Abstract
Background
Treatment monitoring is subjective and disease relapse is common in cats with histoplasmosis. The Histoplasma antigen enzyme immunoassay (EIA) is a noninvasive test used for determining disease remission and detecting disease relapse in humans with histoplasmosis. The utility of the antigen EIA for these purposes in cats remains unknown.
Hypothesis/Objectives
Those Histoplasma antigen concentrations in urine and serum would decline with antifungal treatment and that antigen elimination would be an indicator of clinical remission in cats with histoplasmosis treated with antifungal treatment.
Animals
Fifteen client‐owned cats with histoplasmosis.
Methods
Masked observational study. Cats were monitored monthly during antifungal treatment. Time of clinical remission and serum and urine antigen elimination were determined for each cat.
Results
Twelve of 15 cats achieved clinical remission. At the time of diagnosis, antigen was detectable in urine in 14/15 (93%) cats and in serum in 11/15 (73%) cats. Both serum (P < .0005) and urine (P < .0001) antigen concentrations significantly decreased over time with effective treatment. Antigen elimination was sensitive [urine, 90.0% (95% CI 72.3–97.4%); serum, 90.4% (68.2–98.3%)] but less specific [urine, 64.6% (51.7–75.8%); serum, 52.1% (37.4–66.5%)] for disease remission. Urine antigen was positive in both cats and serum antigen was positive in 1 cat at the time of disease relapse.
Conclusions and Clinical Importance
Measurement of Histoplasma antigen in urine and serum might be useful tests for determining disease remission and relapse in cats with histoplasmosis. Further research is needed to investigate the importance of low‐level antigenemia and antigenuria.
Keywords: Feline, Fungal, Histoplasma, Mycosis
Abbreviations
- EIA
enzyme immunoassay
- IDSA
Infectious Disease Society of America
- FeLV
feline leukemia virus
- FIV
feline immunodeficiency virus
Histoplasmosis, caused by the thermally dimorphic soil‐born fungus, Histoplasma capsulatum is found in temperate and subtropical regions throughout the world. Diagnosis is most often made through identification of H. capsulatum yeasts in fluid or tissue samples.1, 2 Fungal culture of infected tissue or fluid samples has also been used, although culture is rarely performed in veterinary medicine due to long turn‐around time (2–4 weeks), relatively low sensitivity, and risk to laboratory personnel.3, 4, 5, 6, 7 In humans, histoplasmosis is commonly diagnosed via a Histoplasma antigen enzyme immunoassay (EIA).8 The Histoplasma antigen EIA is noninvasive and common test samples include serum, urine, and bronchoalveolar lavage fluid. The Histoplasma antigen EIA has recently been shown to be a sensitive test for the diagnosis of disseminated histoplasmosis in cats.9
Itraconazole, fluconazole, and amphotericin B are the most commonly used antifungal agents for the treatment of histoplasmosis.1, 2, 10, 11 In cats, antifungal treatment is costly for the pet‐owner and can require extended treatment duration.1, 2, 11 The veterinarian often faces challenges in determining the optimum antifungal treatment endpoint to reduce relapse occurrence while mitigating overall cost of treatment.
Commonly, a group of tests is used to determine the time of clinical remission. These include some combination of serial reassessment of clinical signs, physical examination, imaging studies, and cytologic examination of fluid or tissue samples.1, 7 All of these tests have inherent limitations. For example, due to permanent tissue changes (scarring), persistent imaging abnormalities can be present that are difficult to differentiate from active disease. Repeated imaging can be stressful to the cat and costly to the pet‐owner. During antifungal treatment, the morphology of H. capsulatum organisms can be drastically altered, most notably the nuclear material fades and becomes less distinct.12 This can complicate cytologic identification of yeasts.12 In addition, the viability and thus the clinical significance of these organisms are unknown. Finally, depending on the organ sampled, repeated fluid or tissue sampling can be invasive and stressful to the cat.
A reliable, noninvasive test for disease remission and relapse is desirable. In humans, the Histoplasma antigen EIA is used to aid in determining clinical remission status and detecting disease relapse.13, 14, 15 The Infectious Disease Society of America (IDSA) recommends monitoring antigen levels during antifungal treatment before, during, and for at least 12 months after discontinuation of treatment.15 There remains a paucity of information concerning how antifungal treatment affects antigen concentrations in cats during antifungal treatment for histoplasmosis, and if antigen concentrations can be used as a test for disease remission or relapse. The primary tested hypothesis in this study was that urine and serum Histoplasma antigen concentrations would decrease with antifungal treatment and that antigen elimination would be an indicator of clinical remission. A secondary tested hypothesis was that urine and serum antigen concentrations would increase and thus be an indicator of disease relapse.
Materials and Methods
Cats
Cats were recruited from September 2012 through December 2013 from the patient population of the Veterinary Medical Teaching Hospital at Oklahoma State University. Inclusion criteria included clinical signs consistent with histoplasmosis (dyspnea, tachypnea, weight loss, anorexia, vomiting, diarrhea, or lameness); H. capsulatum organisms and pyogranulomatous inflammation in fluid or tissue samples; and a positive Histoplasma urine or serum antigen EIA.1 Exclusion criteria included previous antifungal treatment; comorbidity that was expected to be life‐limiting; or a cat with a fractious nature in which repeated restraint or handling would endanger medical personnel or the cat. The study was approved by the Institutional Animal Care and Use Committee of Oklahoma State University (Animal Care and Use Protocol VM‐12‐12).
Treatment
Cats were treated PO with fluconazole or itraconazole alone or after treatment with amphotericin B. In general itraconazole was prescribed at approximately 10 mg/kg/d and fluconazole was prescribed at approximately 15 mg/kg/d. Liposomal encapsulated amphotericin B was administered at 1–1.5 mg/kg IV q48h to a maximum cumulative dose of up to 12 mg/kg. Cats unable to tolerate itraconazole due to gastrointestinal adverse effects or hepatotoxicosis were switched to fluconazole and vice versa. In addition, the antifungal was switched if deemed ineffective due to a lack of clinical improvement after 1–2 months of treatment. This determination was subjective based on the entire clinical picture. Blood azole levels were not measured in cats that failed to respond clinically. Cats were treated with antifungal treatment at least 1 month after clinical remission. Cats with pulmonary, gastrointestinal, or bone or joint involvement also received prednisolone. In general, prednisolone was prescribed at 0.5–1.0 mg/kg/d for ≤3 weeks in total duration.
Treatment monitoring
Clinical remission was defined as the first monthly hospital visit at which there was resolution of clinical signs, resolution of imaging abnormalities, and the absence of H. capsulatum organisms on cytology. Resolution of imaging abnormalities was defined as a return to normalcy or minimal abnormality that was static on 2 consecutive visits. This consideration was to account for permanent tissue change or scarring most notably of the lungs or bones/joints.
Recheck examinations were scheduled monthly during antifungal treatment and at least once, 6 months after discontinuation of antifungal treatment when possible. Physical examination and historical information were recorded at each hospital visit. Historical information recorded included ongoing clinical signs, compliance with medication administration, and possible drug adverse effects.
Blood and urine was collected for antigen testing at each visit. Urine was collected via prepubic cystocentesis with a 22 gauge, 1‐inch needle attached to a 6 mL syringe. Urine was immediately refrigerated and frozen at −80°C within 4 hours of collection. Blood was collected via peripheral venipuncture with a 1 inch, 22 gauge needle attached to a 6 mL syringe or a 1 inch, 21 gauge butterfly catheter attached to a 3 mL syringe. Serum biochemistry analysis and complete blood count was performed at the initial visit.2 If a cat had not been tested for feline leukemia virus (FeLV) or feline immunodeficiency virus (FIV) in the past month, a point‐of‐care ELISA3 for FeLV/FIV was performed at the initial visit. For antigen testing, whole blood was allowed to sit at room temperature for approximately 20 minutes followed by centrifugation for 10 minutes. Serum was immediately separated and refrigerated. Serum was frozen at −80°C within 4 hours of collection.
Thoracic radiographs were taken at the initial visit. In cats with radiographic abnormalities consistent with histoplasmosis, thoracic radiographs were repeated at each visit. In cats without thoracic radiographic changes consistent with histoplasmosis, alternative imaging such as bone/joint radiographs or abdominal ultrasound were utilized to monitor treatment response. All radiographic images were reviewed by 1 investigator (AH). Consultation with the veterinary radiologist on clinical duty was sought when images were considered equivocal. All written radiograph reports were reviewed for consistency with initial radiographic impressions. At each visit, ultrasonographic images were reviewed by 1 investigator (AH) who routinely performs diagnostic abdominal ultrasonography. Cytologic examination of samples collected via fine needle aspirate, rectal scrape, or impression smear was used to monitor treatment when possible. All cytology samples were submitted to a commercial veterinary diagnostic laboratory3 and reviewed by a board certified veterinary clinical pathologist.
Histoplasma Antigen Enzyme Immunoassay
All antigen testing was performed in 1 batch at a commercial laboratory1 using a previously published method.6, 16 Before testing, all samples were stored at −80°C for ≤2 years before being shipped on ice overnight to the laboratory. Excluding the initial urine or serum antigen concentrations, all investigators and other medical personnel were masked to the antigen test results until study completion. The Histoplasma antigen EIA has a lower and upper limit of quantification of 0.40 and 19.0 ng/mL, respectively. Positive antigen EIA results below 0.4 ng/mL (but above the cutoff optical density) are reported as “positive, below the limit of quantification (BLQ)”. Results above the limit of quantification are reported as “above the limit of quantification (ALQ)”. For study purposes, BLQ and ALQ results were considered to be 0.4 or 19.0 ng/mL, respectively. Antigen elimination was defined as no detectable Histoplasma antigen.
Statistical Analysis
Statistical analysis was performed with commercial software.4 Descriptive statistics were reported as median (range). Generalized linear mixed models were used to detect significant changes in the urine and serum antigen concentrations over time. Dunnett's test was used to compare urine and serum antigen concentrations at each recheck visit to that of baseline. Generalized linear mixed models were used to compare time to urine antigen elimination and serum antigen elimination to time to clinical remission. For cats that achieved clinical remission and had a positive antigen concentration at baseline, antigen results at each visit were classified as a true positive (TP), false positive (FP), true negative (TN), or false negative (FN). Clinical remission as defined above was used as the ‘gold standard’. For example, if antigenuria was not detected, but the cat had evidence of active disease, this was classified as a false‐negative antigen test result. Sensitivity and specificity with 95% confidence intervals were determined for antigen elimination as an indicator of clinical remission. Sensitivity was defined as TP/(TP + FN) and specificity was defined as TN/(FP + TN). Correlation between initial serum and initial urine antigen concentrations and time to clinical remission were determined by a Spearman correlation coefficient. Correlation between initial serum antigen concentration and time to serum antigen elimination and initial urine antigen concentration and time to urine antigen elimination was determined by a Spearman correlation coefficient. Significance was set at P ≤ .05.
Results
Signalment and Clinical Signs
Twenty cats were diagnosed with histoplasmosis during the study enrollment period. Five cats were excluded from the study due to not having a pet‐owner (n = 2), pet‐owner declined study participation (1), fractious nature (1), or not finding fungal organisms (1). Fifteen cats were enrolled in the study. There were 10 spayed females and 5 castrated males. Breeds included 11 domestic shorthairs and 1 each of domestic longhair, Birman, Himalayan, and Oriental shorthair. The median age was 5 years (range 2–17 years) and the median body weight was 3.4 kg (2.5–7.8 kg). Seven cats were housed exclusively indoors and 8 cats were housed indoors but also allowed outside of the house unsupervised. Clinical signs reported at the time of diagnosis included; lethargy/decreased activity (n = 11), anorexia/decreased appetite (9), weight loss (7), tachypnea/dyspnea (5), lameness (3), nasal discharge (2), vomiting (2), and 1 cat each with diarrhea, coughing, ocular discharge, and polyphagia. The median duration of clinical signs at the time of diagnosis was 8.6 weeks (1–39 weeks).
Laboratory Data
All cats were negative for circulating FeLV antigen and FIV antibody titer. Serum biochemistry abnormalities were uncommon with the most common being hypocholesterolemia (n = 2) and hypomagnesemia (2) (Table 1). One cat was mildly hypercalemic (11.0 mg/dL; reference interval 8.2–10.8 mg/dL). The most common changes on CBC included a leukocytosis (n = 4), increased segmented neutrophils (5), increased band neutrophils (3), mild to moderate anemia as assessed by hematocrit (4), lymphopenia (3), and neutropenia (2) (Table 1).
Table 1.
Biochemistry and complete blood count results from 15 cats with histoplasmosis at the time of diagnosis. Only variables for which, at least 2 abnormal values were obtained are presented
| Variable | Unit | Normal Reference Interval | Median (Range) | Above Reference Interval, Number (%) | Below Reference Interval, Number (%) |
|---|---|---|---|---|---|
| Biochemistry | |||||
| Magnesium | mEq/L | 1.5–2.5 | 1.7 (1.3–1.9) | 0/13 (0) | 2/13 (15) |
| Cholesterol | mg/dL | 75–220 | 129 (63–186) | 0/13 (0) | 2/13 (15) |
| Triglyceride | mg/dL | 25–160 | 61 (22–184) | 1/13 (8) | 1/13 (8) |
| Complete Blood Count | |||||
| White blood cell count | 103/μL | 3.5–16.0 | 9.7 (2.4–28.1) | 4/15 (27) | 2/15 (13) |
| Hematocrit | % | 29–48 | 31 (20–40) | 0/15 (0) | 4/15 (27) |
| Neutrophils | /μL | 2,500–8,500 | 5,925 (1,368–25,290) | 5/15 (33) | 2/15 (13) |
| Bands | /μL | 0–150 | 0 (0–546) | 3/15 (20) | 0/15 (0) |
| Lymphocytes | /μL | 1,200–8,000 | 2,528 (693–4,512) | 0/15 (0) | 3/15 (20) |
Diagnosis
Organisms were found via cytology in 14 cats via fine needle aspirate (n = 13) and rectal scrape (1). Sites of organisms found via cytology include: synovial fluid (n = 1), synovial fluid and cutaneous lesion (2), liver and spleen (2), spleen (2) peripheral lymph node (2) and 1 cat each in bone marrow, lung, kidney, subcutaneous mass, and rectal mucosa. Organisms were found in 1 cat on histopathology of the duodenal mucosa obtained via gastrointestinal endoscopy.
Treatment Monitoring
Ten cats had abnormalities on thoracic radiographs consistent with histoplasmosis including a diffuse structured interstitial pattern (n = 3), a diffuse structured interstitial and bronchial pattern (2), single or multiple pulmonary masses (2), and 1 cat each with a diffuse unstructured interstitial pattern and a diffuse bronchial pattern. One cat with normal pulmonary parenchyma had sternal lymphadenopathy. Another cat with a pulmonary mass also had sternal lymphadenopathy. Repeat thoracic radiographs were used for treatment monitoring in the 10 cats with pulmonary histoplasmosis, sternal lymphadenopathy, or both pulmonary histoplasmosis and sternal lymphadenopathy. In addition to thoracic radiographs, repeated needle aspiration and cytology of a peripheral lymph node (n = 1), impression smear and cytology of a cutaneous lesion (1), humero‐radioulnar radiographs (1), and abdominal ultrasound (1) were also used for treatment monitoring. Modalities used for treatment monitoring in cats with normal thoracic radiographs included abdominal ultrasound and rectal scrape and cytology (n = 1), abdominal ultrasound and needle aspirate and cytology of the kidney (1), abdominal ultrasound alone (1), and carpal and tarsal radiographs (1). One cat had no imaging abnormalities. In this cat repeat needle aspirate and cytology of a palpable subcutaneous mass was used for treatment monitoring.
Treatment
Three cats received liposomal encapsulated amphotericin B at 1.0–1.5 mg/kg IV q48h to a cumulative dose of 4, 6, and 9 mg/kg, respectively. Seven cats were started on itraconazole at a median dose of 10.0 mg/kg/d (8.6–11.1 mg/kg/d) and 8 cats were started on fluconazole at a median dose of 13.9 mg/kg/d (8.5–16.7 mg/kg/d). Two cats were switched from itraconazole to fluconazole due to hepatotoxicosis as evidenced by increased serum activity of liver enzymes (alanine transaminase > alkaline phosphatase) after 6 and 15 weeks of treatment, respectively. Two cats were switched from fluconazole to itraconazole due to apparent lack of clinical improvement after 4 and 8 weeks of treatment, respectively. Thirteen cats received prednisolone at a median starting dose of 0.5 mg/kg/d PO (0.19–1.39 mg/kg/d) for a median duration of 20 days (7–120 days). One cat received a longer course of prednisolone (120 days) at a higher starting dose (1.39 mg/kg/d) for pemphigus foliaceus which was diagnosed years prior to histoplasmosis. Prednisolone dose tapering was attempted after the diagnosis of histoplasmosis was made but relapse of skin lesions necessitated a very slow taper.
Clinical Remission and Disease Relapse
Two cats were euthanized at 6 and 8 weeks after diagnosis due to the lack of clinical improvement. A third cat was euthanized 17 weeks after diagnosis of multicentric lymphoma. This cat showed improvement of clinical signs of histoplasmosis until soon before the diagnosis of lymphoma. A fourth cat was euthanized 3 months after achieving clinical remission. The reason for euthanasia is unknown in this cat. Necropsy was not performed on any cat. Twelve cats achieved clinical remission with a median treatment duration of 23.9 weeks (16.6–73.3 weeks). The median duration of antifungal treatment was 33.3 weeks (18.8–101.4 weeks). Two cats had suspected relapse based on return of tachypnea/dyspnea and thoracic radiographs showing a structured interstitial lung pattern. Relapse occurred at 29 and 48 weeks after discontinuation of antifungal treatment, respectively. Initial antifungal treatment duration in both cats was approximately 19 weeks.
Antigen Concentrations
A total of 218 urine and serum samples were tested for H. capsulatum antigen. At the time of diagnosis urine antigen was positive in 14/15 cats with a median (range) concentration of 8.71 ng/mL (0.4–19.0 ng/mL). Serum antigen was positive in 11/15 cats with a median concentration of 1.37 ng/mL (0.4–7.0 ng/mL). In cats that were positive at baseline, at the time of clinical remission the urine and serum antigen was negative in 9/11 and 6/8, respectively. Urine antigen concentrations (0.51 and 0.40 ng/mL, respectively) and serum antigen concentrations (0.60 and 0.40 ng/mL, respectively) were still positive in 2 cats each at the time of clinical remission, although had reduced to near the lower limit of detection for the assay (Fig 1). Three cats that had a positive urine or serum antigen at the time of clinical remission were negative at the 6 month recheck. The fourth cat did not have a 6 month recheck. None of these cats had detectable disease relapse.
Figure 1.
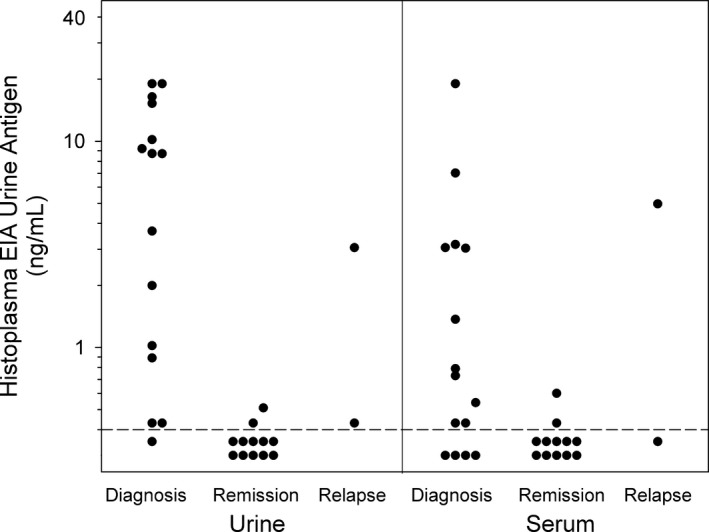
Urine and serum Histoplasma antigen concentrations in 15 cats with histoplasmosis treated with antifungal treatment. The dashed line is the lowest quantifiable concentration. A dot above the dashed line indicates a positive test and a dot below the dotted line indicates a negative test (Reference interval = none detected).
Urine antigen elimination occurred at a median of 13.6 weeks (3.9–31.6 weeks). Serum antigen elimination occurred at a median of 12.7 weeks (4.7–28.4 weeks). The time to urine antigen elimination (13.6 weeks) was significantly shorter than the time to clinical remission (23.9 weeks; P = .01). The time to serum antigen elimination (12.7 weeks) was significantly shorter than the time to clinical remission (23.9 weeks; P = .007). The time to urine antigen elimination (13.6 weeks) was not significantly different from the time to serum antigen elimination (12.7 weeks; P = .68). Both the urine (P < .0001) and serum (P < .0005) antigen concentrations significantly decreased over time. The urine antigen concentrations were significantly decreased compared with baseline from 2 to 6 months after diagnosis (Fig 2). The serum antigen concentrations were significantly decreased compared with baseline at 4–5 months after diagnosis (Fig 2).
Figure 2.
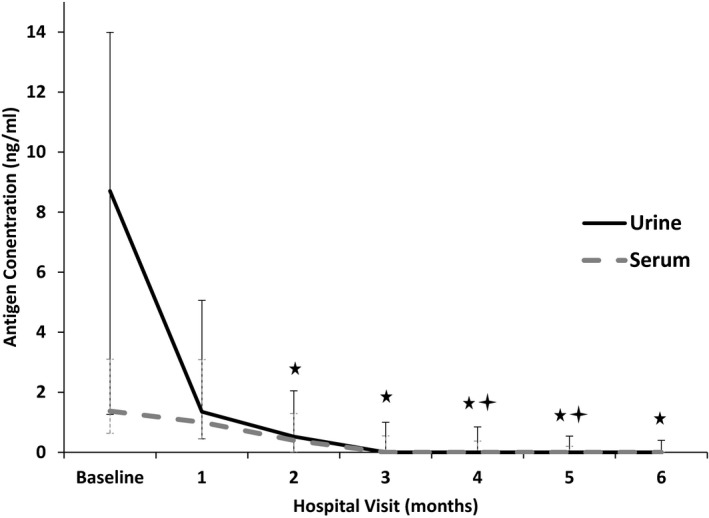
Median (IQR) urine and serum Histoplasma antigen concentrations in 15 cats with histoplasmosis treated with antifungal treatment (Reference interval = none detected).  Median urine antigen concentration significantly decreased from baseline (P < .05).
Median urine antigen concentration significantly decreased from baseline (P < .05).  Median serum antigen concentration significantly decreased from baseline (P < .05).
Median serum antigen concentration significantly decreased from baseline (P < .05).
There was a significant and strong positive linear correlation between initial urine antigen concentration and the time to urine antigen elimination (R = 0.95; P = .0004). There was a significant and strong positive linear correlation between initial serum antigen concentration and the time to serum antigen elimination (R = 0.99; P < .0001). There was a significant positive linear correlation between urine antigen concentration at baseline and time to clinical remission (R = 0.61; P = .04). There was not a significant correlation between serum antigen concentrations at baseline and time to clinical remission (R = 0.24; P = .57).
As a test for disease remission urine antigen elimination had a sensitivity of 90.0% (95% CI 72.3–97.4%) and specificity of 64.6% (51.7–75.8%) and serum antigen elimination had a sensitivity of 90.4% (68.2–98.3%) and specificity of 52.1% (37.4–66.5%).
Excluding disease relapse, antigen concentrations increased on subsequent visits on 3 occasions. Increases were seen in urine (n = 1), serum (1), and both serum and urine (1). All 3 occasions occurred in different cats and all 3 cats were euthanized during the study period. This included 1 cat that was euthanized after clinical remission for unknown reasons. Fourth cat was euthanized 4 weeks after diagnosis. This cat did not have repeat antigen testing to detect a possible increase in antigen concentrations. Urine or serum antigen concentrations never increased in 11 cats that were alive at the end of the study period.
In the 2 cats with disease relapse, urine antigen was positive (3.05 and 0.40 ng/mL, respectively) in both cats and serum antigen was positive (4.97 ng/mL) in 1 cat and negative in 1 cat at the time of relapse (Fig 1). Both of these cats were negative for urine and serum antigen at the time of clinical remission.
Discussion
This study demonstrates the effects of antifungal treatment on antigen concentrations in cats with histoplasmosis. Histoplasma antigen concentrations significantly decreased during antifungal treatment, were negative or low at the time of clinical remission, and increased in 2 cats, at the time of disease relapse. Antigen elimination was sensitive but not specific for clinical remission. Collectively, these findings suggest that the Histoplasma antigen EIA might be a useful clinical test to aid in the determination of clinical remission and detection of disease relapse in cats with histoplasmosis treated with antifungal treatment.
The finding that antigen concentrations decreased with effective antifungal treatment was expected. A decrease in antigen concentrations during effective treatment has been documented in humans and experimentally induced histoplasmosis in rodents.13, 17 In this study urine antigen concentrations were significantly decreased as compared with baseline by 2 months after starting antifungal treatment. A decrease in serum antigen concentrations did not reach statistical significance until 4 months after starting antifungal treatment. This was likely related to the fact that at diagnosis more cats had a positive urine antigen test and that the average urine antigen concentration was higher. This finding is in contrast to a human study that showed a more rapid decrease in serum antigen concentration as compared with urine antigen concentration when treated with antifungal treatment.13 As such, that study concluded that serum antigen concentrations are a better indicator of early treatment response. The fact that, in this study, a significant difference was not found at 6 and 7 months of treatment in serum antigen concentrations as compared to baseline is most likely due to the low number of serum antigen tests performed at these time points.
Of the 218 samples tested in this study, antigen concentration increased during antifungal treatment in only 4 samples. The increases were seen in urine (n = 1), serum (1), and both urine and serum (1). All 3 cats were ultimately euthanized during the study period. In addition, 2 of the 3 cats required a change of azole antifungal. None of the 11 cats without an increase in antigen concentration were euthanized during the study period. This suggests that further investigation of the clinical importance of an increase in antigen concentration during antifungal treatment is warranted. Based on the apparent low occurrence of increasing antigen concentrations during antifungal treatment, investigating this will require a much larger study population.
Antigen elimination commonly preceded clinical remission which made it a relatively sensitive but not specific test for remission. At the time of clinical remission urine and serum antigen concentrations were most commonly negative or less commonly at low concentrations. Considering the relatively high sensitivity as a test for remission (90%), a positive antigen test is reason to consider continuing antifungal treatment. The low specificity for clinical remission indicates that, even with antigen elimination, other clinical indicators of remission must also be considered when determining when to discontinue antifungal treatment. As such, antigen testing augments but does not replace the traditional tests of disease remission in cats with histoplasmosis. The value of monitoring antigen concentrations might be most evident when there are discordant results of tests for clinical remission.
The significance of low antigen concentrations at the time of clinical remission remains unknown. The challenge of interpreting low‐level antigen concentrations is not unique to cats. A recent report in humans recommends cautious interpretation of a urine antigen concentration of <0.6 ng/mL in the absence of clinical signs.18 None of the 4 cats with low antigen concentrations at the time of remission had evidence of disease relapse at study completion. In addition, antigen tests were negative in the 3 cats that were tested 6 months after discontinuation of treatment. It should be noted that these cats were treated for at least 1 month after the time of clinical remission. Collectively, this indicates that discontinuing antifungal treatment with low‐level antigen concentrations might be acceptable when antifungal treatment is continued at least 1 month after clinical remission and all other tests are supportive of clinical remission. The IDSA states that humans who have received appropriate treatment might do well even when antifungal treatment is discontinued in the presence of low‐level antigenuria.15 This recommendation is supported by an observational study including 32 humans who had low‐level antigenuria at the time of treatment discontinuation.19 In that study no occurrence of disease relapse was detected in any patient with a median follow‐up duration of 24 months.19 It should be noted that in the aforementioned study, minimum antifungal treatment duration was 12 months. That is longer than the median treatment duration of approximately 8 months in this study. Until low antigen concentrations can be investigated in a larger number of cats it seems prudent to continue antifungal treatment until antigen elimination and at least 1 month after other indicators of clinical remission.
There was a significant positive correlation between the initial serum and urine antigen concentrations and the time to antigen elimination and initial urine antigen concentrations and time to clinical remission. This suggests that a higher urine antigen concentration at the time of diagnosis could be indicative of more severe disease. In a recent study of humans receiving TNF‐α blocker treatment with histoplasmosis, urine Histoplasma antigen concentrations at the time of diagnosis were found to be an independent predictor of disease severity (OR 1.14 [1.03–1.25]).3 In that study antigen concentrations were presumed to be related to fungal burden. Until the relationship between Histoplasma antigen concentration and disease severity in cats can be further studied, other clinical indicators of disease severity should be utilized.
In this study, at the time of diagnosis, the urine antigen EIA was positive in more cats as compared with the serum antigen EIA (14/15 versus 11/15). The sensitivity of the Histoplasma urine antigen EIA in this study is consistent with what has been previously reported in cats.9 In addition, urine antigen concentrations were significantly positively correlated with time to clinical remission whereas serum antigen concentrations were not. Finally, the urine antigen EIA was positive in both cats with disease relapse whereas serum antigen EIA was positive in 1/2. These findings collectively suggest that urine is the superior sample for antigen testing. This should not be interpreted as an indication for antigen testing of a single sample in every situation. The importance of testing both urine and serum was demonstrated in 1 cat that had positive serum antigen but had negative urine antigen. To maximize sensitivity for diagnosis, serum should be tested if urine is negative and histoplasmosis is still considered likely. The advantage of testing both urine and serum for antigen has been demonstrated in humans with acute pulmonary histoplasmosis, a form of disease that presents a diagnostic challenge.20 In that study the sensitivity of 64.6% with urine alone and 68.6% with serum alone was increased to 82.8% when both samples were tested.
Antigen concentrations increase in 90% of humans with disease relapse.14 Relapse in the 2 cats in this study was supported by the return of clinical signs and thoracic radiographic abnormalities. Thus, true disease relapse was considered likely. Histoplasma antigen was detectable in the urine of both cats at the time of disease relapse. Both were antigen negative at the time of clinical remission. This suggests that the Histoplasma antigen EIA could be a useful test for detecting disease relapse. This also indicates that disease relapse can occur even when continuing antifungal treatment until antigen elimination. Using the antigen EIA for detection of disease relapse could avoid other more invasive, risky, or expensive diagnostic procedures. In addition it could lead to earlier detection, which is intuitively desirable. Early detection of disease relapse is a monitoring goal in humans; therefore the IDSA recommends monitoring antigen concentrations for at least 12 months after discontinuation of antifungal treatment.15 These recommendations include measuring antigen levels before treatment is started, at 2 weeks, at 1 month, and then every 3 months during treatment.15 It should be noted that while monitoring antifungal treatment after disease relapse, monitoring antigen concentrations over time will have the same limitations as discussed above. Most notably, with a low specificity as a test for disease remission, antigen testing does not replace but rather augments other traditionally used tests.
This study has some important limitations. The first limitation is the absence of a single ideal test for the determination of clinical remission in cats with histoplasmosis. As is traditionally done in clinical practice, in this study, a combination of tests were used to determine clinical remission. This included consideration of clinical signs and physical examination in addition to serial imaging and tissue sampling. All of these tests have inherent limitations and are at times subjective. For example, a cat could have been determined to be in remission when active disease was still present, as H. capsulatum organisms could have been missed on cytologic examination, very small imaging changes could have been interpreted as static or normal, and mild clinical signs or subtle physical examination abnormalities could not have been reported by the pet‐owner or detected by the veterinarian, respectively. Alternatively interpreting intracellular vacuoles as nonviable Histoplasma organisms or permanent tissue change (scarring) as active disease could have led to cats in remission being classified as having active disease. To minimize variability in image interpretation all images were reviewed at the time of visit by 1 clinician. When the results were equivocal a second opinion was sought. In each case, consideration was given to prior imaging studies. All cytologic examinations were reviewed by a boarded veterinary clinical pathologist in a laboratory, where histoplasmosis is a relatively common diagnosis. As such, all pathologists had experience with locating and identifying H. capsulatum organisms. In addition, all clinical pathologists were aware of the diagnosis of histoplasmosis at the time of recheck cytologic examinations. Perhaps most importantly, all medical personnel were masked to knowledge of the antigen concentrations in cats. Thus, the time to clinical remission was determined without any prior knowledge of the time to antigen elimination.
Requiring all of the clinical tests to be supportive of remission could have been overly conservative. It is possible that antigen elimination is superior to the combination of tests traditionally used to determine clinical remission. If so, the combination of tests used in this study could have led to excessive treatment duration. While possibly minimizing the chance of disease relapse excessive treatment duration has disadvantages. These possibly include increased cost of treatment and risk of drug adverse effects. Further research is needed comparing traditional tests for clinical remission with antigen elimination considering outcome measures such as survival, cost‐effectiveness, and incidence of disease relapse.
A second limitation is the small number of cats included in the study. Histoplasmosis is relatively common in Oklahoma as compared with other locations in the United States, but remains a relatively uncommon disease of cats overall.21, 22 Every cat diagnosed with histoplasmosis during the enrollment period were assessed for study inclusion. For various reasons, only 15/20 cats were included in the study and 12/15 cats achieved clinical remission. The use of low study numbers inherently leads to more uncertainty. This is demonstrated in the relatively wide confidence intervals associated with the sensitivity and specificity calculated for the antigen EIA as a test for disease remission. In addition, the small number of cats included limits the number of less common occurrences (most notably disease relapse and low antigen concentrations at the time of clinical remission). A larger study will be required to fully describe the utility of the Histoplasma antigen EIA as it relates to these less commonly occurring events.
A third limitation of this study is that it included a heterogeneous population of cats. This study was not designed to investigate the utility of antigen testing in relation to a specific form of histoplasmosis. The prospective recruitment of cats with a specific form of disease would require a longer enrollment period or a larger study population. It is possible that utility of antigen testing is dependent upon the form of histoplasmosis. This has been demonstrated in humans, where the sensitivity of the Histoplasma urine antigen EIA for the diagnosis of histoplasmosis ranged from 30.4 to 91.8% pending the form of disease.6 In this study, all organ systems were not imaged or sampled and thus the true extent of disease is unknown. Acknowledging this limitation, it is interesting that the 4 cats (not including the cat with negative urine antigen) with the lowest initial Histoplasma urine antigen concentrations had disease found only in the GI tract (n = 2), skin (1), and bone/joints (1). In the authors' experience, cats with disease isolated to these anatomic locations, or with disease isolated to the eye(s), are more likely to have negative or low antigen concentrations.
This study did not include a rigid treatment protocol. A recent retrospective study of cats with histoplasmosis failed to detect a significant difference in survival or disease relapse when cats were treated with fluconazole or itraconazole.1 All cats in this study received either fluconazole or itraconazole along with other standard treatment. The duration of treatment was also somewhat variable. As is the case with any client‐owned study participant, the ultimate treatment was to some extent at the pet‐owners discretion. Several pet‐owners, in this study were uncomfortable with the unknowns of determining the time of clinical remission and elected to continue antifungal treatment beyond that recommended. Thus, the median treatment duration was approximately 10 weeks longer than the median time to clinical remission. This limitation is expected to affect this study only insofar as it could have affected the number of cats achieving clinical remission or potentially suffering from disease relapse during the study period.
The majority of cats in this study also received a short course of corticosteroids. Currently there are no published data concerning the use of corticosteroids in the treatment of histoplasmosis in cats. The IDSA recommends the use of corticosteroids with severe disease of multiple different forms of histoplasmosis when nonsteroidal anti‐inflammatory therapy has failed.15 There is a published report of the use of corticosteroids in 16 dogs with tracheobronchial lymphadenopathy secondary to histoplasmosis.23 There was a benefit to the use of corticosteroids with or without antifungal therapy in these dogs. None of the dogs were believed to have active infection. The use of varying treatments, such as corticosteroids, would be expected to affect the results of this study only insofar as it could have affected the number of cats achieving clinical remission. This study was designed to investigate the relationship between clinical remission and antigen elimination. That relationship was expected to be independent of the specific treatment utilized.
Finally, the study was limited by a relatively short follow‐up period and low compliance for the 6‐month recheck. The 6 month recheck was intended to better assess for early disease relapse. Previously reported relapse rates in cats with histoplasmosis are 25 and 40%.1, 11 In the aforementioned studies, disease relapse occurred from 4 to 74 months after discontinuation of antifungal treatment. As such, relapse in the future remains possible for cats included in this study.
Conclusion
Histoplasma urine and serum antigen concentrations decrease with effective antifungal treatment and increase with disease relapse in cats with histoplasmosis. Antigen elimination commonly occurs before clinical remission as determined by traditional means. As such, antigen testing is a sensitive but not specific test for clinical remission. Increasing antigen concentrations during antifungal treatment or after discontinuation of treatment could be a cause for concern. Further research is needed to determine the clinical importance of low antigen concentrations at the time of clinical remission.
Acknowledgments
The authors acknowledge Jocelyn Ireland and Nalani Yamada for their technical assistance and Drs Lauren Cunningham, Alicia Bangert, and Anthony Jarchow for their assistance in clinical management.
Conflict of Interest Declaration: Drs Wheat and Renschler are employees of MiraVista Diagnostics, which offers the Histoplasma antigen assay described in the study reported herein.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at Oklahoma State University.
Footnotes
MVista® Histoplasma Quantitative Antigen EIA, MiraVista Diagnostics, Indianapolis, IN 46241.
Antech Diagnostics, Stillwater, OK 74078.
SNAP FIV/FeLV Combo Test, Idexx Laboratories Inc, Westbrook, ME 04092.
SAS Institute Inc, Cary, NC 27513.
References
- 1. Reinhart JM, KuKanich KS, Jackson T, et al. Feline histoplasmosis: Fluconazole therapy and identification of potential sources of Histoplasma species exposure. J Feline Med Surg 2012;14:841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aulakh HK, Aulakh KS, Troy GC. Feline histoplasmosis: A retrospective study of 22 cases (1986–2009). J Am Anim Hosp Assoc 2012;48:182–187. [DOI] [PubMed] [Google Scholar]
- 3. Vergidis P, Avery RK, Wheat LJ, et al. Histoplasmosis complicating tumor necrosis factor‐alpha blocker therapy: A retrospective analysis of 98 cases. Clin Infect Dis 2015;61:409–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Porter BM, Comfort BK, Menges RW, et al. Correlation of fluorescent antibody, histopathology, and culture on tissues from 372 animals examined for histoplasmosis and blastomycosis. J Bacteriol 1965;89:748–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turner C, Smith CD, Furcolow ML. The efficiency of serologic and cultural methods in the detection of infection with Histoplasma and Blastomyces in mongrel dogs. Sabouraudia 1972;10:1–5. [DOI] [PubMed] [Google Scholar]
- 6. Hage CA, Ribes JA, Wengenack NL, et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin Infect Dis 2011;53:448–454. [DOI] [PubMed] [Google Scholar]
- 7. Bromel C, Greene CE. Histoplasmosis In: Greene CE, ed. Infecious Diseases of the Dog and Cat. St. Louis, MO: Elsevier; 2012:614–621. [Google Scholar]
- 8. Kauffman CA. Diagnosis of histoplasmosis in immunosuppressed patients. Curr Opin Infect Dis 2008;21:421–425. [DOI] [PubMed] [Google Scholar]
- 9. Cook AK, Cunningham LY, Cowell AK, et al. Clinical evaluation of urine Histoplasma capsulatum antigen measurement in cats with suspected disseminated histoplasmosis. J Feline Med Surg 2012;14:512–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clinkenbeard KD, Wolf AM, Cowell RL. Feline disseminated histoplasmosis. Compend Cont Educ Pract Vet 1989;11:1223–1233. [Google Scholar]
- 11. Hodges RD, Legendre AM, Adams LG, et al. Itraconazole for the treatment of histoplasmosis in cats. J Vet Intern Med 1994;8:409–413. [DOI] [PubMed] [Google Scholar]
- 12. Meinkoth JH, Mitchell C, Cowell R, et al. What is your diagnosis? Cytology of post‐treatment histoplasmosis. Vet Clin Pathol 1997;26:118–134.12658588 [Google Scholar]
- 13. Hage CA, Kirsch EJ, Stump TE, et al. Histoplasma antigen clearance during treatment of histoplasmosis in patients with AIDS determined by a quantitative antigen enzyme immunoassay. Clin Vaccine Immunol 2011;18:661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wheat LJ, Connolly‐Stringfield P, Blair R, et al. Histoplasmosis relapse in patients with AIDS: Detection using Histoplasma capsulatum variety capsulatum antigen levels. Ann Intern Med 1991;115:936–941. [DOI] [PubMed] [Google Scholar]
- 15. Wheat LJ, Freifeld AG, Kleiman MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis 2007;45:807–825. [DOI] [PubMed] [Google Scholar]
- 16. Connolly PA, Durkin MM, Lemonte AM, et al. Detection of Histoplasma antigen by a quantitative enzyme immunoassay. Clin Vaccine Immunol 2007;14:1587–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wheat LJ, Cloud G, Johnson PC, et al. Clearance of fungal burden during treatment of disseminated histoplasmosis with liposomal amphotericin B versus itraconazole. Antimicrob Agents Chemother 2001;45:2354–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Theel ES, Ramanan P. Clinical significance of low‐positive Histoplasma urine antigen results. J Clin Microbiol 2014;52:3444–3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldman M, Zackin R, Fichtenbaum CJ, et al. Safety of discontinuation of maintenance therapy for disseminated histoplasmosis after immunologic response to antiretroviral therapy. Clin Infect Dis 2004;38:1485–1489. [DOI] [PubMed] [Google Scholar]
- 20. Swartzentruber S, Rhodes L, Kurkjian K, et al. Diagnosis of acute pulmonary histoplasmosis by antigen detection. Clin Infect Dis 2009;49:1878–1882. [DOI] [PubMed] [Google Scholar]
- 21. Davies C, Troy GC. Deep mycotic infections in cats. J Am Anim Hosp Assoc 1996;32:380–391. [DOI] [PubMed] [Google Scholar]
- 22. Clinkenbeard KD, Cowell RL, Tyler RD. Disseminated histoplasmosis in cats: 12 cases (1981–1986). J Am Vet Med Assoc 1987;190:1445–1448. [PubMed] [Google Scholar]
- 23. Schulman RL, McKiernan BC, Schaeffer DJ. Use of corticosteroids for treating dogs with airway obstruction secondary to hilar lymphadenopathy caused by chronic histoplasmosis: 16 cases (1979–1997). J Am Vet Med Assoc 1999;214:1345–1348. [PubMed] [Google Scholar]


