Abstract
Kisspeptin regulates reproduction via signaling through the receptor, Kiss1r, in GnRH neurons. However, both kisspeptin and Kiss1r are produced in several peripheral tissues, and recent studies have highlighted a role for kisspeptin signaling in metabolism and glucose homeostasis. We recently reported that Kiss1r knockout (KO) mice display a sexually dimorphic metabolic phenotype, with KO females displaying obesity, impaired metabolism, and glucose intolerance at 4–5 months of age. However, it remains unclear when this metabolic phenotype first emerges in development, or which aspects of the pleiotropic phenotype underlie the metabolic defects and which are secondary to the obesity. Here, we studied Kiss1r KO females at different ages, including several weeks before the emergence of body weight (BW) differences and later when obesity is present. We determined that at young adult ages (6 wk old), KO females already exhibit altered adiposity, leptin levels, metabolism, and energy expenditure, despite having normal BWs at this time. In contrast, food intake, water intake, and glucose tolerance are normal at young ages and only show impairments at older adult ages, suggesting that these impairments may be secondary to earlier alterations in metabolism and adiposity. We also demonstrate that, in addition to BW, all other facets of the adult metabolic phenotype persist even when gonadal sex steroids are similar between genotypes. Collectively, these data highlight the developmental emergence of a metabolic phenotype induced by disrupted kisspeptin signaling and reveal that multiple, but not all, aspects of this phenotype are already disrupted before detectable changes in BW.
The neuropeptide kisspeptin (encoded by Kiss1) regulates reproduction by stimulating GnRH neurons via its receptor, Kiss1r (formerly known as GPR54). Humans, mice, and rats with mutations in these genes show impaired puberty, hypogonadism, and infertility (1–6). In addition to being expressed in reproductive areas of the brain, kisspeptin is also expressed in multiple peripheral tissues (7–10). The receptor, Kiss1r, is also expressed in multiple non-GnRH brain areas and in several peripheral tissues (9–11), including metabolic tissues such as fat, liver, and pancreas. The presence of kisspeptin and its receptor outside of reproductive tissues suggests that kisspeptin signaling has additional uncharacterized roles beyond reproduction, perhaps relating to metabolism or glucose control (12, 13). However, until recently, virtually all research on kisspeptin signaling has focused on reproductive regulation.
We recently reported that, in addition to stimulating the reproductive axis, the kisspeptin system is also an important player in body weight (BW), energy balance, and glucose regulation (14). We found that, compared with wildtype (WT) littermates, adult Kiss1r knockout (KO) females maintained on a standard chow diet displayed dramatically higher BWs, but this phenotype did not emerge until early adulthood, beginning around 10 weeks of age. By 4–5 months of age, KO females weighed a robust 30% more than WT females. In addition to becoming obese in adulthood, KO females also had increased adiposity, leptin levels, and impaired glucose tolerance, all measured around 4–5 months of age. This phenotype was sexually dimorphic, as male Kiss1r KO mice had normal BW and glucose regulation, even out to 6 months of age. Despite their obesity, adult Kiss1r KO females ate less than WT females. However, adult KO females displayed markedly reduced metabolic parameters, including lower respiratory rates and energy expenditure. Importantly, the BW and metabolic phenotype in Kiss1r KO females was not solely reflective of absent gonadal sex steroids, because chronically ovariectomized Kiss1r KO females still developed obesity, hyperleptinemia, reduced metabolism, and glucose intolerance compared with ovariectomized WT females.
Our previous study demonstrated that absent kisspeptin signaling can be an important factor in BW, adiposity, glucose intolerance, and metabolism in adulthood. However, other than BW, which was normal at younger adult ages, all of the metabolic parameters examined were done so exclusively in midadulthood, around 4–5 months of age. Thus, it remains unknown when the various aspects of the metabolic phenotype first emerge, or if different parameters show impairments before others. Indeed, which aspects of the KO's metabolic phenotype are secondary to the eventual obesity or which may underlie the pleiotropic phenotype remains undetermined. In the present study, we hypothesized that some aspects of the Kiss1r KO female phenotype, such as decreased feeding and glucose intolerance, would emerge only after (ie, as a consequence of) the onset of overt BW differences, whereas other facets, such as elevated adiposity and reduced metabolic rate, would be present before BW differences, suggesting a causal relationship between the former and the latter. To assess this issue, we examined multiple metabolic parameters in Kiss1r KO females at several different ages, including at 6 weeks old, multiple weeks before the onset of the BW phenotype, at 10 weeks of age, around the time the BW difference first becomes detectable, and later around 20 weeks of age, when midadult KO females show robust metabolic impairments and marked obesity.
Materials and Methods
Animals and experimental ages
Kiss1r KO mice, described previously (14–17), were generated by mating heterozygous breeders and maintained on a mixed C57Bl6 × 129S1/SvImJ background. All mice were genotyped and sexed by PCR of tail DNA. In each experiment, Kiss1r KO mice were compared with control littermates (siblings). Weaned littermates (∼3 wk old) were housed at 2–3 per cage (mixed genotype) in a 12-hour light, 12-hour dark cycle (lights off at 6 pm) with ad libitum water and standard rodent chow (3.5 kcal/g, 45.2% available carbohydrate, 11.4% fat, and 17.2% crude protein). All experiments were approved by the University of California, San Diego Institutional Animal Care and Use Committee.
KO and control mice were studied at 3 specific ages: 6 weeks old (“young adults,” several weeks before any detectable changes in BW in the KOs), 10 weeks old (around the age when BW differences first start to become apparent, with KOs weighing more than controls), and 18–20 weeks old (when BWs, feeding, metabolic rates, and glucose tolerance are all significantly impaired in the KOs). Mice at each age were subjected to BW and body composition analyses, glucose tolerance testing (GTT), hormone analysis, feeding and water intake measures, and metabolic analyses in Comprehensive Laboratory Animal Monitoring System (CLAMS) cages.
Our prior study indicated that adult BW was still dramatically higher in KO than control females even when both genotypes had been chronically without gonadal sex steroids. However, additional measures beyond BW were not assessed in that study. Here, we also studied a cohort of adult mice at 18–20 weeks old that were first ovariectomized (OVX) months earlier, before the normal pubertal period and any significant sex steroid secretion. In this case, both KOs and control siblings were bilaterally ovariectomized at 2.5 weeks old, thereby equalizing gonadal sex steroid secretion (ie, making it absent) in both genotypes for the remaining 16–18 weeks until time of metabolic analyses.
Body composition analyses
Body composition was determined in female Kiss1r KO and control mice by dual energy x-ray absorptiometry. Mice were single housed and fasted for 4–6 hours and then anesthetized (ip injection; ketamine 100 mg/kg and xylazine 10 mg/kg). BW was measured, and lean muscle mass and fat mass were determined by scanning with a GE Lunar Pixi densitometer machine (n = 7–17/group).
Leptin assays
Blood was collected retroorbitally and the serum stored at −20°C. Serum samples were assayed for leptin using a Mouse/Rat Leptin Quantikine ELISA kit (n = 5–7/group, MOB00; R&D Systems), per the manufacturer's instructions. The assay sensitivity is 0.02 ng/mL, intraassay coefficient of variant is 4%, and interassay coefficient of variation is 7%.
Metabolic and energy expenditure analyses
Indirect calorimetry was performed at each age using a 12-cage equal flow CLAMS calorimeter system (Columbus Instruments) coupled with photosensors to detect movement. Females were habituated to the metabolic cages (single-housed) for 2 days before data acquisition (n = 6–9/genotype). O2 consumption (VO2) and CO2 production (VCO2) were measured every 12 minutes per cage. In addition, feeding and drinking were measured in 12-minute intervals and calculated for hourly consumption.
Glucose tolerance tests
GTTs were performed on Kiss1r KO and control mice on a standard chow diet. Mice were fasted for 6 hours beforehand with free access to water. Blood glucose was measured using a handheld glucometer (One Touch UltraMini; LifeScan, Inc) just before ip glucose injection (time 0; 2-g/kg BW in saline) and subsequently at 15, 30, 45, 60, 90, and 120 minutes after administration (n = 8–13/group).
Statistical analyses
All data are expressed as the mean ± SEM for each group. For data at single points (nonrepeated measures), comparisons were made using unpaired 2-tailed t tests. For repeated measures (GTT), two-way repeated-measures ANOVA was performed, with Bonferroni post hoc tests directly comparing genotypes at specific points. Statistical significance was set at P < .05.
Results
Kiss1r KO females already have elevated adiposity and leptin levels at young adult ages, before detectable differences in BW
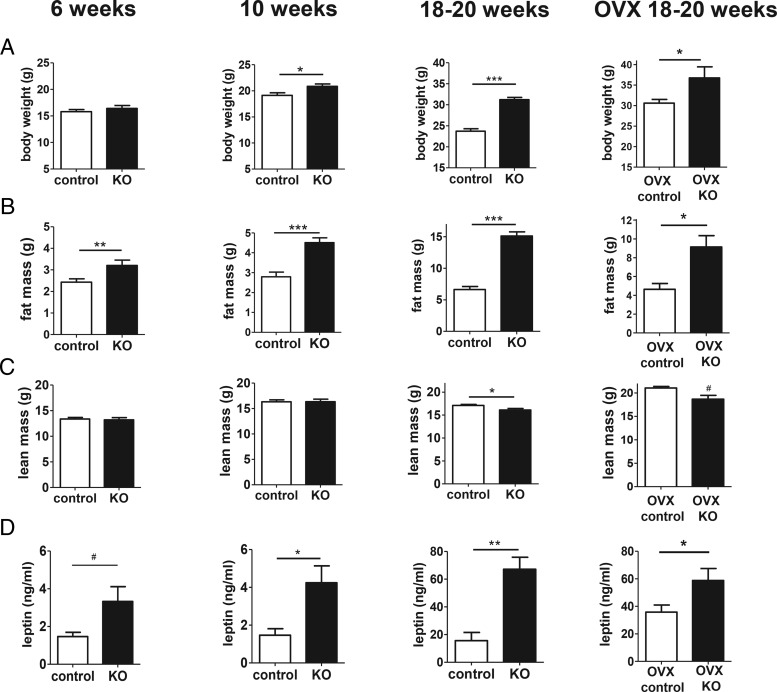
Female Kiss1r KO mice weighed the same as control littermate females at 6 weeks (Figure 1A), with a small (10%) but statistically significant difference in BW at 10 weeks (P < .05) (Figure 1A), compared with a more robust 32% difference in BW in adults (18–20 wk; P < .001) (Figure 1A). However, even though there was no difference in BW at 6 weeks of age, adiposity was already significantly increased in KOs by over 30% at this age (P < .01) (Figure 1B). By 10 weeks of age, adiposity was increased by 60% (P < .001) (Figure 1B), which then rose to 125% in adulthood (P < .001) (Figure 1B). In contrast to fat mass, lean mass was not significantly different between KOs and controls at 6 or 10 weeks of age and showed only a minor decrease (6%) in KOs in adulthood, as previously reported (Figure 1C). Similar to fat mass, serum leptin levels (Figure 1D) were also significantly elevated in KOs in adulthood (P < .01; 300%) and at 10 weeks (P < .05; 140%), with a nonsignificant trend (P = .058) at 6 weeks (80%), despite their BWs being normal at 6 weeks of age and only slightly higher at 10 weeks of age.
Figure 1.
BW, body composition, and leptin levels in Kiss1r KO females fed a standard diet. BW (A), fat mass (B), lean mass (C), and serum leptin (D) of KO and control females at 6, 10, or 18–20 weeks of age; n = 5–17 per group. For the cohort of 18–20 week OVX mice, both genotypes were ovariectomized at 2.5 weeks of age and remained without gonadal sex steroids for the rest of the study. Note the differing scales of the y-axes for younger vs. older animals. *, P < .05; **, P < .01; ***, P < .001; #, nonsignificant trend for genotype difference (P < .06).
We previously reported that BW at 18–20 weeks old was still markedly higher in KO than control females even when both genotypes had been chronically without gonadal sex steroids (14). However, additional measures besides BW were not previously assessed. Here, we further studied a cohort of 18- to 20-week-old adult females of both genotypes that were ovariectomized earlier at 2.5 weeks old. These OVX KOs still weighed more than OVX controls, by approximately 22%, and also still displayed higher fat mass and circulating leptin levels at 18–20 weeks of age (P < .05 for all measures; Figure 1, A, B, and D, far right column). Lean mass showed a nonsignificant trend to be slightly lower in OVX females at 18–20 weeks old (Figure 1C).
Food and water intake are not different at young adult ages before increased BW
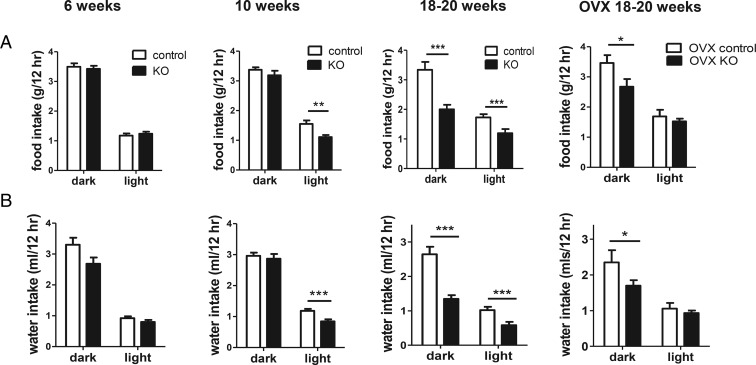
We previously discovered that adult KO females have decreased food intake, despite their higher BWs. We measured feeding in younger animals to determine whether this aspect of the KO phenotype was linked or whether it was secondary (or compensatory) to the obese phenotype. Unlike in adulthood, food consumption of KO females was normal in both dark and light cycles at 6 weeks of age (Figure 2A) and was only slightly decreased in the light cycle at 10 weeks of age (Figure 2A). By comparison, in adult KO females, food intake was strongly decreased in both the dark and light portions of the light-dark cycle (P < .001) (Figure 2A). We also examined water intake, which was previously not examined in our prior study. As with feeding, adult KOs showed significantly decreased water intake in both dark and light phases (P < .001) (Figure 2B). However, at 6 weeks of age, there was no significant genotype difference in water intake (Figure 2B), and at 10 weeks of age, the KOs displayed a minor, although significant, decrease in water intake only during the light phase (Figure 2B).
Figure 2.
Food and water intake during light and dark cycles. Food intake (A) and water intake (B) in 6-, 10-, and 18- to 20-week-old Kiss1r KO and control females. For the cohort of 18- to 20-week OVX mice, both genotypes were ovariectomized at 2.5 weeks of age. The gonad-intact 18- to 20-week-old food intake graph is reused with permission from our previous publication (14) and is shown for comparison purposes to the other ages; n = 6–9 per group; **, P < .01; ***, P < .001; #, nonsignificant trend for genotype difference (P < .07).
To assess potential influence of sex steroids on these measures, feeding and drinking were also measured in a cohort of 18- to 20-week-old mice of both genotypes that had been ovariectomized earlier at 2.5 weeks old. As in gonad-intact mice, 18- to 20-week-old OVX KO females still consumed significantly less food and water than OVX controls during the dark phase of the light-dark cycle, when mice are most active (P < .05 for both measures; Figure 2, far right column).
Metabolism and energy homeostasis are already impaired at younger ages before detectable changes in BW
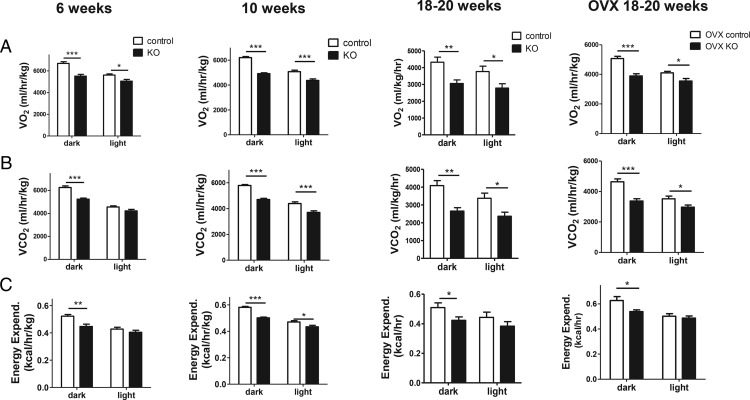
Next, we used CLAMs metabolic cages to measure metabolic gas exchange in KOs and controls at different ages. Similar to adiposity and leptin levels, and in contrast to food intake, gas exchange was already significantly decreased at 6 weeks of age. Specifically, VO2 was significantly decreased in both dark and light phases (P < .001 in dark cycle, P < .05 in light cycle) (Figure 3A), and VCO2 was strongly decreased during the dark phase (P < .001) (Figure 3B). Energy expenditure was also already notably decreased at 6 weeks of age (P < .01) (Figure 3C). By 10 weeks of age, VO2, VCO2, and energy expenditure were all substantially decreased in both dark (P < .001) and light phases (P < .05–0.001) of the light-dark cycle (Figure 3, A–C), similar to what was observed previously in adult 18- to 20-week KO females (Figure 3) (14).
Figure 3.
CLAMS cage assessments of gas exchange and energy expenditure in light and dark cycles. VO2 (A), VCO2 (B), and energy expenditure (C) in 6-, 10-, and 18- to 20-week-old Kiss1r KO and control females; n = 7–9 per group. For the cohort of 18-to 20-week OVX mice, both genotypes were ovariectomized at 2.5 weeks of age and remained without gonadal sex steroids for the rest of the study. The gonad-intact 18- to 20-week-old CLAMS data (third column of graphs) is reused with permission from our previous publication (14) and is shown for comparison purposes to the other ages; *, P < .05; **, P < .01; ***, P < .001.
To determine the potential influence, or lack thereof, of gonadal steroids on these metabolic measures in the KOs, we also studied 18- to 20-week-old females of both genotypes that were chronically ovariectomized at 2.5 weeks old. As in gonad-intact mice, 18- to 20-week-old OVX KOs still displayed significantly reduced VO2, VCO2, and energy expenditure compared with their OVX control siblings (P < .05 to P < .001, depending on the measure; Figure 3, A–C, far right column).
Glucose tolerance in Kiss1r KO females is normal at young adult ages but disrupted at older ages
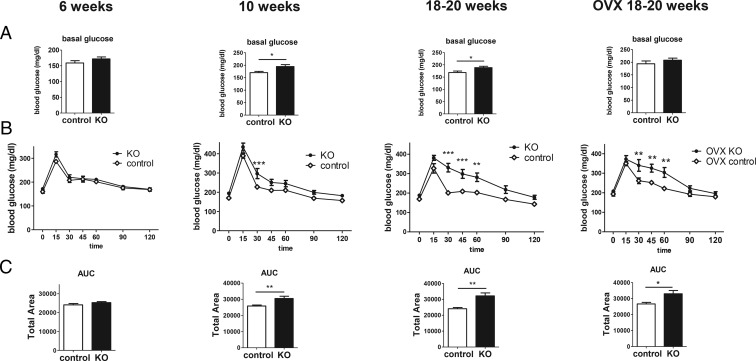
Decreased glucose tolerance can be caused by innate defects in glucose processing or secondary defects due to the inflammatory state caused by obesity. To determine whether the aberrant glucose homeostasis previously seen in adult KO females is secondary to obesity, we performed GTTs at 6 and 10 weeks. In contrast to adult KO females, which showed elevated basal glucose (P < .05) (Figure 4A) and severely impaired glucose tolerance (area under the curve [AUC] P < .01) (Figure 4, B and C), at 6 weeks of age, there were no genotype differences in either basal glucose levels (Figure 4A) or the response to glucose injection (Figure 4, B and C), indicating normal glucose homeostasis at this younger age. However, at 10 weeks of age, when the BW differences are becoming significantly different, basal glucose was elevated (P < .05) (Figure 4A), and glucose tolerance was now significantly decreased (AUC P < .01) (Figure 4, B and C). Thus, like food intake, impairments in glucose homeostasis are not present at younger ages but emerge later in adulthood when BWs are significantly elevated.
Figure 4.
Determination of glucose homeostasis with ip GTT. Basal glucose (A), GTT (B), and AUC (C) at 6, 10, and 18–20 weeks of age. For the cohort of 18- to 20-week OVX mice, both genotypes were ovariectomized at 2.5 weeks of age and remained without gonadal sex steroids for the rest of the study; n = 8–13 per group; *, P < .05; **, P < .01; ***, P < .001.
To ascertain whether absent gonadal steroids influence the glucose intolerance in the adult KOs, we also studied 18- to 20-week-old females of both genotypes that were ovariectomized at 2.5 weeks old. As in gonad-intact mice, 18- to 20-week-old OVX KOs still displayed significantly impaired glucose tolerance compared with their OVX control siblings (P < .05) (Figure 4, right column).
Discussion
Puberty and fertility in mammals are highly dependent on functionally intact kisspeptin signaling in the brain, via kisspeptin's stimulation of GnRH neurons (1–3, 5, 6), with disrupted kisspeptin signaling causing hypogonadal hypogonadism. Because of this role, the vast majority of kisspeptin studies have focused on its actions in controlling reproduction, overlooking possible other functions of this peptide. However, the expression of kisspeptin and its receptor in several peripheral tissues implies other undiscovered roles for kisspeptin signaling. Indeed, some evidence suggested that kisspeptin signaling might play a role in energy balance (13, 18–20), and our recent study discovered a sexually dimorphic BW phenotype in Kiss1r KO mice that was not detectable until the females reached adulthood, first notably emerging around 10 weeks of age and increasing dramatically thereafter into older adult ages (14). We previously found that the KO females also display greatly increased adiposity and leptin levels, decreased glucose tolerance, and markedly disrupted metabolism and energy expenditure. However, other than BWs, all these measures were taken only in midadulthood, around 4–5 months of age. Thus, the developmental timing of the various aspects of the metabolic phenotype, as well as which of these aspects might be secondary to the obesity vs which might causally underlie the development of obesity, remained unknown. Here, in comparison with older adults (18–20 wk old), we examined multiple metabolic and glucose parameters in Kiss1r KO mice at 6 and 10 weeks of age, the former being young adulthood, multiple weeks before the onset of the BW phenotype, and the latter being concurrent with the emergence of the BW phenotype. Our results demonstrate that adiposity, elevated leptin, and decreased metabolism are already altered at younger ages, well before overt changes in BW, whereas impaired glucose tolerance and feeding develop later in adulthood, after significant increases in BW have been established (Table 1).
Table 1.
Metabolic and BW Parameters in Kiss1r KO Females at Different Ages or in Adult OVX Mice
| Phenotypic Parameter | 6 Weeks | 10 Weeks | 18–20 Weeks | 18–20 Weeks (OVX) |
|---|---|---|---|---|
| Increased BW | No | √ (10%) | √ (32%) | √ (22%) |
| Increased adiposity | √ (35%) | √ (60%) | √ (125%) | √ (100%) |
| Increased leptin | √ (80%) | √ (140%) | √ (300%) | √ (65%) |
| Decreased VO2 | √ (20%) | √ (22%) | √ (28%) | √ (22%) |
| Decreased CO2 | √ (20%) | √ (22%) | √ (30%) | √ (25%) |
| Decreased energy expenditure | √ (15%) | √ (15%) | √ (20%) | √ (15%) |
| Decreased food intake | No | No | √ (35%) | √ (25%) |
| Decreased water intake | No | No | √ (45%) | √ (25%) |
| Glucose intolerance (increased AUC) | No | √ (18%) | √ (32%) | √ (25%) |
“No” indicates that there was no significant difference in this measure between control and KO females at this age; a check mark indicates that this measure was significantly different from controls. Percentage values in parentheses indicate approximate % increase or % decrease relative to control females at same age. Percentage values for adult OVX KOs are in comparison with adult OVX control mice similarly ovariectomized at 2.5 weeks of age. Food intake, water intake, VO2, VCO2, and energy expenditure values are for the dark period of the light-dark cycle, when mice are most active. Note that food and water intake at 10 weeks were both normal in the dark cycle although moderately reduced in the light portion.
This study confirms our previous report of a dramatic BW phenotype in adult Kiss1r KO female mice, which does not become first apparent until around 10 weeks of age, and which gets progressively worse as the animals age beyond that point. We found no genotype difference in BW in younger adults, at 6 weeks of age, as previously reported. However, when body composition was examined at 6 weeks of age, we were surprised to find that adiposity was already elevated, despite normal BWs. The degree of increased adiposity was smaller at 6 than 10 weeks, which was also smaller than in adulthood, suggesting a gradual increase in adiposity that begins at least right after puberty, and which likely causes the elevated BW that emerges later around 10 weeks of age. Due to the technical limitations of our available metabolic equipment, we were unable to examine ages younger than 6 weeks old, so it remains unknown how early the increased adiposity begins to manifest. Consistent with the increased fat mass at all ages, circulating leptin was elevated as well. The small difference in lean mass that is observed in adult females was not present at 6 or 10 weeks, so this difference may be due to the chronic lack of sex steroids.
We previously showed that multiple measures of metabolic rate are significantly decreased in adult KO females (14), suggesting the possibility that the eventual emergence of obesity is caused by underlying decreases in metabolism and energy expenditure. However, in our original report, we only measured metabolic rats in older adults (4–5 mo old). Here, we demonstrate that metabolic rates and energy expenditure are also significantly reduced to a substantial degree at both 6 and 10 weeks of age. These data support the likelihood that permanent or early life decreases in metabolism and energy expenditure underlie the eventual emergence of the obese phenotype of adult Kiss1r KO females.
Unexpectedly, even though they weigh much more, adult Kiss1r KO females display decreased food intake rather than the expected hyperphagia, and we report here for the first time that adult KO females also have significantly decreased water intake as well. Interestingly, although adiposity, leptin levels, metabolic rates, and energy expenditure are all already showing significant elevations or decreases at 6 weeks of age, we found that the decreases in feeding and water intake are not yet present in young adults at that age, and only minor changes are present (primarily during the light phase only) for 10-week-old females. These findings suggest that these feeding changes may be secondary side effects to the onset of obesity, perhaps as compensatory response to the higher adiposity. In fact, we hypothesize that the elevated leptin levels in KOs act in the brain to lower food intake, as is well established, but this does not emerge in the KOs until older ages when the adiposity and leptin levels are high enough to provide robust leptin feedback on feeding circuits.
Like feeding and water intake, we found that defects in glucose metabolism were not present yet in KOs at 6 weeks of age, and only very minor impairments were seen at 10 weeks of age. Like the food intake data, these findings imply that the impairments in glucose homeostasis present in older adult KOs are secondary (ie, a side effect) to the higher BW phenotype, and may be due to the increased inflammation commonly seen in obese states. Indeed, increasing evidence suggests that chronic low-grade inflammation caused by increased macrophage infiltration of adipose tissue may directly cause insulin resistance, leading to glucose intolerance and type 2 diabetes (21–24).
In our previous study, we determined that the high BWs of 18- to 20-week-old KO females were still present relative to control females even when both genotypes had been chronically without gonadal sex steroids since an early juvenile age (14). This suggested that the BW phenotype was not simply a product of the KOs hypogonadism. However, additional metabolic measures beyond BW were not assessed in that study. In the present study, we similarly studied 18- to 20-week-old females of both genotypes that had been chronically ovariectomized earlier at 2.5 weeks old. These adult OVX KOs still demonstrated all the metabolic impairments observed in the gonad-intact KOs, including higher fat mass, elevated leptin levels, reduced VO2, lower VCO2, decreased energy expenditure, lower food and water intake, and significantly impaired glucose tolerance. Thus, all facets of the KO's metabolic phenotype were recapitulated even when both genotypes were similarly without gonadal sex steroids since a very young juvenile age. This further emphasizes that the obesity and metabolic status of these mice is not simply due to chronic lack of gonadal sex steroids but rather reflects a role for kisspeptin signaling outside the reproductive axis, supported by the presence of kisspeptin and Kiss1r in several metabolic tissues.
Based on our present data on the developmental timing of different measures, we propose that impaired kisspeptin signaling inherently causes lower metabolism and energy expenditure (by mechanisms yet to be elucidated), which thereby drives increased adiposity. This increased adiposity eventually accumulates enough over development and young adulthood to later produce an overweight and, eventually, an obese state in older adult ages. Thus, the obesity phenotype in Kiss1r KO females is likely due to early developmental defects in metabolism that manifest before detectable BW differences. Conversely, significant alterations in feeding, water intake, and glucose homeostasis emerge at much later ages in KOs, after they have already displayed higher adiposity, BWs, and leptin levels. We therefore hypothesize that the “primary” changes in metabolism, BW, and adiposity (and, by association, leptin levels) then lead to eventual “secondary” responses in food consumption and impairments in glucose homeostasis later in mid to older adulthood. This model would suggest that kisspeptin signaling itself is not a major direct regulator of food intake, water intake, or glucose processing but rather can influence these physiological processes indirectly by altering metabolism, adiposity/leptin, and inducing an obese state. Future studies examining the role of kisspeptin signaling in metabolic health should focus on ascertaining the mechanistic role of kisspeptin in metabolism and energy expenditure, as the other parameters may be secondary to these primary effects.
Acknowledgments
We thank Elena Luo, Matthew Poling, and Shannon Stephens for their technical and experimental support.
This work was supported by the National Science Foundation Grant IOS-1025893 and National Institutes of Health (NIH) Grants R01 HD082567 and P30 DK063491. This work was also supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant U54-HD012303. K.P.T. was supported by the NIH Grant F32 HD076606.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BW
- body weight
- GTT
- glucose tolerance testing
- KO
- knockout
- VCO2
- CO2 production
- VO2
- O2 consumption
- WT
- wildtype.
References
- 1. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 2. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366:629–635. [DOI] [PubMed] [Google Scholar]
- 3. Uenoyama Y, Nakamura S, Hayakawa Y, et al. Lack of pulse and surge modes and glutamatergic stimulation of luteinising hormone release in Kiss1 knockout rats. J Neuroendocrinol. 2015;27:187–197. [DOI] [PubMed] [Google Scholar]
- 4. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. [DOI] [PubMed] [Google Scholar]
- 5. Kirilov M, Clarkson J, Liu X, et al. Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nat Commun. 2013;4:2492. [DOI] [PubMed] [Google Scholar]
- 6. Novaira HJ, Sonko ML, Hoffman G, et al. Disrupted kisspeptin signaling in GnRH neurons leads to hypogonadotrophic hypogonadism. Mol Endocrinol. 2014;28:225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brown RE, Imran SA, Ur E, Wilkinson M. KiSS-1 mRNA in adipose tissue is regulated by sex hormones and food intake. Mol Cell Endocrinol. 2008;281:64–72. [DOI] [PubMed] [Google Scholar]
- 8. Hauge-Evans AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role for kisspeptin in islet function. Diabetologia. 2006;49:2131–2135. [DOI] [PubMed] [Google Scholar]
- 9. Ohtaki T, Shintani Y, Honda S, et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. [DOI] [PubMed] [Google Scholar]
- 10. Kotani M, Detheux M, Vandenbogaerde A, et al. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. [DOI] [PubMed] [Google Scholar]
- 11. Herbison AE, de Tassigny Xd, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151:312–321. [DOI] [PubMed] [Google Scholar]
- 12. Hussain MA, Song WJ, Wolfe A. There is kisspeptin - and then there is kisspeptin. Trends Endocrinol Metab. 2015;26:564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Song WJ, Mondal P, Wolfe A, et al. Glucagon regulates hepatic kisspeptin to impair insulin secretion. Cell Metab. 2014;19:667–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tolson KP, Garcia C, Yen S, et al. Impaired kisspeptin signaling decreases metabolism and promotes glucose intolerance and obesity. J Clin Invest. 2014;124:3075–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Poling MC, Kauffman AS. Sexually dimorphic testosterone secretion in prenatal and neonatal mice is independent of kisspeptin-Kiss1r and GnRH signaling. Endocrinology. 2012;153:782–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dungan HM, Gottsch ML, Zeng H, et al. The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci. 2007;27:12088–12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dror T, Franks J, Kauffman AS. Analysis of multiple positive feedback paradigms demonstrates a complete absence of LH surges and GnRH activation in mice lacking kisspeptin signaling. Biol Reprod. 2013;88:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fu LY, van den Pol AN. Kisspeptin directly excites anorexigenic proopiomelanocortin neurons but inhibits orexigenic neuropeptide Y cells by an indirect synaptic mechanism. J Neurosci. 2010;30:10205–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stengel A, Wang L, Goebel-Stengel M, Taché Y. Centrally injected kisspeptin reduces food intake by increasing meal intervals in mice. Neuroreport. 2011;22:253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Backholer K, Smith JT, Rao A, et al. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151:2233–2243. [DOI] [PubMed] [Google Scholar]
- 21. Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. [DOI] [PubMed] [Google Scholar]
- 22. Heilbronn LK, Campbell LV. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Curr Pharm Des. 2008;14:1225–1230. [DOI] [PubMed] [Google Scholar]
- 23. Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]