Abstract
Although the requirement of pituitary-derived LH for ovulation is well documented, the intrafollicular paracrine and autocrine processes elicited by LH necessary for follicle rupture are not fully understood. Evaluating a published rhesus macaque periovulatory transcriptome database revealed that mRNA encoding leukemia inhibitory factor (LIF) and its downstream signaling effectors are up-regulated in the follicle after animals receive an ovulatory stimulus (human chorionic gonadotropin [hCG]). Follicular LIF mRNA and protein levels are below the limit of detection before the administration of hCG but increase significantly 12 hours thereafter. Downstream LIF receptor (LIFR) signaling components including IL-6 signal transducer, the receptor associated Janus kinase 1, and the transcription factor signal transducer and activator of transcription 3 also exhibit increased expression in the rhesus macaque follicle 12 hours after administration of an ovulatory hCG bolus. A laparoscopic ovarian evaluation 72 hours after the injection of a LIF antagonist (soluble LIFR) into the rhesus macaque preovulatory follicle and hCG administration revealed blocking LIF action prevented ovulation (typically occurs 36–44 h after hCG). Moreover, ovaries removed 52 hours after both hCG and intrafollicular soluble LIFR administration confirmed ovulation was blocked as evidenced by the presence of an intact follicle and a trapped cumulus-oocyte complex. These findings give new insight into the role of LIF in the primate ovary and could lead to the development of new approaches for the control of fertility.
In mammalian species, the ovulatory process consists of highly coordinated events within the dominant follicle during the periovulatory interval. The midcycle surge of pituitary-derived LH is the key endocrine regulator of the cellular and molecular events necessary for ovulation, including follicle rupture, cumulus-oocyte expansion (C-OE), reinitiation of oocyte meiosis, and the development of the corpus luteum. Such events occur through LH binding to its receptor (LH/chorionic gonadotropin [CG] receptor [LHCGR]) located on the granulosa and theca cells in the ovulatory follicle (1). LH actions may be direct, because they are regulated through the downstream signaling pathways that are orchestrated by LHCGR activation (1, 2). Alternatively, certain LH-dependent actions are indirect, manifested through the synthesis of paracrine/autocrine acting factors. As examples, progesterone (P4), prostaglandins, and epidermal growth factor-related ligands (eg, amphiregulin, epiregulin, and betacellulin) are LH-inducible substances that coordinate and facilitate events required for the release of a fertilizable oocyte (3–5). The full complement of LH-inducible factors involved in coordinating primate ovulatory events is not yet fully defined.
From the analysis of a recently published Affymetrix microarray database, mRNAs that are differentially expressed in the naturally selected macaque follicle in response to an ovulatory stimulus, ie, human CG (hCG), were systematically identified (see Ref. 6 for details regarding data acquisition and analyses). From the resultant genomic database (NCBI GEO accession number GSE22776), it was observed that leukemia inhibitory factor (LIF) and its downstream signaling components are highly expressed in the rhesus macaque follicle during the periovulatory interval. Upon LIF binding to the LIF receptor (LIFR), LIFR forms a complex with IL-6 signal transducer (IL6ST) that subsequently activates members of the Janus kinase (JAK)1–3/tyrosine kinase (TYK) family. LIF signaling is primarily dependent on JAK1 activation (7), wherein it phosphorylates signal transducer and activator of transcription (STAT)3 on tyrosine 705 (pY705-STAT3), enabling it to dimerize, enter the nucleus, and subsequently regulate gene transcription (8). Although studies in mice have shown that LIF is necessary for implantation but not rupture of the preovulatory follicle (9), the coordinated regulation of LIF and its downstream signaling components in the rhesus macaque periovulatory interval suggests it may play a yet undiscovered role in primate ovulation that is distinct from its action in rodent species. Thus, studies were conducted to assess the regulation of LIF expression and signaling in the periovulatory follicle, as well as its role in ovulation in a nonhuman primate model (eg, rhesus macaques).
Materials and Methods
Animal protocols and hormone assays
All protocols involving animals were approved by the Oregon National Primate Research Center (ONPRC) Institutional Animal Care and Use Committee and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The housing and general care of rhesus macaques (Macaca mulatta) was previously described (10). Adult females monkeys were monitored daily for menses. Beginning 4 days after the start of menses, 1.5-mL blood samples were collected daily by saphenous venipuncture and serum was assayed for estradiol and P4 concentrations by specific electrochemiluminescence assays using an automated Roche platform (Roche Diagnostics) by the Endocrine Technologies Support Core Laboratory (ETSL) at the ONPRC.
Controlled ovulation (COv)
The COv protocol, which was performed as previously described (6, 11), allows for the selection and development of a single follicle and the ability to precisely time the administration of an ovulatory stimulus and the subsequent collection of the follicle. Briefly, the protocol was initiated when morning estradiol levels reach 95–120 pg/mL during the mid to late follicular phase of the natural cycle (d 6–13 after menses). A GnRH antagonist (Antide, 1.0 mg/kg; Salk Institute for Biological Studies) was administered at 4 pm to prevent a spontaneous LH surge. hFSH and hLH (Repronex, 30 IU each; Ferring Pharmaceuticals) were administered to initiate maturation and development of the ovulatory follicle. The next day, Antide (0.50 mg/kg) as well as hFSH and hLH (30 IU each) were administered at 8 am, with an additional hFSH and hLH (30 IU each) injection given at 4 pm. The females received an ovulatory bolus of hCG (Novarel; Ferring Pharmaceuticals; 1000-IU hCG at 8 am) on the third day. Ovaries with the dominant follicle were removed before (0 h) or 12, 24, 36, and 52 hours after hCG injection (n = 3–6 per time point). Because ovulation occurs 36–44 hours after hCG, the 36-hour time point was divided into 2 groups: those that had intact follicles (36-h-unruptured) and those with follicles that had ruptured (36-h-ruptured). The ovulatory follicle was isolated and processed as previously described (6).
Intrafollicular injection
Intrafollicular injection was performed during a laparotomy on anesthetized animals as previously described (12, 13) and the exposed ovary bearing the ovulatory follicle received vehicle (PBS) or soluble LIFR (sLIFR) (LIF antagonist). An insulin syringe and needle containing 50-μL treatment solution was inserted through the tunica of the ovary opposite of the follicle apex. Once the syringe and needle was positioned inside the follicle, 50 μL of follicular fluid was drawn into the syringe and allowed to mix, diluting the solution by half before injecting 50 μL back into the follicle. This protocol is performed routinely at ONPRC and injection of vehicle alone does not interfere with timely ovulation or normal luteal development (12, 13). At the time of laparotomy, hCG (1000 IU) was administered to initiate ovulatory events. The experimental design was sequential in that animals first received an intrafollicular injection of vehicle (PBS) followed by intrafollicular injection of sLIFR (10 μg/follicle; R&D Systems), with at least 1 recovery menstrual cycle between each protocol.
Immunoassays
Follicular fluid from periovulatory follicles was collected with a 1-mL syringe and 25-gauge needle before follicle dissection (0, 12, and 24 h after hCG). The follicular fluid was centrifuged to remove granulosa cells to assess LIF concentrations. The ONPRC ETSL performed the LIF ELISA (IBL America) according to the manufacturer's instructions. The ETSL also performed an LH RIA on serum samples obtained from animals as they underwent a COv protocol, to ensure a spontaneous LH surge did not occur.
Quantitative real-time PCR (qPCR)
RNA isolated from periovulatory follicles was converted into cDNA and synthesized as described previously (14). The genes of interest were initially selected from a published Affymetrix GeneChip Rhesus Macaque Genome Array database (6) wherein the corresponding probe set sequences from the microarray were used to BLAST the rhesus macaque genome to identify the full-length cDNA sequences. These cDNA sequences were then used to design primers and probes as previously described (14). All primer and probe sequences are provided in Supplemental Table 1. Relative levels of expression were normalized to the gene encoding ribosomal protein S10 (MRPS10).
Immunohistochemistry (IHC) and histology
Ovaries collected via laparoscopy were fixed in 4% paraformaldehyde overnight, placed in 5% sucrose for 24 hours, followed by dehydration in a series of ethanol solutions (50%, 70%, and 100%) for subsequent embedding in paraffin and serial sectioning. Whole ovarian sections (5 μm) were used for IHC as described previously (15). All antibodies were produced in rabbit and can be found in Table 1. Images were taken using an Olympus BX40 microscope equipped with a digital Olympus DP12 camera. A negative control (isotype matched irrelevant rabbit antibody, see Table 1) was used to determine background-staining levels within a Photoshop contact sheet created for each antibody. Any white balance adjustment was made uniformly across all images.
Table 1.
Antibody Table
| Protein Target | Antigen Sequence | Antibody Name | Manufacturer and Catalog Number | Species Raised in; Clonality | Dilution Used |
|---|---|---|---|---|---|
| LIFR | KLH conjugated from the middle of human LIFR | LIFR/CD118 | Bioss, bs-1458R | Rabbit; polyclonal | 1:200 |
| pY705-STAT3 | Phosphopeptide containing human STAT3 Y705 site | Human phosho-STAT3 (Y705) | R&D Systems, AF4607 | Rabbit; polyclonal | 1:50 |
| IL6ST | Phosphorylation site of serine 782 | CD130 (gp130) | Abcam, ab59389 | Rabbit; polyclonal | 1:100 |
| JAK1 | Phosphorylation site of tyrosine 1022 | JAK1 | Abcam, ab47435 | Rabbit; polyclonal | 1:100 |
| STAT3 | Residues surrounding serine 727 of human STAT3 | STAT3 | Abcam, ab32500 | Rabbit; monoclonal | 1:100 |
| pS727-STAT3 | Human STAT3 700 to the C terminus | STAT3 (phospho-S727) | Abcam, ab32143 | Rabbit; monoclonal | 1:650 |
| Isotype control | Proprietary isotype control | Rabbit IgG | Abcam, ab172730 | Rabbit; monoclonal | 1:50, 100, 200, and 650 |
Laparoscopic and histological evaluation of follicle rupture
The presence or absence of an ovulatory stigmata was determined by laparoscopy at 72 hours after intrafollicular injection of vehicle (n = 3) or sLIFR (n = 4) and hCG (16). All evaluations were digitally recorded. In a subset of animals, a hemi-oophorectomy was performed 52 hours after intrafollicular injection of vehicle (n = 2) or sLIFR (n = 3) and hCG to assess the follicle for the presence or absence of a rupture site and/or trapped oocytes (15). Images were taken using an Olympus BX40 microscope equipped with a digital Olympus DP12 camera.
Statistical analysis
SigmaStat software (SPSS, Inc) was used for all statistical comparisons. For parametric data, one-way ANOVA was used to determine differences between groups coupled with the Student Newman-Keuls test, significance level was set at P < .05, and data were transformed accordingly.
Results
LIF and its downstream effectors are induced in the primate periovulatory follicle after an ovulatory stimulus
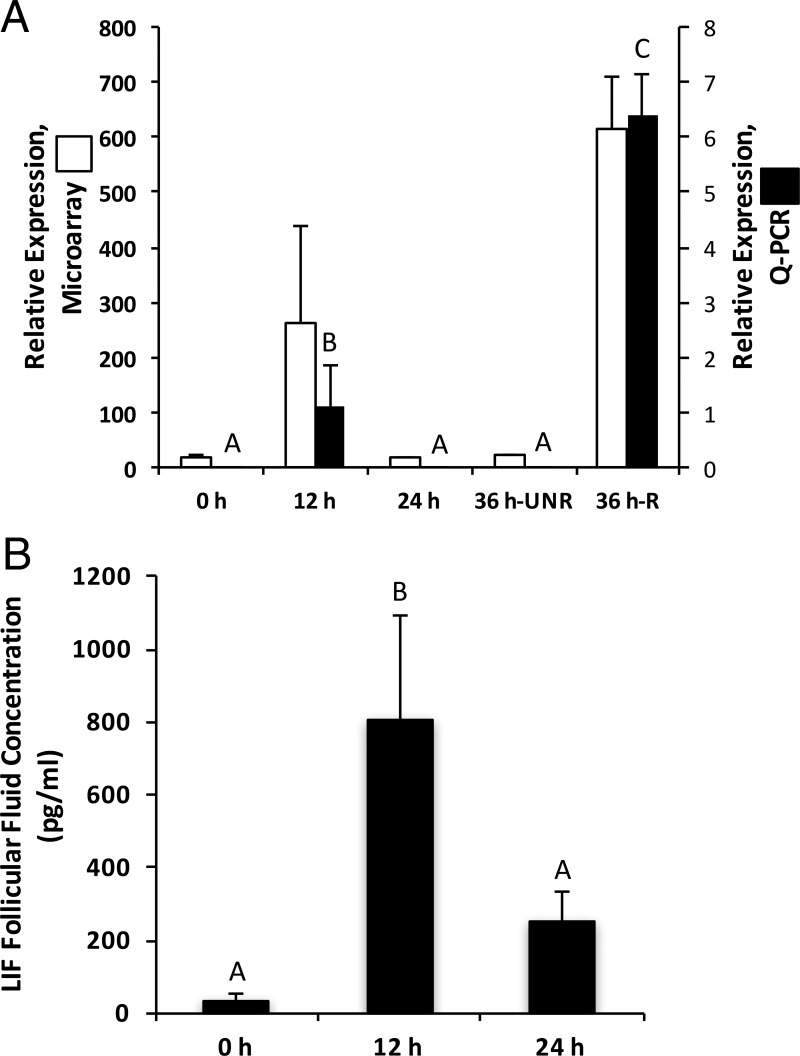
In a previous study, the level of individual mRNAs within periovulatory follicles of rhesus macaques undergoing a COv protocol was assessed using Affymetrix GeneChip Rhesus Macaque Genome Arrays (6). The COv protocol creates a controlled environment where the naturally selected mature follicle can be evaluated at desired time points before and after an ovulatory stimulus (hCG). A comprehensive assessment of genes whose mRNAs were differentially expressed before and after administration of an ovulatory bolus of hCG was performed (6). One subgroup chosen for further analysis included genes such as LIF that were induced significantly after hCG treatment relative to those not receiving hCG (pre-hCG; 0-h group). qPCR was used to confirm this expression pattern, which revealed that the levels of mRNA encoding LIF increased 167-fold (P < .05) 12 hours after hCG injection relative to the 0-hour time point. LIF mRNA expression returned to pre-hCG levels in the unruptured follicle at 24 and 36 hours after administration of hCG. Follicles that had ruptured at 36 hours after hCG demonstrated a 518-fold increase (P < .05) in LIF mRNA levels relative to unruptured follicles at that same time point (Figure 1A). Intrafollicular LIF protein (Figure 1B) and mRNA levels shared a similar pattern, wherein a significant increase (25-fold; P < .05) in LIF protein within follicular fluid was observed 12 hours after hCG relative to the pre-hCG time point. LIF is a member of the IL-6 cytokine superfamily (8); thus, the mRNA levels of all other IL-6 family members were assessed. No other IL-6 superfamily ligand-receptor pairs were significantly expressed in the rhesus macaque periovulatory follicle with the exception of IL-11 (Supplemental Table 2).
Figure 1.
LIF mRNA and protein levels (mean ± SEM) in the rhesus macaque follicle increase after administration of an ovulatory bolus of hCG. A, LIF mRNA levels in rhesus macaque follicles removed before (0 h) or 12, 24, and 36 hours after the administration of an ovulatory bolus of hCG were determined from a Affymetrix DNA array database (NCBI GEO GSE2277; Ref. 6) and qPCR (n = 4–6/time point). The 36-hour post-hCG time point includes follicles that were unruptured (36-h UNR) and those that had ruptured (36-h R). B, LIF protein levels were assessed in follicular fluid collected before (0 h), as well as 12 and 24 hours after hCG administration (n = 3–4/group). Columns with different letters are significantly different (P < .05).
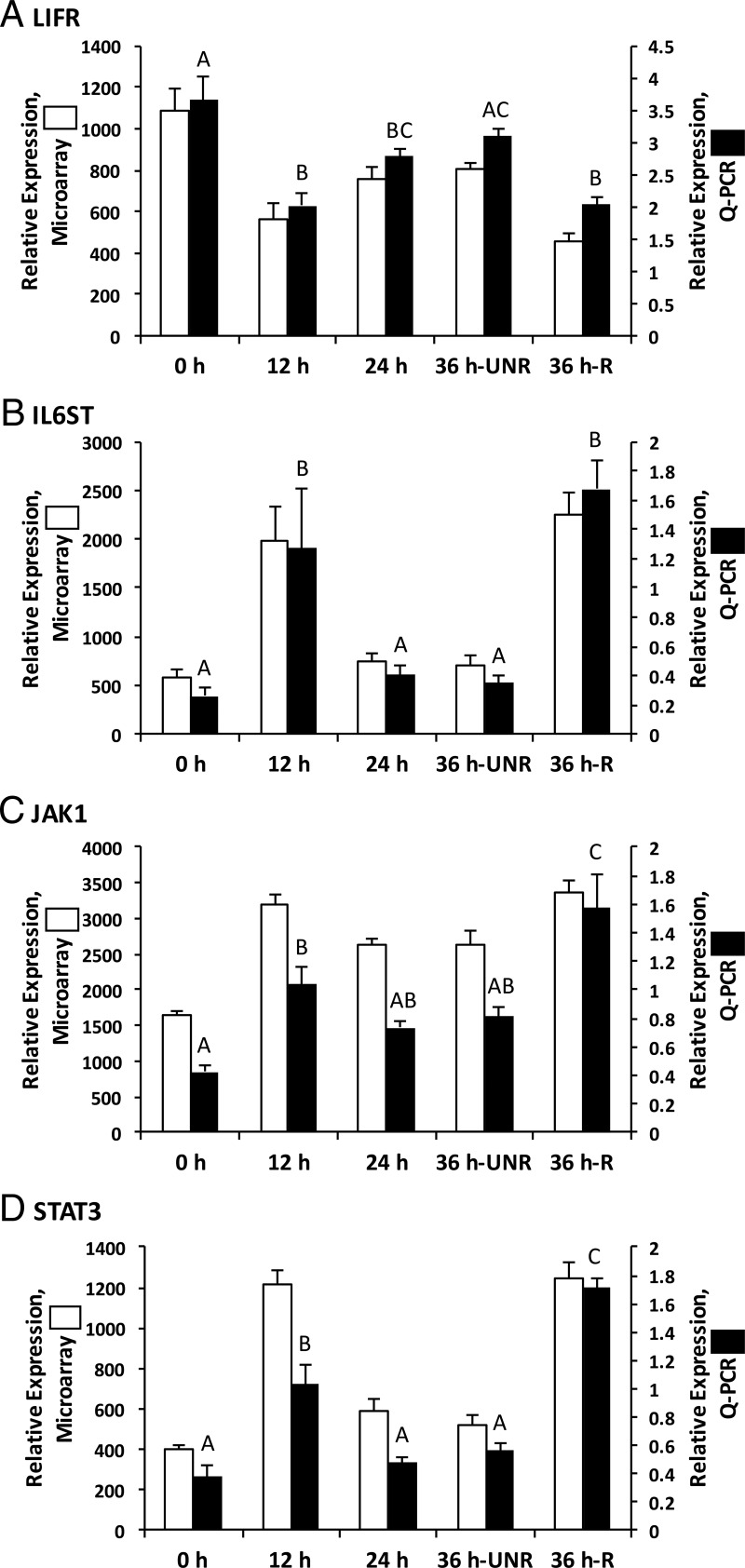
The levels of mRNA encoding downstream LIF effectors, including LIFR, IL6ST, JAK1, and STAT3 in the rhesus macaque periovulatory follicle were also assessed (Figure 2). LIFR mRNA levels decreased 1.8-fold (P < .05; qPCR results) in both 12-hour post-hCG unruptured and 36-hour-ruptured follicles, relative to the 0-hour follicles. LIFR mRNA levels did not differ significantly between follicles obtained before hCG administration (0 h) and before rupture at 36 hours after hCG injection (36-h-unruptured). In contrast, the mRNA levels for IL6ST, JAK1, and STAT3 increased 4.9-, 2.7-, and 2.5-fold, respectively, 12 hours after hCG administration relative to the pre-hCG (0 h) time point (P < .05). IL6ST and STAT3 mRNA displayed similar expression patterns as LIF, decreasing 3.3- and 2-fold, respectively (P < .05), at 24 hours after hCG administration relative to the 12-hour group. IL6ST, JAK1, and STAT3 mRNA levels remained low until rupture at 36 hours after hCG (36-h-ruptured), at which point they increased 6.4-, 4.5-, and 3.7-fold, respectively (P < .05), relative to pre-hCG (0 h) levels. The mRNA levels for the other JAK family members (eg, JAK2, JAK3, and TYK2) did not significantly increase after hCG administration (Supplemental Figure 1).
Figure 2.
The mRNAs corresponding to LIFR and its associated downstream signaling components are expressed in the rhesus macaque periovulatory follicle. Although LIFR mRNA levels show a moderate decline (1.8-fold) in the macaque follicle 12 hours after hCG administration (A), mRNAs encoding downstream LIF signaling components, including IL6ST (B), JAK1 (C), and STAT3 (D), exhibit a significant (P < .05) increase 12 hours after hCG administration relative to the pre-hCG time point. A secondary increase in the level of mRNAs encoding IL6ST, JAK1, and STAT3 was observed in ruptured rhesus macaque follicles obtained 36 hours after hCG administration. Columns with different letters are significantly different from one another (P < .05).
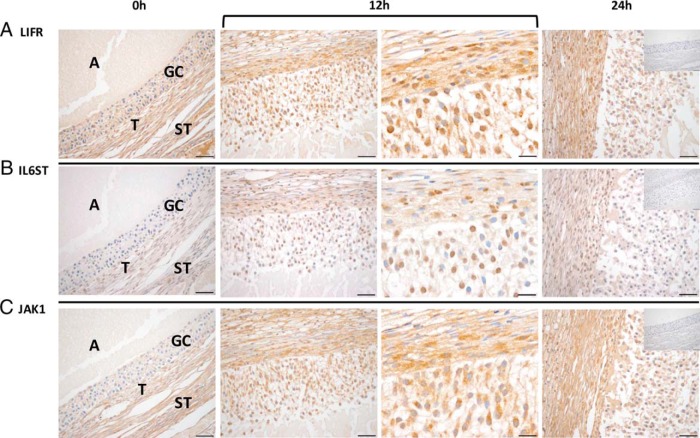
Cellular localization of LIFR, IL6ST, and JAK1 was determined by IHC using follicles collected from rhesus macaques undergoing COv protocols at 0-, 12-, and 24-hour post-hCG time points (Figure 3). LIFR expression was notable in all follicle cell types (eg, granulosa, theca, and stroma cells) throughout the periovulatory interval (Figure 3A). Increased IL6ST immunostaining intensity was observed at 12 hours after hCG relative to the 0-hour time point, particularly in the granulosa and theca cells (Figure 3B). At 24 hours after hCG administration, IL6ST immunostaining levels had decreased and more closely resembled that of the 0-hour time point. JAK1 was localized primarily in the granulosa and theca cells of the rhesus macaque follicle at 12 and 24 hours, with the immunostaining intensity being increased at these time points relative to follicles obtained before hCG administration (Figure 3C).
Figure 3.
LIFR, IL6ST, and JAK1 proteins are expressed in the rhesus macaque follicle. LIFR (A) immunostaining was evident before (0 h; ×40 objective) as well as 12 hours (bracket, left and right panels: ×40 and ×100 objectives, respectively) and 24 hours (×40 objective) after the injection of an ovulatory bolus of hCG. In contrast, both IL6ST (B) and JAK1 (C) immunostaining intensity increased primarily within the granulosa cells (GCs) 12 hours after hCG administration. The insets located in the 24-hour panel are representative negative controls for each primary antibody used, which consisted of a species and isotype matched irrelevant antibody. The various ovarian cell types and compartments are indicated in the 0-hour panels and include antrum (A), GCs, theca cells (T), and stroma (ST). Images are representative of ovaries obtained at each time point (0, 12, and 24 h after hCG) from 2–3 animals undergoing COv protocols. Scale bar, 50 and 20 μm for ×40 and ×100 magnification, respectively.
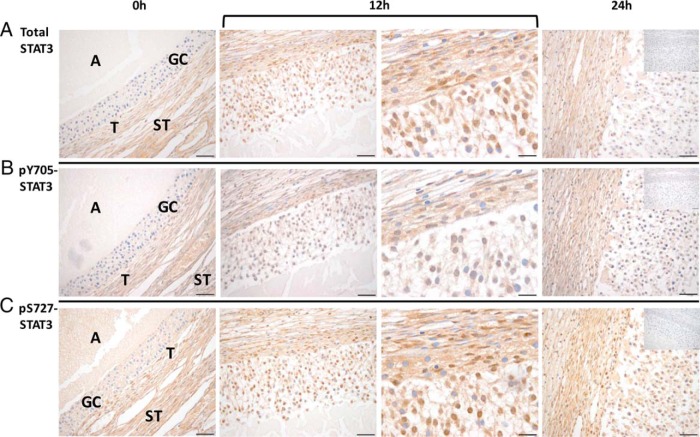
Immunostaining intensity and localization of total and phosphorylated STAT3 (pY705-STAT3 and pS727-STAT3) also changed after hCG administration. Before hCG injection, STAT3 immunostaining was restricted to the theca and stroma but was minimal in granulosa cells (Figure 4). By 12 hours after hCG administration, significant total STAT3 staining was notable in the nuclei of granulosa cells in the rhesus macaque follicle. At 24 hours after hCG injection, total STAT3 staining intensity returned to pre-hCG levels in granulosa cells. A similar increase in anti-pY705-STAT3 and pS727-STAT3 staining intensity and cellular distribution was observed 12 hours after hCG administration (Figure 4). These results indicate that in addition to an increase in the level of total STAT3 expression in the granulosa cells of the rhesus macaque follicle after an ovulatory stimulus, phosphorylation occurs on key residues that regulate its entry into the nucleus (pY705) and stabilization in a conformation (pS727) that promotes target gene transcription (17).
Figure 4.
Total and phosphorylated STAT3 levels increase in the rhesus macaque follicle after an ovulatory stimulus. Immunostaining intensity of total STAT3 (A), STAT3 phosphorylated at tyrosine 705 (pY705) (B), and STAT3 phosphorylated at serine 727 (pS727) (C) is increased in the rhesus macaque follicle 12 hours (bracket, left and right panels: ×40 and ×100 objectives, respectively) and 24 hours after the injection of an ovulatory bolus of hCG relative to that of follicles obtained before hCG administration (0 h). The insets located in the 24-hour panel include the negative controls (eg, irrelevant isotype-matched antibody) for each primary antibody used. The various ovarian cell types and compartments are indicated in the panels, including antrum (A), granulosa cells (GCs), theca cells (T), and stroma (ST). Images are representative of 2–3 ovaries obtained at each time point (0, 12, and 24 h after hCG) from animals undergoing COv protocols. Scale bar, 50 and 20 μm for ×40 and ×100 magnification, respectively.
LIF is necessary for rhesus macaque ovulation
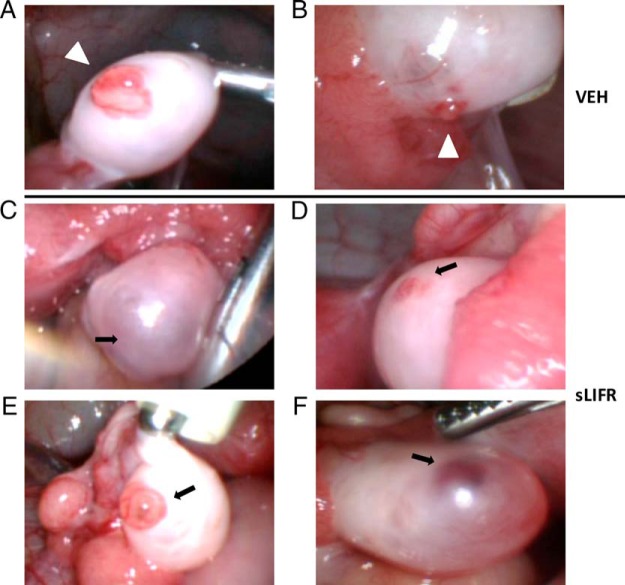
To ascertain whether LIF is responsible for orchestrating events critical for ovulation in the primate ovary in vivo, sLIFR was injected into the naturally selected rhesus macaque follicle at the time that animals received an ovulatory bolus of hCG during a COv protocol. The ability of sLIFR to block LIF action was first assessed using LIF-responsive TF-1 erythroleukemic cells (Supplemental Figure 2), which demonstrated that sLIFR blocked LIF-dependent STAT3 phosphorylation (0.33-ng/mL LIF) with an IC50 of 3–10 μg/mL. Based on these findings, 10 μg of sLIFR were used for intrafollicular injection of the preovulatory follicle in rhesus macaques undergoing a COv protocol. Laparoscopic ovarian evaluations of the injected follicle were performed at 72 hours after hCG injection to visualize the presence or absence of a rupture site or follicular stigma, which typically occurs 36–44 hours after an ovulatory stimulus (6). Ovaries injected with vehicle possessed a clear rupture site (Figure 5, A and B), whereas ovaries injected with sLIFR failed to ovulate by 72 hours after an ovulatory stimulus (Figure 5, C–F). The follicle of one animal receiving an intrafollicular sLIFR injection exhibited a thinning of the tunica (Figure 5E). However, the follicle did not possess the typical postrupture stigma, rather an intact antrum filled with follicular fluid was noted indicating ovulation had not occurred.
Figure 5.
Intrafollicular injection of sLIFR, a LIF antagonist, blocks ovulation. Ovulatory stigmata were observed 72 hours after hCG administration in rhesus macaques receiving intrafollicular injection of vehicle (follicle rupture typically occurs between 36 and 44 h after an ovulatory stimulus) (A and B), whereas those receiving sLIFR (10 μg/follicle) (C–F) lacked a definitive rupture site. In 1 animal (E), there was a protruding, unruptured follicle. Intrafollicular injection of either vehicle or sLIFR occurred at the time of hCG administration. White arrowheads indicate ovulatory stigmata (vehicle injection; n = 3), and black arrows indicate unruptured follicles (sLIFR injection; n = 4).
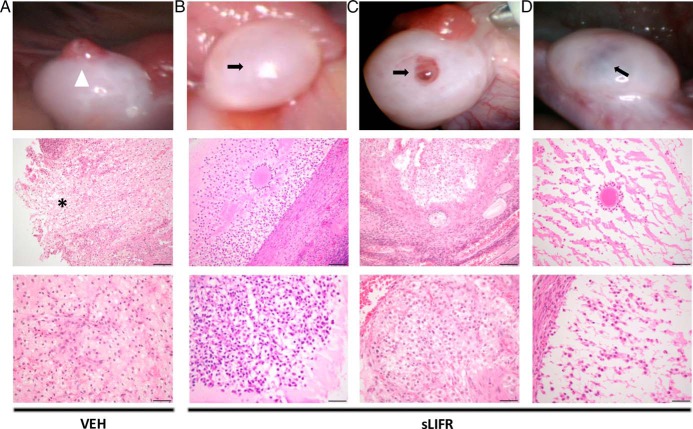
To confirm that blocking LIF action prevents ovulation of the rhesus macaque follicle, ovaries were removed 52 hours after injection of vehicle (PBS) or sLIFR (10 μg/follicle) in a subset of animals and analyzed for the presence of a trapped oocyte in hematoxylin and eosin stained sections. Vehicle-injected follicles possessed a clearly visible rupture site in the follicle wall and no cumulus-oocyte complex (Figure 6A). In contrast, 2 out of 3 sLIFR-injected ovaries possessed an intact follicle wall (Figure 6, B and D), and all 3 had a trapped oocyte (Figure 6, B–D). The cumulus cells surrounding the trapped oocyte were expanded in animals receiving intrafollicular sLIFR injections (Figure 6, B–D), similar to what is observed in rhesus macaque follicles just before ovulation (24 h after hCG; Ref. 18). These findings suggest that C-OE does not require LIF signaling in vivo. Of the 3 oocytes trapped within the sLIFR-injected follicles, 1 oocyte appeared to be degenerated and the other 2 lacked germinal vesicles as well as obvious polar bodies, suggesting reinitiation of meiosis occurred but was not completed. Despite consistently blocking follicle rupture, the degree of luteinization appears to be variable at 52 hours after sLIFR and hCG administration. For example, the cellular constituents of the follicle shown in Figure 6C appear to be undergoing luteinization as evidenced by extensive granulosa cell hypertrophy and cellular reorganization. In contrast, the follicle shown in Figure 6D exhibits minimal signs of granulosa cell hypertrophy and cellular reorganization, whereas the follicle section shown in Figure 6B is intermediate in terms of its apparent level of luteinization. Despite the variability of the cellular indicators of luteinization in ovarian sections obtained 52 hours after sLIFR and hCG administration, long-term impact on luteal development and function are minimal based on the lack of a significant effect of intrafollicular sLIFR on luteal phase P4 levels or luteal phase length (Supplemental Figure 3).
Figure 6.
Histological analysis confirms the presence of a trapped oocyte in follicles treated with sLIFR but not in those receiving vehicle. Rhesus macaque ovaries removed 52 hours after vehicle (PBS) (A) or sLIFR (10 μg/follicle) (B–D) intrafollicular injection were serially sectioned through the entire follicle. The top panels include images taken of the ovary before its removal by laparoscopy. A white arrowhead and asterisk indicate an ovulatory stigma and the site of follicle rupture, respectively (vehicle injection, n = 2; see also Ref. 15) (A), whereas black arrows denote unruptured follicles (sLIFR injection; n = 3). The middle panel revealed a discontinuous stroma indicative of a rupture site that was observed in the vehicle treated follicle (asterisk) (A), whereas a trapped oocyte was observed in follicles from all sLIFR treated animals (B–D). The bottom panels include a higher magnification of the follicle wall from vehicle (A)- and sLIFR (B–D)-injected follicles. Scale bars, 50 μm (middle panels) and 20 μm (bottom panels).
Discussion
The present study is novel in its examination of the role that LIF plays in primate ovulation. In contrast to rodents, LIF is a critical LH inducible intermediate necessary for rhesus macaque ovulation. LIF action was previously shown to be critical for fertility in rodents through its ability to regulate events that occurred after ovulation, including implantation and decidualization (9). Homozygous mutant Lif−/− female mice produced blastocysts at a rate comparable with that obtained from wild type females after mating with males (9), demonstrating that release of fertilizable oocytes in mice is not dependent on LIF action. Although previous studies only assessed the postovulatory role of LIF (19–21) in primate reproductive physiology, we show herein that up-regulation of the transcription and translation of LIF occurs within the follicle before ovulation in response to an ovulatory stimulus. Our studies also demonstrate for the first time that ablation of LIF signaling within the follicle through the use of a LIF antagonist prevents ovulation in a primate species.
LIF is a member of the IL-6 cytokine superfamily that affects multiple tissue types through its ability to regulate cellular differentiation, proliferation, and metabolism (8). LIF action is mediated by binding to LIFR/IL6ST, which through JAK/TYK kinase-dependent tyrosine phosphorylation (pY705-STAT3) and activation of STAT3, regulates the expression of LIF-responsive genes (22). STAT3 can also be phosphorylated on serine 727 (pS727-STAT3) and, depending on the cell type, leads to a modulation in the level of target gene transcription (23). LIF mRNA levels in the rhesus macaque follicle and LIF protein concentration in follicular fluid were significantly up-regulated at 12 hours after hCG administration, with minimal or background levels being observed before the administration of an ovulatory bolus of hCG. LIF mRNA levels returned to baseline thereafter until rupture of the follicle at 36 hours after hCG administration, at which point peak levels of LIF mRNA were observed. Significantly increased levels of IL6ST, JAK1, and STAT3 mRNA were detected 12 hours after hCG in the rhesus macaque follicle after an ovulatory stimulus, similar to what was observed for LIF mRNA levels. Immunolocalization of IL6ST, JAK1, and STAT3 revealed increased staining intensity 12 hours after hCG administration, primarily in the mural granulosa cell layer. Although declining after hCG administration, LIFR mRNA levels remained significant throughout the periovulatory interval. LIFR immunostaining also revealed LIFR protein is present in follicular theca and granulosa cells before and after hCG administration. These results indicate that LIF synthesis and the expression of LIF signaling components (eg, IL6ST, JAK1, and STAT3) increase in the naturally selected rhesus macaque follicle after an ovulatory stimulus.
Intrafollicular injection of sLIFR, which functions as a LIF antagonist, was shown to prevent the release of the oocyte from the rhesus macaque periovulatory follicle after the animals received an ovulatory bolus of hCG. Administration of sLIFR prevents ovulation; however, there is a possibility that follicle rupture is blocked by sLIFR interference with the action of other IL-6 family members. For example, cardiotrophin 1, ciliary neurotrophic factor, and oncostatin-M can form complexes with LIFR (24). The ability of sLIFR to block ovulation via interference with non-LIF cytokines is unlikely, because none of these IL-6 family members or their corresponding receptors showed significant coexpression in the follicle before or after an ovulatory stimulus (Supplemental Table 2). This is in contrast to what was reported using mice, where IL-6 was reported to serve as a regulator of oocyte competence and C-OE (25). According to our microarray database (6), IL-11 and its receptor (IL-11Rɑ) exhibited similar changes in mRNA levels to LIF and LIFR, respectfully (Supplemental Figure 4). However, based on the ability of sLIFR to block ovulation, it appears that IL-11 is not capable of replacing LIF action in the primate follicle.
LIF-dependent regulation of cellular function is mostly associated with the activation of JAK1 and increased pY705-STAT3 levels (6). STAT3 dimerizes after being phosphorylated, allowing for its entry into the nucleus, binding to specific DNA elements and the regulation of target gene transcription (8). Depending on the cell type analyzed, LIF signaling also results in STAT1 and STAT5 phosphorylation (26, 27). It is unlikely that LIF-dependent phosphorylation and activation of STAT1 or STAT5 is involved in regulating events necessary for the rupture of the rhesus macaque follicle because their mRNA levels are only slightly increased above background and do not change through the periovulatory interval (6). Before hCG treatment, there is limited immunostaining of total STAT3, pY705-STAT3, and pS727-STAT3, particularly in granulosa and theca cells. In contrast, marked increases in nuclear total- and phosho-STAT3 staining within both cell types in rhesus macaque follicles removed 12 hours after hCG administration were observed, indicating its activation occurred through the IL6ST/JAK pathway. By 24 hours after hCG administration, the nuclear staining of STAT3, pY705-STAT3, and pS727-STAT3 diminishes, returning to levels that are similar to those observed in the follicles obtained pre-hCG. These findings suggest there is a rapid (12 h, or earlier) induction of LIF synthesis and signaling, which in turn leads to the regulation of LIF/STAT3-responsive genes that are critical for ovulation in the primate ovary. In addition to signaling through the JAK-STAT3 pathway, LIF binding to the LIFR-IL6ST complex activates MAPK and the phosphatidylinositol-3 phosphate kinase pathways (22). Both pathways were reported to be involved in regulating events necessary for ovulation in nonprimate species (28–31). Studies are currently underway to determine the relative importance of LIF signaling through STAT and/or MAPK/phosphatidylinositol-3 phosphate kinase signaling intermediates in regulating events necessary for follicle rupture.
Although blocking LIF action in vivo prevented follicle rupture, it did not prevent all LHCGR-dependent actions, including C-OE. Based on histological evaluation of rhesus macaque ovaries removed 52 hours after intrafollicular injection of sLIFR and hCG administration, inhibiting LIF signaling did not prevent C-OE. Although follicles receiving sLIFR possessed a trapped cumulus-oocyte complex, expansion of the cumulus cells surrounding the oocyte was evident. Previous in vitro studies indicate that LIF can induce C-OE in human and mouse oocytes and that it improves mouse fertilization rates when present during mouse oocyte maturation (32). Our data suggest that blocking LIF action in vivo does not affect C-OE but may negatively impact the completion of oocyte meiosis. A single intrafollicular injection of sLIFR at the time of hCG administration did not significantly affect P4 production and luteal phase length (Supplemental Figure 3). However, based on histological findings, intrafollicular sLIFR injection led to the formation of an unruptured follicle with variable levels of luteinization. The inconsistent short-term effect of intrafollicular sLIFR injection (eg, at 52 h after hCG administration) and the lack of an effect of intrafollicular sLIFR injection in subsequent luteal development and P4 synthesis (eg, several days after hCG administration) may be due to the delivery of only a single dose of sLIFR at the time the animals received an ovulatory stimulus. A more prolonged delivery of sLIFR through the periovulatory interval or after rupture needs to be performed to definitively determine whether the secondary increase in expression of LIF and its downstream signaling components after ovulation regulates luteal development and P4 synthesis.
The downstream effectors of LIF critical for primate ovulation are unknown. Lee et al. demonstrated that Stat3−/− mice have reduced P4 receptor (PGR) expression in the uterus during decidualization (33). Previous studies in primates have shown that PGR is necessary for ovulation (34, 35). If STAT3 regulates PGR expression in the rhesus macaque ovary, then LH induction of PGR expression may be mediated through the LIF pathway. Previously published data indicate an up-regulation in select metalloproteinases in the periovulatory rhesus macaque follicle after the administration of hCG (15) and that their proteolytic activity is required for follicle rupture (36). The expression profile of these proteinases in the rhesus macaque periovulatory follicle resembles that of LIF and its downstream effectors. The ability of LIF to directly increase metalloproteinase expression in nonovarian cells suggests LIF action may be critical for the increased proteolytic activity that allows for follicle rupture. Although the increased expression of LIF and LIF signaling components in the rhesus macaque follicle at 12 hours after hCG also correlates with peak levels of PGR and specific metalloproteinases in the periovulatory interval, data regarding their kinetics of expression between 0 and 12 hours do not exist. It is possible that peak LIF expression could be occurring rapidly after hCG administration, preceding the expression of key effectors necessary for follicle rupture. Thus, further research is needed to determine more precisely the kinetics of LIF expression and action after an ovulatory stimulus and to define the cellular processes regulated by LIF that are critical for rupture of the primate follicle.
Acknowledgments
We thank the members of the Division of Comparative Medicine for their excellent animal care and research support, the Surgical Services Unit for their outstanding surgical expertise that is critical to our work, and the Endocrine Technology and Services Laboratory for their excellent technical support in assessing cytokine and hormone levels. We also thank Byung Park of the Oregon National Primate Research Center Biostatistics and Bioinformatics Unit for his statistical expertise in contributing to this manuscript.
This work was supported by the National Institutes for Health Grants OD011092 (to J.D.H.) and R21HD072528 (to J.D.H.).
Present address for P.A.R.: Department of Obstetrics and Gynecology, University of Utah, 30 North 1900 East, Salt Lake City, UT 84132.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 4209
- CG
- chorionic gonadotropin
- C-OE
- cumulus-oocyte expansion
- COv
- controlled ovulation
- ETSL
- Endocrine Technologies Support Core Laboratory
- hCG
- human CG
- IHC
- immunohistochemistry
- IL6ST
- IL-6 signal transducer
- JAK
- Janus kinase
- LHCGR
- LH/CG receptor
- LIF
- leukemia inhibitory factor
- LIFR
- LIF receptor
- ONPRC
- Oregon National Primate Research Center
- P4
- progesterone
- PGR
- P4 receptor
- qPCR
- quantitative real-time PCR
- sLIFR
- soluble LIFR
- STAT
- signal transducer and activator of transcription
- TYK
- tyrosine kinase.
References
- 1. Richards JS, Pangas SA. The ovary: basic biology and clinical implications. J Clin Invest. 2010;120:963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richards JS. Ovulation: new factors that prepare the oocyte for fertilization. Mol Cell Endocrinol. 2005;234:75–79. [DOI] [PubMed] [Google Scholar]
- 3. Conneely OM. Progesterone receptors and ovulation. Handb Exp Pharmacol. 2010;198:37–44. [DOI] [PubMed] [Google Scholar]
- 4. Kim SO, Harris SM, Duffy DM. Prostaglandin E2 (EP) receptors mediate PGE2-specific events in ovulation and luteinization within primate ovarian follicles. Endocrinology. 2014;155:1466–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- 6. Xu F, Stouffer RL, Müller J, et al. Dynamics of the transcriptome in the primate ovulatory follicle. Mol Hum Reprod. 2011;17:152–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. [DOI] [PubMed] [Google Scholar]
- 8. Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015;26:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. [DOI] [PubMed] [Google Scholar]
- 10. Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: the technique and its applications. Mol Reprod Dev. 1990;27:261–280. [DOI] [PubMed] [Google Scholar]
- 11. Young KA, Chaffin CL, Molskness TA, Stouffer RL. Controlled ovulation of the dominant follicle: a critical role for LH in the late follicular phase of the menstrual cycle. Hum Reprod. 2003;18:2257–2263. [DOI] [PubMed] [Google Scholar]
- 12. Duffy DM, Stouffer RL. Follicular administration of a cyclooxygenase inhibitor can prevent oocyte release without alteration of normal luteal function in rhesus monkeys. Hum Reprod. 2002;17:2825–2831. [DOI] [PubMed] [Google Scholar]
- 13. Xu F, Stouffer RL. Local delivery of angiopoietin-2 into the preovulatory follicle terminates the menstrual cycle in rhesus monkeys. Biol Reprod. 2005;72:1352–1358. [DOI] [PubMed] [Google Scholar]
- 14. Bogan RL, Murphy MJ, Stouffer RL, Hennebold JD. Systematic determination of differential gene expression in the primate corpus luteum during the luteal phase of the menstrual cycle. Mol Endocrinol. 2008;22:1260–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Peluffo MC, Murphy MJ, Baughman ST, Stouffer RL, Hennebold JD. Systematic analysis of protease gene expression in the rhesus macaque ovulatory follicle: metalloproteinase involvement in follicle rupture. Endocrinology. 2011;152:3963–3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rawson JM, Dukelow WR. Observation of ovulation in Macaca fascicularis. J Reprod Fertil. 1973;34:187–190. [DOI] [PubMed] [Google Scholar]
- 17. Wen Z, Zhong Z, Darnell JE., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. [DOI] [PubMed] [Google Scholar]
- 18. Peluffo MC, Stanley J, Braeuer N, et al. A prostaglandin E2 receptor antagonist prevents pregnancies during a preclinical contraceptive trial with female macaques. Hum Reprod. 2014;29:1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lédée-Bataille N, Laprée-Delage G, Taupin JL, Dubanchet S, Frydman R, Chaouat G. Concentration of leukaemia inhibitory factor (LIF) in uterine flushing fluid is highly predictive of embryo implantation. Hum Reprod. 2002;17:213–218. [DOI] [PubMed] [Google Scholar]
- 20. Aschenbach LC, Hester KE, McCann NC, Zhang JG, Dimitriadis E, Duffy DM. The LIF receptor antagonist PEGLA is effectively delivered to the uterine endometrium and blocks LIF activity in cynomolgus monkeys. Contraception. 2013;87:813–823. [DOI] [PubMed] [Google Scholar]
- 21. Sengupta J, Lalitkumar PG, Najwa AR, Ghosh D. Monoclonal anti-leukemia inhibitory factor antibody inhibits blastocyst implantation in the rhesus monkey. Contraception. 2006;74:419–425. [DOI] [PubMed] [Google Scholar]
- 22. Graf U, Casanova EA, Cinelli P. The role of the leukemia inhibitory factor (LIF) - pathway in derivation and maintenance of murine pluripotent stem cells. Genes. 2011;2:280–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. [DOI] [PubMed] [Google Scholar]
- 24. Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. [DOI] [PubMed] [Google Scholar]
- 25. Liu Z, de Matos DG, Fan HY, Shimada M, Palmer S, Richards JS. Interleukin-6: an autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology. 2009;150:3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leduc K, Bourassa V, Asselin E, Leclerc P, Lafond J, Reyes-Moreno C. Leukemia inhibitory factor regulates differentiation of trophoblastlike BeWo cells through the activation of JAK/STAT and MAPK3/1 MAP kinase-signaling pathways. Biol Reprod. 2012;86:54. [DOI] [PubMed] [Google Scholar]
- 27. Piekorz RP, Nemetz C, Hocke GM. Members of the family of IL-6-type cytokines activate Stat5a in various cell types. Biochem Biophys Res Commun. 1997;236:438–443. [DOI] [PubMed] [Google Scholar]
- 28. Fan HY, Liu Z, Shimada M, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sekar N, Veldhuis JD. Concerted transcriptional activation of the low density lipoprotein receptor gene by insulin and luteinizing hormone in cultured porcine granulosa-luteal cells: possible convergence of protein kinase a, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase signaling pathways. Endocrinology. 2001;142:2921–2928. [DOI] [PubMed] [Google Scholar]
- 30. Siddappa D, Beaulieu E, Gevry N, Roux PP, Bordignon V, Duggavathi R. Effect of the transient pharmacological inhibition of Mapk3/1 pathway on ovulation in mice. PLoS One. 2015;10:e0119387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng W, Nagaraju G, Liu Z, Liu K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol Cell Endocrinol. 2012;356:24–30. [DOI] [PubMed] [Google Scholar]
- 32. De Matos DG, Miller K, Scott R, et al. Leukemia inhibitory factor induces cumulus expansion in immature human and mouse oocytes and improves mouse two-cell rate and delivery rates when it is present during mouse in vitro oocyte maturation. Fertil Steril. 2008;90:2367–2375. [DOI] [PubMed] [Google Scholar]
- 33. Lee JH, Kim TH, Oh SJ, et al. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J. 2013;27:2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bishop CV, Hennebold JD, Kahl CA, Stouffer RL. Knockdown of progesterone receptor (PGR) in macaque granulosa cells disrupts ovulation and progesterone production. Biol Reprod. 2016;94:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hibbert ML, Stouffer RL, Wolf DP, Zelinski-Wooten MB. Midcycle administration of a progesterone synthesis inhibitor prevents ovulation in primates. Proc Natl Acad Sci USA. 1996;93:1897–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Curry TE, Jr, Osteen KG. The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev. 2003;24:428–465. [DOI] [PubMed] [Google Scholar]