Abstract
Introduction
Crohn’s disease (CD) is a chronic and progressive disease in which the long-term management is important. This study sought to assess treatment persistence and dose escalation in the maintenance phase with adalimumab (ADA) or infliximab (IFX) in a Japanese real-world setting.
Methods
A retrospective analysis was conducted using the Japan Medical Data Center database. CD patients with either ADA or IFX prescriptions between January 2012 and February 2015 were included. Outcomes of interest were (1) failure in the induction phase (defined as switch or discontinuation) and (2) persistence in the maintenance phase (defined as the absence of switch or discontinuation over 12 months since maintenance initiation).
Results
Overall, 133 patients (53 ADA; 80 IFX) were included. Of them, treatment failed in 26 patients (19.6%) in the induction phase. During the induction phase, there was a trend towards fewer treatment failures with ADA than IFX (88.7% vs. 75.0%; p = 0.051). Of those who completed induction, 64 patients (33 ADA; 31 IFX) had at least 12 months of valid insurance enrolment after the initiation of maintenance and 13 (5 ADA; 8 IFX) had either switch or discontinuation within 12 months after the initiation of maintenance. Probabilities of switch or discontinuation over 12 months after the maintenance date were 15.2% and 20.9% for ADA and IFX groups, respectively (p-log rank = 0.7764).
Conclusion
Japanese patients have a high primary response to anti-tumor necrosis factor therapy in the real-world setting, in line with the results of clinical trials. This initial therapeutic advantage can be lost during the maintenance phase, leading to dose escalation, treatment switch, or discontinuation. This study suggests that those events occurred in comparable proportions of patients treated with either ADA or IFX. However, these findings should be considered with caution given the retrospective nature and small size of the study.
Funding
Abbvie GK, Tokyo, Japan.
Electronic supplementary material
The online version of this article (doi:10.1007/s12325-016-0406-6) contains supplementary material, which is available to authorized users.
Keywords: Adalimumab, Anti-TNF, Crohn’s disease, Discontinuation, Dose escalation, Gastroenterology, Infliximab, Persistence, Switch
Introduction
Crohn’s disease (CD) is a chronic inflammatory condition of the gastrointestinal tract belonging to the group of Inflammatory Bowel Diseases (IBD) that results in a considerably decreased quality of life. The incidence and prevalence of CD are increasing with time and in different regions around the world [1]. The highest reported prevalence rates per 100,000 persons were 322 in Europe, 318.5 in North America, and 67.9 in Asia and the Middle East [1]. In Japan, the prevalence of CD reached 26.8 per 100,000 in 2010 [2].
Given the chronic and progressive nature of the disease, the main goal of the treatment is to stop progression, reduce and control symptoms, reduce the risks of complication, and maintain nourishment to improve the quality of life of patients [3]. Therefore, the long-term management as well as treatment persistence is very important.
CD management includes drug, nutritional and surgical therapies. However, it has been shown that the standard therapies for CD patients have several limitations; treatment with immunosuppressants is associated with adverse events risk [4], and treatment with corticosteroids is not adequate for the maintenance of clinical remission [5]. Monoclonal antibodies, targeting tumor necrosis factor (TNF)-α, are considered the treatment of choice in patients with CD [6].
In Japan, the available anti-TNF treatments for CD are infliximab (IFX) and adalimumab (ADA). IFX was the first TNF-α chimeric monoclonal antibody introduced for the treatment of CD in 2002. It is administered intravenously at a dose of 5 mg/kg body weight at weeks 0, 2, and 6 for induction. The standard dose of maintenance IFX therapy is 5 mg/kg of body weight administered every 8 weeks [7]. ADA is administered subcutaneously and is prescribed at an induction dose of 160 mg at week 0 and 80 mg at week 2. The standard maintenance dose is 40 mg administered every 2 weeks [8–10].
Clinical trials have demonstrated the efficacy and safety of ADA and IFX in the induction and maintenance of remission in patients with moderate to severe CD, in particular in Japanese patients [7, 8, 11–13]. However, treatment outcomes in the real-world setting might differ from clinical trials, as patients’ profiles may be more diverse and patients may not strictly adhere to a regular injection schedule. Several real-world studies have demonstrated that despite the high response rate, the response to ADA or IFX is lost over time, leading the efficacy of treatment waning (and consequently dose increase) to treatment discontinuation or to treatment switch [14–22]. However, to date, there is limited real-world Japan-specific evidence in the literature regarding loss of response and treatment persistence.
The primary objectives of this study were: (1) to estimate the probability of treatment failure in the induction phase with ADA or IFX and (2) to estimate the probability of persistence in the maintenance phase with ADA or IFX. The secondary objective was to estimate the probability of dose escalation in the maintenance phase with ADA or IFX. Exploratory objectives were to estimate the probabilities of adverse events and surgical procedures in the maintenance phase.
Methods
Study Design and Data Source
This study was a retrospective observational cohort analysis of CD patients enrolled in the Japan Medical Data Center (JMDC) database.
The JMDC database is an administrative database comprising medical and pharmacy claims data for salaried workers of medium-to large-scale companies and their family members. This database included up to 2.3 million insured persons between 2005 and 2015, representing approximately 1.8% of the Japanese population. A database extract was used in the current study that included patients with at least one health insurance claim associated with a CD diagnosis and at least one prescription of ADA or IFX between December 2011 and May 2015.
The database was completely anonymized and contained patient demographic information (gender, age, insurance type, and reason of withdrawal), inpatient and outpatient medical and pharmacy claims data, with clinical diagnoses coded under the International Classification of Diseases 10th revision (ICD-10) classification [23], Japan-specific standard disease codes [24], drug prescriptions information coded according to the Anatomical Therapeutic Chemical (ATC) classification, as well as health care procedures defined using Japan-specific standardized procedure codes [25, 26].
Ethical Considerations
The protocol was submitted to Kitasato University Hospital’s Ethics Committee, but the study was exempted from ethical review, since no personally identifiable data were used in the JMDC extraction for the current study. Patients were identified via a unique anonymized code assigned by JMDC and the authors did not have access to the original data containing personal information.
Study Population
The study population was identified based on members’ medical and pharmacy claims data. To meet the objectives of the study, two populations of interest were identified. The population #1—called induction analyses population—included patients who: (1) had a prescription of ADA or IFX between January 2012 and February 2015 (the first prescription date of ADA or IFX was called the “index date”); who (2) had a claim associated with a confirmed diagnosis of CD before or at the same time than the index date, between January 2012 and February 2015; who had (3) no prescription of ADA or IFX, initiated or ongoing, in the 6 months before the index date, and (4) a minimum of 17 weeks of valid insurance status after the index date. A 17-week follow-up was required, as the discontinuation of IFX treatment was assessed up to 17 weeks after the index date.
The population #2—called maintenance analyses population—was a subpopulation of population #1 who (1) completed the induction phase of ADA or IFX and entered the maintenance phase with the same treatment and who (2) had at least 12 months of valid insurance status after the initiation of maintenance. In addition, two subgroups of interest were defined: patients with or without immunosuppressant therapy after the index date.
Definitions of Variables and Outcomes
A confirmed CD diagnosis was identified in medical claims data, using the ICD-10 code “K50” and a diagnosis without a suspicious flag in the database. Prescriptions of ADA or IFX were identified in the pharmacy claims data based on ATC codes “L04B-”.
Patients’ demographic characteristics were directly available in the database, and included age, gender, type of insurance policy holder (primary policy holder or family member), and length of follow-up. Pharmacy claim records included prescribed dose (first, second, and third), average dose intake in induction phase or maintenance phase, and average time between prescriptions.
Treatment Failure in the Induction Phase
Treatment failure in the induction phase was assessed in the population #1, and was defined as switch or discontinuation in the induction phase. Switch in the induction phase was defined as at least one new prescription of ADA within 17 weeks among patients who had initiated induction on IFX treatment or vice versa.
For the IFX group, discontinuation in the induction phase was defined as: (1) the absence of the fourth IFX injection, which is theoretically scheduled on week 16, over 17 weeks after the index date and (2) (a) no second IFX prescription up to 4 weeks after the index date, or (b) no third IFX prescription up to 6 weeks after the second IFX injection, or (c) no fourth IFX prescription up to 10 weeks after the third IFX injection. In short, patients were considered to discontinue in the case of deviation from the theoretical injection schedule by more than 2 weeks. For ADA prescriptions, discontinuation in induction phase was defined as: (1) the absence of the third ADA injection up to 2 weeks after the expected date of the third injection and (2) (a) no second ADA injection up to 4 weeks after the index date or (b) no third ADA injections up to 4 weeks after the second ADA injection.
In addition, if an event, such as infection, surgery, or immunostimulant(s) therapy, occurred between two prescription dates, then patients were allowed an additional 2 weeks of delay before the next ADA or IFX prescription (for example, if a patient had an infection between the second and third injections, and the third injection was within 8 weeks of the second one, then the patient was not classified as discontinued IFX treatment).
Persistence in the Maintenance Phase
Persistence in the maintenance phase was assessed among the population #2 and was defined as the absence of switch or discontinuation in the post-induction phase.
Switch in the maintenance phase was defined as at least one prescription of ADA within 2 weeks following an IFX prescription among patients initiating maintenance treatment with IFX, or vice versa.
For the IFX group, discontinuation in the maintenance phase was defined as: (1) no IFX prescription up to 70 days (10 weeks) after the last prescription date and (2) no infections, surgeries, or immunostimulant therapy within 12 weeks from the last prescription date.
For the ADA group, a patient was considered to have discontinued treatment after the ‘nth’ prescription at time tn with a quantity qn if there was: (1) no ADA prescription renewal up to (t n + 1 + 14) days (t n + 1 was defined as the expected next prescription date, according to the quantity prescribed q n) and (2) no infections, surgeries, or immunostimulant therapy within 12 weeks from the last prescription date tn. In other words, discontinuation was assumed to occur if no prescription renewal occurred within 2 weeks following the expected date of renewal and there was no infection justifying the delay to prescription renewal.
Dose Escalation in Maintenance Phase
Dose escalation in the maintenance phase was assessed among population #2 and was defined as an increase in maintenance therapy dosage or a reduction in the interval between maintenance therapy prescriptions.
For IFX prescriptions, dose escalation was defined as: (1) at least two successive prescriptions with a number of vials exceeding by 2 the number previously prescribed (e.g., three vials to five vials, three vials to six vials, etc.), or at least two successive intervals between injections of less than 6 weeks (5 mg/kg every 6 weeks or shorter instead of 5 mg/kg every 8 weeks). For ADA prescriptions, dose escalation was defined as: (1) at least two successive prescriptions which showed an increase in maintenance therapy dosage (e.g., from 40 mg every 2 weeks to 80 mg every 2 weeks) or (2) at least two successive reductions in the frequency of prescriptions, defined as an increase in the number of prescribed syringes per prescribed duration.
Exploratory Outcomes
Adverse events were identified in medical claims data using ICD-10 codes; these included infections, anemia, neoplasms, nutritional and metabolic diseases, diseases of the respiratory system, and diseases of the digestive system. Adverse events were defined as confirmed diagnosis (without a suspicious flag) occurring for the first time in patient history, after the index date. Surgery was identified using Japan-specific procedure codes [26]. Immunostimulants, immunosuppressant, leucocyte removal therapy, and enteral nutrition therapies were identified using ATC codes. Complications (fistulas or obstructions) were assessed in the pre-index period (3 months prior to the index date), and were defined using ICD-10 codes with a confirmed diagnosis. The full list of codes (ATC, ICD-10, and procedure codes) is provided in the Supplementary Materials (Tables S5, S6, and S7).
Statistical Analyses
Categorical variables were compared between treatment groups using the Chi-square test or Fisher’s exact test, and continuous variables were compared using the Student’s t or Wilcoxon tests. Study outcomes were described over 12 months and 24 months of follow-up, respectively. Times from maintenance date to switch, to discontinuation, and to dose escalation were described using Kaplan–Meier survival curves, stratified by treatment group and for all patients (ADA or IFX). Kaplan–Meier curves were compared between treatments using the Log-rank test.
Only the month and year were available for the date of claims and diagnoses in the JMDC database. However, the full date including the day (DD/MM/YYYY) was available for the majority of prescriptions (dates were missing in 6% of ADA and IFX prescriptions) and health care procedures (dates were missing in 9% of procedures). An imputation algorithm was created to complete missing dates of ADA and IFX prescriptions, based on the theoretical delay between prescriptions (the detailed algorithm is provided in the Supplementary Material [Fig. S10]). The other missing days (dates of prescriptions other than ADA or IFX and dates of procedures and diagnoses) were imputed using the corresponding claim date when available; alternatively, the 15th of the month was used.
A sensitivity analysis was conducted to assess the impact of changing the definition of discontinuation on the results of persistence in the maintenance phase. The time window in the definition was varied by ±7 days (i.e., for the IFX group, the time widow was varied from 63 to 77 days; and for the ADA group, it was varied from (t n + 1 + 7) days to (t n + 1 + 21) days).
Analyses were conducted using the SAS statistical software version 9.3 (SAS Inc., Cary, NC, USA).
Results
Study Population
From January 2012 to February 2015, 150 CD patients treated with ADA or IFX met the inclusion and exclusion criteria. Of these patients, 133 (53 ADA; 80 IFX) had at least 17 weeks of follow-up after the index date (Fig. S1, Supplementary Material).
The majority of patients were male (84.2%), with individual-level insurance, and the mean age was 34.4 years (SD = 13.0. The mean follow-up time was 18.4 months (SD = 9.7). The average number of biologics prescriptions after the index date was 17.1 (SD = 12.5) for patients initiating their treatment with ADA and 10.3 (SD = 5.8) for the IFX group (Table 1).
Table 1.
Patient demographic and clinical characteristics
| Population #1 (n = 133) | Population #2 (n = 107) | |||||||
|---|---|---|---|---|---|---|---|---|
| IFX (n = 80) | ADA (n = 53) | Total (n = 133) | p value* | IFX (n = 60) | ADA (n = 47) | Total (n = 107) | p value* | |
| Gender, n (%) | ||||||||
| Female | 11 (13.8%) | 10 (18.9%) | 21 (15.8%) | 0.43 | 8 (13.3%) | 8 (17.0%) | 16 (15.0%) | 0.60 |
| Male | 69 (86.3%) | 43 (81.1%) | 112 (84.2%) | 52 (86.7%) | 39 (83.0%) | 91 (85.1%) | ||
| Age at the index date, years | ||||||||
| Mean (SD) | 33.9 (13.2) | 35.2 (12.9) | 34.4 (13.0) | 0.58 | 32.9 (12.6) | 33.7 (12.7) | 33.2 (12.6) | 0.76 |
| Median | 32.5 | 34 | 33 | 32 | 33 | 32 | ||
| Type of insurance, n (%) | ||||||||
| Family | 24 (30.0%) | 20 (37.7%) | 44 (33.1%) | 0.35 | 19 (31.7%) | 18 (38.3%) | 37 (34.6%) | 0.47 |
| Individual | 56 (70.0%) | 33 (62.3%) | 89 (66.9%) | 41 (68.3%) | 29 (61.7%) | 70 (65.4%) | ||
| Number of prescriptions of ADA or IFX after the index date (index date included) | ||||||||
| Mean (SD) | 10.3 (5.8) | 17.1 (12.5) | 13.0 (9.7) | 0.001 | 10.8 (5.3) | 18.5 (12.4) | 14.2 (9.9) | 0.001 |
| Median | 8 | 14 | 11 | 9 | 15 | 12 | ||
| Follow-up time after the index date, months | ||||||||
| Mean (SD) | 17.8 (9.5) | 19.2 (9.8) | 18.40 (9.6) | 0.41 | 17.9 (9.6) | 19.4 (9.7) | 18.6 (9.6) | 0.43 |
| Median | 17.5 | 18.1 | 18 | 16.9 | 18.6 | 18.1 | ||
| Surgery after the index date, n (%) | ||||||||
| 15 (18.8%) | 9 (17.0%) | 24 (18.1%) | 0.80 | 10 (16.7%) | 8 (17.0%) | 18 (16.8%) | 0.96 | |
| Immunostimulants after the index date, n (%) | ||||||||
| 0 | 1 (1.9%) | 1 (0.8%) | 0.40 | – | – | – | 0.96 | |
| Immunosuppressant prescription after the index date, n (%) | ||||||||
| 26 (32.5%) | 17 (32.1%) | 43 (32.3%) | 0.96 | 17 (28.3%) | 14 (29.8%) | 31 (29.0%) | 0.87 | |
| Enteral nutrition prescription after the index date, n (%) | ||||||||
| 53 (66.3%) | 38 (71.7%) | 91 (68.4%) | 0.51 | 40 (66.7%) | 35 (74.5%) | 75 (70.1%) | 0.38 | |
| Time between the first and second prescriptions, days | ||||||||
| Mean (SD) | 23.2 (25.8) | 14.3 (9.6) | 19.7 (21.3) | 0.02 | 16.6 (9.1) | 13.8 (7.7) | 15.4 (8.6) | 0.10 |
| Median | 14 | 14 | 14 | 14 | 14 | 14 | ||
| Time between the second and the third prescriptions, days | ||||||||
| Mean (SD) | 37.7 (24.4) | 22.4 (17.7) | 31.6 (23.1) | 0.0001 | 30.3 (7.4) | 21.7 (12.5) | 26.5 (10.8) | <0.0001 |
| Median | 28 | 15 | 28 | 28 | 16 | 28 | ||
| Average time between two successive prescriptions during maintenance phase, days | ||||||||
| Mean (SD) | – | – | – | – | 31.1 (14.4) | 27.5 (12.3) | 29.5 (13.6) | 0.18 |
| Median | – | – | – | – | 33.5 | 27 | 28.3 | |
| First dosea | ||||||||
| Mean (SD) | 3.3 (0.9) | 3.7 (0.9) | – | – | 3.2 (0.7) | 3.8 (0.8) | – | – |
| Median | 3 | 4 | – | – | 3 | 4 | – | |
| Second dosea | ||||||||
| Mean (SD) | 3.3 (1.0) | 2.5 (1.3) | – | – | 3.2 (0.7) | 2.6 (1.2) | – | – |
| Median | 3 | 2 | – | – | 3 | 2 | – | |
| Third dosea | ||||||||
| Mean (SD) | 3.2 (1.3) | 2.2 (1.5) | – | – | 3.2 (0.8) | 2.3 (1.4) | – | – |
| Median | 3 | 2 | – | – | 3 | 2 | – | |
| Average induction dosea | ||||||||
| Mean (SD) | 3.3 (1.0) | 3.1 (0.8) | – | – | 3.2 (0.7) | 3.2 (0.8) | – | – |
| Median | 3 | 3 | – | – | 3 | 3 | – | |
| Average maintenance dosea | ||||||||
| Mean (SD) | – | – | – | – | 2.3 (1.1) | 2.1 (0.9) | – | – |
| Median | – | – | – | – | 2 | 2 | – | |
ADA adalimumab, IFX infliximab, SD standard deviation
* Continuous variables were compared using the student test or the Wilcoxon test; categorical variables were compared using the Chi-square test or the Fisher’s exact test
aDose unit: for ADA, 1 dose = Injection 40 mg Syringe 0.8 mL and for IFX, 1 dose = I.V Infusion 100 mg
Around 32% of patients were prescribed immunosuppressant therapy after the index date in both treatment groups. Nutrition prescriptions were frequent; 71.7% of patients who initiated their treatment with ADA had enteral nutrition prescription after the index date, compared to 66.2% in IFX group (Table 1).
Failure in Induction Phase
Among patients who initiated their treatment with ADA or IFX (population #1—133 patients), 26 patients (19.6%) switched or discontinued their treatment during the induction phase.
Among patients who initiated their treatment with ADA, 88.7% completed induction phase and moved to maintenance phase with the same treatment, compared to 75.0% for IFX group (p = 0.051) (Table 2). Treatment discontinuation occurred in 11.3% and 21.3% of patients in the ADA and IFX groups, respectively. No patient in the ADA group switched treatment during induction phase (Table 2).
Table 2.
Probabilities of failure and persistence in induction phase by treatment group
| IFX (n = 80) | ADA (n = 53) | Total (n = 133) | p value* | |
|---|---|---|---|---|
| Discontinuation, n (%) | ||||
| No | 63 (78.8%) | 47 (88.7%) | 110 (82.7%) | 0.14 |
| Yes | 17 (21.3%) | 6 (11.3%) | 23 (17.3%) | |
| Switch, n (%) | ||||
| No | 77 (96.3%) | 53 (100.0%) | 130 (97.7%) | 0.28 |
| Yes | 3 (3.8%) | 0 | 3 (2.3%) | |
| Persistence, n (%) | ||||
| No | 20 (25.0%) | 6 (11.3%) | 26 (19.6%) | 0.051 |
| Yes | 60 (75.0%) | 47 (88.7%) | 107 (80.5%) | |
ADA adalimumab, IFX infliximab
* Continuous variables were compared using the student test or the Wilcoxon test; categorical variables were compared using the Chi-square test or the Fisher’s exact test
Persistence in Maintenance Phase
Among patients who had completed induction phase and entered maintenance phase with the same treatment (107 patients), 64 patients (33 ADA, 31 IFX) had at least 12 months of valid insurance enrolment after the initiation of maintenance (population #2). Of these, 13 patients (5 ADA, 8 IFX) had at least either a switch or discontinuation event within 12 months after the initiation of maintenance (Table S1, Supplementary Material).
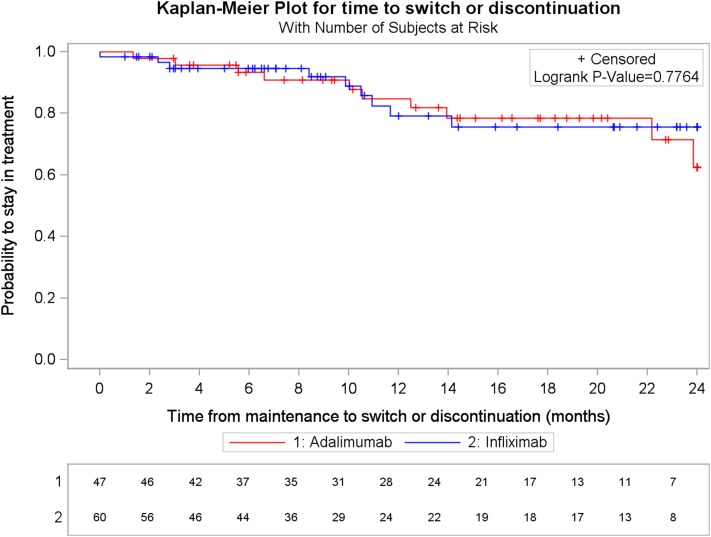
Over 12 months following the initiation of maintenance, the probability of a switch or discontinuation was 15.2% for the ADA group versus 20.9% for IFX group (p-log rank = 0.7764) (Fig. 1).
Fig. 1.
Kaplan–Meier plot of time to switch or discontinuation by treatment group. ADA adalimumab, IFX infliximab
The sensitivity analyses showed that the difference in the probability of switch or discontinuation remained non-significant when the time window was decreased or increased by 7 days (decreased time window: 28.0% vs 23.4% for ADA and IFX, respectively, p-log rank = 0.2142; increased time window: 13.1% vs 19.0% for ADA and IFX, respectively, p-log rank = 0.7572).
Dose Escalation in Maintenance Phase
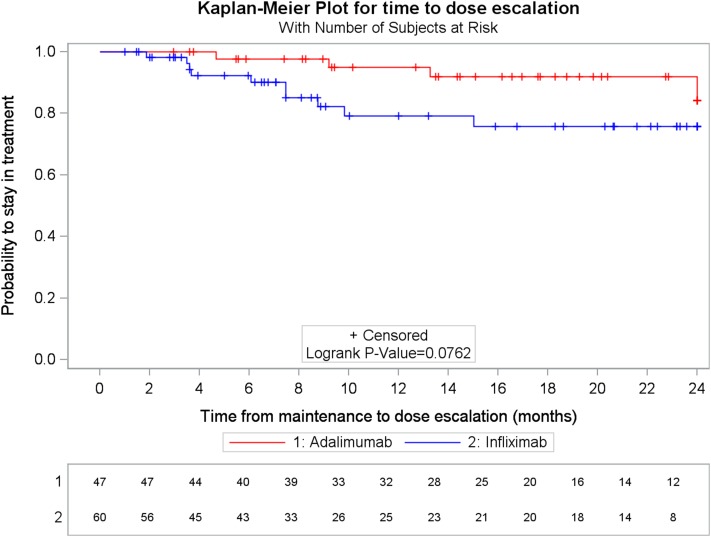
Among population #2, eight patients (12.5%) had dose escalation within 12 months after the initiation of maintenance. The probabilities of dose escalation at 12 months after the initiation of maintenance were 5.0% and 20.7% for ADA and IFX, respectively (p-log rank = 0.0762) (Fig. 2).
Fig. 2.
Kaplan–Meier plot of time to dose escalation by treatment group. ADA adalimumab, IFX infliximab
For each treatment group, dose escalation was assessed for patients treated with or without immunosuppressant therapy after the index date. For patients treated with IFX, the 12-month probability of dose escalation was significantly higher in patients treated with immunosuppressant compared to patients not treated with immunosuppressant (60.6% vs. 7.1%, respectively; p-log rank <0.0001). Dose escalation occurred in one ADA patient without immunosuppressant prescription, and in no patients treated with immunosuppressant (Table S2, Supplementary Material).
Complications and Concomitant Therapies
The probability of surgical procedures over 12 months following the index date was 15.4% and 17.5% for ADA and IFX groups, respectively. Two patients in the IFX group had leucocyte removal therapy within 12 months after the index date (beginning of the induction phase), the data provided in the Supplementary Material (Figs. S7 and S8). The probabilities of any adverse event within 12 months after the maintenance start date were 51.4% and 69.4%, respectively, in ADA and IFX groups. The most frequent adverse events were infections (24.4% of patients), anemia (20.9%), and diseases of the respiratory system (12.7%) (Table 3).
Table 3.
Adverse events occurrence over 12 months after the index date (patients with at least 12 months of follow-up after ADA or IFX treatment initiation)
| IFX (n = 49) | ADA (n = 37) | Total (n = 86) | p value* | |
|---|---|---|---|---|
| Adverse events (at least one event), n (%) | 34 (69.4%) | 19 (51.4%) | 53 (61.6%) | 0.09 |
| Certain infectious and parasitic diseases, n (%) | 15 (30.6%) | 6 (16.2%) | 21 (24.4%) | 0.12 |
| Intestinal infectious diseases | 5 (10.2%) | 1 (2.7%) | 6 (7.0%) | 0.23 |
| Mycoses | 3 (6.1%) | 5 (13.5%) | 8 (9.3%) | 0.28 |
| Other infection | 8 (16.3%) | 0 | 8 (9.3%) | 0.01 |
| Anemia, n (%) | 10 (20.4%) | 8 (21.6%) | 18 (20.9%) | 0.89 |
| Diseases of the digestive system, n (%) | 3 (6.1%) | 1 (2.7%) | 4 (4.7%) | 0.63 |
| Diseases of the respiratory system, n (%) | 8 (16.3%) | 3 (8.1%) | 11 (12.8%) | 0.34 |
| Malignant neoplasms, n (%) | 0 | 1 (2.7%) | 1 (1.2%) | 0.43 |
| Other adverse events, n (%)a | 10 (20.4%) | 5 (13.5%) | 15 (17.4%) | 0.40 |
ADA adalimumab, IFX infliximab
* Continuous variables were compared using the student test or the Wilcoxon test; categorical variables were compared using the Chi-square test or the Fisher’s exact test
aList of other adverse events: Endocrine, nutritional and metabolic diseases, diseases of the skin and subcutaneous tissue, diseases of the musculoskeletal system and connective tissue, diseases of the genitourinary system, symptoms, signs and abnormal clinical and laboratory findings, injury, poisoning, and certain other consequences of external causes
Discussion
This retrospective analysis of data from the JMDC claims database for 150 Japanese patients with CD treated with ADA or IFX between 2012 and 2015 showed trends towards less instances of treatment failure during the induction phase and higher persistence and lower probability of dose escalation during the maintenance phase in ADA, compared to IFX. However, the differences were not statistically significant (p = 0.051).
To the best of our knowledge, this is the first retrospective study that describes ADA and IFX treatment-related events based on Japan-specific claims data. The added value of this study is the provision of real-world data which could be seen to be of higher relevance in terms of understanding actual clinical practice compared to clinical trial or single center studies.
In the current study, patients were considered to have failed induction treatment if they switched or discontinued index treatment within 4 or 14 weeks, for ADA and IFX initiators, respectively. In the literature, failure in the induction phase was most often called primary non-response and defined as no clinical improvement at the end of the anti-TNF induction phase [22, 27]. Our data showed that ≈20% of patients did not complete induction therapy on ADA or IFX, which is in agreement with previously published clinical data on the efficacy of anti-TNFs [22, 23, 28]. A recent study by Viazis et al. (Greece) reported that among 161 CD patients treated with ADA of IFX, 29 (18%) were considered as primary non responders to induction therapy [22]. Farkas et al. (Hungary) reported that 88% of CD patients completed induction therapy with IFX [28]. In Vermeire et al. (Belgium), 74% of CD patients responded to IFX induction therapy [23]. This suggests that our definition of treatment failure, based on claims data only, provides a good proxy of primary non-response.
However, our analysis also showed that frequent changes in treatment occurred during the maintenance phases: switches, discontinuations, or dose escalations. Overall, the probability of switch or discontinuation over 12 months following the initial response to induction therapy was 20.3% (15.2% for ADA group and 25.8% for IFX group). These rates are consistent with published data on anti-TNF persistence from retrospective studies in other countries—rates of discontinuation ranged from 15% to 35% [15, 20, 29]. Gonzaga et al. (US) reported that 27% of 153 CD patients, who received maintenance therapy with IFX, discontinued their treatment within 12 months of maintenance therapy [15]. A recent multicenter retrospective study in the US by Rosh et al. showed that the 12-month probability of discontinuation after ADA initiation in IBD patients was approximately 15% (Kaplan–Meir estimate) [20]. A recent Italian administrative database study in CD adult patients treated with anti-TNFs (ADA, IFX, and etanercept) showed that treatment persistence (defined as the absence of switches or treatment interruptions after anti-TNF treatment initiation) was somewhat lower than in our study for ADA patients: 78% for IFX and 65% for ADA [29].
The secondary outcome assessed in the current study was the rate of dose escalation, which may be interpreted as a proxy of secondary loss of response. In our study, eight patients, representing 12.5% of the study population entering the maintenance phase, required dose increase and or interval reduction between ADA or IFX prescriptions during a 12-month follow-up period. This rate is lower than that obtained from previously published clinical trial data (12-month rates ranged between 23% and 46%) [30]. This wide variability in loss of response rates across studies could be explained by the diversity of definitions of this outcome. In 2011, a literature review by Ben-Horin et al. discussed the disparity in the definitions of loss of response across studies on anti-TNF efficacy; some authors defined loss of response as a quantifiable re-emergence of symptoms (for example, a change in the CD Activity Index [CDAI]), and others qualified dose escalation as a “decisive definition”, while others characterized it by switch, discontinuation, or an occurrence of a surgical intervention [30]. On the other hand, when we compared our study results to real-world published data, where authors adopted dose escalation as a definition of loss of response, we found also higher rates ranging from 23% to 55% [14, 16, 17, 19, 21, 22, 31], but the majority of those studies had a longer follow-up compared to our study. The rate of dose escalation for IFX (22.6% over 12 months) is close to the lower end of the range reported above. Under the current definition of dose escalation, patients treated with IFX who started their dosage at 10 mg/kg were not considered as dose escalated, since the weight was not available in JMDC database. For ADA group, the rate of dose escalation was found to be lower, which likely results from the fact that the higher dose has not yet been approved in Japan [32, 33].
Although treatment switches, discontinuations or dose escalations were interpreted as events of failure or non-response to the treatment and that the definition of those events took into account intercurrent infections, concomitant surgeries, or immunostimulant therapies, it should be noted that a proportion of these events could have occurred for other factors, such as patient compliance, medication reactions, choices made by practitioners, or patient preference.
In the current study, a minority of patients had immunosuppressant prescriptions after anti-TNF treatment initiation (32% approximately in both groups). Several studies suggested that the risk–benefit profile of immunosuppressants therapy is different in Japanese patients compared to Western patients [24, 25, 34], and thus, in the real-world setting, physicians may be cautious on adding immunosuppressant. Furthermore, the results showed that among patients treated with IFX, there was a significantly higher probability of dose escalation in patients who were prescribed immunosuppressant therapy compared to those without immunosuppressants. The addition of immunosuppressants, such as azathioprine, is expected to reduce the risk of loss of response [35]. However, the higher rate of dose escalation in our study, interpreted as proxy of loss of response, in patients treated with the combination could be explained by the fact that physicians may have prescribed immunosuppressants before increasing the IFX dose in patients with worsening symptoms (in other words, patients treated with the combination and IFX only were not comparable). In addition, IFX patients not treated with immunosuppressants could have been switched to other treatments instead of having their IFX dose increased.
The male-to-female ratio was 4:1 in our JMDC extract, and 5:1 in the 133 patients meeting selection criteria. This ratio is higher than those reported by Japan-specific epidemiological studies (2.6:1–2.3:1) [36, 37]. However, given that the JMDC data contain 26% more males compared to Japan census, we would expect to have a 3-to-1 male-to-female ratio approximately. The higher proportion of males in our sample is probably attributable to sampling variability.
Besides the small sample size, several limitations of our study should be noted. First, the sensitivity analysis demonstrated that the results were sensitive to a change in the definition of discontinuation during maintenance phase. As expected, the probability of discontinuation increased in both the groups when the time window decreased. However, the difference in discontinuation rate between the treatment groups remained non-significant.
Second, given the nature of the data available in the JMDC database, we did not have any clinical record, such as CDAI or physician’s assessment of severity, and could only develop proxies of treatment failure in induction phase or loss of response in maintenance phase. However, the comparison of results to the literature suggests that those proxies were actually informative. Besides, although surgeries might indicate a loss of response [38], it was not possible to make the distinction between surgeries performed for treating intestinal complications due to loss of response or for treating lesion which pre-existed before the index date. Although the rate of pre-index date surgical treatments was similar to both the groups (Table S4, Supplementary Material), these factors may have led to an underestimation of loss of response rates observed in this study compared to other studies showing higher rates of loss of response [14, 16–19, 21, 22, 30, 31].
In the current study, the probability of adverse events was investigated in an exploratory manner, based on the claims database. Due to the nature of the JMDC data, the oldest reported diagnosis of an adverse event did not necessarily correspond the first date of the symptoms appearance, which would have led an overestimation of adverse events. Nevertheless, further evaluation of this outcome is required based on data from prospective studies or chart reviews.
Another limitation of this study is that the results were based on crude comparison between ADA and IFX groups. The possibility of performing a matched comparison was considered, but the sample size was too small. Although the clinical status of patients could not be obtained, patients’ demographic and clinical characteristics were similar between treatment groups (Table 1 and Table S4). In addition, given the retrospective nature of the study and the small sample size, the results should be interpreted with caution.
Finally, some limitations related to the nature of claims databases should also be mentioned; diagnostic codes are for a reimbursement purpose which may be different from diagnosis in a chart. Only the month and the year of medical and claims data were available. The full dates were missing for 6% of the prescriptions and 9% of the procedures, respectively. These issues, however, were unlikely to differ across groups or outcomes.
Conclusion
This is the first retrospective study that describes ADA and IFX treatment-related events based on a real-life claims database in Japan. The current study demonstrated that Japanese CD patients have a high primary response to ADA and IFX therapy in the real-world setting, and suggests that outcomes achieved in the real world are comparable to those observed in clinical trials. However, response can be lost during the maintenance phase in a certain number of patients, leading to treatment switch, discontinuation, or dose escalation. This study further suggests that treatment persistence was similar to patients treated with ADA compared to those treated with IFX. However, the results should be considered with caution due to the retrospective nature and small size of the study. Given that CD is a chronic and a progressive disease, further research on the causes of discontinuations and ways to improve persistence are recommended for the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship, article processing charges and the open access charge for this study were funded by Abbvie GK, Tokyo, Japan. The study sponsor was involved in all the stages of the study research and manuscript preparation, but all the authors participated in the design of the study and contributed to the manuscript development. All the authors had full access to all the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given the final approval to the version to be published.
Disclosures
Kaoru Yokoyama has served as a speaker for AbbVie GK, Kyowa Hakko Kirin, Tanabe Mitsubishi, Asahi Kasei, and EA Pharma, and consulting fee from Kyorin and her institution received research grant from JIMRO, Yakult, Eisai, Tsumura, Chugai, MDS, Taiho, Tanabe Mitsubishi, and Shionogi outside the submitted work.
Kiyotaka Yamazaki is a full-time employee of Abbvie GK, which funded the study.
Miiko Katafuchi is a full-time employee of Abbvie GK, which funded the study.
Sameh Ferchichi is a full-time employee of Creativ-Ceutical, which received funding from Abbvie GK.
Compliance with Ethics Guidelines
The protocol was submitted to Kitasato University Hospital’s Ethics Committee, but the study was exempted from ethical review, since no personally identifiable data were used in the JMDC extraction for the current study. Patients were identified via a unique anonymized code assigned by JMDC, and the authors did not have access to the original data containing personal information.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/9E76F06048096649.
References
- 1.Molodecky NA, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142(1):46–54 e42. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Nakano T, et al. Inflammatory bowel disease, epidemiology. Seijinbyo to Seikatsushukanbyo. 2014;44(3):251–5. [Google Scholar]
- 3.Ueno F, et al. Evidence-based clinical practice guidelines for Crohn’s disease, integrated with formal consensus of experts in Japan. J Gastroenterol. 2013;48(1):31–72. doi: 10.1007/s00535-012-0673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manz M, et al. Therapy of steroid-resistant inflammatory bowel disease. Digestion. 2012;86(Suppl 1):11–15. doi: 10.1159/000341952. [DOI] [PubMed] [Google Scholar]
- 5.Summers RW, et al. National cooperative Crohn’s disease study: results of drug treatment. Gastroenterology. 1979;77(4 Pt 2):847–869. [PubMed] [Google Scholar]
- 6.Rubin DT, Uluscu O, Sederman R. Response to biologic therapy in Crohn’s disease is improved with early treatment: an analysis of health claims data. Inflamm Bowel Dis. 2012;18(12):2225–2231. doi: 10.1002/ibd.22925. [DOI] [PubMed] [Google Scholar]
- 7.Hanauer SB, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 8.Colombel JF, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 9.Hanauer SB, et al. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130(2):323–333. doi: 10.1053/j.gastro.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Sandborn WJ, et al. Adalimumab for maintenance treatment of Crohn’s disease: results of the CLASSIC II trial. Gut. 2007;56(9):1232–1239. doi: 10.1136/gut.2006.106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogata H, et al. Safety of adalimumab and predictors of adverse events in 1693 Japanese patients with Crohn’s disease. J Crohns Colitis. 2016;10(9):1033–41. doi: 10.1093/ecco-jcc/jjw060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe M, et al. Adalimumab for the induction and maintenance of clinical remission in Japanese patients with Crohn’s disease. J Crohns Colitis. 2012;6(2):160–173. doi: 10.1016/j.crohns.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe M, et al. Long-term safety and efficacy of adalimumab in Japanese patients with moderate to severe Crohn’s disease. J Crohns Colitis. 2014;8(11):1407–1416. doi: 10.1016/j.crohns.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Dupont-Lucas C, et al. Identifying patients at high risk of loss of response to infliximab maintenance therapy in paediatric Crohn’s disease. J Crohns Colitis. 2016;10(7):795–804 [DOI] [PubMed]
- 15.Gonzaga JE, et al. Durability of infliximab in Crohn’s disease: a single-center experience. Inflamm Bowel Dis. 2009;15(12):1837–1843. doi: 10.1002/ibd.20974. [DOI] [PubMed] [Google Scholar]
- 16.Ma C, et al. Adalimumab dose escalation is effective for managing secondary loss of response in Crohn’s disease. Aliment Pharmacol Ther. 2014;40(9):1044–1055. doi: 10.1111/apt.12940. [DOI] [PubMed] [Google Scholar]
- 17.O’Donnell S, et al. Higher rates of dose optimisation for infliximab responders in ulcerative colitis than in Crohn’s disease. J Crohns Colitis. 2015;9(10):830–836. doi: 10.1093/ecco-jcc/jjv115. [DOI] [PubMed] [Google Scholar]
- 18.Qazi T, et al. The tolerability and efficacy of rapid infliximab infusions in patients with inflammatory bowel disease. Dig Dis Sci. 2016;61(2):589–596. doi: 10.1007/s10620-015-3893-4. [DOI] [PubMed] [Google Scholar]
- 19.Regueiro M, et al. Infliximab dose intensification in Crohn’s disease. Inflamm Bowel Dis. 2007;13(9):1093–1099. doi: 10.1002/ibd.20177. [DOI] [PubMed] [Google Scholar]
- 20.Rosh JR, et al. Retrospective evaluation of the safety and effect of adalimumab therapy (RESEAT) in pediatric Crohn’s disease. Am J Gastroenterol. 2009;104(12):3042–3049. doi: 10.1038/ajg.2009.493. [DOI] [PubMed] [Google Scholar]
- 21.Swaminath A, et al. Early clinical experience with adalimumab in treatment of inflammatory bowel disease with infliximab-treated and naive patients. Aliment Pharmacol Ther. 2009;29(3):273–278. doi: 10.1111/j.1365-2036.2008.03878.x. [DOI] [PubMed] [Google Scholar]
- 22.Viazis N, et al. Azathioprine discontinuation earlier than 6 months in Crohn’s disease patients started on anti-TNF therapy is associated with loss of response and the need for anti-TNF dose escalation. Eur J Gastroenterol Hepatol. 2015;27(4):436–441. doi: 10.1097/MEG.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 23.Vermeire S, et al. Demographic and clinical parameters influencing the short-term outcome of anti-tumor necrosis factor (infliximab) treatment in Crohn’s disease. Am J Gastroenterol. 2002;97(9):2357–2363. doi: 10.1111/j.1572-0241.2002.05991.x. [DOI] [PubMed] [Google Scholar]
- 24.Komiyama T, et al. Lower doses of 6-mercaptopurine/azathioprine bring enough clinical efficacy and therapeutic concentration of erythrocyte 6-mercaptopurine metabolite in Japanese IBD patients. J Crohns Colitis. 2008;2(4):315–321. doi: 10.1016/j.crohns.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Kakuta Y, et al. NUDT15 R139C causes thiopurine-induced early severe hair loss and leukopenia in Japanese patients with IBD. Pharmacogenomics J. 2016;16(3):280–5. doi: 10.1038/tpj.2015.43. [DOI] [PubMed] [Google Scholar]
- 26.The medical procedure index. Health Service Bureau, Ministry of Health, Labor and Welfare.; http://www.iryohoken.go.jp/shinryohoshu/downloadMenu/. Accessed 10 June 2016.
- 27.Schnitzler F, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58(4):492–500. doi: 10.1136/gut.2008.155812. [DOI] [PubMed] [Google Scholar]
- 28.Farkas K, et al. Efficacy of the new infliximab biosimilar CT-P13 induction therapy in Crohn’s disease and ulcerative colitis—experiences from a single center. Expert Opin Biol Ther. 2015;15(9):1257–1262. doi: 10.1517/14712598.2015.1064893. [DOI] [PubMed] [Google Scholar]
- 29.Esposti LD, et al. Adherence and resource use among patients treated with biologic drugs: findings from BEETLE study. Clinicoecon Outcomes Res. 2014;6:401–407. doi: 10.2147/CEOR.S66338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33(9):987–995. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 31.Peyrin-Biroulet L, et al. Anti-TNF monotherapy for Crohn’s disease: a 13-year multicentre experience. J Crohns Colitis. 2016;10(5):516–524. doi: 10.1093/ecco-jcc/jjw008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.IFX: Package insert (30th version) D21, REMICADE for I.V. Infusion100 August 2015 11th May, 2016]. http://www.info.pmda.go.jp/go/pack/2399402F1026_1_37/. Accessed 10 June 2016.
- 33.ADA: Package insert (20th version), HUMIRA subcutaneous infusion 20 mg/syringe 0.4 mL, 40 mg/syringe 0.8 mL. June 2015 11th May, 2016]; http://www.info.pmda.go.jp/go/pack/3999426G1024_2_07/. Accessed 10 June 2016.
- 34.Takatsu N, et al. Adverse reactions to azathioprine cannot be predicted by thiopurine S-methyltransferase genotype in Japanese patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2009;24(7):1258–1264. doi: 10.1111/j.1440-1746.2009.05917.x. [DOI] [PubMed] [Google Scholar]
- 35.Colombel JF, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362(15):1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 36.Ishige T, et al. Inflammatory bowel disease in children: epidemiological analysis of the nationwide IBD registry in Japan. J Gastroenterol. 2010;45(9):911–917. doi: 10.1007/s00535-010-0223-7. [DOI] [PubMed] [Google Scholar]
- 37.Takebayashi T. Descriptive epidemiology of inflammatory bowel disease in Japan. Nihon Rinsho. 2012;70(Suppl 1):39–43. [PubMed] [Google Scholar]
- 38.de Ridder L, et al. Infliximab dependency in pediatric Crohn’s disease: long-term follow-up of an unselected cohort. Inflamm Bowel Dis. 2008;14(3):353–358. doi: 10.1002/ibd.20329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.