The characterization of imaging biomarkers associated with psychiatric illness can facilitate diagnostic and therapeutic practice by providing an objective way to select patients for optimal therapies and to track treatment effects on brain systems.
Abstract
Unlike neurologic conditions, such as brain tumors, dementia, and stroke, the neural mechanisms for all psychiatric disorders remain unclear. A large body of research obtained with structural and functional magnetic resonance imaging, positron emission tomography/single photon emission computed tomography, and optical imaging has demonstrated regional and illness-specific brain changes at the onset of psychiatric disorders and in individuals at risk for such disorders. Many studies have shown that psychiatric medications induce specific measurable changes in brain anatomy and function that are related to clinical outcomes. As a result, a new field of radiology, termed psychoradiology, seems primed to play a major clinical role in guiding diagnostic and treatment planning decisions in patients with psychiatric disorders. This article will present the state of the art in this area, as well as perspectives regarding preparations in the field of radiology for its evolution. Furthermore, this article will (a) give an overview of the imaging and analysis methods for psychoradiology; (b) review the most robust and important radiologic findings and their potential clinical value from studies of major psychiatric disorders, such as depression and schizophrenia; and (c) describe the main challenges and future directions in this field. An ongoing and iterative process of developing biologically based nomenclatures with which to delineate psychiatric disorders and translational research to predict and track response to different therapeutic drugs is laying the foundation for a shift in diagnostic practice in psychiatry from a psychologic symptom–based approach to an imaging-based approach over the next generation. This shift will require considerable innovations for the acquisition, analysis, and interpretation of brain images, all of which will undoubtedly require the active involvement of radiologists.
© RSNA, 2016
Introduction
Psychoradiology is a term that describes a growing intersection between the fields of psychiatry and radiology, and it is an emerging branch of radiology that is closely associated with neuroradiology and neurology. In clinical practice, psychoradiology indicates the use of radiologic approaches in patients with major psychiatric disorders and spans from diagnosis to treatment planning and monitoring. Psychiatric disorders traditionally have been classified as broad syndromes defined by patient complaint and behavioral observation rather than in terms of their underlying neurobiologic substrate. This has led to a lack of specificity when describing psychiatric syndromes and to limited success in drug development. Clearly, there is a pressing need for precision medicine in the treatment of psychiatric disorders.
One of the greatest difficulties in diagnosing and treating psychiatric disorders is that human behaviors are complex; thus, psychiatric disorders can be difficult to study in animal models when trying to understand the pathogenesis. These challenges have slowed progress in psychiatry as a field of clinical medicine; however, in recent decades, progress primarily from clinical brain imaging but also from human postmortem brain studies and genetic research has greatly increased biologic understanding of the neural substrate of these conditions. These advances provide a direction for the development of objective quantitative measures of patterns of brain abnormalities in common psychiatric disorders, such as depression and schizophrenia. Thus, neuroimaging used in the nascent field of psychoradiology is being considered not only as a way to facilitate diagnosis in terms of current standards but also as a way to add neurobiologic information to fundamentally change how the disorders themselves are defined and understood. Although psychoradiology is not yet validated to the degree necessary for clinical practice, it is a rapidly evolving field and one in which the active involvement of radiologists will be important to ensure its success.
It is commonly believed that patients with psychiatric disorders seldom have cerebral deficits that are visible at traditional diagnostic imaging examinations, such as radiography, computed tomography (CT), or conventional magnetic resonance (MR) imaging. Consequently, the role of radiology in diagnosing psychiatric disorders has been generally regarded as limited. In fact, neuroimaging research in psychiatry has yielded objective intelligible evidence to support the view that major mental illnesses are associated with intrinsic brain disorders. In 1976, the first imaging study of schizophrenia with CT revealed bilaterally enlarged ventricles, which was an important confirmation of the neuropathology of the disorder (1). Since then, many psychiatry researchers have used brain imaging to elucidate the profile of brain abnormalities associated with different psychiatric disorders. This effort accelerated considerably in recent years because of the rapid and extensive growth and development in MR imaging, molecular imaging, and other diagnostic imaging techniques.
Increased understanding of neurobiologic mechanisms of psychiatric disorders, increased technical capacities of human brain imaging, and many compelling demonstrations of differences between patients and control subjects in terms of brain anatomy and function provide the basis for the emergence of the clinical subspecialty of psychoradiology. Although clinical application of psychoradiology is not on the immediate horizon, the opportunity to leverage developments in psychiatry and radiology comes at a time when there is growing recognition of an urgent need to improve diagnostic practice for serious mental illnesses to classify patients on the basis of their biologic characteristics, clinical history, and symptoms. Furthermore, the definition of psychiatric illness has changed in the past few decades according to various versions of the Diagnostic and Statistical Manual of Mental Disorders (DSM). There are active efforts within psychiatry to shift toward the use of neurobiologic parameters to diagnose disease and individualize treatment. Given the large number of patients with psychiatric conditions and the fact that most major disorders have prevalence greater than 1%, advances in psychoradiology could have considerable effects on health care by improving the lives of many patients.
This review article will present the state of the art in this field, including (a) an overview of the imaging and analysis methods for psychoradiology; (b) a review of the most robust and important radiologic findings from studies of major psychiatric disorders, such as depression and schizophrenia, as well as the potential clinical value of these findings; and (c) a discussion of the main challenges and future directions in this field. We will restrict our discussion to selected psychiatric disorders that have been studied most extensively, including major depressive disorder (MDD), schizophrenia, bipolar disorder (BD), attention deficit hyperactivity disorder (ADHD), posttraumatic stress disorder (PTSD), and autism spectrum disorder. Diverse substance abuse effects will not be discussed. Our focus will be on anatomic and functional MR imaging studies, instead of on MR spectroscopy or positron emission tomography (PET)/single photon emission CT (SPECT) studies, for which findings are better established.
Methodologic Considerations
Two key features of the brain disorders seen in psychiatry are of special note. Cerebral deficits primarily relate to brain function rather than gross anatomic alterations, and changes are modest, requiring quantitative analysis rather than visual inspection of images. Thus, development and use of noninvasive quantitative methods to observe patterns of structural and functional cerebral changes in patients with psychiatric disorders in vivo are the primary tasks of psychoradiology.
High-spatial-resolution T1-weighted structural MR imaging is used to detect alterations in gray matter morphometry, including regional volume, cortical thickness, and shape of gyral and subcortical structures. Voxel-based analysis, a computer-based technique that can be used to identify changes in given indexes in any part of the whole brain without a prior hypothesis, is commonly used to explore gray matter changes in patients with psychiatric disorders. Moreover, the gross anatomic alterations thought to be the intrinsic aspects of psychiatric disorders are now known to change after treatment (2). White matter deficits are characterized primarily with diffusion-tensor (DT) imaging and magnetization transfer imaging. With DT imaging, MR imaging studies target regions of interest (ROIs), and by using voxel-based analysis and tract-based spatial statistics, investigators have quantified parameters, including fractional anisotropy and mean diffusivity, to identify changes in the physical properties of the fiber bundles, such as packing density, myelination, and axon diameter, in patients with psychiatric disorders (3). Both gray and white matter changes in patients with psychiatric disorders are minor and are rarely judged to be of clinical importance based on visual inspection of images. However, many studies now show that these alterations differ across patient groups and are related to illness severity and treatment outcome (4). The causes of these deficits are unclear and may include genetic factors, life experiences, prenatal challenges, medication effects, and social economic factors. However, it is of note that many abnormalities have been seen in “first-episode” patients (ie, those patients who experience the first episode of the illness before any treatment), as well as in unaffected family members of affected patients, so they appear to be fundamental to the illnesses.
Besides structural MR imaging, functional MR imaging has been widely used to identify brain functional or physiologic abnormalities in patients with psychiatric disorders. On the basis of changes in the blood oxygen level–dependent signal evoked by specific tasks, a large body of literature documents disruptions in sensory, cognitive, and affective brain circuitry, with task-related change of neural activity reflected by the increase or decrease in the blood oxygen level–dependent signal. Functional MR imaging provides an important noninvasive opportunity to evaluate neuronal activity and neural circuitry in vivo. Its use has greatly expanded understanding of human brain and behavior systems. A large number of functional MR imaging studies with different tasks have been used to identify the brain mechanisms of specific symptoms and neurocognitive impairments in patients with psychiatric disorders (5,6).
Task-based functional MR imaging, which involves an examination of changes in brain activity as a person performs a particular psychologic task, was almost exclusively used in early psychiatric imaging studies. The rationale for this approach parallels that of an exercise stress echocardiographic test, as specific functional brain systems supporting a particular psychologic process can be incrementally stressed to evaluate their functional integrity.
More recently, resting-state functional MR imaging has become widely used, as well. Evaluation of connectivity and activity of the brain while subjects rest quietly has the advantage of enabling one to probe whole-brain functional integrity, while not requiring the patient to perform a cognitive task. Low-frequency fluctuations of the blood oxygen level–dependent signal during rest have been measured to investigate temporal correlations across cortical areas that form functionally integrated brain networks and support different neurobehavioral functions. MR spectroscopy and PET/SPECT are used to obtain information about brain metabolism and chemistry. The detection of neurotransmitters, such as γ-aminobutyric acid and glutamine or glutamate, with quantitative methods is one of the most important applications of MR spectroscopy in patients with psychiatric disorders, since most psychiatric disorders are thought to involve deficits in one or both of these neurotransmitter systems. MR perfusion-weighted imaging, including the endogenous arterial spin-labeling technique, and PET/SPECT can reveal changes in resting brain perfusion as an alternative approach to evaluate changes in regional blood supply of the brain.
Although the previously mentioned modalities have been used to study most psychiatric disorders, there is no evidence that one technique is more clinically useful than the others because each modality is sensitive to different cerebral deficits. For example, gray matter atrophy of the hippocampus has been consistently reported in structural studies in patients with depressive disorder, while other studies have shown reduced activity of the hippocampus at functional MR imaging, lower concentration of N-acetyl-l-aspartic acid and glutamine at MR spectroscopy, and disrupted functional connectivity involving the hippocampus at DT imaging and resting-state functional MR imaging. Thus, studies with different modalities provide convergent evidence of hippocampal deficits in patients with depressive disorder.
To date, MR imaging has proved to be the most widely used and informative strategy for psychiatric imaging; however, other modalities may provide useful complementary information. For instance, CT has been used in patients with schizophrenia to depict ventricular enlargement (1), which in turn has been related to poorer acute treatment response and longer term clinical outcome. PET/SPECT has been useful because metabolic or perfusion changes can be detected with this modality more sensitively than with current MR techniques. This has led to the development of various hybrid forms of imaging equipment that combine the strengths of different imaging techniques, such as SPECT/CT, PET/CT, and PET/MR imaging. Magnetoencephalography and optical imaging approaches, such as functional near-infrared spectroscopy, may also be complementary. The former enables sensitive detection of cortical activity with high temporal resolution, while the latter is useful in noninvasive measurement of changes in hemoglobin concentration as an indirect measure of changes in brain function.
While image acquisition methods in psychoradiology are, for the most part, similar to those in clinical neuroradiology, albeit involving more use of functional MR imaging and different PET ligands, image analysis methods in psychoradiology are rather different. Two main strategies are used to explore the neural mechanism of psychiatric disorders. The first is to use knowledge of functional brain systems to hypothesize what is wrong in the brain on the basis of observed behavioral problems. Use of task-based functional MR imaging to probe the functional integrity of a particular brain system is the prototypical example of this approach. This approach tailors image acquisition to the problems of a particular patient and is more inherently interdisciplinary. For example, attention deficit is common in patients with ADHD, and we used the modified Stroop task (a classic task to test attention) to examine the function of the anterior cingulate cortex (a core structure for attention) in patients with ADHD (7). Although activity is typically measured across the brain, the selection of behavioral tasks of course predefines which brain regions will show increased activity. The second approach is to use standard imaging protocols to explore brain deficits in patients from whole-brain studies and then determine after the fact whether imaging findings are likely relevant for behavioral problems associated with the disorder. For example, we used resting-state functional MR imaging with voxel-based analysis to explore the functional deficits in patients with schizophrenia and found decreased activity in the medial prefrontal cortex, which was correlated with and is believed to be related to the severity of symptoms (8). The former strategy has been common in psychiatric research, especially for functional MR imaging, and the latter strategy is more standard in clinical radiology. Both strategies are widely used and have different advantages and limitations, and in time, this field will need to determine how to best exploit both approaches for psychoradiology clinical evaluations.
Since neural activity in the brain to support complex psychologic processes depends on integrated activity in widely distributed neural networks, researchers have begun to study psychiatric illness from the perspective of neural networks rather than evaluate the integrity of brain regions in isolation. Functional connectivity measurements, which reflect the interactions between brain regions, and graph theory analysis, which is a formalism to quantify topologic properties of brain networks, have shown potential in this area. Connectivity alterations are complex, and available evidence from observing patients after treatment initiation suggests that they are state dependent to a degree. Thus, they may be useful for pharmacodynamic measurements of drug effect on the brain; thus, they may serve as biomarkers in the evaluation of treatment outcome. We recently identified hyper- and hypoconnectivity of different prefrontal cortical regions in what is currently the largest sample of patients with treatment-naive schizophrenia studied in this way (n = 129) (9). Furthermore, patterns of functional connectivity changes have been related to symptom severity of schizophrenia and have shown promise in diagnostic classification (9). Also, some alterations of connectivity are seen in unaffected family members of patients with schizophrenia and may be related to genetic liability for illness (10). However, the understanding of the reliability, pathophysiologic basis, and clinical importance of these circuitry-level changes remains at an early stage of progress.
Revealing the Neural Substrate of Psychiatric Disorders
Use of anatomic and functional brain imaging has had a great effect on psychiatry, providing some of the most robust and direct demonstrations of morphologic and functional brain alterations in disorders that many had considered psychologic in nature. Over time, these approaches have yielded information on common and overlapping neural mechanisms of psychiatric disorders, including MDD, schizophrenia, BD, ADHD, PTSD, and autism spectrum disorder (Table E1 [online]). Most of the following cited articles used DSM IV as diagnostic criteria, but there have been no drastic changes in DSM criteria for the major disorders since DSM III.
Major Depressive Disorder
Anatomic and functional deficits are revealed in multiple brain regions in patients with MDD, especially prefrontal-limbic circuits (Fig 1). Our recent meta-analysis of voxel-based morphometry studies of medication-free patients with MDD identified robust gray matter decreases in prefrontal and limbic regions, mainly including the bilateral superior frontal gyrus, lateral middle temporal and inferior frontal gyri, and bilateral parahippocampal gyrus and hippocampus (11). Subcortical brain alterations have included smaller hippocampal volumes in patients with MDD, which appear to be moderated by age of onset and to be greater in patients with recurrent episodes than in those experiencing their first episode (12). Notably, the interesting finding of smaller hippocampal volume has been linked to the “neurotrophic hypothesis of depression,” which proposes that elevated glucocorticoid levels associated with chronic hyperactivity of the hypothalamic-pituitary-adrenal axis in patients with MDD may induce brain atrophy via remodeling and downregulation of growth factors, including brain-derived neurotrophic factor (13). Moreover, these anatomic changes are different (a) between patients experiencing their first episode and those with chronic disease (14) and (b) between remitted patients with MDD and those who are currently depressed (15). The variable pattern of increased and decreased cortical thickness across the brain suggests that a profile analysis of changes across the brain may be more useful for diagnosis than measured changes in any particular brain region.
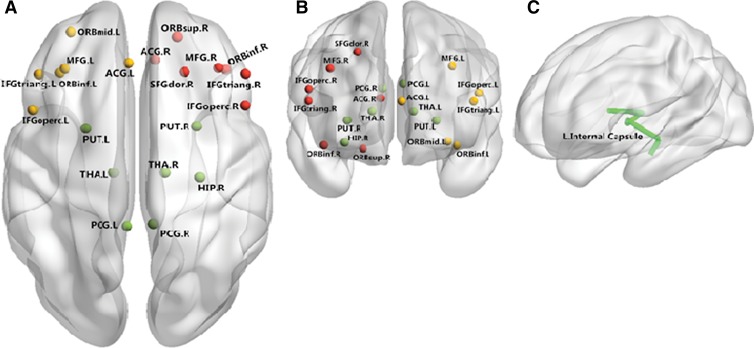
Figure 1:
Neural networks involved in patients with MDD mainly include the medial frontal cortex, temporal cortex, and superior occipital cortex. Both anatomic and functional changes are shown in the, A, superior view and, B, anterior view. Red and yellow spots represent changes in gray matter volume and function, respectively, and green spots identify regions with both functional and anatomic changes. C, Main white matter bundles (green line), with changes of integrities revealed by DT imaging. ACG.L = left anterior cingulate gyrus; ACG.R = right anterior cingulate gyrus; HIP.R = right hippocampus; IFGoperc.L = left inferior frontal gyrus, pars opercularis; IFGoperc.R = right inferior frontal gyrus, opercular part; IFGtriang.L = left inferior frontal gyrus, pars triangularis; IFGtriang.R = right inferior frontal gyrus, triangular part; MFG.L = left middle frontal gyrus; MFG.R = right middle temporal gyrus; ORBinf.L = orbital part of left Inferior frontal gyrus; ORBinf.R = orbital part of right inferior frontal gyrus; ORBmid.L = orbital part of left middle frontal gyrus; ORBsup.R = orbital part of right superior frontal gyrus; PCG.L = left posterior cingulate gyrus; PCG.R = right posterior cingulate gyrus; PUT.L = left lenticular nucleus, putamen; PUT.R = right lenticular nucleus, putamen; SFGdor.R = right superior frontal gyrus, dorsolateral; THA.L = left thalamus; THA.R = right thalamus.
White matter deficits, especially those revealed by DT imaging, have been observed in patients with mood disorders, mainly within emotion regulation circuitry (Fig 1); this finding is consistent with gray matter findings. For example, patients with depressive disorder exhibited substantially lower fractional anisotropy (FA) values in the white matter of the right middle frontal gyrus, the left lateral occipitotemporal gyrus, and the angular gyrus of the right parietal lobe than did healthy comparison subjects. Thus, white matter abnormalities may contribute to disruptions in neural circuits involved in mood regulation and therefore may contribute to the neuropathology of MDD (16). Furthermore, decreased FA in the left anterior limb of the internal capsule may reflect disease in its frontostriatal and frontothalamic projections, which could increase risk for impulsive and emotionally disinhibited behavior, such as suicide (17,18). If replicated, these findings may provide an objective biomarker for suicide risk, which is highly elevated in patients with depression or other major psychiatric disorders and represents the only major cause of mortality in psychiatry. Neuroimaging biomarkers might help identify the specific patients in need of early preventive intervention and intensive monitoring to reduce their suicide risk.
Functional and metabolic studies have provided further evidence demonstrating abnormalities in multiple distributed neural circuits in patients with depression, especially those supporting emotion regulation and reward processing. The most consistent findings involve two patterns of distinct functional abnormalities: (a) those in serotonergically modulated implicit emotion regulation neural circuitry, including the amygdala and regions in the medial prefrontal cortex, and (b) those in dopaminergically modulated reward processing circuitry, including the ventral striatum and medial prefrontal cortex (19). Previous studies used graph theory–based approaches and found that depression was characterized by lower path length and higher global efficiency, implying a shift toward randomization in brain networks that could contribute to disturbances in mood and cognition in patients with MDD (20). In addition, by using resting-state functional connectivity analysis, we discovered treatment refractory depression is associated with disrupted functional connectivity mainly in thalamocortical circuits, while nonrefractory depression is associated with more distributed decreased connectivity in the limbic-striatal-pallidal-thalamic circuit. These results suggest that nonrefractory and refractory depression may represent two distinct MDD subtypes characterized by distinct functional deficits in distributed brain networks (21). Such approaches could be of importance in the early detection of patients who are not likely to respond to first-line treatments and who require adjunctive medical and psychosocial therapies.
The potential clinical value of imaging features has begun to be systematically investigated. For example, a multisite study yielded evidence that frontotemporal functional near-infrared spectroscopy may be a useful tool with which to diagnose depression (22) and showed that this modality can be used to accurately distinguish MDD (74.6%) and two other disorders (85.5%, BD and schizophrenia). A recent study using a multiparametric classification approach based on high-resolution structural images to distinguish first-episode medication-naive adult patients with MDD from healthy control subjects showed that both volumetric and geometric parameters could be used to discriminate patients with MDD from healthy control subjects, with cortical thickness in the right hemisphere yielding the greatest diagnostic classification accuracy (78%, P < .001). These findings extend current understanding of the neuropathologic underpinnings of MDD and yield preliminary support for the use of neuroanatomic examinations in the early detection of MDD (23). Both anatomic and functional imaging features show potential to separate patients from healthy individuals, and there is overlap of the regions with anatomic and functional changes, especially in limbic regions.
Besides diagnosis, studies of treatment response prediction in depression indicate an important role for analyses focused on the amygdala. One study of cognitive behavioral therapy reported that greater pretreatment amygdala activity predicted better outcome (24), while a study of the rapid antidepressant ketamine reported the opposite effect (25), suggesting potential use of amygdala activity to guide treatment with psychosocial approaches instead of with antidepressant medication. Another study reported that greater amygdala activation in response to emotional facial expressions predicted greater reduction in depressive symptoms 8 months after different types of treatment (26). Besides the amygdala, other regions within brain circuitry supporting emotion processing, including the dorsal anterior cingulate cortex (27) and the ventrolateral prefrontal cortex (28,29), were found to have potential in predicting clinical outcome in patients with MDD (30).
Schizophrenia
Schizophrenia is another common psychiatric disorder, affecting approximately 1% of the population, and it is characterized by delusions, flat affect, hallucinations, social withdrawal, and bizarre behavior. Studies of patients with treatment-naive first-episode schizophrenia have revealed brain deficits at the onset of illness (8,31,32). Furthermore, these anatomic deficits may affect functional networks, which subsequently may represent the proximate cause of the clinical symptoms (8). Findings from longitudinal studies of first-episode schizophrenia and comparison of findings in patients with first-episode schizophrenia and those with chronic disease suggest considerable variability in patterns of anatomic changes at the early phase of illness, smaller deficits in patients with first-episode schizophrenia than in those with chronic disease, and some regional progression of brain changes over the longer term course of illness (33). Some of these changes appear to relate to clinical manifestations. For example, patients with prominent negative symptoms, such as affective flattening, avolition, and apathy, have been reported to show greater reductions of gray matter volume in the temporal lobe (31). Regions such as the medial prefrontal cortex, striatum, and thalamus are within the dopamine pathway, which is both a treatment target and a system implicated in the pathogenesis of schizophrenia (Fig 2). There are other regions where illness effects have been seen, including the parietal and occipital regions, that do not receive prominent dopaminergic innervation (Fig 2). This suggests a complex pathophysiologic process with diverse brain effects.
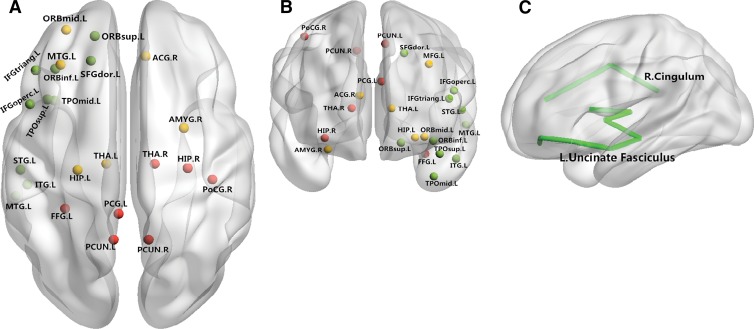
Figure 2:
Neural networks involved in schizophrenia mainly include the prefrontal cortex, temporal cortex, and thalamus. Both anatomic and functional changes are shown in the, A, superior view and, B, anterior view. Red and yellow spots represent changes in gray matter volume and function respectively; green spots indicate regions with both functional and anatomic changes. C, Main white matter bundles (green line), with changes of integrities revealed by DT imaging. ACG.R = right anterior cingulate gyrus; FFG.L = left fusiform gyrus; HIP.L = left hippocampus; HIP.R = right hippocampus; IFGoperc.L = left inferior frontal gyrus, pars opercularis; IFGtriang.L = left inferior frontal gyrus, pars triangularis; ITG.L = left inferior temporal gyrus; MFG.L = left middle frontal gyrus; MTG.L = left middle temporal gyrus; ORBinf.L = orbital part of left inferior frontal gyrus; ORBmid.L = orbital part of left middle frontal gyrus; ORBsup.L = orbital part of left superior frontal gyrus; PCG.L = left posterior cingulate gyrus; PCUN.L = left precuneus; PCUN.R = right precuneus; PoCG.R = right postcentral gyrus; SFGdor.L = left superior frontal gyrus, dorsolateral; STG.L = left superior temporal gyrus; THA.L = left thalamus; TPOmid.L = left temporal pole, middle temporal gyrus; TPOsup.L = left temporal pole, superior temporal gyrus.
Similarly, studies of white matter in patients with first-episode schizophrenia indicate widespread abnormalities across white matter tracts (34), with evidence for reductions in FA in the uncinate (35), cingulum (36), fornix (37), corpus callosum (38), and inferior longitudinal fasciculus (39); however, negative results also have been reported (40). The inconsistent localization of findings is reflected in a relatively recent meta-analysis of FA findings in patients with schizophrenia, as reported in 23 published articles, where nonoverlapping and scattered findings were seen throughout white matter tracts (41). As with morphometric studies, this variability is likely due to factors, such as differences in image acquisition and analysis, small sample sizes, variable duration of illness, and illness heterogeneity.
Findings also suggest that the most robust changes in brain function occur primarily in regions different from those where anatomic findings have been identified (42–44). Hypofunction of the medial prefrontal cortex and hyperactivity of the hippocampus and striatum were reported in patients with first-episode schizophrenia before treatment and may in time provide biomarkers for the disorder and targets for treatment (42). While apparently beneficial and adverse functional changes have been seen after antipsychotic treatment in some brain regions, such as increased activity of the medial prefrontal cortex and disrupted connectivity within the prefrontal-parietal network (45), other alterations in anatomy and function appear to remain relatively stable early in the course of illness after treatment and clinical stabilization, as do cognitive deficits (46). While progression of brain deficits in patients with schizophrenia is not substantial in the early course of illness, they do appear to occur in some brain regions in the decades after illness onset (33). Recent evidence is beginning to identify associations between neuroimaging findings and genetic factors, and the clarification of these associations is among the most important directions for future research in this area (47).
Some relatively recent studies have examined the potential role of imaging features in the clinical diagnosis of schizophrenia. Three studies (48–50) have shown that volume reduction in prefrontal and temporal regions was the main anatomic difference between patients and control subjects, separating groups with a classification accuracy of 75%–90%. Although these findings are encouraging, the interpretation of the results is hindered by potential confounding effects of drug treatments, long duration of illness, and small sample sizes. MR imaging studies have shown that antipsychotic drugs can cause gray matter loss in the neocortex, although potential compensatory effects involving increased striatal volumes have been observed (51,52). Although the effects of different antipsychotic drugs on brain anatomy and function are not well established, these imaging changes have been used to predict the treatment response (53–56).
Bipolar Disorder
BD, known previously as manic-depressive illness, is characterized by periods of elevated mood and periods of depression. Recovery between episodes of acute emotional disturbance is variable. BD shares numerous clinical features with both depressive disorder and schizophrenia. Imaging findings indicate some common cerebral deficits across patients with BD and those with schizophrenia, especially in the approximately 50% of patients with BD who have a history of psychosis. Imaging also reveals some disease-specific abnormalities that enable readers to distinguish BD and schizophrenia. At morphometric analysis, gray matter has been shown to be reduced in the area of the posterior cingulate and retrosplenial cortex and in the superior temporal gyrus in unmedicated subjects with BD relative to these same areas in medicated subjects with BD, as well as in the lateral orbital cortex in medicated subjects with BD relative to control subjects (57) (Fig 3). Some of the anatomic changes in BD, including changes in the amygdala and hippocampal regions, are common findings in patients with MDD (58,59). These findings support the hypothesis that the limbic system, particularly the hippocampus, may be involved in the pathophysiology of affective disorders more generally (58). A resting-state functional MR imaging study revealed that both BD and schizophrenia shared regional and connectivity deficits within striatal-thalamo-cortical networks, while patients with schizophrenia showed more and greater regional functional deficits in the thalamocortical systems (10). The pattern of both overlapping and distinctive abnormalities in patients with schizophrenia and those with BD suggests a model for rapprochement between continuum views of psychosis and views that the disorders are fully distinct.
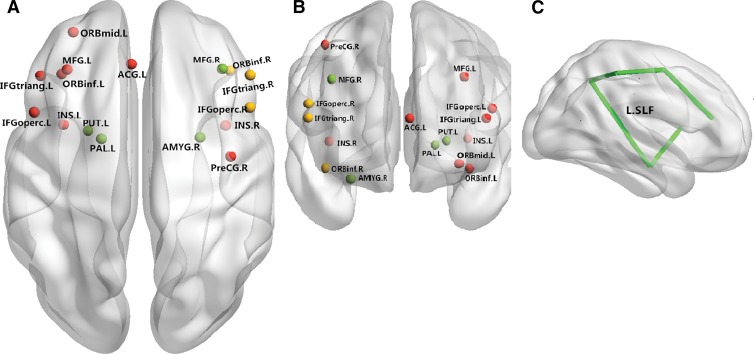
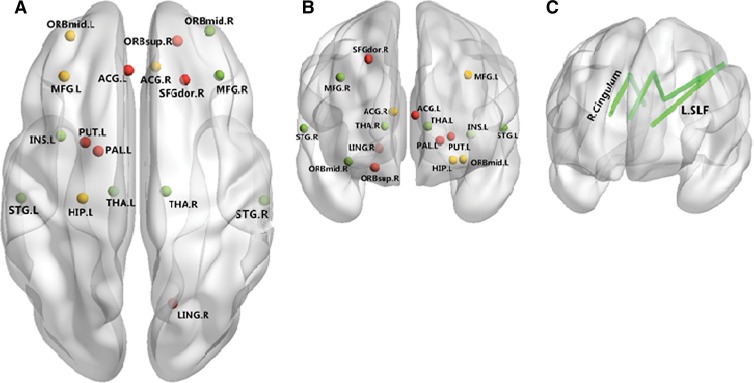
Figure 3:
Neural networks involved in BD mainly include the inferior frontal cortex and limbic areas. Both anatomic and functional changes are shown in the, A, superior view and, B, anterior view. Red spots and yellow spots represent changes in gray matter volume and function, respectively; green spots indicate regions with both functional and anatomic changes. C, Main white matter bundles (green line), with changes of integrities revealed by DT imaging. ACG.L = left anterior cingulate gyrus; AMYG.R = right amygdala; IFGoperc.L = left inferior frontal gyrus, pars opercularis; IFGoperc.R = right inferior frontal gyrus, pars opercularis; IFGtriang.L = left inferior frontal gyrus, pars triangularis; IFGtriang.R = right inferior frontal gyrus, pars triangularis; INS.L = left insula; INS.R = right insula; L.SLF = left superior longitudinal fasciculus; MFG.L = left middle frontal gyrus; MFG.R = right middle frontal gyrus; ORBinf.L = orbital part of left inferior frontal gyrus; ORBinf.R = orbital part of right inferior frontal gyrus; ORBmid.L = orbital part of left middle frontal gyrus; PAL.L = left lenticular nucleus, pallidum; PreCG.R = right precentral gyrus; PUT.L = left lenticular nucleus, putamen.
White matter deficits, including decreased FA in the corpus callosum (60), posterior cingulum (61), superior-frontal white matter tracts (62), and anterior corona radiata (3), have been reported in patients with BD. The consistent finding of abnormalities in white matter tracts of the genu of the corpus callosum is similar to findings in patients with MDD, suggesting a disconnectivity of the bilateral prefrontal cortex related to mood disregulation in both patients with BD and those with MDD. Of note, white matter alterations appear to have a different pattern depending on whether the illness has an onset in adolescence or adulthood (63), and at the time of illness onset, white matter changes can be as pronounced as those in patients with schizophrenia (63).
Consistent with the anatomic findings, functional brain deficits in patients with BD also have been reported; these mainly include reductions in activation in the right ventral lateral prefrontal cortex, amygdala, and anterior cingulate cortex (Table E1 [online]). The amygdala dysfunction may represent a state marker of bipolar illness, whereas ventral lateral prefrontal cortex dysfunction may be independent of mood state and may represent a trait marker of the illness (64); however, this pattern is less evident in pediatric patients (65). The functional connectivity between the posterior anterior cingulate cortex and the amygdala in patients with BD also has been found to be disrupted during emotion processing, which may be caused by disruptions in white matter connectivity of the posterior anterior cingulate cortex and amygdala (66).
MR imaging has proven to have high value in understanding and evaluating brain alterations in pediatric patients with psychiatric disorders. With task-based functional MR imaging studies, emotion processing problems implicated in pediatric patients with BD have been identified, mainly involving the activity of the amygdala and prefrontal regions (67,68). Apart from the illness-related deficits, treatment effect also has been revealed. For example, Pavuluri et al (69) found that second-generation antipsychotics showed enhanced prefrontal and temporal lobe activity in patients with adolescent BD. These deficits have promise as potential biomarkers in the clinical diagnosis of and treatment planning for BD (70).
Imaging features also appear to have potential clinical value in patients with BD, including in the prediction of illness onset and response to different treatment strategies. For example, a functional MR imaging study investigated BD probands and their relatives and reported that increased activity levels in the orbitofrontal cortex and amygdala were related to heightened sensitivity to reward and deficient prediction error signals, suggesting these neural alterations disrupt positive and negative reward signals and that they may represent candidate familial endophenotypes of BD. The results support a role of motivational processing in the risk architecture of BD (71) and enable us to identify a new systems-level therapeutic target for the illness. The right inferior frontal gyrus volume could aid in identification of subjects at risk for BD even before any behavioral manifestations (72). These findings suggest that focusing on genes controlling white matter integrity and function may be a fruitful strategy in the quest to discover vulnerability genes for BD and to develop novel treatment strategies (73).
Attention Deficit Hyperactivity Disorder
ADHD is a common neurodevelopmental psychiatric disorder with childhood onset that is characterized by attention deficit, hyperactivity, and behavioral impulsiveness. Children with ADHD have been shown to have decreased gray matter volume in the bilateral dorsolateral prefrontal cortex and cerebellum and progressive symptom-related volume loss in the inferior-posterior lobule of the cerebellum (74). Regional gray matter volume deficits in the right orbitofrontal cortex, right primary motor or premotor cortex, left anterior cingulate cortex, and left posterior midcingulate cortex in children with ADHD who were drug naive and were without comorbidities might result in executive cognitive dysfunction and therefore might underlie some behavioral manifestations of ADHD (75). DT imaging also showed disrupted structural integrity of white matter neural pathways, which connect frontostriatal and frontoparietal circuits and thus may underpin disturbances in associated functional networks in children with ADHD. Some studies have shown that FA of frontostriatal tracts was decreased in patients with ADHD when compared with that in control subjects; this finding is consistent with the view that frontostriatal alterations are relevant to ADHD-related behaviors (76). These findings add to an emerging picture of abnormal development within frontoparietal cortical networks that may underpin the cognitive and attentional disturbances associated with ADHD (77).
As for functional changes, patients with ADHD have been shown to have abnormal activity in the orbitofrontal, middle temporal, and dorsolateral prefrontal cortex (Fig 4). Hypoactivation in the inferior frontal cortex in patients with ADHD has been related to impaired behavioral response inhibition, which is a central neurobehavioral feature of ADHD (78). Hyperactivation in the striatum and mediotemporal areas during working memory tasks suggests that atypical corticostriatal circuitry may be an important component in the pathyphysiology of ADHD (7). Besides cerebral functional changes, default mode network regions in the cerebellum have shown positive functional connectivity (in contrast to what is typically negative functional connectivity in control subjects), with widespread regions of salience, dorsal attention, and sensorimotor networks. This provides evidence of impaired cerebellar default mode network coupling with cortical networks in patients with ADHD and highlights a role of cerebrocerebellar interactions in patients with this disease. This circuitry has been proposed as a target for therapeutic interventions in patients with ADHD (79). By using multivariate analysis, one previous study applied pattern classification to task-based functional MR imaging of behavioral inhibition to accurately identify 77% of patients with ADHD (80).
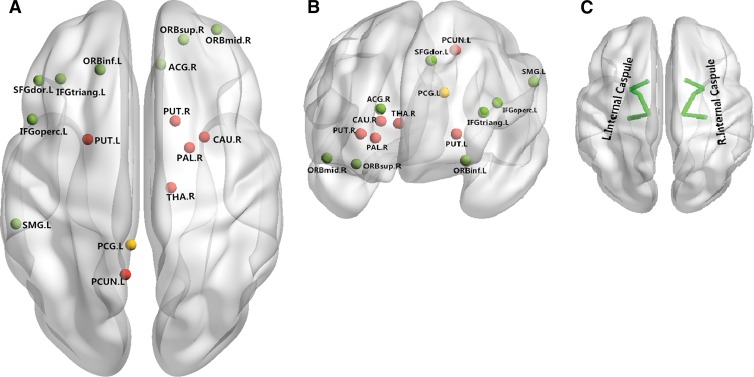
Figure 4:
Neural networks involved in ADHD mainly include the superior frontal cortex, inferior frontal cortex, and basal ganglia. Both anatomic and functional changes are shown in the, A, superior view and, B, anterior view. Red and yellow spots represent changes in gray matter volume and function, respectively; green spots indicate regions with both functional and anatomic changes. C, Main white matter bundles (green line), with changes of integrities revealed by DT imaging. ACG.R = right anterior cingulate gyrus; CAU.R = right caudate nucleus; IFGoperc.L = left inferior frontal gyrus, opercular part; IFG triang.L = left inferior frontal gyrus, triangular part; ORB inf.L = orbital part of left inferior frontal gyrus; ORBmid.R = orbital part of right middle frontal gyrus; ORB.sup.R = orbital part of right superior frontal gyrus; PAL.R = right lenticular nucleus, pallidum; PCG.L = left posterior cingulate gyrus; PCUN.L = left precuneus; PUT.L = left lenticular nucleus, putamen; PUT.R = right lenticular nucleus, putamen; SFGdor.L = left superior frontal gyrus; SMG.L = left supramarginal gyrus; THA.R = right thalamus.
Posttraumatic Stress Disorder
PTSD is a debilitating psychiatric disorder that follows a severe stressful life experience, such as military combat or a natural disaster. The symptoms of flashbacks and anxiety can persist for years after the trauma. In patients with PTSD, the most studied structure is the hippocampus because of established effects of stress on this brain region in animal models. PTSD is associated with abnormalities in multiple frontal-limbic system structures (81). Most studies have found that patients with PTSD have a smaller volume of the hippocampus, which has been related to symptom severity and illness duration (82–85) (Table E1 [online]). However, early in the course of PTSD, increased cortical thickness in the right superior temporal gyrus, inferior parietal lobule, and left precuneus were observed; these may have resulted from neuroinflammatory or other trophic processes related to endocrine changes or functional compensation (86). In pediatric patients with maltreatment-related PTSD, the decreased hippocampal volumes were not seen (87); instead, these patients had smaller intracranial, cerebral, and prefrontal and right temporal cortex volumes, as well as white matter alterations in the prefrontal cortex and subregions of the corpus callosum (88). Our team recently conducted a meta-analysis, which revealed that PTSD symptom severity was negatively correlated with gray matter in the left anterior cingulate cortex and positively correlated with gray matter in the left insula (89). We have used DT imaging to investigate children with PTSD, and our findings suggest that pediatric PTSD is accompanied by a connectivity disequilibrium between the salience and default-mode networks, which is a finding of potential pathophysiologic importance (90).
Functional studies have shown reduced activation of the thalamus, anterior cingulate gyrus, and medial frontal gyrus relative to those in healthy control subjects (91). In contrast, increased activation has been seen in the left hippocampus (92), amygdala (93), and visual cortex; these findings are positively correlated with re-experiencing trauma or avoidance symptoms in patients with PTSD (94). Furthermore, our team used relevance vector regression to examine the relationship between resting-state functional MR imaging data and symptom scores and found that accurate identification of patients with PTSD was based on functional activation in a number of prefrontal, parietal, and occipital regions; this finding enabled us to confirm that PTSD is a disorder specific to the frontolimbic networks (95). The pattern of observed network alterations largely overlapped with the salience, central executive, and default mode networks (96,97), as well as with the somatomotor, auditory, and visual networks (98) (Fig 5). We also found substantial alterations in brain function that are similar in many ways to those observed in patients with PTSD in individuals shortly after major traumatic experiences, highlighting the need for early evaluation and intervention in survivors of trauma (99). However, we also found long-term changes in neural networks involved in core aspects of self-processing and cognitive and emotional functioning in disaster survivors that were independent of anxiety symptoms (100).
Figure 5:
Neural networks involved in PTSD include the dorsolateral frontal cortex and inferior frontal cortex. Both anatomic and functional changes are shown in the, A, superior view and, B, anterior view. Red and yellow spots represent changes in gray matter volume and function, respectively; green spots indicate regions with both functional and anatomic changes. C, Main white matter bundles (green line), with changes of integrities revealed by DT imaging. ACG.L = left anterior cingulate gyrus; ACG.R = right anterior cingulate gyrus; HIP.L = left hippocampus; INS.L = left insula; LING.R = right lingual gyrus; L.SLF = left superior longitudinal fasciculus; MFG.L = left middle frontal gyrus; MFG.R = right middle frontal gyrus; ORBmid.L = orbital part of left middle frontal gyrus; ORBmid.R = orbital part of right middle frontal gyrus; ORB sup.R = orbital part of right superior frontal gyrus; PAL.L = left lenticular nucleus, pallidum; PUT.L = left lenticular nucleus, putamen; SFGdor.R = right superior frontal gyrus, dorsolateral; STG.L = left superior temporal gyrus; STG.R = right superior temporal gyrus; THA.L = left thalamus; THA.R = right thalamus.
These imaging features also showed potential value in prediction of illness onset. For example, Shin et al (101) reported that hyperresponsivity in the dorsal anterior cingulate appears to be a familial risk factor for the development of PTSD after psychologic trauma. In one of our previous studies, we found that patterns of neuroanatomic alternations could be used to identify trauma survivors with PTSD and those without (102). Volume reductions in the hippocampus may hold promise as an approach to monitor therapeutic outcomes (103).
Critical Challenges and Future Directions
Several key questions for psychiatry researchers included how to characterize disease-specific neural deficits to understand illness mechanisms, how to develop a neurobiologically based diagnostic system for major mental illness, and how to use mechanistic understanding coupled with new diagnostic classifications based on shared neural system deficits to advance precision medicine. Psychoradiology will play a key role in all three aspects, as findings from psychiatric imaging research are translated to reshape clinical practice.
Progress in this area will continue as several challenges are addressed. It is now clear that unlike imaging findings of many neurologic diseases in young adults, imaging findings in patients with psychiatric disorders indicate the disease affects multiple widespread brain regions. While regions affected overlap across psychiatric syndromes (Table E1 [online]), some distinct patterns of deficits have been identified that are related to the severity of specific symptoms. Examples of illness-specific alterations include the smaller hippocampus in patients with MDD, hypoactivity of ventral medial prefrontal regions in patients with schizophrenia, hypoactivity of the anterior cingulate cortex in patients with ADHD, and hyperactivity of the amygdala in patients with PTSD or anxiety disorders.
The observation that several psychiatric disorders, including MDD, schizophrenia, and ADHD, share anatomic and functional deficits in brain networks in the default mode network, salience network, emotional regulation network, and central executive network is perhaps not surprising. Psychiatric syndromes have similar neurocognitive deficits (104) and overlapping patterns of emotional disturbances. In particular, the emotion network, which mainly involves the prefrontal limbic circuit and the default mode network were the most vulnerable networks in all of the psychiatric disorders. It has become widely accepted that psychiatric syndromes as currently defined represent heterogeneous and overlapping syndromes clinically, neurobiologically, and genetically. Developments in psychoradiology offer a pathway to directly address this problem. One promising way forward is to identify distinct patterns of brain abnormalities that define neurobiologically distinct subtypes of the disorders and to relate them to different cognitive, affective, and behavioral clinical manifestations and to differential treatment outcome profiles. For example, Kitis et al (105) reported FA reduction in the left uncinate fasciculus, and Rowland et al (106) found lower FA localized to the right superior longitudinal fasciculus and middle frontal white matter in patients with deficit syndrome schizophrenia; such patients are particularly functionally impaired and unresponsive to treatment.
Current symptom-based subtyping strategies for schizophrenia, depression, and autism have been criticized for their instability over time and for their lack of distinct neural system alterations and treatment implication (107). Thus, an alternative approach resolving the heterogeneity with neurobiologic parameters, such as anatomic and functional imaging features, may represent an advantageous strategy to identify subgroups of patients, after which the clinical dimensions of these subgroups in terms of symptoms and treatment response to different therapies can be examined. Recently, Sun et al (108) have used a data-driven patient-clustering method to identify two distinct patterns of white matter abnormalities in the early phase of schizophrenia, suggesting qualitatively distinct genetic influences or neurodevelopmental alterations. This is consistent with the broad aims of the Research Domain Criteria project from the National Institute of Mental Health, which focus on using measurable units of analysis to resolve neurobiologic heterogeneity and on incorporating such strategies into clinical diagnostic practice. The potential to combine imaging findings with neurophysiologic and neurocognitive deficits also appears promising.
From a clinical practice perspective, the primary challenge in developing the field of psychoradiology is to establish the clinical utility of imaging biomarkers for differential diagnoses and therapies. The clinical relevance of neuroimaging biomarkers that have been identified over 2 decades of MR imaging research in psychiatry now needs to be validated for use in clinical practice by establishing the contribution of these biomarkers to differential diagnosis and treatment planning. However, MR imaging data can vary across sites, as can patient populations; a major challenge will be to validate these imaging biomarkers by using large data sets obtained via multisite studies. The aim will be to refine proof-of-concept findings available to date into calibrated diagnostic, prognostic, and predictive laboratory tests. This effort will require a combination of data from different sites and the use of advanced quantitative image analysis techniques. The development of standard imaging protocols across imagers and the use of macromolecular tissue volume techniques can help reduce confounding variables from different imagers at different sites (109). Standardized procedures for patient selection and clinical assessment also will be needed. Standardized data analysis, including data preprocessing methods, whole-brain and ROI analyses, novel quantitative approaches, and advanced statistical methods, will be required. The development of fast multimodal imaging can maximize the use of multimodality data within a clinically acceptable image acquisition time. For example, MR T1 and T2 mapping data can be acquired much more quickly with an MR fingerprinting sequence developed by Ma et al (110), and multiband techniques can also considerably improve the temporal resolution for functional MR imaging (111).
Investigators in psychoradiology need to explore the relationships in a vast amont of imaging data. A challenge that lies ahead is how to maximize the data mining for very large imaging data sets and other types of biomedical data sets to best advance patient care (112,113). New analysis methods have been developed to integrate multimodal information from big data sets for programs such as the Human Connectome Project, the Enhancing Neuro Imaging Genetics by Meta-Analysis, and the Alzheimer’s Disease Neuroimaging Initiative. One possible way to integrate multimodal findings is to study psychiatric disease from the perspective of neural networks, since neural activity in the brain supporting complex psychologic processes depends on integrated activity in widely distributed networks.
Changes in functional brain networks have been proven to be sensitive to therapeutic interventions with medications (114). Brain connectome analysis may help us identify depressed subjects (20), and brain connectivity patterns may enable the early differentiation of treatment refractory from treatment-responsive patients (115).
Interdisciplinary teams involving psychiatry, psychology, physics, biochemistry, mathematics, computer science, and psychoradiology are needed to integrate MR studies into clinical trials so that the utility of the quantitative imaging measures can be determined clinically.
Finally, characterization of imaging biomarkers associated with psychiatric illness can facilitate diagnostic and therapeutic practice by providing an objective way to select patients for optimal therapies and to track treatment effects on brain systems. This will involve not only drug therapies but also nonpharmacologic treatments that affect disrupted neural circuits. For example, vagal nerve stimulation, rapid transcranial magnetic stimulation, and deep brain stimulation have been used to modulate the function of emotional circuitry, including subgenual cingulate cortex in the brain of patients with depression (116).
Taken together, on the basis of thousands of imaging studies of psychiatric disorders in recent years, more active involvement of diagnostic and interventional radiology in investigating the neural mechanisms of psychiatric disorders and applying imaging tools to advance diagnosis and therapeutic intervention has become increasingly urgent (43,44,116). Although there are many problems to be solved, we appear to be at the cusp of having the opportunity to translate new MR research findings into clinical practice for psychiatric disorders. By combining MR imaging with PET/SPECT, magnetoencephalography, and other imaging modalities, psychoradiology has great promise as a new branch of radiology. This will call for new collaborative teams in a very exciting and promising line of work, and it will attract researchers from multiple disciplines. The success of this effort could greatly improve medical care for millions of patients and could incrementally transform psychiatric diagnostic practice from a set of symptom-based syndromes to an imaging-based nosology over the next generation.
Essentials
■ A primary task of psychoradiology is to develop and use noninvasive quantitative methods to observe patterns of structural and functional cerebral changes in patients with psychiatric disorders in vivo.
■ Examples of illness-specific alterations include a smaller hippocampus in patients with major depressive disorder, hypoactivity of ventral medial prefrontal regions in patients with schizophrenia, and hyperactivity of the amygdala in patients with posttraumatic stress disorder or an anxiety disorder.
■ The observation that several psychiatric disorders, including major depressive disorder, schizophrenia, and attention deficit hyperactivity disorder, share anatomic and functional deficits in brain networks in the default mode network, salience network, emotional regulation network, and central executive cognitive network is perhaps not surprising, given the overlap in emotional and cognitive deficits across disorders.
■ Psychoradiology can improve medical care for millions of patients and can incrementally transform psychiatric diagnostic practice from a set of broad symptom-based syndromes to an imaging-based nosology over the next generation.
SUPPLEMENTAL TABLE
Acknowledgments
Acknowledgment
Presented in part by Dr Gong as the NIBIB New Horizons Lecture at the 2015 ISMRM Annual Meeting and Exhibition.
Received October 2, 2015; revision requested November 16; revision received December 31; accepted January 14, 2016; final version accepted January 20; final review July 14.
Supported by the National Natural Science Foundation of China (81621003, 81222018, 81220108013, 81227002, 81030027), the von Humboldt Foundation, the National Institutes of Health (MH077862), and the Changjiang Scholar Professorship Awards of China (Q2015154, T2014190).
Current address: Department of Psychiatry, University of Cincinnati, Cincinnati, Ohio.
Disclosures of Conflicts of Interest: S.L. disclosed no relevant relationships. X.J.Z. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: received a grant from Abbvie Pharmaceuticals and is an owner of Horizon Medical Physics Services. Other relationships: disclosed no relevant relationships. J.A.S. disclosed no relevant relationships. Q.G. disclosed no relevant relationships.
Abbreviations:
- ADHD
- attention deficit hyperactivity disorder
- BD
- bipolar disorder
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- DT
- diffusion tensor
- FA
- fractional anisotropy
- MDD
- major depressive disorder
- PTSD
- posttraumatic stress disorder
- ROI
- region of interest
References
- 1.Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L. Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 1976;2(7992):924–926. [DOI] [PubMed] [Google Scholar]
- 2.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 3.Pavuluri MN, Yang S, Kamineni K, et al. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention-deficit/hyperactivity disorder. Biol Psychiatry 2009;65(7):586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med 2010;40(9):1409–1422. [DOI] [PubMed] [Google Scholar]
- 5.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. a systematic review of neuroimaging studies. Neurosci Biobehav Rev 2013;37(10 Pt 2):2529–2553. [DOI] [PubMed] [Google Scholar]
- 6.McCleery A, Lee J, Joshi A, Wynn JK, Hellemann GS, Green MF. Meta-analysis of face processing event-related potentials in schizophrenia. Biol Psychiatry 2015;77(2):116–126. [DOI] [PubMed] [Google Scholar]
- 7.Li Y, Li F, He N, et al. Neural hyperactivity related to working memory in drug-naive boys with attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry 2014;53:116–122. [DOI] [PubMed] [Google Scholar]
- 8.Lui S, Deng W, Huang X, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry 2009;166(2):196–205. [DOI] [PubMed] [Google Scholar]
- 9.Anticevic A, Hu X, Xiao Y, et al. Early-course unmedicated schizophrenia patients exhibit elevated prefrontal connectivity associated with longitudinal change. J Neurosci 2015;35(1):267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lui S, Yao L, Xiao Y, et al. Resting-state brain function in schizophrenia and psychotic bipolar probands and their first-degree relatives. Psychol Med 2015;45(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao YJ, Du MY, Huang XQ, et al. Brain grey matter abnormalities in medication-free patients with major depressive disorder: a meta-analysis. Psychol Med 2014;44(14):2927–2937. [DOI] [PubMed] [Google Scholar]
- 12.Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016;21(6):806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell S, Macqueen G. The role of the hippocampus in the pathophysiology of major depression. J Psychiatry Neurosci 2004;29(6):417–426. [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu L, Lui S, Kuang W, et al. Regional increases of cortical thickness in untreated, first-episode major depressive disorder. Transl Psychiatry 2014;4:e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salvadore G, Nugent AC, Lemaitre H, et al. Prefrontal cortical abnormalities in currently depressed versus currently remitted patients with major depressive disorder. Neuroimage 2011;54(4):2643–2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma N, Li L, Shu N, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry 2007;164(5):823–826. [DOI] [PubMed] [Google Scholar]
- 17.Jia Z, Huang X, Wu Q, et al. High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry 2010;167(11):1381–1390. [DOI] [PubMed] [Google Scholar]
- 18.Jia Z, Wang Y, Huang X, et al. Impaired frontothalamic circuitry in suicidal patients with depression revealed by diffusion tensor imaging at 3.0 T. J Psychiatry Neurosci 2014;39(3):170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillips ML, Chase HW, Sheline YI, et al. Identifying predictors, moderators, and mediators of antidepressant response in major depressive disorder: neuroimaging approaches. Am J Psychiatry 2015;172(2):124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Wang J, Wu Q, et al. Disrupted brain connectivity networks in drug-naive, first-episode major depressive disorder. Biol Psychiatry 2011;70(4):334–342. [DOI] [PubMed] [Google Scholar]
- 21.Lui S, Wu Q, Qiu L, et al. Resting-state functional connectivity in treatment-resistant depression. Am J Psychiatry 2011;168(6):642–648. [DOI] [PubMed] [Google Scholar]
- 22.Takizawa R, Fukuda M, Kawasaki S, et al. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage 2014;85(Pt 1):498–507. [Published correction appears in Neuroimage 2015;109:530.] [DOI] [PubMed] [Google Scholar]
- 23.Qiu L, Huang X, Zhang J, et al. Characterization of major depressive disorder using a multiparametric classification approach based on high resolution structural images. J Psychiatry Neurosci 2014;39(2):78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry 2006;163(4):735–738. [DOI] [PubMed] [Google Scholar]
- 25.Salvadore G, Cornwell BR, Colon-Rosario V, et al. Increased anterior cingulate cortical activity in response to fearful faces: a neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol Psychiatry 2009;65(4):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canli T, Cooney RE, Goldin P, et al. Amygdala reactivity to emotional faces predicts improvement in major depression. Neuroreport 2005;16(12):1267–1270. [DOI] [PubMed] [Google Scholar]
- 27.Chen CH, Ridler K, Suckling J, et al. Brain imaging correlates of depressive symptom severity and predictors of symptom improvement after antidepressant treatment. Biol Psychiatry 2007;62(5):407–414. [DOI] [PubMed] [Google Scholar]
- 28.Light SN, Heller AS, Johnstone T, et al. Reduced right ventrolateral prefrontal cortex activity while inhibiting positive affect is associated with improvement in hedonic capacity after 8 weeks of antidepressant treatment in major depressive disorder. Biol Psychiatry 2011;70(10):962–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry 2008;13(9):829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Ren L, Womer FY, et al. Alterations in amplitude of low frequency fluctuation in treatment-naïve major depressive disorder measured with resting-state fMRI. Hum Brain Mapp 2014;35(10):4979–4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry 2013;170(11):1308–1316. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Y, Lui S, Deng W, et al. Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia. Schizophr Bull 2015;41(1):201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang W, Deng W, Yao L, et al. Brain structural abnormalities in a group of never-medicated patients with long-term schizophrenia. Am J Psychiatry 2015;172(10):995–1003. [DOI] [PubMed] [Google Scholar]
- 34.Szeszko PR, Ardekani BA, Ashtari M, et al. White matter abnormalities in first-episode schizophrenia or schizoaffective disorder: a diffusion tensor imaging study. Am J Psychiatry 2005;162(3):602–605. [DOI] [PubMed] [Google Scholar]
- 35.Mandl RC, Rais M, van Baal GC, et al. Altered white matter connectivity in never-medicated patients with schizophrenia. Hum Brain Mapp 2013;34(9):2353–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubicki M, Westin CF, Nestor PG, et al. Cingulate fasciculus integrity disruption in schizophrenia: a magnetic resonance diffusion tensor imaging study. Biol Psychiatry 2003;54(11):1171–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuroki N, Kubicki M, Nestor PG, et al. Fornix integrity and hippocampal volume in male schizophrenic patients. Biol Psychiatry 2006;60(1):22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotarska-Jagiela A, Schönmeyer R, Oertel V, Haenschel C, Vogeley K, Linden DE. The corpus callosum in schizophrenia: volume and connectivity changes affect specific regions. Neuroimage 2008;39(4):1522–1532. [DOI] [PubMed] [Google Scholar]
- 39.Ashtari M, Cottone J, Ardekani BA, et al. Disruption of white matter integrity in the inferior longitudinal fasciculus in adolescents with schizophrenia as revealed by fiber tractography. Arch Gen Psychiatry 2007;64(11):1270–1280. [DOI] [PubMed] [Google Scholar]
- 40.Price G, Bagary MS, Cercignani M, Altmann DR, Ron MA. The corpus callosum in first episode schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 2005;76(4):585–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melonakos ED, Shenton ME, Rathi Y, Terry DP, Bouix S, Kubicki M. Voxel-based morphometry (VBM) studies in schizophrenia: can white matter changes be reliably detected with VBM? Psychiatry Res 2011;193(2):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong Q, Lui S, Sweeney JA. A selective review of cerebral abnormalities in patients with first-episode schizophrenia before and after treatment. Am J Psychiatry 2016;173(3):232–243. [DOI] [PubMed] [Google Scholar]
- 43.Sarpal DK, Lencz T, Malhotra AK. In support of neuroimaging biomarkers of treatment response in first-episode schizophrenia. Am J Psychiatry 2016;173(7):732–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gong Q. Response to Sarpal et al: importance of neuroimaging biomarkers for treatment development and clinical practice. Am J Psychiatry 2016;173(7):733–734. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Lui S, Yao L, et al. Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional MR imaging study. Radiology 2016;279(3):867–875. [DOI] [PubMed] [Google Scholar]
- 46.Hill SK, Schuepbach D, Herbener ES, Keshavan MS, Sweeney JA. Pretreatment and longitudinal studies of neuropsychological deficits in antipsychotic-naïve patients with schizophrenia. Schizophr Res 2004;68(1):49–63. [DOI] [PubMed] [Google Scholar]
- 47.Meda SA, Ruaño G, Windemuth A, et al. Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc Natl Acad Sci U S A 2014;111(19):E2066–E2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellani U, Rossato E, Murino V, et al. Classification of schizophrenia using feature-based morphometry. J Neural Transm (Vienna) 2012;119(3):395–404. [DOI] [PubMed] [Google Scholar]
- 49.Fan Y, Liu Y, Wu H, et al. Discriminant analysis of functional connectivity patterns on Grassmann manifold. Neuroimage 2011;56(4):2058–2067. [DOI] [PubMed] [Google Scholar]
- 50.Pohl KM, Sabuncu MR. A unified framework for MR based disease classification. Inf Process Med Imaging 2009;21:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 2011;68(2):128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? a systematic and critical review of MRI findings. Psychol Med 2009;39(11):1763–1777. [DOI] [PubMed] [Google Scholar]
- 53.Bodnar M, Malla AK, Joober R, et al. Neural markers of early remission in first-episode schizophrenia: a volumetric neuroimaging study of the parahippocampus. Psychiatry Res 2012;201(1):40–47. [DOI] [PubMed] [Google Scholar]
- 54.Fung G, Cheung C, Chen E, et al. MRI predicts remission at 1 year in first-episode schizophrenia in females with larger striato-thalamic volumes. Neuropsychobiology 2014;69(4):243–248. [DOI] [PubMed] [Google Scholar]
- 55.Szeszko PR, Narr KL, Phillips OR, et al. Magnetic resonance imaging predictors of treatment response in first-episode schizophrenia. Schizophr Bull 2012;38(3):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reis Marques T, Taylor H, Chaddock C, et al. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain 2014;137(Pt 1):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nugent AC, Milham MP, Bain EE, et al. Cortical abnormalities in bipolar disorder investigated with MRI and voxel-based morphometry. Neuroimage 2006;30(2):485–497. [DOI] [PubMed] [Google Scholar]
- 58.Frazier JA, Chiu S, Breeze JL, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 2005;162(7):1256–1265. [DOI] [PubMed] [Google Scholar]
- 59.Wang Y, Chan RC, Hong X, et al. Prospective memory in schizophrenia: further clarification of nature of impairment. Schizophr Res 2008;105(1-3):114–124. [DOI] [PubMed] [Google Scholar]
- 60.Chen Q, Lui S, Wang JG, et al. Diffusion tensor imaging of two unrelated Chinese men with hereditary spastic paraplegia associated with thin corpus callosum. Neurosci Lett 2008;441(1):21–24. [DOI] [PubMed] [Google Scholar]
- 61.Pavuluri MN, Yang S, Kamineni K, Passarotti AM, Srinivasan G, Harral E, Sweeney JA, Zhou XJ. Diffusion tensor imaging study of white matter fiber tracts in pediatric bipolar disorder and attention deficit hyperactivity disorder. Biological Psychiatry 2009;65(7): 586–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Adler CM, Adams J, DelBello MP, et al. Evidence of white matter pathology in bipolar disorder adolescents experiencing their first episode of mania: a diffusion tensor imaging study. Am J Psychiatry 2006;163(2):322–324. [DOI] [PubMed] [Google Scholar]
- 63.Lu LH, Zhou XJ, Fitzgerald J, et al. Microstructural abnormalities of white matter differentiate pediatric and adult-onset bipolar disorder. Bipolar Disord 2012;14(6):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Foland-Ross LC, Bookheimer SY, Lieberman MD, et al. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage 2012;59(1):738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl) 2011;216(4):485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry 2009;66(5):516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2009;48(3):308–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pavuluri MN, O’Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol Psychiatry 2007;62(2):158–167. [DOI] [PubMed] [Google Scholar]
- 69.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J Clin Psychiatry 2010;71(11):1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magioncalda P, Martino M, Conio B, et al. Functional connectivity and neuronal variability of resting state activity in bipolar disorder: reduction and decoupling in anterior cortical midline structures. Hum Brain Mapp 2015;36(2):666–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Linke J, King AV, Rietschel M, et al. Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry 2012;169(3):316–325. [DOI] [PubMed] [Google Scholar]
- 72.Hajek T, Cullis J, Novak T, et al. Brain structural signature of familial predisposition for bipolar disorder: replicable evidence for involvement of the right inferior frontal gyrus. Biol Psychiatry 2013;73(2):144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Schot AC, Vonk R, Brans RG, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry 2009;66(2):142–151. [DOI] [PubMed] [Google Scholar]
- 74.Hoekzema E, Carmona S, Ramos-Quiroga JA, et al. Training-induced neuroanatomical plasticity in ADHD: a tensor-based morphometric study. Hum Brain Mapp 2011;32(10):1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.He N, Li F, Li Y, et al. Neuroanatomical deficits correlate with executive dysfunction in boys with attention deficit hyperactivity disorder. Neurosci Lett 2015;600:45–49. [DOI] [PubMed] [Google Scholar]
- 76.de Zeeuw P, Mandl RC, Hulshoff Pol HE, van Engeland H, Durston S. Decreased frontostriatal microstructural organization in attention deficit/hyperactivity disorder. Hum Brain Mapp 2012;33(8):1941–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp 2009;30(9):2757–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morein-Zamir S, Dodds C, van Hartevelt TJ, et al. Hypoactivation in right inferior frontal cortex is specifically associated with motor response inhibition in adult ADHD. Hum Brain Mapp 2014;35(10):5141–5152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kucyi A, Hove MJ, Biederman J, Van Dijk KR, Valera EM. Disrupted functional connectivity of cerebellar default network areas in attention-deficit/hyperactivity disorder. Hum Brain Mapp 2015;36(9):3373–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hart H, Chantiluke K, Cubillo AI, et al. Pattern classification of response inhibition in ADHD: toward the development of neurobiological markers for ADHD. Hum Brain Mapp 2014;35(7):3083–3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karl A, Schaefer M, Malta LS, Dörfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev 2006;30(7):1004–1031. [DOI] [PubMed] [Google Scholar]
- 82.Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003;160(5):924–932. [DOI] [PubMed] [Google Scholar]
- 83.Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002;52(11):1089–1101. [DOI] [PubMed] [Google Scholar]
- 84.Wignall EL, Dickson JM, Vaughan P, et al. Smaller hippocampal volume in patients with recent-onset posttraumatic stress disorder. Biol Psychiatry 2004;56(11):832–836. [DOI] [PubMed] [Google Scholar]
- 85.Chao LL, Yaffe K, Samuelson K, Neylan TC. Hippocampal volume is inversely related to PTSD duration. Psychiatry Res 2014;222(3):119–123. [DOI] [PubMed] [Google Scholar]
- 86.Li S, Huang X, Li L, et al. Posttraumatic stress disorder: structural characterization with 3-T MR imaging. Radiology 2016;280(2):537–544. [DOI] [PubMed] [Google Scholar]
- 87.De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 2001;50(4):305–309. [DOI] [PubMed] [Google Scholar]
- 88.De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry 2002;52(11):1066–1078. [DOI] [PubMed] [Google Scholar]
- 89.Meng Y, Qiu C, Zhu H, et al. Anatomical deficits in adult posttraumatic stress disorder: a meta-analysis of voxel-based morphometry studies. Behav Brain Res 2014;270:307–315. [DOI] [PubMed] [Google Scholar]
- 90.Lei D, Li L, Li L, et al. Microstructural abnormalities in children with post-traumatic stress disorder: a diffusion tensor imaging study at 3.0T. Sci Rep 2015;5:8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lanius RA, Williamson PC, Densmore M, et al. Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 2001;158(11):1920–1922. [DOI] [PubMed] [Google Scholar]
- 92.Thomaes K, Dorrepaal E, Draijer NP, et al. Increased activation of the left hippocampus region in Complex PTSD during encoding and recognition of emotional words: a pilot study. Psychiatry Res 2009;171(1):44–53. [DOI] [PubMed] [Google Scholar]
- 93.Yan X, Brown AD, Lazar M, et al. Spontaneous brain activity in combat related PTSD. Neurosci Lett 2013;547:1–5. [DOI] [PubMed] [Google Scholar]
- 94.Zhu H, Zhang J, Zhan W, et al. Altered spontaneous neuronal activity of visual cortex and medial anterior cingulate cortex in treatment-naïve posttraumatic stress disorder. Compr Psychiatry 2014;55(7):1688–1695. [DOI] [PubMed] [Google Scholar]
- 95.Gong Q, Li L, Du M, et al. Quantitative prediction of individual psychopathology in trauma survivors using resting-state FMRI. Neuropsychopharmacology 2014;39(3):681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev 2012;36(9):2130–2142. [DOI] [PubMed] [Google Scholar]
- 97.Bruce SE, Buchholz KR, Brown WJ, Yan L, Durbin A, Sheline YI. Altered emotional interference processing in the amygdala and insula in women with Post-Traumatic Stress Disorder. Neuroimage Clin 2012;2:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shang J, Lui S, Meng Y, et al. Alterations in low-level perceptual networks related to clinical severity in PTSD after an earthquake: a resting-state fMRI study. PLoS One 2014;9(5):e96834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lui S, Huang X, Chen L, et al. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proc Natl Acad Sci U S A 2009;106(36):15412–15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Du MY, Liao W, Lui S, et al. Altered functional connectivity in the brain default-mode network of earthquake survivors persists after 2 years despite recovery from anxiety symptoms. Soc Cogn Affect Neurosci 2015;10(11):1497–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin LM, Bush G, Milad MR, et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry 2011;168(9):979–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gong Q, Li L, Tognin S, et al. Using structural neuroanatomy to identify trauma survivors with and without post-traumatic stress disorder at the individual level. Psychol Med 2014;44(1):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Admon R, Leykin D, Lubin G, et al. Stress-induced reduction in hippocampal volume and connectivity with the ventromedial prefrontal cortex are related to maladaptive responses to stressful military service. Hum Brain Mapp 2013;34(11):2808–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hill SK, Reilly JL, Keefe RS, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry 2013;170(11):1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kitis O, Ozalay O, Zengin EB, et al. Reduced left uncinate fasciculus fractional anisotropy in deficit schizophrenia but not in non-deficit schizophrenia. Psychiatry Clin Neurosci 2012;66(1):34–43. [DOI] [PubMed] [Google Scholar]
- 106.Rowland LM, Spieker EA, Francis A, Barker PB, Carpenter WT, Buchanan RW. White matter alterations in deficit schizophrenia. Neuropsychopharmacology 2009;34(6):1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McGlashan TH, Fenton WS. Subtype progression and pathophysiologic deterioration in early schizophrenia. Schizophr Bull 1993;19(1):71–84. [DOI] [PubMed] [Google Scholar]
- 108.Sun H, Lui S, Yao L, et al. Two patterns of white matter abnormalities in medication-naive patients with first-episode schizophrenia revealed by diffusion tensor imaging and cluster analysis. JAMA Psychiatry 2015;72(7):678–686. [DOI] [PubMed] [Google Scholar]
- 109.Mezer A, Yeatman JD, Stikov N, et al. Quantifying the local tissue volume and composition in individual brains with magnetic resonance imaging. Nat Med 2013;19(12):1667–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ma D, Gulani V, Seiberlich N, et al. Magnetic resonance fingerprinting. Nature 2013;495(7440):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feinberg DA, Moeller S, Smith SM, et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS One 2010;5(12):e15710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Teipel SJ, Wegrzyn M, Meindl T, et al. Anatomical MRI and DTI in the diagnosis of Alzheimer’s disease: a European multicenter study. J Alzheimers Dis 2012;31(Suppl 3):S33–S47. [DOI] [PubMed] [Google Scholar]
- 113.Gollub RL, Shoemaker JM, King MD, et al. The MCIC collection: a shared repository of multi-modal, multi-site brain image data from a clinical investigation of schizophrenia. Neuroinformatics 2013;11(3):367–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry 2010;67(8):783–792. [DOI] [PubMed] [Google Scholar]
- 115.Gong Q, He Y. Depression, neuroimaging and connectomics: a selective overview. Biol Psychiatry 2015;77(3):223–235. [DOI] [PubMed] [Google Scholar]
- 116.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007;10(9):1116–1124. Ahae [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.