Abstract
The amplification of YWHAZ was commonly seen in bladder cancer. We explore the biological significance of YWHAZ amplification on bladder cancer, and the correlation with important other molecular events. The Cancer Genome Atlas (TCGA) database was exploited to study the impact of YWHAZ amplification on either CDKN2A or TP53 mutations. The Database for Annotation, Visualization and Integrated Discovery (DAVID) was also exploited to clustering of enriched genes in the cBioPortal Enrichment tests. There were 127 cases with available mutation and CNV data in the corresponding TCGA bladder cancer dataset, 20% of them had YWHAZ alteration. Patients with both YWHAZ amplification and CDKN2A loss demonstrated significantly better overall survival (OS) compared with CDKN2A loss alone. Patients with both YWHAZ amplification and TP53 mutation demonstrated significantly better overall survival (OS) and disease-free survival (DFS) compared with TP53 mutation alone. The amplification of YWHAZ, along with alteration of CDKN2A or TP53, predict better survival in bladder cancers that only had CDKN2A or TP53 alteration. The protective role of YWHAZ in bladder cancer deserve insightful further studies.
Keywords: YWHAZ, copy number alteration, amplification, prognosis, bladder cancer
INTRODUCTION
Urinary bladder cancer remains one of the most commonly diagnosed cancers in the world. Around 75% of bladder cancer were non-muscle invasive, which is characterized by a high risk of recurrence and progression while the remaining 25% of bladder cancers were muscle-invasive, which is characterize by a high risk of metastasis and poor prognosis [1]. However, treatment progress of bladder cancer has been stagnant for the past decades [2].
One strategy to improve the care of bladder cancer patients is to explore the molecular pathogenesis and novel tumor markers to predict prognosis. Genetic alteration has been known to contribute to the occurrence and development of bladder cancer [3]. By integrated analysis of genomic event, we could define some subgroups of cancer type and found correlations between clinical information, which could provide robust prognosis markers.
Recent report using whole-genome sequencing techniques have identified varieties of focal copy number alterations of bladder cancer, including YWHAZ, located at 8q22, a gene involved in a wide range of biological processes [4]. The cocomitment gain of YWHAZ was found to be associated with some common molecular events, including TP53 mutation [5]. Up till now, the link between YWHAZ and biological characteristics of bladder cancer remains poorly understood. In our study, we reported using the online analytical tools of The Cancer Genome Atlas (TCGA) database, to explore the biological significance of YWHAZ amplification on bladder cancer, and the correlation with important other molecular events.
RESULTS
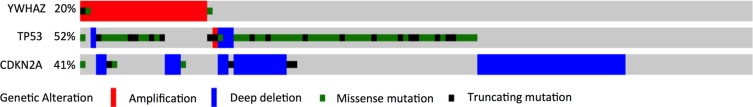
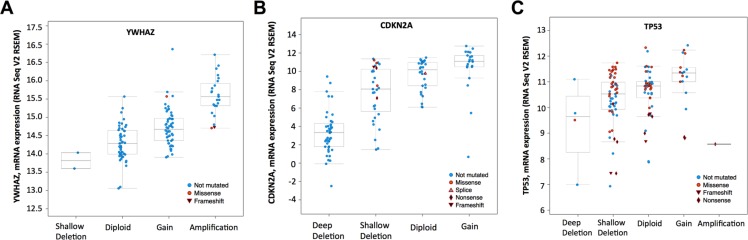
There were 127 cases with available mutation and CNV data in the corresponding TCGA bladder cancer dataset. Amplification/mutation of YWHAZ was found in ~20% of cases (Figure 1). Amplification was the predominant type of alteration for YWHAZ. Deep deletion/mutation of CDKN2A was found in ~41% of cases, whilst Deep deletion/mutation of TP53 was found in ~52% of cases (Figure 1). There were 9 patients (7%) with YWHAZ and CDKN2A alterations, and 16 patients (12.5%) with both YWHAZ and TP53 alterations (Table 1 and Table 2). Functional plotting of the corresponding mRNA level in relation to genetic status of YWHAZ, TP53, and CDKN2A revealed that amplification of YWHAZ was associated with increased mRNA expression (Figure 2A); deletion of CDKN2A was associated with decreased mRNA expression (Figure 2B); mutation of TP53 was not obviously associated with mRNA expression but loss of copy number of was associated with lowered mRNA expression (Figure 2C).
Figure 1. Gene alteration of YWHAZ, TP53 and CDKN2A in bladder cancer patients.
Table 1. The characteristics of patients with TP53 alteration (+: altered; −: unaltered).
| YWHAZ (−) TP53 (+) | YWHAZ (+) TP53 (+) | P Value | ||
|---|---|---|---|---|
| Age (Mean ± SD) | 67.69 ± 9.12 | 66.33 ± 11.11 | 0.823 | |
| Gender (n%) | Male | 37 (56.1) | 13 (19.7) | 0.556 |
| Female | 13 (19.7) | 3 (4.5) | ||
| T (n%) | 2 | 14 (21.2) | 4 (6.1) | 0.925 |
| 3 | 22 (33.3) | 8 (12.1) | ||
| 4 | 7 (10.6) | 2 (3.0) | ||
| Tx | 7 (10.6) | 2 (3.0) | ||
| N (n%) | 0 | 28 (42.4) | 6 (9.1) | 0.215 |
| 1 | 4 (6.1) | 3 (4.5) | ||
| 2 | 9 (13.6) | 5 (7.6) | ||
| 3 | 4 (6.1) | 0 (0) | ||
| Nx | 5 (7.6) | 2 (3.0) | ||
| M (n%) | 0 | 26 (39.3) | 10 (15.1) | 0.385 |
| 1 | 2 (3.0) | 0 (0) | ||
| Mx | 22 (33.3) | 6 (9.1) | ||
| Living Status (n%) | Living | 21 (31.8) | 10 (15.1) | 0.153 |
| Decreased | 29 (43.9) | 6 (9.1) |
Table 2. The characteristics of patients with CDKN2A alteration (+: altered; −: unaltered).
| YWHAZ (−) CDKN2A (+) | YWHAZ (+) CDKN2A (+) | P Value | ||
|---|---|---|---|---|
| Age (Mean ± SD) | 68.07 ± 9.50 | 68.33 ± 8.90 | 0.934 | |
| Gender (n%) | Male | 32 (61.5) | 7 (13.5) | 0.802 |
| Female | 11 (21.2) | 2 (3.8) | ||
| T (n%) | 2 | 11 (21.2) | 1 (1.9) | 0.404 |
| 3 | 22 (42.3) | 5 (9.6) | ||
| 4 | 6 (11.5) | 0 (0) | ||
| Tx | 4 (7.7) | 3 (5.8) | ||
| N (n%) | 0 | 26 (50.0) | 5 (9.6) | 0.618 |
| 1 | 4 (7.7) | 1 (1.9) | ||
| 2 | 8 (15.4) | 0 (0) | ||
| 3 | 1 (1.9) | 0 (0) | ||
| Nx | 4 (7.7) | 3 (5.8) | ||
| M (n%) | 0 | 26 (50.0) | 3 (5.8) | 0.252 |
| 1 | 2 (3.8) | 1 (1.9) | ||
| Mx | 15 (28.8) | 5 (9.6) | ||
| Living Status (n%) | Living | 19 (36.5) | 6 (11.5) | 0.22 |
| Decrease | 24 (46.2) | 3 (5.8) | ||
Figure 2. mRNA level was consistent with gene change.
Functional plotting of the corresponding mRNA level in relation to genetic status of (A) YWHAZ, (B) CDKN2A and (C) TP53.
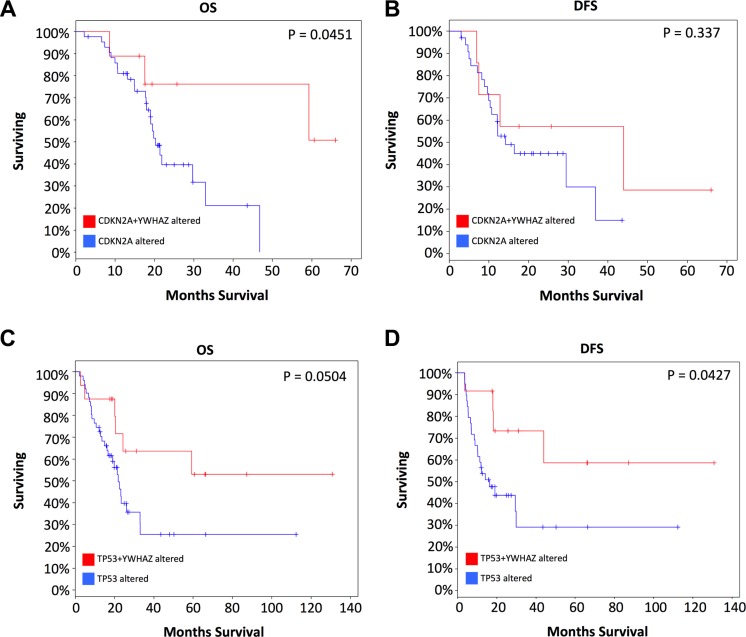
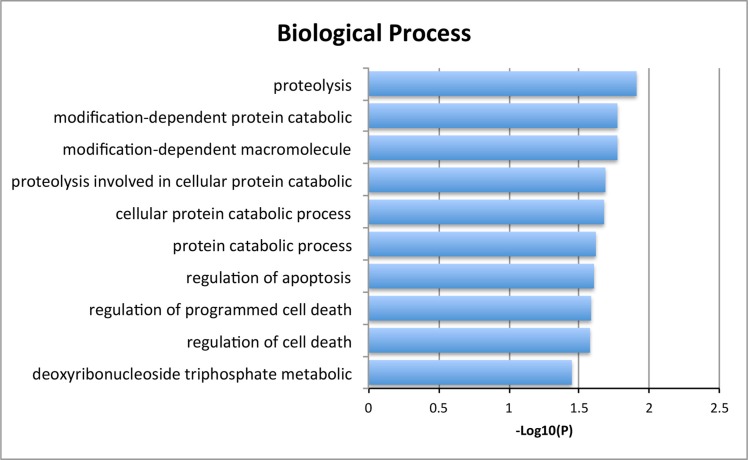
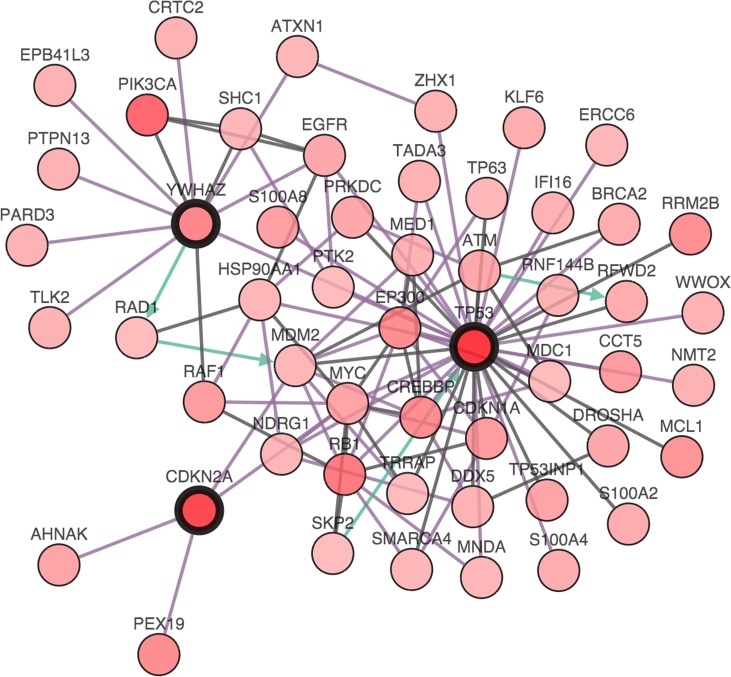
We then studied cases with alterations in either gene, namely CDKN2A, or TP53 and grouped cases according to co-occurrence of YWHAZ amplification. Demographic and clinicopathological parameters were summarized in Tables 1 and 2, and age, gender, T stage, nodal involvement, metastasis, or living status was not significantly different between TP53 mutated or CDKN2A deleted patients with or without YWHAZ amplification (Table 1 and Table 2). Patients with both YWHAZ amplification and CDKN2A loss demonstrated significantly better overall survival (OS) compared with CDKN2A loss alone (Figure 3A). Nonetheless, the disease-free survival (DFS) was not significantly different (Figure 3B). Patients with both YWHAZ amplification and TP53 mutation demonstrated significantly better overall survival (OS) and disease-free survival (DFS) compared with TP53 mutation alone (Figure 3C–3D). For CDKN2A, cases with both alterations versus single alteration did not demonstrate significant differences in enrichments at mRNA, protein, CNV, or mutation level (data not shown). For TP53, cases with both alterations versus single alteration demonstrated a series of significantly enriched genes at mRNA expression level. When those genes were input into DAVID, we noticed that proteolysis was the top enriched biological process between patients with both TP53 mutation and YWHAZ amplification, and patients with solely TP53 mutation (Figure 4). We then tried to analysis the interactions between TP53, CDKN2A, and YWHAZ alteration via computation, yet the strength of direct interaction between the nodes was solely moderate (Figure 5).
Figure 3. Coexistence of YWHAZ amplification contribute to better prognosis with CDKN2A or TP53 loss.
Figure 4. Biological process of the significantly enriched genes between both YWHAZ and TP53 alteration and single TP53 alteration.
Figure 5. The interactions between TP53, CDKN2A, and YWHAZ alteration.
We summarized the CNA and expression status of YWHAZ based on the study of Cancer Cell Line Encyclopedia. A total of 54 breast cancer, 7 prostate cancer and 24 urinary tract cancer cell lines were included. The profile of YWHAZ change and its mRNA expression status in this three cancer types was showed in Table 3. Details in different cell lines was showed in Supplementary Table 1.
Table 3. The profile of YWHAZ gene and expression status in breast, prostate and urinary tract cancer cell lines.
| cancer type | YWHAZ status | Cell lines count |
|---|---|---|
| breast | amplification | 9 |
| mRNA upregulation | 5 | |
| mRNA downregulation | 1 | |
| unchanged | 45 | |
| prostate | amplification | 1 |
| unchanged | 6 | |
| Urinary tract | mRNA upregulation | 3 |
| unchanged | 21 |
DISCUSSION
This study revealed that the amplification of YWHAZ, along with alteration of CDKN2A or TP53, predict better survival in bladder cancers that only had CDKN2A or TP53 alteration. The finding implicated that YWHAZ might play protective roles in bladder cancer. This was interesting because in previous studies, the overexpression of YWHAZ, has been implicated in the initiation and progression of multiple cancers [6]. In prostate cancer, 14-3-3ζ was upregulated by androgen and contributes to cell proliferation and resistance to etoposide-induced apoptosis in LNCaP cells. The higher expression is associated with malignancy and lymph node metastasis [7]. In breast cancers, 14-3-3ζ overexpression occurred in nearly half of breast tumors and was determined to be an independent prognostic factor for reduced disease-free survival. By using stable transfection or siRNA technique, increased 14-3-3ζ expression was found to enhanced cell growth and inhibited apoptosis, whereas downregulation of 14-3-3ζ reduced growth and sensitized cells apoptosis in breast cell lines [8].
TP53, also known as tumor protein 53, is a well-known tumor suppressor gene. The mutation or deletion of TP53 gene is commonly seen in bladder cancer patients [9]. In our cohort, TP53 mutation or deletion is seen in 52% of the patients. CDKN2A, also known as cyclin-dependent kinase inhibitor 2A, codes for two proteins, including p16 an p14arf, both act as tumor suppressors by regulating the cell cycle. The genetic alteration of CDKN2A is most common clinically relevant in the advanced bladder cancer [10]. Both TP53 and CDKN2A alteration contributed to the unfavorable clinical and survival outcomes [11].
In muscle invasive bladder tumor, gain on 8q was paralleled with TP53 mutation. Here using TCGA database we found this association positively contributed to the overall survival. Also the survival benefits were observed in the combination of YWHAZ and CDKN2A alteration. As both TP53 and CDKN2A were cell cycle checkpoint genes, we assume YWHAZ function in the cell cycle related pathway. 14-3-3 zeta regulates cell cycle progression through various ligands and processes. Previous study reported that 14-3-3 zeta controls cellular senescence by complexing with BIS to chaperone protein folding of STAT3 and activate the signaling pathway [12]. Also, 14-3-3 zeta can negatively regulate the G2-M phase checkpoint by binding and sequestering the cyclin-dependent kinases to the cytoplasm, thus inhibiting their activity [13]. Another study reported 14-3-3 zeta binding to Cdc25B and inhibits its interaction with CyclinB/Cdk1 [14]. Cdc25 dual-specificity phosphatases are essential regulators that activate cyclin-dependent kinases(CDKs) at critical stages of the cell cycle, acting both in S phase and G2/M in mammalian cells [15]. Thus, developing selective inhibitors for Cdc25 family proteins could provided novel therapeutic strategies for cancer therapy [16–18]. This might account for how 14-3-3 zeta exerts its protective function. As a tumor suppressor, CDKN2A is involved in the p53 pathway, arrest growth by holding the cell cycle at G1/S checkpoint on DNA damage recognition. When this mechanism fails, 14-3-3 zeta could inhibit Cdc25B and arrest the cell cycle at G2/M phase. All these speculations however still warrant insightful studies both in vivo and in vitro.
MATERIALS AND METHODS
An in silico reproduction using TCGA dataset was performed in the current study, as previously reported [19–21]. The TCGA bladder cancer (Provisional) dataset was chosen on the cBioPortal online platform [22, 23]. Cases with CDKN2A or TP53 mutations were respectively chosen, amongst which concurrent YWHAZ amplification was queried using the OncoPrint function. The Plots function illustrated the correlation of CNV/mutation versus mRNA expression. The Enrichment function explored alterations including mutations, copy number alterations, mRNA expression changes, and protein expression changes that were enriched in either altered or unaltered queried samples. The Survival illustrated Kaplan-Meier curves for overall survival and cancer-free survival. The Network illustrated the interactions between queried genes and in our cohort. All statistical analyses were performed automatically by the cBioPortal platform and the P value of < .05 and Q value of < .05 were accepted as statistically significant, respectively.
To explore the copy number alteration and mRNA expression status of YWHAZ on different cancer type, the Cancer Cell Line Encyclopedia (CCLE) datasets was chosen on cBioPortal online platform. Cell lines of breast cancer, prostate cancer, and urinary tract cancer was selected, amongst which YWHAZ was queried using OncoPrint function.
The Database for Annotation, Visualization and Integrated Discovery (DAVID) v6.7 was used for clustering of enriched genes passing both p (< .05) and q (< .05) values in the cBioPortal Enrichment tests. Clinicopathological parameters of the patients within TCGA cohort were retrieved and grouped according to alterations in single or double query genes and were compared using the Fisher's exact test. The P value of < .05 was accepted as statistically significant.
SUPPLEMENTARY MATERIALS TABLE
Acknowledgments
We thank all the authors for contribution to this manuscript.
Footnotes
CONFLICTS OF INTEREST
All authors declare no conflicts of interest.
REFERENCES
- 1.Lughezzani G, Sun M, Shariat SF, Budaus L, Thuret R, Jeldres C, Liberman D, Montorsi F, Perrotte P, Karakiewicz PI. A population-based competing-risks analysis of the survival of patients treated with radical cystectomy for bladder cancer. Cancer. 2011;117:103–109. doi: 10.1002/cncr.25345. [DOI] [PubMed] [Google Scholar]
- 2.Knollman H, Godwin JL, Jain R, Wong YN, Plimack ER, Geynisman DM. Muscle-invasive urothelial bladder cancer: an update on systemic therapy. Therapeutic advances in urology. 2015;7:312–330. doi: 10.1177/1756287215607418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J, Akbani R, Creighton CJ, Lerner SP, Weinstein JN, Getz G, Kwiatkowski DJ. Invasive Bladder Cancer: Genomic Insights and Therapeutic Promise. Clin Cancer Res. 2015;21:4514–4524. doi: 10.1158/1078-0432.CCR-14-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghazadeh Y, Papadopoulos V. The role of the 14–3-3 protein family in health, disease, and drug development. Drug Discov Today. 2015 doi: 10.1016/j.drudis.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Hurst CD, Platt FM, Taylor CF, Knowles MA. Novel tumor subgroups of urothelial carcinoma of the bladder defined by integrated genomic analysis. Clin Cancer Res. 2012;18:5865–5877. doi: 10.1158/1078-0432.CCR-12-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matta A, Siu KW, Ralhan R. 14–3-3 zeta as novel molecular target for cancer therapy. Expert Opin Ther Targets. 2012;16:515–523. doi: 10.1517/14728222.2012.668185. [DOI] [PubMed] [Google Scholar]
- 7.Murata T, Takayama K, Urano T, Fujimura T, Ashikari D, Obinata D, Horie-Inoue K, Takahashi S, Ouchi Y, Homma Y, Inoue S. 14–3-3zeta, a novel androgen-responsive gene, is upregulated in prostate cancer and promotes prostate cancer cell proliferation and survival. Clin Cancer Res. 2012;18:5617–5627. doi: 10.1158/1078-0432.CCR-12-0281. [DOI] [PubMed] [Google Scholar]
- 8.Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H, Lan KH, Ensor J, Hittelman W, Hung MC, Yu D. 14–3-3zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res. 2009;69:3425–3432. doi: 10.1158/0008-5472.CAN-08-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazrafshani MR, Nowshadi PA, Shirian S, Daneshbod Y, Nabipour F, Mokhtari M, Hosseini F, Dehghan S, Saeedzadeh A, Mosayebi Z. Deletion/duplication mutation screening of TP53 gene in patients with transitional cell carcinoma of urinary bladder using multiplex ligation-dependent probe amplification. Cancer Med. 2016;5:145–52. doi: 10.1002/cam4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JS, Wang K, Khaira D, Ali SM, Fisher HA, Mian B, Nazeer T, Elvin JA, Palma N, Yelensky R, Lipson D, Miller VA, Stephens PJ, et al. Comprehensive genomic profiling of 295 cases of clinically advanced urothelial carcinoma of the urinary bladder reveals a high frequency of clinically relevant genomic alterations. Cancer. 2016;122:702–11. doi: 10.1002/cncr.29826. [DOI] [PubMed] [Google Scholar]
- 11.Kim PH, Cha EK, Sfakianos JP, Iyer G, Zabor EC, Scott SN, Ostrovnaya I, Ramirez R, Sun A, Shah R, Yee AM, Reuter VE, Bajorin DF, et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. European urology. 2015;67:198–201. doi: 10.1016/j.eururo.2014.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JJ, Lee JS, Cui MN, Yun HH, Kim HY, Lee SH, Lee JH. BIS targeting induces cellular senescence through the regulation of 14–3-3 zeta/STAT3/SKP2/p27 in glioblastoma cells. Cell Death Dis. 2014;5:e1537. doi: 10.1038/cddis.2014.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Li Y, Lin C, Ding J, Liao G, Tang B. Aberrant upregulation of 14-3-3sigma and EZH2 expression serves as an inferior prognostic biomarker for hepatocellular carcinoma. PLoS One. 2014;9:e107251. doi: 10.1371/journal.pone.0107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mils V, Baldin V, Goubin F, Pinta I, Papin C, Waye M, Eychene A, Ducommun B. Specific interaction between 14–3-3 isoforms and the human CDC25B phosphatase. Oncogene. 2000;19:1257–1265. doi: 10.1038/sj.onc.1203419. [DOI] [PubMed] [Google Scholar]
- 15.Bouldin CM, Kimelman D. Cdc25 and the importance of G2 control: insights from developmental biology. Cell Cycle. 2014;13:2165–2171. doi: 10.4161/cc.29537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiyokawa H, Ray D. In vivo roles of CDC25 phosphatases: biological insight into the anti-cancer therapeutic targets. Anticancer Agents Med Chem. 2008;8:832–836. doi: 10.2174/187152008786847693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bana E, Sibille E, Valente S, Cerella C, Chaimbault P, Kirsch G, Dicato M, Diederich M, Bagrel D. A novel coumarin-quinone derivative SV37 inhibits CDC25 phosphatases, impairs proliferation, and induces cell death. Mol Carcinog. 2015;54:229–241. doi: 10.1002/mc.22094. [DOI] [PubMed] [Google Scholar]
- 18.Lavecchia A, Di Giovanni C, Novellino E. Inhibitors of Cdc25 phosphatases as anticancer agents: a patent review. Expert Opin Ther Pat. 2010;20:405–425. doi: 10.1517/13543771003623232. [DOI] [PubMed] [Google Scholar]
- 19.Feng C, Sun Y, Ding G, Wu Z, Jiang H, Wang L, Ding Q, Wen H. PI3Kbeta inhibitor TGX221 selectively inhibits renal cell carcinoma cells with both VHL, SETD2 mutations and links multiple pathways. Sci Rep. 2015;5:9465. doi: 10.1038/srep09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng C, Ding G, Jiang H, Ding Q, Wen H. Loss of MLH1 confers resistance to PI3Kbeta inhibitors in renal clear cell carcinoma with SETD2 mutation. Tumour biology. 2015;36:3457–3464. doi: 10.1007/s13277-014-2981-y. [DOI] [PubMed] [Google Scholar]
- 21.Feng C, Xiong Z, Jiang H, Ding Q, Fang Z, Hui W. Genetic alteration in notch pathway is associated with better prognosis in renal cell carcinoma. BioFactors (Oxford, England) 2016;42:41–8. doi: 10.1002/biof.1250. [DOI] [PubMed] [Google Scholar]
- 22.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Science signaling. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.