated in microvascular dysfunction, was recently shown to regulate neurodegeneration and may be involved in glaucoma (Yao et al., 2016). Another study of lncRNA in the eye demonstrated that a myocardial infarction associated transcript (MIAT) knockdown could suppress tumor necrosis factor-alpha-induced abnormal proliferation of human lens epithelial cells (Shen et al., 2016). Promoter activity of a lncRNA encoded on the opposite strand of LOXL1 is influenced by cellular stressors associated with exfoliation syndrome (Hauser et al., 2015). As these examples highlight, lncRNAs may carry out both gene inhibition and gene activation through a range of diverse mechanisms that are made even more complex by the fact that 40% of coding genes have an overlapping antisense transcription. Could it be possible that a highly variable post transcriptional regulation contributes to the range of glaucoma phenotypes? LncRNAs might play a key role because they are poorly conserved between species, and with about 30,000 different transcripts, are estimated to be present in humans at 100 fold the number of messenger RNA. Our view of the human genome and understanding of human diseases is constantly evolving. The research tools and strategies presented here will help to explore cause and effect in the highly complex pathogenesis of glaucoma that has the simple endpoint of elevated IOPs.
Acknowledgements
Many scientists have contributed to the development of tools and techniques that allow gene transfer and transfer of non-coding material to the outflow tract. Space constraints limited a more complete discussion. Omission of credit was not intended. This work was supported in part the National Institutes of Health research grant EY022737, the Eye and Ear Foundation of Pittsburgh and by the Louis J. Fox Center for Vision Regeneration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 293 [HEK-293] ATCC ® CRL-1573™ Homo sapiens embryonic kidney [WWW Document], n.d. URL http://www.atcc.org/products/all/CRL-1573.aspx (accessed 4.22.16).
- Aga M, Bradley JM, Wanchu R, Yang Y-F, Acott TS, Keller KE. Differential effects of caveolin-1 and -2 knockdown on aqueous outflow and altered extracellular matrix turnover in caveolin-silenced trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2014;55:5497–5509. doi: 10.1167/iovs.14-14519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, McKibbin M, Booth A, Parry DA, Jain P, Riazuddin SA, Hejtmancik JF, Khan SN, Firasat S, Shires M, Gilmour DF, Towns K, Murphy A-L, Azmanov D, Tournev I, Cherninkova S, Jafri H, Raashid Y, Toomes C, Craig J, Mackey DA, Kalaydjieva L, Riazuddin S, Inglehearn CF. Null mutations in LTBP2 cause primary congenital glaucoma. Am. J. Hum. Genet. 2009;84:664–671. doi: 10.1016/j.ajhg.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado JA, Chau P, Wu J, Juster R, Shifera AS, Geske M. Profiling of Cytokines Secreted by Conventional Aqueous Outflow Pathway Endothelial Cells Activated In Vitro and Ex Vivo With Laser Irradiation. Invest. Ophthalmol. Vis. Sci. 2015;56:7100–7108. doi: 10.1167/iovs.15-17660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armentano D, Yu SF, Kantoff PW, von Ruden T, Anderson WF, Gilboa E. Effect of internal viral sequences on the utility of retroviral vectors. J. Virol. 1987;61:1647–1650. doi: 10.1128/jvi.61.5.1647-1650.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronovich EL, McIvor RS, Hackett PB. The Sleeping Beauty transposon system: a non-viral vector for gene therapy. Hum. Mol. Genet. 2011;20:R14–20. doi: 10.1093/hmg/ddr140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo-Pinto S, Pereira-Silva P, Rocha-Sousa A. Ghrelin in ocular pathophysiology: from the anterior to the posterior segment. Peptides. 2013;47:12–19. doi: 10.1016/j.peptides.2013.06.011. [DOI] [PubMed] [Google Scholar]
- Bainbridge JWB, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Barde I, Salmon P, Trono D. Production and titration of lentiviral vectors. Curr. Protoc. Neurosci. 2010 doi: 10.1002/0471142301.ns0421s53. Chapter 4, Unit 4.21. [DOI] [PubMed] [Google Scholar]
- Barraza RA, McLaren JW, Poeschla EM. Prostaglandin pathway gene therapy for sustained reduction of intraocular pressure. Mol. Ther. 2010;18:491–501. doi: 10.1038/mt.2009.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraza RA, Rasmussen CA, Loewen N, Cameron JD, Gabelt BT, Teo W-L, Kaufman PL, Poeschla EM. Prolonged transgene expression with lentiviral vectors in the aqueous humor outflow pathway of nonhuman primates. Hum. Gene Ther. 2009;20:191–200. doi: 10.1089/hum.2008.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JB, Podetz-Pedersen KM, Aronovich EL, Belur LR, McIvor RS, Hackett PB. Preferential delivery of the Sleeping Beauty transposon system to livers of mice by hydrodynamic injection. Nat. Protoc. 2007;2:3153–3165. doi: 10.1038/nprot.2007.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belur LR, Podetz-Pedersen K, Frandsen J, McIvor RS. Lung-directed gene therapy in mice using the nonviral Sleeping Beauty transposon system. Nat. Protoc. 2007;2:3146–3152. doi: 10.1038/nprot.2007.460. [DOI] [PubMed] [Google Scholar]
- Berkowitz RD, Ilves H, Plavec I, Veres G. Gene transfer systems derived from Visna virus: analysis of virus production and infectivity. Virology. 2001;279:116–129. doi: 10.1006/viro.2000.0659. [DOI] [PubMed] [Google Scholar]
- Berkowitz R, Ilves H, Lin WY, Eckert K, Coward A, Tamaki S, Veres G, Plavec I. Construction and molecular analysis of gene transfer systems derived from bovine immunodeficiency virus. J. Virol. 2001;75:3371–3382. doi: 10.1128/JVI.75.7.3371-3382.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessis N, GarciaCozar FJ, Boissier M-C. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl 1):S10–7. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- Bogner B, Boye SL, Min SH, Peterson JJ, Ruan Q, Zhang Z, Reitsamer HA, Hauswirth WW, Boye SE. Capsid Mutated Adeno-Associated Virus Delivered to the Anterior Chamber Results in Efficient Transduction of Trabecular Meshwork in Mouse and Rat. PLoS One. 2015;10:e0128759. doi: 10.1371/journal.pone.0128759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrás T, Buie LK, Spiga M-G, Carabana J. Prevention of nocturnal elevation of intraocular pressure by gene transfer of dominant-negative RhoA in rats. JAMA Ophthalmol. 2015;133:182–190. doi: 10.1001/jamaophthalmol.2014.4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrás T, Gabelt BT, Klintworth GK, Peterson JC, Kaufman PL. Non-invasive observation of repeated adenoviral GFP gene delivery to the anterior segment of the monkey eye in vivo. J. Gene Med. 2001;3:437–449. doi: 10.1002/jgm.210. [DOI] [PubMed] [Google Scholar]
- Borrás T, Matsumoto Y, Epstein DL, Johnson DH. Gene transfer to the human trabecular meshwork by anterior segment perfusion. Invest. Ophthalmol. Vis. Sci. 1998;39:1503–1507. [PubMed] [Google Scholar]
- Borrás T, Rowlette LL, Erzurum SC, Epstein DL. Adenoviral reporter gene transfer to the human trabecular meshwork does not alter aqueous humor outflow. Relevance for potential gene therapy of glaucoma. Gene Ther. 1999;6:515–524. doi: 10.1038/sj.gt.3300860. [DOI] [PubMed] [Google Scholar]
- Borrás T, Xue W, Choi VW, Bartlett JS, Li G, Samulski RJ, Chisolm SS. Mechanisms of AAV transduction in glaucoma-associated human trabecular meshwork cells. J. Gene Med. 2006;8:589–602. doi: 10.1002/jgm.886. [DOI] [PubMed] [Google Scholar]
- Budenz DL, Bennett J, Alonso L, Maguire A. In vivo gene transfer into murine corneal endothelial and trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 1995;36:2211–2215. [PubMed] [Google Scholar]
- Buie LK, Karim MZ, Smith MH, Borrás T. Development of a model of elevated intraocular pressure in rats by gene transfer of bone morphogenetic protein 2. Invest. Ophthalmol. Vis. Sci. 2013;54:5441–5455. doi: 10.1167/iovs.13-11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buie LK, Rasmussen CA, Porterfield EC, Ramgolam VS, Choi VW, Markovic-Plese S, Samulski RJ, Kaufman PL, Borrás T. Self-complementary AAV virus (scAAV) safe and long-term gene transfer in the trabecular meshwork of living rats and monkeys. Invest. Ophthalmol. Vis. Sci. 2010;51:236–248. doi: 10.1167/iovs.09-3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel II, Kaplowitz K, Schuman JS, Loewen NA, Group TS. Outcomes of ab interno trabeculectomy with the trabectome after failed trabeculectomy. Br. J. Ophthalmol. 2014;99:258–262. doi: 10.1136/bjophthalmol-2013-304717. Others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussel II, Kaplowitz K, Schuman JS, Loewen NA, Trabectome Study Group Outcomes of ab interno trabeculectomy with the trabectome by degree of angle opening. Br. J. Ophthalmol. 2015;99:914–919. doi: 10.1136/bjophthalmol-2014-305577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am. J. Ophthalmol. 2011;152:340–344. e2. doi: 10.1016/j.ajo.2011.05.029. [DOI] [PubMed] [Google Scholar]
- Cavet ME, Vittitow JL, Impagnatiello F, Ongini E, Bastia E. Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest. Ophthalmol. Vis. Sci. 2014;55:5005–5015. doi: 10.1167/iovs.14-14515. [DOI] [PubMed] [Google Scholar]
- Chalberg TW, Genise HL, Vollrath D, Calos MP. φC31 Integrase Confers Genomic Integration and Long-Term Transgene Expression in Rat Retina. Invest. Ophthalmol. Vis. Sci. 2005;46:2140–2146. doi: 10.1167/iovs.04-1252. [DOI] [PubMed] [Google Scholar]
- Challa P, Luna C, Liton PB, Chamblin B, Wakefield J, Ramabhadran R, Epstein DL, Gonzalez P. Lentiviral mediated gene delivery to the anterior chamber of rodent eyes. Mol. Vis. 2005;11:425–430. [PMC free article] [PubMed] [Google Scholar]
- Chang JYH, Stamer WD, Bertrand J, Read AT, Marando CM, Ethier CR, Overby DR. Role of nitric oxide in murine conventional outflow physiology. Am. J. Physiol. Cell Physiol. 2015;309:C205–14. doi: 10.1152/ajpcell.00347.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay M, Wolfe D, Mata M, Huang S, Glorioso JC, Fink DJ. Long-term neuroprotection achieved with latency-associated promoter-driven herpes simplex virus gene transfer to the peripheral nervous system. Mol. Ther. 2005;12:307–313. doi: 10.1016/j.ymthe.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Coffin J, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor Laboratory Press; Plainview, NY: 1997. p. xv. [PubMed] [Google Scholar]
- Comes N, Borrás T. Functional delivery of synthetic naked siRNA to the human trabecular meshwork in perfused organ cultures. Mol. Vis. 2007;13:1363–1374. [PubMed] [Google Scholar]
- Compton AA, Malik HS, Emerman M. Host gene evolution traces the evolutionary history of ancient primate lentiviruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120496. doi: 10.1098/rstb.2012.0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Cai X, Naash MI. Nonviral ocular gene therapy: assessment and future directions. Curr. Opin. Mol. Ther. 2008;10:456–463. [PMC free article] [PubMed] [Google Scholar]
- De Groef L, Van Hove I, Dekeyster E, Stalmans I, Moons L. MMPs in the trabecular meshwork: promising targets for future glaucoma therapies? Invest. Ophthalmol. Vis. Sci. 2013;54:7756–7763. doi: 10.1167/iovs.13-13088. [DOI] [PubMed] [Google Scholar]
- DelloRusso C, Scott JM, Hartigan-O'Connor D, Salvatori G, Barjot C, Robinson AS, Crawford RW, Brooks SV, Chamberlain JS. Functional correction of adult mdx mouse muscle using gutted adenoviral vectors expressing full-length dystrophin. Proc. Natl. Acad. Sci. U. S. A. 2002;99:12979–12984. doi: 10.1073/pnas.202300099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva S, Lotta LT, Jr, Burris CA, Bowers WJ. Virion-associated cofactor high-mobility group DNA-binding protein-1 facilitates transposition from the herpes simplex virus/Sleeping Beauty amplicon vector platform. Hum. Gene Ther. 2010;21:1615–1622. doi: 10.1089/hum.2010.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Yun H, Yang E, Schuman JS. Stem cells from trabecular meshwork home to TM tissue in vivo. Invest. Ophthalmol. Vis. Sci. 2013;54:1450–1459. doi: 10.1167/iovs.12-11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eljarrat-Binstock E, Orucov F, Aldouby Y, Frucht-Pery J, Domb AJ. Charged nanoparticles delivery to the eye using hydrogel iontophoresis. J. Control. Release. 2008;126:156–161. doi: 10.1016/j.jconrel.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Ellis DZ, Sharif NA, Dismuke WM. Endogenous regulation of human Schlemm's canal cell volume by nitric oxide signaling. Invest. Ophthalmol. Vis. Sci. 2010;51:5817–5824. doi: 10.1167/iovs.09-5072. [DOI] [PubMed] [Google Scholar]
- Ethier CR, Wada S, Chan D, Stamer WD. Experimental and numerical studies of adenovirus delivery to outflow tissues of perfused human anterior segments. Invest. Ophthalmol. Vis. Sci. 2004;45:1863–1870. doi: 10.1167/iovs.03-1133. [DOI] [PubMed] [Google Scholar]
- Fan BJ, Wang DY, Lam DSC, Pang CP. Gene mapping for primary open angle glaucoma. Clin. Biochem. 2006;39:249–258. doi: 10.1016/j.clinbiochem.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flachsbarth K, Kruszewski K, Jung G, Jankowiak W, Riecken K, Wagenfeld L, Richard G, Fehse B, Bartsch U. Neural Stem Cell--Based Intraocular Administration of Ciliary Neurotrophic Factor Attenuates the Loss of Axotomized Ganglion Cells in Adult MiceNS Cell--Based Intraocular Administration of CNTF. Invest. Ophthalmol. Vis. Sci. 2014;55:7029–7039. doi: 10.1167/iovs.14-15266. [DOI] [PubMed] [Google Scholar]
- Francis AW, Kagemann L, Wollstein G, Ishikawa H, Folz S, Overby DR, Sigal IA, Wang B, Schuman JS. Morphometric analysis of aqueous humor outflow structures with spectral-domain optical coherence tomography. Invest. Ophthalmol. Vis. Sci. 2012;53:5198–5207. doi: 10.1167/iovs.11-9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Quigley HA, Gelb L, Tan J, Margolis J, Shah SN, Kim EE, Zimmerman T, Hahn SR. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest. Ophthalmol. Vis. Sci. 2007;48:5052–5057. doi: 10.1167/iovs.07-0290. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Stephan DA, Russell P, Tamm ER. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: Identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp. Eye Res. 2009;88:1020–1032. doi: 10.1016/j.exer.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchshofer R, Tamm ER. The role of TGF-β in the pathogenesis of primary open-angle glaucoma. Cell Tissue Res. 2012;347:279–290. doi: 10.1007/s00441-011-1274-7. [DOI] [PubMed] [Google Scholar]
- Fuchshofer R, Yu AHL, Welge-Lüssen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest. Ophthalmol. Vis. Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- [1.10.16];Gene Therapy Clinical Trials Worldwide [WWW Document] URL http://www.abedia.com/wiley/indications.php.
- Gerometta R, Spiga M-G, Borrás T, Candia OA. Treatment of sheep steroid-induced ocular hypertension with a glucocorticoid-inducible MMP1 gene therapy virus. Invest. Ophthalmol. Vis. Sci. 2010;51:3042–3048. doi: 10.1167/iovs.09-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. J. Gene Med. 2013;15:65–77. doi: 10.1002/jgm.2698. [DOI] [PubMed] [Google Scholar]
- Giovingo M, Nolan M, McCarty R, Pang I-H, Clark AF, Beverley RM, Schwartz S, Stamer WD, Walker L, Grybauskas A, Skuran K, Kuprys PV, Yue BYJT, Knepper PA. sCD44 overexpression increases intraocular pressure and aqueous outflow resistance. Mol. Vis. 2013;19:2151–2164. [PMC free article] [PubMed] [Google Scholar]
- Goins WF, Huang S, Cohen JB, Glorioso JC. Engineering HSV-1 vectors for gene therapy. Methods Mol. Biol. 2014;1144:63–79. doi: 10.1007/978-1-4939-0428-0_5. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Tan JCH. Tissue-based caldesmon silencing by naked siRNA increases F-actin in the human trabecular meshwork. Invest. Ophthalmol. Vis. Sci. 2014;55:5653–5653. [Google Scholar]
- Gonzalez P, Caballero M, Liton PB, Stamer WD, Epstein DL. Expression analysis of the matrix GLA protein and VE-cadherin gene promoters in the outflow pathway. Invest. Ophthalmol. Vis. Sci. 2004;45:1389–1395. doi: 10.1167/iovs.03-0537. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Li G, Qiu J, Wu J, Luna C. Role of microRNAs in the trabecular meshwork. J. Ocul. Pharmacol. Ther. 2014;30:128–137. doi: 10.1089/jop.2013.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieger JC, Choi VW, Samulski RJ. Production and characterization of adeno-associated viral vectors. Nat. Protoc. 2006;1:1412–1428. doi: 10.1038/nprot.2006.207. [DOI] [PubMed] [Google Scholar]
- Hann CR, Bentley MD, Vercnocke A, Ritman EL, Fautsch MP. Imaging the aqueous humor outflow pathway in human eyes by three-dimensional micro-computed tomography (3D micro-CT). Exp. Eye Res. 2011;92:104–111. doi: 10.1016/j.exer.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser MA, Aboobakar IF, Liu Y, Miura S, Whigham BT, Challa P, Wheeler J, Williams A, Santiago-Turla C, Qin X, Rautenbach RM, Ziskind A, Ramsay M, Uebe S, Song L, Safi A, Vithana EN, Mizoguchi T, Nakano S, Kubota T, Hayashi K, Manabe S-I, Kazama S, Mori Y, Miyata K, Yoshimura N, Reis A, Crawford GE, Pasutto F, Carmichael TR, Williams SEI, Ozaki M, Aung T, Khor C-C, Stamer WD, Ashley-Koch AE, Allingham RR. Genetic variants and cellular stressors associated with exfoliation syndrome modulate promoter activity of a lncRNA within the LOXL1 locus. Hum. Mol. Genet. 2015;24:6552–6563. doi: 10.1093/hmg/ddv347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husso T, Ylä-Herttuala S, Turunen MP. A New Gene Therapy Approach for Cardiovascular Disease by Non-coding RNAs Acting in the Nucleus. Mol. Ther. Nucleic Acids. 2014;3:e197. doi: 10.1038/mtna.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivics Z, Hackett PB, Plasterk RH, Izsvák Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell. 1997;91:501–510. doi: 10.1016/s0092-8674(00)80436-5. [DOI] [PubMed] [Google Scholar]
- Ivics Z, Izsvák Z. Nonviral gene delivery with the sleeping beauty transposon system. Hum. Gene Ther. 2011;22:1043–1051. doi: 10.1089/hum.2011.143. [DOI] [PubMed] [Google Scholar]
- Johnen S, Izsvák Z, Stöcker M, Harmening N, Salz AK, Walter P, Thumann G. Sleeping Beauty transposon-mediated transfection of retinal and iris pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 2012;53:4787–4796. doi: 10.1167/iovs.12-9951. [DOI] [PubMed] [Google Scholar]
- Johnson M, Erickson K. Mechanisms and routes of aqueous humor drainage. Principles and Practice of Ophthalmology. 2000;4:2577–2595. [Google Scholar]
- Junglas B, Kuespert S, Seleem AA, Struller T, Ullmann S, Bösl M, Bosserhoff A, Köstler J, Wagner R, Tamm ER, Fuchshofer R. Connective tissue growth factor causes glaucoma by modifying the actin cytoskeleton of the trabecular meshwork. Am. J. Pathol. 2012;180:2386–2403. doi: 10.1016/j.ajpath.2012.02.030. [DOI] [PubMed] [Google Scholar]
- Kajaste-Rudnitski A, Naldini L. Cellular innate immunity and restriction of viral infection: implications for lentiviral gene therapy in human hematopoietic cells. Hum. Gene Ther. 2015;26:201–209. doi: 10.1089/hum.2015.036. [DOI] [PubMed] [Google Scholar]
- Kaplowitz K, Bussel II, Honkanen R, Schuman JS, Loewen NA. Review and meta-analysis of abinterno trabeculectomy outcomes. Br. J. Ophthalmol. 2016 doi: 10.1136/bjophthalmol-2015-307131. doi:10.1136/bjophthalmol-2015-307131. [DOI] [PubMed] [Google Scholar]
- Kaplowitz K, Schuman JS, Loewen NA. Techniques and outcomes of minimally invasive trabecular ablation and bypass surgery. Br. J. Ophthalmol. 2014;98:579–585. doi: 10.1136/bjophthalmol-2013-304256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz K, Wang S, Bilonick R, Oatts JT, Grippo T, Loewen NA. Randomized Controlled Comparison of Titanium-Sapphire Versus Standard Q-Switched Nd: YAG Laser Trabeculoplasty. J. Glaucoma. 2015 doi: 10.1097/IJG.0000000000000317. doi:10.1097/IJG.0000000000000317. [DOI] [PubMed] [Google Scholar]
- Keller KE, Bradley JM, Vranka JA, Acott TS. Segmental versican expression in the trabecular meshwork and involvement in outflow facility. Invest. Ophthalmol. Vis. Sci. 2011;52:5049–5057. doi: 10.1167/iovs.10-6948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Sun YY, Yang Y-F, Bradley JM, Acott TS. Perturbation of hyaluronan synthesis in the trabecular meshwork and the effects on outflow facility. Invest. Ophthalmol. Vis. Sci. 2012;53:4616–4625. doi: 10.1167/iovs.12-9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Vranka JA, Haddadin RI, Kang M-H, Oh D-J, Rhee DJ, Yang Y-F, Sun YY, Kelley MJ, Acott TS. The effects of tenascin C knockdown on trabecular meshwork outflow resistance. Invest. Ophthalmol. Vis. Sci. 2013;54:5613–5623. doi: 10.1167/iovs.13-11620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khare PD, Loewen N, Teo W, Barraza RA, Saenz DT, Johnson DH, Poeschla EM. Durable, safe, multi-gene lentiviral vector expression in feline trabecular meshwork. Mol. Ther. 2008;16:97–106. doi: 10.1038/sj.mt.6300318. [DOI] [PubMed] [Google Scholar]
- Kimura H, Sakamoto T, Cardillo JA, Spee C, Hinton DR, Gordon EM, Anderson WF, Ryan SJ. Retrovirus-mediated suicide gene transduction in the vitreous cavity of the eye: feasibility in prevention of proliferative vitreoretinopathy. Hum. Gene Ther. 1996;7:799–808. doi: 10.1089/hum.1996.7.7-799. [DOI] [PubMed] [Google Scholar]
- Kizhatil K, Ryan M, Marchant JK, Henrich S, John SWM. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014;12:e1001912. doi: 10.1371/journal.pbio.1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppel F. Production of high-capacity adenovirus vectors. Methods Mol. Biol. 2014;1089:211–229. doi: 10.1007/978-1-62703-679-5_15. [DOI] [PubMed] [Google Scholar]
- Kumar S, Shah S, Tang HM, Smith M, Borrás T, Danias J. Tissue plasminogen activator in trabecular meshwork attenuates steroid induced outflow resistance in mice. PLoS One. 2013;8:e72447. doi: 10.1371/journal.pone.0072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing JM, Gober MD, Golembewski EK, Thompson SM, Gyure KA, Yarowsky PJ, Aurelian L. Intranasal Administration of the Growth-Compromised HSV-2 Vector ΔRR Prevents Kainate-Induced Seizures and Neuronal Loss in Rats and Mice. Mol. Ther. 2006;13:870–881. doi: 10.1016/j.ymthe.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence SD, Buie LK, Borras T. Direct Delivery of Synthetic Naked siRNA to Trabecular Meshwork in Living Rats. Invest. Ophthalmol. Vis. Sci. 2011;52:6616–6616. [Google Scholar]
- Lee A, Nolan A, Watson J, Tristem M. Identification of an ancient endogenous retrovirus, predating the divergence of the placental mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;368:20120503. doi: 10.1098/rstb.2012.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Gabelt BT, Faralli JA, Peters DM, Brandt CR, Kaufman PL, Bhattacharya SK. COCH transgene expression in cultured human trabecular meshwork cells and its effect on outflow facility in monkey organ cultured anterior segments. Invest. Ophthalmol. Vis. Sci. 2010;51:2060–2066. doi: 10.1167/iovs.09-4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee ES, Rasmussen CA, Filla MS, Slauson SR, Kolb AW, Peters DM, Kaufman PL, Gabelt BT, Brandt CR. Prospects for lentiviral vector mediated prostaglandin F synthase gene delivery in monkey eyes in vivo. Curr. Eye Res. 2014;39:859–870. doi: 10.3109/02713683.2014.884593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J. Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim S-H, Tran-Viet K-N, Yanovitch TL, Freedman SF, Klemm T, Call W, Powell C, Ravichandran A, Metlapally R, Nading EB, Rozen S, Young TL. CYP1B1, MYOC, and LTBP2 mutations in primary congenital glaucoma patients in the United States. Am. J. Ophthalmol. 2013;155:508–517. e5. doi: 10.1016/j.ajo.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SC. Endoscopic and transscleral cyclophotocoagulation for the treatment of refractory glaucoma. J. Glaucoma. 2008;17:238–247. doi: 10.1097/IJG.0b013e31815f2539. [DOI] [PubMed] [Google Scholar]
- Lipps HJ, Jenke ACW, Nehlsen K, Scinteie MF, Stehle IM, Bode J. Chromosome-based vectors for gene therapy. Gene. 2003;304:23–33. doi: 10.1016/s0378-1119(02)01215-5. [DOI] [PubMed] [Google Scholar]
- Liton PB, Liu X, Stamer WD, Challa P, Epstein DL, Gonzalez P. Specific targeting of gene expression to a subset of human trabecular meshwork cells using the chitinase 3-like 1 promoter. Invest. Ophthalmol. Vis. Sci. 2005;46:183–190. doi: 10.1167/iovs.04-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu ML, Winther BL, Kay MA. Pseudotransduction of hepatocytes by using concentrated pseudotyped vesicular stomatitis virus G glycoprotein (VSV-G)-Moloney murine leukemia virus-derived retrovirus vectors: comparison of VSV-G and amphotropic vectors for hepatic gene transfer. J. Virol. 1996;70:2497–2502. doi: 10.1128/jvi.70.4.2497-2502.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Brandt CR, Gabelt BT, Bryar PJ, Smith ME, Kaufman PL. Herpes simplex virus mediated gene transfer to primate ocular tissues. Exp. Eye Res. 1999;69:385–395. doi: 10.1006/exer.1999.0711. [DOI] [PubMed] [Google Scholar]
- Liu X, Hu Y, Filla MS, Gabelt BT, Peters DM, Brandt CR, Kaufman PL. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol. Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- Loewen N, Bahler C, Teo W-L, Whitwam T, Peretz M, Xu R, Fautsch MP, Johnson DH, Poeschla EM. Preservation of aqueous outflow facility after second-generation FIV vector-mediated expression of marker genes in anterior segments of human eyes. Invest. Ophthalmol. Vis. Sci. 2002;43:3686–3690. [PubMed] [Google Scholar]
- Loewen N, Fautsch MP, Peretz M, Bahler CK, Cameron JD, Johnson DH, Poeschla EM. Genetic modification of human trabecular meshwork with lentiviral vectors. Hum. Gene Ther. 2001;12:2109–2119. doi: 10.1089/10430340152677449. [DOI] [PubMed] [Google Scholar]
- Loewen N, Fautsch MP, Teo W-L, Bahler CK, Johnson DH, Poeschla EM. Long-term, targeted genetic modification of the aqueous humor outflow tract coupled with noninvasive imaging of gene expression in vivo. Invest. Ophthalmol. Vis. Sci. 2004a;45:3091–3098. doi: 10.1167/iovs.04-0366. [DOI] [PubMed] [Google Scholar]
- Loewen N, Leske DA, Cameron JD, Chen Y, Whitwam T, Simari RD, Teo W-L, Fautsch MP, Poeschla EM, Holmes JM. Long-term retinal transgene expression with FIV versus adenoviral vectors. Mol. Vis. 2004b;10:272–280. [PubMed] [Google Scholar]
- Loewen N, Leske DA, Chen Y, Teo W-L, Saenz DT, Peretz M, Holmes JM, Poeschla EM. Comparison of wild-type and class I integrase mutant-FIV vectors in retina demonstrates sustained expression of integrated transgenes in retinal pigment epithelium. J. Gene Med. 2003;5:1009–1017. doi: 10.1002/jgm.447. [DOI] [PubMed] [Google Scholar]
- Loewen N, Poeschla EM. Lentiviral vectors. Adv. Biochem. Eng. Biotechnol. 2005;99:169–191. doi: 10.1007/10_007. [DOI] [PubMed] [Google Scholar]
- Loewen RT, Roy P, Park DB, Jensen A, Scott G, Cohen-Karni D, Fautsch MP, Schuman JS, Loewen NA. A Porcine Anterior Segment Perfusion and Transduction Model With Direct Visualization of the Trabecular Meshwork. Invest. Ophthalmol. Vis. Sci. 2016;57:1338–1344. doi: 10.1167/iovs.15-18125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losa C, Calvo P, Castro E, Vila-Jato JL, Alonso MJ. Improvement of ocular penetration of amikacin sulphate by association to poly(butylcyanoacrylate) nanoparticles. J. Pharm. Pharmacol. 1991;43:548–552. doi: 10.1111/j.2042-7158.1991.tb03534.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Deng Z-L, Luo X, Tang N, Song W-X, Chen J, Sharff KA, Luu HH, Haydon RC, Kinzler KW, Vogelstein B, He T-C. A protocol for rapid generation of recombinant adenoviruses using the AdEasy system. Nat. Protoc. 2007;2:1236–1247. doi: 10.1038/nprot.2007.135. [DOI] [PubMed] [Google Scholar]
- Mangeot PE, Nègre D, Dubois B, Winter AJ, Leissner P, Mehtali M, Kaiserlian D, Cosset FL, Darlix JL. Development of minimal lentivirus vectors derived from simian immunodeficiency virus (SIVmac251) and their use for gene transfer into human dendritic cells. J. Virol. 2000;74:8307–8315. doi: 10.1128/jvi.74.18.8307-8315.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi P, Fraefel C, Epstein AL. Herpes simplex virus type 1 (HSV-1)-derived recombinant vectors for gene transfer and gene therapy. Methods Mol. Biol. 2015;1254:269–293. doi: 10.1007/978-1-4939-2152-2_20. [DOI] [PubMed] [Google Scholar]
- Martin E, Jiang Y, Johnstone MA. Schlemm's canal (SC) and Distal Aqueous Outflow Pathways: New scanning EM (SEM) Preparation Technique Permits Identifying Unique Structural Relationships. Invest. Ophthalmol. Vis. Sci. 2014;55:5683–A0385. [Google Scholar]
- Mattick JS, Rinn JL. Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 2015;22:5–7. doi: 10.1038/nsmb.2942. [DOI] [PubMed] [Google Scholar]
- McDowell CM, Tebow HE, Wordinger RJ, Clark AF. Smad3 is necessary for transforming growth factor-beta2 induced ocular hypertension in mice. Exp. Eye Res. 2013;116:419–423. doi: 10.1016/j.exer.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- Millar JC, Pang I-H, Wang W-H, Wang Y, Clark AF. Effect of immunomodulation with anti-CD40L antibody on adenoviral-mediated transgene expression in mouse anterior segment. Mol. Vis. 2008;14:10–19. [PMC free article] [PubMed] [Google Scholar]
- Milligan MJ, Lipovich L. Pseudogene-derived lncRNAs: emerging regulators of gene expression. Front. Genet. 2014;5:476. doi: 10.3389/fgene.2014.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa Y, Marino P, Verlengia G, Uchida H, Goins WF, Yokota S, Geller DA, Yoshida O, Mester J, Cohen JB, Glorioso JC. Herpes simplex viral-vector design for efficient transduction of nonneuronal cells without cytotoxicity. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E1632–41. doi: 10.1073/pnas.1423556112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM. Development of a self-inactivating lentivirus vector. J. Virol. 1998;72:8150–8157. doi: 10.1128/jvi.72.10.8150-8157.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai H, Montini E, Fuess S, Storm TA, Grompe M, Kay MA. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nat. Genet. 2003;34:297–302. doi: 10.1038/ng1179. [DOI] [PubMed] [Google Scholar]
- Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatts JT, Zhang Z, Tseng H, Shields MB, Sinard JH, Loewen NA. In vitro and in vivo comparison of two suprachoroidal shunts. Invest. Ophthalmol. Vis. Sci. 2013;54:5416–5423. doi: 10.1167/iovs.13-11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh D-J, Kang MH, Ooi YH, Choi KR, Sage EH, Rhee DJ. Overexpression of SPARC in human trabecular meshwork increases intraocular pressure and alters extracellular matrix. Invest. Ophthalmol. Vis. Sci. 2013;54:3309–3319. doi: 10.1167/iovs.12-11362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JC. Gene transfer vectors derived from equine infectious anemia virus. Gene Ther. 1998;5:1481–1487. doi: 10.1038/sj.gt.3300768. [DOI] [PubMed] [Google Scholar]
- Peschansky VJ, Wahlestedt C. Non-coding RNAs as direct and indirect modulators of epigenetic regulation. Epigenetics. 2014;9:3–12. doi: 10.4161/epi.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeschla EM, Wong-Staal F, Looney DJ. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- Puthenveetil G, Scholes J, Carbonell D, Xia P, Qureshi N, Zeng L, Li S, Yu Y, Hiti AL, Yee JK, Malik P. Successful Correction of the Human Beta-Thalassemia Major Phenotype Using a Lentiviral Vector. Blood. 2004 doi: 10.1182/blood-2004-04-1427. [DOI] [PubMed] [Google Scholar]
- Ramírez JM, Ramírez AI, Salazar JJ, Rojas B, De Hoz R, Triviño A. Schlemm's canal and the collector channels at different developmental stages in the human eye. Cells Tissues Organs. 2004;178:180–185. doi: 10.1159/000082248. [DOI] [PubMed] [Google Scholar]
- Rao PV, Deng P, Maddala R, Epstein DL, Li C-Y, Shimokawa H. Expression of dominant negative Rho-binding domain of Rho-kinase in organ cultured human eye anterior segments increases aqueous humor outflow. Mol. Vis. 2005;11:288–297. [PubMed] [Google Scholar]
- Robertson JV, Golesic E, Gauldie J, West-Mays JA. Ocular gene transfer of active TGF-beta induces changes in anterior segment morphology and elevated IOP in rats. Invest. Ophthalmol. Vis. Sci. 2010;51:308–318. doi: 10.1167/iovs.09-3380. [DOI] [PubMed] [Google Scholar]
- Romero Z, Campo-Fernandez B, Wherley J, Kaufman ML, Urbinati F, Cooper AR, Hoban MD, Baldwin KM, Lumaquin D, Wang X, Senadheera S, Hollis RP, Kohn DB. The human ankyrin 1 promoter insulator sustains gene expression in a β-globin lentiviral vector in hematopoietic stem cells. Mol Ther Methods Clin Dev. 2015;2:15012. doi: 10.1038/mtm.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr. Eye Res. 1989;8:1233–1240. doi: 10.3109/02713688909013902. [DOI] [PubMed] [Google Scholar]
- Saenz D, Barraza R, Loewen N, Teo W, Poeschla E. Production and use of feline immunodeficiency virus (FIV)-based lentiviral vectors. Gene transfer: a Cold Spring. 2006 [Google Scholar]
- Saenz DT, Loewen N, Peretz M, Whitwam T, Barraza R, Howell KG, Holmes JM, Good M, Poeschla EM. Unintegrated lentivirus DNA persistence and accessibility to expression in nondividing cells: analysis with class I integrase mutants. J. Virol. 2004;78:2906–2920. doi: 10.1128/JVI.78.6.2906-2920.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber AT, Toris CB. Effects of a Schlemm canal scaffold on collector channel ostia in human anterior segments. Exp. Eye Res. 2013;30:e7. doi: 10.1016/j.exer.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Schuman JS, Chang W, Wang N, de Kater AW, Allingham RR. Excimer laser effects on outflow facility and outflow pathway morphology. Invest. Ophthalmol. Vis. Sci. 1999;40:1676–1680. [PubMed] [Google Scholar]
- Selbach JM, Posielek K, Steuhl K-P, Kremmer S. Episcleral venous pressure in untreated primary open-angle and normal-tension glaucoma. Ophthalmologica. 2005;219:357–361. doi: 10.1159/000088378. [DOI] [PubMed] [Google Scholar]
- Shen Y, Dong L-F, Zhou R-M, Yao J, Song Y-C, Yang H, Jiang Q, Yan B. Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: a clinical and in vitro study. J. Cell. Mol. Med. 2016;20:537–548. doi: 10.1111/jcmm.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard AR, Millar JC, Pang I-H, Jacobson N, Wang W-H, Clark AF. Adenoviral gene transfer of active human transforming growth factor-{beta}2 elevates intraocular pressure and reduces outflow facility in rodent eyes. Invest. Ophthalmol. Vis. Sci. 2010;51:2067–2076. doi: 10.1167/iovs.09-4567. [DOI] [PubMed] [Google Scholar]
- Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR. Herpes simplex virus-mediated gene delivery to the rodent visual system. Invest. Ophthalmol. Vis. Sci. 2000;41:1392–1401. [PubMed] [Google Scholar]
- Spiga M-G, Borrás T. Development of a gene therapy virus with a glucocorticoid-inducible MMP1 for the treatment of steroid glaucoma. Invest. Ophthalmol. Vis. Sci. 2010;51:3029–3041. doi: 10.1167/iovs.09-4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springelkamp H, Iglesias AI, Cuellar-Partida G, Amin N, Burdon KP, van Leeuwen EM, Gharahkhani P, Mishra A, van der Lee SJ, Hewitt AW, Rivadeneira F, Viswanathan AC, Wolfs RCW, Martin NG, Ramdas WD, van Koolwijk LM, Pennell CE, Vingerling JR, Mountain JE, Uitterlinden AG, Hofman A, Mitchell P, Lemij HG, Wang JJ, Klaver CCW, Mackey DA, Craig JE, van Duijn CM, MacGregor S. ARHGEF12 influences the risk of glaucoma by increasing intraocular pressure. Hum. Mol. Genet. 2015;24:2689–2699. doi: 10.1093/hmg/ddv027. [DOI] [PubMed] [Google Scholar]
- Stamer WD, Acott TS. Current understanding of conventional outflow dysfunction in glaucoma. Curr. Opin. Ophthalmol. 2012;23:135–143. doi: 10.1097/ICU.0b013e32834ff23e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Lei Y, Boussommier-Calleja A, Overby DR, Ethier CR. eNOS, a pressure-dependent regulator of intraocular pressure. Invest. Ophthalmol. Vis. Sci. 2011;52:9438–9444. doi: 10.1167/iovs.11-7839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int. Rev. Immunol. 2002;21:123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–670. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- Sultan M, Blondeau P. Episcleral venous pressure in younger and older subjects in the sitting and supine positions. J. Glaucoma. 2003;12:370–373. doi: 10.1097/00061198-200308000-00013. [DOI] [PubMed] [Google Scholar]
- Sumida GM, Stamer WD. S1P2 receptor regulation of sphingosine-1-phosphate effects on conventional outflow physiology. Am. J. Physiol. Cell Physiol. 2011;300:C1164–71. doi: 10.1152/ajpcell.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Hewitt AW, Masson G, Helgason A, DeWan A, Sigurdsson A, Jonasdottir A, Gudjonsson SA, Magnusson KP, Stefansson H, Lam DSC, Tam POS, Gudmundsdottir GJ, Southgate L, Burdon KP, Gottfredsdottir MS, Aldred MA, Mitchell P, St Clair D, Collier DA, Tang N, Sveinsson O, Macgregor S, Martin NG, Cree AJ, Gibson J, Macleod A, Jacob A, Ennis S, Young TL, Chan JCN, Karwatowski WSS, Hammond CJ, Thordarson K, Zhang M, Wadelius C, Lotery AJ, Trembath RC, Pang CP, Hoh J, Craig JE, Kong A, Mackey DA, Jonasson F, Thorsteinsdottir U, Stefansson K. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat. Genet. 2010;42:906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turunen TAK, Laakkonen JP, Alasaarela L, Airenne KJ, Ylä-Herttuala S. Sleeping Beauty-baculovirus hybrid vectors for long-term gene expression in the eye. J. Gene Med. 2014;16:40–53. doi: 10.1002/jgm.2756. [DOI] [PubMed] [Google Scholar]
- Ueyama K, Mori K, Shoji T, Omata H, Gehlbach PL, Brough DE, Wei LL, Yoneya S. Ocular localization and transduction by adenoviral vectors are serotype-dependent and can be modified by inclusion of RGD fiber modifications. PLoS One. 2014;9:e108071. doi: 10.1371/journal.pone.0108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buskirk EM. Trabeculotomy in the immature, enucleated human eye. Invest. Ophthalmol. Vis. Sci. 1977;16:63–66. [PubMed] [Google Scholar]
- van Koolwijk LME, Ramdas WD, Ikram MK, Jansonius NM, Pasutto F, Hysi PG, Macgregor S, Janssen SF, Hewitt AW, Viswanathan AC, ten Brink JB, Hosseini SM, Amin N, Despriet DDG, Willemse-Assink JJM, Kramer R, Rivadeneira F, Struchalin M, Aulchenko YS, Weisschuh N, Zenkel M, Mardin CY, Gramer E, Welge-Lüssen U, Montgomery GW, Carbonaro F, Young TL, DCCT/EDIC Research Group. Bellenguez C, McGuffin P, Foster PJ, Topouzis F, Mitchell P, Wang JJ, Wong TY, Czudowska MA, Hofman A, Uitterlinden AG, Wolfs RCW, de Jong PTVM, Oostra BA, Paterson AD, Wellcome Trust Case Control Consortium 2. Mackey DA, Bergen AAB, Reis A, Hammond CJ, Vingerling JR, Lemij HG, Klaver CCW, van Duijn CM. Common genetic determinants of intraocular pressure and primary open-angle glaucoma. PLoS Genet. 2012;8:e1002611. doi: 10.1371/journal.pgen.1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittitow JL, Garg R, Rowlette L-LS, Epstein DL, O'Brien ET, Borrás T. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol. Vis. 2002;8:32–44. [PubMed] [Google Scholar]
- Wald A, Corey L. Persistence in the population: epidemiology, transmission. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore PS, Roizman B, Whitley R, Yamanishi K, editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge: 2011. [Google Scholar]
- Wang J, Liu X, Zhong Y. Rho/Rho-associated kinase pathway in glaucoma (Review). Int. J. Oncol. 2013;43:1357–1367. doi: 10.3892/ijo.2013.2100. [DOI] [PubMed] [Google Scholar]
- Wang W-H, McNatt LG, Pang I-H, Millar JC, Hellberg PE, Hellberg MH, Steely HT, Rubin JS, Fingert JH, Sheffield VC, Stone EM, Clark AF. Increased expression of the WNT antagonist sFRP-1 in glaucoma elevates intraocular pressure. J. Clin. Invest. 2008;118:1056–1064. doi: 10.1172/JCI33871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock JN, Daigre C, Al-Rubeai M. Introduction to viral vectors. Methods Mol. Biol. 2011;737:1–25. doi: 10.1007/978-1-61779-095-9_1. [DOI] [PubMed] [Google Scholar]
- Wilson JM. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2009;96:151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Fleenor DL, Hellberg PE, Pang I-H, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest. Ophthalmol. Vis. Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- Wordinger RJ, Sharma T, Clark AF. The role of TGF-β2 and bone morphogenetic proteins in the trabecular meshwork and glaucoma. J. Ocul. Pharmacol. Ther. 2014;30:154–162. doi: 10.1089/jop.2013.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749–1751. doi: 10.1126/science.1083413. [DOI] [PubMed] [Google Scholar]
- Yáñez-Muñoz RJ, Balaggan KS, MacNeil A, Howe SJ, Schmidt M, Smith AJ, Buch P, MacLaren RE, Anderson PN, Barker SE, Duran Y, Bartholomae C, von Kalle C, Heckenlively JR, Kinnon C, Ali RR, Thrasher AJ. Effective gene therapy with nonintegrating lentiviral vectors. Nat. Med. 2006;12:348–353. doi: 10.1038/nm1365. [DOI] [PubMed] [Google Scholar]
- Yan Z, Lei-Butters DCM, Zhang Y, Zak R, Engelhardt JF. Hybrid Adeno-Associated Virus Bearing Nonhomologous Inverted Terminal Repeats Enhances Dual-Vector Reconstruction of Minigenes In Vivo. Hum. Gene Ther. 2006;0:061221035427001. doi: 10.1089/hum.2006.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Zak R, Zhang Y, Engelhardt JF. Inverted terminal repeat sequences are important for intermolecular recombination and circularization of adeno-associated virus genomes. J. Virol. 2005;79:364–379. doi: 10.1128/JVI.79.1.364-379.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Wang X-Q, Li Y-J, Shan K, Yang H, Wang Y-N-Z, Yao M-D, Liu C, Li X-M, Shen Y, Liu J-Y, Cheng H, Yuan J, Zhang Y-Y, Jiang Q, Yan B. Long non-coding RNA MALAT1 regulates retinal neurodegeneration through CREB signaling. EMBO Mol. Med. 2016 doi: 10.15252/emmm.201505725. doi:10.15252/emmm.201505725. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zehnpfennig D, Deissler H, Polack A, Herr D, Bornkamm GW, Kurzeder C. B-cell-specific elements enhance sustained gene expression mediated by self-replicating extrachromosomal vectors. Mol. Med. Rep. 2010;3:689–692. doi: 10.3892/mmr_00000318. [DOI] [PubMed] [Google Scholar]
- Zhang G-R, Zhao H, Cao H, Geller AI. Overexpression of either lysine-specific demethylase-1 or CLOCK, but not Co-Rest, improves long-term expression from a modified neurofilament promoter, in a helper virus-free HSV-1 vector system. Brain Res. 2012;1436:157–167. doi: 10.1016/j.brainres.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Maddala R, Rao PV. Novel molecular insights into RhoA GTPase-induced resistance to aqueous humor outflow through the trabecular meshwork. Am. J. Physiol. Cell Physiol. 2008;295:C1057–70. doi: 10.1152/ajpcell.00481.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dhaliwal AS, Tseng H, Kim JD, Schuman JS, Weinreb RN, Loewen NA. Outflow tract ablation using a conditionally cytotoxic feline immunodeficiency viral vector. Invest. Ophthalmol. Vis. Sci. 2014;55:935–940. doi: 10.1167/iovs.13-12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Nair A, Weng H, Tsai Y-T, Hu Z, Tang L. Intraocular pressure changes: an important determinant of the biocompatibility of intravitreous implants. PLoS One. 2011;6:e28720. doi: 10.1371/journal.pone.0028720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulliger R, Conley SM, Naash MI. Non-viral therapeutic approaches to ocular diseases: An overview and future directions. J. Control. Release. 2015;219:471–487. doi: 10.1016/j.jconrel.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]