SUMMARY
The fate of mosquito sperm in the female reproductive tract has been addressed sporadically and incompletely, resulting in significant gaps in our understanding of sperm‐female interactions that ultimately lead to fertilization. As with other Diptera, mosquito sperm have a complex journey to their ultimate destination, the egg. After copulation, sperm spend a short time at the site of insemination where they are hyperactivated and quickly congregate near the entrance of the spermathecal ducts. Within minutes, they travel up the narrow ducts to the spermathecae, likely through the combined efforts of female transport and sperm locomotion. The female nourishes sperm and maintains them in these permanent storage organs for her entire life. When she is ready, the female coordinates the release of sperm with ovulation, and the descending egg is fertilized. Although this process has been well studied via microscopy, many questions remain regarding the molecular processes that coordinate sperm motility, movement through the reproductive tract, maintenance, and usage. In this review, we describe the current understanding of a mosquito sperm's journey to the egg, highlighting gaps in our knowledge of mosquito reproductive biology. Where insufficient information is available in mosquitoes, we describe analogous processes in other organisms, such as Drosophila melanogaster, as a basis for comparison, and we suggest future areas of research that will illuminate how sperm successfully traverse the female reproductive tract. Such studies may yield molecular targets that could be manipulated to control populations of vector species. Mol. Reprod. Dev. 83: 897–911, 2016. © 2016 The Authors. Molecular Reproduction and Development Published by Wiley Periodicals, Inc.
INTRODUCTION
The battle to control mosquito disease vectors is among the greatest public health challenges of our time. Anopheles mosquitoes cause nearly 200 million cases of malaria annually, and kill approximately 600,000 people each year (World Health Organization, 2014). Aedes aegypti and Aedes albopictus transmit dengue viruses, which cause more than 100 million infections (Bhatt et al., 2013), as well as emerging viral threats such as chikungunya (Staples and Fischer, 2014) and Zika (Fauci and Morens, 2016). Many mosquito‐borne pathogens have no commercially licensed vaccine or cure; even for those with a cure, such as malaria, drug resistance poses a serious challenge (Murai et al., 2015). The most effective tools against mosquito‐borne pathogens therefore remain those focused on regulating vector populations.
While most mosquito‐control strategies focus on killing adults or larvae, strategies that target mosquito reproduction hold significant promise. To date, studies of reproduction have focused primarily on female biology and aspects of egg development and deposition, but relatively little work has been conducted on male contributions to reproduction. A deeper understanding of sperm motility and interactions with the female reproductive tract will be valuable to devising field‐ready, practical targets for use in new control strategies.
Studies of mosquito sperm may also advance our understanding of sperm biology in other animals, including humans. Many aspects of sperm biology are conserved across Animalia. Drosophila melanogaster is the model organism of choice for many biological phenomena, but the unusual length of their sperm (1.9 mm) makes some aspects of their biology—such as sperm motility—difficult to study. Mosquitoes are an excellent surrogate model, as their sperm are about one‐eighth the length (depending on species) and much more tractable for investigation.
Here, we describe the journey of mosquito sperm through the female reproductive tract to its ultimate destination, the egg. We first describe modulators of sperm motility, as these are critical to understanding how sperm move through the reproductive tract. A sperm's course throughout the female mosquito has been well characterized via microscopy, but little attention has been paid to molecular interactions among sperm, seminal fluid, and the environment within the female that assist sperm on their voyage. Here, we assemble what is known of mosquito sperm and highlight future avenues of research, paying special attention to areas where molecular mechanisms are likely critical to sperm viability and male reproductive success. Much of our knowledge in mosquitoes comes from the important vector A. aegypti, thus we focus on this species as a model but include other mosquito species for which information is available. We also glean insight from D. melanogaster, other insects, or other organisms whose biology has been studied in greater detail for phenomena that have not been adequately evaluated in mosquitoes.
REPRODUCTIVE TRACT MORPHOLOGY
A description of the arena that sperm traverse is necessary in order to understand their journey through the female reproductive tract (Figs. 1 and S1). Males transfer sperm and semen directly into a sac‐like organ inside the female gonotreme, or vagina (Giglioli, 1963; Spielman, 1964). This organ acts as a temporary holding site for sperm. After a ∼40‐sec delay (Spielman, 1964; Jones and Wheeler, 1965a), sperm begin to travel from this organ up narrow ducts into one to three spermathecae. The spermathecae are chitinous, spherical reservoirs in which sperm are stored long‐term. Sperm are maintained in these capsules for a female's entire life, nourished by glandular cells adjoining the spermathecae (Clements and Potter, 1967; Pascini et al., 2012, 2013). Ultimately, sperm travel back down these same ducts and fertilize eggs that pass down the common oviduct and out the gonotreme (Fig. 2).
Figure 1.

Typical reproductive tract of female Culicinae mosquitoes. A: Simplified sagittal diagram with relevant anatomy labeled. For detailed anatomical drawing of the final abdominal segments, see Figure S1. B: Dissected reproductive tract of virgin (left) and mated (right) Aedes aegypti females. The bursa is inflated by semen in the mated female. Scale bar, 200 μm.
Figure 2.

Frame‐by‐frame model of sperm path through Culicinae female reproductive tract from insemination (A) to fertilization (F).
Two mosquito subfamilies are currently recognized within Culicidae: Culicinae and Anophelinae (Mitchell et al., 2002; Harbach, 2007). Members of each subfamily possess slightly different reproductive tract morphology. In Culicinae, the sac‐like organ into which sperm are deposited is called a bursa (also referred to as a bursa copulatrix or bursa inseminalis), a reservoir that is adjacent to and attaches at the base of the common oviduct (Fig. 1). In Anophelinae, the organ with the same function is termed the atrium, and is simply an expanded region of the common oviduct that accommodates the ejaculate when initially transferred. In contrast to Culicinae, a mating plug quickly forms in the atrium of most Anophelinae via a combination of the male ejaculate and female secretions (Giglioli and Mason, 1966; Mitchell et al., 2015); a noted exception is the New World species Anopheles albimanus, in which a mating plug does not form after copulation (Mitchell et al., 2015). The number of spermathecae varies by subfamily as well: Anophelinae has only one, whereas most (but not all) Culicinae have three (Edwards, 1941; Yuval, 2006).
SPERM MORPHOLOGY
Mosquito sperm are long and slender. Unlike mammalian sperm, mosquito sperm heads are as wide as the tail, with a diameter of 0.5–0.6 μm (Clements and Potter, 1967; Tongu, 1968). The head contains the nucleus and is identifiable by its rigidity, in contrast to the undulating flagellum (Fig. 3). The flagellum consists of two mitochondrial derivatives, which extend the length of most of the flagellum, and an axoneme, a microtubular structure responsible for motility (Clements and Potter, 1967; Bao et al., 1992). Mosquito sperm axonemes have 19 microtubules arranged in two concentric circles of nine around a lone central tubule (9 + 9 + 1) (Breland et al., 1966; Clements and Potter, 1967; Swan, 1981)—an arrangement that contrasts with most other insect axonemes studied to date, which have a pair of central tubules (9 + 9 + 2) (Phillips, 1969). The functional significance of this deviation in mosquitoes is unknown. Average mosquito sperm length varies by species, from 100 μm in A. albimanus (Klowden and Chambers, 2004) to 570 μm in Culiseta inornata (Breland et al., 1968). Klowden and Chambers (2004) found that average sperm length across six mosquito species correlated with spermathecal volume, implying that these measurements may have sexually coevolved. Experimental selection of D. melanogaster sperm length and seminal receptacle size yielded similar insights (Miller and Pitnick, 2002).
Figure 3.

Aedes aegypti sperm. Rigid heads are stained with ethidium bromide. Scale bar, 50 μm.
Klowden and Chambers (2004) also identified significant variation in sperm length within Anopheles gambiae, Anopheles quadriannulatus, and Anopheles darlingi, whereas sperm size was uniform in other species, such as Anopheles freeborni and A. aegypti. In one study with A. gambiae, average sperm length varied with body size (Voordouw et al., 2008). While these few studies have identified intriguing variation in sperm length for some species, the contribution of different length sperm to fertilization remains unknown. In contrast to Lepidoptera, which produce a class of short sperm without nuclei (Friedlander and Gitay, 1972; reviewed in Friedlander, 1997), all mosquito sperm contain nuclear DNA, suggesting that even short sperm are capable of fertilization (Klowden and Chambers, 2004). Some Drosophila species produce both long and short nucleated sperm, but only long sperm fertilize eggs (Snook et al., 1994; Snook and Karr, 1998). Several hypotheses have been proposed for other insects to explain the adaptive significance of having a class of sperm that does not contribute to fertilization, including nutritional provisioning by extra sperm, facilitating sperm transport, or preventing the receipt or storage of another male's sperm (reviewed in Swallow and Wilkinson, 2002). If and how polymorphic sperm may be adaptive in mosquitoes is an intriguing and underexplored topic of research.
SPERM MOTILITY
Mosquito Sperm Motility
Thaler et al. (2013) provide the most comprehensive description of in vitro mosquito sperm motility to date using an elegant series of experiments. They described three discrete flagellar wave patterns in Culex quinquefasciatus sperm: a long‐wavelength, low‐amplitude flagellar wave (type A); a double‐wave consisting of a short‐wavelength, low‐amplitude wave superimposed over a long‐wavelength, high‐amplitude wave (type B); and a rapid, helical wave (type C). Such a range of motility is consistent with that observed in the sperm of A. aegypti (Jones and Wheeler, 1965b), and is similar to the waveform diversity described in D. melanogaster (Yang and Lu, 2011). Thaler et al. (2013) further found that C. quinquefasciatus sperm are capable of swimming both forward and backward. Bidirectional locomotion has been described in several other Dipterans, including the hump‐backed fly, Megaselia scalaris (Curtis and Benner, 1991); three flies in the family Tephritidae—Ceratitis capitata, Bactrocera dorsalis, Bactrocera oleae (Baccetti et al., 1989); and D. melanogaster (Kottgen et al., 2011). The significance of backwards swimming by mosquito sperm has not been examined, whereas it likely helps orient sperm correctly for fertilization in D. melanogaster (Kottgen et al., 2011).
Thaler et al. (2013) also revealed molecular regulators of the different waveforms and direction of C. quinquefasciatus sperm motility. Sperm are immotile in the male's seminal vesicle prior to ejaculation, and sperm dissected from the seminal vesicle remain weakly motile. Upon mixing with semen from the accessory glands, however, sperm progress sequentially from A‐ to B‐ to C‐type motility. This progression is calcium‐dependent; without this cation, very few sperm are motile, and those that are motile exhibit backwards (tail‐leading) type‐A motility. In the absence of accessory glands, complete progression to type‐C motility could be induced by the addition of trypsin, suggesting that a similar serine protease is present in semen that may be critical for full sperm activation. Transition from type‐B to type‐C motility is mediated by protease‐dependent activation of the mitogen‐activated protein kinase (MAPK) phosphorylation pathway; sperm incubated with MAPK inhibitors are incapable of type‐C motility. A similar process of activation has also been described in the water strider, Aquarius remigis, in which trypsin activates the MAPK pathway via the protease‐activated receptor PAR2 (Miyata et al., 2012). Protease‐dependent activation of sperm has also been described in other distantly related insect orders, including Lepidoptera (Shepherd, 1974; Aigaki et al., 1987; Osanai and Baccetti, 1993; Aigaki et al., 1994) and Orthoptera (Osanai and Baccetti, 1993), suggesting a highly conserved mechanism of sperm activation across Insecta.
Calcium Dependence of Sperm Motility
Intracellular calcium concentration is a highly conserved regulator of sperm motility in animals (Suarez et al., 1993; Bannai et al., 2000; Gao et al., 2003; reviewed in Darszon et al., 2006). In insects, specific proteins that control sperm calcium concentrations are best described in D. melanogaster. The calcium channel Pkd2 is localized at sperm heads and the tips of sperm tails, and is required for successful sperm storage in the D. melanogaster seminal receptacle (Gao et al., 2003; Watnick et al., 2003). Pkd2 also plays a role in hyperactivation of sperm (Kottgen et al., 2011). The gene CG34110 produces a protein predicted to be an upstream signaler of Pkd2 (Yang et al., 2011), as mutations of either CG34110 or Pkd2 result in similar sperm dysfunctions. Similar calcium channels have yet to be identified in mosquitoes, although an odorant‐gated ion channel, Orco, localizes to the sperm flagella and may play a similar role in the regulation of intracellular osmolarity and motility in the mosquitoes A. aegypti and A. gambiae (Pask et al., 2011; Pitts et al., 2014). Orco forms a complex with an accompanying odorant receptor (Sato et al., 2008), and has been shown to activate mosquito sperm in the presence of various ligands (Pitts et al., 2014).
The importance of calcium channels in sperm movement raises the question of whether or not females can control fluxes of extracellular calcium or other ions throughout their reproductive tract to modulate sperm movement—a possibility that should be considered in mosquitoes. Kaneuchi et al. (2015) recently used a transgenic fly expressing a fluorescent calcium sensor (GCaMP3) to image calcium fluxes in vivo in D. melanogaster oocytes; adapting their method to mosquitoes could elucidate the osmotic environment that sperm encounter in females.
Other Parameters That May Modulate Motility
Substrate viscosity may also modulate sperm motility. Curtis and Benner (1991) found that sperm velocity of the humpbacked fly, M. scalaris, tripled when substrate viscosity was increased with 1% methylcellulose, and sperm converted to a more linear swimming form. Higher viscosity elicits a different waveform in sperm of the tunicate Ciona intestinalis, whose flagellar movement converts from a planar wave to a helical wave (Brokaw, 1966). It is unclear how discrete waveforms may be induced by substrate viscosity, although Werner and Simmons (2008) hypothesized that increased mechanical stress on the sperm tail is converted into a biochemical signal (such as an influx of ions) by membrane proteins (Watson, 1991).
Physical interactions with the reproductive tract itself may also be critical for sperm movement. Werner et al. (2007) and Werner and Simmons (2008) discussed the possibility that helical waveforms may maximize contact with narrow spermathecal ducts to assist sperm locomotion, allowing sperm to propel themselves by pushing against the duct rather than just the fluid in the lumen of the reproductive tract. Nosrati et al. (2015) report that mammalian sperm may change their flagellar motion from a free‐swimming, helical wave to a two‐dimensional “slither” when within 1 μm of a solid surface. Similar interactions are easy to imagine in mosquitoes: In A. aegypti, for example, 250 μm‐long sperm travel up 1.2–3.5 μm‐wide ducts into spermathecae that are half as wide as a sperm's total length (Jones and Wheeler, 1965b; Clements and Potter, 1967; Linley and Simmons, 1981). Such crowded conditions lead to intimate contact with the reproductive tract and may facilitate locomotion.
SPERM AGGREGATION AT THE SPERMATHECAL VESTIBULE
Most female mosquitoes pair with males in flight and copulate shortly thereafter, during which time insemination occurs. A. aegypti males transfer seminal fluid and sperm into the bursa. Spielman (1964) tracked the movement of sperm in freshly inseminated females by flash‐freezing females after insemination, and observing sperm location in these fixed specimens by light microscopy. He noted that sperm are initially dispersed throughout the bursa, but quickly aggregate at and orient towards the spermathecal vestibule (Fig. 2), a small invagination of the reproductive tract near the entrance to the bursa and leading to the spermathecal ducts (Fig. 1). Jones and Wheeler (1965a) noted that sperm displayed vigorous activity concentrated around the spermathecal vestibule. Our observations in A. aegypti support these accounts of strong, directed localization to the spermathecal vestibule with robust locomotion (Video S1). These results are consistent with studies that report hyperactivation in D. melanogaster (Kottgen et al., 2011). Of the waveforms described by Thaler et al. (2013), it is unknown which are used in guiding sperm to the spermathecal vestibule, and it is possible that sperm switch from one form to another within the bursa. Due to the mass movement of sperm in the confined spaces of the bursa, we have thus far been unable to identify discrete waveforms of individual sperm in our observations. To our knowledge, detailed descriptions of sperm activity in the atrium of Anophelinae do not exist, although such studies would further our understanding of sperm in this medically important subfamily.
Getting sperm to the spermathecal vestibule may be accomplished by chemotaxis, consistent with the results of Pitts et al. (2014), which demonstrated chemical activation of sperm in response to different ligands. Possible origins of the stimulus responsible for localization to the vestibule include the female accessory gland, the spermathecal duct glands, or the spermathecal glands (Clements and Potter, 1967; Pascini et al., 2012, 2013). Yet Jones and Wheeler (1965a) discounted all three possibilities, as they did not observe any in vitro activation of sperm in response to these organs—although these observations should be verified in vivo to truly understand sperm activation. Schnakenberg et al. (2011) showed that products from D. melanogaster spermathecal secretory cells contribute to the motility of sperm in a separate storage organ, the seminal receptacle. They eliminated these cells by using the promoter of genes specific to the spermathecal secretory cells to drive expression of a protein that induces apoptosis. With this tissue disabled, sperm in the seminal receptacle lost motility. Thus, secretion from one tissue of the reproductive tract can modulate sperm motility in a different organ. If specific sperm‐activating molecules in the female mosquito reproductive tract are discovered, knowing where and when they are expressed will be important for understanding how they affect sperm. Heifetz et al. (2014) developed antibody‐based methods to map the expression of several neurohormones throughout the D. melanogaster reproductive tract over several hours; their methods may be adapted to identify reproductive molecules in mosquitoes.
Coagulation of semen has been reported for mosquitoes such as A. albopictus (Oliva et al., 2013), and a mating plug forms in many Anophelinae species (Giglioli and Mason, 1966; Mitchell et al., 2015). Such changes in the seminal mass may create a viscous environment that influences sperm motility, as has been demonstrated in other organisms (Brokaw, 1966; Curtis and Benner, 1991). If confirmed, such a mechanism of sperm activation would complement a study by Rogers et al. (2009) that showed the importance of a key seminal fluid protein in A. gambiae. They demonstrated that the mating plug ensures successful sperm storage, and sperm storage was severely inhibited without the plug‐forming enzyme transglutaminase. Similarly, the seminal fluid protein PEBme is required for effective mating plug coagulation and sperm storage in D. melanogaster (Avila et al., 2015).
MECHANISMS OF SPERMATHECAL FILLING
Sperm are rapidly stored in the spermathecae. A. aegypti sperm begin to enter the spermathecae as soon as 30 sec after mating, and spermathecal filling is completed in 300 sec (Spielman, 1964; Jones and Wheeler, 1965b). In most Culicinae, sperm typically only fill one of the lateral spermathecae and the median spermatheca (Jones and Wheeler, 1965b; Oliva et al., 2013; personal observation). How the spermathecae are filled with sperm remains unclear, although sperm translocation to the spermathecae is broadly attributed to either sperm locomotion or active transport by the female reproductive tract—which are not mutually exclusive. To our knowledge, the process of spermathecal filling in mosquitoes has only been examined in detail for A. aegypti.
Jones and Wheeler (1965a,1965b) performed several experiments in an attempt to elucidate how sperm reach the spermathecae. They noted that sperm moved rapidly in the bursa, and thus concluded that sperm locomotion alone is sufficient to fill the spermathecae. Yet, they also found that dead females did not store sperm, even if the bursa was inseminated. Therefore, some cooperation from the female was evident (Jones and Wheeler, 1965a). Spielman (1964) suggests that the ventral tuft, a valve‐like projection of the female reproductive tract ending in delicate hairs, may act as a gate to the spermathecal vestibule, preventing storage of a male's sperm until a female opens this valve (Fig. 4). The stimulus that causes a female to grant this access is unknown, but this checkpoint may serve as a guard against interspecific matings (Carrasquilla and Lounibos, 2015b).
Figure 4.
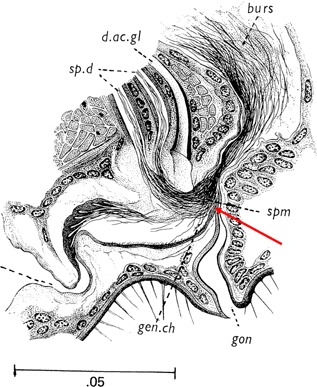
Sagittal section of the Aedes aegypti female reproductive tract (Jobling and Lewis, 1987). Red arrow indicates the ventral tuft, proposed by Spielman (1964) to act as a valve that guards access to the spermathecal ducts. Scale bar, 50 μm; burs, bursa; d. ac. gl, female accessory gland duct; gon, gonotreme; sp. d, spermathecal ducts; spm, sperm. Used with permission from the Wellcome Trust (London, UK).
In addition to opening the ventral tuft, Linley and Simmons (1981) suggest that active female transport is necessary for sperm storage. Contrary to Jones and Wheeler (1965b), they asserted that sperm locomotion alone cannot move sperm into the spermathecae as quickly as has been observed. Linley and Simmons (1981) argue that the rate of storage in the spermathecae is impossible given the measured sperm swimming speed (25 μm/s) versus the minimum diameter (1.2 μm) of the spermathecal ducts. According to their morphometric calculations, the only way for sperm to reach the spermathecae as quickly as they do is if females actively moved them. Yet Jones and Wheeler (1965b) noted no contractions of the spermathecal ducts (even though the ducts are surrounded by a helical musculature), and that the spermathecal volume was static. Therefore, Linley and Simmons (1981) concluded that rapid fluid transport out of the spermathecae was the most likely mechanism, presumably accomplished by the spermathecal glandular cells. Linley (1981) concluded the same mechanism in the biting midge, Culicoides melleus, based on a similar argument. Clements (1999) endorses this idea, suggesting that the microvillar structure of the spermathecal glandular cells in A. aegypti is consistent with an ability to rapidly transport liquid. Further evidence for liquid export from the spermatheca is the fact that dyes injected into the bursa are sometimes observed in the spermathecae, suggesting that negative pressure draws in bursal contents (Jones and Wheeler, 1965a). Exactly what triggers this process is unknown, although seminal fluid molecules may be involved since male‐transferred seminal proteins and hormones play critical roles in modulating many other aspects of the female post‐mating response (reviewed in Baldini et al., 2012).
Based on the above arguments, fluid transport likely plays some role in sperm transport, although we postulate that the role of sperm locomotion in the storage process may be underestimated. A. aegypti in vitro sperm swimming speed was measured in a saline medium (Linley and Simmons, 1981), but, based on what is known in other insects (Curtis and Benner, 1991; Yang and Lu, 2011), mosquito sperm may exhibit different swimming behaviors in the reproductive tract, as described above. The rapidly spinning aggregates of sperm described by Jones and Wheeler (1965a), and shown in our video (Video S1), are consistent with the idea of mosquito sperm being hyperactivated in the bursa before storage. Spermathecal ducts have a high elasticity due to their resilin content (Giglioli, 1963; Clements and Potter, 1967), which may allow for expansion of the ducts as a mass of sperm passes through. Additionally, even though Jones and Wheeler did not observe contractions of the spermathecal duct, this possibility should not be discounted. Indeed, the musculature surrounding the spermathecal duct may pump sperm peristaltically, as suggested in Anopheles melas (Giglioli, 1963); the yellow dung fly, Scatophaga stercoraria (Hosken and Ward, 2000); and the ant, Crematogaster opuntiae (Wheeler and Krutzsch, 1994).
The seemingly coordinated, rapid whirling motion of sperm in the bursa is suggestive of sperm cooperation. Sperm likely encounter each other frequently, as females receive over one thousand sperm in a single insemination (Ponlawat and Harrington, 2009), and these sperm are in intimate proximity to each other. The interactions of sperm and the potential for sperm cooperation have been demonstrated in D. melanogaster by Yang and Lu (2011), who described sperm moving through the seminal receptacle in parallel, intertwined bundles. Sperm cooperation has also been documented in the fishfly, Parachauliodes japonicus. In these insects, sperm are transferred in bundles, with their heads glued together by a trypsin‐degradable protein (Hayashi, 1997). Bundles with more sperm had a higher aggregate velocity than bundles with fewer sperm (Hayashi, 1998). While mosquito sperm do not form conjugated groups like other taxa (Hayashi, 1998; Higginson et al., 2012), it is possible that mosquito sperm reduce drag by swimming in parallel, allowing for their faster storage in the spermathecae. Females of some mosquitoes may occasionally mate more than once (Boyer et al., 2012; Richardson et al., 2015), especially soon after their first mating (Degner and Harrington, 2016). Therefore, males whose sperm fill the spermathecae quickly may reduce their risk of sharing paternity with a second male.
MAINTENANCE AND STORAGE IN THE SPERMATHECAE
Once sperm reach the spermathecae, they are stored and maintained for the female's life. Remarkably, one study documented fertilization in more than 100‐day‐old females (Styer et al., 2007). While some authors suggest that fertility is reduced after multiple egg‐laying cycles (Young and Downe, 1982), the consensus remains that mosquitoes maintain and utilize sperm from a single insemination without increased sterility throughout their lifetime (Styer et al., 2007; Oliva et al., 2013; Shaw et al., 2014). The mechanisms and molecules that sustain viable sperm in mosquitoes long‐term remain poorly understood, but this is an important area for future study.
A. aegypti sperm are often seen swirling around the perimeter of spermathecae (personal observation). A. gambiae sperm, on the other hand, were rarely motile in the spermathecae of recently mated females, although two thirds of the spermathecae contained motile sperm if they were dissected 24 hr or more after mating, suggesting that sperm may become activated after they have been stored for some time (Verhoek and Takken, 1994). One caveat is that both of these observations were made in spermathecae immersed in saline. In the honeybee, Apis mellifera, reduced concentrations of sodium and potassium can activate sperm (Verma, 1973), so it is possible that the osmotic environment in mosquito spermathecae changes after dissection. Indeed, Verhoek and Takken (1994) reported that A. gambiae sperm become motile in the spermathecae after immersion in saline. Nonetheless, moving sperm were recently observed in the spermathecae of an intact A. aegypti female (Carrasquilla and Lounibos, 2015a). How soon sperm become motile and what stimulates their movement in the spermathecae in vivo remains untested.
Maintaining live, motile sperm is energetically costly. Energy storage substrates, such as glycogen, have not been detected inside mosquito sperm, nor have they been described for sperm of D. melanogaster or other insects (Anderson and Personne, 1970), so the energy needed to maintain sperm in storage is likely provisioned by the female. Mosquito sperm contain two long mitochondrial derivatives (Clements and Potter, 1967; Bao et al., 1992), but the function of these organelles in aerobic metabolism is dubious. In C. quinquefasciatus, activity of cytochrome C oxidase, a necessary enzyme for oxidative phosphorylation, was weak in mature sperm (Bao et al., 1992). Perotti (1973) reported a similar absence of activity in Drosophila. In the rove beetle, Aleochara bilineata, cytochrome C oxidase was active in spermatozoa, but potassium cyanide, an inhibitor of this enzyme, did not reduce sperm motility (Werner et al., 1999). Therefore, anaerobic metabolism is most likely what powers sperm, but whether or not the mitochondrial derivatives in mosquito sperm function to provide energy for sperm deserves further investigation.
Secretory cells associated with each spermatheca nourish sperm. These cells connect to the lumen of the spermatheca via ductules, and form a gland situated around the juncture of the spermathecal duct and capsule. Their ultrastructure has been extensively characterized by both light and electron microscopy in A. aegypti and A. aquasalis (Clements and Potter, 1967; Pascini et al., 2012, 2013). The secretions of these glands have not been examined directly in mosquitoes, but proteomic studies of the spermathecal fluid in the honey bee, A. mellifera, may provide insight to mosquito spermathecal function. Honey bees, like mosquitoes, store sperm for life without later replenishment, and therefore the spermatheca of this hymenopteran plays an analogous supporting role. Indeed, the proteome of spermathecal fluid in A. mellifera is enriched for energy metabolism enzymes (Baer et al., 2009). While male accessory gland secretions also produce metabolic enzymes, the network of enzymes produced by the female is more extensive. Further, the metabolic networks derived from males versus females show very little overlap (Baer et al., 2009). Therefore, energetic sustenance of sperm may be partially achieved by male seminal fluid in the short term, whereas female secretions likely take over in the long term (Baer et al., 2009). Glycoproteins and lipoproteins, which are present in the spermathecae of all Diptera examined to date, may act as energy sources for sperm (reviewed in Heifetz and Rivlin, 2010).
In addition to requiring energetic nourishment, sperm must be protected from oxidative stress. One enzyme that provides this service in A. gambiae is heme peroxidase (Shaw et al., 2014). Expression of heme peroxidase in the spermatheca was up‐regulated after mating, and females with RNA‐interference‐induced knockdown of heme peroxidase suffered partial infertility that worsened with successive batches of eggs. Controlling oxidative damage is important in other insects as well. Proteins with antioxidant function have been described in the spermathecal fluid of A. mellifera (Baer et al., 2009), as well as both the spermathecae and seminal receptacle of D. melanogaster (Prokupek et al., 2010, 2009), implying that minimizing oxidative stress is important for long‐term sperm viability across insect taxa.
SPERM MODIFICATION IN STORAGE
Do mosquito sperm undergo modifications while in storage in order for fertilization to take place? One clue to the answer comes from a study that uncoupled the receipt of semen and sperm. Adlakha and Pillai (1975) surgically removed accessory glands or seminal vesicles from A. aegypti and C. quinquefasciatus to produce males that only transfer sperm or seminal fluid, respectively, during mating. Sperm from males without accessory glands were capable of reaching the spermathecae, but fertilization did not take place; fertilization was rescued in females that were subsequently mated to a male that only transferred seminal fluid. A comparable study in D. melanogaster reported similar results (Xue and Noll, 2000). Although semen receipt is necessary for fertilization, the mechanism by which semen acts to facilitate fertilization remains unknown. It is possible that semen directly modifies sperm to prepare them for fertilization. Alternatively, it may trigger a physiological change in females that is necessary for fertilization to occur (e.g., enabling sperm release mechanisms).
Mammalian sperm become fertilization‐competent while in the female through structural and molecular modifications. This process, called capacitation (Austin, 1952; Visconti et al., 1995a), resembles semen‐mediated activation of sperm motility described in mosquitoes. Both processes are calcium‐dependent (Yanagimachi and Usui, 1974; Thaler et al., 2013), and both result in protein tyrosine phosphorylation (Visconti et al., 1995a,1995b; Thaler et al., 2013). The capacitation process in mammals is initiated by removal of cholesterol from the plasma membrane (Visconti et al., 1999a,1999b). To our knowledge, cholesterol removal has not been described in insect sperm, although some limited evidence suggests that the mosquito sperm glycocalyx undergoes structural modification while in storage (Ndiaye et al., 1997). Whether a process analogous to mammalian capacitation exists in insects remains uncertain but warrants investigation, and comparison to mammals may guide investigations of this phenomenon in mosquito sperm.
RELEASE FROM THE SPERMATHECAE
Sperm leave the spermathecae and travel down the spermathecal duct en route to their final destination, the egg. Sperm release is likely controlled by the muscles encircling the spermathecal duct, which act as a sphincter (Pascini et al., 2012). Indeed, the duct musculature is well‐innervated (Clements, 1999). The inside diameter and shape of the duct varies, but is a mere 1.2 μm and stellate in cross‐section at its narrowest point (Clements and Potter, 1967; Linley and Simmons, 1981). Such a narrow passageway probably serves to limit the number of sperm released per fertilization. Curtin and Jones (1961) noted that spermathecae move “vigorously and repeatedly dorsally and posteriorward” while laying eggs, but it remains to be determined if this is due to contractions of the spermathecal ducts or a consequence of an egg moving down the oviduct.
Sperm release in other insects is controlled neurohormonally. In the locust, Locusta migratoria, octopamine induces spermathecal contractions involved in sperm release. An octopamine receptor, Octβ2R, is also present in the oviduct and spermatheca of D. melanogaster. Mutants without octβ2R lay few eggs, and any eggs laid are not fertilized, suggesting that this receptor coordinates sperm release and ovulation (Li et al., 2015). Sex peptide, a male seminal fluid protein, also mediates sperm release in D. melanogaster (Avila et al., 2010). This peptide localizes to sperm tails (Peng et al., 2005), but must be cleaved from sperm in order for sperm to exit from storage; indeed, sperm of mutant males whose sex peptide binds irreversibly to sperm do not leave storage properly (Avila et al., 2010). No male‐derived sperm‐bound proteins have been identified in mosquitoes to date. Additionally, nothing is known about the specific neural signals that coordinate ovulation in mosquitoes; however, a recent neurotranscriptome of A. aegypti (Matthews et al., 2016) should assist future investigations of this critical reproductive process.
The number of sperm released for fertilization is an important factor in estimating reproductive potential of females, since releasing too many sperm per egg may deplete the sperm cache prematurely—a phenomenon that has been scarcely examined in mosquitoes. Harber and Mutchmor (1970) reported finding three sperm heads in the eggs of C. inornata, and Davis (1967) counted 6–10 sperm in the micropyle of Culex fatigans. In Culex pipiens, one or two sperm enter the egg, but rarely more (Jost, 1971). This is in contrast to D. melanogaster, in which only one sperm penetrates the egg (reviewed in Hildreth and Lucchesi, 1963; Loppin et al., 2015). It is possible that the number of sperm released depends on the number of sperm a female stores: A variable sperm allocation strategy has been demonstrated in the yellow dung fly, S. stercoraria, as females released more sperm when the number stored was high than when it was low (Sbilordo et al., 2009). Releasing more than one sperm per egg may be adaptive if sperm are plentiful, since multiple sperm provides increased assurance of fertilization.
ENTRY INTO THE EGG
Fertilization in mosquitoes occurs as eggs are oviposited (Fig. 2). Eggs protrude halfway from the gonotreme, but pause briefly when the micropyle, a small opening for sperm entry on the anterior pole of the egg, aligns with the egress of the spermathecal ducts (Curtin and Jones, 1961; Giglioli, 1963). Similar alignment of sperm and micropyle was also recently visualized in D. melanogaster (Mattei et al., 2015), and is a strategy thought to give the female stringent control of the fertilization of each egg, preventing gamete wastage (Downes, 1968; Bloch Qazi et al., 2003).
Sperm‐egg recognition is probably mediated by interactions between sperm proteins and egg carbohydrates (reviewed in Mengerink and Vacquier, 2001; Perotti et al., 2001). Two likely egg recognition proteins have been identified in D. melanogaster sperm. A mutant strain, casanova, lacks β‐N‐acetylhexosaminidase on the sperm head and is unable to fertilize eggs, despite successful arrival of sperm at the egg (Perotti et al., 2001). The Drosophila micropyle contains α‐L‐fucose, and its sperm have the glycoconjugate enzyme α‐L‐fucosidase (Pasini et al., 2008; Intra et al., 2015). This pairing is conserved within Drosophila, whereas the same enzyme is absent from sperm of the closely related genus Scaptodrosophila (Pasini et al., 2008), indicating sperm‐egg recognition mechanisms are taxon‐specific. Another glycosidic enzyme, α‐D‐mannosidase, localizes to the D. melanogaster egg and has been suggested to further contribute to sperm‐egg recognition (Perotti et al., 2001). α‐D‐mannosidase was also identified in the semen proteome of A. aegypti (Sirot et al., 2011), indicating that some shared lock‐and‐key mechanisms could mediate mosquito gamete interactions—although these models require experimental support.
A laid mosquito egg contains an oocyte whose development has been arrested at metaphase I of meiosis (Jost, 1971). Jost (1971) assumed that the presence of sperm activates the resumption of meiosis in C. pipiens, as is the case in most vertebrates (reviewed in Ducibella et al., 2006). Yet, a recent study demonstrated that unfertilized Anopheles stephensi eggs are activated by immersion in distilled water. Yamamoto et al. (2013) used DAPI staining to show that meiosis resumed after the egg was immersed in water—even those eggs dissected from the ovaries. Whether this is the primary activating stimulus or acts as a fail‐safe mechanism to resume development is unknown. Activation by immersion likely does not apply to all mosquitoes, especially since some genera, such as Aedes, generally do not lay their eggs in water (Clements, 1999). Therefore, sperm's role in mosquito egg activation has not been fully illuminated, and activating stimuli may depend on the ecology of different taxa.
A conserved component of egg activation in animals is a calcium wave that propagates through the egg (Roux et al., 2006; Miao et al., 2012; Kaneuchi et al., 2015). In D. melanogaster, eggs are activated while they pass through the oviduct (Heifetz et al., 2001), when extracellular calcium is imported (Kaneuchi et al., 2015; York‐Andersen et al., 2015). Given calcium's role in sperm motility, an intriguing possibility is that the sudden influx of calcium into the egg prior to sperm arrival assists in the sperm's entry via activation of motility, ensuring the entire sperm tail enters the egg (Karr and Pitnick, 1996). Whether or not egg activation facilitates sperm's penetration of the egg in mosquitoes has not yet been determined. Once safely inside, however, fertilization occurs and sperm's journey is complete.
CONCLUSIONS AND PERSPECTIVES
Many questions about mosquito sperm remain to be adequately answered (Fig. 5), particularly since the phenomena studied to date are often described in only one species. Most detailed accounts of reproductive processes in mosquitoes are from A. aegypti, owing to its importance as a disease vector and its ease of culture in the lab. Similar investigations of sperm in other mosquitoes lag severely behind A. aegypti, even in the critically important Anopheles vectors of malaria. Given the differing reproductive tract morphologies between Culicinae and Anophelinae, descriptions of sperm biology may not always be generalizable across subfamilies. Therefore, future work should investigate murky areas of mosquito sperm biology in vectors other than A. aegypti.
Figure 5.

Analog of Figure 2, with text indicating unexplored, under‐studied, and poorly understood aspects of mosquito sperm as they move through the female reproductive tract.
Most studies of sperm movement have relied on in vitro assays that test sperm response to potential activating molecules or other conditions (Jones and Wheeler, 1965b; Linley and Simmons, 1981; Swan, 1981; Thaler et al., 2013; Pitts et al., 2014). Such studies are important first steps for understanding what drives sperm locomotion, but the results of these studies are unlikely to be generalizable to in vivo conditions. Investigators should therefore consider the environment sperm are likely to face in vivo, paying particular attention to osmolarity, pH, viscosity, and spatial constraints. Work by Manier et al. (2010) and Yang and Lu (2011) provides excellent descriptions of sperm motility and locomotion within the D. melanogaster female reproductive tract. Such elegant systems of visualizing sperm in vivo (Lu, 2013) could be adapted to mosquitoes using lines with fluorescently labeled sperm, such as those produced by Smith et al. (2007). Other recent techniques in D. melanogaster may allow detailed studies of the molecular environment sperm experience. These include the methods of Kaneuchi et al. (2015) to visualize calcium fluxes in vivo and of Heifetz et al. (2014) to map expression of specific molecules across the reproductive tract.
Research involving comparisons with other insects will shed light on under‐studied aspects of mosquito sperm. The abundance of work on D. melanogaster sperm motility, storage, and usage is a substantial resource for investigations of mosquitoes. As both mosquitoes and D. melanogaster are in the order Diptera, homology of proteins or processes may further our understanding of mechanisms utilized by mosquitoes. Spermathecal filling and sperm usage have also been well described in other flies, such as S. stercoraria (Sbilordo et al., 2009) and Dryomyza anilis (Otronen, 1997; Otronen et al., 1997), and the morphological similarity of these species' spermathecae to those of mosquitoes may provide insights into the mechanisms of sperm storage and usage. Species that store sperm for life without replenishment, such as A. mellifera and other hymenopterans, may offer clues to how viable sperm can be maintained so efficiently for so long in mosquitoes. Finally, contrasting the unique 9 + 9 + 1 structure of the mosquito sperm axoneme to other insects may provide insight for the mechanics of flagellar motility.
In conclusion, the details of how mosquito sperm interact with the female on a mechanistic and molecular level is a largely unexplored, yet fascinating, field awaiting future investigation. Here, we have discussed the foundations for this evaluation and highlight areas that could yield the most insightful discoveries. Spielman (1964), Jones and Wheeler (1965a,1965b), Clements and Potter (1967), and Giglioli (1963) provided a firm initial foundation of knowledge about mosquito sperm movement in the female, but they lacked adequate methods to delve deeper than basic morphological descriptions. Today, a vast array of genetic and molecular tools and resources has made it possible to answer questions that were once untouchable. For example, the sequenced genomes of A. aegypti (Nene et al., 2007), A. albopictus (Chen et al., 2015), C. quinquefasciatus (Arensburger et al., 2010), and 16 Anopheles species (Neafsey et al., 2015) provides a resource to mine for sperm‐relevant genes that are homologous to D. melanogaster or other organisms. Transcriptomics of reproductive tissues in A. aegypti (Akbari et al., 2013; Alfonso‐Parra et al., 2016) and A. gambiae (Rogers et al., 2008; Baker et al., 2011) and proteomics of semen and sperm in A. aegypti (Sirot et al., 2011) and A. albopictus (Boes et al., 2014) provide databases from which specific genes of interest can be investigated. Finally, targeted mutagenesis has been accomplished in mosquitoes using rapidly advancing genome editing techniques, such as transcription activator‐like effector nuclease (TALENs) (Aryan et al., 2013; Smidler et al., 2013), zinc‐finger nucleases (DeGennaro et al., 2013; McMeniman et al., 2014), and, most recently, the CRISPR‐Cas9 system (Dong et al., 2015; Kistler et al., 2015). Such resources and methods will allow the identification of proteins in sperm and in the female that play integral roles in activating sperm motility, guiding sperm through the reproductive tract, and enabling fertilization—ultimately placing a deeper understanding of mosquito sperm's journey from male ejaculate to the female's egg within our reach.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Figure S1. Detailed anatomical drawing from which Figure 1A is derived (Jobling and Lewis 1987).
Video 1. Sperm motility within recently inseminated Aedes aegypti female reproductive tract.
Supplementary Legend.
ACKNOWLEDGMENTS
We thank Frank Avila and Susan Villarreal for helpful comments; Alongkot Ponlawat for photographing Figure 3; Nicolas Buchon for microscope use; and the Wellcome Trust for use of Jobling's anatomical drawings. This work was supported by NIH/NIAID grant R01AI095491 and a Cornell University Graduate School fellowship awarded to ECD.
Details of how mosquito sperm interact with the female on a mechanistic and molecular level is a largely unexplored, yet fascinating, field.
REFERENCES
- Adlakha V, Pillai MK. 1975. Involvement of male accessory gland substance in the fertility of mosquitoes. J Insect Physiol 21:1453–1455. [DOI] [PubMed] [Google Scholar]
- Aigaki T, Kasuga H, Nagaoka S, Osanai M. 1994. Purification and partial amino acid sequence of initiatorin, a prostatic endopeptidase of the silkworm, Bombyx mori . Insect Biochem Mol Biol 24:969–975. [DOI] [PubMed] [Google Scholar]
- Aigaki T, Kasuga H, Osanai M. 1987. A specific endopeptidase, BAEE esterase, in the glandula prostatica of the male reproductive system of the silkworm, Bombyx mori . Insect Biochem 17:323–328. [Google Scholar]
- Akbari OS, Antoshechkin I, Amrhein H, Williams B, Diloreto R, Sandler J, Hay BA. 2013. The developmental transcriptome of the mosquito Aedes aegypti, an invasive species and major arbovirus vector. G3‐Genes Genom Genet 3:1493–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfonso‐Parra C, Ahmed‐Braimah YH, Degner EC, Avila FW, Villarreal SM, Pleiss JA, Wolfner MF, Harrington LC. 2016. Mating‐induced transcriptome changes in the reproductive tract of female Aedes aegypti . PLoS Negl Trop Dis 10:e0004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson WA, Personne P. 1970. The localization of glycogen in the spermatozoa of various invertebrate and vertebrate species. J Cell Biol 44:29–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arensburger P, Megy K, Waterhouse RM, Abrudan J, Amedeo P, Antelo B, Bartholomay L, Bidwell S, Caler E, Camara F, Campbell CL, Campbell KS, Casola C, Castro MT, Chandramouliswaran I, Chapman SB, Christley S, Costas J, Eisenstadt E, Feschotte C, Fraser‐Liggett C, Guigo R, Haas B, Hammond M, Hansson BS, Hemingway J, Hill SR, Howarth C, Ignell R, Kennedy RC, Kodira CD, Lobo NF, Mao CH, Mayhew G, Michel K, Mori A, Liu NN, Naveira H, Nene V, Nguyen N, Pearson MD, Pritham EJ, Puiu D, Qi YM, Ranson H, Ribeiro JMC, Roberston HM, Severson DW, Shumway M, Stanke M, Strausberg RL, Sun C, Sutton G, Tu ZJ, Tubio JMC, Unger MF, Vanlandingham DL, Vilella AJ, White O, White JR, Wondji CS, Wortman J, Zdobnov EM, Birren B, Christensen BM, Collins FH, Cornel A, Dimopoulos G, Hannick LI, Higgs S, Lanzaro GC, Lawson D, Lee NH, Muskavitch MAT, Raikhel AS, Atkinson PW. 2010. Sequencing of Culex quinquefasciatus establishes a platform for mosquito comparative genomics. Science 330:86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryan A, Anderson MAE, Myles KM, Adelman ZN. 2013. TALEN‐based gene disruption in the dengue vector Aedes aegypti . PLoS ONE 8:e60082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin CR. 1952. The capacitation of the mammalian sperm. Nature 170:326. [DOI] [PubMed] [Google Scholar]
- Avila FW, Cohen AB, Ameerudeen FS, Duneau D, Suresh S, Mattei AL, Wolfner MF. 2015. Retention of ejaculate by Drosophila melanogaster females requires the male‐derived mating plug protein PEBme. Genetics 200:1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila FW, Ravi Ram K, Bloch Qazi MC, Wolfner MF. 2010. Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccetti B, Gibbons BH, Gibbons IR. 1989. Bidirectional swimming in spermatozoa of Tephritid flies. J Submicr Cytol Path 21:619–625. [PubMed] [Google Scholar]
- Baer B, Eubel H, Taylor NL, O'Toole N, Millar AH. 2009. Insights into female sperm storage from the spermathecal fluid proteome of the honeybee Apis mellifera . Genome Biol 10:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Nolan T, Fischer B, Pinder A, Crisanti A, Russell S. 2011. A comprehensive gene expression atlas of sex‐ and tissue‐specificity in the malaria vector, Anopheles gambiae . BMC Genomics 12:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini F, Gabrieli P, Rogers DW, Catteruccia F. 2012. Function and composition of male accessory gland secretions in Anopheles gambiae: A comparison with other insect vectors of infectious diseases. Pathog Glob Health 106:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannai H, Yoshimura M, Takahashi K, Shingyoji C. 2000. Calcium regulation of microtubule sliding in reactivated sea urchin sperm flagella. J Cell Sci 113:831–839. [DOI] [PubMed] [Google Scholar]
- Bao SN, Lins U, Farina M, Desouza W. 1992. Mitochondrial derivatives of Culex quinquefasciatus (Culicidae) spermatozoon: Some new aspects evidenced by cytochemistry and image processing. J Struct Biol 109:46–51. [Google Scholar]
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GRW, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch Qazi MC, Heifetz Y, Wolfner MF. 2003. The developments between gametogenesis and fertilization: Ovulation and female sperm storage in Drosophila melanogaster . Dev Biol 256:195–211. [DOI] [PubMed] [Google Scholar]
- Boes KE, Ribeiro JMC, Wong A, Harrington LC, Wolfner MF, Sirot LK. 2014. Identification and characterization of seminal fluid proteins in the Asian tiger mosquito, Aedes albopictus . PLoS Neglect Trop D 8:e2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer S, Toty C, Jacquet M, Lemperiere G, Fontenille D. 2012. Evidence of multiple inseminations in the field in Aedes albopictus . PLoS ONE 7:e42040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breland OP, Eddleman CD, Biesele JJ. 1968. Studies of insect spermatozoa. I. Entomol News 79:197–216. [PubMed] [Google Scholar]
- Breland OP, Gassner G, Riess RW, Biesele JJ. 1966. Certain aspects of centriole adjunct spermiogenesis and mature sperm of insects. Can J Genet Cytol 8:759–&. [Google Scholar]
- Brokaw CJ. 1966. Effects of increased viscosity on the movements of some invertebrate spermatozoa. J Exp Biol 45:113–139. [DOI] [PubMed] [Google Scholar]
- Carrasquilla MC, Lounibos LP. 2015a. Detection of insemination status in live Aedes aegypti females. J Insect Physiol 75:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasquilla MC, Lounibos LP. 2015b. Satyrization without evidence of successful insemination from interspecific mating between invasive mosquitoes. Biol Lett 11:20150527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XG, Jiang XT, Gu JB, Xu M, Wu Y, Deng YH, Zhang C, Bonizzoni M, Dermauw W, Vontas J, Armbruster P, Huang X, Yang YL, Zhang H, He WM, Peng HJ, Liu YF, Wu K, Chen JH, Lirakis M, Topalis P, Van Leeuwen T, Hall AB, Jiang XF, Thorpe C, Mueller RL, Sun C, Waterhouse RM, Yan GY, Tu ZJJK, Fang XD, James AA. 2015. Genome sequence of the Asian tiger mosquito, Aedes albopictus, reveals insights into its biology, genetics, and evolution. Proc Natl Acad Sci USA 112:E5907–E5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AN. 1999. The biology of mosquitoes: Sensory reception and behavior. Wallingford, United Kingdom: CAB Publishing. [Google Scholar]
- Clements AN, Potter SA. 1967. The fine structure of the spermathecae and their ducts in the mosquito Aedes aegypti . J Insect Physiol 13:1825–1836. [DOI] [PubMed] [Google Scholar]
- Curtin TJ, Jones JC. 1961. The mechanics of ovulation and oviposition in Aedes aegypti . Ann Entomol Soc Am 54:298–313. [Google Scholar]
- Curtis SK, Benner DB. 1991. Movement of spermatozoa of Megaselia scalaris (Diptera, Brachycera, Cyclorrhapha, Phoridae) in artificial and natural fluids. J Morphol 210:85–99. [DOI] [PubMed] [Google Scholar]
- Darszon A, Acevedo JJ, Galindo BE, Hernandez‐Gonzalez E, Nishigaki T, Trevino CL, Wood C, Beltran C. 2006. Sperm channel diversity and functional multiplicity. Reproduction 131:977–988. [DOI] [PubMed] [Google Scholar]
- Davis CWC. 1967. A comparative study of larval embryogenesis in the mosquito Culex fatigans Wiedemann (Diptera: Culicidae) and the sheep‐fly Lucilia sericata Meigen (Diptera: Calliphoridae). Aust J Zool 15:547–549. [Google Scholar]
- DeGennaro M, McBride CS, Seeholzer L, Nakagawa T, Dennis EJ, Goldman C, Jasinskiene N, James AA, Vosshall LB. 2013. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature 498:487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degner EC, Harrington LC. 2016. Polyandry depends on postmating time interval in the dengue vector Aedes aegypti . Am J Trop Med Hyg 94:780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong SZ, Lin JY, Held NL, Clem RJ, Passarelli AL, Franz AWE. 2015. Heritable CRISPR/Cas9‐mediated genome editing in the yellow fever mosquito, Aedes aegypti . PLoS ONE 10:e0122353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes JA. 1968. Notes on organs and processes of sperm‐transfer in Lower Diptera. Can Entomol 100:608–617. [Google Scholar]
- Ducibella T, Schultz RM, Ozil J‐P. 2006. Role of calcium signals in early development. Semin Cell Dev Biol 17:324–332. [DOI] [PubMed] [Google Scholar]
- Edwards FW. 1941. Mosquitoes of the Ethiopian region. London: The Oxford University Press. [Google Scholar]
- Fauci AS, Morens DM. 2016. Zika virus in the Americas—Yet another arbovirus threat. N Engl J Med 374:601–604. [DOI] [PubMed] [Google Scholar]
- Friedlander M. 1997. Control of the eupyrene‐apyrene sperm dimorphism in Lepidoptera. J Insect Physiol 43:1085–1092. [DOI] [PubMed] [Google Scholar]
- Friedlander M, Gitay H. 1972. The fate of the normal‐anucleated spermatozoa in inseminated females of the silkworm Bombyx mori . J Morphol 138:121–129. [DOI] [PubMed] [Google Scholar]
- Gao Z, Ruden DM, Lu X. 2003. PKD2 cation channel is required for directional sperm movement and male fertility. Curr Biol 13:2175–2178. [DOI] [PubMed] [Google Scholar]
- Giglioli ME. 1963. The female reproductive system of Anopheles gambiae melas. I. The structure and function of the genital ducts and associated organs. Riv Malariol 42:149–176. [PubMed] [Google Scholar]
- Giglioli ME, Mason GF. 1966. Mating plug in anopheline mosquitoes. Proc R Entomol Soc A 41:123–129. [Google Scholar]
- Harbach RE. 2007. The Culicidae (Diptera): A review of taxonomy, classification and phylogeny. Zootaxa 1668:591–638. [Google Scholar]
- Harber PA, Mutchmor JA. 1970. Early embryonic development of Culiseta inornata (Diptera‐Culicidae). Ann Entomol Soc Am 63:1609–1614. [Google Scholar]
- Hayashi F. 1997. A trypsin‐degradable protein agglutinates fishfly sperm‐bundles (Megaloptera: Corydalidae). Int J Insect Morphol 26:63–66. [Google Scholar]
- Hayashi F. 1998. Sperm co‐operation in the fishfly, Parachauliodes japonicus . Funct Ecol 12:347–350. [Google Scholar]
- Heifetz Y, Lindner M, Garini Y, Wolfner MF. 2014. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr Biol 24:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifetz Y, Rivlin PK. 2010. Beyond the mouse model: Using Drosophila as a model for sperm interaction with the female reproductive tract. Theriogenology 73:723–739. [DOI] [PubMed] [Google Scholar]
- Heifetz Y, Yu J, Wolfner MF. 2001. Ovulation triggers activation of Drosophila oocytes. Dev Biol 234:416–424. [DOI] [PubMed] [Google Scholar]
- Higginson DM, Miller KB, Segraves KA, Pitnick S. 2012. Convergence, recurrence and diversification of complex sperm traits in diving beetles (Dytiscidae). Evolution 66:1650–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth PE, Lucchesi JC. 1963. Fertilization in Drosophila. 1. Evidence for regular occurrence of monospermy. Dev Biol 6:262–278. [DOI] [PubMed] [Google Scholar]
- Hosken DJ, Ward PI. 2000. Copula in yellow dung flies (Scathophaga stercoraria): Investigating sperm competition models by histological observation. J Insect Physiol 46:1355–1363. [DOI] [PubMed] [Google Scholar]
- Intra J, Veltri C, De Caro D, Perotti ME, Pasini ME. 2015. Drosophila sperm surface alpha‐L‐fucosidase interacts with the egg coats through its core fucose residues. Insect Biochem Mol Biol 63:133–143. [DOI] [PubMed] [Google Scholar]
- Jobling B, Lewis DJ. 1987. Anatomical drawings of biting flies. London, UK: British Museum (Natural History). [Google Scholar]
- Jones JC, Wheeler RE. 1965a. Studies on spermathecal filling in Aedes aegypti (Linnaeus). 2. experimental. Biol Bull 129:532–545. [DOI] [PubMed] [Google Scholar]
- Jones JC, Wheeler RE. 1965b. Studies on spermathecal filling in Aedes aegypti (Linnaeus) I. description. Biol Bull 129:134–150. [DOI] [PubMed] [Google Scholar]
- Jost E. 1971. Meiosis in the male of Culex pipiens and Aedes albopictus and fertilization in the Culex pipiens‐complex. Can J Genet Cytol 13:237–250. [DOI] [PubMed] [Google Scholar]
- Kaneuchi T, Sartain CV, Takeo S, Horner VL, Buehner NA, Aigaki T, Wolfner MF. 2015. Calcium waves occur as Drosophila oocytes activate. Proc Natl Acad Sci USA 112:791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr TL, Pitnick S. 1996. The ins and outs of fertilization. Nature 379:405–406. [DOI] [PubMed] [Google Scholar]
- Kistler KE, Vosshall LB, Matthews BJ. 2015. Genome engineering with CRISPR‐Cas9 in the mosquito Aedes aegypti . Cell Rep 11:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klowden MJ, Chambers GM. 2004. Production of polymorphic sperm by anopheline mosquitoes and their fate within the female genital tract. J Insect Physiol 50:1163–1170. [DOI] [PubMed] [Google Scholar]
- Kottgen M, Hofherr A, Li W, Chu K, Cook S, Montell C, Watnick T. 2011. Drosophila sperm swim backwards in the female reproductive tract and are activated via TRPP2 ion channels. PLoS ONE 6:e20031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fink C, El‐Kholy S, Roeder T. 2015. The octopamine receptor octß2R is essential for ovulation and fertilization in the fruit fly Drosophila melanogaster . Arch Insect Biochem Physiol 88:168–178. [DOI] [PubMed] [Google Scholar]
- Linley JR. 1981. Emptying of the spermatophore and spermathecal filling in Culicoides melleus (Coq) (Diptera, Ceratopogonidae). Can J Zool 59:347–356. [Google Scholar]
- Linley JR, Simmons KR. 1981. Sperm motility and spermathecal filling in Lower Diptera. Int J Inver Rep Dev 4:137–145. [Google Scholar]
- Loppin B, Dubruille R, Horard B. 2015. The intimate genetics of Drosophila fertilization. Open Biol 5:150076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY. 2013. Fluorescent imaging of Drosophila melanogaster sperm in the reproductive tract: A new model of flagellar motility. Method Enzymol 525:131–148. [DOI] [PubMed] [Google Scholar]
- Manier MK, Belote JM, Berben KS, Novikov D, Stuart WT, Pitnick S. 2010. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster . Science 328:354–357. [DOI] [PubMed] [Google Scholar]
- Mattei AL, Riccio ML, Avila FW, Wolfner MF. 2015. Integrated 3D view of postmating responses by the Drosophila melanogaster female reproductive tract, obtained by micro‐computed tomography scanning. Proc Natl Acad Sci USA 112:8475–8480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews BJ, McBride CS, DeGennaro M, Despo O, Vosshall LB. 2016. The neurotranscriptome of the Aedes aegypti mosquito. BMC Genomics 17:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMeniman CJ, Corfas RA, Matthews BJ, Ritchie SA, Vosshall LB. 2014. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156:1060–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengerink KJ, Vacquier VD. 2001. Glycobiology of sperm‐egg interactions in deuterostomes. Glycobiology 11:37r––43r. [DOI] [PubMed] [Google Scholar]
- Miao YL, Stein P, Jefferson WN, Padilla‐Banks E, Williams CJ. 2012. Calcium influx‐mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci USA 109:4169–4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GT, Pitnick S. 2002. Sperm‐female coevolution in Drosophila . Science 298:1230–1233. [DOI] [PubMed] [Google Scholar]
- Mitchell A, Sperling FAH, Hickey DA. 2002. Higher‐level phylogeny of mosquitoes (Diptera: Culicidae): mtDNA data support a derived placement for Toxorhynchites . Insect Syst Evol 33:163–174. [Google Scholar]
- Mitchell SN, Kakani EG, South A, Howell PI, Waterhouse RM, Catteruccia F. 2015. Evolution of sexual traits influencing vectorial capacity in anopheline mosquitoes. Science 347:985–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata H, Thaler CD, Haimo LT, Cardullo RA. 2012. Protease activation and the signal transduction pathway regulating motility in sperm from the water strider Aquarius remigis . Cytoskeleton 69:207–220. [DOI] [PubMed] [Google Scholar]
- Murai K, Culleton R, Hisaoka T, Endo H, Mita T. 2015. Global distribution of polymorphisms associated with delayed Plasmodium falciparum parasite clearance following artemisinin treatment: Genotyping of archive blood samples. Parasitol Int 64:267–273. [DOI] [PubMed] [Google Scholar]
- Ndiaye M, Mattei X, Thiaw OT. 1997. Maturation of mosquito spermatozoa during their transit throughout the male and female reproductive systems. Tissue Cell 29:675–678. [DOI] [PubMed] [Google Scholar]
- Neafsey DE, Waterhouse RM, Abai MR, Aganezov SS, Alekseyev MA, Allen JE, Amon J, Arca B, Arensburger P, Artemov G, Assour LA, Basseri H, Berlin A, Birren BW, Blandin SA, Brockman AI, Burkot TR, Burt A, Chan CS, Chauve C, Chiu JC, Christensen M, Costantini C, Davidson VLM, Deligianni E, Dottorini T, Dritsou V, Gabriel SB, Guelbeogo WM, Hall AB, Han MV, Hlaing T, Hughes DST, Jenkins AM, Jiang X, Jungreis I, Kakani EG, Kamali M, Kemppainen P, Kennedy RC, Kirmitzoglou IK, Koekemoer LL, Laban N, Langridge N, Lawniczak MKN, Lirakis M, Lobo NF, Lowy E, MacCallum RM, Mao C, Maslen G, Mbogo C, McCarthy J, Michel K, Mitchell SN, Moore W, Murphy KA, Naumenko AN, Nolan T, Novoa EM, O'Loughlin S, Oringanje C, Oshaghi MA, Pakpour N, Papathanos PA, Peery AN, Povelones M, Prakash A, Price DP, Rajaraman A, Reimer LJ, Rinker DC, Rokas A, Russell TL, Sagnon NF, Sharakhova MV, Shea T, Simao FA, Simard F, Slotman MA, Somboon P, Stegniy V, Struchiner CJ, Thomas GWC, Tojo M, Topalis P, Tubio JMC, Unger MF, Vontas J, Walton C, Wilding CS, Willis JH, Wu Y‐C, Yan G, Zdobnov EM, Zhou X, Catteruccia F, Christophides GK, Collins FH, Cornman RS, Crisanti A, Donnelly MJ, Emrich SJ, Fontaine MC, Gelbart W, Hahn MW, Hansen IA, Howell PI, Kafatos FC, Kellis M, Lawson D, Louis C, Luckhart S, Muskavitch MAT, Ribeiro JM, Riehle MA, Sharakhov IV, Tu Z, Zwiebel LJ, Besansky NJ. 2015. Highly evolvable malaria vectors: The genomes of 16 Anopheles mosquitoes. Science 347:1258522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nene V, Wortman JR, Lawson D, Haas B, Kodira C, Tu ZJ, Loftus B, Xi ZY, Megy K, Grabherr M, Ren QH, Zdobnov EM, Lobo NF, Campbell KS, Brown SE, Bonaldo MF, Zhu JS, Sinkins SP, Hogenkamp DG, Amedeo P, Arensburger P, Atkinson PW, Bidwell S, Biedler J, Birney E, Bruggner RV, Costas J, Coy MR, Crabtree J, Crawford M, deBruyn B, DeCaprio D, Eiglmeier K, Eisenstadt E, El‐Dorry H, Gelbart WM, Gomes SL, Hammond M, Hannick LI, Hogan JR, Holmes MH, Jaffe D, Johnston JS, Kennedy RC, Koo H, Kravitz S, Kriventseva EV, Kulp D, LaButti K, Lee E, Li S, Lovin DD, Mao CH, Mauceli E, Menck CFM, Miller JR, Montgomery P, Mori A, Nascimento AL, Naveira HF, Nusbaum C, O'Leary S, Orvis J, Pertea M, Quesneville H, Reidenbach KR, Rogers YH, Roth CW, Schneider JR, Schatz M, Shumway M, Stanke M, Stinson EO, Tubio JMC, VanZee JP, Verjovski‐Almeida S, Werner D, White O, Wyder S, Zeng QD, Zhao Q, Zhao YM, Hill CA, Raikhel AS, Soares MB, Knudson DL, Lee NH, Galagan J, Salzberg SL, Paulsen IT, Dimopoulos G, Collins FH, Birren B, Fraser‐Liggett CM, Severson DW. 2007. Genome sequence of Aedes aegypti, a major arbovirus vector. Science 316:1718–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosrati R, Driouchi A, Yip CM, Sinton D. 2015. Two‐dimensional slither swimming of sperm within a micrometre of a surface. Nat Commun 6:8703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva CF, Damiens D, Vreysen MJB, Lemperiere G, Gilles J. 2013. Reproductive strategies of Aedes albopictus (Diptera: Culicidae) and implications for the sterile insect technique. PLoS ONE 8:e78884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai M, Baccetti B. 1993. 2‐Step acquisition of motility by insect spermatozoa. Experientia 49:593–595. [Google Scholar]
- Otronen M. 1997. Sperm numbers, their storage and usage in the fly Dryomyza anilis . P Roy Soc B‐Biol Sci 264:777–782. [Google Scholar]
- Otronen M, Reguera P, Ward PI. 1997. Sperm storage in the yellow dung fly Scathophaga stercoraria: Identifying the sperm of competing males in separate female spermathecae. Ethology 103:844–854. [Google Scholar]
- Pascini TV, Ramalho‐Ortigao JM, Martins GF. 2012. Morphological and morphometrical assessment of spermathecae of Aedes aegypti females. Mem I Oswaldo Cruz 107:705–712. [DOI] [PubMed] [Google Scholar]
- Pascini TV, Ramalho‐Ortigao JM, Martins GF. 2013. The fine structure of the spermatheca in Anopheles aquasalis (Diptera: Culicidae). Ann Entomol Soc Am 106:857–867. [Google Scholar]
- Pasini ME, Intra J, Pavesi G. 2008. Expression study of an alpha‐L‐fucosidase gene in the Drosophilidae family. Gene 420:23–33. [DOI] [PubMed] [Google Scholar]
- Pask GM, Jones PL, Rutzler M, Rinker DC, Zwiebel LJ. 2011. Heteromeric anopheline odorant receptors exhibit distinct channel properties. PLoS ONE 6:e28774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, Chen S, Busser S, Liu H, Honegger T, Kubli E. 2005. Gradual release of sperm bound sex‐peptide controls female postmating behavior in Drosophila . Curr Biol 15:207–213. [DOI] [PubMed] [Google Scholar]
- Perotti ME. 1973. Mitochondrial derivative of spermatozoon of Drosophila before and after fertilization. J Ultrastruct Res 44:181–198. [DOI] [PubMed] [Google Scholar]
- Perotti ME, Cattaneo F, Pasini ME, Verni F, Hackstein JHP. 2001. Male sterile mutant casanova gives clues to mechanisms of sperm‐egg interactions in Drosophila melanogaster . Mol Reprod Dev 60:248–259. [DOI] [PubMed] [Google Scholar]
- Phillips DM. 1969. Exceptions to prevailing pattern of tubules (9 + 9 + 2) in sperm flagella of certain insect species. J Cell Biol 40:28–&. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Liu C, Zhou X, Malpartida JC, Zwiebel LJ. 2014. Odorant receptor‐mediated sperm activation in disease vector mosquitoes. Proc Natl Acad Sci USA 111:2566–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponlawat A, Harrington LC. 2009. Factors associated with male mating success of the dengue vector mosquito, Aedes aegypti . Am J Trop Med Hyg 80:395–400. [PubMed] [Google Scholar]
- Prokupek AM, Eyun SI, Ko L, Moriyama EN, Harshman LG. 2010. Molecular evolutionary analysis of seminal receptacle sperm storage organ genes of Drosophila melanogaster . J Evol Biol 23:1386–1398. [DOI] [PubMed] [Google Scholar]
- Prokupek AM, Kachman SD, Ladunga I, Harshman LG. 2009. Transcriptional profiling of the sperm storage organs of Drosophila melanogaster . Insect Mol Biol 18:465–475. [DOI] [PubMed] [Google Scholar]
- Richardson JB, Jameson SB, Gloria‐Soria A, Wesson DM, Powell J. 2015. Evidence of limited polyandry in a natural population of Aedes aegypti . Am J Trop Med Hyg 93:189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DW, Baldini F, Battaglia F, Panico M, Dell A, Morris HR, Catteruccia F. 2009. Transglutaminase‐mediated semen coagulation controls sperm storage in the malaria mosquito. PLoS Biol 7:e1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DW, Whitten MMA, Thailayil J, Soichot J, Levashina EA, Catteruccia F. 2008. Molecular and cellular components of the mating machinery in Anopheles gambiae females. Proc Natl Acad Sci USA 105:19390–19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux MM, Townley IK, Raisch M, Reade A, Bradham C, Humphreys G, Gunaratne HJ, Killian CE, Moy G, Su YH, Ettensohn CA, Wilt F, Vacquier VD, Burke RD, Wessel G, Foltz KR. 2006. A functional genomic and proteomic perspective of sea urchin calcium signaling and egg activation. Dev Biol 300:416–433. [DOI] [PubMed] [Google Scholar]
- Sato K, Pellegrino M, Nakagawa T, Nakagawa T, Vosshall LB, Touhara K. 2008. Insect olfactory receptors are heteromeric ligand‐gated ion channels. Nature 452:1002–1006. [DOI] [PubMed] [Google Scholar]
- Sbilordo SH, Schafer MA, Ward PI. 2009. Sperm release and use at fertilization by yellow dung fly females (Scathophaga stercoraria). Biol J Linn Soc 98:511–518. [Google Scholar]
- Schnakenberg SL, Matias WR, Siegal ML. 2011. Sperm‐storage defects and live birth in Drosophila females lacking spermathecal secretory cells. PLoS Biol 9:e1001192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw WR, Teodori E, Mitchell SN, Baldini F, Gabrieli P, Rogers DW, Catteruccia F. 2014. Mating activates the heme peroxidase HPX15 in the sperm storage organ to ensure fertility in Anopheles gambiae . Proc Natl Acad Sci USA 111:5854–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JG. 1974. Sperm activation in saturniid moths: Some aspects of mechanism of activation. J Insect Physiol 20:2321–2328. [DOI] [PubMed] [Google Scholar]
- Sirot LK, Hardstone MC, Helinski MEH, Ribeiro JMC, Kimura M, Deewatthanawong P, Wolfner MF, Harrington LC. 2011. Towards a semen proteome of the dengue vector mosquito: Protein identification and potential functions. PLoS Neglect Trop D 5:e989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidler AL, Terenzi O, Soichot J, Levashina EA, Marois E. 2013. Targeted mutagenesis in the malaria mosquito using TALE nucleases. PLoS ONE 8:e74511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Walter MF, Hice RH, O'Brochta DA, Atkinson PW. 2007. Testis‐specific expression of the β2 tubulin promoter of Aedes aegypti and its application as a genetic sex‐separation marker. Insect Mol Biol 16:61–71. [DOI] [PubMed] [Google Scholar]
- Snook RR, Karr TL. 1998. Only long sperm are fertilization‐competent in six sperm‐heteromorphic Drosophila species. Curr Biol 8:291–294. [DOI] [PubMed] [Google Scholar]
- Snook RR, Markow TA, Karr TL. 1994. Functional nonequivalence of sperm in Drosophila pseudoobscura . Proc Natl Acad Sci USA 91:11222–11226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman A. 1964. The mechanics of copulation in Aedes aegypti . Biol Bull 127:324–344. [Google Scholar]
- Staples JE, Fischer M. 2014. Chikungunya virus in the Americas—What a vectorborne pathogen can do. New Engl J Med 371:887–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Minnick SL, Sun AK, Scott TW. 2007. Mortality and reproductive dynamics of Aedes aegypti (Diptera: Culicidae) fed human blood. Vector Borne Zoonotic Dis 7:86–98. [DOI] [PubMed] [Google Scholar]
- Suarez SS, Varosi SM, Dai X. 1993. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci USA 90:4660–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow JG, Wilkinson GS. 2002. The long and short of sperm polymorphisms in insects. Biol Rev Camb Philos Soc 77:153–182. [DOI] [PubMed] [Google Scholar]
- Swan MA. 1981. The generation and propagation of double waves in mosquito (Aedes notoscriptus) sperm‐tails. Gamete Res 4:241–250. [Google Scholar]
- Thaler CD, Miyata H, Haimo LT, Cardullo RA. 2013. Waveform generation is controlled by phosphorylation and swimming direction is controlled by Ca2+ in sperm from the mosquito Culex quinquefasciatus . Biol Reprod 89:135. [DOI] [PubMed] [Google Scholar]
- Tongu Y. 1968. The ultrastructure of mosquitoes. 1. Spermatozoa in Culex pipiens pallens . Jpn J Sanit Zool 19:215–217. [Google Scholar]
- Verhoek BA, Takken W. 1994. Age effects on the insemination rate of Anopheles gambiae s. l. in the laboratory. Entomol Exp Appl 72:167–172. [Google Scholar]
- Verma LR. 1973. An ionic basis for a possible mechanism of sperm survival in the spermatheca of the queen honey bee (Apis mellifera L.). Comp Biochem Physiol 44A:1325–1331. [Google Scholar]
- Visconti PE, Bailey JL, Moore GD, Pan D, Olds‐Clarke P, Kopf GS. 1995a. Capacitation of mouse spermatozoa. I. Correlation between the capacitation state and protein tyrosine phosphorylation. Development 121:1129–1137. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Galantino‐Homer H, Ning X, Moore GD, Valenzuela JP, Jorgez CJ, Alvarez JG, Kopf GS. 1999a. Cholesterol efflux‐mediated signal transduction in mammalian sperm. beta‐cyclodextrins initiate transmembrane signaling leading to an increase in protein tyrosine phosphorylation and capacitation. J Biol Chem 274:3235–3242. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, Pan D, Olds‐Clarke P, Kopf GS. 1995b. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP‐dependent pathway. Development 121:1139–1150. [DOI] [PubMed] [Google Scholar]
- Visconti PE, Ning X, Fornes MW, Alvarez JG, Stein P, Connors SA, Kopf GS. 1999b. Cholesterol efflux‐mediated signal transduction in mammalian sperm: Cholesterol release signals an increase in protein tyrosine phosphorylation during mouse sperm capacitation. Dev Biol 214:429–443. [DOI] [PubMed] [Google Scholar]
- Voordouw MJ, Koella JC, Hurd H. 2008. Intra‐specific variation of sperm length in the malaria vector Anopheles gambiae: Males with shorter sperm have higher reproductive success. Malaria J 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watnick TJ, Jin Y, Matunis E, Kernan MJ, Montell C. 2003. A flagellar polycystin‐2 homolog required for male fertility in Drosophila . Curr Biol 13:2179–2184. [DOI] [PubMed] [Google Scholar]
- Watson PA. 1991. Function follows form: Generation of intracellular signals by cell deformation. FASEB J 5:2013–2019. [DOI] [PubMed] [Google Scholar]
- Werner M, Gack C, Speck T, Peschke K. 2007. Queue up, please! Spermathecal filling in the rove beetle Drusilla canaliculata (Coleoptera, Staphylinidae). Naturwissenschaften 94:837–841. [DOI] [PubMed] [Google Scholar]
- Werner M, Simmons LW. 2008. Insect sperm motility. Biol Rev Camb Philos Soc 83:191–208. [DOI] [PubMed] [Google Scholar]
- Werner M, Zissler D, Peschke K. 1999. Structure and energy pathways of spermatozoa of the rove beetle Aleochara bilineata (Coleoptera, Staphylinidae). Tissue Cell 31:413–420. [DOI] [PubMed] [Google Scholar]
- Wheeler DE, Krutzsch PH. 1994. Ultrastructure of the spermatheca and its associated gland in the ant Crematogaster opuntiae (Hymenoptera, Formicidae). Zoomorphology 114:203–212. [Google Scholar]
- World Health Organization. 2014. World Malaria Report 2014. Geneva, Switzerland: World Health Organization. [Google Scholar]
- Xue L, Noll M. 2000. Drosophila female sexual behavior induced by sterile males showing copulation complementation. Proc Natl Acad Sci USA 97:3272–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto DS, Hatakeyama M, Matsuoka H. 2013. Artificial activation of mature unfertilized eggs in the malaria vector mosquito, Anopheles stephensi (Diptera, Culicidae). J Exp Biol 216:2960–2966. [DOI] [PubMed] [Google Scholar]
- Yanagimachi R, Usui N. 1974. Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp Cell Res 89:161–174. [DOI] [PubMed] [Google Scholar]
- Yang Y, Cochran DA, Gargano MD, King I, Samhat NK, Burger BP, Sabourin KR, Hou Y, Awata J, Parry DAD, Marshall WF, Witman GB, Lu X. 2011. Regulation of flagellar motility by the conserved flagellar protein CG34110/Ccdc135/FAP50. Mol Biol Cell 22:976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lu X. 2011. Drosophila sperm motility in the reproductive tract. Biol Reprod 84:1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York‐Andersen AH, Parton RM, Bi CJ, Bromley CL, Davis I, Weil TT. 2015. A single and rapid calcium wave at egg activation in Drosophila . Biol Open 4:553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young ADM, Downe AER. 1982. Renewal of sexual receptivity in mated female mosquitos, Aedes aegypti. Physiol Entomol 7:467–471. [Google Scholar]
- Yuval B. 2006. Mating systems of blood‐feeding flies. Annu Rev Entomol 51:413–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Figure S1. Detailed anatomical drawing from which Figure 1A is derived (Jobling and Lewis 1987).
Video 1. Sperm motility within recently inseminated Aedes aegypti female reproductive tract.
Supplementary Legend.


