Abstract
Obstructive sleep apnea (OSA) is one of the systemic risk factors for glaucoma which causes irreversible visual field (VF) damage. We reviewed the published data of all types of studies on the association between these two conditions and papers regarding functional and structural changes related to glaucomatous damage using Scopus, web of science, and PubMed databases. There is evidence that the prevalence of glaucoma is higher in OSA patients, which independent of intraocular pressure (IOP). Studies have reported thinning of retinal nerve fiber layer (RNFL), alteration of optic nerve head, choroidal and macular thickness, and reduced VF sensitivity in patients of OSA with no history glaucoma. A negative correlation of apnea-hypopnea index with RNFL and VF indices has been described in some studies. Raised IOP was noted which is possibly related to obesity, supine position during sleep, and raised intracranial pressure. Diurnal fluctuations of IOP show more variations in OSA patients before and after continuous positive airway pressure (CPAP) therapy when compared with the normal cases. The vascular factors behind the pathogenesis include recurrent hypoxia with increased vascular resistance, oxidative stress damage to the optic nerve. In conclusion, comprehensive glaucoma evaluation should be recommended in patients with OSA and should also periodically monitor IOP during CPAP treatment which may trigger the progression of glaucomatous damage.
Keywords: Choroidal thickness, glaucoma, intraocular pressure, macular thickness, obstructive sleep apnea, retinal nerve fiber layer, visual fields
Introduction
Glaucoma is a progressive optic neurodegenerative disease with specific characteristics of structural optic nerve head (ONH) and with changes in the inner retinal layer (ganglion cell complex) along with the presence of corresponding functional visual field (VF) changes that are irreversible.[1] Raised intraocular pressure (IOP) is one of the major risk factors for glaucoma. It causes mechanical damage to ONH and inner retinal layers, which eventually leads to progressive, irreversible functional VF loss. Some patients show deterioration of VFs even when IOP is normal which provokes to look for other risk factors such as systemic disorders which will cause neurodegenerative diseases.[2]
Obstructive sleep apnea (OSA) is characterized by recurrent complete or partial interruption of normal breathing due to functional occlusion or collapse of upper airway during sleep that leads to apnea or hypopnea and hypoxia.[3,4] This causes decrease in the arterial oxygen (O2) saturation and a rise in the carbon dioxide saturation during sleep[5] and results in transient hypoxia and increased vascular resistance in body tissues. In case of ocular tissues, this results in a reduction of ocular perfusion pressure and decreased oxygenation to the optic nerve which eventually leads to glaucomatous optic neuropathy.[5] It shows specific symptoms and signs such as excessive daytime sleepiness or fatigue and obesity. The prevalence of OSA in community screening is 2%-6% for moderate and above and 14% for mild form. The prevalence increased to 21%-90% when patients were referred for sleep evaluation.[6] Ethnicity-wise prevalence of OSA in middle-aged population was 4% in men and 2% in women in the US, 0.5%-1.5% in the middle aged men in the UK,[7] and 4.1%-7.5% and 2.1%-3.2% in men and women in Asians, respectively.[4] However, all these studies used different criteria and variation in definition to diagnose the condition.
The more clinical importance of OSA is due to apart from symptoms such as excessive daytime sleepiness, it increases the risk of severity of life-threatening diseases with significant mortality and morbidity. Clinical representations of OSA is habitual loud snoring, nocturnal gasping, reduced cognitive abilities, lack of energy, reduced concentration, memory impairment, dry throat on waking up, and morning headache. Moreover, the risk factors are obesity with high body mass index (BMI), alcohol consumption, male gender, thick neck, and abnormal craniofacial abnormalities such as enlargement of tonsils, tongue, and uvula with increasing Mallampati airway classification. All these abnormalities may lead to collapsible upper airway which predisposes to OSA. Population-based epidemiological studies showed that obesity with high BMI significantly associated with OSA positively.[3,4,6]
OSA is significantly associated with several life-threatening cardiovascular diseases such as stroke associated with atrial fibrillation, coronary artery disease, hypertension and arrhythmias,[8] neurovascular and cerebrovascular diseases such as cerebrovascular insult, impaired neurocognition, and poorly controlled mood disorder, raised intracranial pressure and increased motor vehicle accidents,[9] metabolic disorders such as effect on glucose tolerance and lipid metabolism,[10] and endocrine disorders such as acromegaly, hypothyroidism, and diabetes mellitus.[3] OSA diagnosis is done by overnight polysomnography evaluation. The severity of the disease is calculated by apnea-hypopnea index (AHI) score per hour which is the sum of apnea and hypopnea episodes. Apnea is defined as cessation of oronasal flow for a duration of 10 s or more, and hypopnea is defined as reduction of oronasal flow for greater than 50% with a 3%-4% of oxygen desaturation. Most of the studies reported that if AHI score ≥5, it is diagnosed as OSA. The severity of the disease classified as mild – AHI between 5 and 15, moderate – AHI between 15 and 30, and severe OSA – AHI >30.[11,12,13] Apart from glaucoma, OSA is associated with several other ophthalmic disorders including floppy eyelid syndrome (FES), nonarteritic ischemic optic neuropathy, papilledema, optic neuropathy, idiopathic intracranial hypertension, diabetic retinopathy, geographic atrophy, age-related macular degeneration, retinal vein occlusion, and central serous retinopathy.[3,9,14,15] There are various studies which have reported the association between OSA and glaucoma, its associated conditions. However, the exact cause and pathophysiology behind association is still unclear. This article aims to review its association and proposed pathophysiology mechanisms of glaucoma in OSA till now.
Literature Review and Study Selection
We reviewed the published data on association between OSA and glaucoma, glaucomatous structural and functional changes in OSA. Literature review conducted by searching Scopus, Web of Science, Embase, IndMed, and PubMed databases to find relevant published data till June, 2016, using the following combined search terms: “Obstructive sleep apnea” or “OSA”, “glaucoma,” “Intra ocular pressure,” “retinal nerve fiber layer,” “visual fields,” “macular thickness,” and “choroidal thickness.” We have reviewed reference list of retrieved articles so as to expand the search.
We have included studies which show (1) prevalence of glaucoma in OSA or vice versa, (2) structural changes of retina and ONH in OSA, for example, retinal nerve fiber layer (RNFL), macular and choroidal changes, (3) functional changes of retina in OSA, for example, electrophysiological tests and VF tests results.
Pathophysiology of Glaucoma in Obstructive Sleep Apnea
There are multiple factors involved in pathogenesis of glaucoma and structural changes of retina, ONH in OSA. Pathophysiology of glaucoma in OSA is complex, and various altered physiological activities such as hypoxia, vascular, and mechanical factors may be involved.
Hypoxia
Oxygen is important for all living cells for cellular function and tissue formation. Especially, neuronal cells need more oxygen and glucose consumption to generate continuous energy in the form of adenosine triphosphate (ATP). Mitochondria in cells need oxygen to breakdown glucose through a process of oxidative phosphorylation to generate ATP. Hence, repetitive apnea-hypopnea episodes or reduction in ventilator drive will cause hypoxia and hypercapnia which will decrease in pO2 and an increase in pCO2. These prolonged episodes of hypoxia directly damage the ONH, retinal ganglion cells (RGCs), and its axons. Hypoxia will cause oxidative stress and inflammation by increasing of reactive oxygen species and inflammatory markers which subsequently leads to mitochondrial dysfunction of RGCs and glaucoma.[2,3,16]
Vascular factors
OSA causes definite vascular changes and vascular dysregulation of ONH. Studies showed that OSA is associated with hypertension, atherosclerosis of carotid artery, vascular endothelial dysfunction, autonomic dysfunction, and raised intracranial pressure.[2,12] Retina and optic nerve require constant blood flow to meet their high metabolic needs. OSA causes insufficient blood supply and nourishment to the RNFL and optic nerve.[17]
Carotid artery (internal) supplies blood to the brain through each side of neck. OSA will cause vascular cell wall changes in carotid artery by building the plaques formation inside the wall. It will narrow the blood vessel and subsequently may end up with ONH ischemia.[12] Intermittent hypoxia will cause increase of sympathetic nervous system activity which leads to vasoconstriction and systemic hypertension.[18] Raising sympathetic activity during daytime causes autonomic dysfunction. Raise of sympathetic activity and autonomic dysfunction may alter the cerebral and ocular circulation, especially during night. The reason behind this is that at night, normally sympathetic activity and blood pressure decreases. However, ocular perfusion pressure remains stable because episcleral venous pressure around eye increases during night in supine position. Hence, raise of sympathetic activity and autonomic dysfunction may alter ocular perfusion pressure during night.[2,12]
Hypoxia causes oxidative stress, inflammation and decreases availability of nitric oxide which is vasodilator and damages the endothelium. It will increase the level of endothelin-1 which is a powerful vasoconstrictor. Production of endothelin-1 also found to be higher in OSA and normal tension glaucoma (NTG) patients, which leads to severe impairment of vasodilator response of blood vessels. Hypoxia-induced endothelin-1 and nitric oxide imbalance in OSA causes vascular dysregulation and affects blood flow of ONH and retina.[2,17,19] It was also found that hypoxia indirectly causes the increase of intracranial pressure. It leads to decrease of cerebral perfusion pressure and may disturb the blood flow of ONH, especially during nocturnal systemic hypotension.[3,12,19]
Mechanical factors
Raising of IOP causes RGCs death, development of glaucoma, and its progression.[17] However, among OSA patients, it was reported that the role of IOP in the development of glaucoma is very minimal and unclear.[2] As a result of vascular dysregulation, ischemia and abnormal perfusion pressure optic nerve may be more sensitive even for normal IOP to get damage.[13] Apart from supine position at night during sleep, obesity also one of the risk factors for high IOP due to excessive intraorbital adipose tissue and increased episcleral venous pressure.[3] As mentioned earlier, intracranial pressure will increase during apnea–hypopnea episodes and alters the cerebral perfusion pressure [Figure 1].[3,12,19]
Figure 1.
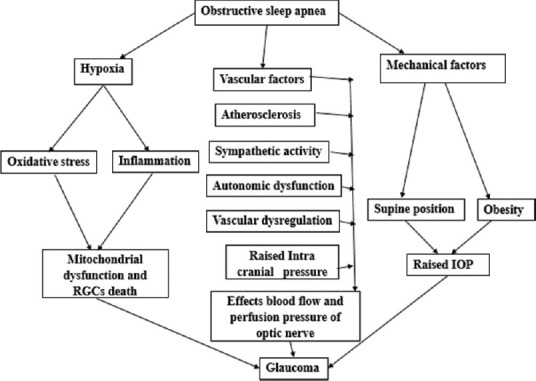
Flowchart explains the mechanism's involved pathophysiology of glaucoma in obstructive sleep apnea
Prevalence of Glaucoma in Patients with Obstructive Sleep Apnea
Walsh and Montplaisir[20] initially described the association of OSA and glaucoma in 1982. They diagnosed glaucoma in five patients of two generations in the same family with OSA. Among those, three surviving members had heavy snoring with maximum IOP in the morning time. Then, in 1999, a study was published by Mojon et al.[21] which reported a high prevalence of glaucoma in OSA patients. In this study, 114 consecutive patients with suspect of OSA were included after polysomnography evaluation. Sixty-nine patients were diagnosed to have OSA based on respiratory disturbance index (RDI) ≥10. The prevalence of glaucoma is 7.2% (5/69). Among those 5 glaucoma patients, 3 patients had primary open-angle glaucoma (POAG), and 2 patients had NTG. Apart from this, it was also reported that RDI is positively correlated with IOP (P = 0.025), VF loss variance (P = 0.03), glaucomatous optic disc changes (P = 0.001), and diagnosis of glaucoma (P = 0.01).
Later, several large population-based studies and other prevalence studies have shown the association between the glaucoma and OSA. Results of the most of these studies showed significant prevalence of glaucoma in OSA patients. Here, we briefly discuss few important studies while rest of the studies are reported in Table 1.
Table 1.
Studies reported the prevalence of glaucoma in obstructive sleep apnea patients
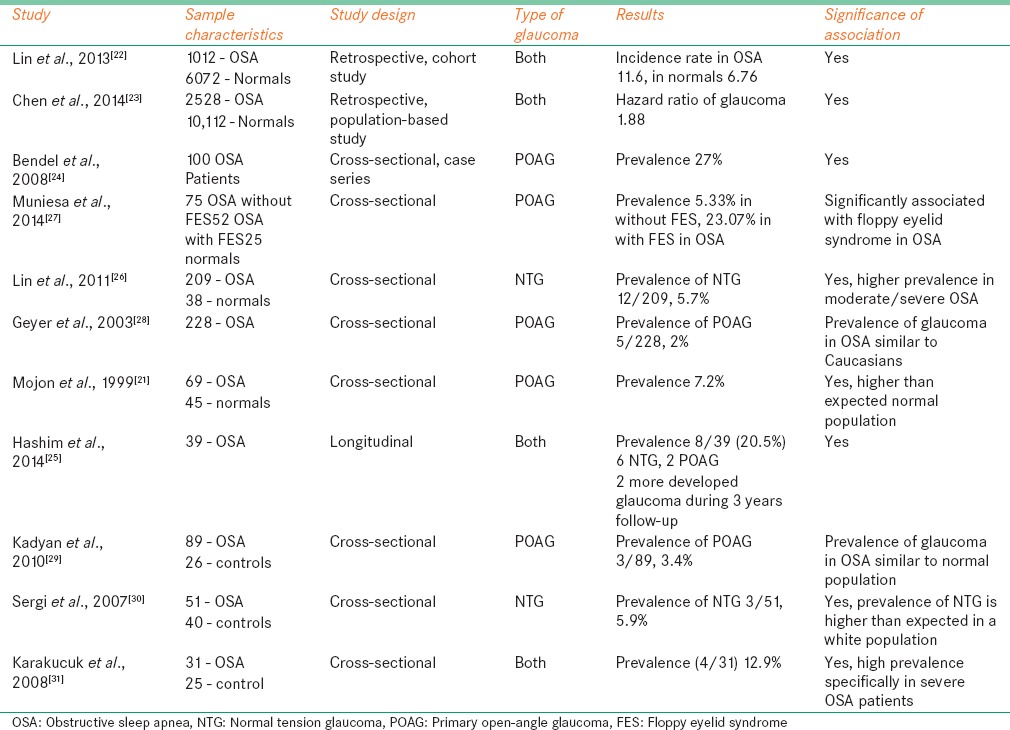
Lin et al.[22] did a large sample retrospective cohort study to analyze incidence rate and risk of open-angle glaucoma (OPA) in OSA patients over a 5-year follow-up period after diagnosed as OSA. Data were collected from the data source of the longitudinal health insurance database 2000 in Taiwan population. Total 1012 patients in disease cohort and 6072 randomly selected controls were studied. All cases traced for next 5 years period to identify who subsequently developed OPA in both disease and control cohorts. The incident rate of glaucoma per 1000 person-years was 11.26 (95% confidence interval [CI]: 8.61–14.49) for OSA patients and 6.76 (95% CI: 5.80–7.83) for control cohort, respectively. After adjusting for systemic abnormalities and socioeconomic status, OSA patients were associated with 1.67 times more risk of developing OAG within first 5-year period of initial diagnosis.
In a retrospective cohort study in Taiwanese Population using the Longitudinal Health Insurance database 2000, Chen et al. showed the treatment options for OSA and its associated risk of developing glaucoma. They have included larger sample of 2528 OSA patients and 10112 randomly selected age-matched controls. The study included OSA cohort collected based on diagnosis with polysomnography evaluation made between 2000 and 2009. They also excluded patients with previous history of glaucoma and analyzed the risk of glaucoma due to the treatment. The risk of glaucoma investigated in patients with OSA was assessed for various OSA treatment options such as with surgical treatment, with continuous positive airway pressure (CPAP) with multiple modalities and without treatment. Results showed that adjusted hazard ratio of glaucoma in OSA was 1.88 (with 95% CI: 1.46–2.42) compared with controls. Adjusted hazard ratio increased to 2.15 (with 95% CI: 1.60–2.88) in patients with OSA without treatment. However, in patients with OSA, treatment risk of glaucoma was not significantly different from controls except in CPAP therapy patients where hazard ratio is 1.65 (with 95% CI: 1.09–2.49) compared with controls. These estimated hazard ratios were adjusted for after for sex, age, hypertension, diabetes, and other systemic conditions.
There are several other cross-sectional and case-control studies with small samples that investigate association between OSA and glaucoma. Some studies reported the prevalence of POAG in OSA while some other report on NTG. Bendel et al.[24] evaluated 100 patients with OSA to study the prevalence of glaucoma and association of glaucoma and IOP with these patients. The prevalence of glaucoma is 27% (27 patients), of them 14 patients were diagnosed based on both optic nerve and VFs, 9 based on optic nerve appearance only, and 4 were already diagnosed from history and ophthalmic examination. Glaucoma appears to be associated with age (P = 0.014), and IOP is more associated BMI (P = 0.006). There is no correlation found between AHI and IOP as well as between AHI and glaucoma.
Hashim et al.[25] conducted a study to analyze the prevalence and progression of glaucoma in treated OSA patients over a follow-up of 3 years period. Total 39 patients (12 patients with moderate OSA and 27 patients with severe OSA) with moderate to severe OSA were investigated. Glaucoma found in 8 patients (20.5% 95% CI: 9.9%-37%), of them 6 patients had NTG and 2 patients had POAG. If we observe the prevalence of glaucoma in severity wise, 7 (25.9% 95% CI: 8%-34%) of 27 severe OSA and 1 (8.3% 95% CI: 0.1%-15%) of 12 moderate OSA patients had glaucoma. It showed that severe OSA is very important risk factor for glaucoma. During the follow-up period of 3 years under the treatment of OSA, only 2 patients developed NTG and 2 patients showed glaucoma deterioration which indicates proper and adequate treatment of OSA with ophthalmic care showed better control of glaucoma.
Lin et al.[26] evaluated 209 patients with OSA and 38 normal controls to study the prevalence of NTG in each group and risk of severity of OSA for developing glaucoma. The prevalence of NTG found to have 5.7% (12/209). In 12 NTG patients, 1 was in the mild OSA group, 3 were in the moderate OSA group, and 8 were in the severe OSA group. The prevalence of NTG in moderate/severe OSA found to have 7.1% (11/153) which is significantly higher (P = 0.033) than normal/mild OSA cases. This study also shows that disease severity of OSA is an important risk factor for developing glaucoma.
Muniesa et al.[27] investigated the prevalence of glaucoma in OSA with and without FES. Glaucoma was defined as asymmetric cupping, glaucomatous optic disc damage with corresponding VFs, thinner neuroretinal rim, and/or optic disc hemorrhage and/or with RNFL defects. If untreated, IOP > 21 mmHg is considered as POAG, <22 mmHg as NTG. The prevalence of glaucoma in the OSA patients without FES was 5.33% (4/75), and in the OSA patients with FES, it was 23.07% (12/52). The difference between these two was statistically significant (P = 0.004). Of 12 patients in OSA with FES, 6 had NTG, 5 had POAG, and one patient was already diagnosed as glaucoma before.
There were some studies which reported less prevalence rates also. A study done by Geyer et al.[28] showed that it was included a total of 228 patients with OSA, of those only 5 patients found to have POAG, and the prevalence rate is 2% (95% CI: 0.7%-5%). This is equal to the prevalence of glaucoma in general Caucasian population (1.7%-3%). In another study, Kadyan et al.[29] reported that the prevalence of glaucoma in OSA was 3.4% (3/89) which is similar to normal population 2% (P = 0.429).
Overall, all these studies showed that there is increased prevalence of glaucoma in OSA and it is significantly associated with severity of the disease. However, glaucoma and IOP are not significantly associated with AHI or RDI.
Prevalence of Obstructive Sleep Apnea in Patients with Glaucoma
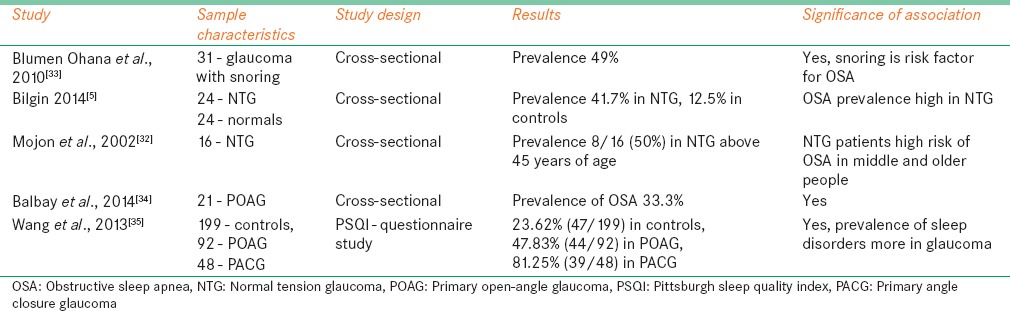
Studies have reported the prevalence of OSA and other related conditions among glaucoma patients. These studies also showed higher prevalence rates of OSA, especially more among NTG than POAG patients. Mojon et al.[32] evaluated 16 Caucasian patients with NTG to study the prevalence of OSA. All patients underwent overnight polysomnography examination to make a diagnosis of OSA. Patients with RDI more >10 were diagnosed as having OSA. 50% (8/16) patients diagnosed to have OSA after polysomnography evaluation. Among those 4 with mild OSA, 2 were with moderate OSA and 2 with severe OSA. The prevalence rate was more in middle and older age people which is significantly high (P < 0.025).
Another study done by Ohana et al.[33] reported the prevalence of OSA in POAG and snoring. 31 snoring POAG patients had polysomnography evaluation to rule out clinical diagnosis of OSA. The prevalence of OSA found to have 49%, 15 of 31 total cases. There is no significant difference found in ocular characteristics among patients with and without OSA. This study explained that POAG with snoring is an important sign to screen and make clinical diagnosis of OSA.
Bilgin et al.[5] studied the association between OSA and NTG. They recruited 24 established NTG patients and similar number of age-matched controls. All patients underwent overnight polysomnography evaluation for the diagnosis of OSA. Patients with AHI score ≥20/h were diagnosed as OSA. Total 10 patients (41.7%) were diagnosed to have OSA in NTG group, 3 cases (12.5) in age-matched controls. There was a significant difference (P < 0.05) in the prevalence of OSA between the groups, and relative risk of OSA in NTG is 3.34 times more compared with age-matched controls.
In another study, Balbay et al.[34] investigated the prevalence of POAG in OSA patients. Twenty-one POAG patients underwent polysomnography examination for the diagnosis of OSA. AHI criteria were used to grade the severity of the disease. OSA diagnosis confirmed in 33.3% (7/21) of patients, 14.3% with mild and 19.0% with moderate OSA. Age (P = 0.047) and neck (P = 0.024) circumference of OSA patients was significantly higher.
A questionnaire study was done by Wang et al.[35] to study a sleep quality in POAG, PACG, and controls. Pittsburgh sleep quality index was used to evaluate the glaucoma effect on sleep quality. Patients who score more than >7 were considered as disturbed sleep. Sleep quality decreased with age in all cases. People with sleep disorders were 23.62% (47/199) in controls, 47.83% (44/92) in POAG, and 81.25% (39/48) in PACG. The prevalence of sleep disturbances significantly higher in POAG and PACG compared with controls. PACG shows higher prevalence of sleep disturbances (P = 0.04) compared with POAG in age range of 61–80 years. This study explains that glaucoma and loss of intrinsically photosensitive RGCs may have influence on regular circadian rhythm effect sleep/wake cycle. Hence, glaucoma patients may have more chance to get sleep-related disorders [Table 2].
Table 2.
Studies reported the prevalence obstructive sleep apnea in glaucoma patients
Structural Changes of Retina and Optic Nerve Head in Obstructive Sleep Apnea
Retinal nerve fiber layer
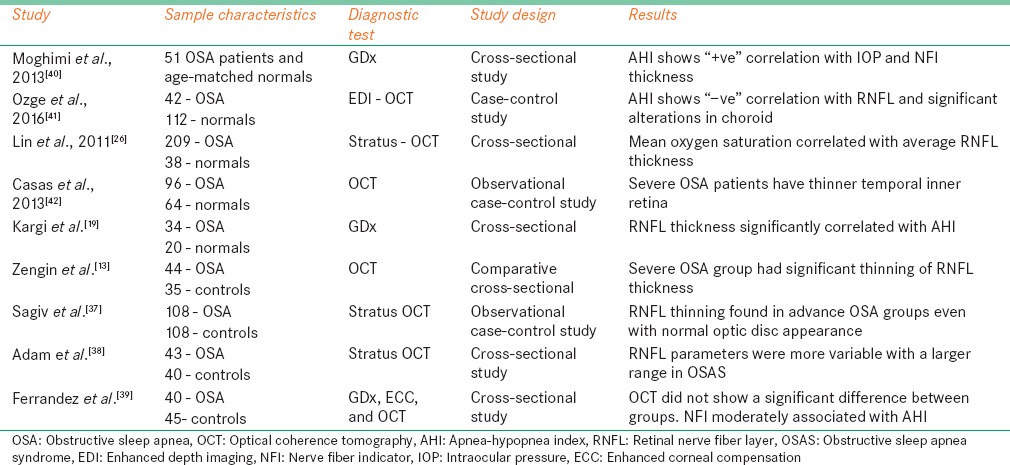
Several studies have evaluated RNFL thickness in OSA patients using different diagnostic techniques and it was found that RNFL is thinner in OSA patients compared with normal controls. It was also reported that RNFL thickness negatively correlated with AHI or RDI and positive correlation with oxygen saturation in OSA patients. Lowest saturation of oxygen significantly associated with decreased RNFL thickness.[36] The mechanism behind the RNFL thinning is that OSA is associated with several vascular disorders such as imbalance of vasoconstriction and vasodilation. These vascular disturbances may result in thinning of RNFL. When these vascular disturbances are associated with systemic nocturnal hypotension, it will cause further more damage and thinning of RNFL.[2,12] Kargi et al.[19] first reported RNFL thinning in OSA patients. In this study, RNFL thickness was measured in 34 patients with OSA with 19 mild and 15 severe conditions, 20 age-matched controls using GDx scanning laser polarimetry (SLP). RNFL thickness significantly reduced in OSA patients compared with normals in superior average (P = 0.001) and inferior average (P = 0.002). Moreover, it is also noted that thinning RNFL thickness is significantly correlated with severity of the disease (P = 0.01). This study proposed two possible mechanisms for RNFL thinning in OSA.
Sagiv et al.[37] measured RNFL thickness in large sample, 108 patients with moderate (n = 62), severe (n = 46) OSA and 108 age-matched controls with optical coherence tomography (OCT). All patients underwent overnight polysomnography examination. In OSA group average (P = 0.003), superior (P = 0.022), inferior (P = 0.011), and temporal (P = 0.029) quadrants of RNFL thickness was significantly lower and reduced than control group. Average, superior, and inferior RNFL thickness in both OSA group ((P = 0.004, P = 0.034, and P = 0.003) and control group ((P = 0.001, P = 0.003, and P = 0.008) is significantly negatively associated with age, which explains that RNFL thickness will decrease with age. Based on multiple linear regression model, in OSA group, it showed average RNFL thinner by 4.20 mm (95% CI [−6.97, −1.43], P = 0.003), superior quadrant RNFL thinner by 4.83 mm (95% CI [−9.14, −0.52], P = 0.028), and inferior quadrant RNFL thinner by 5.19 mm (95% CI [−9.39, −0.99], P = 0.016) compared with controls.
A study published by Adam et al.[38] showed different results. In this study, RNFL thickness was measured in 43 newly diagnosed with mild (n = 4), moderate (n = 23), and severe (n = 16) OSA patients and in 40 healthy controls using stratus OCT. Results showed that there is no significant difference of average (P = 0.81), superior (P = 0.73), inferior (P = 0.68), temporal (P = 0.82), and nasal (P = 0.37) RNFL thickness between OSA and control groups. However, OSA patients showed large range of variables in RNFL parameters in average 84.45–131.25 μ, superior 52–165 μ, inferior 86–200 μ, nasal 56- 127 μ, and temporal quadrants 53–123 μ compared with controls same as average 93.7–119 μ, superior 97–162 μ, inferior 115–164 μ, nasal 54–115 μ, and temporal quadrants 53–113 μ. This study explains that duration of the disease is more important than the severity of the disease; hence, long-term follow-up of monitoring of the OSA patients is necessary.
Zengin et al.[13] investigated the RNFL thickness measurement over a period of 1 year for every 3 months with OCT in 44 OSA patients with 13 mild, 17 moderate, and 14 severe disease and 35 age-matched controls. It showed that last measurements of average RNFL in OSA were found to be significantly reduced compared with the first visit (P = 0.001) and with controls (P = 0.002). All four quadrants of RNFL thickness in OSA showed a significant reduction in the last visit compared with the initial visit and controls. RNFL measurements also significantly correlated with AHI (P < 0.05). OSA patients should be followed up regularly to screen for glaucomatous changes, glaucoma development, and for detection of possible progression of the disease.
Another recent study by Ferrandez et al.[39] evaluated RNFL thickness using SLP-enhanced corneal compensation (ECC) and OCT. It is a cross-sectional study included 40 OSA patients and 45 age-matched controls. OCT parameters showed no significant difference of RNFL thickness among control and OSA patients at all clock hour positions, in 4 quadrants and in average RNFL thickness. SLP-ECC parameters showed nerve fiber indicator (NFI) higher and superior average RNFL thickness lower in OSA group. NFI moderately correlated with AHI in OSA group. Some of SLP-ECC parameters were significantly reduced in OSA, but OCT did not show any neurodegenerative change. Several other studies reported RNFL thinning and its relation with the severity of the OSA [Table 3].
Table 3.
Studies reported the retinal nerve fiber layer thickness in obstructive sleep apnea patients
Optic nerve head and macula
Some studies have evaluated the ONH measurements in patients with OSA and found that there is a significant increase in morphological characteristics of ONH and thickness changes in inner macula. Increase in disc area and other parameters are maybe due to the mild optic nerve swelling caused by intracranial vascular dysfunction. Increase in macular thickness explained by RGC is sensitive to mild systemic hypoxic stress. Hypoxia will cause swelling of cell body, disruption of plasma membrane, and alterations in nuclear DNA. This swelling may be the reason for an increased macular thickness, and then it will precede to atrophy secondary to neuronal death. This may be the reason that mild-moderate OSA patients show higher inner macular thickness compared with control and severe OSA patients.[11,36,42]
Tsang et al.[11] first measured the optic nerve cup: disc ratio in OSA patients using slit lamp biomicroscopy by single examiner. Thirty-six patients with moderate and severe OSA, 30 age-matched controls were included in the study. The incidence of suspicious discs with glaucomatous changes in OSA (26.39%) was 4 times higher when compared to controls (6.78%), which is statistically significant difference (P = 0.001, 99% CI). Casas et al.[42] assessed RNFL thickness and ONH parameters in 50 OSA patients and 33 age-matched controls using Stratus OCT. Results showed that ONH measurements are significantly higher in OSA patients, and mean vertical integrated rim area is 0.67 ± 0.41 mm3 in OSA and 0.55 ± 0.29 mm3 in controls which is statistically significant (P = 0.043). Mean horizontal integrated rim width is 1.87 ± 0.31 mm2 in OSA and 1.8 ± 0.25 mm2 in controls, P = 0.039. Disc area is 2.74 ± 0.62 mm2 in OSA and 2.48 ± 0.42 mm2 in controls, P = 0.002. Severe OSA patients had significant higher disc area 2.8 ± 0.7 mm2 than controls 2.5 ± 0.4 mm2 ; P = 0.016. Temporal inner macular thickness was significantly higher in mild-moderate OSA patients 270 ± 12 μm compared with severe OSA patients 260 ± 19 μm, P = 0.021.
Lin et al.[36] reported ONH parameters in 105 OSA patients and 22 controls. This study found that there is no significant difference in ONH parameters and macula thickness profiles (P > 0.1) between OSA and age-matched controls. Another study by Sergi et al.[30] reported that AHI is significantly correlated with cup-disc ratio of both right and left eye (P = 0.001 and P = 0.01).
Choroid thickness
Choroid is one of the highly vascularized tissues in the body with rich vascular components. Significant vascular changes and imbalance between nitric oxide and endothelin in OSA may cause decrease in choroidal blood flow and blood supply of the choroid because endothelin-1 is a major component of choroid blood flow. It may be the possible mechanism behind thinner and structural alterations in choroid.[2,43]
Bayhan et al.[43] studied macular choroid thickness in OSA patients and controls using RTVue spectral-domain-OCT (SD-OCT). Ninety-two patients with OSA and 32 age-matched controls were included in the study. The choroidal thickness measurements at 1.5 mm and 3 mm nasal to the fovea were significantly thinner in severe OSA patients (both P < 0.05) compared with controls, and severe OSA patients had significant thinner choroid at 3 mm nasal to fovea compared with mild OSA patients. However, there is no significant difference of choroid thickness at subfoveal and temporal area between the OSA and controls.
Ozge et al.[41] studied macular choroid thickness and peripapillary choroid thickness in 42 OSA patients and 56 age-matched controls using enhanced depth imaging – OCT (SD-OCT). OSA patients had significantly thicker choroid at 0.5 and 1.5 mm nasal to the fovea in both eyes compared with the control group (P < 0.05). There were no significant differences of choroid thickness in the subfoveal and temporal regions. Moreover, peripapillary choroidal thickness was significantly higher in OSA patients at all segments (P < 0.05) except at temporal and superotemporal regions. As earlier mentioned in this article hypoxic related swelling associated with OSA, also may be the reason for an altered choroid thickness at macular and peripapillary regions.
Xin et al.[44] measured choroidal thickness in 53 OSA and 12 age-matched controls. It reported that subfoveal choroid thickness in severe OSA group was significantly thinner than control, mild, and moderate groups (P = 0.023, 0.006, and 0.036), and choroid thickness 1 mm nasal to the fovea was significantly thinner in severe group compared with control and mild groups (P = 0.013 and 0.010). Another study by Karaca et al.[45] measured macular choroid thickness in 74 OSA and 33 controls. Results showed that there is no significant difference of subfoveal choroidal thickness among control and OSA groups. There is no significant correlation between severity of disease and choroid thickness. However, moderate OSA group showed reduced choroid thickness at all areas compared with control, mild, and severe groups.
Functional Changes of Retina in Obstructive Sleep Apnea
Visual fields
Hypoxia, oxidative stress, vascular dysregulation, and compromise ONH perfusion are the risk factors for Ganglion cell death in OSA. This neuronal death leads to a thinning of RNFL and reduced sensitivity at corresponding VF areas. Ferrandez et al.[46] studied retinal sensitivity in OSA patients using standard automated perimeter (SAP). Eighty OSA patients and 111 age-matched controls were prospectively enrolled. Results showed that mean deviation (MD) of SAP was −0.23 ± 0.8 dB in the control group and −1.74 ± 2.8 dB in the OSA group, and VF index (VFI) was 99.52 in controls and 97.51 in OSA patients which is statistically significant (both P < 0.001). Of 52 points, most of points showed reduced sensitivity in OSA patients (P < 0.001) compared with controls. AHI was significantly correlated with MD, pattern standard deviation (PSD), and VFI.
Several other prevalence studies have reported VF sensitivity changes in OSA patients. Tsang et al.[11] reported that both MD and PSD showed a significant difference between OSA and controls which is statistically significant (P < 0.01) and moderate to severe OSA patients associated with higher incidence of VF defects. Study by Moghimi et al.[40] reported that MD −2.19 in OSA and −1.15 in controls which is a significant difference (P = 0.04). Another study by Casas et al.[42] showed MD −0.64 ± 1.38 in OSA, 0.07 ± 1.01 in controls with significance difference (P < 0.05) and VFI 98.5 ± 2.3 in OSA, 99.2 ± 0.8 in controls. In a study by Sergi et al.[30] right eyes showed MD of −3.7 ± 6.4 in OSA, 0.25 ± 1 in controls (P = 0.01); left eyes showed MD of −2.84 ± 3.7 in OSA, 0.36 ± 1.8 in controls (P = 0.01) and AHI significantly correlated with MD in both right and left eyes (P = 0.01 and P = 0.001).
Electrophysiological tests
Objective functional tests are very effective for measuring the retinal sensitivity compared with subjective perimetry tests which has its own related issues. Gutiιrrez-Dνaz et al.[47] measured retinal function using multifocal visual-evoked potential (mfVEP) in twenty newly diagnosed OSA patients with and without NTG. mfVEP amplitude responses showed abnormal cluster in 40% of the eyes in the nonglaucoma group and in 90% of eyes of the NTG patients (P = 0.019). mfVEP latency analysis showed latency delays in 30% of the nonglaucoma group eyes and in 60% of eyes of the NTG group. Overall, 60% of eyes in the nonglaucoma group and all NTG eyes showed amplitude and/or latency defects in the mfVEP. In OSA patients with glaucoma, mfVEP amplitude and latency were significantly correlated with systolic blood pressure, sleep efficiency, arousal index, mean and minimum arterial oxygen saturation (SaO2), time SaO2 <90%, oxyhemoglobin desaturation index, number of central and mixed apneas, and apnea-hypopneas index (P < 0.05).
Another study by Sergi et al.[30] performed VEP and pattern electroretinography (PERG) in 51 patients with OSA and 40 controls. Of 51 patients, 3 had NTG. VEP and PERG were abnormal in 23 and 19 OSA patients and none of control group had pathologic VEP or PERG. Abnormal VEP amplitude and latency, abnormal PERG amplitude and latency found in both eyes of OSA patients compared with controls which is statistically significant difference (P = 0.001).
Intraocular pressure
Several studies have reported that IOP is significantly associated with AHI and BMI. As reported earlier, the possible mechanism behind high IOP in OSA with high BMI is because of excessive adipose in intraorbital region which will increase episcleral venous pressure.[3] Lin et al.,[26] showed significant difference of IOP (P = 0.01) between patients with AHI <15 and AHI >15 groups. Casas et al.,[42] reported significant difference of IOP (P = 0.003) between OSA and control groups. Moghimi et al.[40] reported that among 51 OSA patients, 7 patients had IOP >21 mmHg and significance difference of IOP (P < 0.001) between control and OSA group patients. Sergi et al.[30] reported in right eye IOP 15.4 ± 2.7 in OSA, 12.5 ± 2 in controls (P = 0.05) and in left eye IOP 16.5 ± 2.8 in OSA, 13.4 ± 3 in controls (P = 0.05). Some other studies showed no significant difference between IOP and OSA or AHI. Adam et al.[38] reported that there is no significant difference of IOP (P = 0.11) between OSA group and control group.
Continuous positive airway pressure therapy
The gold standard technique for treating OSA is CPAP which administered through a nasal or face mask. This therapy prevents upper airway collapse at night. Studies have reported that diurnal variations IOP higher in OSA patients and it will increase even more after CPAP therapy. The exact mechanism behind higher diurnal variations in OSA patients and CPAP therapy is unknown. Kiekens et al.[48] measured 24 h IOP for every 2 h in 21 newly diagnosed OSA patients and after 1 month of CPAP therapy. 24 h IOP fluctuations (difference between trough and peak IOP) ≥8 mmHg noted in 7 patients at baseline visit and the number increased to 12 patients after 1 month of CPAP therapy. The mean difference between trough and peak IOP was 6.7 ± 1.5 mmHg at baseline and 9.0 ± 2.0 mmHg after CPAP therapy.
Another study by Cohen et al.[49] measured 24 h IOP in two different groups. One is OSA with non-CPAP treated group another one is CPAP-treated group. The mean IOP of the CPAP and non-CPAP groups measured in sitting position before the sleep period was 13.33 ± 2.04 mmHg and 14.02 ± 2.44 mmHg, respectively (P = 0.9). After 1 min of supine position, IOP increased by 1.93 mmHg and 2.13 mmHg for both the non-CPAP and CPAP groups (P = 0.02, P = 0.001). The IOP increased significantly after 7 h of sleep in the supine position, and the mean IOP of the CPAP and non-CPAP groups was 19.2 ± 5.68 mmHg and 19.69 ± 5.61 mmHg, respectively. Three OSA patients with glaucoma treated with CPAP had mean IOP of 23.75 mmHg after 7 h of sleep.
Conclusion
OSA is the major risk factor for developing glaucoma. Studies show strong association between OSA and glaucoma. Complete ophthalmic evaluation should be advised at every follow-up for patients with OSA. Glaucoma patients with obesity and progressive VF damage even under low eye pressure (NTG) should be evaluated for OSA and other sleep disorders. OSA patients treating with CPAP therapy should undergo regular glaucoma screening and IOP monitoring because CPAP therapy may trigger the glaucoma damage and its progression by raising the IOP.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.George R, Ve RS, Vijaya L. Glaucoma in India: Estimated burden of disease. J Glaucoma. 2010;19:391–7. doi: 10.1097/IJG.0b013e3181c4ac5b. [DOI] [PubMed] [Google Scholar]
- 2.Faridi O, Park SC, Liebmann JM, Ritch R. Glaucoma and obstructive sleep apnoea syndrome. Clin Exp Ophthalmol. 2012;40:408–19. doi: 10.1111/j.1442-9071.2012.02768.x. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Rico C, Gutiérrez-Díaz E, Mencía-Gutiérrez E, Díaz-de-Atauri MJ, Blanco R. Obstructive sleep apnea-hypopnea syndrome (OSAHS) and glaucomatous optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2014;252:1345–57. doi: 10.1007/s00417-014-2669-4. [DOI] [PubMed] [Google Scholar]
- 4.Lam B, Lam DC, Ip MS. Obstructive sleep apnoea in Asia. Int J Tuberc Lung Dis. 2007;11:2–11. [PubMed] [Google Scholar]
- 5.Bilgin G. Normal-tension glaucoma and obstructive sleep apnea syndrome: A prospective study. BMC Ophthalmol. 2014;14:27. doi: 10.1186/1471-2415-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Myers KA, Mrkobrada M, Simel DL. Does this patient have obstructive sleep apnea? The Rational Clinical Examination systematic review. JAMA. 2013;310:731–41. doi: 10.1001/jama.2013.276185. [DOI] [PubMed] [Google Scholar]
- 7.Stradling JR, Davies RJ. Sleep 1: Obstructive sleep apnoea/hypopnoea syndrome: Definitions, epidemiology, and natural history. Thorax. 2004;59:73–8. doi: 10.1136/thx.2003.007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivas M, Ratra A, Nugent K. Obstructive sleep apnea and its effects on cardiovascular diseases: A narrative review. Anatol J Cardiol. 2015;15:944–50. doi: 10.5152/AnatolJCardiol.2015.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNab AA. The eye and sleep. Clin Exp Ophthalmol. 2005;33:117–25. doi: 10.1111/j.1442-9071.2005.00969.x. [DOI] [PubMed] [Google Scholar]
- 10.Stansbury RC, Strollo PJ. Clinical manifestations of sleep apnea. J Thorac Dis. 2015;7:E298–310. doi: 10.3978/j.issn.2072-1439.2015.09.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsang CS, Chong SL, Ho CK, Li MF. Moderate to severe obstructive sleep apnoea patients is associated with a higher incidence of visual field defect. Eye (Lond) 2006;20:38–42. doi: 10.1038/sj.eye.6701785. [DOI] [PubMed] [Google Scholar]
- 12.Fraser CL. Obstructive sleep apnea and optic neuropathy: Is there a link? Curr Neurol Neurosci Rep. 2014;14:465. doi: 10.1007/s11910-014-0465-5. [DOI] [PubMed] [Google Scholar]
- 13.Zengin MO, Tuncer I, Karahan E. Retinal nerve fiber layer thickness changes in obstructive sleep apnea syndrome: One year follow-up results. Int J Ophthalmol. 2014;7:704–8. doi: 10.3980/j.issn.2222-3959.2014.04.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khurana RN, Porco TC, Claman DM, Boldrey EE, Palmer JD, Wieland MR. Increasing sleep duration is associated with geographic atrophy and age-related macular degeneration. Retina. 2016;36:255–8. doi: 10.1097/IAE.0000000000000706. [DOI] [PubMed] [Google Scholar]
- 15.Stein JD, Kim DS, Mundy KM, Talwar N, Nan B, Chervin RD, et al. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011;152:989–98.e3. doi: 10.1016/j.ajo.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Gupta SK, Agarwal P, Saxena R, Agrawal SS. Current concepts in the pathophysiology of glaucoma. Indian J Ophthalmol. 2009;57:257–66. doi: 10.4103/0301-4738.53049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: Implications for obstructive sleep apnoea. Exp Physiol. 2007;92:51–65. doi: 10.1113/expphysiol.2006.035204. [DOI] [PubMed] [Google Scholar]
- 19.Kargi SH, Altin R, Koksal M, Kart L, Cinar F, Ugurbas SH, et al. Retinal nerve fibre layer measurements are reduced in patients with obstructive sleep apnoea syndrome. Eye (Lond) 2005;19:575–9. doi: 10.1038/sj.eye.6701582. [DOI] [PubMed] [Google Scholar]
- 20.Walsh JT, Montplaisir J. Familial glaucoma with sleep apnoea: A new syndrome? Thorax. 1982;37:845–9. doi: 10.1136/thx.37.11.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mojon DS, Hess CW, Goldblum D, Fleischhauer J, Koerner F, Bassetti C, et al. High prevalence of glaucoma in patients with sleep apnea syndrome. Ophthalmology. 1999;106:1009–12. doi: 10.1016/S0161-6420(99)00525-4. [DOI] [PubMed] [Google Scholar]
- 22.Lin CC, Hu CC, Ho JD, Chiu HW, Lin HC. Obstructive sleep apnea and increased risk of glaucoma: A population-based matched-cohort study. Ophthalmology. 2013;120:1559–64. doi: 10.1016/j.ophtha.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 23.Chen HY, Chang YC, Lin CC, Sung FC, Chen WC. Obstructive sleep apnea patients having surgery are less associated with glaucoma. J Ophthalmol. 2014;2014:838912. doi: 10.1155/2014/838912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bendel RE, Kaplan J, Heckman M, Fredrickson PA, Lin SC. Prevalence of glaucoma in patients with obstructive sleep apnoea – A cross-sectional case-series. Eye (Lond) 2008;22:1105–9. doi: 10.1038/sj.eye.6702846. [DOI] [PubMed] [Google Scholar]
- 25.Hashim SP, Al Mansouri FA, Farouk M, Al Hashemi AA, Singh R. Prevalence of glaucoma in patients with moderate to severe obstructive sleep apnea: Ocular morbidity and outcomes in a 3 year follow-up study. Eye (Lond) 2014;28:1304–9. doi: 10.1038/eye.2014.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin PW, Friedman M, Lin HC, Chang HW, Wilson M, Lin MC. Normal tension glaucoma in patients with obstructive sleep apnea/hypopnea syndrome. J Glaucoma. 2011;20:553–8. doi: 10.1097/IJG.0b013e3181f3eb81. [DOI] [PubMed] [Google Scholar]
- 27.Muniesa M, Sánchez-de-la-Torre M, Huerva V, Lumbierres M, Barbé F. Floppy eyelid syndrome as an indicator of the presence of glaucoma in patients with obstructive sleep apnea. J Glaucoma. 2014;23:e81–5. doi: 10.1097/IJG.0b013e31829da19f. [DOI] [PubMed] [Google Scholar]
- 28.Geyer O, Cohen N, Segev E, Rath EZ, Melamud L, Peled R, et al. The prevalence of glaucoma in patients with sleep apnea syndrome: Same as in the general population. Am J Ophthalmol. 2003;136:1093–6. doi: 10.1016/s0002-9394(03)00709-8. [DOI] [PubMed] [Google Scholar]
- 29.Kadyan A, Asghar J, Dowson L, Sandramouli S. Ocular findings in sleep apnoea patients using continuous positive airway pressure. Eye (Lond) 2010;24:843–50. doi: 10.1038/eye.2009.212. [DOI] [PubMed] [Google Scholar]
- 30.Sergi M, Salerno DE, Rizzi M, Blini M, Andreoli A, Messenio D, et al. Prevalence of normal tension glaucoma in obstructive sleep apnea syndrome patients. J Glaucoma. 2007;16:42–6. doi: 10.1097/01.ijg.0000243472.51461.24. [DOI] [PubMed] [Google Scholar]
- 31.Karakucuk S, Goktas S, Aksu M, Erdogan N, Demirci S, Oner A, et al. Ocular blood flow in patients with obstructive sleep apnea syndrome (OSAS) Graefes Arch Clin Exp Ophthalmol. 2008;246:129–34. doi: 10.1007/s00417-007-0656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mojon DS, Hess CW, Goldblum D, Boehnke M, Koerner F, Gugger M, et al. Normal-tension glaucoma is associated with sleep apnea syndrome. Ophthalmologica. 2002;216:180–4. doi: 10.1159/000059625. [DOI] [PubMed] [Google Scholar]
- 33.Blumen Ohana E, Blumen MB, Bluwol E, Derri M, Chabolle F, Nordmann JP. Primary open angle glaucoma and snoring: Prevalence of OSAS. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127:159–64. doi: 10.1016/j.anorl.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Balbay EG, Balbay O, Annakkaya AN, Suner KO, Yuksel H, Tunç M, et al. Obstructive sleep apnoea syndrome in patients with primary open-angle glaucoma. Hong Kong Med J. 2014;20:379–85. doi: 10.12809/hkmj134021. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Zhang Y, Ding J, Wang N. Changes in the circadian rhythm in patients with primary glaucoma. PLoS One. 2013;8:e62841. doi: 10.1371/journal.pone.0062841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin PW, Friedman M, Lin HC, Chang HW, Pulver TM, Chin CH. Decreased retinal nerve fiber layer thickness in patients with obstructive sleep apnea/hypopnea syndrome. Graefes Arch Clin Exp Ophthalmol. 2011;249:585–93. doi: 10.1007/s00417-010-1544-1. [DOI] [PubMed] [Google Scholar]
- 37.Sagiv O, Fishelson-Arev T, Buckman G, Mathalone N, Wolfson J, Segev E, et al. Retinal nerve fibre layer thickness measurements by optical coherence tomography in patients with sleep apnoea syndrome. Clin Exp Ophthalmol. 2014;42:132–8. doi: 10.1111/ceo.12145. [DOI] [PubMed] [Google Scholar]
- 38.Adam M, Okka M, Yosunkaya S, Bozkurt B, Kerimoglu H, Turan M. The evaluation of retinal nerve fiber layer thickness in patients with obstructive sleep apnea syndrome. J Ophthalmol. 2013;2013:292158. doi: 10.1155/2013/292158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrandez B, Ferreras A, Calvo P, Abadia B, Marin JM, Pajarin AB. Assessment of the retinal nerve fiber layer in individuals with obstructive sleep apnea. BMC Ophthalmol. 2016;16:40. doi: 10.1186/s12886-016-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moghimi S, Ahmadraji A, Sotoodeh H, Sadeghniat K, Maghsoudipour M, Fakhraie G, et al. Retinal nerve fiber thickness is reduced in sleep apnea syndrome. Sleep Med. 2013;14:53–7. doi: 10.1016/j.sleep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 41.Ozge G, Dogan D, Koylu MT, Ayyildiz O, Akincioglu D, Mumcuoglu T, et al. Retina nerve fiber layer and choroidal thickness changes in obstructive sleep apnea syndrome. Postgrad Med. 2016;128:317–22. doi: 10.1080/00325481.2016.1159118. [DOI] [PubMed] [Google Scholar]
- 42.Casas P, Ascaso FJ, Vicente E, Tejero-Garcés G, Adiego MI, Cristóbal JA. Retinal and optic nerve evaluation by optical coherence tomography in adults with obstructive sleep apnea-hypopnea syndrome (OSAHS) Graefes Arch Clin Exp Ophthalmol. 2013;251:1625–34. doi: 10.1007/s00417-013-2268-9. [DOI] [PubMed] [Google Scholar]
- 43.Bayhan HA, Aslan Bayhan S, Intepe YS, Muhafiz E, Gürdal C. Evaluation of the macular choroidal thickness using spectral optical coherence tomography in patients with obstructive sleep apnoea syndrome. Clin Exp Ophthalmol. 2015;43:139–44. doi: 10.1111/ceo.12384. [DOI] [PubMed] [Google Scholar]
- 44.Xin C, Wang J, Zhang W, Wang L, Peng X. Retinal and choroidal thickness evaluation by SD-OCT in adults with obstructive sleep apnea-hypopnea syndrome (OSAS) Eye (Lond) 2014;28:415–21. doi: 10.1038/eye.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karaca EE, Ekici F, Yalçin NG, Çiftçi TU, Özdek S. Macular choroidal thickness measurements in patients with obstructive sleep apnea syndrome. Sleep Breath. 2015;19:335–41. doi: 10.1007/s11325-014-1025-6. [DOI] [PubMed] [Google Scholar]
- 46.Ferrandez B, Ferreras A, Calvo P, Abadia B, Fogagnolo P, Wang Y, et al. Retinal sensitivity is reduced in patients with obstructive sleep apnea. Invest Ophthalmol Vis Sci. 2014;55:7119–25. doi: 10.1167/iovs.14-14389. [DOI] [PubMed] [Google Scholar]
- 47.Gutiérrez-Díaz E, Pérez-Rico C, de Atauri MJ, Mencía-Gutiérrez E, Blanco R. Evaluation of the visual function in obstructive sleep apnea syndrome patients and normal-tension glaucoma by means of the multifocal visual evoked potentials. Graefes Arch Clin Exp Ophthalmol. 2012;250:1681–8. doi: 10.1007/s00417-012-1982-z. [DOI] [PubMed] [Google Scholar]
- 48.Kiekens S, De Groot V, Coeckelbergh T, Tassignon MJ, van de Heyning P, De Backer W, et al. Continuous positive airway pressure therapy is associated with an increase in intraocular pressure in obstructive sleep apnea. Invest Ophthalmol Vis Sci. 2008;49:934–40. doi: 10.1167/iovs.06-1418. [DOI] [PubMed] [Google Scholar]
- 49.Cohen Y, Ben-Mair E, Rosenzweig E, Shechter-Amir D, Solomon AS. The effect of nocturnal CPAP therapy on the intraocular pressure of patients with sleep apnea syndrome. Graefes Arch Clin Exp Ophthalmol. 2015;253:2263–71. doi: 10.1007/s00417-015-3153-5. [DOI] [PubMed] [Google Scholar]


