Abstract
The regulation of blood gases in mammals requires precise feedback mechanisms including chemoreceptor feedback from the carotid bodies. Carotid body denervation (CBD) leads to immediate hypoventilation (increased PaCO2) in adult rats, but over a period of days and weeks ventilation normalizes due in part to central (brain) mechanisms. Here, we tested the hypothesis that functional ventilatory recovery following CBD correlated with significant shifts in medullary raphe gene expression of molecules/pathways associated with known or novel forms of neuroplasticity. Tissue punches were obtained from snap frozen brainstems collected from rats 1–2 days or 14–15 days post-sham or post-bilateral CBD surgery (verified by physiologic measurements), and subjected to mRNA sequencing to identify, quantify, and statistically compare gene expression level differences among these groups of rats. We found the greatest number of gene expression changes acutely after CBD (154 genes), with fewer changes in the weeks after CBD (69–80 genes) and the fewest changes in expression among the time control groups (39 genes). Little or no changes were observed for multiple genes associated with serotonin- or glutamate receptor-dependent forms of neuroplasticity. However, an unbiased assessment of gene expression changes using a bioinformatics pathway analysis highlighted multiple changes in gene expression in signaling pathways associated with immune function. These included several growth factors and cytokines associated with peripheral and innate immune systems. Thus, these medullary raphe gene expression data support a role for immune-related signaling pathways in the functional restoration of blood gas control after CBD, but little or no role for serotonin- or glutamate receptor-mediated plasticity.
Keywords: Respiratory plasticity, Carotid body denervation (CBD), Medullary raphe, Neural control of breathing, Serotonin, RNA Sequencing, Neuro-immune response
1. Introduction
Continuous gas exchange through ventilation is critical to maintain homeostatic levels of oxygen, CO2 and pH to sustain mammalian life. Disease processes that compromise the ventilatory system at the level of the lung, circulation, and/or neural control centers in the brainstem can ultimately lead to ventilatory insufficiency. However, the ventilatory control system appears to have an innate capacity for compensation, as evidenced by partial or complete recovery from experimental and/or therapeutic neural lesions. This partial or complete restoration of ventilatory function is a form of respiratory neuroplasticity, for which there are several examples and potential mechanisms (Forster, 2003; Kinkead et al., 2001; Mitchell et al., 2001).
Carotid body denervation (CBD) has been performed in multiple mammalian species including humans (Dahan et al., 2007; Dahan et al., 2008), and in most cases this leads to hypoventilation and elimination of hypoxic ventilatory responses (Miller et al., 2013; Mouradian et al., 2012; Bisgard et al., 1976). However, the degree and duration of eupneic hypoventilation (increase in PaCO2) was variable among species, lasting for only a few weeks (Mouradian et al., 2012) up to 20 or more years (Honda, 1992). Bilateral CBD in adult rats also elicits a significant and immediate hypoventilation, but in contrast to many other species the CBD-induced hypoventilation was rapidly and completely resolved within 2 weeks. Bilateral CBD in adult rats also elicits a significant and immediate hypoventilation, but in contrast to many other species the CBD-induced hypoventilation was rapidly and completely resolved within 2 weeks (Mouradian et al., 2012). In contrast, hypoventilation after CBD in ponies persisted for up to 2 years, at which time there was 30–40% recovery of their hypoxic ventilatory responses which was not due to reinnervation or a return of function of the denervated carotid chemoreceptors (Bisgard et al., 1980). Subsequent aortic arch denervation eliminated the recrudescent hypoxic response, but failed to alter eupneic PaCO2. Thus, increased activity of aortic body chemoreceptors and not a reinnervation of the carotid bodies accounted for plasticity in the hypoxic ventilatory responses, but did not account for the plasticity in eupneic breathing which may be occurring within the CNS.
Medullary serotonergic (5-HT) raphe neurons are embedded in the neural network generating ventilatory pattern and rhythm and provide a major source of excitatory neuromodulation to pre-motor and motor nuclei throughout the CNS (Hodges and Richerson, 2010). 5-HT receptor activation alone is sufficient to induce and is involved in the maintenance of central respiratory plasticity (Hodges and Richerson, 2008), and contributes to the recovery of eupneic breathing after peripheral nerve injury (Mitchell et al., 2000) (Kinkead et al., 1998). 5-HT can initiate the long-lasting increase in respiratory motor output that follows acute intermittent hypoxia (i.e. phrenic long-term facilitation, pLTF) (Fuller et al., 2001), and the upregulation of 5-HT innervation has been shown after recovery of respiratory function after cervical dorsal rhizotomy (CDR) (Kinkead et al., 1998) and thoracic dorsal rhizotomy (TDR) (Mitchell et al., 2000). Furthermore, the 5-HT receptors appear to mediate the establishment of oxygen chemosensitivity in the aorta following carotid body denervation (CBD) in developing piglets (Serra et al., 2002a). These and other data highlight an important role of the 5-HT system in facilitating specific forms of respiratory neuroplasticity and/or recovery following perturbations.
In addition, activity-dependent neuroplasticity is a major mechanism governing neuroplasticity in higher brain regions. However, it may also contribute to recovery of function within the brainstem under conditions of altered respiratory activity with chronic hypoxia (Kline et al., 2007) or following injury. Activity-dependent neuroplasticity, or homeostatic plasticity, is thought to be a cell-autonomous process that restores the activity of a neuron or neural network from a reduced state to normal through upregulation of specific glutamatergic (AMPA and NMDA) receptor subunits. This process may theoretically occur in brainstem nuclei following experimental peripheral deafferentation like that after CDR, TDR, or CBD. Indeed, we have recently demonstrated that CBD in adult goats leads to severe hypoventilation 3–5 days post-CBD, which modestly but significantly recovered within 30 days post-CBD (Miller et al., 2014). Expression of glutamatergic receptor subunits GluA2 and GluN1 was decreased in several brainstem nuclei acutely after CBD, but then were restored to pre-CBD levels by 30 days post-CBD. This suggests that despite an initial decrease, the compensatory increase in AMPA and NMDA receptor levels may contribute to the functional restoration of eupneic breathing after peripheral nerve sectioning.
Given that the rat exhibits a relatively robust ventilatory recovery/plasticity after CBD, it is an ideal model for studies aiming to elucidate brainstem-specific mechanisms of neuroplasticity following peripheral nerve injury/sectioning. Herein we utilized mRNA Sequencing (Puissant et al., 2015) (RNASeq) to measure gene expression changes in the 5-HT neuron-rich medullary raphe nuclei at two time points after CBD in rats to gain insights into mechanisms driving the restoration of breathing following CBD. Here we tested the hypothesis that the restoration of eupneic breathing acutely or chronically after CBD depends on 5-HT-specific and/or glutamate receptor-dependent changes in medullary raphe gene expression. Concomitantly, we sought to determine if there are additional genes and/or signaling pathways that may contribute to the restoration of ventilation after CBD in adult rats.
2. Methods
Adult (7–8 weeks) male Sprague Dawley rats ((Harlan) SD; n = 39) were used in this study. All rats were housed in the Biomedical Research Center, allowed access to low salt chow (Dyets 0.4% NaCl) and water ad libitum, and maintained on a 12:12 h light/dark cycle. All experimental protocols were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee prior to commencing the study.
2.1. Experimental design
All rats were chronically instrumented with indwelling femoral artery and vein catheters as previously described (Mouradian et al., 2012). Three or more days later, ventilation was measured and arterial blood was sampled while breathing room air (RA; FIO2 = 0.21, bal. N2) for 20 min or hypoxic gas (FIO2 = 0.12, bal. N2) in a 10 L flow-through, whole body plethysmograph. Arterial blood was sampled (0.4 mL) during the last 3 min of RA or hypoxia. In additional studies, ventilatory responses to venous injections of NaCN (3 mg mL−1) or saline (0.9% NaCl) were also measured in RA as previously described (Mouradian et al., 2012). Rats then underwent sham or CBD surgery and breathing studies were repeated at regular intervals thereafter (1–2, 4–5, 9–10, and 14–15 days post-surgery). Hypoxic and NaCN ventilatory responses and corresponding blood gases were measured pre- and 1 or 2 days post-surgery. RA ventilation and blood gases were measured at each of the indicated intervals. A total of four groups of rats were used. The first group of rats was denervated and euthanized 1–2 days post-CBD (“CBD Acute”) and a second group of rats was denervated and euthanized 14–15 days post-CBD (“CBD Chronic”). Each denervation group had a corresponding time control, sham-denervated group of rats (“Sham Acute” and “Sham Chronic”). This study design permitted subsequent gene and biological function comparisons associated with peak hypoventilation (CBD Acute vs Sham Acute), the recovery process (CBD Acute vs CBD Chronic), and significant recovery of ventilation (CBD Chronic vs Sham Chronic) while controlling for time and surgery (Sham Acute vs Sham Chronic; Fig. 1).
Fig. 1.
Study design showing the four raphe transcriptome comparisons employed to identify gene expression changes and biological functions associated with peak hypoventilation (#1, Acute CBD vs Sham Acute), the recovery process (#2, CBD Acute vs CBD Chronic), and significant recovery of ventilation (#3, CBD Chronic vs Sham Chronic) while controlling for time and surgery (#4, Sham Acute vs Sham Chronic).
2.2. Surgical protocols
All catheterization, sham, and CBD surgeries were carried out using aseptic technique as previously described (Mouradian et al., 2012). Rats received intraoperative injections of Carprofen (Rimadyl; 5 mg kg−1 i.p.) for analgesia and enrofloxacin (Baytril; 1 mg (100 g)−1) to prevent infection. Additional Carprofen was given BID for 48 h, and Baytril (1 mg/100 mL) supplied in the drinking water continuously after surgery.
2.3. Tissue collection and mRNA sequencing
Upon completion of physiological protocols, the rats were deeply anesthetized (20% isoflurane in propylene glycol) and upon respiratory arrest the brain extracted, embedded with embedding media and flash frozen and stored (−80 °C) until being placed into a chilled stainless steel matrix for ~1 mm serial sectioning (Puissant et al., 2015). Frozen tissue punches (~0.85 mm in diameter) were collected from the ventral midline from 2 serial sections beginning with the one containing Obex as previously described (Puissant et al., 2015), and stored in Eppendorf tubes (−80 °C) until RNA extraction.
Total RNA was extracted using standard Trizol-chloroform procedures, and was qualified and quantified (Pico or Nano Assay, Agilent 2100 Bioanalyzer). 32 of 36 samples had an RNA integrity number (RIN) of 7.5 or greater indicative of high integrity/quality of RNA. 4 samples had RIN values between 6 and 7.3. Total RNA samples were processed and made into cDNA libraries per manufacturer instruction (Illumina) for mRNA sequencing (HiSeq 2000) as described previously. Sequence data were aligned to the rat reference genome for identification and quantification of transcripts, and differential expression determined using an in-house pipeline described previously (Puissant et al., 2015; Liu et al., 2014).
2.4. Statistical analyses
Statistical significance of differential expression of transcripts was first set to a q value of 0.05 (a 0.05 q value represents a P value normalized for multiple comparisons and indicates a 5% false discovery rate), and later relaxed to a q value of 0.10 (equates to an un-normalized P value of 0.001) to provide more insight into represented biological functions of differentially expressed genes. Ingenuity Pathway Analysis (IPA) was used for its “core analysis” and “core comparison analysis” bioinformatics tools to gain insight into the biological functions represented by the differentially expressed genes (based on a q value of 0.10) and then to identify which biological functions were more or less represented during each phase after CBD (peak hypoventilation, recovering, recovered, over time). Core analyses yielded lists of biological functions predicted to be activated or de-activated based on a predicted activation Z-score (calculated based on number and direction of expression of genes known or associated with each function).
Two way repeated measure ANOVAs using GraphPad Prism v6.05 were used to assess statistical significance (P < 0.05) of resting blood gases and ventilatory measurements between pre- and post-surgery across all 4 groups of rats. Average values and SEM are expressed when applicable.
3. Results
3.1. Raphe tissues obtained from physiologically distinct phases after CBD
Resting PaCO2 was not statistically different among pre-CBD values across Sham Acute (30.7 ± 0.64 mm Hg; n = 5), CBD Acute (32.1 ± 0.57 mm Hg; n = 9), Sham Chronic (33.0 ± 0.84 mm Hg; n = 9), and CBD Chronic (30.7 ± 0.35 mm Hg; n = 9; P > 0.05; Fig. 2A) groups, and the values measured were similar to previous reports in adult rats (Mouradian et al., 2012; Hodges et al., 2002).Eupneic PaCO2 was increased in the CBD Acute (42.7 ± 0.76 mm Hg; n = 10) group relative to pre-CBD control (P < 0.05) 1–2 days after CBD. We found no difference in PaCO2 among Sham Acute (30.6 ± 0.74 mm Hg) and CBD Acute (32.1 ± 0.55) groups compared to pre-CBD values (P > 0.05; Fig. 2A, left). We did find a small yet significant difference in PaCO2 among Sham Chronic (33.0 ± 0.84 mm Hg), and CBD Chronic (30.6 ± 1.06 mm Hg) groups compared to pre-CBD values (P < 0.05; Fig. 2A, right). All rats within the CBD Chronic group demonstrated an elevated resting PaCO2 1–2 days after CBD compared to pre-CBD values (40.0 ± 0.5 mm Hg; P < 0.001; Fig. 2A, right). The elevated resting PaCO2 1–2 days after CBD in CBD Chronic rats was significantly decreased at 14–15 days post-CBD (symbols not shown on graph; P < 0.001; Fig. 2A, right) indicative of ventilatory recovery. We found similar differences in resting PaO2 among the various groups. Resting PaO2 1–2 days after CBD in the Acute CBD group (70.8 ± 2.41 mm Hg) was significantly different compared to both pre-CBD values (83.4 ± 2.74 mm Hg; P < 0.001) and Sham Acute values 1–2 days after surgery (82.9 ± 1.66 mm Hg; P < 0.01; Fig. 2B, left). CBD Chronic and Sham Chronic rats had significantly different PaO2 pre-surgery values (91.3 ± 2.19 vs 80.8 ± 1.49 mm Hg, respectively; Fig. 2B, right). 1–2 days after surgery, CBD Chronic rats significantly decreased resting PaO2 (P < 0.0001; 67.5 ± 2.17 mm Hg), which returned to pre-surgery values between 14 and 15 days (P > 0.05; 77.0 ± 1.57 mm Hg; Fig. 2B, right).
Fig. 2.
A–C. Resting blood gases and ventilation before and after surgery. Resting PaCO2 (A), PaO2 (B) and minute ventilation/100 g (C) pre-surgery and 1–2 days post-surgery in Acute CBD and Acute Sham groups and 1–2, 4–5, and 14–15 days post-surgery in Chronic CBD and Chronic Sham groups. CBD elicited significant hypoventilation 1–2 days after surgery in the Acute CBD group indicated by elevated resting PaCO2, decreased PaO2, and decreased resting ventilation (A–C, left hand side). Chronic CBD rats elicited significant hypoventilation 1–2 days after surgery indicated by a decrease in resting PaCO2 and PaO2 which returned to pre-CBD values by 14–15 days after surgery (A–B, right hand side). Resting minute ventilation normalized to 100 g of body weight (C). *, significantly (P < 0.05) different compared to pre-surgery and #, significantly (P < 0.05) different compared to respective sham value within time point.
Eupneic VE was decreased to 70.5 ± 7.1 VE/100 g acutely after CBD in the CBD Acute group compared to pre-CBD values (P < 0.05; 104.7 ± 6.3 VE/100 g; Fig. 2C, left). Although eupneic VE was not different between pre-CBD and 1–2 days post-CBD in CBD Chronic (P > 0.05), eupneic VE was significantly less 1–2 days post-surgery between CBD Chronic (91.1 ± 9.1 VE/100 g) and Sham Chronic groups (P < 0.05; 140.9 ± 17.6 VE/100 g; Fig. 2C, right). The HVR was decreased relative to pre-CBD values in the CBD Acute (P < 0.05; 73.7 ± 9.5%) and CBD Chronic (P < 0.05; 92.6 ± 9.2%) groups (Fig. 3A), consistent with the previously documented effects of CBD in rats (Mouradian et al., 2012). The ventilatory response to NaCN, which is a chemical test of peripheral chemoreceptor sensitivity (Bisgard et al., 1976; Lowry et al., 1999), was significantly attenuated after CBD in both CBD Acute and CBD Chronic rats (P < 0.05), but not in Sham Acute and Sham Chronic rats 1–2 days after surgery (P > 0.05; Fig. 3B). The residual NaCN ventilatory response that we did detect (values above 1) in CBD groups has been reported by others, and suggests hypoxic chemosensitivity at sites other than the carotid bodies (Martin-Body et al., 1985; Martin-Body et al., 1986; Serra et al., 2002b).
Fig. 3.
A–B. Confirmation of denervation by hypoxic and NaCN ventilatory responses. Hypoxic ventilatory response as a percent of room air (RA) ventilation (A) and NaCN response ratio (B) before and 2 days after surgery. *, significantly (P < 0.05) different compared to pre-surgery/intact.
3.2. Does the expression of 5-HT- and glutamatergic signaling-related genes change acutely and/or chronically after CBD?
More than 32 million reads were sequenced for all 12 libraries with only 2 libraries (both CBD Acute) slightly below 30 million reads. The mean quality score was 35 out of 40 representing a 99.95% base calling accuracy for all 12 libraries. ~95% of all bases sequenced had a mean quality score of ≥30, indicating a 99.9% base calling accuracy in all 12 libraries. Approximately 90% of all reads were mapped to the reference Brown Norway rat genome for all libraries (Table 1). A stringent false discovery rate of 5% (q < 0.05) was set as a first pass analysis to assess differentially expressed genes between groups, including at peak hypoventilation (CBD Acute vs Sham Acute; 108 genes), during recovery from CBD (Acute CBD vs Chronic CBD; 62 genes), at a significantly recovered state (CBD Chronic vs Sham chronic; 49 genes), and in time controls (Sham Acute vs Sham Chronic; 25 genes). However, we found that using a more inclusive statistical significance threshold (P < 0.001, corresponds to ~q < 0.10), the numbers of differentially expressed genes increased slightly in the peak hypoventilation (154 genes), recovering (69), recovered states (80), and time control (39 genes) groups (Fig. 4).
Table 1.
Summary of RNA Sequencing and quality of bases and reads for each group of rats used. There were 3 cDNA libraries sequenced per group (Column 1). On average, each group had over 30 million reads (FPKM; Column 3). Column 3, 94% of all bases sequenced had a quality score (PF) higher than 30 indicating 99.9% base calling accuracy or better. On average the base calling accuracy was 35 (Column 4). Column 5, ~90% of all reads were mapped to the Brown Norway Reference Genome.
| Group | Avg # reads (FPKM) | % of ≥Q30 bases (PF) | Mean quality score (PF) | % of mapping rate |
|---|---|---|---|---|
| CBD Acute | 30,471,037 | 94.4 | 35.2 | 89.3 |
| Sham Acute | 34,385,343 | 94.2 | 35.2 | 89.6 |
| CBD Chronic | 32,622,701 | 94.2 | 35.2 | 89.7 |
| Sham Chronic | 32,582,579 | 94.1 | 35.1 | 89.7 |
Fig. 4.
Unique and total number of differentially expressed genes for each of four transcriptome comparisons using a more inclusive statistical significance threshold. Differentially expressed genes represent significantly up- and down-regulated genes. The greatest numbers of differentially expressed genes were found 1–2 days post-CBD.
3.2.1. Serotonergic plasticity-related gene expression after CBD
There were 10 genes related to the 5-HT system with high expression (FPKM > 1) including those important for cell fate determination (Fev (Pet-1)), 5-HT biosynthesis (Tph2 and Ddc), 5-HT transport (Slc6a4 and Slc18a2), 5-HT receptors (Htr5b and Htr2c), co-released neuropeptides (Trh and Tac1) and associated receptors (Tacr1). However, all but 2 of these transcripts were unchanged after CBD. Htr5b (the 5-HT5B receptor) was significantly reduced, and Htr2c (the 5-HT2C receptor) was increased at peak hypoventilation and chronically after CBD, respectively (Fig. 5A). Thus, there was a strong representation of 5-HT specific genes (Tph2, Fev, Ddc) confirming that raphe genes were well represented in the tissue punches, but the lack of differential expression among all groups is counter to our hypothesis and data from goats after CBD (Miller et al., 2013; Miller et al., 2014).
Fig. 5.
Serotonergic and glutamatergic genes were abundant though few showed differential expression after CBD. There were 10 serotonergic (A) and 13 glutamatergic (B) related gene transcripts, some with high abundance (log2(1 + FPKM)) across the four different transcriptomes. Note, only 2 serotonergic receptor subunits, Htr5b and Htr2c, and 1 glutamatergic receptor subunit, Gria2, were significantly different in abundance relative to respective sham groups. *, significantly (P < 0.05) different compared to respective sham group.
3.2.2. Glutamatergic plasticity-related gene expression after CBD
Given the importance of glutamatergic receptors in known forms of neuroplasticity (e.g. Hebbian and Homeostatic plasticity), we analyzed the changes in gene expression related to AMPA and NMDA receptors and associated molecules involved with trafficking these receptors as highlighted in the review by Wang et al. (2012) We found 13 genes involved with AMPA receptor and NMDA receptor trafficking that were represented in the datasets, including specific molecules needed for AMPA receptor internalization (Arc, Dnm1, Pick1, Ubb). Activity deprivation elicits signaling cascades to up-regulate AMPA receptor expression and mediate new AMPA receptor synthesis. Many of the molecules important for this process were identified in the data, including the TNFα receptor 1 (Tnfsrf1a), the TrkB receptor (Ntrk2), and the alpha retinoic acid receptor (Rara). Furthermore, major AMPA and NMDA receptor subunits were represented, such as GluA2 (Gria2), GluA4 (Gria4), and NR1 (Grin1), where Gria2 was upregulated in the chronic CBD dataset consistent with mechanisms driven by activity deprivation (Fig. 5B). However, despite the presence of these plasticity-related transcripts in the datasets, the only CBD-related change in gene expression was in Gria2, suggesting that the ventilatory recovery from CBD is not dominated by major changes in gene expression of these glutamatergic systems.
3.3. Unbiased bioinformatics analyses of the altered raphe gene expression point to potential novel mechanisms that may drive ventilatory recovery after CBD
Overall, there were no major effects of CBD on serotonergic or glutamatergic gene expression in the medullary raphé as we originally hypothesized. Therefore, we approached the gene expression analysis using a global, unbiased approach by first looking at overall trends in gene expression between experimental groups and then gene and pathway associations in bioinformatics analyses (IPA).
Each comparison of genes identified and quantified in each experimental group led to slightly different numbers of commonly expressed genes (~17,000 transcripts/comparison; (Fig. 6A–D)). Most transcripts (>99%) of these common transcripts were not differentially expressed among the groups (gray data in Fig. 6A–D). However, the greatest numbers of differentially expressed genes were among the acute CBD and acute sham comparison, where 73 transcripts were upregulated and 81 transcripts were downregulated (Fig. 6A). Comparing the changes in gene expression during the chronic phase after CBD with that which occurs during peak hypoventilation we found 52 downregulated genes and 24 upregulated genes (Fig. 6B), where the fold-change was generally greater for those downregulated. In contrast, there were 27 transcripts upregulated and 54 downregulated comparing CBD Chronic to Sham Chronic groups (Fig. 6C), where the magnitude of the detected changes were small relative to the comparisons to the Acute CBD group. We found only 2 upregulated and 43 downregulated transcripts in the control group comparison (Fig. 6D), representing the fewest number of differentially expressed transcripts in all comparisons.
Fig. 6.
Acute hypoventilation has the most up- and down-regulated genes among four different transcriptome comparisons. Transcriptome comparisons were carried out between CBD Acute and Sham Acute (A) CBD Chronic and CBD Acute (B), CBD Chronic and Sham Chronic (C), and Sham chronic and Sham Acute (D). Top 10 up- and downregulated genes per comparison are listed to the right of each graph (expressed in fold-change).
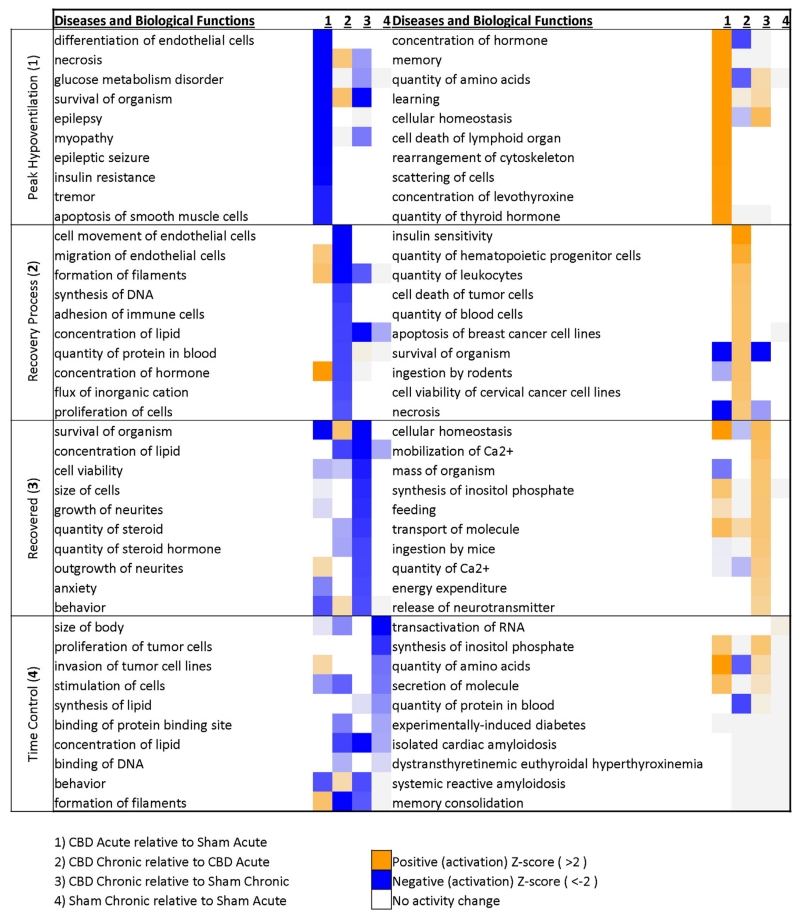
Bioinformatics analyses led to the identification of potential correlations among all differentially expressed genes in each comparison first by the identification of predicted Biological Functions affected and then whether those biological functions were predicted to be activated or deactivated (z-score; Fig. 7). We first noted that for any given set of deactivated (blue) or activated (orange) Disease or Biological Function identified in each comparison, that it was relatively unique to that experimental group comparison. For example, the top 3 predicted activated Biological Functions during peak hypoventilation (comparing CBD Acute and Sham Acute; column 1) included “concentration of hormone”, “memory”, and “quantity of amino acids”. These predicted activated functions are unique to peak hypoventilation as column 2 (comparing CBD Chronic to CBD Acute), 3 (comparing CBD Chronic to Sham Chronic), and 4 (comparing Sham Chronic with Sham Acute) do not have these functions predicted. In addition, there were few associations made with the few changes in gene expression among the Sham Acute and Sham Chronic control comparisons, and where associations were identified these were not consistent among other experimental groups. Thus, each set of differentially expressed genes among experimental groups were associated with unique activated or deactivated Biological Functions, suggesting that the changes in gene expression were not uniform across groups and the strength of associations of these changes were greatest in comparisons including CBD animals.
Fig. 7.
Distinct biological functions are associated with each phase of recovery following CBD. Bioinformatics analysis of biological functions for peak hypoventilation (upper panel and column #1) were associated with a significant change in endothelial cells and hormone related functions. These were unique to peak hypoventilation as other phases of recovery did not share similar z-scores (columns 2–4). The recovery process (middle upper panel and column #2) was associated with changes in immune related processes and also unique to other phases of recovery. The recovered state (middle bottom panel and column #3) was associated with general functions pertaining to general cellular phenomenon.
We first focused on the predicted Biological Functions identified with differentially expressed genes during peak hypoventilation after CBD. Predicted deactivated functions/diseases included “epilepsy”, “epileptic seizures”, “necrosis” and “apoptosis of smooth muscle cells” (P < 0.001;Fig. 7, upper left), suggesting reduced probability of a hyperexcitable state and reduced cell death. Predicted activated functions included increased concentrations and/or quantities of “hormone” and “amino acids”, increased “memory” and “learning”, and increased “cellular homeostasis” and “rearrangement of cytoskeleton” (P < 0.001; Fig. 7, upper right). During the recovery process comparing CBD Acute to CBD Chronic groups, there were 3 different immune response-related functions predicted to be activated, including increases in the “quantity of hematopoietic progenitor cells”, the “quantity of leukocytes”, and the “quantity of blood cells” (P < 0.005; Fig. 7, second row, right) among others. During this same time, deactivated functions identified included a reduced “movement/migration of endothelial cells” and “formation of filaments”, and a reduced “adhesion of immune cells” and “proliferation of cells” (P < 0.005; Fig. 7, second row left). Comparing the Chronic CBD to the Sham Chronic group, “cellular homeostasis” was predicted to be increased (P < 0.05; Fig. 7, third row, right), similar to that in the Acute CBD comparison. Additional functions predicted to be activated during this phase were increased “mobilization” and “quantity of Ca2+ “and increased “release of neurotransmitters” (P < 0.005), while a decrease was predicted for “cell viability”, “cell size”, “growth of neurites”, and the “quantity of steroids” (P < 0.005; Fig. 7, mid lower panel).
While some pathways and/or functions identified above appeared not to be related, an accounting of functions across the experimental process begin to hint to a more complete picture. Shown in Fig. 8 are identified biological functions associated with gene expression changes at multiple points after CBD. Note that brainstem gene expression at peak hypoventilation indicated changes in hormone levels, migration and proliferation of monocyte and endothelial cells. After additional time, gene expression changes from acute to chronic CBD states were predictive of changes in the quantity and maturation of several cell types, including highlighted hematopoietic progenitor, leukocytes, granulocytes and T lymphocytes. In the chronic state, after ventilatory recovery from CBD has occurred, gene expression profiles were indicative of a state of homeostasis and general viability, with processes centered on ion homeostasis and intracellular calcium. Thus, it appears that raphe gene expression profiles and associated biological functions (based on Z-scores) during each “state” following CBD provide a novel, hypothesized series of changes centered on hormones and shifts in immune-related signaling pathways, influx of immune related cells (recovery process), and increases in functions pertaining to general cellular phenomenon (recovered state). To better gauge the representation of these functions in our data sets we curated all related biological functions with activation scores (Fig. 8). Curation of gene lists associated with each biological function incorporates genes that are upregulated (bolded) or downregulated (not bolded) from the data sets (Fig. 8, right column). In doing so, there are a significant number of other, related biological functions aside from those in the top 10 (highlighted by an *), especially pertaining to immune related processes during recovery and a core set of genes are often listed across related though different biological functions.
Fig. 8.
In depth curation of all biological functions emphasize the role of immune-related processes in driving the recovery of breathing. Key themes from Fig. 6 were investigated further by manually curating all related biological functions associated with the unbiased bioinformatics analysis of the differentially expressed genes. Peak hypoventilation is associated with 9 additional functions pertaining to endothelial cells/blood vessels, and the involvement of an immune mediated recovery is supported with the addition of 12 functions related to immune functions. Biological functions from Fig. 6 are noted with an asterisk (*). Genes related to all listed biological functions are noted in the right column. Bolded gene names are upregulated and non-bolded gene names are downregulated.
3.4. Predicted upstream regulators of raphe gene expression point to the potential importance of increased growth factors and cytokines during different phases of CBD-induced ventilatory plasticity
The unbiased bioinformatics analyses of the differentially-expressed genes also provided lists of predicted molecules that could act as upstream regulators for the resulting changes in expression. There were 7 growth factors and 3 receptors predicted to be activated acutely after CBD (Fig. 9; top row, right), including Fgf2 and Fgf receptors 1 and 2, Tgfβ and Tgfβ1, and bone morphogenic factors (Bmp2 and Bmp6). However, the top 10 predicted decreased upstream regulators were more variable, including transcriptional regulators (Hdac1, Hdac, and Pou4f2) and a cytokine (IL2) among others (Fig. 9, top row, left). In contrast, when comparing gene expression between CBD Acute and CBD Chronic groups there were 7 cytokines, cytokine receptors or groups that were predicted to be increased (INFγ, INFAR, INFβ1, TNF, IFNα2, Ifnα, IFN, and IL-27), along with the cytokine-regulated NFκB. In this same comparison, top predicted regulators reduced were growth factors (EGF and NGF) and receptors (EGFR), a cytokine (IL-100 and a microRNA (Mir-21; Fig. 9, second row, left). Similarly, 7 of the top 10 regulators predicted to be activated comparing the CBD Chronic to Sham Chronic groups were also growth factor and cytokine-related molecules (Fig. 9, third row, right), with a variable list of molecules predicted to be reduced (Fig. 9, third row, left). In summary, it appears that the gene expression changes are predictive of general shifts in upstream regulators that are activated acutely after CBD (growth factors and receptors) to a more chronic state (cytokines and receptors).
Fig. 9.
Cytokines are predicted upstream regulators driving the recovery process. Bioinformatics analysis of upstream regulators predicted various growth factors to be upregulated at peak hypoventilation (upper panel and column #1). The recovery process (middle upper panel and column #2) was predicted to have upstream regulators in the form of different cytokines. The recovered state (middle bottom panel and column #3) had a mix of cytokines and growth factors.
4. Discussion
Carotid body denervation has been performed experimentally in several animal species, and has been performed in humans as a form of therapeutic surgical intervention to alleviate disease-related symptoms. The effects of CBD on eupneic ventilation are essentially uniform across these species in causing hypoventilation and CO2 retention, but are highly variable in terms of how long it takes for eupneic breathing to return to pre-CBD levels (if at all). It has been long hypothesized that the time-dependent restoration of eupneic ventilation after CBD is driven by a combination of peripheral and central mechanisms, including changes in the glutamatergic and serotonergic systems that underlie various forms of neural plasticity in the brainstem and spinal cord. Herein, we investigated the potential central mechanisms via time-dependent transcriptional changes over the course of ventilatory recovery within the meduallary raphe nucleus. We did not investigate whether the transcriptional changes were primary or secondary to extracarotid peripheral chemoreceptor adaptation. Our study design measured acute and chronic gene expression changes but not the progression of gene expression changes over the course of hypoventilation to recovery. Nonetheless, CBD in the rat elicited significant, time-dependent shifts in medullary raphe gene expression suggestive of major changes in the adaptive and innate immune systems and limited or no changes in serotonin- or glutamate-dependent mechanisms important for other forms of neuroplasticity.
4.1. Unbiased bioinformatics analyses point to immune-related changes in the brainstem after CBD
Among the many genes differentially expressed acutely after CBD, the expression profiles provided evidence of structural changes and/or disruption of the blood brain barrier and were predictive of the release of major growth factors (e.g. TgfB and FGFs) and other hormones (TRH and others). Growth factors play critical roles in neuronal development and survival in response to injury, including processes that pertain to cellular and synaptic structural changes. Major transcripts identified herein contribute to the survival of neurons and include fibronectin (Fn1), connective tissue growth factor (Ctgf), medium and heavy neuro-filaments (Nefm and Nefh, respectively), fibroblast growth factor 1 and 13 (Fgf1 and −13), and others. Representative of these transcript changes are biological processes specific to structural changes (morphology of nervous system, outgrowth of neurites, and differentiation of neurons, for example). Although these changes are features during brain development, such processes are also activated to drive recovery after various types of physiologic insults (Finger and Almli, 1985). Indeed, fibroblast growth factors (Fgf), nerve growth factor (Ngf), transforming growth factor beta (Tgfβ), and many others are critical in regulating developmental features (cell growth, axon regeneration, etc.) all of which are predicted to be upstream regulators of the transcript changes measured acutely after CBD.
The sources of the above factors include neurons, astrocytes, and glial cells. Glial cells are critical components of the CNS as they regulate blood flow and the blood brain barrier, extra-synaptic neurotransmitter concentrations, synaptic morphology, and facilitate neural plasticity through their continuous monitoring of neuronal activity (Hanisch and Kettenmann, 2007; Buffo et al., 2010). Additionally, microglia cells are the innate immune system of the brain which can induce immune responses due to neural injury in the form of an M1 (pro-inflammatory) and a subsequent M2 (resolving/anti-inflammatory) phenotype (Schwartz et al., 2013). Because markers of astrocytes and M2 microglial activation (glial fibrillary acidic protein, Gfap and CD74 and Hla-dra, respectively) are downregulated acutely after CBD, it is likely that glial cells are expressing the M1, pro-inflammatory phenotype, which persists during the recovery period as indicated by the influx of cytokines. Indeed, peak hypoventilation and the recovery process have elevated levels of Il1B and Tnfa transcripts, markers of the M1 phenotype (Schwartz et al., 2013). Although there is a significant increase in Tgfβ, a potent anti-inflammatory cytokine, the activation, initiation, and resolution of an immune response is complex and dynamic.
Whereas glial cells appeared to demonstrate only the M1-pro-inflammatory phenotype acutely after CBD, it is quite apparent that glial cells are activated (both M1 and M2 phenotypes) and immune responses are mounted during the longer-term recovery process following CBD. Immune responses generated by microglia can be detrimental and exacerbate neural pathologies but paradoxically can have neuroprotective affects by promoting trophic factors and remyelination (Nguyen et al., 2002). The innate immune system responds first to neuronal injury and as mentioned in the previous section, is represented at peak hypoventilation (Il1β) and during the recovery process (Tnfα) in the form of the M1 phenotype producing growth factors (acutely after CBD) and cytokines (observed during recovery). Resolution of the M1 phenotype is critical to facilitating neural repair and requires circulating monocytes (upregulated in the data set) that express the M2 phenotype (Schwartz et al., 2013). Characteristic of the M2 phenotype is the expression of MHCII (upregulated in the data set, Hla-dra). The recruitment of inflammatory cells supports evidence for axonal regeneration (Yin et al., 2006), cell renewal (Martino et al., 2011), and resolution of inflammation (Shechter et al., 2009). For example, recruited macrophages facilitate the resolution of inflammation following spinal cord injury in mice (Shechter et al., 2009). The recruitment of circulating blood-derived macrophages and leukocytes from the blood and to the site of injury through the blood brain barrier occurs at the choroid plexus via T cells, permitting these circulating cells to cross into the brain only when stimulated by interferon gamma. Herein, Ifnγ is predicted to be upregulated and is a major cytokine released from glial cells and, our results suggest an influx of leukocytes and blood cells. The targeting of leukocytes to the site of injury likely occurs through the elevated expression of two chemokines, Cxcl10 and 11, both significantly upregulated in our data sets (during recovery and upon recovery) and have been shown to recruit leukocytes and generate T cells (Dufour et al., 2002). The recruitment of T cells is critical for the subsequent targeting and accumulation of the M2 monocyte-derived macrophages which helps resolve the inflammation as demonstrated by the reduced number of molecules and biological functions related to a pro-inflammatory phenotype (Shechter et al., 2009). The recruitment of leukocytes is further suggested based on our observation that rats lacking the ability to generate mature T and B Cells demonstrate a significant disruption in the recovery process (unpublished observations). Thus, these gene expression data suggest major changes in adaptive and innate immune response-related molecules, giving rise to a new working model and hypothesis to be further tested.
4.2. CBD induces minimal changes to serotonergic and homeostatic neural plasticity related transcripts
Serotonin modulates the excitability of both central (Manzke et al., 2003; Johnson et al., 2001; Hodges and Richerson, in press) and peripheral chemoreceptor sensitization (Serra et al., 2002a). Furthermore, serotonin is an initiating factor of respiratory plasticity (pLTF) (Fuller et al., 2001; Baker-Herman and Mitchell, 2002), and is upregulated upon ventilatory recovery after spinal cord injury (Mitchell et al., 2000; Kinkead et al., 1998). Glutamatergic molecules, especially AMPA and NMDA receptors are key molecules in homeostatic plasticity, a concept used to describe a neuron’s or network’s ability to re-establish/maintain a target firing rate given increased or decreased synaptic activity (Turrigiano and Nelson, 2004). A target firing rate is attained through trafficking of AMPA receptors to or from the synaptic terminal by coordinated signaling cascades as highlighted by Wang et al. (2012). Given the hallmark hypoventilation that ensues following CBD, we simultaneously tested whether the serotonergic system and/or AMPA receptor trafficking and related molecules would be up-regulated during the recovery of breathing following CBD. To do so, we first collected and confirmed physiologic data demonstrating the acute hypoventilation and recovery following bilateral CBD. Using medullary raphe tissue from these rats, RNA sequencing was employed to test our hypotheses.
Many serotonergic related genes were identified and highly expressed in all four groups of rats (CBD Acute and Chronic, and Sham Acute and Chronic) confirming the sampling of the medullary raphe. The 5-HT related genes measured are involved in 5-HT synthesis, vesicular packaging, reuptake and breakdown in addition to co-released neuropeptides and receptors (Fig. 5A). However, only two genes were differentially expressed over time (Htr5b and Htr2c), and one (Htr5b) in the opposite direction that was hypothesized. The lack of changes to the serotonergic system is inconsistent with previous work showing up regulation of 5-HT terminals after spinal cord injury (Mitchell et al., 2000; Kinkead et al., 1998) and downregulation of both the serotonin transporter (SERT, gene Slc6a4) and the rate limiting 5-HT synthesis enzyme, tryptophan hydroxylase (Tph2, gene Tph2), after partial ventilatory recovery from CBD in adult goats (Miller et al., 2013). However, this disparity may stem from comparing transcriptional effects after CBD with changes in actual protein as in previous studies. Indeed, in a subset of rats we quantified 5-HT turnover ratio in whole brainstem using HPLC and found peak hypoventilating rats have an elevated 5-HT turnover ratio compared to control and recovered rats (unpublished findings). Nonetheless, transcriptional 5-HT changes do not seem to be a major point of regulation in driving the recovery of breathing following bilateral CBD.
Next, potential transcriptional changes associated with the glutamatergic and homeostatic plasticity related gene transcripts were investigated. We assumed that CBD leads to a reduction in excitatory inputs into breathing centers, including the medullary raphe, based on the hypoventilation observed within 1–2 days post-CBD. This assumption was not corroborated as IPA predicted an increase in inhibitory processes including GABA receptor signaling and an increase in synaptic long-term depression during peak-hypoventilation and not upon significant recovery of breathing. This finding suggests that CBD induces a shift towards an inhibitory signaling phenotype rather an overall reduction of neural activity. However, the potential for an overall reduction of neural activity cannot be ruled out. As mentioned in the results, many genes pertaining to homeostatic plasticity (as described by Wang et al. (2012)) and the glutamatergic system were represented across the four groups of rats. A single gene was significantly upregulated, Gria2, which encodes for the GluA2 AMPA receptor subunit. Also, there was a predicted increase in calcium signaling during peak hypoventilation. Although changes in GluA2 subunits and calcium flux are hallmark features of homeostatic plasticity, there are no other supporting pieces of evidence from our transcriptional data suggesting that homeostatic plasticity is involved in the recovery from CBD induced hypoventilation. Furthermore, the phenomenon of synaptic scaling (a major form of homeostatic plasticity) occurs within 4 to 24 h of activity deprivation (Ibata et al., 2008; Sutton et al., 2006; Thiagarajan et al., 2005; Turrigiano et al., 1998) and is associated with a drop, not an increase, in calcium influx as predicted by our bioinformatics analysis. Furthermore, we are assuming CBD decreases neuronal activity, but it is also plausible that normal neural activity is maintained due to a shift away from excitatory signaling and towards greater inhibitory signaling.
Although our transcriptional (mRNA) data sets do not support the implication of homeostatic plasticity mechanisms, these mechanisms may be present at the protein level. Furthermore, we did not investigate whether the transcriptional changes were primary or secondary to extracarotid peripheral chemoreceptor adaptation, such as an increase in aortic chemoreceptor activity as demonstrated in piglets following CBD (Serra et al., 2002b). Additionally, the respiratory neural network may leverage mechanisms of homeostatic plasticity at other brainstem sites, particularly at the site of carotid input like the caudal portion of nucleus of the solitary tract (NTS) where the carotid sinus makes first order, glutamatergic synapses into the neural respiratory network. Nonetheless, our RNA Seq data do not implicate the involvement of homeostatic neural plasticity mechanisms (defined by others (Turrigiano and Nelson, 2004))in the recovery of breathing following CBD within the region of the medullary raphe. Additional experiments are needed to definitively determine if homeostatic plasticity in the form of synaptic scaling is or is not involved in the recovery of breathing after CBD both at the protein level and in other primary regions of carotid sinus input such as the NTS.
5. Summary and conclusions
Analyses of medullary raphe gene expression data acutely and chronically after CBD in rats suggests that the restoration of eupneic ventilation correlates to major shifts in gene expression profiles that regulate dynamic structural and immune-related CNS functions. However, the raphe expression data do not support the hypothesis that mechanisms related to glutamatergic or serotonergic systems are involved in the recovery of breathing within the medullary raphe following CBD. Based on the data herein, we conclude that immune-related mechanisms, whether from infiltrating peripheral immune cells or microglial-mediated functions, could contribute to the functional restoration of ventilation following CBD in rats.
Acknowledgments
Thank you to Dr. Bert Forster for discussions and revisions to the manuscript. Dr. Mingyu Liang and Chun Yang for assistance designing and preparing for the RNA Sequencing experiment. Funding sources, HL097033 and HL007852.
Abbreviations
- CBD
carotid body denervation
- SD
Sprague Dawley
- VE
minute ventilation
- VT
tidal volume
- 5-HT
serotonin
References
- Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J. Neurosci. 2002;22:6239–6246. doi: 10.1523/JNEUROSCI.22-14-06239.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgard GE, Forster HV, Orr JA, Buss DD, Rawlings CA, Rasmussen B. Hypoventilation in ponies after carotid body denervation. J. Appl. Physiol. 1976;40:184–190. doi: 10.1152/jappl.1976.40.2.184. [DOI] [PubMed] [Google Scholar]
- Bisgard GE, Forster HV, Klein JP. Recovery of peripheral chemoreceptor function after denervation in ponies. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980;49:964–970. doi: 10.1152/jappl.1980.49.6.964. [DOI] [PubMed] [Google Scholar]
- Buffo A, Rolando C, Ceruti S. Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem. Pharmacol. 2010;79:77–89. doi: 10.1016/j.bcp.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Dahan A, Nieuwenhuijs D, Teppema L. Plasticity of central chemoreceptors: effect of bilateral carotid body resection on central CO2 sensitivity. PLoS Med. 2007;4:e239. doi: 10.1371/journal.pmed.0040239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan A, Sarton E, Teppema L. Plasticity in the brain: influence of bilateral carotid body resection (bCBR) on central CO2 sensitivity. Adv. Exp. Med. Biol. 2008;605:312–316. doi: 10.1007/978-0-387-73693-8_54. [DOI] [PubMed] [Google Scholar]
- Dufour JH, Dziejman M, Liu MT, Leung JH, Lane TE, Luster AD. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002;168:3195–3204. doi: 10.4049/jimmunol.168.7.3195. [DOI] [PubMed] [Google Scholar]
- Finger S, Almli CR. Brain damage and neuroplasticity: mechanisms of recovery or development? Brain Res. 1985;357:177–186. doi: 10.1016/0165-0173(85)90023-2. [DOI] [PubMed] [Google Scholar]
- Forster HV. Plasticity in the control of breathing following sensory denervation. J. Appl. Physiol. 2003;94:784–794. doi: 10.1152/japplphysiol.00602.2002. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J. Appl. Physiol. 2001;90:2001–2006. doi: 10.1152/jappl.2001.90.5.2001. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir. Physiol. Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception, and thermoregulation. J. Appl. Physiol. 2010a;108:1425–1432. doi: 10.1152/japplphysiol.01270.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. The role of medullary serotonin (5-HT) neurons in respiratory control: contributions to eupneic ventilation, CO2 chemoreception and thermoregulation. J. Appl. Physiol. 2010b doi: 10.1152/japplphysiol.01270.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J. Appl. Physiol. 2002;93:974–983. doi: 10.1152/japplphysiol.00019.2002. [DOI] [PubMed] [Google Scholar]
- Honda Y. Respiratory and circulatory activities in carotid body-resected humans. J. Appl. Physiol. 1992;73:1–8. doi: 10.1152/jappl.1992.73.1.1. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Wilkerson JE, Henderson DR, Wenninger MR, Mitchell GS. Serotonin elicits long-lasting enhancement of rhythmic respiratory activity in turtle brain stems in vitro. J. Appl. Physiol. 2001;91:2703–2712. doi: 10.1152/jappl.2001.91.6.2703. [DOI] [PubMed] [Google Scholar]
- Kinkead R, Zhan WZ, Prakash YS, Bach KB, Sieck GC, Mitchell GS. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J. Neurosci. 1998;18:8436–8443. doi: 10.1523/JNEUROSCI.18-20-08436.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinkead R, Bach KB, Johnson SM, Hodgeman BA, Mitchell GS. Plasticity in respiratory motor control: intermittent hypoxia and hypercapnia activate opposing serotonergic and noradrenergic modulatory systems. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;130:207–218. doi: 10.1016/s1095-6433(01)00393-2. [DOI] [PubMed] [Google Scholar]
- Kline DD, Ramirez-Navarro A, Kunze DL. Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J. Neurosci. 2007;27:4663–4673. doi: 10.1523/JNEUROSCI.4946-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Liu P, Yang C, Cowley AW, Jr., Liang M. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in Dahl S rats: effect of salt and genomic sequence. Hypertension. 2014;63:827–838. doi: 10.1161/HYPERTENSIONAHA.113.02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry TF, Forster HV, Pan LG, et al. Effect of carotid body denervation on breathing in neonatal goats. J. Appl. Physiol. 1999;87:1026–1034. doi: 10.1152/jappl.1999.87.3.1026. [DOI] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, et al. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Martin-Body RL, Robson GJ, Sinclair JD. Respiratory effects of sectioning the carotid sinus glossopharyngeal and abdominal vagal nerves in the awake rat. J. Physiol. 1985;361:35–45. doi: 10.1113/jphysiol.1985.sp015631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Body RL, Robson GJ, Sinclair JD. Restoration of hypoxic respiratory responses in the awake rat after carotid body denervation by sinus nerve section. J. Physiol. 1986;380:61–73. doi: 10.1113/jphysiol.1986.sp016272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino G, Pluchino S, Bonfanti L, Schwartz M. Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol. Rev. 2011;91:1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Neumueller S, Muere C, et al. Changes in neurochemicals within the ventrolateral medullary respiratory column in awake goats after carotid body denervation. J. Appl. Physiol. 2013;115:1088–1098. doi: 10.1152/japplphysiol.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Neumueller S, Muere C, et al. Changes in glutamate receptor subunits within the medulla in goats after section of the carotid sinus nerves. J. Appl. Physiol. 2014;116:1531–1542. doi: 10.1152/japplphysiol.00216.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Bach KB, Martin PA, et al. Increased spinal monoamine concentrations after chronic thoracic dorsal rhizotomy in goats. J. Appl. Physiol. 2000;89:1266–1274. doi: 10.1152/jappl.2000.89.4.1266. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, et al. Invited review: intermittent hypoxia and respiratory plasticity. J. Appl. Physiol. 2001;90:2466–2475. doi: 10.1152/jappl.2001.90.6.2466. [DOI] [PubMed] [Google Scholar]
- Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation on ventilation and chemoreflexes in three rat strains. J. Physiol. 2012;590:3335–3347. doi: 10.1113/jphysiol.2012.234658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat. Rev. Neurosci. 2002;3:216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Puissant MM, Echert AE, Yang C, et al. RNASeq-derived transcriptome comparisons reveal neuromodulatory deficiency in the CO2 insensitive brown Norway rat. J. Physiol. 2015;593:415–430. doi: 10.1113/jphysiol.2014.285171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J. Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Simeon T, et al. Serotonin and serotonin receptor expression in the aorta of carotid intact and denervated newborns. Respir. Physiol. Neurobiol. 2002a;132:253–264. doi: 10.1016/s1569-9048(02)00119-2. [DOI] [PubMed] [Google Scholar]
- Serra A, Brozoski D, Hodges M, Roethle S, Franciosi R, Forster HV. Effects of carotid and aortic chemoreceptor denervation in newborn piglets. J. Appl. Physiol. 2002b;92:893–900. doi: 10.1152/japplphysiol.00819.2001. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 2009;6:e1000113. doi: 10.1371/journal.pmed.1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Wang G, Gilbert J, Man HY. AMPA receptor trafficking in homeostatic synaptic plasticity: functional molecules and signaling cascades. Neural Plast. 2012;2012:825364. doi: 10.1155/2012/825364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci. 2006;9:843–852. doi: 10.1038/nn1701. [DOI] [PubMed] [Google Scholar]