Abstract
Albuminuria class transition (normo- to micro- to macroalbuminuria) is used as an intermediate end point to assess renoprotective drug efficacy. However, definitions of such class transition vary between trials. To determine the most optimal protocol, we evaluated the approaches used in four clinical trials testing the effect of renin-angiotensin-aldosterone system intervention on albuminuria class transition in patients with diabetes: the BENEDICT, the DIRECT, the ALTITUDE, and the IRMA-2 Trial. The definition of albuminuria class transition used in each trial differed from the definitions used in the other trials by the number (one, two, or three) of consecutively collected urine samples at each study visit, the time interval between study visits, the requirement of an additional visit to confirm the class transition, and the requirement of a percentage increase in albuminuria from baseline in addition to the class transition. In Cox regression analysis, neither increasing the number of urine samples collected at a single study visit nor differences in the other variables used to define albuminuria class transition altered the average drug effect. However, the SEM of the treatment effect increased (decreased precision) with stricter end point definitions, resulting in a loss of statistical significance. In conclusion, the optimal albuminuria transition end point for use in drug intervention trials can be determined with a single urine collection for albuminuria assessment per study visit. A confirmation of the end point or a requirement of a minimal percentage change in albuminuria from baseline seems unnecessary.
Keywords: albuminuria, diabetes mellitus, microalbuminuria, randomized controlled trials
Transition in albuminuria class (normo- to microalbuminuria or micro- to macroalbuminuria) is a hallmark of progression of diabetic kidney disease.1,2 This hallmark has been used as an end point in clinical trials to assess renoprotective efficacy of clinical interventions. Because there is no standardized protocol of how to measure an albuminuria class transition, different definitions have been used in past trials3–8 that vary in the way urine was collected (first morning void versus spot urine),3,8 how albuminuria was expressed (urinary albumin excretion or albumin-to-creatinine ratio),3,6,8 the frequency and interval of urine collections to define a class transition,3,4,6 and the requirement for a confirmatory visit.3,4 Confirmation at a subsequent visit may be important, because albuminuria is known to show a large day to day variability.9,10 To ensure that a class change is not caused by chance, the end point definition is often complemented with a requirement that albuminuria increase should be greater than a predefined percentage of baseline.3,4
Harmonization of the end point criteria for the optimal definition of class transition in albuminuria is important for the design of future clinical trials. Defining a transition by a single measurement without a predefined percentage change may increase the number of end points and thereby, the statistical power to assess drug effects. However, a predefined percentage change and a subsequent confirmatory visit may decrease the number of end points but may increase the precision of the transition and thereby, increase statistical power to assess drug effects.
The aim of this study was, therefore, to weigh the importance of all of these opposing effects in a post hoc analysis of completed clinical trials, which tested the effects of renin-angiotensin-aldosterone system (RAAS) inhibition on albuminuria class transitions. The objective was to determine the optimal definition for a transition in albuminuria class regarding the frequency of urine collections, the interval between study visits for urine collections, the requirement of confirmatory visits, and the cutoff threshold for the percentage change from baseline.
Results
Baseline characteristics of included trials are published elsewhere and summarized in Table 1.3,11–13
Table 1.
Baseline characteristics of the clinical trial populations
| BENEDICT, n=742 | DIRECT (T1D), n=3093 | DIRECT (T2D), n=1758 | ALTITUDE, n=3654 | IRMA-2 Trial, n=565 | |
|---|---|---|---|---|---|
| Men, n (%) | 397 (53.5) | 1771 (57.3) | 858 (48.1) | 2497 (68.3) | 391 (69.2) |
| Age, yr, n (%) | 61.9 (8.0) | 31.0 (8.4) | 56.9 (7.7) | 65.8 (9.4) | 58.1 (8.6) |
| Diastolic BP, mmHg, n (%) | 88.8 (7.7) | 73.1 (6.9) | 78.0 (7.0) | 74.1 (10.0) | 89.6 (9.3) |
| Systolic BP, mmHg, n (%) | 152.4 (14.0) | 116.9 (9.6) | 132.7 (13.5) | 138.2 (16.3) | 152.7 (14.6) |
| Albuminuriaa | 5.2 [3.5–8.8]b | 4.5 [3.5–7.0]b | 5 [3.5–8.0]b | 23.7 [4.11–73.6]c | 58.0 [33.0–102.0]c |
| Normoalbuminuria, n (%) | 742 (100) | 3093 (100) | 1758 (100) | 1020 (27.9) | 0 (0) |
| Microalbuminuria, n (%) | 0 (0) | 0 (0) | 0 (0) | 2634 (72.1) | 565 (100) |
| Hemoglobin A1c, n (%) | 3.0 (1.4) | 8.3 (1.6) | 8.2 (1.6) | 7.9 (1.7) | 7.0 (1.7) |
| Total cholesterol, mg/dl, n (%) | 211.8 (36.5) | 184.8 (37.5) | 204.9 (42.5) | 170.5 (46.4) | 223.2 (44.4) |
| eGFR, ml/min per 1.73 m2, n (%) | 81.4 (15.3) | 80.8 (13.7) | 70.0 (14.2) | 55.4 (20.6) | 70.5 (12.4) |
T1D, type 1 diabetes; T2D, type 2 diabetes.
Presented as median and interquartile range.
Urinary albumin excretion rate: micrograms per minute.
UACR: milligrams per gram.
Number of End Points
Increasing the number of urine collections per visit reduced the number of end points (Table 2). The number of end points further decreased in each trial when the transition end point required a confirmation at a subsequent visit (Table 2). Adding increasing percentages of albuminuria change to the end point definition only marginally reduced the number of end points in each trial (Table 2).
Table 2.
Number of end points and event rates (events per 100 patient-years) for each clinical trial and end point definition
| Single Visit | Confirmationa: One Measurement | |||
|---|---|---|---|---|
| One Measurement | Mean of Two Measurements | Mean of Three Measurements | ||
| Baseline | ||||
| Normo- to microalbuminuria | ||||
| BENEDICT | 123 (5.9) | 107 (5.1) | 98 (4.1) | 14 (0.7) |
| DIRECT (T1D) | 441 (3.5) | 344 (2.7) | NA | 100 (1.0) |
| DIRECT (T2D) | 431 (6.5) | 380 (5.6) | NA | 159 (2.9) |
| Micro- to macroalbuminuria | ||||
| IRMA-2 Trialb | 114 (12.6) | NA | NA | 26 (3.8) |
| Normo- to microalbuminuria and micro- to macroalbuminuria | ||||
| ALTITUDE | 1861 (33.1) | 1738 (29.4) | 1717 (29.0) | 1087 (21.0) |
| 10% Change in albuminuria in addition to a transition | ||||
| Normo- to microalbuminuria | ||||
| BENEDICT | 123 (5.9) | 107 (5.1) | 98 (4.1) | 14 (0.7) |
| DIRECT (T1D) | 440 (3.5) | 344 (2.7) | NA | 100 (1.0) |
| DIRECT (T2D) | 431 (6.5) | 380 (5.6) | NA | 159 (2.9) |
| Micro- to macroalbuminuria | ||||
| IRMA-2 Trial | 114 (12.6) | NA | NA | 26 (3.8) |
| Normo- to microalbuminuria and micro- to macroalbuminuria | ||||
| ALTITUDE | 1858 (33.0) | 1731 (29.3) | 1713 (28.9) | 1078 (20.7) |
| 30% Change in albuminuria in addition to a transition | ||||
| Normo- to microalbuminuria | ||||
| BENEDICT | 120 (5.7) | 103 (4.9) | 95 (4.0) | 13 (0.7) |
| DIRECT (T1D) | 437 (3.4) | 341 (2.6) | NA | 99 (1.0) |
| DIRECT (T2D) | 428 (6.4) | 378 (5.6) | NA | 154 (2.8) |
| Micro- to macroalbuminuria | ||||
| IRMA-2 Trial | 113 (12.5) | NA | NA | 25 (3.6) |
| Normo- to microalbuminuria and micro- to macroalbuminuria | ||||
| ALTITUDE | 1826 (32.0) | 1704 (28.6) | 1684 (28.1) | 1045 (19.8) |
| 50% Change in albuminuria in addition to a transition | ||||
| Normo- to microalbuminuria | ||||
| BENEDICT | 112 (5.3) | 100 (4.7) | 88 (3.7) | 13 (0.7) |
| DIRECT (T1D) | 431 (3.4) | 339 (2.6) | NA | 99 (1.0) |
| DIRECT (T2D) | 421 (6.3) | 372 (5.5) | NA | 146 (2.6) |
| Micro- to macroalbuminuria | ||||
| IRMA-2 Trial | 109 (12.0) | NA | NA | 22 (3.2) |
| Normo- to microalbuminuria and micro- to macroalbuminuria | ||||
| ALTITUDE | 1754 (30.1) | 1654 (27.2) | 1636 (26.8) | 989 (18.4) |
| 100% Change in albuminuria in addition to a transition | ||||
| Normo- to microalbuminuria | ||||
| BENEDICT | 95 (4.5) | 80 (3.8) | 73 (3.0) | 8 (0.4) |
| DIRECT (T1D) | 409 (3.2) | 316 (2.4) | NA | 93 (0.9) |
| DIRECT (T2D) | 391 (5.8) | 351 (5.1) | NA | 131 (2.3) |
| Micro- to macroalbuminuria | ||||
| IRMA-2 Trial | 95 (10.4) | NA | NA | 20 (2.9 |
| Normo- to microalbuminuria and micro- to macroalbuminuria | ||||
| ALTITUDE | 1628 (26.5) | 1504 (23.5) | 1493 (23.3) | 849 (15.3) |
T1D, type 1 diabetes; NA, not available; T2D, type 2 diabetes.
Confirmation of transition end point at a subsequent study visit. The confirmation is on the basis of a single urine sample obtained at the initial and confirmation visits.
In the IRMA-2 Trial, only single urine collections were performed at each visit.
Number of Urine Collections at a Single Visit and Time Interval between Visits
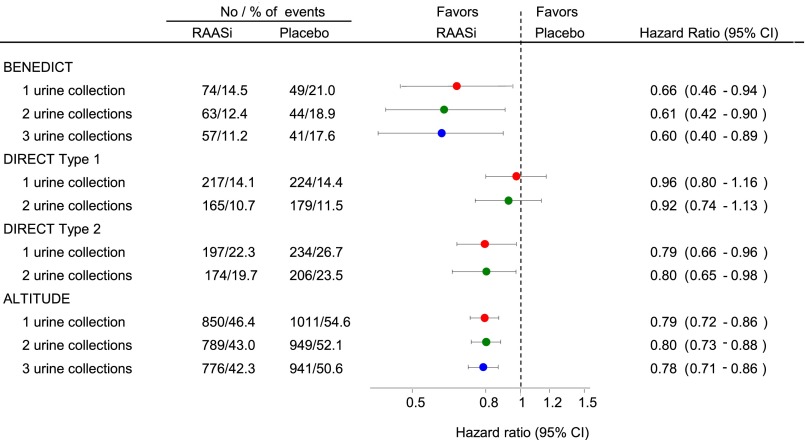
Increasing the number of urine collections at a single visit only had minimal effect on the average treatment effect and 95% confidence interval (95% CI) in the Bergamo Nephrologic Diabetic Complications Trial (BENEDICT), the Diabetic Retinopathy Candesartan Trials (DIRECT), and the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE) (Figure 1). For example, in the BENEDICT, the hazard ratio (HR) was 0.60 (95% CI, 0.40 to 0.89) when the transition end points were calculated from the geometric mean of three consecutive urine samples at each visit and 0.66 (95% CI, 0.46 to 0.99) on the basis of single urine samples. Increasing the time interval between study visits for albuminuria assessment had no effect on the magnitude of the average treatment effect, whereas the precision of the drug effect estimate decreased somewhat as indicated by the wider 95% CIs (Supplemental Figure 1).
Figure 1.
Increasing the number of urine samples at a study visit does not affect the average drug effect and 95% CI. Magnitude and precision of the drug effect estimate according the number of urine collections at a single study visit. Circles represents the estimate of the treatment effect, and the horizontal line indicates the 95% CI. RAASi, renin-angiotensin-aldosterone system intervention.
Requirement for Confirmation Visits and Time Interval of the Confirmation Visit
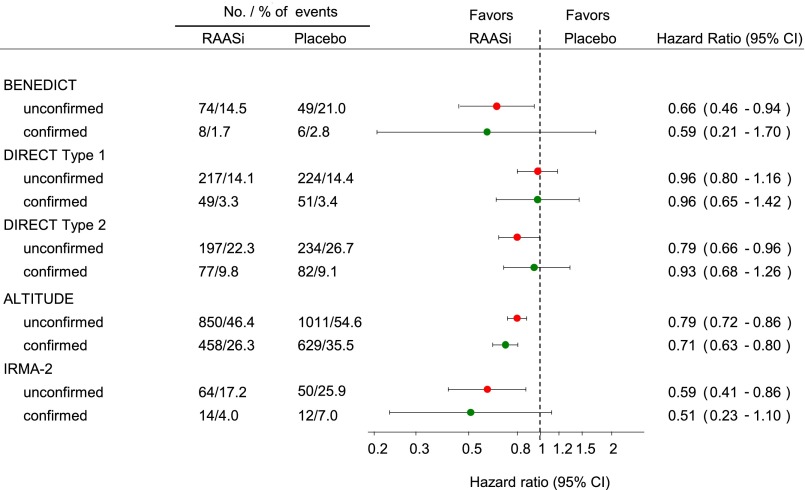
Addition of a confirmation visit to firmly establish the class transition only minimally affected the average treatment effects of trandolapril, candesartan, irbesartan, and aliskiren (Figure 2). However, the precision of the drug effect estimate markedly decreased as reflected by the wider 95% CI in all trials. For example, in the BENEDICT, the HR was 0.66 (95% CI, 0.46 to 0.99) when the transition end points were not confirmed and 0.60 (95% CI, 0.21 to 1.70) with confirmation. Despite the different time intervals of the confirmatory visit, a decrease in precision occurred in all trials, suggesting that the timing of the confirmatory visit had no effect on the result.
Figure 2.
Addition of a confirmatory visit to the end point definition had minimal effect on the average drug effect and increases the 95% CI. Magnitude and precision of the drug effect estimate with a single urine collection with and without a confirmatory visit. Circles represents the estimate of the treatment effect, and the horizontal line indicates the 95% CI. RAASi, renin-angiotensin-aldosterone system intervention.
Percentage Increase in Albuminuria
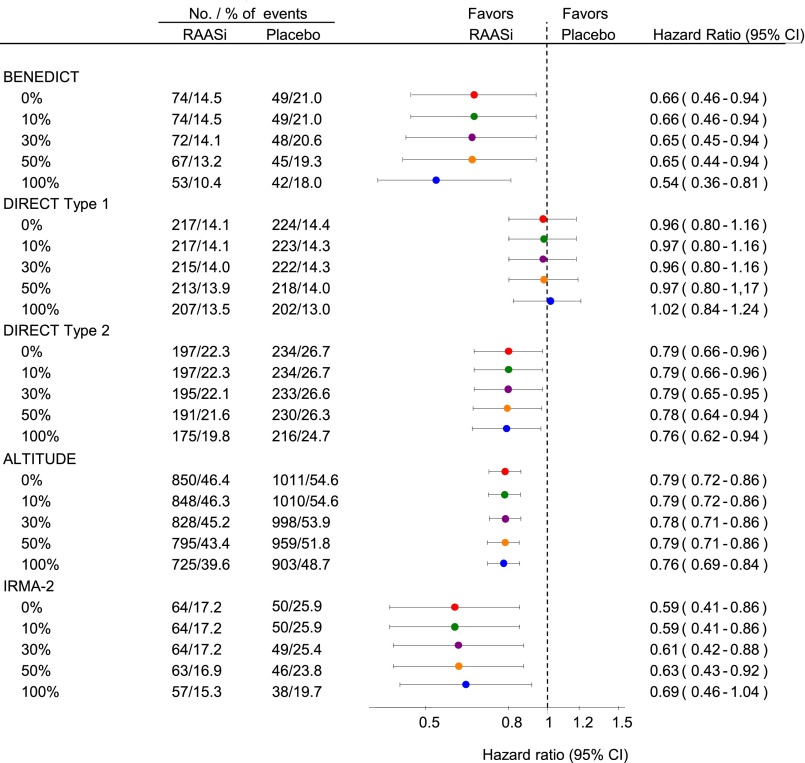
Enhancing the level of minimal percentage change in albuminuria from baseline from 10% to 100% had minimal effect on the average and SEM of the treatment effects of trandolapril, candesartan, irbesartan, and aliskiren (Figure 3). For example, in the BENEDICT, the HR was 0.66 (95% CI, 0.46 to 0.94) with a 10% increase in albuminuria in addition to the class transition, 0.65 (95% CI, 0.44 to 0.94) with a 50% increase in albuminuria, and 0.54 (95% CI, 0.36 to 0.81) with a 100% increase in albuminuria. HRs were somewhat higher (smaller treatment effect) in most trials if the end point was on the basis of a 50% or 100% increase without class transition (Supplemental Figure 2).
Figure 3.
Addition of a percentage change in albuminuria to the end point definition has no effect on the average treatment effect and 95% CI. Magnitude and precision of the drug effect estimate with a single urine collection and various percentage albuminuria increases on top of the class transition without a confirmatory visit. Circles represent the estimate of the treatment effect, and the horizontal line indicates the 95% CI. RAASi, renin-angiotensin-aldosterone system intervention.
Association between Albuminuria Class Transition End Points and Clinical Outcomes
The ALTITUDE database was used to determine the association between a class transition in albuminuria and an intermediate end point with subsequent clinical renal and cardiovascular outcomes. A transition in albuminuria stage from normo- to microalbuminuria or micro- to macroalbuminuria on the basis of a single urine sample at a single visit was independently associated with a higher risk of the renal (HR, 1.92; 95% CI, 1.09 to 3.40; P=0.03) and cardiovascular end points (HR, 1.45; 95% CI, 1.15 to 1.83; P=0.002) (Table 3). HRs only marginally changed when the class transition was on the basis of the average of multiple urine samples at a single visit or when a confirmation visit or a percentage albuminuria increase to the end point definition was added to define the class transition (Table 3).
Table 3.
Association between a transition in albuminuria class (normo- to microalbuminuria transition or micro- to macroalbuminuria transition) during the first year of the ALTITUDE and subsequent renal (doubling serum creatinine, ESRD, or renal death) and cardiovascular (cardiovascular death, resuscitated sudden death, myocardial infarction, stroke, or unplanned hospitalization for heart failure) outcomes
| Class Transitions | Renal Outcome | Cardiovascular Outcome | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | Chi-Squared | P Value | HR (95% CI) | Chi-Squared | P Value | |
| Single visit | ||||||
| Single urine sample | 1.92 (1.09 to 3.40) | 5.02 | 0.03 | 1.45 (1.15 to 1.83) | 9.81 | 0.002 |
| Two urine samples | 1.80 (1.01 to 3.20) | 3.98 | 0.05 | 1.30 (1.02 to 1.65) | 4.46 | 0.04 |
| Three urine samples | 1.82 (1.02 to 3.24) | 4.12 | 0.04 | 1.35 (1.06 to 1.72) | 6.00 | 0.01 |
| Addition of confirmation visit | 2.47 (1.36 to 4.49) | 8.81 | 0.003 | 1.42 (1.09 to 1.85) | 6.84 | <0.01 |
| Transition and 10% UACR increase | 1.94 (1.10 to 3.43) | 5.19 | 0.02 | 1.44 (1.14 to 1.82) | 9.45 | 0.002 |
| Transition and 30% UACR increase | 2.06 (1.17 to 3.64) | 6.20 | 0.01 | 1.39 (1.10 to 1.76) | 7.47 | <0.01 |
| Transition and 50% UACR increase | 2.22 (1.26 to 3.91) | 7.59 | <0.01 | 1.36 (1.07 to 1.72) | 6.39 | 0.01 |
| Transition and 100% UACR increase | 1.98 (1.11 to 3.51) | 5.42 | 0.02 | 1.30 (1.02 to 1.66) | 4.39 | 0.04 |
The definitions of albuminuria class transitions are similar as those that were used to assess the drug effect. The presented HRs are derived from a Cox proportional hazard models and adjusted for age, sex, albumin-to-creatinine ratio, eGFR, systolic and diastolic BPs, hemoglobin A1c, and cardiovascular disease history. The composite renal end point was defined as ESRD defined as the need for chronic dialysis or renal transplantation, renal death defined as the need for RRT with no dialysis or transplantation available or initiated, or doubling of serum creatinine from baseline sustained for at least 1 month. The composite cardiovascular end point was defined as death from cardiovascular causes or the first occurrence of cardiac arrest with resuscitation, nonfatal myocardial infarction, nonfatal stroke, or unplanned hospitalization for heart failure. All clinical end points were adjudicated by a central end point committee using standard definitions.
Discussion
Transition of albuminuria class may become a more and more important (surrogate) end point to evaluate renoprotective drug effects. Its current use shows a wide variety of definitions of such an albuminuria class transition. The variations concern the number of albuminuria collections per visit, the interval of study visits for albuminuria collection, the addition of a class change conformation visit, and the addition of a minimal percentage albuminuria change with the class transition. We established that, in a post hoc analysis of four randomized, controlled clinical trials, none of these variations had a significant effect on most of the outcome parameters in the different studies. These data suggest that single urine collections at a study visit are sufficient to define a transition in albuminuria as an end point in clinical trials.
Because of the within–individual day to day fluctuations in albuminuria, prior clinical trials have used the average albuminuria from three consecutive visits instead of one urine collection at each study visit to determine a transition in albuminuria stage. In our study, we found that decreasing the number of urine samples at a single visit led to a small increase in the number of end points, but the average drug effect and the precision of the drug effect did not change. It is likely that, when using single urine samples, a potential increase in the precision of the drug effect estimate (as a result of the higher number of end points) is balanced by introducing random noise and false–positive end points, so that the average drug effect and precision do not change.
Another approach to minimize the effect of the day to day fluctuations in albuminuria on the transition end point has been the requirement of a confirmation of the albuminuria transition. The addition of a confirmatory visit is expected to decrease the random noise in the treatment effect because of albuminuria fluctuations and false-positive transitions. However, our analysis showed that adding a confirmatory visit to the end point definition led to a marked decrease in end points and a decrease in precision of the drug effect estimates. The marked decrease in end points apparently overruled the potential gain in statistical power because of the removal of false-positive transitions. Previous trials have added a 30% increase in albuminuria when the transition occurs as a third strategy to reduce the influence of day to day albuminuria fluctuations and end point occurrence because of chance, particularly for patients whose baseline albuminuria levels are close to the transition threshold. The first trial that added a 30% albuminuria increase to the transition end point was a trial on the effect of captopril in patients with type 1 diabetes.14 Although it seems logical to add a percentage increase in albuminuria to the end point definition, our results indicate that it had no effect on the number of end points, the average drug effect, or the precision of the drug effect.
The question is whether a transition in albuminuria class, a surrogate, represents a hard outcome, and if so, whether a single urine–determined transition is as good as two or three consecutive urine–determined transitions for hard outcome prediction. Indeed, in a post hoc analysis of the ALTITUDE, we found that a transition in albuminuria predicts renal and cardiovascular outcomes. In addition, the association was independent of all different definitions that we used for the albuminuria class transition. These data support using single urine samples at a single visit to define a transition end point.
Standardization of clinical trial end point definitions is important for drug regulators, physicians, and patients to assess and compare clinical trial results and interpret drug efficacy. Surprisingly, all past clinical trials have used different definitions for a class transition in albuminuria end point. Because of the different definitions, we unified the end point definitions across all included trials to compare different trial results. Because we standardized the end point definitions for the included trials, our results may differ from the published results in the original publications.
These results may, at first sight, contrast our previous findings, in which we concluded that increasing the frequency of urine collections during follow-up of a clinical trial increases the precision and statistical power to detect an albuminuria–lowering drug effect.15 However, there are important differences between these studies. First, the end points between the studies were different. In this study, the drug effect on a dichotomous outcome was determined. In trials with dichotomous outcomes, the statistical power is determined by the proportion of patients who reach the outcome. In our prior study, we evaluated the drug effect on the continuous outcome of percentage albuminuria change. In these trials, the power is determined by the magnitude of change and SD of the change. Second, in addition, the populations were different. In this study, we included patients with normo- and microalbuminuria, because these individuals may progress in albuminuria stage. In our previous study, patients with macroalbuminuria were included. Noteworthy, using single urine samples for albuminuria measurements without confirmation may increase the sensitivity to detect a transition but decrease specificity, resulting in false-positive and/or false-negative results. In the context of a randomized, controlled clinical trial, this may increase data variability without affecting the overall result of the trial. For the individual patient treated in clinical practice, the increase in false-positive and/or false-negative results may hamper diagnostic and prognostic performance and lead to erroneous conclusions on whether the individual is at increased renal or cardiovascular risk. The limitations of our study are that we were only able to include clinical trials that assessed the effect of RAAS intervention in patients with type 1 or type 2 diabetes. We do not know whether the results will be similar with other drugs or in other populations. To calculate a percentage increase in albuminuria, we used the albuminuria values collected at a single baseline visit. Albuminuria was not assessed at multiple visits before randomization. We, therefore, do not know whether multiple baseline visits improved the precision in the percentage increase in albuminuria and the potential effect on the results. Third, urine collections did not occur more frequently than every 6 months in all trials. We were, therefore, unable to determine whether shorter time intervals between visits would lead to more significant treatment effects. Fourth, these results were derived post hoc. We encourage clinical trialists to prospectively validate these findings.
In conclusion, increasing the number of urines collected at a single study visit, inclusion of a confirmation visit, time to the confirmation visit, and addition of a minimal required percentage albuminuria change did not alter the average drug effect. Therefore, we conclude that future clinical trials in diabetic nephropathy using albuminuria transitions as end points should consider single urine collections per study visit spaced ≤6 months apart for albuminuria assessment.
Concise Methods
Patients and Clinical Trials
Data from four randomized, controlled clinical trials in patients with type 1 or type 2 diabetes were analyzed: the BENEDICT, the DIRECT, the ALTITUDE, and the Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria (IRMA-2) Trial were included in this study. All trials assessed the effect of intervention in the RAAS on progression of albuminuria. The study designs and results of each trial are reported elsewhere and briefly described here.3,6,11,12,16
In the BENEDICT, 1209 patients with type 2 diabetes, normoalbuminuria (urinary albumin excretion rate <20 μg/min), and serum creatinine ≤1.5 mg/dl were randomly allocated to placebo or active treatment with verapamil (240 mg/d), trandolapril (2 mg/d), or the combination of verapamil (180 mg/d) and trandolapril (2 mg/d). Because the effects of trandalopril and trandolapril/verapamil on delaying progression to microalbuminuria were similar, we combined both treatment arms to increase statistical power and compared them against placebo. Patients with microalbuminuria at baseline were excluded from analysis. Urinary albumin excretion was assessed at randomization and every 6 months during the 3.6 years of median follow-up.4,11
The DIRECT was a clinical trial program consisting of three clinical trials in 3326 patients with type 1 diabetes with or without retinopathy and 1905 patients with type 2 diabetes and retinopathy. Patients had normoalbuminuria (urinary albumin excretion rate <20 μg/min) and serum creatinine ≤1.5 and ≤1.1mg/dl (for men and women, respectively). In the DIRECT, patients were randomly allocated to placebo or treatment with candensartan (16 mg/d; increasing to 32 mg/d according to tolerability) and followed for a median time of 4.7 years.12 Albuminuria was assessed at randomization and yearly during follow-up.5 Patients with missing baseline albuminuria or microalbuminuria at baseline were excluded from analysis, leaving 3095 patients with type 1 diabetes and 1795 patients with type 2 diabetes available for analysis.
The ALTITUDE was a randomized, double–blind, placebo–controlled trial enrolling 8561 patients with type 2 diabetes mellitus at high risk for cardiovascular and renal events. Eligible patients had either persistent macroalbuminuria (defined in this trial as urinary albumin-to-creatinine ratio [UACR] ≥200 mg/g) or an eGFR≥30 to ≤60 ml/min per 1.73 m2 combined with either persistent microalbuminuria (UACR≥20 to ≤200 mg/g) or a history of cardiovascular disease. Patients were randomly assigned to aliskiren (300 mg/d) or matched placebo in addition to an optimal recommended dose of an angiotensin–converting enzyme inhibitor or angiotensin receptor blocker. For the purpose of this study, we excluded patients in the ALTITUDE who had UACR>300 mg/g at baseline (n=4493) and those with missing baseline albuminuria measurements or no follow-up measurements (n=384), leaving 3654 patients available for analysis. Albuminuria was assessed at randomization, every 3 months during the first year, and every 6 months thereafter. Randomized patients were followed for a median of 32.9 months for occurrence of cardiovascular and renal events.6,16
In the IRMA-2 Trial, 590 patients with type 2 diabetes, microalbuminuria (urinary albumin excretion rate >20–200 μg/min), and serum creatinine ≤1.5 and ≤1.1 mg/dl (for men and women, respectively) were enrolled. Patients were randomly allocated to either placebo or active treatment with irbesartan (150 or 300 mg/d). For the purpose of analysis, we combined the two irbesartan treatment arms to increase statistical power. Urinary albumin excretion was assessed at randomization and every 6 months during the 2 years of follow-up.3 We included 565 patients without macroalbuminuria at baseline.
Urine Collections and Albuminuria Measurements
The frequency of urine collections (single, double, or triple) per study visit as well as albuminuria assessment (i.e., spot or timed collection) differed in the four trials. In the BENEDICT and the ALTITUDE, three consecutive urine collections for albuminuria assessment were performed at each study visit. In the DIRECT, two consecutive urine collections were performed at each study visit starting the day before the study visit. In the IRMA-2 Trial, only single urine collections were performed at each study visit. In the BENEDICT and the ALTITUDE, urinary albumin and creatinine were measured in first morning void urine collections. In the DIRECT and the IRMA-2 Trial, timed overnight urine collections were performed, and urinary albumin excretion was measured. In each trial, urinary albumin and creatinine were measured in a central laboratory. In this study, we defined microalbuminuria as a UACR=30 mg/g or urinary albumin excretion of 20 μg/min. Macroalbuminuria was defined as a UACR=300 mg/g or urinary albumin excretion of 200 μg/min.
Statistical Analyses
Baseline characteristics are presented as means and SDs for continuous variables and counts and proportions for discrete variables. Nonparametric data are presented as medians and interquartile ranges. The number of albuminuria class transitions (progression from either normo- to microalbuminuria or from micro- to macroalbuminuria) was recorded, and incidence rate per 100 patient-years was calculated. The effects of randomized treatment on all end point definitions were estimated from unadjusted Cox proportional hazard models on the basis of the intention to treat principle. For participants who experienced more than one event during follow-up, survival time to the first relevant end point was used in each analysis. Participants were censored at their date of death or for those still alive, the end of follow-up. The magnitude of the treatment effect is reflected by the HR, and the precision of the treatment effect is reflected by its 95% CI. We first determined whether increasing the number of consecutive urine collections at a single visit would lead to a more significant treatment effect. We subsequently determined the effect of the time interval between visits, the addition of a confirmatory visit, and the addition of a percentage change in albuminuria from baseline using the number of urine collections as defined in the first step. Unscheduled visits for albuminuria measurements were not included in the drug efficacy analyses.
The association between a transition in albuminuria class (progression to micro- or macroalbuminuria or regression to micro- or normoalbuminuria) during the first year of the trial and subsequent renal and cardiovascular outcomes was estimated using a multivariable Cox regression model. The Cox model was adjusted for age, sex, albuminuria, eGFR, systolic and diastolic BPs, hemoglobin A1c, and history of cardiovascular disease (yes or no). All analyses were performed using SAS 9.3 for Windows (SAS Institute Inc., Cary, NC). A two–sided P value <0.05 was considered to indicate statistical significance.
Disclosures
T.F.K. reports no conflicts of interests. D.d.Z. has consultancy agreements with the following companies: Abbvie, Astellas, Bristol-Myers Squibb (Princeton, NJ), Fresenius, Hemocue, Johnson & Johnson, Merck GmbH (Darmstadt, Germany), Merck Sharpe & Dohme, Novartis (Basel, Switzerland), Reata Pharmaceuticals, and Vitae. All honoraria are paid to his institution. G.R. has consultancy agreements with Dompé farmaceutici S.pA., AbbVie, Alexion Pharmaceuticals, Bayer HealthCare (Whippany, NJ), Reata Pharmaceuticals, Novartis Pharma, AstraZeneca Pharmaceuticals (Wilmington, DE), Otsuka Pharmaceutical Europe, and Concert Pharmaceuticals. No personal remuneration is accepted from AbbVie, Alexion Pharmaceuticals, Bayer HealthCare, Reata Pharmaceuticals, Novartis Pharma, AstraZeneca Pharmaceuticals, Otsuka Pharmaceutical Europe, and Concert Pharmaceuticals, and compensations are paid to his institution for research and educational activities. R.B. reports honoraria from Roche (Basel, Switzerland), Novo Nordisk, Boehringer Ingelheim (Mannheim, Germany), and Animas. He serves on data safety monitoring committees of clinical trials sponsored by Abbvie and Mitsubishi. H.-H.P. consults for Abbvie. H.J.L.H. has consultancy agreements with the following companies: Abbvie, Astellas, Astra Zeneca, Boehringer Ingelheim, Janssen Biotech (Horsham, PA), and ZS Pharma (Coppell, TX). He has a policy that all honoraria are paid to his institution.
Supplementary Material
Acknowledgments
The authors acknowledge the supportive role of all investigators, support staff, and participating patients in all trials.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015101150/-/DCSupplemental.
References
- 1.Roscioni SS, Lambers Heerspink HJ, de Zeeuw D: Microalbuminuria: Target for renoprotective therapy PRO. Kidney Int 86: 40–49, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Lambers Heerspink HJ, Gansevoort RT: Albuminuria is an appropriate therapeutic target in patients with CKD: The pro view. Clin J Am Soc Nephrol 10: 1079–1088, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parving HH, Lehnert H, Bröchner-Mortensen J, Gomis R, Andersen S, Arner P Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group : The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878, 2001 [DOI] [PubMed] [Google Scholar]
- 4.BENEDICT Group : The BErgamo NEphrologic DIabetes Complications Trial (BENEDICT): Design and baseline characteristics. Control Clin Trials 24: 442–461, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Chaturvedi N, Sjoelie AK, Svensson A DIRECT Programme Study Group : The DIabetic Retinopathy Candesartan Trials (DIRECT) Programme, rationale and study design. J Renin Angiotensin Aldosterone Syst 3: 255–261, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Parving H-H, Brenner BM, McMurray JJV, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Ghadanfar M, Weissbach N, Xiang Z, Armbrecht J, Pfeffer MA: Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE): Rationale and study design. Nephrol Dial Transplant 24: 1663–1671, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Makino H, Haneda M, Babazono T, Moriya T, Ito S, Iwamoto Y, Kawamori R, Takeuchi M, Katayama S INNOVATION Study Group : Prevention of transition from incipient to overt nephropathy with telmisartan in patients with type 2 diabetes. Diabetes Care 30: 1577–1578, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G ROADMAP Trial Investigators : Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med 364: 907–917, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Smulders YM, Slaats EH, Rakic M, Smulders FT, Stehouwer CD, Silberbusch J: Short-term variability and sampling distribution of various parameters of urinary albumin excretion in patients with non-insulin-dependent diabetes mellitus. J Lab Clin Med 132: 39–46, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Witte EC, Lambers Heerspink HJ, de Zeeuw D, Bakker SJL, de Jong PE, Gansevoort R: First morning voids are more reliable than spot urine samples to assess microalbuminuria. J Am Soc Nephrol 20: 436–443, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, Brusegan V, Rubis N, Gherardi G, Arnoldi F, Ganeva M, Ene-Iordache B, Gaspari F, Perna A, Bossi A, Trevisan R, Dodesini AR, Remuzzi G Bergamo Nephrologic Diabetes Complications Trial (BENEDICT) Investigators : Preventing microalbuminuria in type 2 diabetes. N Engl J Med 351: 1941–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Bilous R, Chaturvedi N, Sjølie AK, Fuller J, Klein R, Orchard T, Porta M, Parving HH: Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: Three randomized trials. Ann Intern Med 151: 11–20, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Parving H-H, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Nicolaides M, Richard A, Xiang Z, Armbrecht J, Pfeffer MA; ALTITUDE Investigators: Baseline characteristics in the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints (ALTITUDE). J Renin Angiotensin Aldosterone Syst 13: 387–393, 2012 [DOI] [PubMed]
- 14.Viberti G, Mogensen CE, Groop LC, Pauls JF European Microalbuminuria Captopril Study Group : Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. JAMA 271: 275–279, 1994 [PubMed] [Google Scholar]
- 15.Kröpelin TF, de Zeeuw D, Andress DL, Bijlsma MJ, Persson F, Parving HH, Heerspink HJ: Number and frequency of albuminuria measurements in clinical trials in diabetic nephropathy. Clin J Am Soc Nephrol 10: 410–416, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parving H-H, Brenner BM, McMurray JJV, de Zeeuw D, Haffner SM, Solomon SD, Chaturvedi N, Persson F, Desai AS, Nicolaides M, Richard A, Xiang Z, Brunel P, Pfeffer MA ALTITUDE Investigators : Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213, 2012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.