Abstract
Objectives:
The coprevalence, severity, and biomarkers for seizures and arrhythmias in long QT syndrome (LQTS) remain incompletely understood.
Methods:
Using the Rochester-based LQTS Registry, this study included large cohorts of LQTS1–3 participants (LQTS+, n = 965) and those without a LQTS mutation (LQTS−, n = 936).
Results:
Compared to LQTS− participants, there was a higher prevalence of LQTS1, LQTS2, and LQTS+ participants classified as having seizures (p < 0.001, i.e., history of seizures/epilepsy or antiseizure medication). LQTS+ participants with longer corrected QT interval (QTc) durations were more likely to have seizures. LQTS2 mutations in the KCNH2 pore domain were positive predictors for both arrhythmias and seizures. In contrast, mutations in the cyclic nucleotide binding domain (cNBD) of KCNH2 conferred a negative risk of seizures, but not arrhythmias. LQTS2, KCNH2-pore, KCNH2-cNBD, QTc duration, and sex were independent predictors of seizures. LQTS+ participants with seizures had significantly longer QTc durations, and a history of seizures was the strongest independent predictor of arrhythmias (hazard ratio 4.09, 95% confidence interval 2.63–6.36, p < 0.001).
Conclusions:
This study highlights potential biomarkers for neurocardiac electrical abnormalities in LQTS.
Patients with genetic ion channel diseases develop electrical disturbances in the brain (seizures) and heart (arrhythmias) that can lead to sudden death.1–5 Congenital long QT syndrome (LQTS) is a classically studied genetic cardiac disease affecting 1:2,000 people.6 LQTS mutations disrupt the cardiac activation-recovery process, due to sustained depolarizing current (e.g., LQTS3) or reduced repolarizing current (e.g., LQTS1 and LQTS2). Type of LQTS, age, sex, corrected QT interval (QTc) duration, mutation region, autonomic nervous system (ANS) tone, and channel expression and biophysical properties are risk factors for arrhythmias and sudden cardiac death (SCD).7–10 A subpopulation of LQTS+ patients also develop seizures.1,3 One possible link for neurocardiac electrical disturbances is that the genes mutated in LQTS1–3 are expressed in the heart and brain.11–13
Young adults with epilepsy have a 24-fold higher risk of sudden death.14 Potential mechanisms for sudden unexpected death in epilepsy (SUDEP) include cardiac arrhythmias, respiratory dysfunction, cerebral hypoperfusion, and other ANS perturbations.2 In a cohort of SUDEP patients, genetic analysis identified many genes responsible for cardiac arrhythmias.15
The Rochester-based LQTS Registry was used to assess LQTS mutations and their relationship to neurocardiac disease manifestations and the risk of SCD/SUDEP. We hypothesized that participants with LQTS mutations have an increased seizure susceptibility, and LQTS+ participants with seizures are at an increased risk of cardiac arrhythmias and SCD. The results demonstrate that seizures are an additional LQTS comorbidity. Seizures were the strongest independent risk factor for lethal cardiac arrhythmias in LQTS.
METHODS
Rochester-based LQTS Registry.
The Rochester-based LQTS Registry included detailed clinical and genetic information from 1,901 genotyped consented participants (61% women) with follow-up (birth to 40 years old or June 2014, no ethnic or sex restrictions). Analyses were limited to participants genotype positive for a single KCNQ1 (LQTS1), KCNH2 (LQTS2), or SCN5A (LQTS3) mutation (LQTS+, n = 965), or their family members without a LQTS mutation (LQTS−, n = 936). Thus, only one known LQTS mutation was present in each family. The groups were based on genotype, and subanalyses included subdividing the groups based on QTc duration (see the definitions section below) or adjusting for QTc duration in the multivariate analysis.
Outcome measures included seizures, QTc duration, and cardiac arrhythmias. Analyses were performed to assess the prevalence of seizures based on LQTS+ vs LQTS−, LQTS mutant gene, region of LQTS gene mutation, QTc duration, sex, and age. Differences in the cardiac manifestations were compared in those with vs without seizures.
Definitions.
Similar to the inclusion criteria used previously by others,1 participants were classified as having seizures if patient or physician documents noted either a personal history of seizures/epilepsy or antiseizure medication. Baseline QTc duration was classified as follows: normal QTc (male <430 milliseconds [ms], female <450 ms), borderline QTc prolongation (male 430–449 ms, female 450–469 ms), and QTc prolongation (male ≥450 ms, female ≥470 ms). Cardiac arrhythmias were defined as ventricular tachycardia, torsades de pointes, ventricular fibrillation, or aborted cardiac arrest (ACA), which were gathered from yearly follow-up reports.
LQTS gene topology.
KCNQ1 (LQTS1) and KCNH2 (LQTS2) missense mutations were classified by the region of the mutation on the protein topology.7,10 Specifically, KCNH2 amino acid (aa) mutations were divided into N-terminus (≤403 aa), transmembrane (404–547 aa), pore (548–659 aa), cyclic nucleotide binding domain (cNBD, 750–870 aa), and C-terminus (≥660 aa, excluding cNBD).
Study approval.
The LQTS Registry was approved by the University of Rochester Research Subject Review Board (RSRB00025305). All data were handled in accordance with applicable laws. HIPAA (Health Insurance Portability and Accountability Act) requirements for accounting of disclosure, consent, and withdrawal of consent were followed. Written informed consent was obtained from all participants or guardians in the study.
Statistics.
Comparisons were tested for statistical significance using the χ2 test. The Cochran-Mantel-Haenszel test was applied to assess the correlation between QTc vs seizures or cardiac arrhythmias. Multivariate Cox proportional hazards regression analysis was used to identify the independent predictors of seizures and cardiac arrhythmias,7,10 adjusting for LQTS genotype, region of the mutation, QTc per 100 ms, sex (age <15 and 15–40 years), time-dependent β-blocker usage, and history of seizures, and stratified by decade of birth. The t test with Welch correction and analysis of variance with the Tukey post hoc test were used to test for differences in QTc among groups.10 Significance was defined as p < 0.05, and all statistical tests were 2-sided. Where the difference was highly significant, p < 0.001 was listed.
RESULTS
LQTS+, type of LQTS, QTc prolongation, and female sex were positive risk factors for seizures.
Baseline characteristics, listed in table 1, indicated that compared to LQTS− participants, LQTS+ participants were significantly younger, with longer R-R and QTc intervals, and a higher percentage of them received antiarrhythmic therapy. Despite different mutant genes in LQTS1–3, other than antiarrhythmic therapy, the baseline characteristics were similar.
Table 1.
Baseline characteristics

Based on these inclusion criteria, 182 of 1,901 participants had seizures (figure 1), which included history of seizures/epilepsy (80 participants) and antiseizure medication (153 participants), with 51 reporting yes to both criteria (high-sensitivity analysis; table e-1 at Neurology.org). Individuals with LQTS are at an increased risk of cardiac arrhythmias, which is one mechanism for seizures.8,16 Despite this possibility, results from this study indicate that seizures were not purely cardiogenic in origin (see limitation 2 in the discussion section). Of note, as shown previously,8,10 we confirmed that β-blockers reduced the risk of cardiac arrhythmias in LQTS+ participants (hazard ratio [HR] 0.463, 95% confidence interval [CI] 0.273–0.784, p = 0.004), yet β-blockers did not alter the risk of seizures (HR 0.787, CI 0.49–1.27, p = 0.324).
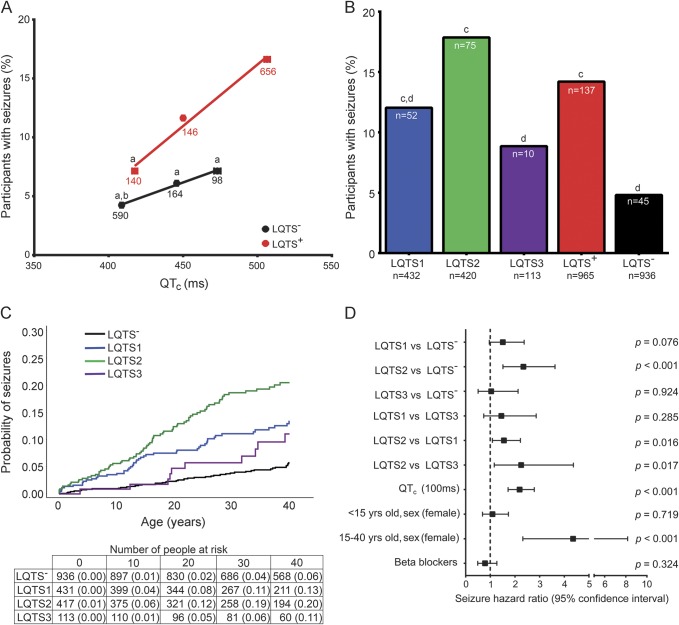
Figure 1. Higher prevalence of LQTS+ and female participants with seizures.
Number and percentage of participants with seizures based on genotype and sex. LQTS = long QT syndrome.
The LQTS+ and LQTS− seizure cohorts each had a higher percentage of females and longer QTc durations compared to those without seizures. LQTS+ participants were 3.0-fold more likely to have seizures, compared to LQTS− participants (p < 0.001, figure 1). Female LQTS+ and LQTS− participants were more likely than male participants of the same genotype to have seizures (p < 0.05).
Within each QTc window, LQTS+ vs LQTS− participants were 1.7- to 2.3-fold more likely to have seizures. Also, with increasing QTc duration, there was a higher prevalence of LQTS+ participants with seizures (figure 2A, p < 0.05). Even accounting for the normal QTc duration being longer in females, within each QTc window, there was a significantly higher prevalence of female participants with seizures.
Figure 2. Biomarkers for seizure status.
(A) Positive correlation of QTc to percentage with seizures (n = participants; CMH test, LQTS+ p < 0.05, LQTS− p = not significant, χ2, p < 0.001; ap < 0.05 vs LQTS+ QTc prolongation; bp < 0.001 vs LQTS+ borderline QTc prolongation; LQTS+ R2 = 0.9844, slope = 0.1045; LQTS− R2 = 0.9944, slope = 0.0454). (B) Prevalence of participants with seizures based on type of LQTS (N = total participants, n = participants with seizures, χ2: LQTS1 vs LQTS2 vs LQTS3 vs LQTS−, p < 0.001; LQTS1 vs LQTS2 vs LQTS3, p < 0.05; cp < 0.001 vs LQTS−; dp < 0.05 vs LQTS2). (C) Cumulative probability of seizures (log rank p < 0.001). (D) Multivariate analysis of seizure risk. CMH = Cochran-Mantel-Haenszel; LQTS = long QT syndrome; QTc = corrected QT interval.
The type of LQTS conferred a differential risk of seizures. LQTS1 and LQTS2 cohorts had a higher prevalence of seizures compared to the LQTS− group (figure 2B, p < 0.001). Despite no difference in QTc duration between each type of LQTS (table 1, p = not significant), the LQTS2 group had the highest percentage of participants with seizures (p < 0.05 vs each LQTS1, LQTS3, and LQTS−). Also, with increasing QTc, there was an increased prevalence of LQTS2 participants with seizures (p < 0.001).
LQTS1 and LQTS2 participants exhibited a steady increase in the cumulative probability of seizures from birth to age 40 years (figure 2C, log rank p < 0.001). Similar to sex- and age-dependent differences in the cumulative probability of cardiac events,8,10 there was a sex- and age-dependent crossover of seizures occurring at 15 years of age when female LQTS2 participants were at a higher risk (z test, p < 0.05 at 30 and 40 years).
Time-dependent multivariate analyses confirmed that LQTS2 (vs each LQTS−, LQTS1, and LQTS3), QTc prolongation, and female sex between 15 and 40 years of age were each independent predictors of seizures (figure 2D). In contrast to protection from arrhythmias,10,16 time-dependent β-blocker treatment did not alter the risk of seizures (figure 2D).
Region of the LQTS mutation predicts seizure status.
While the location of missense mutations in KCNQ1 (LQTS1) and KCNH2 (LQTS2) gene products are predictive of cardiac events,7,9,10 its ability to predict seizures remained unknown. None of the regions of the KCNQ1 gene product (n = 350) conferred a difference in the prevalence or risk of seizures.
Consistent with previous studies,7,9 KCNH2 pore mutations were an independent predictor of cardiac arrhythmias (HR 2.01, 95% CI 1.11–3.61, p = 0.021). The percentage of LQTS2 participants with seizures was highest in those with mutations in the KCNH2 pore region (figure 3A; S5-pore-S6: 32.1%, n = 81; vs all other regions: 10.9%, n = 193; p < 0.001). The pore region of KCNH2 was an independent positive predictor of seizures (figure 3B; HR 3.06, 95% CI 1.68–5.56, p < 0.001). In contrast, the percentage of LQTS2 participants with mutations in the cNBD who had seizures was lower compared to the sum of all other regions (figure 3A; cNBD: 8.8%, n = 80; vs all other regions: 20.6%, n = 194; p < 0.05). The cNBD was a negative predictor of seizures (figure 3B; HR 0.39, 95% CI 0.17–0.88, p < 0.05) but not arrhythmias.
Figure 3. Region of the KCNH2 (LQTS2) mutation predicted seizures.
(A) Percent of LQTS2 participants with mutations in each topological region with seizures vs sum of all other regions (χ2: ratio of seizure cohort—total with mutations in that region). (B) Multivariate analysis indicated that the pore and cNBD regions were independent predictors of seizures. C-term = C-terminus; cNBD = cyclic nucleotide binding domain; N-term = N-terminus; LQTS = long QT syndrome; QTc = corrected QT interval; Trans = transmembrane.
A history of seizures was predictive of cardiac arrhythmias.
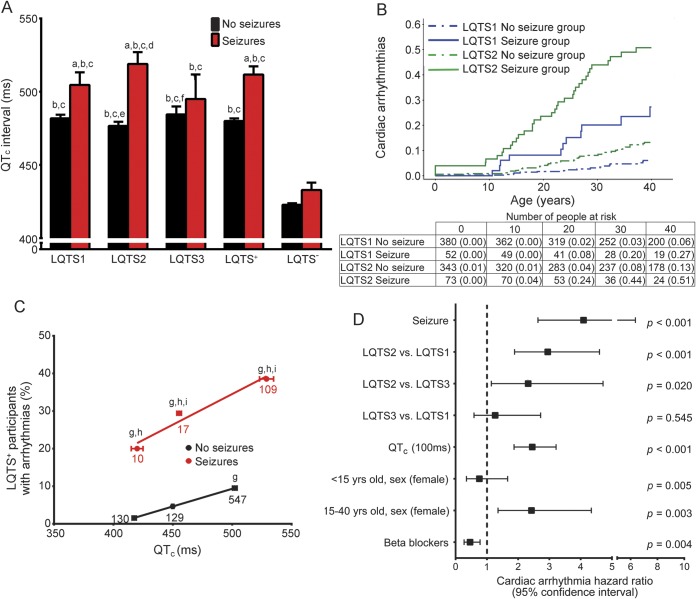
While QTc prolongation and increased risk of arrhythmias are hallmarks of LQTS, those with seizures had more severe cardiac ECG manifestations (table 1). LQTS1, LQTS2, and LQTS+ participants with seizures each had significantly longer QTc durations and a 5-fold higher rate of lethal cardiac arrhythmias (figure 4).
Figure 4. LQTS+ participants with seizures exhibited more severe ECG manifestations.
(A) Seizure cohort exhibited longer QTc durations (analysis of variance with Tukey test: LQTS− and LQTS+ with and without seizures, p < 0.001; LQTS1, 2, and 3, and LQTS− with and without seizures, p < 0.001; ap < 0.05 vs genotype no seizure; bp < 0.05 vs LQTS− no seizure; cp < 0.05 vs LQTS− seizure; dp < 0.05 vs LQTS1 no seizure; ep < 0.05 vs LQTS1 seizure; fp < 0.05 vs LQTS2 seizure). (B) Higher cumulative probability of cardiac arrhythmias in LQTS1 and LQTS2 participants with vs without seizures (log rank p < 0.001). (C) Within the same QTc window, there was a higher percentage of LQTS+ seizure participants who developed arrhythmias (n = participants; Cochran-Mantel-Haenszel test: LQTS+ no seizure; p < 0.001; LQTS+ seizure, p = not significant. Chi square: p < 0.001; gp < 0.05 vs LQTS+ no seizure; normal QTc; hp < 0.05 vs LQTS+ no seizure; borderline QT prolongation; ip < 0.05 vs LQTS+ no seizure; QTc prolongation. LQTS− no seizure; R2 = 0.9999, slope = 0.0939. LQTS+ seizure, R2 = 0.9568, slope = 0.1631). (D) Multivariate analysis indicated seizures were the strongest independent positive predictor of arrhythmias. LQTS = long QT syndrome; QTc = corrected QT interval.
Nearly 20% of LQTS+ participants with seizures underwent ACA, in which they experienced a lethal cardiac arrhythmia that required external defibrillation. LQTS1, LQTS2, and LQTS+ participants with seizures had a higher prevalence and cumulative probability of cardiac arrhythmias compared to those without seizures for each genotype (figure 4B; p < 0.001; z test, p < 0.05 LQTS1 at 30 and 40 years, LQTS2 at 10, 20, 30, and 40 years). Even after stratifying LQTS+ participants by QTc window, the seizure cohort had a higher percentage of participants with cardiac arrhythmias (figure 4C; p < 0.05).
After adjustment for genotype, QTc duration, sex, and β-blockers, seizures were the strongest independent predictor of cardiac arrhythmias (figure 4D; HR 4.09, 95% CI 2.63–6.36, p < 0.001). In summary, participants with a history of seizures exhibited more pronounced cardiac manifestations, as evidenced by greater QTc prolongation and a higher prevalence of lethal cardiac arrhythmias.
High-sensitivity analysis: Seizure status.
There were potential limitations to the seizure group inclusion criteria (see limitation 2 in the discussion section). However, repeating the analyses for separate cohorts for either only a history of seizures/epilepsy or only having received antiseizure medication yielded results similar to those observed above (table e-1). Of note, an analysis using a more stringent high-sensitivity inclusion criterion (i.e., both a history of seizure/epilepsy and antiseizure medication) revealed similar differences in seizure prevalence due to genotype, QTc prolongation, and pore/cNBD LQTS2 mutations (p < 0.05; figure e-1, A and C). Time-dependent multivariate analyses confirmed that in contrast to β-blockers, LQTS2, QTc prolongation, and LQTS2 pore mutations were each independent predictors of an increased risk of seizures (figure e-1, B and D). In contrast to figure 2D, sex (age 15–40 years) was not a risk factor for seizures in this high-sensitivity analysis.
High-sensitivity analysis further indicated that LQTS+ and LQTS2 participants with seizures had greater QTc prolongation compared to those without seizures (figure e-2A). LQTS1, LQTS2, and LQTS+ participants with seizures had a higher prevalence of arrhythmias (figure e-2, B and C), and seizure status was associated with an increased risk of cardiac arrhythmias (figure e-2D; HR 2.613, 95% CI 1.394–4.898, p = 0.0027).
DISCUSSION
LQTS and seizure disorders lead to multisystem pathologies. LQTS1 patients with Jervell and Lange-Nielsen syndrome have hearing loss.17 LQTS3 patients are more likely to develop gastrointestinal symptoms.18 Neurocardiac pathologies have been found in Dravet syndrome,5,19,20 Brugada syndrome,21 CPVT (catecholaminergic polymorphic ventricular tachycardia),22 SCN8A-mediated diseases,23 and HCN2-mediated diseases.24
Results from this study provided novel insights into the risk factors for seizures. LQTS+, type of LQTS, LQTS2 mutation domain, QTc duration, and female sex were important biomarkers for predicting seizure susceptibility. QTc duration was significantly longer in LQTS+ participants with seizures. Seizures were the strongest independent positive risk factor for cardiac arrhythmias.
While a single seizure does not define epilepsy, the prevalence of LQTS− participants with seizures aligns with a 2% to 5% lifetime prevalence of seizures and 1% of Americans have epilepsy (table e-1).25,26 Consistent with LQTS1–3 mutant genes being expressed in the heart and brain,11–13 there was a 3-fold higher prevalence of LQTS+ vs LQTS− participants with seizures. QTc prolongation is a positive risk factor for cardiac arrhythmias, ACA, SCD,7,9 and now seizures. LQTS genotype and phenotype provide potential biomarkers for seizures.
LQTS2 patients have the highest prevalence of a personal or family history of seizures, documented seizure/seizure-like episodes, and EEG recorded epileptiform activity.1,3 Our study considered only a personal history of seizures, a 5.5-fold larger patient cohort, and we conducted high-sensitivity analyses, which yielded similar results. LQTS2 participants were at the highest risk of seizures. In contrast to the findings of Johnson et al. (2009),1 there was a significantly higher prevalence of LQTS1 vs LQTS− participants with seizures. LQTS3 participants were not at a higher risk of seizures, yet these results need to be confirmed with a larger sample size. Despite similar QTc durations in each form of LQTS, the type of LQTS conferred differing risks of seizures, suggesting potential differences in the level and region of LQTS1–3 gene expression in the brain.
More than 20% of people diagnosed with epilepsy and heart rhythm disturbances have pathologic single nucleotide variants in cardiac ion channel genes.27 While a previous study1 did not find an association between seizures and the location of the mutation, likely because of a lack of power, we found that the mutation region altered the risk of cardiac arrhythmias and seizures. Male LQTS2 participants7,9 with KCNH2 pore mutations have longer QTc durations and the highest risk of a cardiac event. We confirmed these previous results, plus demonstrated that KCNH2 pore mutations were associated with an increased risk of seizures.
KCNH2 cNBD mutations were an independent negative predictor of seizures. While cyclic nucleotides do not directly bind to the channel or modulate the current, cNBD couples to the channel pore through a C-linker and directly binds to the Per-Arnt-Sim (PAS) domain in the N-terminus.28 In heterologous cells, cNBD mutations result in abnormal ER processing/retention, Golgi transit, protein degradation, reduced membrane localization, and loss of function.29 Future mechanistic studies will determine the effect of these mutations in native cardiac and neuronal cells.
Sex hormones influence cardiac ion channel expression, action potential morphology, dispersion of repolarization across the myocardium, and the likelihood of cardiac arrhythmias.30 The risk of cardiac events increases in female and diminishes in male LQTS, LQTS1, and LQTS2 individuals after puberty, and diminishes in females after menopause.7,8,10 Sex hormones contribute to the emergence of several forms of epilepsy at puberty and recede after menopause.31 We observed a similar sex-dependent crossover for the cumulative probability of seizures in LQTS1 and LQTS2 participants during adolescence, and female sex (age 15–40 years) was a biomarker for seizures.
Recordings of the events surrounding SUDEP are often not available. Thus, the clinical and genetic biomarkers for SUDEP remain incompletely understood. Since LQTS+ patients are at an increased risk of both cardiac arrhythmias and seizures, they provide an ideal cohort for assessing neurocardiac electrical disturbances, potential triggers, and mechanisms for SUDEP. A novel direction that this study embarked on was to examine the cardiac manifestations in LQTS+ participants with seizures.
The genes that are mutated in LQTS1–3 are expressed throughout the brain, particularly in brainstem ANS regions that provide direct brain–heart connections and a likely pathway for neurocardiac disease progression.11–13 Seizures within cortical limbic regions disturb ANS tone, with changes in heart rate, ECG measures, and increased rates of arrhythmias and SUDEP.32,33 Patients with epilepsy exhibit a progressive reduction in ANS tone.5,34 During preictal, ictal, or postictal periods, there is a surge of sympathetic tone, yet a few studies report parasympathetic dominance.32,33 Thus, it is plausible that seizure-induced sympathetic hyperexcitability potentiates ECG pathologies, particularly since increased sympathetic tone (exercise/arousal) is known to potentiate arrhythmias in LQTS1–2.16
Baseline and interictal QTc durations are prolonged in patients with epilepsy.4,5 There is often QT prolongation, variability, and dispersion during the ictal period,4,35 particularly in SUDEP patients.36 Seizure-induced QTc prolongation, particularly in LQTS+ patients, may tip the balance leading to arrhythmogenesis.
Conduction disturbances, bradycardia, asystole, and atrial/ventricular arrhythmias are frequently observed during ictal and postictal periods.35 Of note, 42% of LQTS1 participants with mutations at the A341 site had a history of seizures (n = 12). LQTS1 mice with the human equivalent of the A341E mutation recapitulate the human phenotype of QTc prolongation, arrhythmias, and seizures.11,37 LQTS1 mice exhibit a high concurrence between seizures and cardiac ECG abnormalities.11
Seizures were the strongest independent predictor of cardiac arrhythmias. Because nearly 20% of LQTS participants with seizures underwent ACA, they were at the highest risk of SCD/SUDEP. Future studies need to assess the efficacy of implantable cardiac defibrillators in LQTS+ participants with seizures.
Genetic background, modifier genes, common polymorphisms, and polygenic burden may augment the risk of arrhythmias and seizures.38,39 These contributing factors were partially accounted for in our analyses by including LQTS+ and LQTS− family members of the proband. Poor seizure control and sodium channel–blocking antiseizure drugs increase the risk of arrhythmias.40
Limitations.
Limitations of this study include the following 2 points. (1) The LQTS Registry is retrospective until enrollment date, so information may be limited. After enrollment, all information is prospective, with improved temporal resolution. (2) Inclusion criteria were based on patient/physician reports. As neurologic records and EEG recordings were not available, we were unable to use an expert panel to confirm seizure status, severity, and etiology. Thus, it is possible that arrhythmias may have been misdiagnosed as seizures, seizures may have been mediated by arrhythmias, and in some patients, antiseizure drugs may have been given for nonseizure reasons (e.g., pain and mood stabilization.) However, the following points minimize this concern: (1) β-blockade reduced cardiac events, but did not alter the risk of seizures; (2) inclusion criteria based solely on seizure/epilepsy or antiseizure medication (table e-1) and high-sensitivity analyses each yielded similar conclusions (table e-1; figures e-1 and e-2); (3) differences were found in the increase in the cumulative probability of seizures vs arrhythmias (figures 2C and 4B); (4) LQTS3 participants had QTc prolongation (table 1) and were at a high risk of arrhythmias (HR 22.682, 95% CI 4.971–103.488, p < 0.001) but not seizures (figure 2B and D); (5) differences in the recovery phase following seizures and syncope41; and (6) previous studies align with our results of the coexistence and prevalence of seizures in LQTS+ and LQTS−.1,3,11,25 While these points do not eliminate the limitation of our inclusion criteria, these finding suggest that seizures are present and likely a distinct clinical manifestation of LQTS.
LQTS+ (particularly LQTS2), QTc prolongation, LQTS2 pore/cNBD mutations, and female sex are independent risk factors for seizures. In LQTS+ participants, seizures are a significant predictor of cardiac arrhythmias. This study establishes the basis for future investigations into the mechanisms for neurocardiac pathologies in LQTS. It remains unknown whether the neurocardiac pathologies are the result of (1) mutant gene coexpression in the heart and brain; (2) seizure-induced ANS dysfunction promoting arrhythmias; (3) cardiac arrhythmias leading to cerebral hypoxia/ischemia-induced seizures; or (4) pro- and antiarrhythmic/epileptic effects of medical therapy. Regardless of the mechanism(s), it is critical to take a multisystem approach to studying the neurocardiac pathologies in genetic ion channel diseases. LQTS includes a complex network of factors, which coalesce to establish a substrate for the development of electrical disturbances in the brain and the heart.
Supplementary Material
ACKNOWLEDGMENT
The authors appreciate Mark Andrews for initial computational support and Martin Ruwald, MD, PhD, for clinical expertise.
GLOSSARY
- aa
amino acid
- ACA
aborted cardiac arrest
- ANS
autonomic nervous system
- CI
confidence interval
- cNBD
cyclic nucleotide binding domain
- HR
hazard ratio
- LQTS
long QT syndrome
- QTc
corrected QT interval
- SCD
sudden cardiac death
- SUDEP
sudden unexpected death in epilepsy
Footnotes
Editorial, page 1638
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
D.S.A.: conceived and designed the research study, analyzed data, interpreted results, drafted the manuscript and figures, principal investigator. S.M.: analyzed data, performed and guided all statistical analyses, interpreted results. R.A.G.: interpreted results, edited the manuscript. W.Z.: maintains LQTS Registry, interpreted results, edited the manuscript. R.T.D.: interpreted results, edited the manuscript. A.J.M.: founded and maintains LQTS Registry, interpreted results, edited the manuscript.
STUDY FUNDING
Supported by a University of Rochester CTSA Career Development Award (NIH-NCATS KL2TR000095, D.S.A.), University of Rochester Preventative Cardiology training grant (NIH 5T32HL007937, D.S.A.), and research grants HL-33843 (A.J.M.), HL-51618 (A.J.M.), HL-123483 (A.J.M.), and U54NS048843 (R.T.D.) from the NIH, Bethesda, MD.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Johnson JN, Hofman N, Haglund CM, Cascino GD, Wilde AA, Ackerman MJ. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 2009;72:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Surges R, Thijs RD, Tan HL, Sander JW. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol 2009;5:492–504. [DOI] [PubMed] [Google Scholar]
- 3.Anderson JH, Bos JM, Cascino GD, Ackerman MJ. Prevalence and spectrum of electroencephalogram-identified epileptiform activity among patients with long QT syndrome. Heart Rhythm 2014;11:53–57. [DOI] [PubMed] [Google Scholar]
- 4.Surges R, Taggart P, Sander JW, Walker MC. Too long or too short? New insights into abnormal cardiac repolarization in people with chronic epilepsy and its potential role in sudden unexpected death. Epilepsia 2010;51:738–744. [DOI] [PubMed] [Google Scholar]
- 5.Delogu AB, Spinelli A, Battaglia D, et al. . Electrical and autonomic cardiac function in patients with Dravet syndrome. Epilepsia 2011;52(suppl 2):55–58. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz PJ, Stramba-Badiale M, Crotti L, et al. . Prevalence of the congenital long-QT syndrome. Circulation 2009;120:1761–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Migdalovich D, Moss AJ, Lopes CM, et al. . Mutation and gender-specific risk in type 2 long QT syndrome: implications for risk stratification for life-threatening cardiac events in patients with long QT syndrome. Heart Rhythm 2011;8:1537–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Locati EH, Zareba W, Moss AJ, et al. . Age- and sex-related differences in clinical manifestations in patients with congenital long-QT syndrome: findings from the International LQTS Registry. Circulation 1998;97:2237–2244. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu W, Moss AJ, Wilde AA, et al. . Genotype-phenotype aspects of type 2 long QT syndrome. J Am Coll Cardiol 2009;54:2052–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barsheshet A, Goldenberg I, O-Uchi J, et al. . Mutations in cytoplasmic loops of the KCNQ1 channel and the risk of life-threatening events: implications for mutation-specific response to beta-blocker therapy in type 1 long-QT syndrome. Circulation 2012;125:1988–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldman AM, Glasscock E, Yoo J, Chen TT, Klassen TL, Noebels JL. Arrhythmia in heart and brain: KCNQ1 mutations link epilepsy and sudden unexplained death. Sci Transl Med 2009;1:2ra6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann HA, Colom LV, Sutherland ML, Noebels JL. Selective localization of cardiac SCN5A sodium channels in limbic regions of rat brain. Nat Neurosci 1999;2:593–595. [DOI] [PubMed] [Google Scholar]
- 13.D'Adamo MC, Catacuzzeno L, Di Giovanni G, Franciolini F, Pessia M. K(+) channelepsy: progress in the neurobiology of potassium channels and epilepsy. Front Cell Neurosci 2013;7:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ficker DM. Sudden unexplained death and injury in epilepsy. Epilepsia 2000;41(suppl 2):S7–S12. [DOI] [PubMed] [Google Scholar]
- 15.Bagnall RD, Crompton DE, Petrovski S, et al. . Exome-based analysis of cardiac arrhythmia, respiratory control, and epilepsy genes in sudden unexpected death in epilepsy. Ann Neurol 2016;79:522–534. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz PJ, Priori SG, Spazzolini C, et al. . Genotype-phenotype correlation in the long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation 2001;103:89–95. [DOI] [PubMed] [Google Scholar]
- 17.Jervell A, Lang-Nielsen F. Congenital deaf-mutism, functional heart disease with prolongation of the Q-T interval and sudden death. Am Heart J 1957;54:59–68. [DOI] [PubMed] [Google Scholar]
- 18.Saito YA, Strege PR, Tester DJ, et al. . Sodium channel mutation in irritable bowel syndrome: evidence for an ion channelopathy. Am J Physiol Gastrointest Liver Physiol 2009;296:G211–G218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Auerbach DS, Jones J, Clawson BC, et al. . Altered cardiac electrophysiology and SUDEP in a model of Dravet syndrome. PLoS One 2013;8:e77843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Santiago LF, Meadows LS, Ernst SJ, et al. . Sodium channel Scn1b null mice exhibit prolonged QT and RR intervals. J Mol Cell Cardiol 2007;43:636–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandorfi G, Clemens B, Csanadi Z. Electrical storm in the brain and in the heart: epilepsy and Brugada syndrome. Mayo Clin Proc 2013;88:1167–1173. [DOI] [PubMed] [Google Scholar]
- 22.Lehnart SE, Mongillo M, Bellinger A, et al. . Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest 2008;118:2230–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noujaim SF, Kaur K, Milstein M, et al. . A null mutation of the neuronal sodium channel NaV1.6 disrupts action potential propagation and excitation-contraction coupling in the mouse heart. FASEB J 2012;26:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ludwig A, Budde T, Stieber J, et al. . Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J 2003;22:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shorvon SD. Epidemiology, classification, natural history, and genetics of epilepsy. Lancet 1990;336:93–96. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Chronic Disease Prevention and Health Promotion/Division of Population Health. Epilepsy fast facts [online]. Available at: http://www.cdc.gov/epilepsy/basics/fast-facts.htm. Accessed April 13, 2016.
- 27.Partemi S, Vidal MC, Striano P, et al. . Genetic and forensic implications in epilepsy and cardiac arrhythmias: a case series. Int J Legal Med 2015;129:495–504. [DOI] [PubMed] [Google Scholar]
- 28.Brelidze TI, Gianulis EC, DiMaio F, Trudeau MC, Zagotta WN. Structure of the C-terminal region of an ERG channel and functional implications. Proc Natl Acad Sci USA 2013;110:11648–11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Akhavan A, Atanasiu R, Noguchi T, Han W, Holder N, Shrier A. Identification of the cyclic-nucleotide-binding domain as a conserved determinant of ion-channel cell-surface localization. J Cell Sci 2005;118:2803–2812. [DOI] [PubMed] [Google Scholar]
- 30.Jonsson MK, Vos MA, Duker G, Demolombe S, van Veen TA. Gender disparity in cardiac electrophysiology: implications for cardiac safety pharmacology. Pharmacol Ther 2010;127:9–18. [DOI] [PubMed] [Google Scholar]
- 31.Koppel BS, Harden CL. Gender issues in the neurobiology of epilepsy: a clinical perspective. Neurobiol Dis 2014;72:193–197. [DOI] [PubMed] [Google Scholar]
- 32.Devinsky O. Effects of seizures on autonomic and cardiovascular function. Epilepsy Curr 2004;4:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moseley B, Bateman L, Millichap JJ, Wirrell E, Panayiotopoulos CP. Autonomic epileptic seizures, autonomic effects of seizures, and SUDEP. Epilepsy Behav 2013;26:375–385. [DOI] [PubMed] [Google Scholar]
- 34.Tomson T, Ericson M, Ihrman C, Lindblad LE. Heart rate variability in patients with epilepsy. Epilepsy Res 1998;30:77–83. [DOI] [PubMed] [Google Scholar]
- 35.Moseley BD. Seizure-related autonomic changes in children. J Clin Neurophysiol 2015;32:5–9. [DOI] [PubMed] [Google Scholar]
- 36.Tavernor SJ, Brown SW, Tavernor RM, Gifford C. Electrocardiograph QT lengthening associated with epileptiform EEG discharges: a role in sudden unexplained death in epilepsy? Seizure 1996;5:79–83. [DOI] [PubMed] [Google Scholar]
- 37.Casimiro MC, Knollmann BC, Yamoah EN, et al. . Targeted point mutagenesis of mouse Kcnq1: phenotypic analysis of mice with point mutations that cause Romano-Ward syndrome in humans. Genomics 2004;84:555–564. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz PJ, Vanoli E, Crotti L, et al. . Neural control of heart rate is an arrhythmia risk modifier in long QT syndrome. J Am Coll Cardiol 2008;51:920–929. [DOI] [PubMed] [Google Scholar]
- 39.Leu C, Balestrini S, Maher B, et al. . Genome-wide polygenic burden of rare deleterious variants in sudden unexpected death in epilepsy. EBioMedicine 2015;2:1063–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bardai A, Blom MT, van Noord C, Verhamme KM, Sturkenboom MC, Tan HL. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart 2014;101:17–22. [DOI] [PubMed] [Google Scholar]
- 41.Hunter JV, Moss AJ. Seizures and arrhythmias: differing phenotypes of a common channelopathy? Neurology 2009;72:208–209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.