Abstract
The etiology for half of congenital hypopituitarism cases is unknown. Our long-term goal is to expand the molecular diagnoses for congenital hypopituitarism by identifying genes that contribute to this condition. We have previously shown that the forkhead box transcription factor, FOXO1, is present in approximately half of somatotropes at embryonic day (e) 18.5, suggesting it may have a role in somatotrope differentiation or function. To elucidate the role of FOXO1 in somatotrope differentiation and function, Foxo1 was conditionally deleted from the anterior pituitary (Foxo1Δpit). Uncommitted progenitor cells are maintained and able to commit to the somatotrope lineage normally based on the expression patterns of Sox2, a marker of uncommitted pituitary progenitors, and Pou1f1 (also known as Pit1), which marks committed progenitors. Interestingly, Foxo1Δpit embryonic mice exhibit delayed somatotrope differentiation as evidenced by an almost complete absence of GH immunoreactivity at e16.5 and reduced expression of Gh at e18.5 and postnatal day (P) 3. Consistent with this conclusion, expression of GHRH receptor, a marker of terminally differentiated somatotropes, is significantly reduced at e18.5 and P3 in the absence of FOXO1. The mechanism of FOXO1 regulation of somatotrope differentiation may involve the basic helix-loop-helix transcription factor, Neurod4, which has been implicated in somatotrope differentiation and is significantly reduced in Foxo1Δpit mice. Foxo1Δpit mice do not exhibit growth defects, and at P21 their pituitary glands exhibit a normal distribution of somatotropes. These studies demonstrate that FOXO1 is important for initial somatotrope specification embryonically but is dispensable for postnatal somatotrope expansion and growth.
Pituitary gland dysfunction, defined by abnormal levels of hormone production, can result from developmental defects, physical trauma, or tumor formation (1). Congenital pituitary hormone deficiencies occur in approximately 1 in 4000 live births (2). Approximately 28% of individuals with traumatic brain injury are diagnosed with hypopituitarism (3). Pituitary hormone deficiency can consist of loss of a single hormone (isolated hormone deficiency) or several hormones (combined pituitary hormone deficiency). Combined pituitary hormone deficiency is most often caused by mutations in transcription factors and tends to be syndromic involving several organ systems (4–6). Genetically engineered and spontaneous mouse mutations with pituitary defects provide models to help understand the regulatory mechanisms that may be involved in these disorders. These models have been used to identify many of the genes regulating pituitary gland development (7, 8). Importantly, known mutations account for only half of congenital pituitary hormone deficiencies in humans.
We sought to identify additional transcription factors that are important for normal pituitary development. Toward this end we have found that the forkhead box transcription factor, FOXO1, is present in the developing pituitary gland (9). FOXO1 regulates cell differentiation in a number of tissues including muscle, bone, and pancreas (10–15). To elucidate the function of FOXO1 during pituitary development, we have used a loss-of-function model. Global deletion of Foxo1 (Foxo1−/−) in mice results in embryonic lethality at embryonic day (e) 10.5 due to vascular and placental defects (16, 17). Foxo1 heterozygous null (Foxo1+/−) mice are phenotypically indistinguishable from Foxo1+/+ littermates, suggesting that one allele of Foxo1 is sufficient for normal development and function. Due to the early embryonic lethality of Foxo1 null mice, many studies have used floxed alleles of Foxo1 to eliminate its expression specifically in a particular tissue (12, 18, 19).
Previously we identified FOXO1 protein and mRNA in the embryonic pituitary. Nuclear FOXO1 protein was detected in approximately 40% of somatotropes and approximately 10% of gonadotropes, corticotropes, and thyrotropes at e18.5 (9). To begin to assess the role of FOXO1 in pituitary development, we conditionally deleted Foxo1 in the pituitary gland using Foxg1-cre mice. Our studies show that deletion of Foxo1 results in delayed terminal differentiation of somatotropes.
Materials and Methods
Mice
Foxo1flox/flox mice contain loxP sites flanking exon 2 of the Foxo1 gene (19). To create the Foxo1 null allele, exon 1 of the Foxo1 gene was replaced with the β-geo cassette (20). The Foxg1cre allele was engineered by replacing nearly the entire coding sequence of Foxg1 with the coding sequence for cre (21). Foxg1+/cre mice were purchased from Jackson Laboratories (stock number 004337). Mice were maintained in a 12-hour dark, 12-hour light cycle and fed Purina Mills Formulab diet 5008 ad libitum. To obtain embryos of the appropriate genotype, Foxo1+/− mice were mated with Foxg1+/cre mice. The resulting Foxo1+/−;Foxg1+/cre mice were mated to Foxo1flox/flox mice (19) to obtain Foxo1flox/−;Foxg1+/cre (Foxo1Δpit), in which Foxo1 is deleted from the pituitary gland. Foxo1flox/−;Foxg1+/+ and Foxo1flox/+;Foxg1+/+ and Foxo1flox/+;Foxg1+/cre were used as wild-type controls. The morning the copulatory plug was detected is designated e0.5 and the day of birth is designated postnatal day (P) 0. Quantitative PCR (qPCR) was used to genotype embryos from this mating as follows: the presence of the Foxo1− allele was detected with the primers 5′-TTCACTGGCCGTCGTTTTACAAGCTCGTGA-3′ and 5′-ATGTGAGCGAGTAACAACCCGTCGGATTCT-3′; the presence of the Foxg1cre allele was detected with the primers 5′-GCGGTCTGGCAGTAAAAACTATC-3′ and 5′-GTGAAACAGCATTGCTGTCACTT-3′. Foxo1flox/flox mice were genotyped by PCR with the primers: 5′-GCTTAGAGCAGAGATGTTCTCACATT-3′, 5′-CCAGAGTCTTTGTATCAGGCAAATAA-3′, which amplify a 149-bp band in the presence of the floxed allele and a 115-bp band in the presence of the wild-type allele. All procedures using mice were approved by the Southern Illinois University Animal Care and Use Committee. All experiments were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guidelines for the Care and Use of Experimental Animals.
Histology and immunohistochemistry
Embryos and pituitary glands were dissected and fixed in 4% paraformaldehyde in PBS (pH 7.2) for 20 minutes to 24 hours (depending on sample size). All samples were washed in PBS, dehydrated in a graded series of ethanol, and embedded in paraffin. Sections (5 μm) were deparaffinized in xylene and rehydrated through a series of graded ethanol washes before immunohistochemistry was performed. Between two and four sections were stained per embryo.
To visualize FOXO1, tissue sections were deparaffinized and rehydrated as described above, and 1.5% peroxide in water was used to remove endogenous peroxidases. After epitopes were unmasked by boiling in 10 mM citric acid for 10 minutes, tissue sections were blocked with blocking reagent from the tyramide signal amplification kit (PerkinElmer). Sections were incubated overnight at 4°C with antibodies that specifically recognize FOXO1 (Cell Signaling; 1:50). Tissue sections were incubated with biotinylated antirabbit secondary (Jackson ImmunoResearch Laboratories, Inc) for 30 minutes at room temperature. Next, sections were incubated sequentially with streptavidin-horseradish peroxidase and fluorescein from the tyramide signal amplification kit (PerkinElmer). After a 5-minute incubation in water, sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (167 nM; Molecular Probes). Immunohistochemistry for POU domain class 1 transcription factor 1 (POU1F1) was performed as described above with antibodies that specifically recognize POU1F1 (generously provided by Dr Simon Rhodes, Indiana University-Purdue University, Indianapolis, Indiana; lot number 1603, 1:2000).
For immunofluorescent labeling of phosphorylated (p)-ERK and p-JNK, pituitary sections were fixed, embedded, and epitopes unmasked, as described above. Sections were washed in Tris-buffered saline (TBS) and blocked with 10% normal goat serum/2× casein in TBS for 30 minutes at room temperature. Sections were then incubated in either rabbit anti-p-ERK (1:200) or mouse anti-p-JNK (1:400) primary antibodies diluted in TBS/1× casein overnight. Normal rabbit and mouse IgG at equivalent concentration (micrograms per milliliter) was used as a negative control. After primary antibody incubation, sections were washed with TBS and incubated with the appropriate Alexa Fluor 488 secondary antibody for 1 hour at room temperature in the dark. Slides were then washed and mounted in Vectashield (Vector Laboratories) with DAPI (Vector Laboratories, VectorLabs.com). Images were obtained on a Zeiss 710 laser-scanning confocal microscope using the appropriate filters.
Visualization of pituitary hormones was performed as described above with the following exceptions: tissue sections were boiled in citric acid for 5 minutes and incubated with antibodies against GH (1:10 000; National Hormone and Peptide Program (NHPP), Torrance, California), prolactin (PRL; 1:10 000; NHPP), ACTH (1:500; NHPP), TSH-β subunit (TSHB; 1:2000; NHPP), or LH-β subunit (LHB; 1:500; NHPP) for 1 hour at room temperature and then the appropriate secondary antibodies, antirabbit-tetramethylrhodamine isothiocyanate (1:100; Jackson ImmunoResearch) or antiguinea pig-fluorescein isothiocyanate (1:100; Jackson ImmunoResearch). To observe GH in tissue sections from e16.5 embryos and PRL in all tissues, a biotinylated antirabbit secondary antibody (1:100; Jackson ImmunoResearch) was used in combination with the tyramide signal amplification kit (PerkinElmer).
In situ hybridization was performed as previously described (22). Briefly, slides were deparaffinized, washed, permeabilized, digested with proteinase-K, acetylated, and incubated in hybridization solution. The Ghrh probe was linearized with appropriate enzymes and transcribed with polymerase in the presence of digoxigenin-labeled nucleotides. Labeled probe was denatured for 3 minutes and incubated overnight at hybridization temperature. Slides were then washed in 0.5× formamide solution, then in 0.5× sodium citrate, and blocked in in situ hybridization blocking solution (10% heat inactivated sheep serum, 2% bovine serum albumin, and 0.1% Triton-X 100 in Tris buffered saline). After blocking, the slides were incubated with antidigoxigenin antibody (1:500; Roche) diluted in in situ hybridization block. Slides were then washed in Tris-buffered saline (pH 7.5 and then pH 9.5) and incubated for 12–36 hours in 4-nitro blue tetrazolium chloride /5-bromo-4-chloro-3-indoyl-phosphate, 4-toluidine salt developing solution (1:50; Roche).
Programmed cell death was detected by the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling method using the in situ cell detection kit POD (Roche). Between six and eight sections were stained per embryo. Three (e16.5) and five (e18.5) litters were analyzed.
Digital images of pituitary sections were captured with a Leica DM 5000B fluorescent microscope and Retiga 2000R digital camera. Fluorescein isothiocyanate, tetramethylrhodamine isothiocyanate, and DAPI pictures were merged using Adobe Photoshop CS3. In some instances, photos were brightened for illustrative purposes. On these occasions, care was taken to treat photographs of controls and null animals in the same manner.
LHB immunohistochemistry was quantified by measuring the area of the LHB staining and DAPI staining using Image J (National Institutes of Health, Bethesda, Maryland). The LHB area was normalized by dividing by DAPI area. Foxo1Δpit values were set relative to wild-type controls.
Real time RT-PCR
Pituitaries were dissected from e16.5 and e18.5 embryos. Total RNA was isolated with the RNAqueous-micro kit (Ambion, Inc) according to the manufacturer's directions. RNA concentrations were determined by spectrophotometry. RNA was treated with deoxyribonuclease (DNase) I and DNase inactivating reagent from the TURBO DNase-free kit (Ambion, Inc) as per the manufacturer's instructions. The RT-qPCR procedure used the TaqMan RNA-to-CT one-step kit (Applied Biosystems by Life Technologies, Inc; number 4392938) according to the manufacturer's directions and CFX96 real time system (Bio-Rad Laboratories, www.bio-rad.com). Expression levels for Foxo1 (Mm00490672_m1), Gh (Mm00433590_g1), Pomc (Mm00435874_m1), Lhb (Mm00656868_g1), Tshb (Mm00437190_m1), Prl (Mm00599949_m1), Pou1f1 (Mm00476852_m1), Neurod4 (Mm00431922_s1), Pitx2 (Mm01316994_m1), Sox2 (Mm03053810_s1), and Ghrhr (Mm01326479_M1) were determined, using Actb (4352933E) and Rn18s (4331182) as controls (all Taqman probes from Applied Biosystems, Inc). Amplification was accomplished using Taqman gene expression assays (Applied Biosystems) as per the manufacturer's instructions. Five-ten nanograms of cDNA were used in a 20-μL reaction volume. Samples and controls were run in triplicate. Data were analyzed by the ΔΔcycle threshold method (23, 24).
Statistical analysis
All results are expressed as mean ± SEM. Data were analyzed using Prism 7 software (GraphPad Software, Inc). Outliers were identified with the Grubbs' test using the online GraphPad software (http://www.graphpad.com/quickcalcs/Grubbs1.cfm). Comparisons of genotype and age effects were performed by a two-way ANOVA and a Sidak's multiple comparisons test. Comparisons of genotype only were made using a Student's t test. Values of P < .05 are considered significant (*). Values of P < .01 are considered very significant (**).
Results
Pituitary morphology is normal in Foxo1Δpit embryos
Our earlier studies demonstrate that FOXO1 is present in approximately half of pituitary somatotropes, suggesting that FOXO1 may be important for somatotrope differentiation and/or function (9). To test for this possibility, we deleted Foxo1 in the pituitary gland using mice carrying the following alleles: cre driven by the Foxg1 promoter (21, 25), a floxed allele of Foxo1 (19), and a global null allele for Foxo1 (20). We refer to these as Foxo1Δpit mice. Foxo1 is efficiently deleted at e16.5, e18.5, and P3 in Foxo1Δpit mice as assessed by immunohistochemistry for FOXO1 and RT-qPCR for Foxo1 in wild-type and Foxo1Δpit littermates (Figure 1). To assess pituitary morphology of mouse embryos lacking Foxo1 in the pituitary gland, we performed hematoxylin and eosin staining of tissue sections from Foxo1Δpit embryos and wild-type littermates at e16.5 and e18.5. No apparent difference in morphology was observed (Figure 2). FOXO1 regulates apoptosis in some tissues (26). We analyzed apoptosis in sections of pituitary from Foxo1Δpit embryos and wild-type littermates at e16.5 and e18.5 embryos by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end labeling. We did not observe any appreciable apoptosis in the absence of FOXO1 or in sections from wild-type littermates at e16.5 or e18.5 (data not shown). These data suggest that FOXO1 is dispensable for normal morphology and apoptosis during pituitary development.
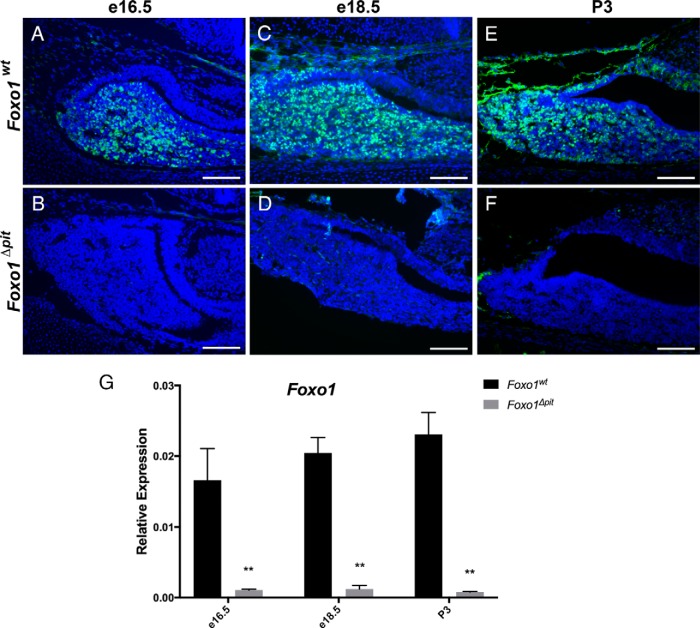
Figure 1.
Foxo1 is efficiently deleted from the pituitary gland in Foxo1Δpit embryos. Immunohistochemical staining for FOXO1 was performed on coronal sections from Foxo1Δpit embryos and wild-type littermate controls at e16.5 (A and B) and e18.5 (C and D) and P3 (E and F). FOXO1 (green)-immunopositive cells are almost completely absent in Foxo1Δpit pituitaries at all ages examined. Images shown are representative of embryos from three different litters for each genotype at each age. Nuclei are stained with DAPI (blue). Scale bars, 100 μm. RT-qPCR also shows the expression of Foxo1 is significantly reduced at e16.5, e18.5, and P3 (G). Values are set relative to Actb for each sample. Data are expressed as mean ± SEM of five animals at each age for each genotype. The data were analyzed by a two-way ANOVA with a Sidak's multiple comparisons test to determine significant differences between Foxo1Δpit embryos and wild-type littermate controls at each age. **, P < .01).
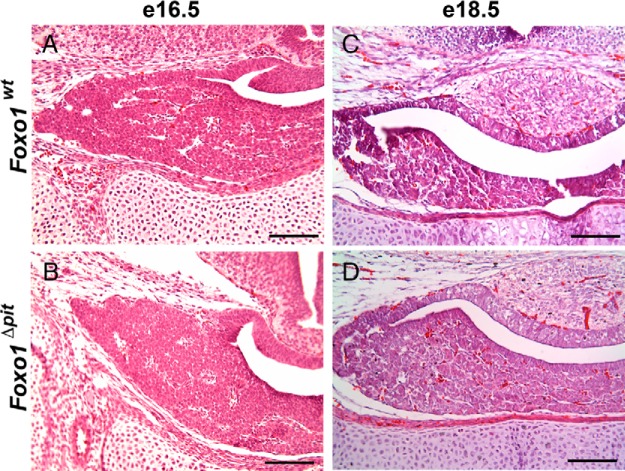
Figure 2.
Loss of FOXO1 does not affect the gross morphology of the pituitary gland during development. Hematoxylin and eosin staining was performed on coronal sections from Foxo1Δpit embryos and wild-type littermate controls at e16.5 (A and B) and e18.5 (C and D). Images shown are representative of embryos from four different litters at e16.5 and two different litters at e18.5 for each genotype. Scale bars, 100 μm.
Progenitor cell commitment occurs normally in Foxo1Δpit embryos
Pituitary cells originate as uncommitted progenitors (4, 5). During the differentiation process, these cells become committed progenitor cells, meaning they have committed to a certain fate, but are not yet terminally differentiated. To determine whether FOXO1 is required for the maintenance of the uncommitted progenitor population, we first investigated Sox2, a marker of uncommitted progenitor cells (27). We find that Sox2 expression is not altered in Foxo1Δpit embryos as compared with wild-type littermate controls at e16.5 or e18.5, suggesting that the maintenance of the uncommitted pituitary progenitor population does not require FOXO1 (Figure 3A).
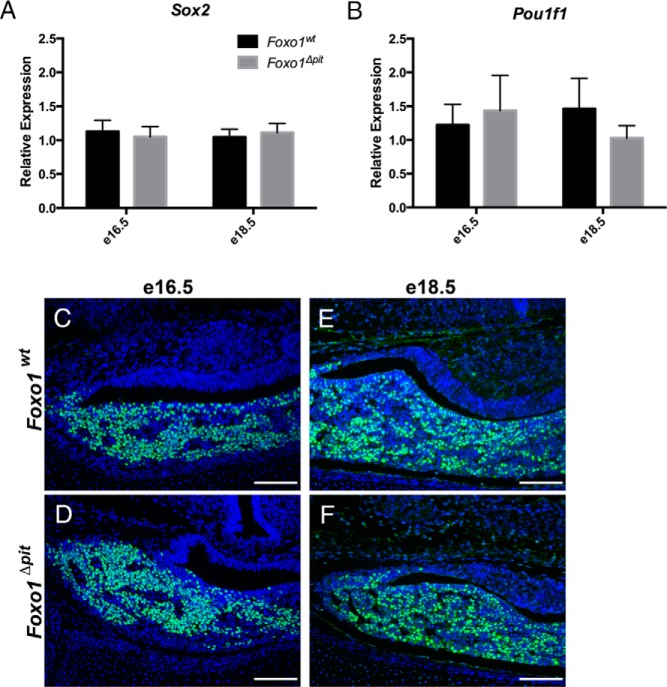
Figure 3.
The transition from uncommitted progenitor to committed progenitor occurs normally in the absence of FOXO1. RT-qPCR was performed for Sox2, a marker of uncommitted progenitor cells (A), and Pou1f1, a marker of cells committed to the somatotrope lineage (B) in Foxo1Δpit embryos and wild-type littermate controls. Values are relative to wild-type controls at e16.5. Data are expressed as mean ± SEM of 16 (e16.5) and nine (e18.5) embryos for Sox2 and nine (e16.5) and nine (e18.5) embryos for each genotype for Pou1f1. The data were analyzed by a two-way ANOVA to determine significant differences between Foxo1Δpit embryos and wild-type littermate controls at e16.5 and e18.5. Immunohistochemistry for POU1F1 (green) was performed on coronal sections from Foxo1Δpit embryos and wild-type littermate controls at e16.5 (C and D) and e18.5 (E and F). All cell nuclei are labeled with DAPI (blue). Images shown are representative of sections from four different litters for each genotype at each age. Scale bars. 100 μm.
We next sought to determine whether uncommitted progenitor cells are able to commit to the somatotrope lineage in the absence of FOXO1. To test this, we examined a marker of committed somatotrope precursors, POU1F1 (originally known as PIT1). POU1F1-immunoreactive cells are committed to differentiating into somatotropes, thyrotropes, or lactotropes. We find that Pou1f1 mRNA levels and POU1F1 protein distribution are similar in Foxo1Δpit embryos and wild-type littermate controls, suggesting that the commitment phase of somatotrope differentiation occurs normally in the absence of FOXO1 (Figure 3, B–F).
Terminal differentiation of somatotropes is delayed in the absence of Foxo1
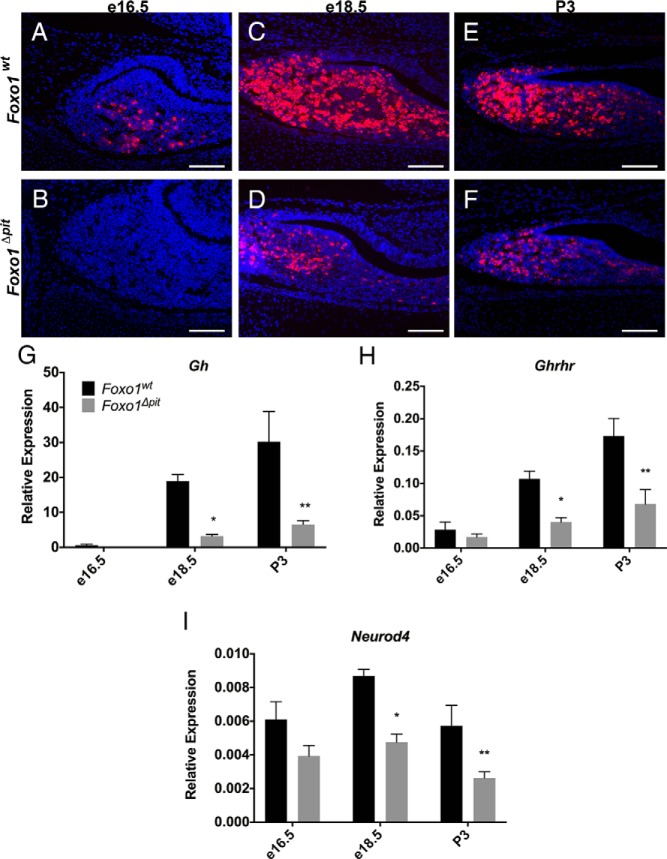
Thus far, we have established that FOXO1 is dispensable for the commitment of pituitary progenitor cells to the somatotrope lineage; however, based on its expression pattern (9), we hypothesized that FOXO1 is important for somatotrope differentiation and/or function. Somatotropes begin to terminally differentiate and are producing detectable amounts of GH by e16.5 (28). To determine whether terminal differentiation of somatotropes occurs normally in the absence of FOXO1, we performed immunohistochemistry and RT-qPCR for GH in pituitary tissue from Foxo1Δpit mice and wild-type littermate controls. Immunohistochemistry for GH reveals an almost complete absence of somatotropes in Foxo1Δpit embryos at e16.5, whereas several GH-immunoreactive somatotropes are present in wild-type littermate controls (Figure 4, A and B). Consistent with these results, at e18.5 and P3, only a few somatotropes are present in the pituitary glands of Foxo1Δpit mice (Figure 4, C–F). To evaluate the mRNA levels of Gh, we dissected pituitary glands from Foxo1Δpit mice and wild-type littermates and performed real-time RT-qPCR. Expression of Gh is quite low in both wild-type and Foxo1Δpit embryos at e16.5. Two days later at e18.5, the expression of Gh is greatly increased in wild-type embryonic pituitary glands; however, expression remains low in Foxo1Δpit embryos (Figure 4G). At P3 the expression of Gh is significantly reduced but is trending upward (Figure 4G). To determine whether FOXO1 is required for terminal differentiation of somatotropes or simply for the normal expression of Gh, we examined the expression of the receptor for GHRH (Ghrhr). Ghrhr is a marker of terminal differentiation in somatotropes (29). Low levels of mRNA for Ghrhr are detectable in wild-type and mutant pituitary glands at e16.5, but the expression is reduced to approximately 40% in Foxo1Δpit embryos at e18.5 and P3, suggesting terminal differentiation of somatotropes is delayed in the absence of Foxo1 (Figure 4H).
Figure 4.
Terminal differentiation of somatotropes is delayed in Foxo1Δpit embryos. Immunohistochemistry for GH (red) was performed on coronal sections from Foxo1Δpit embryos and wild-type littermate controls at e16.5 (A and B), e18.5 (C and D), and P3 (E and F). All cell nuclei are stained with DAPI (blue). Images shown are representative of embryos from three different litters at e16.5, five different litters at e18.5, and four different litters at P3. Scale bars, 100 μm. RT-qPCR was performed for Gh (G), Ghrhr (H), and Neurod4 (I) using total RNA from Foxo1Δpit embryos and wild-type littermate controls at e16.5, e18.5, and P3. Values are relative to Actb for each sample. Data are expressed as mean ± SEM of five (Gh, Ghrhr) and four (Neurod4) animals of each genotype and each age. The data were analyzed by a two-way ANOVA with a Sidak's multiple comparisons test to determine significant differences between Foxo1Δpit embryos and wild-type littermate controls at each age. *, P < .05; **, P < .01.
Our data show that FOXO1 is necessary for the proper timing of somatotrope terminal differentiation. To begin to understand the mechanism thereof, we investigated transcription factors that are known to contribute to this process. Several transcription factors are important for normal Gh expression during embryonic development including PITX2, Prophet of Pit-1 (PROP1), POU1F1, and neurogenic differentiation 4 (NEUROD4). We have previously found that Ames dwarf mice, which have a loss of function mutation in Prop1 and therefore do not produce either PROP1 or its downstream target, POU1F1, still produce FOXO1 (9). These data suggest that FOXO1 is not downstream of PROP1 or POU1F1 in the genetic hierarchy of pituitary development. To determine whether FOXO1 is upstream of Pitx2 or Neurod4, we analyzed their expression in Foxo1Δpit mice. No difference in Pitx2 expression was detected in the absence of Foxo1 at e16.5 or e18.5 (data not shown). Expression of Neurod4 is trending downward at e16.5 but is not significantly reduced. However, Neurod4 expression is significantly reduced at e18.5 and P3 (Figure 4I). Thus, Neurod4 mRNA levels, but not those of Pitx2, are partially dependent on FOXO1. Whether the decrease in Neurod4 expression is causal for the delay in somatotrope differentiation or a result of the decreased number of somatotropes remains to be determined.
Mice with GH deficiency are significantly smaller than wild-type littermates by 3 weeks of age (30, 31). To assess the functional consequences of the somatotrope differentiation defect we observed in Foxo1Δpit embryos, we extended our observations to include mice at this age. Control mice exhibit bright staining for FOXO1 at 3 weeks of age, but only a few dimly fluorescent FOXO1-positive cells are present in pituitary glands of Foxo1Δpit mice (Figure 5, A and B). Unlike earlier ages, however, pituitary glands from Foxo1Δpit mice exhibit a normal distribution of somatotropes in females (Figure 5, C and D) and males (data not shown). Next, we weighed Foxo1Δpit mice and wild-type littermates at 3, 4, and 5 weeks of age. There is no difference in body weight between Foxo1Δpit mice and wild-type littermates for females or males (Figure 5, E and F). Thus, loss of Foxo1 affects embryonic somatotrope differentiation but is dispensable for postnatal somatotrope expansion and growth.
Figure 5.
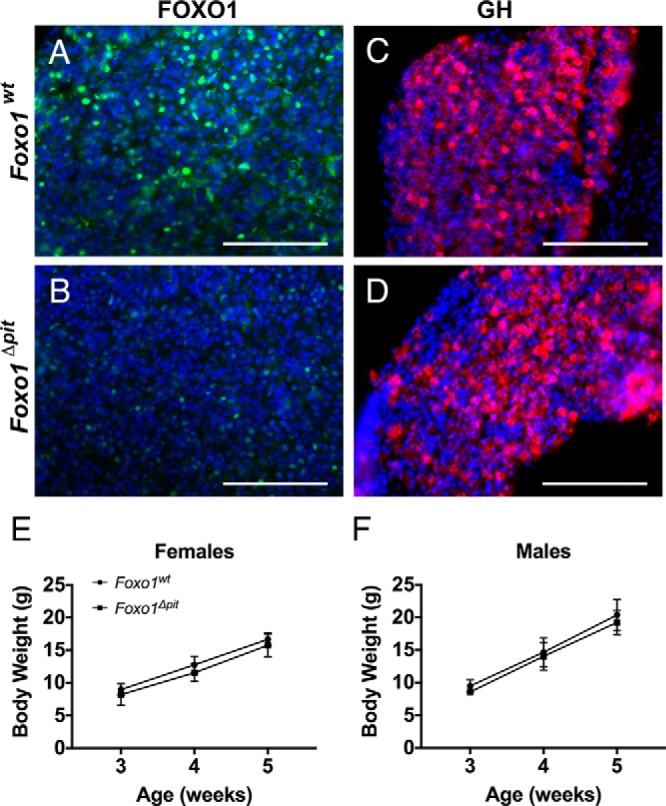
Body weight is normal in Foxo1Δpit mice. Immunohistochemistry for FOXO1 (green)(A, B) or GH (red)(C, D) was performed on coronal sections from Foxo1Δpit mice and wild-type littermate controls at 3 weeks of age. All cell nuclei are stained with DAPI (blue). Images shown are representative of mice from at least three different litters. Scale bars, 100 μm. Foxo1Δpit mice and wild-type littermate control females (E) and males (F) were weighed at 3, 4, and 5 weeks of age. Data are expressed as mean ± SEM of three mice of each genotype. The data were analyzed by a two-way ANOVA with a Sidak's multiple comparisons test to determine significant differences between Foxo1Δpit mice and wild-type littermate controls.
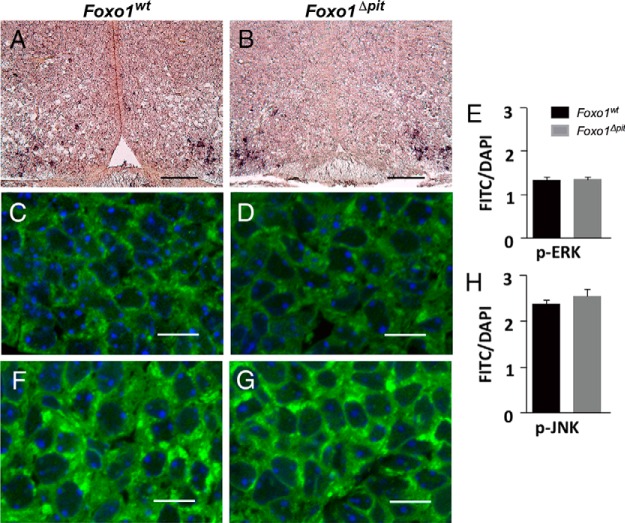
Postnatally, GHRH from the arcuate nucleus of the hypothalamus stimulates GH synthesis and secretion by binding receptors on the surface of somatotropes. It is unlikely, however, that delayed somatotrope differentiation in Foxo1Δpit embryos is due to GHRH deficiency. First, although GHRH signaling stimulates Gh expression postnatally, it is not required for normal somatotrope differentiation in mouse embryos (32, 33). Second, in situ hybridization for Ghrh on tissue sections from the arcuate nucleus of Foxo1Δpit embryos at e18.5 demonstrates that the GHRH neurons are intact (Figure 6, A and B). In support of these data, we find that activated MAPKs, which are a target of GHRH signaling (29, 34), are normal in Foxo1Δpit embryonic pituitary glands at e18.5 (Figure 6, C–H). This conclusion is further supported by the fact that the expression of another target of GHRH signaling, Pou1f1, is unaltered in Foxo1Δpit embryos (Figure 3, B–F). Therefore, several lines of data support our conclusion that the delay in somatotrope differentiation is not due to alterations in GHRH signaling to the pituitary glands of Foxo1Δpit embryos.
Figure 6.
GHRH neurons are present in the arcuate nucleus of Foxo1Δpit embryos at e18.5. In situ hybridization was performed for Ghrh (dark brown) on coronal sections from wild-type embryos (A) and Foxo1Δpit littermates (B) at e18.5. Images shown are representative of embryos from two different litters for each genotype. Scale bars, 100 μm. To assess GHRH signaling, pituitary sections were immunostained for either p-ERK (C and D) or p-JNK (F and G) followed by the appropriate Alexa Fluor 488-conjugated secondary antibody (green) and then stained with DAPI (blue). Sections were imaged by confocal laser-scanning microscopy. Images are representative of sections from three different litters. Scale bars, 10 μm. A ratio of the fluorescence intensity of either p-ERK- (E) or p-JNK (H)-positive cells to total DAPI per pituitary section was calculated at e18.5. Data are expressed as mean ± SEM. Data were analyzed by a Student's t test.
FOXO1 is required for normal expression of Lhb
After analyzing the somatotrope phenotype of Foxo1Δpit mice, we next examined the other pituitary cell types. Corticotrope and thyrotrope cells exhibit normal distribution, and the expression of the genes encoding ACTH (Pomc) and TSHB (Tshb) is similar between Foxo1Δpit embryos and wild-type littermate controls at e16.5 and e18.5 (data not shown), demonstrating that these cell types are able to differentiate in the absence of FOXO1. Similarly, a few PRL-positive cells are apparent in Foxo1Δpit mice and wild-type littermates as determined by immunohistochemistry, and Prl expression is unaffected as determined by RT-qPCR in Foxo1Δpit mice at P3, suggesting that lactotropes differentiate normally in the absence of FOXO1 (data not shown). We next examined the gonadotrope population. Although our analysis shows that the number of gonadotropes is not different in Foxo1Δpit pituitaries and wild-type littermate controls at e18.5 as determined by immunohistochemistry for LHB, mRNA levels of Lhb in Foxo1Δpit embryos are decreased to half that of wild-type littermate controls (Figure 7). These data suggest that FOXO1 is required for the normal expression of Lhb but not for differentiation of gonadotropes.
Figure 7.
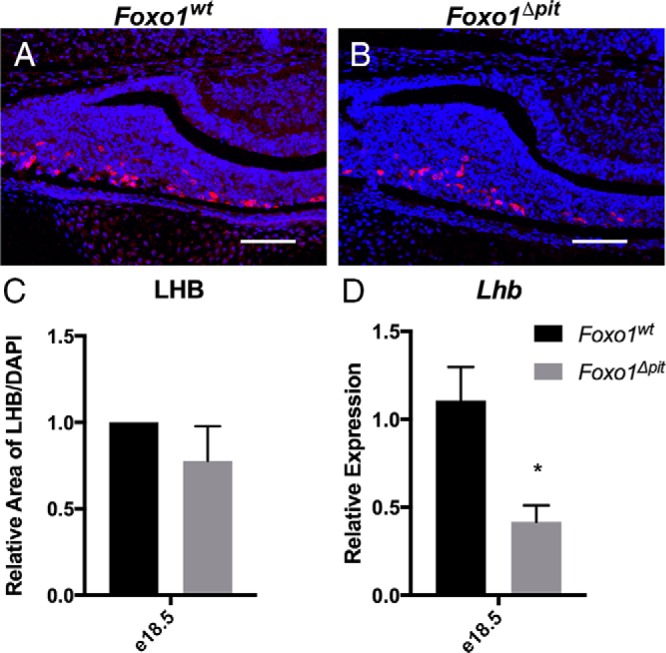
FOXO1 is important for the normal expression of Lhb. Immunohistochemistry for LHB (red) was performed on three different litters at e18.5 (A and B). Nuclei are stained with DAPI (blue). Scale bars, 100 μm. C, Area of LHB staining was measured on at least three different slides from three different litters for both Foxo1Δpit embryos and wild-type littermate controls using Image J (National Institutes of Health). Data are expressed as mean ± SEM. The data were analyzed by a Student's t test to assess significant differences between Foxo1Δpit embryos and wild-type littermate controls. D, RT-qPCR was performed for Lhb using total RNA from Foxo1Δpit embryos and wild-type littermate controls at e18.5. Values are relative to e18.5 wild-type controls. The data were analyzed by a Student's t test to determine significant differences between Foxo1Δpit embryos and wild-type littermate controls. *, P < .05.
Discussion
In many tissues, FOXO1 regulates cell differentiation (12, 13, 15). For example, loss of FOXO1 specifically in muscle results in increased formation of fast-twitch muscle fibers and decreased formation of slow-twitch fibers (12), suggesting that FOXO1 is important for cell fate determination. Interestingly, in the absence of FOXO1, we observe delayed terminal differentiation of somatotropes.
Normal somatotrope development requires uncommitted pituitary progenitor cells to commit to the POU1F1 lineage. We determine that, in the absence of FOXO1, uncommitted progenitor cells are maintained and able to commit to the POU1F1 lineage, indicating FOXO1 is not required for initial commitment of these cells. This conclusion is based on normal expression of Sox2, a marker of uncommitted progenitors, and Pou1f1, a marker of the POU1F1 lineage. However, terminal differentiation of somatotropes is delayed as evidenced by an almost complete absence of GH-positive cells at e16.5 in Foxo1Δpit mice. At e18.5 some GH-positive cells are apparent in Foxo1Δpit mice; however, the number of cells is drastically reduced as compared with wild-type littermates. Additionally, expression of Ghrhr, a marker of terminally differentiated somatotropes, is reduced at e18.5 and P3. We do not observe an increase in other differentiated cell types including gonadotropes, corticotropes, thyrotropes, or lactotropes, suggesting that the reduction of somatotropes in Foxo1Δpit mice is not because somatotropes are erroneously differentiating into these other cell types.
Expression of the transcription factor, Neurod4, is reduced in Foxo1Δpit mice at e18.5 and P3, suggesting that Neurod4 is downstream of FOXO1 in the genetic hierarchy of pituitary developmental control (Figure 8). The effect of FOXO1 on Neurod4 expression may be direct via protein-DNA interactions or may be indirect via an intermediate transcription factor. Studies by Zhu et al (35) indicate that Neurod4 is required for terminal differentiation of somatotropes. Thus, Neurod4 may be one of the intermediates between FOXO1 and terminal differentiation of somatotropes. Developmentally, lactotropes and somatotropes arise from a common progenitor, sometimes referred to as a mammosomatotrope. The fact that we do not see a difference in the lactotrope population suggests that FOXO1 is important for somatotrope differentiation after the mammosomatotrope stage of development. Similarly, mice lacking NEUROD4 have reduced Gh expression but normal Prl expression (35), further supporting a model in which NEUROD4 is involved in the mechanism for FOXO1 regulation of somatotrope development. However, Neurod4 expression is reduced only by approximately half. Thus, additional intermediates likely contribute to the deficit of Gh expression. The decreased number of terminally differentiated somatotropes likely also contributes to the reduction in Neurod4 expression.
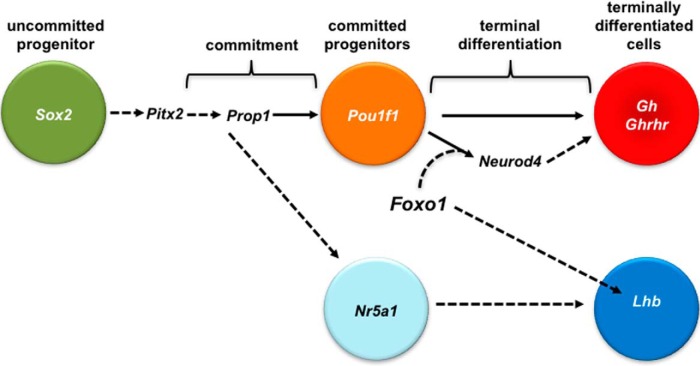
Figure 8.
FOXO1 is required for the normal differentiation of somatotropes in the embryonic pituitary gland. The HMG domain factor, Sox2, is expressed in uncommitted pituitary progenitor cells of Rathke's pouch early in pituitary organogenesis (4, 5). Sox2 is expressed normally in Foxo1Δpit embryonic pituitary glands, suggesting that FOXO1 is not required for the maintenance of the uncommitted progenitor population. Mutations in the bicoid homeodomain factor, Pitx2, result in GH deficiency in humans and mice. However, Pitx2 expression is normal in Foxo1Δpit embryonic pituitary glands, suggesting that Pitx2 is not downstream of FOXO1 in the genetic hierarchy of somatotrope development. PROP1 directly stimulates expression of another pituitary-specific transcription factor, Pou1f1 (also known as Pit1) (45, 62, 63). Pou1f1 is expressed in the POU1F1 lineage consisting of cells committed to differentiate into somatotropes, lactotropes, or thyrotropes (4, 5). We previously demonstrated that Ames dwarf mice, which have a loss of function mutation in Prop1, express FOXO1 (9). This indicates that FOXO1 is not downstream of PROP1 or POU1F1. We do not detect a significant difference in Pou1f1 expression in Foxo1Δpit embryonic pituitary glands, suggesting that FOXO1 is not required for the transition from uncommitted pituitary progenitor cells to cells committed to the POU1F1 lineage. We do observe a significant decrease in expression of the somatotrope markers, Gh and Ghrhr, suggesting that FOXO1 is required for terminal differentiation of somatotrope cells. Expression of Neurod4 (also known as Math3), which is required for normal somatotrope differentiation (35), is also reduced in the absence of FOXO1. Thus, the mechanism of FOXO1 regulation of somatotrope differentiation appears to involve NEUROD4. Furthermore, pituitary cells committed to the gonadotrope lineage express the gene encoding the orphan nuclear receptor, Nr5a1. These cells then differentiate into gonadotropes. FOXO1 is important for the stimulation of Lhb expression in embryonic gonadotropes. Direct regulation is depicted with solid arrows. Regulation that has not been tested or has been found to be indirect is depicted with dotted arrows.
One concern with cre/lox studies is whether the target gene is deleted from tissues other than the tissue in question. This can occur wherever expression of the floxed target gene overlaps with CRE activity. Both Foxo1 and Foxg1 are expressed in tissues other than the pituitary gland. FOXO1 is present in the hypothalamus and is important for the function of Pomc neurons (36–38). In situ studies and reporter mice show Foxg1 is not normally expressed in the hypothalamus (Allen brain atlas) (39), although scattered cells of the hypothalamus show CRE activity in Foxg1-cre mice (21). Interestingly, mice with heterozygous loss of Foxg1 have increased oxytocin and vasopressin expression, suggesting that Foxg1 may have a low level or transient expression in the hypothalamus or may be expressed in tissues that affect hypothalamic function (40). In the following paragraphs, we will discuss several extrapituitary signaling cascades that are known to affect GH synthesis and secretion.
Various morphogenetic signals from the ventral diencephalon and infundibulum are essential for early pituitary gland organogenesis (41). For example, fibroblast growth factor-8 is secreted from the infundibulum early in development and human mutations in FGF8 can cause hypopituitarism with GH insufficiency (42, 43). Sonic hedgehog and the bone morphogenetic proteins are also secreted from the ventral diencephalon and infundibulum and can affect somatotrope number (25, 44). It is unlikely that the phenotype of Foxo1Δpit mice is due to loss of Foxo1 in the ventral diencephalon or infundibulum because Foxg1-cre does not cause recombination in these tissues (25). Also, loss of signaling from the ventral diencephalon or infundibulum often causes abnormalities in pituitary morphology (25, 46, 47), which do not occur in Foxo1Δpit mice.
The hypothalamic-releasing hormone, GHRH, is important for stimulating GH synthesis and secretion postnatally by binding specifically to a G protein-coupled receptor on the surface of somatotropes (1). GHRH signaling stimulates expression of Pou1f1 and activates MAPKs (29, 48). During embryonic development in the mouse, neither somatotrope differentiation nor expression of Gh or Pou1f1 is dependent on GHRH signaling (32, 33). These conclusions are based on studies by Lin et al (32), who found that the number of GH-positive cells is normal before birth in lit mice, which have a loss of function mutation in the Ghrhr gene. Postnatally, lit mice exhibit pituitary hypoplasia and a significant reduction in the number of GH-positive cells (32). Lin et al (32) suggest that embryonic somatotropes are GHRH independent and that GHRH-dependent somatotropes arise after birth. This is consistent with the fact that Foxo1Δpit embryos have normal MAPK signaling and expression of Pou1f1. Furthermore, GHRH treatment of cultured fetal rat pituitary cells does not affect the timing of somatotrope differentiation or the number of somatotropes (49). Additionally, we confirmed that GHRH neurons are present in Foxo1Δpit embryos. Thus, it is unlikely the delay in somatotrope differentiation that occurs in the absence of FOXO1 is due to alterations in GHRH signaling.
Other hormones also affect somatotrope number. Adult hypothyroid mice have elevated TRH and reduced somatotrope and lactotrope populations, possibly due to a shift in differentiation of the POU1F1 lineage toward thyrotropes rather than somatotropes and lactotropes (50, 51). However, Tshb expression is normal in Foxo1Δpit mice, suggesting that the hypothalamic-pituitary-thyroid axis is normal. Similarly, mice lacking D2 dopamine receptors have increased PRL levels and reduced GH levels, possibly due to a shift in differentiation from somatotropes to lactotropes (52, 53). Again we find that Prl expression is normal in Foxo1Δpit mice, suggesting that the dopamine-PRL axis is unperturbed.
Finally, cortisol has been shown to promote somatotrope differentiation (54). However, pituitary Pomc expression and the number of ACTH-immunopositive cells is not altered in Foxo1Δpit mice, suggesting that the hypothalamic-pituitary-adrenal axis is not altered. Together these data lead us to conclude that the reduction in somatotrope number in Foxo1Δpit embryos is likely not caused by indirect effects due to loss of Foxo1 in tissues outside the pituitary gland.
Whereas Foxo1Δpit mice exhibit a delay in terminal differentiation of somatotropes, these cells do begin to differentiate by e18.5. This could be due to several factors. For example, Foxo1 is deleted early in pituitary development. Over time, other factors may be up-regulated to compensate for the loss of FOXO1. In fact, FOXO3 has been shown to compensate for FOXO1 in other tissues such as neural stem cells (55). Further studies will be important for testing this possibility. Another explanation is that the somatotropes that arise in Foxo1Δpit embryos at e18.5 may represent the first GHRH-dependent somatotropes, and these may not require FOXO1 for differentiation.
Previously we demonstrated FOXO1 immunoreactivity in approximately half of somatotropes and approximately one-tenth of gonadotropes and thyrotropes as well as in a fraction of corticotropes and lactotropes in the anterior pituitary (9). We detect FOXO1 primarily in the nucleus of pituitary cells with a few cells containing cytoplasmic FOXO1 (9). However, other groups have detected FOXO1 only in gonadotrope and thyrotrope cells exclusively in the cytoplasm (56–58). In the current study, we find that FOXO1 immunoreactivity is absent in Foxo1Δpit mice. The absence of immunoreactivity in knockout mice is the gold standard for antibody specificity in immunohistochemistry (59). Thus, these data suggest that our immunohistochemistry for FOXO1 is specific and support our hypothesis that FOXO1 is expressed in approximately half of somatotrope cells. These data do not preclude the possibility that there is FOXO1 we are not detecting by immunohistochemistry.
We find that Lhb expression is reduced in Foxo1Δpit mice, whereas the number of gonadotropes are normal at e18.5, suggesting that FOXO1 is not required for gonadotrope differentiation but rather for normal expression of Lhb. Other groups have indicated that expression of Lhb and Fshb is repressed by FOXO1 (57, 58, 60). These discrepancies may be due to the fact that these other studies used gain-of-function models, whereas we are analyzing a loss-of-function model. Finally, studies demonstrating the repression of gonadotropin expression by FOXO1 overexpression have been performed in vitro, whereas we are reporting in vivo data (57, 58, 60). It may be that the absence of normal hormonal influence from the hypothalamus and gonads with in vitro studies accounts for the different effects of FOXO1. In fact, environmental factors can affect posttranslational modifications of FOXO3 to reprogram its activity from a transcriptional activator to a transcriptional repressor (61). Thus, these in vitro studies may not properly recapitulate in vivo systems.
In conclusion, we suggest that FOXO1 is essential for normal somatotrope differentiation as well as gonadotrope function. The mechanism for FOXO1 regulation of somatotrope differentiation appears to involve FOXO1 stimulation of Neurod4 promoter activity. NEUROD4 is required for normal Ghrhr expression and ultimately terminal differentiation of somatotropes (35). Thus, we suggest that FOXO1 is required for normal somatotrope differentiation, in part, by enhancing expression of Neurod4, either directly or indirectly, which then promotes terminal differentiation of somatotropes (Figure 8).
Acknowledgments
We thank Dr Philip J. Jensik (Southern Illinois University) for the critical reading of this manuscript. Foxo1+/LacZ mice were generously provided by Dr Domenico Accili and Dr Utpal Pajvani (Columbia University, New York, New York). Foxo1flox/flox mice were also provided by Dr Domenico Accili and Dr Utpal Pajvani with permission from Dr Ronald A. DePinho (University of Texas M. D. Anderson Cancer Center, Houston, Texas). We also thank Dr A. F. Parlow and the National Hormone and Pituitary Program, the National Institute of Diabetes and Digestive and Kidney Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development for providing antibodies for TSHB, GH, LHB, and proopiomelanocortin. In addition, we thank Dr Simon J. Rhodes (Indiana University-Purdue University, Indianapolis, Indiana) for generously providing us with the POU1F1 antibodies.
This work was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grants R15HD063469 and R15HD078885 (to B.S.El.); National Center for Research Resources Grant P20RR016474 and the National Institute of General Medical Sciences Grant P20GM103432 (to A.M.N.); and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK76647 (to L.T.R.) as well as a REACH Award from Southern Illinois University (to B.E.K.).
Disclosure Summary: The authors have nothing to disclose.
Appendix
Table 1.
Antibody Table
| Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| Phospho-ERK1/ERK2 | p-ERK | Cell Signaling, number 4370 | Rabbit; monoclonal | 1:200 | |
| Phospho-SAPK/JNK | p-JNK | Cell Signaling, number 9255 | Mouse; monoclonal | 1:400 | |
| Rat ACTH | ACTH | National Hormone and Peptide Program | Rabbit; polyclonal | 1:500 | |
| Rat TSHB | TSHB | National Hormone and Peptide Program | Rabbit; polyclonal | 1:2000 | |
| Mouse GH | GH | National Hormone and Peptide Program | Rabbit; polyclonal | 1:10 000 | |
| Rat LHB | LHB | National Hormone and Peptide Program | Guinea pig; polyclonal | 1:500 | |
| Mouse PRL, recombinant | PRL | National Hormone and Peptide Program | Rabbit; polyclonal | 1:10 000 | |
| C terminus of human FOXO1 | FOXO1 | Cell Signaling, number 2880 | Rabbit; monoclonal | 1:50 | |
| POU1F1 | PIT1 | Simon Rhodes, lot 1603 | Rabbit; polyclonal | 1:2000 |
Abbreviations: JNK, c-Jun N-terminal kinase; SAPK, stress-activated protein kinase.
Footnotes
- DAPI
- 4′,6-diamidino-2-phenylindole
- DNase
- deoxyribonuclease
- e
- embryonic day
- FOXO1
- forkhead box transcription factor O1
- LHB
- LH-β subunit
- NHPP
- National Hormone and Peptide Program
- PRL
- prolactin
- p
- phosphorylated
- P
- postnatal day
- POU1F1
- POU domain class 1 transcription factor F1
- PROP1
- Prophet of Pit-1
- qPCR
- quantitative PCR
- TBS
- Tris-buffered saline
- TSHB
- TSH-β subunit.
References
- 1. Kronenberg H, Williams RH. Williams Textbook of Endocrinology. 11th ed Philadelphia: Saunders/Elsevier; 2008. [Google Scholar]
- 2. Procter AM, Phillips JA, 3rd, Cooper DN. The molecular genetics of growth hormone deficiency. Hum Genet. 1998;103:255–272. [DOI] [PubMed] [Google Scholar]
- 3. Fernandez-Rodriguez E, Bernabeu I, Castro AI, Kelestimur F, Casanueva FF. Hypopituitarism following traumatic brain injury: determining factors for diagnosis. Front Endocrinol (Lausanne). 2011;2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Davis SW, Ellsworth BS, Perez Millan MI, et al. Pituitary gland development and disease: from stem cell to hormone production. Curr Topics Dev Biol. 2013;106:1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rizzoti K. Genetic regulation of murine pituitary development. J Mol Endocrinol. 2015;54:R55–R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelberman D, Rizzoti K, Lovell-Badge R, Robinson IC, Dattani MT. Genetic regulation of pituitary gland development in human and mouse. Endocr Rev. 2009;30:790–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burrows HL, Douglas KR, Seasholtz AF, Camper SA. Genealogy of the anterior pituitary gland: tracing a family tree. Trends Endocrinol Metab. 1999;10:343–352. [DOI] [PubMed] [Google Scholar]
- 8. Watkins-Chow DE, Camper SA. How many homeobox genes does it take to make a pituitary gland? Trends Genet. 1998;14:284–290. [DOI] [PubMed] [Google Scholar]
- 9. Majumdar S, Farris CL, Kabat BE, Jung DO, Ellsworth BS. Forkhead Box O1 is present in quiescent pituitary cells during development and is increased in the absence of p27 Kip1. PLoS One. 2012;7:e52136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. [DOI] [PubMed] [Google Scholar]
- 11. Kitamura T, Ido Kitamura Y. Role of FoxO proteins in pancreatic β cells. Endocr J. 2007;54:507–515. [DOI] [PubMed] [Google Scholar]
- 12. Kitamura T, Kitamura YI, Funahashi Y, et al. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest. 2007;117:2477–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. [DOI] [PubMed] [Google Scholar]
- 14. Potente M, Urbich C, Sasaki K, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115:2382–2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teixeira CC, Liu Y, Thant LM, Pang J, Palmer G, Alikhani M. Foxo1, a novel regulator of osteoblast differentiation and skeletogenesis. J Biol Chem. 2010;285:31055–31065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hosaka T, Biggs WH, 3rd, Tieu D, et al. Disruption of forkhead transcription factor (FOXO) family members in mice reveals their functional diversification. Proc Natl Acad Sci USA. 2004;101:2975–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferdous A, Morris J, Abedin MJ, Collins S, Richardson JA, Hill JA. Forkhead factor FoxO1 is essential for placental morphogenesis in the developing embryo. Proc Natl Acad Sci USA. 2011;108:16307–16312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. [DOI] [PubMed] [Google Scholar]
- 19. Paik JH, Kollipara R, Chu G, et al. FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell. 2007;128:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakae J, Biggs WH, 3rd, Kitamura T, et al. Regulation of insulin action and pancreatic β-cell function by mutated alleles of the gene encoding forkhead transcription factor Foxo1. Nat Genet. 2002;32:245–253. [DOI] [PubMed] [Google Scholar]
- 21. Hebert JM, McConnell SK. Targeting of cre to the Foxg1 (BF-1) locus mediates loxP recombination in the telencephalon and other developing head structures. Dev Biol. 2000;222:296–306. [DOI] [PubMed] [Google Scholar]
- 22. Aujla PK, Bogdanovic V, Naratadam GT, Raetzman LT. Persistent expression of activated notch in the developing hypothalamus affects survival of pituitary progenitors and alters pituitary structure. Dev Dyn. 2015;244:921–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-δδC[T]) method. Methods. 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 24. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 25. Wang Y, Martin JF, Bai CB. Direct and indirect requirements of Shh/Gli signaling in early pituitary development. Dev Biol. 2011;348:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hameedaldeen A, Liu J, Batres A, Graves GS, Graves DT. FOXO1, TGF-beta regulation and wound healing. Int J Mol Sci. 2014;15:16257–16269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fauquier T, Rizzoti K, Dattani M, Lovell-Badge R, Robinson IC. SOX2-expressing progenitor cells generate all of the major cell types in the adult mouse pituitary gland. Proc Natl Acad Sci USA. 2008;105:2907–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Japon MA, Rubinstein M, Low MJ. In situ hybridization analysis of anterior pituitary hormone gene expression during fetal mouse development. J Histochem Cytochem. 1994;42:1117–1125. [DOI] [PubMed] [Google Scholar]
- 29. Mayo KE, Miller T, DeAlmeida V, Godfrey P, Zheng J, Cunha SR. Regulation of the pituitary somatotroph cell by GHRH and its receptor. Recent Prog Horm Res. 2000;55:237–266; discussion 266–237. [PubMed] [Google Scholar]
- 30. Gage PJ, Lossie AC, Scarlett LM, Lloyd RV, Camper SA. Ames dwarf mice exhibit somatotrope commitment but lack growth hormone-releasing factor response. Endocrinology. 1995;136:1161–1167. [DOI] [PubMed] [Google Scholar]
- 31. Bartke A. The response of two types of dwarf mice to growth hormone, thyrotropin, and thyroxine. Gen Comp Endocrinol. 1965;5:418–426. [DOI] [PubMed] [Google Scholar]
- 32. Lin SC, Lin CR, Gukovsky I, Lusis AJ, Sawchenko PE, Rosenfeld MG. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–213. [DOI] [PubMed] [Google Scholar]
- 33. Frohman LA, Kineman RD. Growth hormone-releasing hormone and pituitary development, hyperplasia and tumorigenesis. Trends Endocrinol Metab. 2002;13:299–303. [DOI] [PubMed] [Google Scholar]
- 34. Miller TI, Godfrey PA, Dealmeida VI, Mayo KE. The rat growth hormone-releasing hormone receptor gene: structure, regulation, and generation of receptor isoforms with different signaling properties. Endocrinology. 1999;140:4152–4165. [DOI] [PubMed] [Google Scholar]
- 35. Zhu X, Zhang J, Tollkuhn J, et al. Sustained Notch signaling in progenitors is required for sequential emergence of distinct cell lineages during organogenesis. Genes Dev. 2006;20:2739–2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Belgardt BF, Husch A, Rother E, et al. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. [DOI] [PubMed] [Google Scholar]
- 37. Iskandar K, Cao Y, Hayashi Y, et al. PDK-1/FoxO1 pathway in POMC neurons regulates Pomc expression and food intake. Am J Physiol Endocrinol Metab. 2010;298:E787–E798. [DOI] [PubMed] [Google Scholar]
- 38. Kitamura T, Feng Y, Kitamura YI, et al. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. [DOI] [PubMed] [Google Scholar]
- 39. Xuan S, Baptista CA, Balas G, Tao W, Soares VC, Lai E. Winged helix transcription factor BF-1 is essential for the development of the cerebral hemispheres. Neuron. 1995;14:1141–1152. [DOI] [PubMed] [Google Scholar]
- 40. Frullanti E, Amabile S, Lolli MG, et al. Altered expression of neuropeptides in FoxG1-null heterozygous mutant mice. Eur J Hum Genet. 2016;24:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mehta A, Dattani MT. Developmental disorders of the hypothalamus and pituitary gland associated with congenital hypopituitarism. Best Pract Res Clin Endocrinol Metab. 2008;22:191–206. [DOI] [PubMed] [Google Scholar]
- 42. Ericson J, Norlin S, Jessell TM, Edlund T. Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development. 1998;125:1005–1015. [DOI] [PubMed] [Google Scholar]
- 43. Falardeau J, Chung WC, Beenken A, et al. Decreased FGF8 signaling causes deficiency of gonadotropin-releasing hormone in humans and mice. J Clin Invest. 2008;118:2822–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Treier M, Gleiberman AS, O'Connell SM, et al. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev. 1998;12:1691–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gage PJ, Brinkmeier ML, Scarlett LM, Knapp LT, Camper SA, Mahon KA. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol. 1996;10:1570–1581. [DOI] [PubMed] [Google Scholar]
- 46. Cha KB, Douglas KR, Potok MA, Liang H, Jones SN, Camper SA. WNT5A signaling affects pituitary gland shape. Mech Dev. 2004;121:183–194. [DOI] [PubMed] [Google Scholar]
- 47. Davis SW, Camper SA. Noggin regulates Bmp4 activity during pituitary induction. Dev Biol. 2007;305:145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pombo CM, Zalvide J, Gaylinn BD, Dieguez C. Growth hormone-releasing hormone stimulates mitogen-activated protein kinase. Endocrinology. 2000;141:2113–2119. [DOI] [PubMed] [Google Scholar]
- 49. Hemming FJ, Begeot M, Dubois MP, Dubois PM. Fetal rat somatotropes in vitro: effects of insulin, cortisol, and growth hormone-releasing factor on their differentiation: a light and electron microscopic study. Endocrinology. 1984;114:2107–2113. [DOI] [PubMed] [Google Scholar]
- 50. Kendall SK, Samuelson LC, Saunders TL, Wood RI, Camper SA. Targeted disruption of the pituitary glycoprotein hormone alpha-subunit produces hypogonadal and hypothyroid mice. Genes Dev. 1995;9:2007–2019. [DOI] [PubMed] [Google Scholar]
- 51. Stahl JH, Kendall SK, Brinkmeier ML, et al. Thyroid hormone is essential for pituitary somatotropes and lactotropes. Endocrinology. 1999;140:1884–1892. [DOI] [PubMed] [Google Scholar]
- 52. Diaz-Torga G, Feierstein C, Libertun C, et al. Disruption of the D2 dopamine receptor alters GH and IGF-I secretion and causes dwarfism in male mice. Endocrinology. 2002;143:1270–1279. [DOI] [PubMed] [Google Scholar]
- 53. Garcia-Tornadu I, Rubinstein M, Gaylinn BD, et al. GH in the dwarf dopaminergic D2 receptor knockout mouse: somatotrope population, GH release, and responsiveness to GH-releasing factors and somatostatin. J Endocrinol. 2006;190:611–619. [DOI] [PubMed] [Google Scholar]
- 54. Hemming FJ, Aubert ML, Dubois PM. Differentiation of fetal rat somatotropes in vitro: effects of cortisol, 3,5,3′-triiodothyronine, and glucagon, a light microscopic and radioimmunological study. Endocrinology. 1988;123:1230–1236. [DOI] [PubMed] [Google Scholar]
- 55. Paik JH, Ding Z, Narurkar R, et al. FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell. 2009;5:540–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Coss D, Mellon PL, Thackray VG. A FoxL in the Smad house: activin regulation of FSH. Trends Endocrinol Metab. 2010;21:562–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Arriola DJ, Mayo SL, Skarra DV, Benson CA, Thackray VG. FOXO1 inhibits transcription of luteinizing hormone beta in pituitary gonadotrope cells. J Biol Chem. 2012;287:33424–33435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Choi YS, Lee HJ, Ku CR, et al. FoxO1 is a negative regulator of FSH β gene expression in basal and GnRH-stimulated conditions in female. Endocrinology. 2014;155:2277–2286. [DOI] [PubMed] [Google Scholar]
- 59. Fritschy JM. Is my antibody-staining specific? How to deal with pitfalls of immunohistochemistry. Eur J Neurosci. 2008;28:2365–2370. [DOI] [PubMed] [Google Scholar]
- 60. Lannes J, L'Hote D, Garrel G, Laverriere JN, Cohen-Tannoudji J, Querat B. Rapid communication: a microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol Endocrinol. 2015;29:364–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Li Z, Zhao J, Tikhanovich I, et al. Serine 574 phosphorylation alters transcriptional programming of FOXO3 by selectively enhancing apoptotic gene expression. Cell Death Differ. 2016;23:583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sornson MW, Wu W, Dasen JS, et al. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996;384:327–333. [DOI] [PubMed] [Google Scholar]
- 63. Carvalho LR, Brinkmeier ML, Castinetti F, Ellsworth BS, Camper SA. Corepressors TLE1 and TLE3 interact with HESX1 and PROP1. Mol Endocrinol. 2010;24:754–765. [DOI] [PMC free article] [PubMed] [Google Scholar]