Abstract
Background
Patients who undergo colorectal surgery with new ileostomies incur high rates of readmission. Ostomates face a steep learning curve to master the skills and knowledge needed for success at home. We designed and implemented a patient-centered checklist promoting independence and validating self-care knowledge and care skills, and evaluated its effect on readmissions after ileostomy creation.
Methods
On a single inpatient unit, new ileostomy patients were taught and evaluated using a novel postoperative self-care checklist, while perioperative care for ostomates remained unchanged elsewhere in the institution. In a retrospective cohort including all consecutive ileostomy patients from two years before (Period 1) and one year after (Period 2) checklist implementation, we identified univariable predictors of readmission within 30 days of discharge and used a multivariable, difference-in-differences approach to compare trends in readmission between the intervention and control units.
Results
Of the 430 patients in the study period, there were 116 with readmissions (26%). Readmitted patients had significantly higher All Patient Refined-Diagnosis Related Group weights (3.6 vs. 3.3, p=0.006), longer initial length of stay (13.3 vs. 11.3 days, p=0.006) and were more likely to be emergency admissions (49% vs. 38%, p=0.04). The readmission rate on the intervention unit decreased from 28% in Period 1 to 20% in Period 2. The logistic regression-based difference-in-differences approach revealed that implementation of the checklist was an independent negative predictor of readmission (p=0.04).
Conclusion
Implementation of a patient-centered, self-care oriented postoperative education checklist was associated with significantly reduced odds of readmission after ileostomy creation in colorectal surgery.
Background
Patients who undergo colorectal surgery with the creation of a new ileostomy incur high rates of readmission 1–3. These unplanned readmissions are costly and are potentially preventable in nearly half of cases 3, 4. With the advent of Accountable Care Organizations, and the Center for Medicare and Medicaid Services Value Based Purchasing Readmissions Reduction Program, hospitals and payers are facing mounting pressure to reduce unwanted readmissions. Yet, at the same time, there is increasing implementation of fast-track enhanced recovery pathways to reduce postoperative length of stay. For patients with a new ileostomy, a shorter hospital course means less time to acquire the skills and knowledge required for stoma care after discharge. As a result, there is growing interest in promoting stoma self-care efficacy in order to arm patients with the knowledge to combat common challenges associated with ostomy creation 5, 6.
Although perioperative stoma education is considered a standard of care for new ileostomy patients 7, and a key factor in preventing readmissions, there are few studies to inform the optimal timing, content, and effectiveness of ileostomy care pathways in the setting of accelerated postoperative discharge. As a part of enhanced recovery programs, peri-operative stoma teaching reduces postoperative length of stay and complications, but has not been associated with an overall reduction in readmissions 8, 9. Nagle and colleagues reported that an “ileostomy pathway” focused on patient self-management eliminated readmissions for dehydration, but did not significantly change readmission rates overall 6. Stoma self-care efficacy has been shown to improve psychological adaptation following discharge but its impact on managing common complications related to ileostomy creation is unknown 5, 10.
With a goal of reducing readmissions among new ileostomates in an academic colorectal surgery specialty practice, we sought to evaluate a novel postoperative education checklist. Unlike standard educational approaches, this patient-initiated, self-care oriented checklist emphasized development of the independence and autonomy that are required after hospital discharge. While checklists are common in the healthcare setting 11–13, the use of discharge checklists in assessment of patient education has been very limited and no prior studies have assessed the use of checklists in ileostomy patients 14. In this context, we evaluated the effect of this checklist in an observational study, using a difference-in-differences approach to compare trends in readmission for patients on the intervention unit, as compared with similar patients in the same institution who were not exposed to the intervention.
Methods
Checklist and Pathway
Prior to initiation of this project, all new ileostomy patients in our tertiary care hospital were educated about stoma care predominantly through three, approximately one hour each, post-operative teaching sessions with our hospital based certified Wound, Ostomy, and Continence Nurses (WOCN’s), in addition to teaching materials provided by the WOCN’s. In addition, most patients also had a pre-operative education and marking session. A folder of educational materials including information on dehydration, ostomies, and appliances were also given to the patient. Most patients receive home health care support in the initial post-discharge period, though care agencies but logistics vary widely depending on their geographic location.
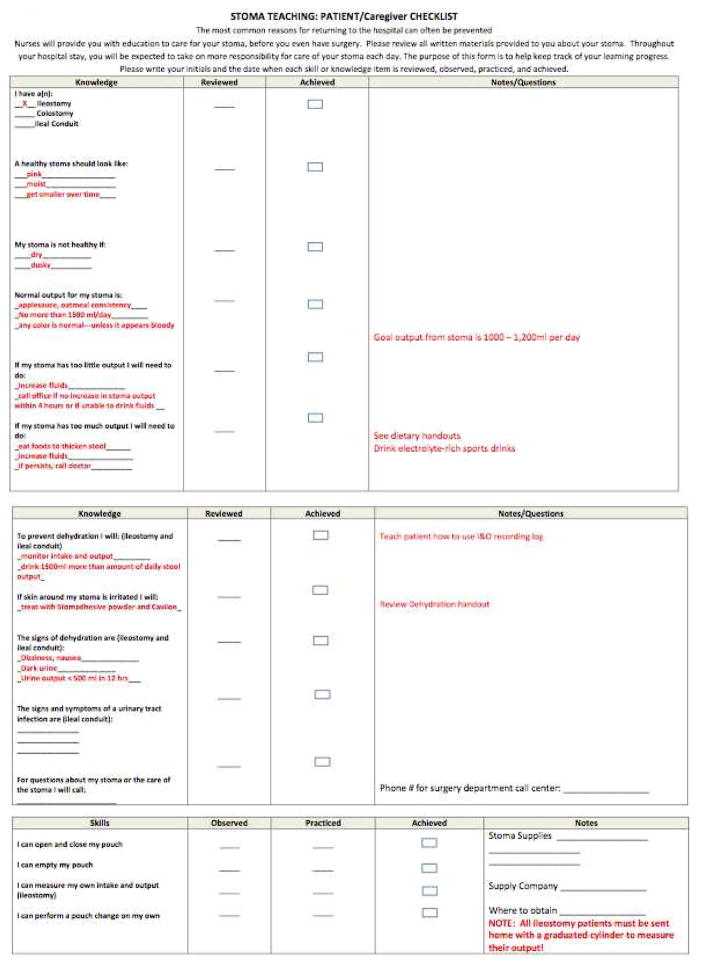
Through our hospital unit-based quality improvement process on a single inpatient unit in the hospital, a multidisciplinary group including physicians, nurses, WOCN’s, social work, and unit managers drafted, ratified, and implemented a patient-centered checklist of knowledge and skills felt to be essential for post-discharge success with an ileostomy. The purpose of the checklist was to ensure that prior to discharge, patients understood the education that was being provided and that they were able to perform the necessary tasks for caring for their ileostomy. The intervention unit is the most common site of admission for the authors’ clinical service (and admitted 47 % of new ileostomy patients during the study period), whereas other surgical services predominated on the control units. On a single inpatient unit, since implementation on December 1, 2013, all new ileostomy patients were responsible for completion of the checklist with a health care provider prior to discharge (Figure 1). An answer key was developed (red portions of Figure 1) of ideal answers to be used by providers when assessing patients with the checklist. Patients were given the checklist and instructed in its use within 24 hours of completion of surgery. Patients would indicate their progress with each component and take graduated responsibility for ileostomy care throughout their stay. Prior to institution of the checklist, nurses, physician’s assistants and house officers on the intervention unit were educated about its use. Progress in patient learning and skills were assessed throughout the patients’ hospital stay and patients were required to have completed the checklist prior to discharge. Care pathways for ostomates on other inpatient units were unchanged during the study period.
Figure 1.
Ileostomy Checklist with Key
Data Source
Patients who underwent surgery that included creation of a new ileostomy at the University of Michigan Hospital from two years prior to institution of the checklist (Period 1) to one year after (Period 2) were included in this retrospective review. We truncated analysis one year after introduction, because informal use of the checklist was increasingly noted in comparison units thereafter, often attributable to house officers who had previously observed its use on the intervention unit. Patients were identified by searching the Health System’s Research Data Warehouse and MiChart Clarity Database (Epic Systems Corporation, Verona, WI) for International Classification of Diseases, Ninth Revision codes including ileostomy creation (46.01, 46.02, 46.20, 46.21, 46.23, 46.24). This database included patient demographics, admission date, unit, and diagnoses, procedure at index admission, All Patients Refined Diagnosis Related Groups weighting (APR-DRGwt – a normalized measure of severity of illness for inpatient stay), age, gender, length of stay, admission class (emergent/urgent/elective), labs at discharge, discharge status (self care, home with home health nursing visits, nursing facility), readmission date, readmission diagnosis, labs at readmission, and procedures at readmission. We defined acute renal failure according to published criteria, as an acute increase in serum creatinine at readmission of 1.5 times baseline 18. Readmission was defined as a secondary inpatient stay in our tertiary care hospital within 30 days after discharge from the primary index procedure. Patients were divided into 4 categories based on admission unit and time period: intervention unit before intervention, intervention unit after intervention, control units before intervention, and control unit after intervention. Patients under the age of 18 were excluded. The study was reviewed and approved for publication by the University of Michigan Institutional Review Board and was deemed exempt because it was undertaken as quality improvement.
Statistical Analysis
In our retrospective cohort, we compared patient characteristics between units and between pre- and post- intervention time periods using univariate analysis. We identified univariate predictors of readmission within 30 days of discharge during the 2 years before, and the year after, checklist implementation. Categorical data were compared using Fisher’s exact test and continuous data were compared using two-tailed t tests.
Multivariable logistic regression-based difference-in-differences analysis was performed to isolate the effect of the checklist, independent of secular trends in readmission on intervention and control units. In the model, we assessed the relationship of the checklist to readmission while adjusting for admission type (scheduled or Urgent/Emergent), unit (intervention or control) and checklist time period (checklist used or not used) 15–17. Candidate predictors were those with a univariable p value less than 0.2 in addition to clinical factors thought to potentially be important such as diagnosis, surgery performed, age, surgeon, and admitting service. Backward selection was then used to eliminate predictors with multivariable p value greater than 0.5. All statistical analyses were conducted using SAS version 9.4 (SAS Corporation, Cary, NC).
Results
Patient characteristics by time period and care unit
There were a total of 430 patients who met inclusion criteria, 255 in Period 1 and 175 in Period 2. The proportion of patients admitted to the intervention unit, versus control units, was greater in Period 2 than in Period 1 (60% vs. 39%). The overall rate of readmission for the entire study period was 25.6%, and did not differ significantly between the two periods (25.9% in Period 1 versus 25.1% in Period 2).
Table 1 compares the characteristics of patients in intervention and control units between Period 1 and Period 2. There were no statistically significant differences in age, weight, acuity or severity of admission, length of stay, or indications for surgery between time periods in either the intervention or control units. However, control units patients were significantly older (53.2 ± 16.6 vs. 46.4 ± 15.9 years, p<0.001), less likely to have a diagnosis of inflammatory bowel disease (22% vs. 46%, p<0.001), more likely to have emergent or urgent admissions, (59% vs. 20%, p<0.001), and had a higher APR-DRGwt (4.2 ± 3.1 vs 2.4 ± 1.3, p<0.001) and longer average length of stay (14.8 ± 14.7 vs 8.6 ± 8.1 days, p<0.001). In addition, patients on control units were more likely to be admitted for a diagnosis in the “other” category (18% vs. 48%, p< 0.001), including intestinal perforation, other digestive system complications, adhesions with obstruction, fistula, septicemia, acute vascular insufficiency, and Clostridium diffcile colitis.
Table 1.
Patient characteristics by unit and time period. P values are for comparisons between time periods, within units, derived from t tests for continuous data and Fisher’s exact test for proportions.
| Intervention Unit | Control Units | |||||
|---|---|---|---|---|---|---|
| Variable (Patients) | Period 1 (n = 99) | Period 2 (n = 105) | P Value | Period 1 (n = 156) | Period 2 (n = 70) | P Value |
| Age | 45.8 ± 15.6 | 46.9 ± 16.3 | 0.64 | 54.6 ± 15.9 | 50.2 ± 17.4 | 0.06 |
| Gender, % Female (N) | 56% (55) | 49% (51) | 0.33 | 52% (81) | 50% (35) | 0.79 |
| % Urgent/Emergent Admission (N) | 19% (19) | 21% (22) | 0.86 | 60% (94) | 57% (40) | 0.66 |
| APR-DRGwt | 2.35 ± 1.24 | 2.41 ± 1.42 | 0.75 | 4.32 ± 3.18 | 4.08 ± 3.07 | 0.60 |
| Admission Diagnosis (N) | ||||||
| Cancer | 32% (32) | 34% (36) | 0.82 | 23% (36) | 29% (20) | 0.74 |
| Diverticulitis | 3% (3) | 2% (2) | 5% (8) | 4% (3) | ||
| IBD | 44% (44) | 48% (50) | 24% (37) | 19% (13) | ||
| Other* | 20% (20) | 16% (17) | 48% (75) | 49% (34) | ||
| Postop LOS days | 8.7 ± 7.8 | 8.5 ± 8.4 | 0.87 | 14.9 ± 15.2 | 14.4 ± 13.9 | 0.82 |
| Readmit % yes (N) | 28% (28) | 20% (21) | 0.19 | 25% (38) | 33% (23) | 0.19 |
APR-DRGwt = All Patient Refined-Diagnosis Related Groups weighting; IBD=inflammatory bowel disease; LOS=Length of Stay
Other diagnoses included: intestinal perforation, other digestive system complications, adhesions with obstruction, fistula, septicemia, acute vascular insufficiency, and Clostridium diffcile colitis.
Predictors of readmission and effect of the checklist
Univariate predictors of readmission are shown in Table 2. Readmitted patients were significantly more likely to have had urgent or emergency operations (49% vs. 38%, p=0.04), had higher APR-DRGwt (3.6 ± 2.1 vs 3.3 ± 2.8, p=0.006), and longer index postoperative length of stay (13.3 ± 11.5 vs 11.3 ± 12.7 days, p=0.006). The distribution of indications for surgery did not differ between patients readmitted and those not readmitted. There was also no difference in the overall distribution of readmissions between the intervention and control units. However, the readmission rate on the intervention unit decreased from 28% in Period 1 to 20% in Period 2, after introduction of the checklist.
Table 2.
Predictors of Readmission. P values are for comparisons between patients who were readmitted and those who were not, derived from t tests for continuous data and Fisher’s exact test for proportions.
| Readmission | |||
|---|---|---|---|
| Variable (Patients) | no (n = 320) | yes (n = 110) | P Value |
| Age | 50.0 ± 16.3 | 49.9 ± 17.2 | 0.96 |
| Gender % Female (N) |
51% (164) | 53% (58) | 0.79 |
| Weight (kg) | 78.4 ± 22.0 | 77.1 ± 20.2 | 0.59 |
| % Urgent/Emergent Admission | 38% (121) | 49% (54) | *0.04 |
| APR-DRGwt | 3.3 ± 2.8 | 3.6 ± 2.1 | *0.006 |
| Diagnosis | |||
| Cancer | 30% (96) | 25% (28) | 0.46 |
| Diverticulitis | 3% (10) | 5% (6) | |
| IBD | 34% (109) | 33% (35) | |
| Other | 33% (105) | 37% (41) | |
| Postop LOS | 11.3 ± 12.7 | 13.3 ± 11.5 | *0.006 |
| Unit | |||
| Unit-checklist | 157 (47%) | 52 (45%) | 0.67 |
| Unit-other | 174 (53%) | 64 (55%) | |
APR-DRGwt = All Patient Refined-Diagnosis Related Groups weighting; IBD=inflammatory bowel disease; LOS=Length of Stay
Difference-in-differences analysis (Table 3), adjusting for time period trends, unit of admission, implementation of the checklist, and significant confounding predictors identified a significant decrease in readmission attributable to the checklist (odds ratio 0.40, p=0.04).
Table 3.
Effect of institution of the checklist on the checklist Unit. This analysis used multivariable modeling with difference-in-differences comparison.
| Variable | Beta | Standard Error | P Value |
|---|---|---|---|
| Intercept | −1.4443 | 0.2483 | *<0.0001 |
| Admit type: Urgent/Emergent vs Elective | 0.4917 | 0.2454 | *0.0451 |
| Intervention Unit | 0.4118 | 0.3116 | 0.1863 |
| Pre vs Post Intervention Period | 0.4383 | 0.3176 | 0.1676 |
| Checklist Effect | −0.9071 | 0.4597 | *0.0445 |
We assessed the diagnosis at readmission on the intervention unit and found that the reasons for readmission were similar in Period 1 and Period 2 (Table 4). The most common cause for readmission was infection, responsible for 43% of intervention unit readmissions in Period 1 and 39% of readmissions in Period 2. Because patients are often readmitted with multiple diagnosis including dehydration but this may not be the primary diagnosis, we assessed dehydration at readmission further. We defined dehydration as an increase in serum creatinine at readmission of 1.5 times baseline 18 and found that on the intervention unit, the rate of dehydration at readmission was not statistically different before versus after institution of the checklist (46.4% vs 33.3%, respectively, p=0.35). The most common “other” causes of readmission in table 4 included: complication of enterostomy, other digestive system complication, pulmonary embolism, attention to ileostomy, and shock.
Table 4.
Reasons for readmission for Intervention Unit patients, by time period. P value for comparison of diagnosis at readmission derived from Fisher’s exact test.
| Readmission Diagnosis | Period 1 (n=21) | Period 2 (n=28) | P Value |
|---|---|---|---|
| ARF | 19% (4) | 14% (4) | 0.8264 |
| Ileus | 14% (3) | 11% (3) | |
| Infection | 43% (9) | 39% (11) | |
| Other | 24% (5) | 36%(10) |
ARF= Acute Renal Failure
Discussion
In this study, we find that a novel, patient-centered, self-care oriented checklist focused on building autonomy and independence significantly improved the rate of readmission after ileostomy creation in a specialty colorectal surgery practice. In a time period with no overall improvement in readmission rates after ileostomy in our institution, the intervention care unit experienced a nearly 30% reduction in rates of readmission. In other ileostomy care studies, it has been difficult, to attribute improved readmission rates to the study intervention, as increased attention to preventing readmissions may already have been present throughout the institution 4, 6. Thus, it was essential to compare the change in rates from Period 1 and Period 2 on the intervention unit against trends on control units in the hospital, and to further account for the most important differences between patients in these areas. A simple pre-intervention versus post-intervention design, as used in other studies would have been susceptible to selection bias, conflation with secular trends, regression to the mean, confounding by surgeon or clinical service characteristics, and chance. Accordingly, we used a difference-in-differences approach to account for these potential sources of bias and isolate the effect of the intervention itself.
The checklist was developed with a goal of rapidly building and assessing skills and knowledge to allow for early postoperative discharge in accordance with modern enhanced recovery principles. Our clinical teams placed the checklist at the patient’s bedside during the post-operative stay, assessed progress during the hospitalization, and required completion before discharge. We found this checklist straightforward to implement and easy to use, and we recognized that readmission rates declined on the intervention unit. It is not possible to know if the checklist itself was responsible for the decrease in readmissions seen in our study or the time it took to use it. Anecdotally, the patients receiving the checklist during the intervention period were better able to report measures of output and skin issues enabling early intervention by phone rather than late by readmission. Subjectively, there was immediate interest in the checklist from nursing staff and from house officers involved in the care of colorectal surgery patients. In fact, our study had to be limited to only one year in Period 2 because during this period, house officers began taking the checklist to other units in the hospital to facilitate postoperative education. The perceived success of the checklist for ileostomies also led to development of a similar checklist for patients with new urostomies on the same care unit.
If this patient-centered approach proves successful in other settings and other institutions, it could substantially improve the rate of postoperative readmission after new ileostomy creation. With the advent of bundled payments for surgery, and potential reimbursement penalties associated with unplanned readmissions in the Center for Medicare and Medicaid Services Readmissions Reduction Program, hospitals will be increasingly dependent on such programs to improve their rate of unwanted readmissions. We sought to further understand the effect of the intervention by examining the reasons for readmission before and after intervention. However, the readmission diagnoses were quite varied and did not clearly differ between periods. Infection was the most common cause of readmission, an etiology not expected to be affected by the checklist. Thus, we concluded that the effect of the intervention was not specific to the prevention of any particular complication or adverse event. Though this was somewhat contrary to our expectation that the checklist would primarily decrease readmissions due to dehydration, the study was not sufficiently powered to confirm differences in etiology of readmission, only the overall rate of readmissions as a whole.
This study is limited by its retrospective, observational design. Beyond the use of control units and a difference-in-differences design, there is the possibility that the effects of the intervention are unique to the unit on which it was developed, and may not be immediately generalizable to other care units, non-specialty practices, or to other institutions. Additionally, because we applied the checklist as a single intervention, we do not know which components of the teaching, or increased attention and care, are responsible for its effects. The use of administrative and electronic clinical data is potentially subject to coding and documentation errors, but we had access to the full detail available in the medical records, and thus, as compared with data from administrative databases alone, we had far greater richness of clinical detail. Finally, we may have missed readmissions to other institutions or other emergency departments, as we only accessed our internal data. However, the practice in our region is generally to transfer patients in the early postoperative period back to our hospital, and thus, the study sample includes the vast majority of clinically relevant readmissions, as indicated by the consistency between these readmission rates and those in other literature. Regardless, it is unlikely that readmissions to other institutions would be differentially distributed between groups or time periods. Thus, our analytic approach makes the failure to identify admissions to other hospitals conservative, and would, if anything, reduce the likelihood of finding an effect of the intervention.
It is impossible to ensure that the improvement in readmission rates is due to the checklist itself, rather than other unmeasured care improvements or a “Hawthorne effect” of greater staff awareness due to the ongoing clinical quality improvement effort that inspired the checklist introduction. Yet, there were no other specific clinical care changes during this period, and thus we attribute the success of this particular initiative to its patient-centeredness and focus on individual skills and autonomy. Before its introduction, patients who underwent new ileostomy creation were noted to have a high rate of readmission, despite attention and education from certified WOCNs. In some patients, this appeared to be due to a lack of comprehension of the education as well as a lack of ownership for their new ostomy. The use of the checklist encouraged patient autonomy with care, greater participation in teaching, as well as testing of knowledge by all care providers including physicians, physician assistants, and nurses beyond the WOCN’s.
There are many ways to perform patient education but if the patients do not comprehend what is being taught, they will not succeed. Our checklist is relatively simple and inexpensive to institute and appears to decrease readmissions in new ileostomy patients. As surgical patients are discharged earlier and earlier, further study is needed to assess the efficacy of this and other patient-centered education tools.
Acknowledgments
Grant Support: Dr. Hardiman is supported by the American Surgical Association Foundation Fellowship. Dr. Regenbogen is supported by the American Society of Colon and Rectal Surgeons Career Development Award CDG-015, National Institute on Aging Grants for Early Medical/Surgical Specialists Transition to Aging, R03-AG047860, and National Institute on Aging K08-AG047252.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kiran RP, Delaney CP, Senagore AJ, Steel M, Garafalo T, Fazio VW. Outcomes and prediction of hospital readmission after intestinal surgery. J Am Coll Surg. 2004;198:877–883. doi: 10.1016/j.jamcollsurg.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 2.Greenblatt DY, Weber SM, O’Connor ES, LoConte NK, Liou JI, Smith MA. Readmission after colectomy for cancer predicts one-year mortality. Ann Surg. 2010;251:659–669. doi: 10.1097/SLA.0b013e3181d3d27c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wick EC, Shore AD, Hirose K, Ibrahim AM, Gearhart SL, Efron J, Weiner JP, Makary MA. Readmission rates and cost following colorectal surgery. Dis Colon Rectum. 2011;54:1475–1479. doi: 10.1097/DCR.0b013e31822ff8f0. [DOI] [PubMed] [Google Scholar]
- 4.Messaris E, Sehgal R, Deiling S, Koltun WA, Stewart D, McKenna K, Poritz LS. Dehydration is the most common indication for readmission after diverting ileostomy creation. Dis Colon Rectum. 2012;55:175–180. doi: 10.1097/DCR.0b013e31823d0ec5. [DOI] [PubMed] [Google Scholar]
- 5.O’Connor G. Teaching stoma-management skills: The importance of self-care. Br J Nurs. 2005;14:320–324. doi: 10.12968/bjon.2005.14.6.17800. [DOI] [PubMed] [Google Scholar]
- 6.Nagle D, Pare T, Keenan E, Marcet K, Tizio S, Poylin V. Ileostomy pathway virtually eliminates readmissions for dehydration in new ostomates. Dis Colon Rectum. 2012;55:1266–1272. doi: 10.1097/DCR.0b013e31827080c1. [DOI] [PubMed] [Google Scholar]
- 7.Hendren S, Hammond K, Glasgow SC, Perry WB, Buie WD, Steele SR, Rafferty J. Clinical practice guidelines for ostomy surgery. Dis Colon Rectum. 2015;58:375–387. doi: 10.1097/DCR.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 8.Younis J, Salerno G, Fanto D, Hadjipavlou M, Chellar D, Trickett JP. Focused preoperative patient stoma education, prior to ileostomy formation after anterior resection, contributes to a reduction in delayed discharge within the enhanced recovery programme. Int J Colorectal Dis. 2012;27:43–47. doi: 10.1007/s00384-011-1252-2. [DOI] [PubMed] [Google Scholar]
- 9.Phatak UR, Kao LS, You YN, Rodriguez-Bigas MA, Skibber JM, Feig BW, Nguyen S, Cantor SB, Chang GJ. Impact of ileostomy-related complications on the multidisciplinary treatment of rectal cancer. Ann Surg Oncol. 2014;21:507–512. doi: 10.1245/s10434-013-3287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekkers MJ, van Knippenberg FC, van den Borne HW, van Berge-Henegouwen GP. Prospective evaluation of psychosocial adaptation to stoma surgery: The role of self-efficacy. Psychosom Med. 1996;58:183–191. doi: 10.1097/00006842-199603000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Haynes AB, Weiser TG, Berry WR, Lipsitz SR, Breizat AH, Dellinger EP, Herbosa T, Joseph S, Kibatala PL, Lapitan MC, Merry AF, Moorthy K, Reznick RK, Taylor B, Gawande AA. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med. 2009;360:491–499. doi: 10.1056/NEJMsa0810119. [DOI] [PubMed] [Google Scholar]
- 12.Basoor A, Doshi NC, Cotant JF, Saleh T, Todorov M, Choksi N, Patel KC, Degregorio M, Mehta RH, Halabi AR. Decreased readmissions and improved quality of care with the use of an inexpensive checklist in heart failure. Congest Heart Fail. 2013;19:200–206. doi: 10.1111/chf.12031. [DOI] [PubMed] [Google Scholar]
- 13.Haugen AS, Softeland E, Almeland SK, Sevdalis N, Vonen B, Eide GE, Nortvedt MW, Harthug S. Effect of the world health organization checklist on patient outcomes: A stepped wedge cluster randomized controlled trial. Ann Surg. 2015;261:821–828. doi: 10.1097/SLA.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 14.Soong C, Daub S, Lee J, Majewski C, Musing E, Nord P, Wyman R, Baker GR, Zacharopoulos N, Bell CM. Development of a checklist of safe discharge practices for hospital patients. J Hosp Med. 2013;8:444–449. doi: 10.1002/jhm.2032. [DOI] [PubMed] [Google Scholar]
- 15.Osborne NH, Nicholas LH, Ryan AM, Thumma JR, Dimick JB. Association of hospital participation in a quality reporting program with surgical outcomes and expenditures for medicare beneficiaries. JAMA. 2015;313:496–504. doi: 10.1001/jama.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryan AM, Burgess JF, Jr, Dimick JB. Why we should not be indifferent to specification choices for difference-in-differences. Health Serv Res. 2015;50:1211–1235. doi: 10.1111/1475-6773.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dimick JB, Ryan AM. Methods for evaluating changes in health care policy: The difference-in-differences approach. JAMA. 2014;312:2401–2402. doi: 10.1001/jama.2014.16153. [DOI] [PubMed] [Google Scholar]
- 18.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (adqi) group. Crit Care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]