Summary
Background
Chromium (Cr) release from Cr‐tanned leather articles is a major cause of Cr contact dermatitis. It has been suggested that Cr(VI) release from leather is not necessarily an intrinsic property of the leather, but is strongly dependent on environmental conditions.
Objectives
To test this hypothesis for long‐term (8 months) simulated use.
Materials and methods
The release of total Cr and Cr(VI) from Cr‐tanned, unfinished leather was analysed in subsequent phosphate buffer (pH 8.0) immersions for a period of 7.5 months. The effect of combined ultraviolet treatment and alkaline solution (pH 12.1) was tested. Dry storage [20% relative humidity (RH)] was maintained between immersions. Atomic absorption spectroscopy, X‐ray fluorescence and diphenylcarbazide tests were used.
Results
Cr(VI) release was dependent on previous dry storage or alkaline treatment, but not on duration or number of previous immersions. Cr(III) release decreased with time. Fifty‐two percent of the total Cr released during the last immersion period was Cr(VI). Cr(VI) release exceeded 9 mg/kg in all immersion periods except in the first 10‐day immersion (2.6 mg/kg).
Conclusions
Cr(VI) release is primarily determined by environmental factors (RH prior to immersion, solution pH, and antioxidant content). The RH should be kept low prior to testing Cr(VI) release from leather.
Keywords: alkaline environment, allergic contact dermatitis, chemical analysis, chromium(III), chromium(VI), humidity, leather, metals, occupational
Contact allergy to chromium (Cr) is the third most common metal allergy, and affects approximately 1–3% of the general adult population 1. Today, the majority of recently Cr‐sensitized patients in Europe are women. Owing to the successful restriction on the use of Cr in cement, occupationally related Cr allergy in male construction workers has decreased 2. Since the 1990s, leather products have attracted an increasing amount of attention as a cause of Cr allergy and dermatitis 2, 3, 4, and have been found to be a major cause in several studies 3, 4. Since 2015, the release of Cr(VI) from leather has been restricted in the EU by the Regulation on Registration, Evaluation, Authorization and Restriction of Chemicals (REACH); testing for control of compliance is based on the ISO 17075 standard 5. Between 7% and 50% of ∼9500 leather products tested and reported since the year 2000 contained Cr(VI) in concentrations above the limit of detection (3 mg/kgleather) of the ISO 17075 standard 6, 7, 8. We have previously questioned this standard, owing to the possibility of false‐negative results, depending on the relative humidity (RH) during storage prior to testing 9.
We found that relatively high amounts of Cr(III) were released from different Cr‐tanned leathers 9, 10, 11, 12. In these studies, Cr(VI) was released at amounts exceeding 3 mg/kg for leather types with low antioxidant contents and in certain solutions. The amounts of Cr(III) and Cr(VI) were higher than what would be needed to induce allergic contact dermatitis in Cr‐allergic patients. Both the RH during storage prior to immersion of the leather in the extraction solution and the solution pH were found to be of substantial importance 9. The combination of alkaline solution at pH 12, ultraviolet (UV) treatments and previous dry storage was found to be the worst scenario. However, the longest duration of immersion tested in our previous studies was 1 week 11. We also found that Cr(III) and Cr(VI) release decreased upon repeated immersions 9, 11.
Our previous conclusion, based on the short‐term study 9, was that Cr(VI) release is mainly caused by environmental factors, and is not necessarily an intrinsic (independent) property of the leather material. The primary objective of this study was therefore to investigate whether this hypothesis is valid for longer immersions (months), which constitute a possible simulation of long‐term use of leather articles.
Materials and Methods
Leather used in this study
The leather in this study was from the same sample, sized approximately 0.5 m2, as has been used in our previous studies, denoted there as ‘from cattle’ 9, CrCr gloves 10, 11, or ‘reference leather’ 12. It was received from a European tannery, and had been produced from cattle according to the normal production process. Triplicate pieces, sized 1.0 × 1.0 × 0.2 cm3, were cut from the larger leather piece. The leather was Cr‐tanned and Cr‐post‐tanned, not coated, without finish (so‐called crust leather), and intended for use as working gloves (generally low‐price leather), as characterized in 10. From previous findings, this leather was chosen because it released Cr(VI) after storage at an RH of ≤35% and in solutions with a pH of ≥7.5 9, 10, 11. This was considered to be suitable for the study of how long‐term Cr leaching affects the ability to form Cr(VI) during dry storage.
Storage conditions and UV treatment
Prior to each immersion, the triplicate samples were dried in an environmental chamber (Weiss WK3‐340/40) at 70°C and 20% RH for 24 h. With this preconditioning, Cr(VI) release was previously found for new leather pieces and for leather pieces that had been exposed for up to 6 h in different solutions 9. The temperature of 70°C was chosen to ensure that 20% RH could be obtained even if the RH outside the environmental chamber was high. The sample pieces were directly immersed in solution in the environmental chamber to avoid any contamination with air of higher RH. The sequence of periods of storage and immersion is shown in Fig. 1. After one storage period, a UV treatment was performed. The UV irradiation emanated from a UV‐light source of 15 W, placed 25 cm from the dry samples, which were irradiated for 3 h and 15 min.
Figure 1.
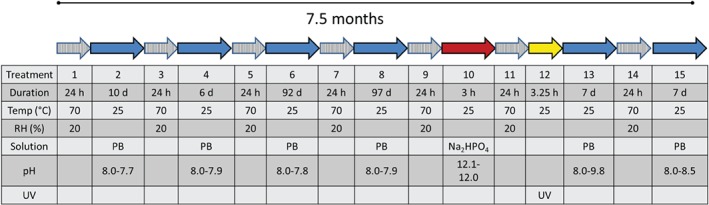
Illustration of the experimental sequence of drying (storage) and immersion, and initial and final pH values of immersions. PB, phosphate buffer; pH, initial–final pH; RH, relative humidity; UV, ultraviolet.
Immersion
Ultrapure water (resistivity of 18.2 MΩ cm; Millipore, Solna, Sweden) was used as the solvent for all solutions, and all equipment was acid‐cleaned (10% HNO3 for at least 24 h) prior to use, and then rinsed four times with ultrapure water. Sequential extraction was conducted in phosphate buffer (PB) of pH 8.0 and in an alkaline solution (denoted Na2HPO4) at pH 12.1, for consecutive time periods as shown in Fig. 1. All chemicals were of analytical grade. The Na2HPO4 solution was composed of 7.85 g/l Na2HPO4 and 1.4 g/l NaOH (pH 12.1, adjusted with approximately 1.2 ml/l 50% NaOH). The PB was composed of 11.8 g/l K2HPO4·3H2O, adjusted to pH 8.0 ± 0.1 with 70 vol% phosphoric acid. Both solutions had been used in 9. The phosphate concentration of 11.8 g/l in the PB is identical to that used in a previous study 9, but lower than the 22.8 g/l specified in the standard test ISO 17075 5 and used in other studies 10, 11, 12. The difference in Cr release from the used leather immersed in 11.8 g/l and 22.8 g/l K2HPO4·3H2O was previously found to be low 9, with <20% difference in released total Cr and Cr(VI). The extraction was performed at room temperature (20–25°C), with bilinear shaking (22 cycles/min, 12° inclination), and in 5 ml of solution (∼50 mg of leather sample in 5 ml of solution), as in 9. After extraction, the solution was centrifuged (704 g) to remove any released leather fibres, and frozen prior to Cr(VI) analysis or acidified (pH < 2) prior to total Cr analysis by atomic absorption spectroscopy (AAS).
Atomic absorption spectroscopy
The total amount of Cr released was determined by the use of AAS (Perkin Elmer AAnalyst 800, Upplands Väsby, Sweden), with calibration standards of concentrations 0, 0.5, 1.5, 5, 10, 15 and 45 mg/l Cr (in 1% HNO3). The limit of determination was estimated to 0.073 mg Cr/l, determined from the highest number of ‘blank concentration (if > 0) + 3 times standard deviation of the blank’ of eight different blank solutions. All sample solution concentrations in this study were significantly higher than the limit of determination. After four samples had been measured, quality controls with known concentrations were measured. If the measured control sample deviated by >10%, recalibration was performed.
Spectrophotometry
The amount of Cr(VI) in the extractant (frozen prior to analysis) was determined by spectrophotometry (Jenway 6300, Staffordshire, UK), utilizing the pink colour of the complex between Cr(VI) and diphenylcarbazide (DPC) 13, with an absorption maximum at 540 nm. As in our previous studies 9, 10, 11, 12 and in accordance with ISO 17075 5, the solutions were mixed in the ratio of 96 vol% sample, 2 vol% phosphoric acid, and 2 vol% DPC solution. The phosphoric acid was 70 vol% in water, and the DPC solution was composed of 1.0 g of 1,5‐diphenylcarbazide in 100 ml of acetone acidified with one drop of glacial acetic acid. It was freshly prepared and colourless (non‐oxidized). The calibration was based on 0, 125, 247.5, 495 and 990 µg Cr(VI)/l in PB. The limit of determination was considered to be 40 µg Cr(VI)/l (highest blank concentration + 3 times highest standard deviation of all blanks). The calibration curves were linear (R 2 = 0.9979 − 0.9986).
DPC spot test
Leather samples were conditioned at 70°C and 20% RH for 24 h in the environmental chamber to investigate whether the RH influences the results of the DPC spot test on dry leather. Samples of unexposed (new) triplicate pieces, one of the exposed (7.5 months) leather pieces and one negative control leather piece [previously found to neither contain nor release any Cr, denoted Vegveg in 10, 11] were investigated. After conditioning, they were taken out of the environmental chamber for DPC spot testing. A similar set of leather pieces was conditioned at 70°C and 35% RH [the upper limit for formation of Cr(VI) 9] for 24 h. These samples were not taken out of the environmental chamber, and spot testing was performed in the chamber. The test was performed by applying a drop (∼100 µl, with a micropipette) of DPC solution directly onto the dry leather, and also by rubbing a white cottonwool stick (cotton swab), which had been entirely moistened (by dipping a part of the stick in the DPC solution), against the leather for 30 seconds, as in 14.
X‐ray fluorescence (XRF) measurements
Three unexposed leather pieces and the three leather pieces that had been exposed for 7.5 months were analysed for their Cr contents at duplicate locations by the use of XRF (handheld XRF analyser, Innov‐X Alpha 4000; Innov‐X Europe, 's‐Hertogenbosch, The Netherlands). The analysis was performed in the mode for thin film analysis for 60 seconds, and the results were reported as µg per 1 cm2 thin film. The relative standard deviation within one measurement was <1% for all measurements.
Presentation of data
The data are presented as mean values with error bars indicating standard deviations of the triplicate samples. The data were normalized either to the surface area (mg/cm2) or to the dry leather mass (mg/kg). The surface area and mass of each piece were measured before the sequence. Statistical significance was evaluated with Student's t‐test for paired data, the word ‘significant’ referring to a p‐value of <0.05.
Results
Cr(VI) and Cr(III) release
Figure 2 shows the amounts of Cr(III) and Cr(VI) released during the sequence, normalized by surface area, and Table S1 gives corresponding values, including statistical significance values. A significant decrease in Cr(III) release was seen during the first four immersions in PB (pH 8.0) for ∼7 months (treatments 2, 4, 6, and 8). After an initial increase, the amount of Cr(VI) released was stable during the same period, meaning that Cr(VI) constituted an increasing proportion (from 0.6% to 34%) of the total Cr released during this period. The immersion for 3 h at pH 12.1 (Na2HPO4 solution) resulted in an increase (30‐fold) in the release of Cr(III) and increase (twofold) in the release of Cr(VI) (Fig. 2, treatment 10), as compared with the previous 97 days of immersion in PB (pH 8.0). After the alkaline treatment (treatment 10), the release of Cr(III) decreased in subsequent immersions in PB (pH 8.0, treatments 13 and 15), despite the UV treatment (treatment 12), whereas the amount of Cr(VI) released remained stable (Fig. 2), resulting in an increase in the percentage of Cr(VI) released from 4.5% (treatment 10) to 51.3% (treatment 15).
Figure 2.

The amount of released Cr(III) and Cr(VI) (mg/cm2) in phosphate buffer (PB, pH 8.0) and Na2HPO4 solution (pH 12.1) in subsequent immersions (according to Fig. 1) at room temperature (25°C) for different time periods. There were only two replicates for treatment 8 [Cr(VI)]; otherwise, there were triplicate samples. Corresponding values in mg/cm2 and mg/kg, and significance values, are given in Table S1.
Comparison with the restriction limit of 3 mg/kg Cr(VI)
Figure 3 shows the amounts of released Cr(VI), normalized per unit mass of dry leather, in comparison with unexposed samples of the same leather type and with identical pretreatment immersed for 3 h in PB and Na2HPO4 solution. Cr(VI) release during all periods, except for the first (treatment 2), was above the restriction limit of 3 mg Cr(VI)/kg (for 3 h of immersion in PB), with maximum average values of 26.4 mg/kg (treatment 15).
Figure 3.

Amount of Cr(VI) released, normalized over dry leather mass (mg/kg), in phosphate buffer (PB, pH 8.0) and Na2HPO4 solution (pH 12.1) in subsequent immersions (according to Fig. 1) at room temperature (25°C) for different time periods. There were only two replicates for treatment 8; otherwise, there were triplicate samples. For comparison, Cr(VI) released from previously unexposed leather samples of the same type and subjected to identical pretreatment and exposure conditions is shown for 3 h of immersion [data from 9]. The red dashed line indicates the Cr(VI) restriction limit of 3 mg/kg. The asterisk represents statistically significant differences between subsequent periods (*p < 0.05; no asterisk, p ≥ 0.05).
Spot testing
The first set of leather pieces (conditioned at 70°C and 20% RH for 24 h) were taken out of the environmental chamber and, after ∼10 min, the spot testing was conducted by applying a drop of DPC onto the leather and rubbing with a cottonwool stick. No signs of pink colour were seen after 2 min and ∼50 min, either directly on the leather, or on the cottonwool sticks. The RH and temperature in the room were therefore measured, and were found to be 46% and 24°C, respectively. After 24 h, a pale pink colour was seen on the unexposed leather pieces, but no colour change was noted on the cottonwool sticks, the exposed sample, or the negative control sample. The second set of leather pieces (conditioned at 70°C and 35% RH for 24 h) were kept in the environmental chamber during the spot testing. The unexposed samples already showed a pale pink colour after 2 min. The cottonwool stick, the exposed sample and the negative control sample did not change colour. Any pink colour on the old (exposed) leather samples may have been masked by their green colour.
XRF analysis
XRF analysis showed 1007 ± 7.2 µg/cm2 Cr for the unexposed samples, and 618 ± 104 µg/cm2 Cr for the exposed samples. The exposed samples had a smaller area than the unexposed samples (approximately 0.5 cm2 versus 1–2 cm2), owing to shrinkage, and the exposed samples could not be laid flat on the analyser, because of shrinkage and stiffness. It is therefore not possible to directly compare these numbers, although it is obvious that the exposed samples still contained significant amounts of Cr.
Discussion
These results suggest that the Cr(VI) released in PB (pH 8.0) was formed during the dry storage period before immersion. This is indicated by the constant amount of Cr(VI) in PB solutions, namely ∼10 mg/kg, independently of immersion duration or previous immersions, and equal to the amount in previously unexposed samples in PB for 3 h 9. The fact that the release of Cr(VI) during the first immersion period of 10 days is lower seems, therefore, to be related either to unintended contact with humid air prior to immersion, or to initially released acids/antioxidants. After the combined alkaline and UV treatment, the release of Cr(VI) was higher, namely ∼25 mg/kg, which is similar to that from similarly treated leather pieces that were not pre‐exposed for 7 months 9. Note that, in all cases, the release of Cr(VI) was dependent on the direct previous treatment(s), but not on the previous long‐term immersions. Previous immersions for 7 months did not have any effect on the Cr(VI) release.
This study confirms our earlier findings of decreasing release of Cr(III) in subsequent immersions 9, 11 for this leather. The amount of Cr(VI) released was independent of the amount of Cr(III) released, and independent of the immersion duration or previous immersions. Earlier studies on previously unexposed leather found that the major proportion of Cr is initially released as Cr(III) 7, 9, 10, 11, 12, 15, 16. A maximum of 15% of the total Cr released was released in the hexavalent form Cr(VI) in PB (pH 8.0, 3 h) 9. In this study, up to 51.3% (last immersion period) of the total Cr released was released as Cr(VI) in PB (pH 8.0). This means that Cr(III) may not necessarily be the major form of Cr released in the case of long‐term exposed/used leather.
The release of Cr(III) and the release of Cr(VI) were slightly higher in this study in the Na2HPO4 solution (pH 12.1) for 3 h after 7 months of pre‐immersion than those from unexposed leather pieces in a similar solution (pH 12.3) for 3 h 9. We have previously speculated that this increase upon alkaline treatment is the result of leather swelling and a negative charge of collagen (above a pH of 8.3) bound to Cr(III) 9. Our findings imply that pre‐exposure of leather does not protect against the release of Cr, at least not when the leather is immersed in alkaline (pH 12) solutions. Exposure of Cr‐tanned leather to alkaline solutions should be avoided, owing to the increased Cr release (even in subsequent immersions or contact). Skin permeation of Cr in vitro is also significantly higher at alkaline pH 17. Examples of leather in contact with alkaline solutions are contact with wet cement 18, alkaline shoe glue 19, alkaline coolants 12, detergents, and other chemicals.
The spot test results in this study suggest that the RH just before or at testing is important for the formation of the pink Cr–DPC complex indicating the presence of Cr(VI). We therefore suggest that spot testing of leather should be performed at a low RH, for example by storing the leather article in a desiccator for at least 24 h prior to testing. This procedure would reduce the risk of false‐negative results.
A few studies 20 have reported that new and high‐quality leather shoes cause less allergic Cr contact dermatitis in Cr‐sensitized persons than old (used) leather shoes. The antioxidants (e.g. dyes), fats and additives in shoes are expected to result in an initial low amount of Cr(VI) release 15, 21, 22. Once the antioxidants have been leached out, Cr(VI) release could increase, as it is a function of the amount of antioxidants in the leather 10, 11, 15, 21, 22. The amount of released Cr(III) is not necessarily affected by antioxidants or additives.
For unfinished leather with a low amount of antioxidants, for example leather for working gloves, which, under certain conditions, releases Cr(VI) even initially 9, 10, 11, 12, 14, the amount of Cr(III) released is expected to decrease with time. The amount of Cr(VI) is, instead, constant for similar prestorage conditions.
Through patch testing, it was previously shown that Cr(III) concentrations of 0.18 µg/cm2 and Cr(VI) concentrations of 0.03 µg/cm2 would elicit eczema in 10% of Cr‐allergic persons [minimum elicitation threshold (MET)10%] 23. The release of Cr(III) in this study exceeded the MET10% value by factors of 50, 14, 5, 2 and 3 during the first four treatments (treatments 2, 4, 6, and 8) and the last treatment (treatment 15), indicating a decrease in the importance of Cr(III) during long‐term exposure. The release of Cr(VI) exceeded the MET10% value by factors of 2, 10, 7, 7 and 19 during the first four treatments (treatments 2, 4, 6, and 8) and the last treatment (treatment 15), which instead indicates an increase in the importance of Cr(VI) during long‐term exposure.
Cr(III) is released in amounts exceeding the MET10% value during several months of this accelerated long‐term release test. This indicates that Cr(III) might be of great importance for contact dermatitis caused by leather. The results also imply that Cr(VI) release is not only an issue for some unused and new leather types; in fact, Cr(VI) release increases in importance in relation to Cr(III) when these leather types are used for long time. Two different heavily used leather working gloves have been investigated previously 9, 12, and they showed similar levels of Cr(VI) and Cr(III) release as found in this study for the initial immersion periods. The results also highlight the importance of dry conditions prior to testing for Cr(VI), both for spot testing and for release testing.
A weakness of this study is its primary applicability for leathers that are not coated or finished, and do not contain large amounts of antioxidants. This is mostly true for Cr‐tanned leather gloves 9, 12, 14. Cr‐tanned leathers containing high amounts of antioxidants may initially only release Cr(III), and not Cr(VI) 10, 11. However, for those leathers it should be investigated whether antioxidants can be leached out with time to such an extent that Cr(VI) could be formed and released.
The strengths of this study are that the leather studied is comparable to that used in typical leather gloves on the market 9, 12, 14, and that we determined long‐term Cr release by using a methodology that considers the major experimental factors affecting Cr(III) and Cr(VI) release from leather.
Conclusions and future perspectives
Although the release of Cr(III) diminished upon repeated immersions over a period of several months in PB (pH 8.0), the release of Cr(VI) was not affected by previous immersions or the duration of immersion. More Cr(VI) than Cr(III) was released after long‐term (months) immersions.
A combination of alkaline (pH 12.1) and UV treatment increased the release of Cr(III) (30‐fold) and Cr(VI) (twofold) after 7 months of pre‐immersion in PB.
The release of Cr(VI) after up to 7.5 months of pre‐immersion in different solutions did not differ significantly from the release of Cr(VI) from the previously unexposed leather. The release of Cr(VI) is mostly dependent on environmental conditions, and is not necessarily an intrinsic property of the leather or its history.
This study strongly suggests that the RH affects not only the release of Cr(VI), but also the spot testing results for Cr‐tanned leather articles.
Further studies should investigate the amount of Cr actually deposited on the skin during contact with Cr‐tanned leather, correlating the amounts of different Cr species on the skin with skin reactions. Used leather should also be investigated further.
Supporting information
Table S1. The amount of released Cr(III) and Cr(VI) (mg/kg and mg/cm2, average and standard deviation of three samples) in phosphate buffer (PB, pH 8.0) and Na2HPO4 solution (pH 12.1) in seven subsequent immersions at room temperature (25°C) for different time periods, as shown in Fig. 2. The experimental conditions are shown in Fig. 1, and include drying periods (70°C, 20% RH, 24 h) in between the immersions. Significance values (p‐value for paired data) are shown for subsequent immersions for the unit mg/cm2 (if significant, in bold).
Acknowledgements
The authors thank Maria‐Elisa Karlsson, KTH, for her experimental help and a language check, and Frederik Mathiason, KTH, for experimental help.
Conflicts of interests: The authors declare that they have no conflicts of interest.
Funding: Swedish Research Council for Health, Working Life, and Welfare (FORTE, postdoc‐grant 2013‐0054), Swedish Asthma and Allergy Association's Research foundation (grant no. 2013014), KI research foundations (grant no. 2013fobi37277), Hudfonden (grant no. 2291), and KTH faculty grants.
References
- 1. Thyssen J P, Menné T. Metal allergy – a review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol 2010: 23: 309–318. [DOI] [PubMed] [Google Scholar]
- 2. Bregnbak D, Johansen J D, Jellesen M S et al. Chromium allergy and dermatitis: prevalence and main findings. Contact Dermatitis 2015: 73: 261–280. [DOI] [PubMed] [Google Scholar]
- 3. Carøe C, Andersen K E, Thyssen J P, Mortz C G. Fluctuations in the prevalence of chromate allergy in Denmark and exposure to chrome‐tanned leather. Contact Dermatitis 2010: 63: 340–346. [DOI] [PubMed] [Google Scholar]
- 4. Thyssen J P, Jensen P, Carlsen B C et al. The prevalence of chromium allergy in Denmark is currently increasing as a result of leather exposure. Br J Dermatol 2009: 161: 1288–1293. [DOI] [PubMed] [Google Scholar]
- 5. ISO . Leather Chemical tests – Determination of Chromium(VI) Content. ISO: 17075, 2007. Geneva, International Organization for Standardization.
- 6. German Federal Institute for Risk Assessment . Chromium (VI) in leather clothing and shoes problematic for allergy sufferers! 2007. Available at: http://www.bfr.bund.de/en/press_information/2007/10/chromium__vi__in_leather_clothing_and_shoes_problematic_for_allergy_sufferers_‐9575.html, (last accessed 25 January 2016).
- 7. Hansen M B, Rydin S, Menné T, Johansen J D. Quantitative aspects of contact allergy to chromium and exposure to chrome‐tanned leather. Contact Dermatitis 2002: 47: 127–134. [DOI] [PubMed] [Google Scholar]
- 8. Thyssen J P, Strandesen M, Poulsen P B et al. Chromium in leather footwear – risk assessment of chromium allergy and dermatitis. Contact Dermatitis 2012: 66: 279–285. [DOI] [PubMed] [Google Scholar]
- 9. Mathiason F, Lidén C, Hedberg Y. Chromium released from leather – II: the importance of environmental parameters. Contact Dermatitis 2015: 72: 275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hedberg Y, Lidén C, Odnevall Wallinder I. Correlation between bulk‐ and surface chemistry of Cr‐tanned leather and the release of Cr(III) and Cr(VI). J Hazard Mater 2014: 280: 654–661. [DOI] [PubMed] [Google Scholar]
- 11. Hedberg Y, Lidén C, Odnevall Wallinder I. Chromium released from leather – I: exposure conditions that govern the release of chromium(III) and chromium(VI). Contact Dermatitis 2015: 72: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hedberg Y S, Lidén C, Lindberg M. Chromium dermatitis in a metal worker due to leather gloves and alkaline coolant. Acta Derm Venereol 2016: 96: 104–106. [DOI] [PubMed] [Google Scholar]
- 13. Pflaum R T, Howick L C. The chromium–diphenylcarbazide reaction. J Am Chem Soc 1956: 78: 4862–4866. [Google Scholar]
- 14. Bregnbak D, Johansen J D, Jellesen M S et al. Chromium (VI) release from leather and metals can be detected with a diphenylcarbazide spot test. Contact Dermatitis 2015: 73: 281–288. [DOI] [PubMed] [Google Scholar]
- 15. Meyndt R, Germann H. Relationships in the formation of hexavalent chrome [Cr(VI)]. World Leather, June/July 2011, pp. 14–17.
- 16. Nygren O E, Wahlberg J. Speciation of chromium in tanned leather gloves and relapse of chromium allergy from tanned leather samples. Analyst 1998: 123: 935–937. [DOI] [PubMed] [Google Scholar]
- 17. Gammelgaard B, Fullerton A, Avnstorp C, Menné T. Permeation of chromium salts through human skin in vitro. Contact Dermatitis 1992: 27: 302–310. [DOI] [PubMed] [Google Scholar]
- 18. Hedberg Y S, Gumulka M, Lind M‐L et al. Severe occupational chromium allergy despite cement legislation. Contact Dermatitis 2014: 70: 321–323. [DOI] [PubMed] [Google Scholar]
- 19. Hauber C. Sources, detection and avoidance of hexavalent chromium in leather and leather products, UNIDO Report. US/RAS/92/120: Vienna, 1999.
- 20. Freeman S. Shoe dermatitis. Contact Dermatitis 1997: 36: 247–251. [DOI] [PubMed] [Google Scholar]
- 21. Graf D. Formation of Cr(VI) traces in chrome tanned leathers: causes, prevention, and latest findings. J Am Leather Chem Assoc 2001: 96: 169–179. [Google Scholar]
- 22. Gong Y, Liu X, Huang L, Chen W. Stabilization of chromium: an alternative to make safe leathers. J Hazard Mater 2010: 179: 540–544. [DOI] [PubMed] [Google Scholar]
- 23. Hansen M B, Johansen J D, Menné T. Chromium allergy: significance of both Cr(III) and Cr(VI). Contact Dermatitis 2003: 49: 206–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The amount of released Cr(III) and Cr(VI) (mg/kg and mg/cm2, average and standard deviation of three samples) in phosphate buffer (PB, pH 8.0) and Na2HPO4 solution (pH 12.1) in seven subsequent immersions at room temperature (25°C) for different time periods, as shown in Fig. 2. The experimental conditions are shown in Fig. 1, and include drying periods (70°C, 20% RH, 24 h) in between the immersions. Significance values (p‐value for paired data) are shown for subsequent immersions for the unit mg/cm2 (if significant, in bold).


