Abstract
Survival of any living organism critically depends on its ability to repair and regenerate damaged tissues and/or organs during its lifetime following injury, disease, or aging. Various animal models from invertebrates to vertebrates have been used to investigate the molecular and cellular mechanisms of wound healing and tissue regeneration. It is hoped that such studies will form the framework for identifying novel clinical treatments that will improve the healing and regenerative capacity of humans. Amongst these models, Xenopus stands out as a particularly versatile and powerful system. This review summarizes recent findings using this model, which have provided fundamental knowledge of the mechanisms responsible for efficient and perfect tissue repair and regeneration.
Keywords: limb regeneration, scar‐free wound healing, tail regeneration, tissue regeneration
1. INTRODUCTION
A prominent question in biomedical research is how organisms respond to injuries and ultimately restore the morphological and functional integrity of tissues and organs, thus ensuring their survival. Humans and most other mammals fail to achieve scar‐free healing or to regenerate complex tissues as adults (Gurtner, Werner, Barrandon, & Longaker, 2008). In contrast, various non‐mammalian vertebrates retain the capacity to heal in a scar‐free manner and to regenerate various organs and appendages even as adults (Seifert & Maden, 2014). For example, fish can regenerate their fins and heart and urodele amphibians can heal wounds perfectly and regenerate a range of complex tissues and organs, including limbs, tails, lenses, and retina (Godwin, 2014). While the ultimate goal in regenerative medicine is to improve wound healing and regeneration in human patients, research on other animal model systems, especially on those that have efficient repair and regeneration abilities, promises to provide invaluable information on the molecular and cellular basis of these processes, which will pave the way toward more efficient and effective treatments that will entice our tissues to repair and regenerate better (Godwin, 2014; Seifert & Maden, 2014).
Amongst the various model systems that have been exploited for investigating the mechanisms of scar‐free healing and appendage regeneration is the anuran amphibian Xenopus laevis and its diploid relative Xenopus tropicalis. These frogs heal epidermal wounds without scar formation throughout embryonic and larval stages, and like urodele amphibians (e.g. newts, axolotls, and salamanders) they are able to regenerate limbs, tails, and lens at the larval stages (Beck, Izpisúa Belmonte, & Christen, 2009). However, unlike urodeles, which maintain regeneration capacity throughout life, post‐metamorphic Xenopus froglets lose their ability to fully regenerate their limbs (Godwin & Rosenthal, 2014). This stage‐dependent regenerative ability provides a powerful model for investigating the progressive loss of regenerative ability through ontogeny and also provides an excellent assay system for identifying mechanisms that prolong regenerative capacity (Beck et al., 2009; Lin, Chen, & Slack, 2013).
Prior to its exploitation as a model system for wound healing and tissue regeneration research, Xenopus has enjoyed a long history as a powerful and highly tractable system for the study of embryonic development. The key advantages of this system include ex utero development, which enables ready observation and manipulation of embryos at all stages of development; easy husbandry; and controllable induction of ovulation at any time of year, resulting in the production of large numbers of eggs (Amaya, 2005). In addition, Xenopus has an extensive array of genomic and genetic tools (reviewed in Harland & Grainger, 2011), including a published genome (Hellsten et al., 2010), extensive expressed sequence tag libraries (Gilchrist et al., 2004), transgenic protocols and reagents (Kroll & Amaya, 1996; Love et al., 2011b), and advanced genetic editing tools (Ishibashi, Cliffe, & Amaya, 2012; Nakayama et al., 2013).
In addition to its value as an experimental embryological system, Xenopus also provides a tractable and powerful system for investigating the mechanisms of tissue repair and regeneration. The large‐sized and easy‐to‐culture Xenopus oocytes have been used to study single‐cell wound healing, a fundamental process that shares many features in common with more complicated multicellular tissue and organ repair mechanisms (Sonnemann & Bement, 2011). Furthermore, the blastula stage embryo with thousands of cells (termed blastomeres) can be used to study multicellular scar‐free wound healing (Davidson, Ezin, & Keller, 2002; Li, Zhang, Soto, Woolner, & Amaya, 2013; Soto et al., 2013). Finally, research on the tadpole and later stages can be explored to investigate more complex tissue repair mechanisms, such as tail, limb, and lens regeneration (reviewed in Beck et al., 2009).
Here we summarize recent findings in both wound healing and tissue regeneration in Xenopus, and we highlight the value and potential of this system for elucidating key fundamental mechanisms that permit efficient scar‐free wound healing and appendage regeneration, with implications for regenerative medicine.
2. SINGLE‐CELL WOUND HEALING IN XENOPUS OOCYTES
Even before the advent of multicellular life, unicellular organisms would have encountered various forms of potential injuries from mechanical, predatory, or chemical insults. Such injuries would have provided strong selective pressures for the advent of rapid and efficient unicellular repair mechanisms. To this day, such repair mechanisms remain critical for the ability of cells to survive both mechanical stresses generated by normal physiological processes (skeletal and cardiac muscle contraction) and those arising from various injuries from the external environment (McNeil & Steinhardt, 1997, 2003). Single‐cell wounds, like multicellular wounds, trigger a rapid wound healing response, aimed at reconstituting the barrier function between the inside and outside of the cell. This is done by rapidly resealing the plasma membrane through rapid exocytosis of intracellular membrane vesicles (Miyake & McNeil, 1995; Terasaki, Miyake, & McNeil, 1997). Research using Xenopus oocyte single‐cell wound healing assays revealed the participation of F‐actin and myosin‐2, two cytoskeletal components extensively involved as force‐generating machineries in cell movement and rearrangement, in single‐cell wound healing (see Fig. 1) (Bement, Mandato, & Kirsch, 1999; Mandato, Weber, Zandy, Keating, & Bement, 2001). Bement and colleagues also showed that a contractile zone of F‐actin and myosin‐2 forms at the wound circumference within seconds post wounding, promoting the constriction of the membrane at the wound margin (Bement et al., 1999; Mandato et al., 2001). By exploiting the benefit of the large size and the availability of high‐resolution live imaging techniques in the Xenopus oocyte system, researchers have been able to visualize the dynamics and spatial organization of key molecular players, such as the activation state of the small Rho GTPases, Cdc42 and RhoA, which underlie the formation and function of the contractile actomyosin array at the wound margin (Benink & Bement, 2005). Using this experimental system, it has also been possible to show that the closure of the actomyosin array is driven by centripetal gradients (i.e. towards the center of the wound) of Rho and Cdc42 activity (Burkel, Benink, Vaughan, Dassow, & Bement, 2012). Rho and Cdc42 are preferentially activated at the wound edge and inactivated away from the trailing edge away from the wound. Moreover, these gradients of Rho and Cdc42 are regulated by the contraction of myosin‐2 and the turnover of F‐actin, revealing a complex two‐way regulation of the power‐generating cytoskeleton and its upstream regulators (Burkel et al., 2012). Studies using the Xenopus oocyte wound healing system have also identified a dual‐functional protein, Abr, in coordinating the spatiotemporal activation of Rho and Cdc42 and consequentially the reorganization of the cytoskeletal machinery at the wound edge (Vaughan, Miller, Yu, & Bement, 2011). These findings have led to a mathematical model that is able to simulate the single‐cell wound healing process and, moreover, predict cellular responses under different patterns of injuries (Simon, Vaughan, Bement, & Edelstein‐Keshet, 2013).
Figure 1.
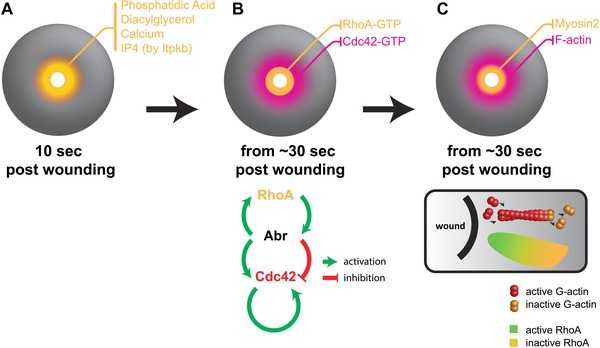
Single‐cell wound responses. (A) Immediate signals, including calcium, IP4, and others, are produced in a gradient at the wound edge, upstream of the cytoskeletal signaling. (B) From 30 sec post wounding onwards, small Rho GTPases Cdc42 and RhoA are activated at the wound edge. Spatial patterning of the circumferential rings of Cdc42 and RhoA is regulated by Abr, a dual‐functional GAP/GEF, which separates active Cdc42 and active RhoA into an outer ring and an inner ring, respectively. (C) Cytoskeletal machinery in single‐cell wound closure. Myosin‐2 and F‐actin also accumulate circumferentially at the wound edge, and the closure of this actomyosin ring is driven by a centripetal gradient of RhoA activity (box). Reciprocally, this RhoA activity is also regulated by treadmilling of the actin filaments at the wound edge
Because the activation of Rho and Cdc42 is a robust response in single‐cell wound healing, researchers have endeavored to identify the molecules that act upstream of these small Rho GTPases during and/or following injury. It has been known for a long time that Ca2+ influx is required for Rho GTPase activation (Benink & Bement, 2005), and it was discovered recently that de novo synthesis and transport of different lipids to different domains at the wound site are also correlated with spatial organization of Rho activity and cytoskeletal machinery (Vaughan et al., 2014). The lipid diacylglycerol accumulates in a zone circumferential to the wound, and by acting through two antagonizing downstream factors, protein kinase β and η, regulates the activation of Rho and Cdc42 (Vaughan et al., 2014). Additional findings using Xenopus oocytes have identified the lipid kinase inositol‐trisphosphate 3‐kinase B (Itpkb), and its enzymatic product inositol 1,3,4,5‐tetrakisphosphate (InsP4), as essential regulators of single‐cell wound healing (Soto et al., 2013). Overexpression of Itpkb or application of InsP4 is able to enhance the activity of Rho and Cdc42, to enhance actin assembly at the wound edge, and to accelerate the speed of wound closure (Soto et al., 2013). The ability of Itpkb and especially its product InsP4 in accelerating wound healing makes it a potential target for improving the speed of acute and chronic wound healing in patients. In addition to the identification of wound healing promoters, a small molecule screen was carried out in the Xenopus oocyte wound healing system aimed at identifying molecules that alter the speed of healing (Clark et al., 2012). Greatly facilitated by the copious availability of oocytes from Xenopus females, their large size and tractability, two small molecules, Sph1 and Sph2, were found to downregulate Rho activation and impair single‐cell wound healing using this system (Clark et al., 2012). Taken together, Xenopus oocytes provide a tractable and powerful system for uncovering the molecular and cellular mechanisms responsible for single‐cell wound healing.
3. MULTICELLULAR WOUND HEALING IN XENOPUS EMBRYOS
Compared with single‐cell wound healing, which mainly consists of repairing disrupted membrane and constriction of an actomyosin array, multicellular wound healing involves simultaneous mobilization of a sheet of cells and subsequent collective movement (reviewed by Sonnemann & Bement, 2011). Thus, unlike single‐cell wounds, multicellular wound healing requires the coordination of both intracellular and intercellular signal transduction pathways and cell behaviors for successful wound repair. Xenopus embryos and larvae provide an excellent system to investigate the molecular and cellular mechanisms underpinning multicellular scar‐free wound healing. First the embryos can be produced in large numbers, and their large size and external development facilitate manipulating and observing wound healing processes. Research using this system has shown that multicellular wound closure shares several mechanisms with those seen in single‐cell wounds, such as a critical role for calcium influx, local activation of Rho GTPases at the wound margin, and formation of an actomyosin array, albeit on a multicellular scale (Stanisstreet, 1982; Clark et al., 2009) (Fig. 2). Constriction of the multicellular actomyosin array closes the wounded area, and intriguingly, in line with the finding in single‐cell wound healing, overexpression of Itpkb or application of InsP4 also enhances actomyosin assembly and wound contraction, implicating a shared molecular and cellular continuum in single‐cell and multicellular wound responses (Soto et al., 2013).
Figure 2.
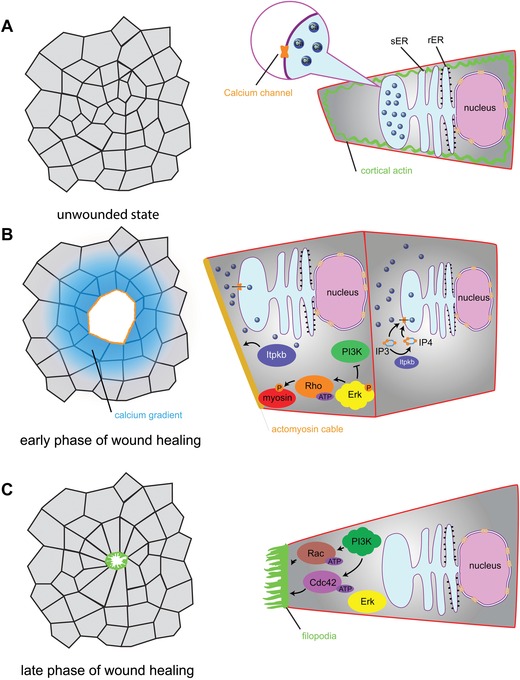
Stages of multicellular wound healing. (A) In an unwounded epithelium, calcium ions are stored in the network of smooth endoplasmic reticulum (ER) and cell shape is maintained by cortical actin. Blue beads, calcium ions. (B) At early stages post wounding, calcium ions are released from the ER storage through calcium channels. The opening of the calcium channels is promoted by both IP3 and the product of Itpkb, IP4. The wave of calcium release propagates planarly across several rows of epithelial cells, mobilizing a larger region of the epithelial sheet for reepithelialization. On the other hand, cortical actin in leading edge cells undergoes reorganization and forms a contractile actomyosin cable at the wound edge. Itpkb regulates the accumulation of F‐actin, whereas Erk signaling regulates myosin‐2 activation, mediated by active RhoA. PI3K activity is inhibited at this stage. (C) Closure of the wound at a later stage is driven by filopodial zippering at the leading edge. PI3K signaling is active, and through Rac and Cdc42 transforms early‐stage actomyosin cable to filopodial protrusions
As is the case during single‐cell wound healing, calcium also acts upstream of Rho GTPase activation and reorganization of the cytoskeleton in multicellular wounds (Clark et al., 2009). However, Ca2+ signaling in multicellular wound healing is more complex in that it involves not only Ca2+ influx in the injured cells but also subsequent Ca2+ wave propagation from cell to cell traveling from the injury site, thereby mobilizing nearby uninjured cells for collective cell movement. Very little is known about the mechanisms that drive the propagation of Ca2+ signaling across the epithelium, although a recent finding showed that Itpkb and its product InsP4, besides enhancing Rho activity and actin assembly, also facilitate Ca2+ propagation across the epithelial tissue from the site of injury (Soto et al., 2013). Although it has been known from studies in other systems that both the actomyosin contraction and filopodial zippering are mechanical forces that help repair the wound (Wood et al., 2002), a recent study in Xenopus embryonic epithelium described a mechanism that temporally coordinates the function of these two distinct force‐generating machineries, thereby facilitating efficient embryonic wound healing (Li et al., 2013). This work showed that an early activation of Erk signaling initiates Rho activation and myosin‐2 phosphorylation, which in turn triggers actomyosin constriction for a quick phase of wound closure. Later on, PI3K signaling takes over, activating Rac and Cdc42, and the mode of cell motility is transformed to filopodial zippering to seal the wound edges (Li et al., 2013). Intriguingly, coordinated actomyosin‐based contraction also participates in the rapid neuroepithelial wound healing in the developing Xenopus brain, which expels damaged neuroepithelial cells from the brain, thereby protecting the tissue from further cell death (Herrgen, Voss, & Akerman, 2014). This contraction is initiated by ATP released from damaged cells and propagated as a calcium wave induced by purinergic receptors (Herrgen et al., 2014).
Tailbud stage embryos heal epithelial wounds in a scar‐free manner and in a comparable timescale to blastula stage embryos (Yoshii, Noda, Matsuzaki, & Ihara, 2005; Fuchigami, Matsuzaki, & Ihara, 2011). Thus, studying the cellular and molecular mechanisms of wound healing at these later stages adds to the growing repertoire of accessible stages for experimentation using Xenopus embryos. The tailbud stages are of particular interest, as these are the stages when inflammatory cells begin to respond to injuries (Costa, Soto, Chen, Zorn, & Amaya, 2008; Chen et al., 2009), and therefore these are the earliest stages when one can begin investigating the role of inflammation during the healing process. Furthermore, one can use these stages, as well as the slightly later tadpole stages, in combination with the establishment and use of transgenic lines, to investigate the mechanisms by which inflammatory cells are recruited to the site of injury and respond to infections (Smith, Kotecha, Towers, Latinkic, & Mohun, 2002; Love et al., 2011b; Paredes, Ishibashi, Borrill, Robert, & Amaya, 2015).
4. WOUND HEALING IN XENOPUS EMBRYOS, FROGLETS AND ADULTS
The mature skin of the post‐metamorphic Xenopus froglet and adult has a highly comparable histology with that of the mammalian skin, containing a layered epidermis and a spongy dermis underneath (Kawasumi, Sagawa, Hayashi, Yokoyama, & Tamura, 2013; Haslam et al., 2014). Unlike adult mammalian wound healing, which generally results in scar formation, wound healing in Xenopus froglets is scarless (Yokoyama et al., 2011). However, this capacity declines as froglets age, such that Xenopus adults heal wounds in a manner that results in scar‐like tissue (Bertolotti, Malagoli, & Franchini, 2013). It has long been noted that scarring and regenerative capacity are inversely related and, as such, scarring is inhibitory to regeneration (Harty, Neff, King, & Mescher, 2003). Changes in both the innate and adaptive immune systems have long been suggested to be responsible for this transition between regenerative capacity and scarring, both within the lifetime of an organism and between organisms (Harty et al., 2003; Mescher, Neff, & King, 2016). Indeed, the maturation state of the immune system and the activation of thymus post wounding have been correlated with increased incidence of scarring as Xenopus adults age (Franchini & Bertolotti, 2014). Thus, this age‐related change in the capacity for scar‐free healing, which largely correlates with decreased regenerative capacity, can be exploited to investigate the critical changes responsible for the switch from scar‐free to scarring events.
Even though, as Xenopus adults age, their propensity for scarring increases, wound healing still proceeds remarkably well in these organisms. Frogs produce many substances, including antimicrobial compounds, which are either not present or are present at much lower concentrations in the mammalian skin (Zasloff, 1987; Berkowitz, Bevins, & Zasloff, 1990). Indeed some of these compounds present in amphibian skin have been shown to promote wound healing (Lipsky, Holroyd, & Zasloff, 2008; Mashreghi et al., 2013; Di Grazia et al., 2015). Intriguingly, another research direction has recently been established that exploits a comparative approach by which Xenopus skin explants and human skin explants are used side by side, as ex vivo organ culture systems, to identify compounds or treatments that improve wound healing (Meier et al., 2013). In this study, thyrotropin‐releasing hormone, a hypothalamic regulator of thyroid hormone production that is abundant in frog skin, was found to stimulate migration, proliferation, and differentiation of keratinocytes in both Xenopus and human skin wounds, thus promoting wound healing (Meier et al., 2013). This pilot assay with Xenopus and human ex vivo skin cultures revealed conserved wound healing responses between these two species and opened new avenues for efficient testing of novel compounds or applications in wound healing research aimed at facilitating the translation of wound promoting mechanisms from amphibians to humans.
5. TAIL REGENERATION IN XENOPUS
The Xenopus tadpole tail comprises various axial tissues, such as a spinal cord, notochord, somites, vasculature, and skin. These tissues are able to regenerate, resulting in a fully restored and functional tail 7−14 days post‐amputation (Love et al., 2011a; Chen, Love, & Amaya, 2014; Love, Ziegler, Chen, & Amaya, 2014) (Fig. 3). Tadpole tail regeneration follows three overlapping stages: an early phase, dominated by scar‐free healing and inflammation; an intermediate phase, dominated by the initiation of proliferation and the formation of the regenerative bud; and a late phase, when clear differentiation of tissues ensues (Love et al., 2011a). Intriguingly, regenerative capacity is stage‐dependent, in that only tadpoles younger than stage 45 or older than stage 48 are capable of regeneration, while tadpoles between stages 46 and 47 are refractory to regeneration (Slack, Beck, Gargioli, & Christen, 2004). The reason for this refractory period remains unclear. However, the period coincides with two important physiological transitions in the tadpole. One is the transition in nutritional sources from maternal yolk stores to food intake and digestion. Thus the refractory period coincides with a major change in metabolism, which may lead to a transient period of limited nutritional availability, required to feed the regenerative process. Another change that occurs during this period is maturation of the immune system, including the development of the adaptive immune system. Indeed, the refractory period can be inhibited by immunosuppression (Fukazawa, Naora, Kunieda, & Kubo, 2009), suggesting that the refractory period may be caused by changes in immunity. However, it remains unclear why older tadpoles regain full regenerative capacity, despite having an increasingly mature immune system. To this end, much remains to be learned to explain fully why the transitory refractory stage exists in the tadpole, and whether the mechanisms that underpin the refractory phase are relevant to why mammals lack full regenerative capacity.
Figure 3.
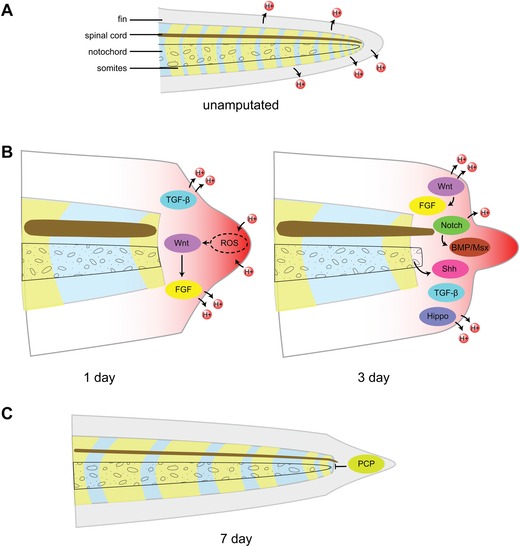
Stages of tadpole tail regeneration. (A) A Xenopus tadpole tail is composed of a number of axial structures including the spinal cord, notochord, and somites. An unamputated tail is in a polarized state, sustained by V‐ATPase pumps in the skin. (B) After amputation, wounded tail is depolarized and simultaneously reactive oxygen species (ROS) are produced at the amputation site. Downstream targets of the ROS include Wnt and FGF, and a number of other signaling pathways are required for successful regeneration such as Shh, TGF‐β, BMP, Notch, and Hippo pathways. V‐ATPases are also upregulated at this stage to repolarize the skin. (C) A fully functional tail is regenerated 7 days after amputation. The growth and termination of a regenerating tail are regulated by PCP signaling. BMP, bone morphogenetic protein; FGF, fibroblast growth factor; TGF‐β, transforming growth factor β; PCP, planar cell polarity
In order to gain insight into the molecular and genetic mechanisms responsible for tadpole tail regeneration, a transcriptomic analysis was carried out during the three phases of tail regeneration, which revealed remarkable changes in gene expression in relation to inflammation and metabolism (Love et al., 2011a, 2014). For example, there is a significant and sustained increase in the expression level of several genes encoding enzymes associated with the production of reactive oxygen species (ROS), including NAD kinase, which phosphorylates NAD+ to NADP+, and glucose‐6‐phosphate dehydrogenase, which generates NADPH from NADP+ (Love et al., 2011a, 2013). NADPH is then used as a substrate for various NADPH oxidases, which generate superoxide and eventually other ROS (Bedard & Krause, 2007). Coincident with the increased expression in the genes encoding these metabolic enzymes, it was found that tail amputation is also associated with an increased and sustained production of ROS, throughout the regenerative response; and if this sustained ROS production is attenuated, using either pharmacological or genetic approaches, tail regeneration does not proceed (Love et al., 2013). Intriguingly, the increased ROS level appears to facilitate growth factor signaling, which is essential for tail regeneration (Love et al., 2013).
More recently, another study compared changes in gene expression profiles in injured spinal cords isolated from regenerative (tadpole) and non‐regenerative (post‐metamorphic froglet) stages (Lee‐Liu et al., 2014). The study found extensive transcriptome changes associated with stress response, metabolism, cell cycle, development, inflammation, and neurogenesis. Interestingly, the regenerative spinal cord takes a significantly shorter time to alter the gene expression level of amputation‐responsive transcripts, and the repertoires of regulated genes are significantly different from that found in non‐regenerative spinal cord tissues (Lee‐Liu et al., 2014). The study also identified many additional genes of unknown function, which also change their expression levels during spinal cord regeneration (Lee‐Liu et al., 2014). It is hoped that this system may provide an easily tractable model system for investigating the mechanisms that permit spinal cord regeneration in vertebrates (Lee‐Liu, Edwards‐Faret, Tapia, & Larraín, 2013; Muñoz et al., 2015).
While the mechanism of spinal cord regeneration is a fascinating and important problem in its own right, it is also of interest to investigate how the spinal cord coordinates the regeneration process as a whole. It has been known for nearly 200 years that appendage regeneration is nerve‐dependent (Todd, 1823; Singer, 1952; Kumar & Brockes, 2012). It has subsequently been shown that appendage regeneration in the Xenopus tadpole tail and limb is also nerve‐dependent (Filoni & Paglialunga, 1990; Taniguchi, Sugiura, Tazaki, Watanabe, & Mochii, 2008). In particular, removal of the spinal cord leads to significant defects in the patterning and growth of the tadpole tail during regeneration (Taniguchi et al., 2008). Furthermore, laser ablations aimed at generating more subtle injuries within the spinal cord at different anteroposterior positions also result in patterning and growth defects during tail regeneration (Mondia et al., 2011). Much work remains to uncover the mechanisms by which the spinal cord coordinates the growth and patterning of the tadpole tail during regeneration.
A signaling pathway that has often been associated with growth regulation and size control in various tissues, organs, and organisms is the Hippo pathway (Yu, Zhao, & Guan, 2015). It is thus perhaps not surprising that this pathway has also been shown to play a critical role in the control of the growth of the tadpole tail during regeneration (Hayashi et al., 2014). Another pathway involved in the growth control and/or termination of growth during development and regeneration is the planar cell polarity (PCP) pathway (Beane, Tseng, Morokuma, Lemire, & Levin, 2012).
Besides the Hippo and PCP pathways, several other signaling pathways have been shown to play important roles during tail regeneration, such as the Wnt, Notch, BMP, FGF, Shh, and TGF‐β pathways (Beck, Christen, & Slack, 2003; Beck, Christen, Barker, & Slack, 2006; Ho & Whitman, 2008; Lin & Slack, 2008; Taniguchi, Watanabe, & Mochii, 2014). Outstanding questions with regard to the regulation of these signaling pathways include, for example, what is the source of the signals? What are their upstream activators? How are the various pathways coordinated during the complex regeneration process?
On the cellular level, a question that has preoccupied researchers for many years is: which cells give rise to the nascent tissue during regeneration and where do they come from? Work performed primarily on urodele amphibians has suggested that blastema cells (mesenchymal stem cells) are the cells that give rise to the nascent tissues in the regenerating appendage, and furthermore that these cells come at least partly from dedifferentiation from adult mesenchymal cells near the amputation site (Brockes & Kumar, 2005; McCusker, Bryant, & Gardiner, 2015). Much interest has been devoted to understanding the biology of blastema cells, as they retain positional memory (cell identity associated with proximal−distal positions within the appendage) and are able to self‐organize. One question that has interested scientists is whether blastema cells are pluripotent or whether they exhibit lineage restriction. Another key question is whether all blastema cells arise from dedifferentiation or whether some arise from activated quiescent stem cell pools. Answering these questions required the advancement of tools which would allow lineage‐tracing experiments to be carried out over long periods of time. Such tools were finally developed in the mid‐1990s with the development of transgenic technologies in amphibians, and given that Xenopus was the first amphibian where such technologies were developed (Kroll & Amaya, 1996), it is not surprising that the first experiments using transgenic lines to investigate the origin and potency of blastema cells during tail regeneration were done in Xenopus tadpoles (Gargioli & Slack, 2004; Slack et al., 2004). Interestingly, these experiments showed that the regenerating tissues arise primarily from lineage‐restricted precursors/stem cells and little or no transdifferentiation or metaplasia is evident (Gargioli & Slack, 2004). Furthermore, the authors found strong evidence that the regenerating muscle arises from the resident stem cell pool of satellite cells rather than from dedifferentiated myofibrils, which are more commonly seen during urodele tail or limb regeneration (Lo, Allen, & Brockes, 1993; Gargioli & Slack, 2004; Tanaka, 2008; Rodrigues, Christen, Martí, & Izpisúa Belmonte, 2012). Overall the authors concluded that appendage regeneration in Xenopus follows mechanisms more similar to those seen during mammalian tissue renewal than those operating during urodele appendage regeneration. Interestingly, more recent findings, using similar approaches of employing transgenic lines to assess the origin and potency of cells in the blastema in axolotls and mammals, have shown that tissue regeneration generally follows lineage restriction, suggesting that dedifferentitation to a pluripotent blastema state is relatively uncommon during appendage regeneration in both amphibians and mammals, and metaplasia in urodeles occurs only in a relatively small subset of tissues, such as within connective tissues (e.g. dermis being able to form both cartilage and tendon) (Kragl et al., 2009; Rinkevich, Lindau, Ueno, Longaker, & Weissman, 2011).
6. LIMB REGENERATION IN XENOPUS
Unlike tail regeneration, limb regeneration in Xenopus has an ontogenic decline, whereby regenerative capacity decreases with age. This makes Xenopus an excellent model for elucidating the mechanisms that may promote regenerative capacity in non‐regenerative stages/organisms (Lin et al., 2013). As in other cases of regeneration, there is considerable interest in investigating whether limb regeneration in Xenopus simply recapitulates the mechanisms of limb development. To this end, it is very important to understand the mechanisms of limb development in Xenopus, so that proper comparisons to the mechanisms of regeneration can be performed. In contrast to other commonly used models of limb development, such as the chick and mouse, much less is known about the mechanisms of limb development and morphogenesis in Xenopus, even though pioneering studies on this model were done nearly 60 years ago (Tschumi, 1957; Keenan & Beck, 2016). Nevertheless, the few studies that have been done on limb development in Xenopus, using modern molecular approaches, have suggested that the mechanisms of limb formation in this model are largely conserved with those found in other tetrapods (Christen & Slack, 1998; Keenan & Beck, 2016). To this end, the spatiotemporal expression patterns of most genes known to play critical roles in limb development in the chick and the mouse are similarly expressed during Xenopus limb development (McEwan, Lynch, & Beck, 2011; Wang & Beck, 2014; Keenan & Beck, 2016), and indeed most of the molecular players involved during limb development partake as well during limb regeneration (Fig. 4).
Figure 4.

Xenopus as a model to compare regenerative and non‐regenerative limbs. Before metamorphosis, Xenopus froglets are capable of regenerating amputated limbs. Listed are required genes and pathways in different processes of limb regeneration, including growth, patterning, joint and muscle development. Post‐metamorphic froglets enter a non‐permissive stage of limb regeneration when amputated limb can only grow into a spike instead of a restored limb. Many of the upregulated genes and activated pathways in the permissive stage are not properly expressed or activated at the non‐permissive stage
One could ask, what is the use of yet another model system for investigating the mechanisms of limb regeneration if several powerful ones are available amongst the urodele amphibians? While urodele amphibians represent the species with the greatest capacity for limb regeneration amongst the vertebrates (McCusker et al., 2015), anuran amphibians such as Xenopus represent a unique model organism which is capable of limb regeneration at the early limb bud stages of development, but this regenerative capacity decreases as the limb development proceeds such that in the post‐metamorphic froglet only a hypomorphic spike regenerates after amputation (Dent, 1962; Muneoka, Holler‐Dinsmore, & Bryant, 1986; Beck et al., 2009). As such, Xenopus is intermediate between the full regenerative ability of urodeles and no regenerative capacity, as seen in birds and mammals, including humans. Thus, Xenopus tadpoles can be explored to investigate the mechanisms that permit limb regeneration during the permissive stages, versus those that impede it during the non‐permissive stages, within the same model organism. Furthermore, one can explore this system in order to investigate the molecular and cellular mechanisms that will prolong or promote regeneration during normally non‐permissive stages. A particularly poignant example of this sort of study was published by Gufa Lin and colleagues in 2013. In that monumental piece of work, the authors performed a series of very careful and technically demanding experiments to ask whether transplantation of blastema‐derived progenitor cells from regenerative stage tadpoles into post‐metamorphic froglet amputated limbs would be able to enhance the regenerative capacity of amputated limbs of non‐regenerative post‐metamorphic froglets. The answer was yes, but to get optimal enhancement of regeneration required that the transplanted cells had active Wnt/β‐catenin signaling and for the transplanted cells to be placed near sources of Shh, FGF10, and thymosin β4 (Lin et al., 2013). These findings suggest that it might be possible, in the future, to enhance the regenerative capacity of mammalian limbs using cell transplantation approaches, but any success will probably depend on a combination of factors, including the origin and age of the transplanted progenitor cells and what signaling pathways are active within them and in their vicinity.
7. CONCLUSIONS
Xenopus is a versatile and highly tractable system for research in wound healing and complex appendage regeneration. One emphasis of future research with this system is to obtain more mechanistic insights of the molecular and cellular bases of repair and regeneration, using the advanced imaging and genomic tools that have been developed in this system in the past decade. Another emphasis of future research is comparative studies between different stages of Xenopus with different wound healing and regeneration competence, as well as comparing healing and regenerating processes in Xenopus and mammals. Both directions have been touched on in previous work, but more detailed examination is still needed. It is hoped that these fundamental understandings of wound healing and regeneration in Xenopus will soon lead to the development of treatments aimed at improving healing, reducing scarring, and promoting functional regeneration of tissues in humans who have experienced traumatic injuries.
ACKNOWLEDGMENTS
This work was supported by two project grants from the Healing Foundation, one project grant from the Medical Research Council, and one Institutional Strategic Support grant from the Wellcome Trust (097820/Z/11/Z). The authors declare no conflicts of interest.
REFERENCES
- Amaya, E. (2005). Xenomics. Genome Research, 15, 1683–1691. [DOI] [PubMed] [Google Scholar]
- Beane, W. S. , Tseng, A. ‐S. , Morokuma, J. , Lemire, J. M. , & Levin, M. (2012). Inhibition of planar cell polarity extends neural growth during regeneration, homeostasis, and development. Stem Cells and Development, 21, 2085–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck, C. W. , Christen, B. , & Slack, J. M. W. (2003). Molecular pathways needed for regeneration of spinal cord and muscle in a vertebrate. Developmental Cell, 5, 429–439. [DOI] [PubMed] [Google Scholar]
- Beck, C. W. , Christen, B. , Barker, D. , & Slack, J. M. W. (2006). Temporal requirement for bone morphogenetic proteins in regeneration of the tail and limb of Xenopus tadpoles. Mechanisms of Development, 123, 674–688. [DOI] [PubMed] [Google Scholar]
- Beck, C. W. , Izpisúa Belmonte, J. C. , & Christen, B. (2009). Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Developmental Dynamics, 238, 1226–1248. [DOI] [PubMed] [Google Scholar]
- Bedard, K. , & Krause, K. ‐H. (2007). The NOX family of ROS‐generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews, 87, 245–313. [DOI] [PubMed] [Google Scholar]
- Bement, W. M. , Mandato, C. A. , & Kirsch, M. N. (1999). Wound‐induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Current Biology, 9, 579–587. [DOI] [PubMed] [Google Scholar]
- Benink, H. A. , & Bement, W. M. (2005). Concentric zones of active RhoA and Cdc42 around single cell wounds. The Journal of Cell Biology, 168, 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz, B. A. , Bevins, C. L. , & Zasloff, M. A. (1990). Magainins: a new family of membrane‐active host defense peptides. Biochemical Pharmacology, 39, 625–629. [DOI] [PubMed] [Google Scholar]
- Bertolotti, E. , Malagoli, D. , & Franchini, A. (2013). Skin wound healing in different aged Xenopus laevis. Journal of Morphology, 274, 956–964. [DOI] [PubMed] [Google Scholar]
- Brockes, J. P. , & Kumar, A. (2005). Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science, 310, 1919–1923. [DOI] [PubMed] [Google Scholar]
- Burkel, B. M. , Benink, H. A. , Vaughan, E. M. , von Dassow, , G. , & Bement, W. M. (2012). A Rho GTPase signal treadmill backs a contractile array. Developmental Cell, 23, 384–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Costa, R. M. B. , Love, N. R. , Soto, X. , Roth, M. , Paredes, R. , & Amaya, E. (2009). C/EBPalpha initiates primitive myelopoiesis in pluripotent embryonic cells. Blood, 114, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Love, N. R. , & Amaya, E. (2014). Tadpole tail regeneration in Xenopus . Biochemical Society Transactions, 42, 617–623. [DOI] [PubMed] [Google Scholar]
- Christen, B. , & Slack, J. M. W. (1998). All limbs are not the same. Nature, 395, 230–231. [DOI] [PubMed] [Google Scholar]
- Clark, A. G. , Miller, A. L. , Vaughan, E. , Yu, H.‐Y. E. , Penkert, R. , & Bement, W. M. (2009). Integration of single and multicellular wound responses. Current Biology, 19, 1389–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, A. G. , Sider, J. R. , Verbrugghe, K. , Fenteany, G. , von Dassow, , G. , & Bement, W. M. (2012). Identification of small molecule inhibitors of cytokinesis and single cell wound repair. Cytoskeleton (Hoboken), 69, 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, R. M. B. , Soto, X. , Chen, Y. , Zorn, A. M. , & Amaya, E. (2008). spib is required for primitive myeloid development in Xenopus . Blood, 112, 2287–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson, L. A. , Ezin, A. M. , & Keller, R. (2002). Embryonic wound healing by apical contraction and ingression in Xenopus laevis. Cell Motil Cytoskeleton, 53, 163–176. [DOI] [PubMed] [Google Scholar]
- Dent, J. N. (1962). Limb regeneration in larvae and metamorphosing individuals of the South African clawed toad. Journal of Morphology, 110, 61–77. [DOI] [PubMed] [Google Scholar]
- Di Grazia, A. , Cappiello, F. , Imanishi, A. , Mastrofrancesco, A. , Picardo, M. , Paus, R. , & Mangoni, M. L. (2015). The frog skin‐derived antimicrobial peptide Esculentin‐1a(1‐21)NH2 promotes the migration of human HaCaT keratinocytes in an EGF receptor‐dependent manner: a novel promoter of human skin wound healing? PLoS One, 10, e0128663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoni, S. , & Paglialunga, L. (1990). Effect of denervation on hindlimb regeneration in Xenopus laevis larvae. Differentiation, 43, 10–19. [DOI] [PubMed] [Google Scholar]
- Franchini, A. , & Bertolotti, E. (2014). The thymus and skin wound healing in Xenopus laevis adults. Acta Histochemica, 116, 1141–1147. [DOI] [PubMed] [Google Scholar]
- Fuchigami, T. , Matsuzaki, T. , & Ihara, S. (2011). Exposure to external environment of low ion concentrations is the trigger for rapid wound closure in Xenopus laevis embryos. Zoological Science, 28, 633–641. [DOI] [PubMed] [Google Scholar]
- Fukazawa, T. , Naora, Y. , Kunieda, T. , & Kubo, T. (2009). Suppression of the immune response potentiates tadpole tail regeneration during the refractory period. Development, 136, 2323–2327. [DOI] [PubMed] [Google Scholar]
- Gargioli, C. , & Slack, J. M. W. (2004). Cell lineage tracing during Xenopus tail regeneration. Development, 131, 2669–2679. [DOI] [PubMed] [Google Scholar]
- Gilchrist, M. J. , Zorn, A. M. , Voigt, J. , Smith, J. C. , Papalopulu, N. , & Amaya, E. (2004). Defining a large set of full‐length clones from a Xenopus tropicalis EST project. Developmental Biology, 271, 498–516. [DOI] [PubMed] [Google Scholar]
- Godwin, J. (2014). The promise of perfect adult tissue repair and regeneration in mammals: learning from regenerative amphibians and fish. Bioessays, 36, 861–871. [DOI] [PubMed] [Google Scholar]
- Godwin, J. W. , & Rosenthal, N. (2014). Scar‐free wound healing and regeneration in amphibians—immunological influences on regenerative success. Differentiation, 87, 66–75. [DOI] [PubMed] [Google Scholar]
- Gurtner, G. C. , Werner, S. , Barrandon, Y. , & Longaker, M. T. (2008). Wound repair and regeneration. Nature, 453, 314–321. [DOI] [PubMed] [Google Scholar]
- Harland, R. M. , & Grainger, R. M. (2011). Xenopus research: metamorphosed by genetics and genomics. Trends in Genetics., 27, 507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harty, M. , Neff, A. W. , King, M. W. , & Mescher, A. L. (2003). Regeneration or scarring: an immunologic perspective. Developmental Dynamics, 226, 268–279. [DOI] [PubMed] [Google Scholar]
- Haslam, I. S. , Roubos, E. W. , Mangoni, M. L. , Yoshizato, K. , Vaudry, H. , Kloepper, J. E. , …, & Paus, R. (2014). From frog integument to human skin: dermatological perspectives from frog skin biology. Biological Reviews, 89, 618–655. [DOI] [PubMed] [Google Scholar]
- Hayashi, S. , Ochi, H. , Ogino, H. , Kawasumi, A. , Kamei, Y. , Tamura, K. , & Yokoyama, H. (2014). Transcriptional regulators in the Hippo signaling pathway control organ growth in Xenopus tadpole tail regeneration. Developmental Biology, 396, 31–41. [DOI] [PubMed] [Google Scholar]
- Hellsten, U. , Harland, R. M. , Gilchrist, M. J. , Hendrix, D. , Jurka, J. , Kapitonov, V. , …, & Rokhsar, D. S. (2010). The genome of the Western clawed frog Xenopus tropicalis . Science, 328, 633–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrgen, L. , Voss, O. P. , & Akerman, C. J. (2014). Calcium‐dependent neuroepithelial contractions expel damaged cells from the developing brain. Developmental Cell, 31, 599–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, D. M. , & Whitman, M. (2008). TGF‐beta signaling is required for multiple processes during Xenopus tail regeneration. Developmental Biology, 315, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi, S. , Cliffe, R. , & Amaya, E. (2012). Highly efficient bi‐allelic mutation rates using TALENs in Xenopus tropicalis . Biology Open, 1, 1273–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasumi, A. , Sagawa, N. , Hayashi, S. , Yokoyama, H. , & Tamura, K. (2013). Wound healing in mammals and amphibians: toward limb regeneration in mammals. Current Topics in Microbiology and Immunology, 367, 33–49. [DOI] [PubMed] [Google Scholar]
- Keenan, S. R. , & Beck, C. W. (2016). Xenopus limb bud morphogenesis. Developmental Dynamics, 245, 233–243. [DOI] [PubMed] [Google Scholar]
- Kragl, M. , Knapp, D. , Nacu, E. , Khattak, S. , Maden, M. , Epperlein, H. H. , & Tanaka, E. M. (2009). Cells keep a memory of their tissue origin during axolotl limb regeneration. Nature, 460, 60–65. [DOI] [PubMed] [Google Scholar]
- Kroll, K. L. , & Amaya, E. (1996). Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development, 122, 3173–3183. [DOI] [PubMed] [Google Scholar]
- Kumar, A. , & Brockes, J. P. (2012). Nerve dependence in tissue, organ, and appendage regeneration. Trends in Neurosciences., 35, 691–699. [DOI] [PubMed] [Google Scholar]
- Lee‐Liu, D. , Edwards‐Faret, G. , Tapia, V. S. , & Larraín, J. (2013). Spinal cord regeneration: lessons for mammals from non‐mammalian vertebrates. Genesis, 51, 529–544. [DOI] [PubMed] [Google Scholar]
- Lee‐Liu, D. , Moreno, M. , Almonacid, L. I. , Tapia, V. S. , Muñoz, R. , von Marées, , J. , …, & Larraín, J. (2014). Genome‐wide expression profile of the response to spinal cord injury in Xenopus laevis reveals extensive differences between regenerative and non‐regenerative stages. Neural Development, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Zhang, S. , Soto, X. , Woolner, S. , & Amaya, E. (2013). ERK and phosphoinositide 3‐kinase temporally coordinate different modes of actin‐based motility during embryonic wound healing. Journal of Cell Science, 126(21), 5005–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, G. , & Slack, J. M. W. (2008). Requirement for Wnt and FGF signaling in Xenopus tadpole tail regeneration. Developmental Biology, 316, 323–335. [DOI] [PubMed] [Google Scholar]
- Lin, G. , Chen, Y. , & Slack, J. M. W. (2013). Imparting regenerative capacity to limbs by progenitor cell transplantation. Developmental Cell, 24, 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsky, B. A. , Holroyd, K. J. , & Zasloff, M. (2008). Topical versus systemic antimicrobial therapy for treating mildly infected diabetic foot ulcers: a randomized, controlled, double‐blinded, multicenter trial of pexiganan cream. Clinical Infectious Diseases, 47, 1537–1545. [DOI] [PubMed] [Google Scholar]
- Lo, D. C. , Allen, F. , & Brockes, J. P. (1993). Reversal of muscle differentiation during urodele limb regeneration. Proceedings of the National Academy of Sciences USA, 90, 7230–7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, N. R. , Chen, Y. , Bonev, B. , Gilchrist, M. J. , Fairclough, L. , Lea, R. , …, & Amaya, E. (2011a). Genome‐wide analysis of gene expression during Xenopus tropicalis tadpole tail regeneration. BMC Developmental Biology, 11, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, N. R. , Chen, Y. , Ishibashi, S. , Kritsiligkou, P. , Lea, R. , Koh, Y. , …, & Amaya, E. (2013). Amputation‐induced reactive oxygen species are required for successful Xenopus tadpole tail regeneration. Nature Cell Biology, 15, 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, N. R. , Thuret, R. , Chen, Y. , Ishibashi, S. , Sabherwal, N. , Paredes, R. , …, & Amaya, E. (2011b). pTransgenesis: a cross‐species, modular transgenesis resource. Development, 138, 5451–5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love, N. R. , Ziegler, M. , Chen, Y. , & Amaya, E. (2014). Carbohydrate metabolism during vertebrate appendage regeneration: what is its role? How is it regulated?: A postulation that regenerating vertebrate appendages facilitate glycolytic and pentose phosphate pathways to fuel macromolecule biosynthesis. Bioessays, 36, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandato, C. A. , Weber, K. L. , Zandy, A. J. , Keating, T. J. , & Bement, W. M. (2001). Xenopus egg extracts as a model system for analysis of microtubule, actin filament, and intermediate filament interactions. Methods in Molecular Biology, 161, 229–239. [DOI] [PubMed] [Google Scholar]
- Mashreghi, M. , Rezazade Bazaz, M. , Mahdavi Shahri, N. , Asoodeh, A. , Mashreghi, M. , Behnam Rassouli, M. , & Golmohammadzadeh, S. (2013). Topical effects of frog “Rana ridibunda” skin secretions on wound healing and reduction of wound microbial load. Journal of Ethnopharmacology, 145, 793–797. [DOI] [PubMed] [Google Scholar]
- McCusker, C. , Bryant, S. V. , & Gardiner, D. M. (2015). The axolotl limb blastema: cellular and molecular mechanisms driving blastema formation and limb regeneration in tetrapods. Regeneration (Oxf), 2, 54–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan, J. , Lynch, J. , & Beck, C. W. (2011). Expression of key retinoic acid modulating genes suggests active regulation during development and regeneration of the amphibian limb. Developmental Dynamics, 240, 1259–1270. [DOI] [PubMed] [Google Scholar]
- McNeil, P. L. , & Steinhardt, R. A. (1997). Loss, restoration, and maintenance of plasma membrane integrity. The Journal of Cell Biology, 137, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, P. L. , & Steinhardt, R. A. (2003). Plasma membrane disruption: repair, prevention, adaptation. Annual Review of Cell and Developmental Biology, 19, 697–731. [DOI] [PubMed] [Google Scholar]
- Meier, N. T. , Haslam, I. S. , Pattwell, D. M. , Emelianov, V. , Paredes, R. , Debus, S. , …, & Paus, R. (2013). Thyrotropin‐releasing hormone (TRH) promotes wound re‐epithelialisation in frog and human skin. PLoS One, 8, e73596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher, A. L. , Neff, A. W. , & King, M. W. (2016). Inflammation and immunity in organ regeneration. Developmental & Comparative Immunology, 2016 Feb 16. doi:10.1016/j.dci.2016.02.015 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Miyake, K. , & McNeil, P. L. (1995). Vesicle accumulation and exocytosis at sites of plasma membrane disruption. The Journal of Cell Biology, 131, 1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondia, J. P. , Levin, M. , Omenetto, F. G. , Orendorff, R. D. , Branch, M. R. , & Adams, D. S. (2011). Long‐distance signals are required for morphogenesis of the regenerating Xenopus tadpole tail, as shown by femtosecond‐laser ablation. PLoS One, 6, e24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneoka, K. , Holler‐Dinsmore, G. , & Bryant, S. V. (1986). Intrinsic control of regenerative loss in Xenopus laevis limbs. Journal of Experimental Zoology, 240, 47–54. [DOI] [PubMed] [Google Scholar]
- Muñoz, R. , Edwards‐Faret, G. , Moreno, M. , Zuñiga, N. , Cline, H. , & Larraín, J. (2015). Regeneration of Xenopus laevis spinal cord requires Sox2/3 expressing cells. Developmental Biology, 408, 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama, T. , Fish, M. B. , Fisher, M. , Oomen‐Hajagos, J. , Thomsen, G. H. , & Grainger, R. M. (2013). Simple and efficient CRISPR/Cas9‐mediated targeted mutagenesis in Xenopus tropicalis . Genesis, 51, 835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes, R. , Ishibashi, S. , Borrill, R. , Robert, J. , & Amaya, E. (2015). Xenopus: an in vivo model for imaging the inflammatory response following injury and bacterial infection. Developmental Biology, 408, 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinkevich, Y. , Lindau, P. , Ueno, H. , Longaker, M. T. , & Weissman, I. L. (2011). Germ‐layer and lineage‐restricted stem/progenitors regenerate the mouse digit tip. Nature, 476, 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, A. M. C. , Christen, B. , Martí, M. , & Izpisúa Belmonte, J. C. (2012). Skeletal muscle regeneration in Xenopus tadpoles and zebrafish larvae. BMC Developmental Biology, 12, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert, A. W. , & Maden, M. (2014). New insights into vertebrate skin regeneration. International Review of Cell and Molecular Biology, 310, 129–169. [DOI] [PubMed] [Google Scholar]
- Simon, C. M. , Vaughan, E. M. , Bement, W. M. , & Edelstein‐Keshet, L. (2013). Pattern formation of Rho GTPases in single cell wound healing. Molecular Biology of the Cell, 24, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer, M. (1952). The influence of the nerve in regeneration of the amphibian extremity. The Quarterly Review of Biology, 27, 169–200. [DOI] [PubMed] [Google Scholar]
- Slack, J. M. W. , Beck, C. W. , Gargioli, C. , & Christen, B. (2004). Cellular and molecular mechanisms of regeneration in Xenopus . Philosophical Transactions of the Royal Society B: Biological Sciences, 359, 745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. J. , Kotecha, S. , Towers, N. , Latinkic, B. V. , & Mohun, T. J. (2002). XPOX2‐peroxidase expression and the XLURP‐1 promoter reveal the site of embryonic myeloid cell development in Xenopus . Mechanisms of Development, 117, 173–186. [DOI] [PubMed] [Google Scholar]
- Sonnemann, K. J. , & Bement, W. M. (2011). Wound repair: toward understanding and integration of single‐cell and multicellular wound responses. Annual Review of Cell and Developmental Biology, 27, 237–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto, X. , Li, J. , Lea, R. , Dubaissi, E. , Papalopulu, N. , & Amaya, E. (2013). Inositol kinase and its product accelerate wound healing by modulating calcium levels, Rho GTPases, and F‐actin assembly. Proceedings of the National Academy of Sciences USA, 110, 11029–11034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisstreet, M. (1982). Calcium and wound healing in Xenopus early embryos. Journal of Embryology and Experimental Morphology, 67, 195–205. [PubMed] [Google Scholar]
- Tanaka, E. M. (2008). Skeletal muscle reconstitution during limb and tail regeneration in amphibians: two contrasting mechanisms In G. J. M. Stienen (Ed.) Skeletal Muscle Repair and Regeneration, Advances in Muscle Research (pp. 181–198). Dordrecht, Netherlands: Springer. [Google Scholar]
- Taniguchi, Y. , Sugiura, T. , Tazaki, A. , Watanabe, K. , & Mochii, M. (2008). Spinal cord is required for proper regeneration of the tail in Xenopus tadpoles. Development, Growth & Differentiation, 50, 109–120. [DOI] [PubMed] [Google Scholar]
- Taniguchi, Y. , Watanabe, K. , & Mochii, M. (2014). Notochord‐derived hedgehog is essential for tail regeneration in Xenopus tadpole. BMC Developmental Biology, 14, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki, M. , Miyake, K. , & McNeil, P. L. (1997). Large plasma membrane disruptions are rapidly resealed by Ca2+‐dependent vesicle−vesicle fusion events. J. Cell Biol., 139, 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, T. J. (1823). On the process of reproduction of the members of the aquatic salamander. Quaterly Journal of Science Literature and the Arts, 16, 84–96. [Google Scholar]
- Tschumi, P. A. (1957). The growth of the hindlimb bud of Xenopus laevis and its dependence upon the epidermis. Journal of Anatomy, 91, 149–173. [PMC free article] [PubMed] [Google Scholar]
- Vaughan, E. M. , Miller, A. L. , Yu, H.‐Y. E. , & Bement, W. M. (2011). Control of local Rho GTPase crosstalk by Abr. Current Biology, 21, 270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, E. M. , You, J. ‐S. , Elsie Yu, H. ‐Y. , Lasek, A. , Vitale, N. , Hornberger, T. A. , & Bement, W. M. (2014). Lipid domain‐dependent regulation of single‐cell wound repair. Molecular Biology of the Cell, 25, 1867–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. ‐H. , & Beck, C. W. (2014). Distal expression of sprouty (spry) genes during Xenopus laevis limb development and regeneration. Gene Expression Patterns, 15, 61–66. [DOI] [PubMed] [Google Scholar]
- Wood, W. , Jacinto, A. , Grose, R. , Woolner, S. , Gale, J. , Wilson, C. , & Martin, P. (2002). Wound healing recapitulates morphogenesis in Drosophila embryos. Nature Cell Biology, 4, 907–912. [DOI] [PubMed] [Google Scholar]
- Yokoyama, H. , Maruoka, T. , Aruga, A. , Amano, T. , Ohgo, S. , Shiroishi, T. , & Tamura, K. , (2011). Prx‐1 expression in Xenopus laevis scarless skin‐wound healing and its resemblance to epimorphic regeneration. Journal of Investigative Dermatology, 131, 2477–2485. [DOI] [PubMed] [Google Scholar]
- Yoshii, Y. , Noda, M. , Matsuzaki, T. , & Ihara, S. (2005). Wound healing ability of Xenopus laevis embryos. I. Rapid wound closure achieved by bisectional half embryos. Development, Growth & Differentiation, 47, 553–561. [DOI] [PubMed] [Google Scholar]
- Yu, F. ‐X. , Zhao, B. , & Guan, K. ‐L. (2015). Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell, 163, 811–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff, M. (1987). Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proceedings of the National Academy of Sciences USA, 84, 5449–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]


