Abstract
Interactions between exogenous and endogenous opioids are not commonly investigated as a basis for sexually dimorphic opioid analgesia. We investigated the influence of spinal endomorphin 2 (EM2), an endogenous mu-opioid receptor (MOR) ligand, on the spinal antinociception produced by intrathecally administered opioids. Activation of spinal MORs facilitated spinal EM2 release. This effect was sexually dimorphic, occurring in males but not females. Although activational effects of testosterone were required for opioid facilitation of spinal EM2 release in males, the absence of this facilitation in females resulted from neither insufficient levels of testosterone nor mitigating effects of estrogens. Strikingly, in males, the contribution of spinal EM2 to the analgesia produced by intrathecally applied MOR agonists depended on their analgesic efficacy relative to that of EM2. Spinal EM2 released by the higher efficacy MOR agonist sufentanil diminished sufentanil’s analgesic effect, whereas EM2 released by the lower efficacy morphine had the opposite effect on spinal morphine antinociception. Understanding antithetical contributions of endogenous EM2 to intrathecal opioid antinociception not only enlightens the selection of opioid medications for pain management, but also helps explain variable sex-dependence of the antinociception produced by different opioids, facilitating the acceptance of sexually dimorphic antinociception as a basic tenet.
Perspective
The male-specific MOR-coupled enhancement of spinal EM2 release implies a parallel ability to harness endogenous EM2 antinociception. The inferred diminished ability of females to utilize the spinal EM2 antinociceptive system could contribute to their greater frequency and severity of chronic pain syndromes.
Keywords: endomorphin, sexual dimorphism, estrogen, testosterone, opioid
Introduction
The growing pressure to provide evidence-based justification for new medicinal therapies underscores the imperative to better understand the sex divide in pain and to optimize pain management in both sexes. Epidemiological studies consistently demonstrate a greater prevalence among women than men of chronic pain disorders.29 These include musculoskeletal pain,33,79,89,97 headaches and migraines,22,54,86,90 fibromyalgia,100 and back pain.41,87 Furthermore, women exhibit higher self-reported severity of pain in clinical studies of osteoarthritis,43 back pain,6 and pain following surgical procedures such as cholecystectomy,24 colonoscopy,50 and knee arthroplasty.77 Moreover, multiple investigations of experimental pain have demonstrated that sexually dimorphic nociception and opioid antinociception are not idiosyncratic to a particular pain modality in either humans30,94 or laboratory animals.5,12,18,19,46,66,67 Although most studies document greater frequency and severity of pain among women than men, there are notable exceptions to this pattern in the epidemiological,25,93 clinical,53,80 and experimental8,72 literature. The underlying biological basis for this complexity remains unknown.
Sex differences are also observed in the effectiveness of opioid analgesics.28,29 There is a general consensus that mu-opioid receptor (MOR) agonists produce greater analgesia in males than females.3,7,13,14,16,42,48,73,74,96 However, human studies of sexual dimorphism in MOR-mediated antinociception are not without controversy, particularly with respect to morphine (a MOR-preferring agonist).2,9,31,32,38,44,45,81 Inconsistencies among human studies of sexually dimorphic analgesic responsiveness impede the development of a conceptual framework for understanding sex-dependent opioid analgesia, underscoring the utility of animal models. As an exemplar, in a study using rats, opioid antinociception qualitatively differed between males and females depending on a complex interaction between sex and pain type (and/or the body region receiving the nociceptive stimulus);57 this could inform at least some of the diametrically opposed findings reported in humans.
In rats, MOR-coupled sequelae also frequently demonstrate sexual dimorphism. For example, acute systemic morphine activates more than twice the number of neurons in the periaqueductal gray-rostral ventromedial medulla of male rats than female rats,60 and chronic systemic morphine upregulates mRNA encoding two MOR splice variants (MOR-1B2 and MOR-1C1) in the spinal cord of male but not female rats.91 Notably, although supraspinal/systemic morphine produces greater analgesia in male rats than female rats,3,7,13,14 effects of morphine on spinal antinociception are equianalgesic in males and females.59 This difference underscores the complexity of sexually dimorphic opioid analgesia on both neuroanatomical and physiological levels (see Loyd and Murphy, 2014 for review61).
Sexual dimorphism in rats also extends to utilization of the endogenous MOR ligand endomorphin (EM) 2 in spinal tissue. EMs are endogenous activators of MOR,102 having a higher affinity (Ki = 360 and 690 pM for EM1 and EM2 respectively) and selectivity for MOR than any other endogenous opioid. Intracerebroventricular or intrathecal (i.t.) administration of EM1 or EM2 in rats produces potent MOR-mediated antinociception.82,102 Notably, the magnitude of in vitro basal and evoked EM2 release from spinal tissue of male rats is ≈50% greater than that from spinal tissue of females,36 a finding that is consistent with the enhanced activation of MOR by experimental pain in human males.104 These data suggest that males and females differentially utilize the endogenous MOR/EM2 antinociceptive system.
One parameter of endogenous EM2 utilization that remains relatively unexplored is its relationship with intrathecally-administered opioids. We previously reported that sufentanil enhances in vitro K+-induced release of EM2, an effect that was observable only following in vitro pretreatment with pertussis toxin10 to eliminate MOR-mediated inhibition of K+-induced EM2 release.10,36 However, the contribution of endogenous EM2 to spinal antinociception resulting from intrathecally administered MOR agonists, and the mechanisms thereof, were not studied. Such knowledge could hold a key to harnessing endogenous opioids for pain management.
Accordingly, we investigated the ability of the highly specific MOR agonist sufentanil to elicit release of EM2 from spinal cords of male and female rats in vivo, as well as the contribution of this endogenously released EM2 to spinal opioid analgesia. We chose a MOR-selective agonist because MOR is the opioid receptor most abundantly expressed in the CNS and most frequently targeted for pain management. We selected EM2, not EM1, because EM2 is the predominant EM in spinal cord.64 Results demonstrate MOR-mediated enhancement of EM2 release in males but not females, as well as sexually dimorphic regulation of this mechanism by gonadal sex steroids. The contribution of EM2 to the antinociception produced by i.t. MOR agonists was also found to be sexually dimorphic. These findings provide, in part, a physiological basis for sex differences in MOR-mediated opioid analgesia.
Materials and Methods
Animals and Housing
Experiments used Sprague-Dawley rats (Charles River Laboratories, Kingston, NY; 250–300 g) maintained in a controlled environment on a 12 hour light/dark cycle. Food and water were available ad libitum. All experimental procedures were reviewed and approved by our Institutional Animal Care and Use Committee.
Determination of Stage of Estrous Cycle
Estrous cycle stage was determined by histology of vaginal smears. Proestrus or diestrus showed a predominance of large round nucleated cells or small leukocytes, respectively. Potential disruptions of the estrous cycle resulting from surgery did not confound data interpretation because stage of cycle was defined by vaginal smear histology rather than predictions assuming regularity of cycling.
Orchiectomy and Ovariectomy in Adult Rats
To determine whether the sex-related differences in the effect of i.t. sufentanil on EM2 release were the result of the activational effects of steroids, groups of adult male and female rats were gonadectomized either 1 or 2 weeks before measurement of EM2 release. In brief, animals were anesthetized with sodium pentobarbital (50 mg/kg i.p.; Abbott Laboratories, North Chicago, IL) after pretreatment with atropine (0.85 mg/kg i.p.; IUX Animal Health, Inc., St. Joseph, MO). Orchiectomy was performed by the removal of the testes together with testicular fat and epididymis, and ovariectomy was performed by removal of the ovaries.63 Activational effects of sex hormones have been reliably altered either 1 or 2 weeks following gonadectomy.11,23,26,85 In the current study, effects of ovariectomy did not differ between groups (1 week vs. 2 weeks). To underscore this point, we include data from both groups. The same experimental protocol (i.t. cannulation, i.t. treatment, in vivo perfusion, analysis of EM2 in the perfusate; see below for methods) was employed for both groups.
Androgenization of Female Adults
One group of adult female rats was ovariectomized, 1 week after which they received testosterone implants. These remained in place for an additional 1 week, at which time i.t. cannulation and perfusion (see below) were performed. This method, previously used to androgenize gonadectomized adult rats, achieves circulating testosterone levels comparable to those of intact adult male rats (11.7 ± 4.7 ng/ml).70,101 In brief, Silastic laboratory tubing (Dow Corning, Midland, MI) was used for testosterone implants, which consisted of two Silastic tubes (i.d. 0.20 cm, o.d. 0.32 cm, length 3 cm) packed with testosterone (Steraloids, Inc., Newport, RI) and sealed with A-100 Medical Silicone Adhesive (Factor II, Inc., Lakeside, AZ). Implants were submerged in 0.9% saline solution at 37°C for 24 ho urs, and then implanted subcutaneously 3 cm distal to the shoulder blade (under 2.5% isoflurane anesthesia).
Androgenization of Female Pups
To determine whether the sex-related differences of i.t. sufentanil on EM2 release were the result of developmental (organizational) effects of steroids, female rats were subjected to gonadal ablation on neonatal day 1 as described previously.15,59 In brief, female pups were taken from their mother and put on a metal plate on top of ice. When they became motionless and pale (about 15 min), they received subcutaneous injections of testosterone propionate dissolved in sesame seed oil (500 μg in 30 μL). The pups were cleaned and put on a warming pad to recover (about 30 min) before being returned to their mother. Pups were weaned at 3 weeks of age. As adults (250–300 g), one week prior to testing, half the rats received Silastic implants filled with testosterone (described above), while the other half received empty Silastic implants.
Implantation of Intrathecal Cannulas
For measurement of acute thermal antinociception, a permanent indwelling cannula was inserted into the lumbar spinal cord subarachnoid space 1 week before experimentation as described previously.59 In brief, animals were anesthetized as described above, and an 8.25 cm PE-10 catheter (Becton, Dickinson and Company, Franklin Lanes, NJ) was inserted through the atlanto-occipital membrane into the spinal subarachnoid space. The cephalic portion of the catheter was secured in place and externalized through the skin on the dorsal side of the neck, where it was relatively inaccessible to the paws. Upon gross inspection, all animals appeared to be free of infection. The righting reflex and the inclined plane test were used to assess motoric integrity; any animals showing motor impairment following surgery were excluded. For in vivo perfusion of the spinal i.t. space, two PE-10 catheters (8.25 cm inflow and 6.75 cm outflow) were implanted immediately before perfusion.
Intrathecal Administration of Drugs
Drugs were administered over a 60-second period via i.t. cannula to the subarachnoid space of the lumbar spinal cord. Each drug was delivered in 5 to 10 μL vehicle with an additional 10 μL to flush the cannula. Responsiveness to nociceptive stimuli or EM2 release was determined at various intervals thereafter and compared with pre-drug values. Narcotics were obtained from NIDA, the androgen receptor antagonist flutamide was obtained from Sigma-Aldrich (St. Louis, MO), and affinity-purified anti-EM2 antibody was obtained from Neuromics (Edina, MN). This antibody has less than 3% cross-reactivity with EM1 and less than 0.01% cross-reactivity with Leu5-enkephalin, Met5-enkephalin, and β-endorphin. I.t. application of preadsorbed anti-EM2 antibody (negative control) failed to alter basal tail flick latency (TFL; described below) and spinal opioid antinociceptive responsiveness. We have reported that male and female rats do not differ in analgesic responsiveness to i.t. sufentanil56 or i.t. morphine.59 Thus, in the current study, the equivalent i.t. doses of sufentanil/morphine administered to males and females were equianalgesic.
Quantification of Acute Thermal Nociception
A radiant heat source (Analgesia Meter; IITC, Woodland Hills, CA) was used to assess thermal nociceptive threshold (i.e., TFL) immediately before and at various intervals after i.t. opioid administration. The radiant heat intensity used was identical for males and females and resulted in a basal TFL between 3 and 4 seconds. A 10-second cutoff was employed to prevent tissue damage. Male and female rats have been reported to differ in thermal pain sensitivity92 as well as cutaneous blood flow.17,51,52 Nevertheless, in the present study, basal TFL did not vary between males and females (F7,54 = 0.70; p = 0.68). Moreover, effects of opioids on thermal nociceptive thresholds were not compared between males and females. Instead, within-sex analysis was used to determine the effect of i.t. anti-EM2 antibody on spinal opioid analgesic responsiveness. Thus, sex-dependent thermal nociception and cutaneous blood flow are not confounding variables.
In Vivo Perfusion of the Spinal Intrathecal Space
The push-pull method was used to perfuse the i.t. space of intact, anesthetized rats as performed previously.58 Two PE-10 catheters (8.25 cm inflow and 6.75 cm outflow) were inserted into the spinal subarachnoid space via the atlanto-occipital membrane under sodium pentobarbital anesthesia (50 mg/kg i.p.). The longer cannula extended to the middle of the lumbar enlargement; the shorter cannula extended to the caudal portion of the thoracic spinal cord. The i.t. space was perfused with Krebs-Ringer buffer (120 mM NaCl, 4.7 mM KCl, 1.2 mM NaH2PO4, 14 mM dextrose, 0.3 mM MgSO4, 25 mM NaHCO3, and 2.5 mM CaCl2) pre-warmed to 37°C and containing protease inhibitors Phenanth roline (1 mM; Sigma-Aldrich, St. Louis, MO) and Actinonin (100 μM; AG Scientific, San Diego, CA) to prevent EM2 degradation. The outflow tubing was placed on ice to cool the perfusate. The i.t. space was equilibrated with the perfusion medium for 10 min. Subsequently, four 10-min perfusate samples (5 μL/min) were collected from each animal for quantification of EM2 release: two before (for basal release) and two after i.t. opioid administration, with a 10-min resting period after each sample collection. Following the initial 10-min equilibration period, the basal rate of spinal EM2 release did not vary significantly over the period of i.t. perfusion. Therefore, EM2 release after drug treatment was compared against the basal rate of release immediately preceding it.
EM2 Competitive Radioimmunoassay
The content of EM2 in i.t. perfusate was quantified using a competitive radioimmunoassay (RIA) as described previously.36 The anti-EM2 antibody used (generously supplied by Dr. James Zadina) is highly selective for EM2; it does not recognize EM1. 125I-labeled EM2 (Phoenix Pharmaceuticals, Burlingame, CA) was used as tracer. Bovine serum albumin (0.1%; Sigma-Aldrich, St. Louis, MO) in the assay buffer minimized nonspecific adherence to the assay tube. Tube contents were transferred to plates coated with IgG and scintillant. Plates were counted with a MicroBeta plate reader (Perkin Elmer, Waltham, MA). A standard curve, plotted as percent inhibition of binding against the log concentration of unlabeled EM2 (0.9–28 fmol), was included with each assay and used to estimate EM2 quantities in perfusate samples using the “forecast” function in Excel (Microsoft, Redmond, WA).
Data Analysis
One-way repeated measures ANOVA was used to determine the effect of treatment at multiple time points after i.t. administration of drugs within each group. Tukey’s post-hoc test identified specific time points showing a significant effect. Two-way ANOVA was used to analyze interactions between sex/group and time after treatment. Bonferroni post-hoc tests identified differences between groups. One-way ANOVA was used to compare basal TFL between groups. P < 0.05 was considered significant. Data are expressed as mean ± SEM.
Results
Regulation of Spinal EM2 Release by I.t. Sufentanil Is MOR-Mediated, Sexually Dimorphic, and Estrous Cycle-Independent
The basal rate of EM2 release (before drug treatment) among males (3.71 ± 0.42 fmol/10 min; n = 9), diestrous females (2.47 ± 0.33 fmol/10 min; n = 6), and proestrous females (2.66 ± 0.44 fmol/10 min; n = 8) did not differ (F2,20 = 2.70; p = 0.09). Basal EM2 release also did not differ among males treated with sufentanil, naloxone, or sufentanil plus naloxone (described below; F2,14 = 1.65; p = 0.23, n = 4–9).
Based on the dose-effect relationship of i.t. sufentanil to generate spinal analgesia, we elected to study effects of 0.7 nmol sufentanil on spinal EM2 release because this dose produces near-maximal antinociception (TFL).56 One-way ANOVA revealed a significant treatment effect of sufentanil in males (F2,16 = 7.45; p < 0.01): 30 to 40 min after the i.t. administration of sufentanil, EM2 release increased to 5.14 ± 0.56 fmol/10 min (p < 0.01), an increase of approximately 39% (Fig. 1). A lower dose of sufentanil (0.35 nmol) failed to enhance EM2 release (F2,8 = 1.79; p = 0.23, n = 5). Since an i.t. dose of 0.7 nmol sufentanil produces near-maximal antinociception,56 effects of doses higher than 0.7 nmol on spinal EM2 release would have dubious relevance to sufentanil analgesic responsiveness and were therefore not studied. In striking contrast to males, sufentanil (0.7 nmol) did not alter EM2 release in either diestrous (F2,10 = 1.23; p = 0.33) or proestrous females (F2,14 = 0.01; p = 0.99).
Figure 1.
Enhancement of spinal EM2 release by sufentanil was sexually dimorphic. Spinal EM2 release increased 30–40 min after i.t. sufentanil in males but not diestrous or proestrous females. The spinal i.t. space was perfused at 5 μL/min using the push-pull method as described in Materials and Methods. Drugs were administered via the inflow spinal cannula. The EM2 content of i.t. perfusate was quantified using a competitive radioimmunoassay. Data are expressed as fmol EM2 per 10-min period. * p < 0.01 for EM2 release in males 30-40 min after i.t. administration of sufentanil. n = 6–9.
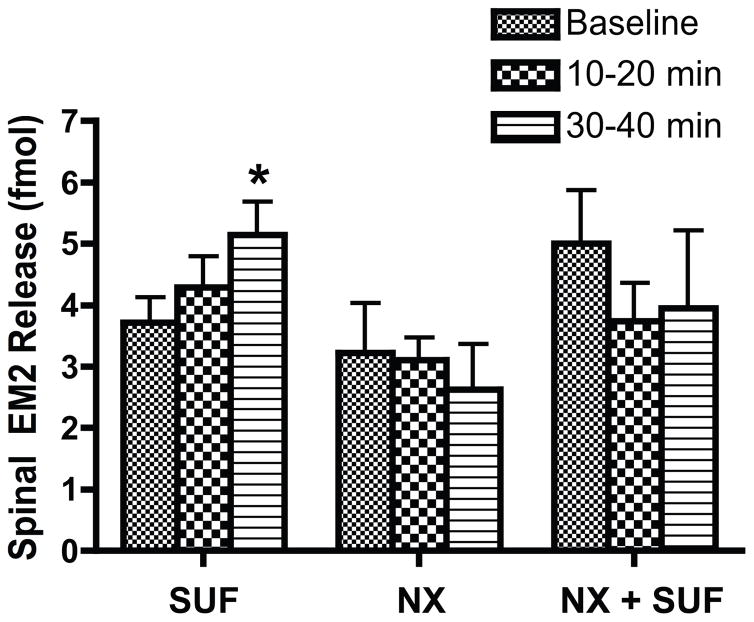
Although the opioid receptor antagonist naloxone (i.t., 100 nmol; Fig. 2) did not alter EM2 release in males (F2,6 = 0.25; p = 0.78, n = 4), naloxone pretreatment (30 min) prevented the facilitation of EM2 release by sufentanil, i.e., basal EM2 release did not differ from release in the presence of both sufentanil and naloxone (F2,6 = 1.26; p = 0.35, n = 4). Notably, the rate of EM2 release 30–40 min after treatment with naloxone plus sufentanil (3.44 ± 1.11 fmol/10 min) was indistinguishable from either EM2 release 30–40 min after treatment with naloxone alone (2.63 ± 0.73 fmol/10 min; t6 = 0.90; p = 0.40) or basal EM2 release among males treated with sufentanil, naloxone, or both (F3,18 = 0.86; p = 0.48, n = 4–9). Naloxone is ≈100-fold more selective for MOR than delta-opioid receptor.75 Moreover, sufentanil is >200-fold more selective for MOR than delta- or kappa-opioid receptor,103 and MOR represents ≈90% of the total opioid binding capacity in lumbosacral spinal cord laminae I and II.83 Thus, it is highly likely that the mechanism by which sufentanil facilitates EM2 release in males is mediated predominantly, if not exclusively, by MOR.
Figure 2.
Facilitation of spinal EM2 release in males by sufentanil was MOR-mediated. The opioid antagonist naloxone (NX) did not itself alter EM2 release but prevented facilitation of EM2 release by sufentanil (SUF). Data are expressed as fmol EM2 per 10-min period. The basal rate of EM2 release did not differ significantly among groups (F2,14 = 1.65; p = 0.23). * p < 0.01 for EM2 release vs. basal in males 30–40 min after i.t. administration of sufentanil. n = 4–9.
Influence of Gonadal Sex Hormones on MOR-Mediated Regulation of Spinal EM2 Release
We determined the contribution of testicular testosterone to MOR-mediated facilitation of spinal EM2 release in males by administering i.t. sufentanil to orchiectomized males. Strikingly, facilitation of EM2 release by sufentanil was no longer manifest following orchiectomy (F2,10 = 0.40; p = 0.68, n = 6), indicating that in males, facilitation of EM2 release by spinal MOR depends on activational effects of testicular testosterone. Consistent with this finding, i.t. 1-hour pretreatment with flutamide (60 μg), a highly specific androgen receptor antagonist,68,69,71,84 also abolished the facilitation of EM2 release by sufentanil in males (F2,6 = 0.35; p = 0.72, n = 4).
To assess whether ovarian factors prevent MOR-mediated facilitation of EM2 release in naïve females, we measured the effect of sufentanil on EM2 release in ovariectomized females. One week following ovariectomy, i.t. sufentanil remained unable to enhance spinal EM2 release (F2,4 = 1.62; p = 0.31, n = 3). To ensure that circulating and tissue levels of estrogens were fully depleted, we repeated this experiment 2 weeks post-ovariectomy. This also failed to reveal an effect of sufentanil on EM2 release (F2,6 = 0.18; p = 0.84, n = 4). We further determined whether approximating male levels of circulating testosterone in females would reveal spinal MOR-mediated facilitation of EM2 release. Administration of chronic testosterone to ovariectomized females did not enable an effect of sufentanil on EM2 release (F2,8 = 0.45; p = 0.66, n = 5). Similarly, neonatal androgenization (via systemic testosterone) also failed to enable an effect of sufentanil on EM2 release in females (F2,8 = 0.33; p = 0.73, n = 5), even when followed by chronic testosterone in adulthood (F2,8 = 0.68; p = 0.53, n = 5). Importantly, using the current protocol, we and others have previously demonstrated that neonatal androgenization eliminates the spinal kappa-opioid receptor component of i.t. morphine analgesia59 and eliminates sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray.49 These earlier studies validate the ability of our neonatal androgenization procedure to masculinize opioid responsiveness in females. Thus, the failure of neonatal androgenization to enable sufentanil facilitation of EM2 release was not idiosyncratic to our particular protocol.
Release of Spinal EM2 by I.t. MOR Agonists Is Pharmacologically Relevant to the Antinociception They Produce
To assess the contribution of spinal EM2 release to the antinociception produced by i.t. sufentanil, we investigated whether the analgesic action of i.t. sufentanil (0.7 nmol) could be reduced by neutralizing endogenously released EM2 via i.t. anti-EM2 antibody. Unexpectedly, i.t. anti-EM2 antibody (200–1000 ng) failed to alter the spinal sufentanil antinociception produced by this dose. In order to avoid the potential confound that 0.7 nmol i.t. sufentanil, which produces near maximum analgesia (TFL), masked contributions from spinal EM2, we repeated this experiment using 0.35 nmol sufentanil. This dose is near the ED50 for spinal sufentanil antinociception (unpublished data) and thus should be optimal for revealing hypothesized contributions from spinal EM2. Indeed, i.t. anti-EM2 antibody (330 ng) significantly altered the antinociception produced by 0.35 nmol sufentanil (F1,56 = 7.13; p < 0.05; Fig. 3A). However, paradoxically, anti-EM2 antibody approximately doubled peak TFL, rather than reducing it, as was hypothesized. Given that anti-EM2 antibody augments sufentanil antinociception, it is not surprising that the antibody had no effect on the near-maximal antinociception resulting from i.t. 0.7 nmol sufentanil.
Figure 3.
I.t. application of anti-EM2 antibody revealed agonist-dependent contributions of spinal EM2 to the antinociception produced by either i.t. sufentanil or i.t. morphine in males but not females. Since data obtained in diestrous and proestrous females did not differ, data is shown for only diestrous females. Thirty minutes following the i.t. application of anti-EM2 antibody (330 ng) or its preadsorbed control, i.t. sufentanil (0.35 nmol) or morphine (7.5 nmol) was injected and antinociception quantified at various time points. I.t. application of anti-EM2 antibody failed to alter spinal sufentanil or morphine antinociception in diestrous females (left panels, A and B). In contrast, in males, i.t. anti-EM2 antibody, but not its preadsorbed control, substantially increased spinal antinociception of sufentanil but significantly attenuated the spinal antinociception of morphine (right panels, A and B). Data are expressed as mean ± S.E.M. n = 4–5 and 9–12 for sufentanil- and morphine-treated groups, respectively.
The unanticipated enhancement of spinal sufentanil antinociception by i.t. anti-EM2 antibody could result from the antibody’s ability to effectively remove EM2, which has a much lower efficacy at MOR than sufentanil.1,27 As a partial agonist, EM2 competes with sufentanil for MOR activation. Thus, EM2 plus sufentanil would produce less antinociception than sufentanil itself. We investigated this possibility by determining the effect of i.t. anti-EM2 antibody on the spinal antinociception produced by i.t. morphine. Since the efficacy of morphine is similar to that of EM2 for MOR G protein activation39 (which correlates with antinociceptive efficacy62), eliminating the EM2 released by i.t. morphine (via anti-EM2 antibody) should effectively lower the aggregate concentration of MOR agonist, and therefore reduce spinal morphine antinociception. As predicted, i.t. anti-EM2 antibody significantly reduced the antinociception resulting from i.t. morphine (7.5 nmol), approximately halving peak antinociception (F1,88 = 5.18; p < 0.05; Fig. 3B).
Since i.t. sufentanil does not alter spinal EM2 release in females, i.t. anti-EM2 antibody (200–1000 ng) was not expected to alter their antinociceptive responsiveness to i.t. sufentanil. This expectation was confirmed in both diestrous (F1,42 = 0.001; p = 0.98; Fig. 3A) and proestrous females (F1,32 = 0.027; p = 0.87; data not shown). I.t. anti-EM2 antibody similarly failed to alter i.t. morphine antinociception in either diestrous (F1,68 = 0.22; p = 0.65; Fig. 3B) or proestrous females (F1,40 = 0.85; p = 0.38; data not shown). Thus, the influence of spinal EM2 on the antinociception produced by MOR agonists is not only agonist-dependent but also sexually dimorphic.
Discussion
Major findings of the present study include: (1) activation of spinal MOR augments endogenous spinal EM2 release in males but not females, irrespective of estrous cycle stage; (2) MOR-mediated facilitation of EM2 release in males depends on the activational effects of testicular (circulating) testosterone via spinal androgen receptors; (3) the absence of MOR-mediated facilitation of EM2 release in females does not result from activational or organizational effects of ovarian steroids; (4) activational effects of testosterone are not sufficient for spinal MOR-mediated facilitation of spinal EM2 release to be manifest in females; (5) the EM2 released by i.t. sufentanil or i.t. morphine influences the spinal antinociception produced by these drugs in males but not females.
The validity of formulations derived from these findings is bolstered by an experimental approach that cross-validates behavioral and neurochemical data. Sexually dimorphic recruitment and regulation of spinal MOR/EM2 antinociception underscore that MOR analgesics differentially activate spinal antinociceptive systems in male and female rats. It remains to be determined whether the sex-based ability of MOR agonists to augment EM2 release and the consequences thereof on analgesic responsiveness extend to humans. If so, this could represent a new dimension of sex-based opioid antinociception in men vs. women, thereby enlightening the selection of MOR agonists in a clinical context.
Augmentation of EM2 release by i.t. sufentanil suggests the presence of a positive feedback mechanism in the male spinal cord that could be activated by endogenous EM2 itself. Such a feed-forward mechanism would enhance the ability of males to harness the EM2 system in response to a noxious stimulus, thereby enhancing physiological antinociception. This could contribute to the higher pain tolerance often observed in males vs. females. Conversely, the failure of i.t. sufentanil to release spinal EM2 in females suggests their compromised ability to physiologically utilize the endogenous spinal MOR/EM2 antinociceptive system when confronted by noxious stimuli. This, combined with estrous cycle-dependent fluctuations in EM2-mediated antinociception reported previously,56 could be causally associated with the greater severity and frequency of some chronic pain syndromes in women (e.g., irritable bowel syndrome, interstitial cystitis, generalized pelvic pain), as well as sex differences in the effectiveness of opioid analgesia.28,29 Interestingly, compromised endogenous pain inhibition is associated with the increased pain sensitivity characteristic of irritable bowel syndrome and temporomandibular joint dysfunction.47 Future pharmacological therapies to increase activity of the MOR/EM2 system could prove useful for pain management in women.
The effects of i.t. anti-EM2 antibodies on the spinal antinociception produced by i.t. sufentanil and morphine not only underscore that endogenous spinal EM2 contributes to the antinociceptive action of these drugs, but also reveal that the direction of this contribution is opioid agonist-dependent. The variable nature of the influence of endogenous spinal EM2 on the antinociception produced by i.t. MOR agonists could explain variability among MOR agonists in the sex-dependence of the analgesia they produce. For example, in males, the negative contribution of endogenous spinal EM2 to the analgesia produced by higher efficacy MOR analgesics (e.g., sufentanil) would be expected to mitigate what might otherwise be more robust antinociception in males vs. females. In contrast, sex-dependent differences in spinal analgesic responsiveness to MOR analgesics of comparable efficacy to EM2 should not be obscured by contributions of endogenous spinal EM2. Rather, such contributions should bolster manifestation of sex-dependent differences. This would explain why high efficacy MOR-selective opioid analgesics such as sufentanil, DAMGO [D-Ala2, N-MePhe4, Gly-ol]-enkephalin, and fentanyl produce comparable antinociception in male vs. female rats,4,21,56,65,88 whereas the sexual dimorphism of lower efficacy MOR-preferring analgesics such as hydromorphone and hydrocodone is considerably more robust.74 Notably, Cook et al.16 directly compared sexually dimorphic antinociception among opioids with high vs. low efficacy at MOR, using a consistently employed nociceptive paradigm (rat tail thermal nociception). They definitively established an inverse relationship between agonist efficacy and the magnitude of sexual dimorphism in analgesic responsiveness. Current findings provide a physiological context for understanding their observations. The variable contribution of EM2 to opioid antinociception could also explain, at least in part, why some opioid analgesics are equally effective in men vs. women while others are not.20,28,29
Diffusion of EM2 from spinal tissue into i.t. perfusate would result in a substantial dilution of EM2 content. Thus, the observed sufentanil-induced increment in EM2 released into i.t. perfusate is undoubtedly an underestimate of the magnitude of change in synaptic EM2. However, irrespective of its magnitude, the increment in synaptic EM2 resulting from i.t. sufentanil or morphine is functionally relevant, as evidenced by the ability of endogenous EM2 to influence opioid analgesic responsiveness (Fig. 3). These considerations are underscored by the ability of anti-EM2 antibody to enhance the spinal antinociception produced by 0.35 nmol sufentanil despite the inability of current methods to quantify a corresponding increment in EM2 release.
The current demonstration that i.t. sufentanil augmented spinal EM2 release contrasts with our previous finding that MOR-mediated modulation of neurotransmitter release and intracellular signaling in vitro is bimodal, with stimulatory (Gs-mediated) and inhibitory (Gi-mediated) effects occurring at low and high concentrations of MOR agonist, respectively.10,34,36,95,99 Multiple factors could account for the current ability of a seemingly high dose of i.t. sufentanil to facilitate EM2 release: (1) An i.t. dose that produces maximum analgesia (e.g., 0.7 nmol) does not necessarily produce high synaptic concentrations of sufentanil at spinal MORs, given the need for tissue diffusion. (2) Given that pentobarbital activates/inhibits specific G proteins in some cell types,35,40,78 it is possible that under in vivo conditions and/or the influence of anesthesia, facilitative (Gs-mediated) effects of MOR predominate, such that a high dose of i.t. sufentanil augments EM2 release. (3) A predominance of facilitative vs. inhibitory effects of MOR in vivo could result from the absence of high K+, which was present in in vitro experiments, and which could influence neuronal state-dependent effects of sufentanil.98
Interestingly, in contrast to our previous finding in vitro that naloxone increases EM2 release from spinal tissue of male rats,36 naloxone itself failed to augment in vivo EM2 release. The suggested ongoing negative feedback regulation of spinal EM2 release observed in vitro, but not in vivo, could be a consequence of the higher basal EM2 release in vitro (≈1.3 fmol/min) vs. in vivo (≈0.4 fmol/min), resulting in greater feedback inhibition in vitro and thus a larger effect of naloxone. Differential basal EM2 release in vivo vs. in vitro could result from depressant effects of the anesthetic in the former and the absence of descending inhibition and/or afferent inputs in the latter.
Sex steroids exert long-term “organizational” effects throughout post-natal development (which can be eliminated by gonadal ablation at birth), as well as “activational” effects via systemic circulation (which can be eliminated by either gonadectomy in adulthood or acute blockade of corresponding hormone receptors).37,76 We assessed whether the ability (or inability) of MOR to facilitate EM2 release results from effects of testosterone in males and estrogens in females.
Orchiectomy eliminated the effect of sufentanil in adult males, demonstrating that MOR-mediated facilitation of EM2 release requires activational effects of testosterone. Pretreatment (1 hour) of intact adult males with the androgen receptor antagonist flutamide also eliminated the effect of sufentanil on EM2 release. The rapid onset of effects of blocking spinal androgen receptors indicates that testosterone acts, at least in part, acutely to enable MOR-mediated facilitation of spinal EM2 release in males. The cellular and molecular mechanisms by which testosterone influences spinal EM2 release remain to be elucidated.
In adult females, the failure of MOR activation to alter EM2 release persisted following ovariectomy, and is therefore independent of presumptive restrictive consequences of activational effects of circulating ovarian sex steroids and/or other ovarian factors. Ovariectomy plus chronic testosterone also failed to unmask a facilitative effect of sufentanil on spinal EM2 release, eliminating the possibility that females possess the cellular machinery to augment EM2 release via MOR, but lack the testosterone to mobilize this mechanism. Lastly, androgenization of female pups (via a bolus injection of testosterone resulting in gonadal ablation) followed by chronic testosterone in adulthood also failed to enable sufentanil facilitation of spinal EM2 release in females. Collectively, these results suggest that the molecular and cellular elements required for MOR-mediated facilitation of spinal EM2 release are hard-wired and presumably sex-linked.
The present study demonstrates sexual dimorphism in the mechanisms regulating spinal EM2 release as well as their hormonal activation. In males, testosterone is a permissive factor, enabling MOR-mediated facilitation of spinal EM2 release, whereas in females, spinal MOR is functionally disconnected from spinal EM2 release, independent of both estrogens and testosterone. These findings bolster previous evidence that steroidal regulation of the MOR/EM2 system is sexually dimorphic. For instance, pregnancy levels of progesterone and estradiol activate MOR-coupled antinociception in orchiectomized males but not ovariectomized females,55 and EM2 antinociception is constant in males but fluctuates with circulating estrogen levels (i.e., the estrous cycle) in females.56
Despite the evidence for sexual dimorphism in nociception and opioid antinociception, sex-specific pharmacotherapies for acute and chronic pain remain elusive. One impediment to improving pain management is that sex differences in nociception and opioid antinociception across human and animal studies are often antithetical. The present demonstration of agonist-dependent and sexually dimorphic contributions of endogenous EM2 to spinal opioid antinociception in rats provides a conceptual framework for investigating the biology of sex-based differences in pain and opioid analgesia in humans. This could enlighten the selection of opioid medications for pain management. Furthermore, the current study identifies a compromised functionality in females (attenuated ability to harness spinal EM2 antinociception), which could serve as a novel pharmacological target for pain management in women.
We studied contributions of spinal endomorphin to opioid analgesia in rats.
Mu-opioids enhance spinal endomorphin release in males but not females.
Released endomorphin reduces analgesia of high efficacy mu-opioids.
Findings explain variable sex-dependence of opioid antinociception.
Findings could inform the selection of opioid analgesics in humans.
Footnotes
Disclosures: This study was supported by a grant from the National Institute on Drug Abuse, R01DA027663, to A.R.G. None of the authors hold any conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alt A, Mansour A, Akil H, Medzihradsky F, Traynor JR, Woods JH. Stimulation of guanosine-5′-O-(3-[35S]thio)triphosphate binding by endogenous opioids acting at a cloned mu receptor. J Pharmacol Exp Ther. 1998;286:282–288. [PubMed] [Google Scholar]
- 2.Aubrun F, Salvi N, Coriat P, Riou B. Sex- and age-related differences in morphine requirements for postoperative pain relief. Anesthesiology. 2005;103:156–160. doi: 10.1097/00000542-200507000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Barrett AC, Smith ES, Picker MJ. Sex-related differences in mechanical nociception and antinociception produced by mu- and kappa-opioid receptor agonists in rats. Eur J Pharmacol. 2002;452:163–173. doi: 10.1016/s0014-2999(02)02274-4. [DOI] [PubMed] [Google Scholar]
- 4.Bartok RE, Craft RM. Sex differences in opioid antinociception. J Pharmacol Exp Ther. 1997;282:769–778. [PubMed] [Google Scholar]
- 5.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- 6.Bingefors K, Isacson D. Epidemiology, co-morbidity, and impact on health-related quality of life of self-reported headache and musculoskeletal pain--a gender perspective. Eur J Pain. 2004;8:435–450. doi: 10.1016/j.ejpain.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Boyer JS, Morgan MM, Craft RM. Microinjection of morphine into the rostral ventromedial medulla produces greater antinociception in male compared to female rats. Brain Res. 1998;796:315–318. doi: 10.1016/s0006-8993(98)00353-9. [DOI] [PubMed] [Google Scholar]
- 8.Bragdon EE, Light KC, Costello NL, Sigurdsson A, Bunting S, Bhalang K, Maixner W. Group differences in pain modulation: pain-free women compared to pain-free men and to women with TMD. Pain. 2002;96:227–237. doi: 10.1016/S0304-3959(01)00451-1. [DOI] [PubMed] [Google Scholar]
- 9.Cepeda MS, Carr DB. Women experience more pain and require more morphine than men to achieve a similar degree of analgesia. Anesth Analg. 2003;97:1464–1468. doi: 10.1213/01.ANE.0000080153.36643.83. [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti S, Liu NJ, Zadina JE, Sharma T, Gintzler A. Pleiotropic opioid regulation of spinal endomorphin 2 release and its adaptations to opioid withdrawal are sexually dimorphic. J Pharmacol Exp Ther. 2012;340:56–63. doi: 10.1124/jpet.111.186874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JR, Wang TJ, Lim SH, Wang YJ, Tseng GF. Testosterone modulation of dendritic spines of somatosensory cortical pyramidal neurons. Brain structure & function. 2013;218:1407–1417. doi: 10.1007/s00429-012-0465-7. [DOI] [PubMed] [Google Scholar]
- 12.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Influences of laboratory environment on behavior. Nat Neurosci. 2002;5:1101–1102. doi: 10.1038/nn1102-1101. [DOI] [PubMed] [Google Scholar]
- 13.Cicero TJ, Nock B, Meyer ER. Gender-related differences in the antinociceptive properties of morphine. J Pharmacol Exp Ther. 1996;279:767–773. [PubMed] [Google Scholar]
- 14.Cicero TJ, Nock B, Meyer ER. Sex-related differences in morphine’s antinociceptive activity: relationship to serum and brain morphine concentrations. J Pharmacol Exp Ther. 1997;282:939–944. [PubMed] [Google Scholar]
- 15.Cicero TJ, Nock B, O’Connor L, Meyer ER. Role of steroids in sex differences in morphine-induced analgesia: activational and organizational effects. J Pharmacol Exp Ther. 2002;300:695–701. doi: 10.1124/jpet.300.2.695. [DOI] [PubMed] [Google Scholar]
- 16.Cook CD, Barrett AC, Roach EL, Bowman JR, Picker MJ. Sex-related differences in the antinociceptive effects of opioids: importance of rat genotype, nociceptive stimulus intensity, and efficacy at the mu opioid receptor. Psychopharmacology (Berl) 2000;150:430–442. doi: 10.1007/s002130000453. [DOI] [PubMed] [Google Scholar]
- 17.Cooke JP, Creager MA, Osmundson PJ, Shepherd JT. Sex differences in control of cutaneous blood flow. Circulation. 1990;82:1607–1615. doi: 10.1161/01.cir.82.5.1607. [DOI] [PubMed] [Google Scholar]
- 18.Coyle DE, Sehlhorst CS, Behbehani MM. Intact female rats are more susceptible to the development of tactile allodynia than ovariectomized female rats following partial sciatic nerve ligation (PSNL) Neuroscience Letters. 1996;203:37–40. doi: 10.1016/0304-3940(95)12259-1. [DOI] [PubMed] [Google Scholar]
- 19.Coyle DE, Sehlhorst CS, Mascari C. Female rats are more susceptible to the development of neuropathic pain using the partial sciatic nerve ligation (PSNL) model. Neuroscience Letters. 1995;186:135–138. doi: 10.1016/0304-3940(95)11304-f. [DOI] [PubMed] [Google Scholar]
- 20.Craft RM. Sex differences in opioid analgesia: “from mouse to man”. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Craft RM, Tseng AH, McNiel DM, Furness MS, Rice KC. Receptor-selective antagonism of opioid antinociception in female versus male rats. Behavioural pharmacology. 2001;12:591–602. doi: 10.1097/00008877-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Dahlof C, Linde M. One-year prevalence of migraine in Sweden: a population-based study in adults. Cephalalgia. 2001;21:664–671. doi: 10.1046/j.1468-2982.2001.00218.x. [DOI] [PubMed] [Google Scholar]
- 23.de Castilhos J, Hermel EE, Rasia-Filho AA, Achaval M. Influence of substitutive ovarian steroids in the nuclear and cell body volumes of neurons in the posterodorsal medial amygdala of adult ovariectomized female rats. Neurosci Lett. 2010;469:19–23. doi: 10.1016/j.neulet.2009.11.036. [DOI] [PubMed] [Google Scholar]
- 24.De Cosmo G, Congedo E, Lai C, Primieri P, Dottarelli A, Aceto P. Preoperative psychologic and demographic predictors of pain perception and tramadol consumption using intravenous patient-controlled analgesia. Clin J Pain. 2008;24:399–405. doi: 10.1097/AJP.0b013e3181671a08. [DOI] [PubMed] [Google Scholar]
- 25.Deleu D, Khan MA, Al Shehab TA. Prevalence and clinical characteristics of headache in a rural community in Oman. Headache. 2002;42:963–973. doi: 10.1046/j.1526-4610.2002.02225.x. [DOI] [PubMed] [Google Scholar]
- 26.Dubal DB, Kashon ML, Pettigrew LC, Ren JM, Finklestein SP, Rau SW, Wise PM. Estradiol protects against ischemic injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:1253–1258. doi: 10.1097/00004647-199811000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Emmerson PJ, Clark MJ, Mansour A, Akil H, Woods JH, Medzihradsky F. Characterization of opioid agonist efficacy in a C6 glioma cell line expressing the mu opioid receptor. J Pharmacol Exp Ther. 1996;278:1121–1127. [PubMed] [Google Scholar]
- 28.Fillingim RB, Gear RW. Sex differences in opioid analgesia: clinical and experimental findings. Eur J Pain. 2004;8:413–425. doi: 10.1016/j.ejpain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 31.Fillingim RB, Ness TJ, Glover TL, Campbell CM, Hastie BA, Price DD, Staud R. Morphine responses and experimental pain: sex differences in side effects and cardiovascular responses but not analgesia. J Pain. 2005;6:116–124. doi: 10.1016/j.jpain.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Gagliese L, Gauthier LR, Macpherson AK, Jovellanos M, Chan VW. Correlates of postoperative pain and intravenous patient-controlled analgesia use in younger and older surgical patients. Pain Med. 2008;9:299–314. doi: 10.1111/j.1526-4637.2008.00426.x. [DOI] [PubMed] [Google Scholar]
- 33.Gerdle B, Bjork J, Henriksson C, Bengtsson A. Prevalence of current and chronic pain and their influences upon work and healthcare-seeking: a population study. The Journal of rheumatology. 2004;31:1399–1406. [PubMed] [Google Scholar]
- 34.Gintzler AR, Xu H. Different G proteins mediate the opioid inhibition or enhancement of evoked [5-methionine]enkephalin release. Proc Natl AcadSci (USA) 1991;88:4741–4745. doi: 10.1073/pnas.88.11.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzales JM. Anesthetic barbiturates enhance Gs alpha-dependent cyclic AMP production in S49 mouse lymphoma cells. J Neurochem. 1995;64:2559–2566. doi: 10.1046/j.1471-4159.1995.64062559.x. [DOI] [PubMed] [Google Scholar]
- 36.Gupta DS, vonGizycki H, Gintzler AR. Sex-/ovarian steroid-dependent release of endomorphin 2 from spinal cord. J Pharmacol Exp Ther. 2007;321:635–641. doi: 10.1124/jpet.106.118505. [DOI] [PubMed] [Google Scholar]
- 37.Guyton A, Hall JE. Guyton and Hall Textbook of Medical Physiology. 12. Philadelphia, PA: Saunders; 2010. [Google Scholar]
- 38.Hirasawa M, Hasegawa J, Nishiyama J, Suzuki T. Utilization of PCIA (patient-controlled intravenous analgesia) for postoperative analgesia of spine fusion. The Tokai journal of experimental and clinical medicine. 2003;28:17–20. [PubMed] [Google Scholar]
- 39.Hosohata K, Burkey TH, Alfaro-Lopez J, Varga E, Hruby VJ, Roeske WR, Yamamura HI. Endomorphin-1 and endomorphin-2 are partial agonists at the human mu-opioid receptor. European journal of pharmacology. 1998;346:111–114. doi: 10.1016/s0014-2999(98)00117-4. [DOI] [PubMed] [Google Scholar]
- 40.Humar M, Andriopoulos N, Pischke SE, Loop T, Schmidt R, Hoetzel A, Roesslein M, Pahl HL, Geiger KK, Pannen BH. Inhibition of activator protein 1 by barbiturates is mediated by differential effects on mitogen-activated protein kinases and the small G proteins ras and rac-1. J Pharmacol Exp Ther. 2004;311:1232–1240. doi: 10.1124/jpet.104.071332. [DOI] [PubMed] [Google Scholar]
- 41.Ihlebaek C, Hansson TH, Laerum E, Brage S, Eriksen HR, Holm SH, Svendsrod R, Indahl A. Prevalence of low back pain and sickness absence: a “borderline” study in Norway and Sweden. Scandinavian journal of public health. 2006;34:555–558. doi: 10.1080/14034940600552051. [DOI] [PubMed] [Google Scholar]
- 42.Ji Y, Murphy AZ, Traub RJ. Sex differences in morphine-induced analgesia of visceral pain are supraspinally and peripherally mediated. American journal of physiology Regulatory, integrative and comparative physiology. 2006;291:R307–314. doi: 10.1152/ajpregu.00824.2005. [DOI] [PubMed] [Google Scholar]
- 43.Jinks C, Jordan K, Croft P. Measuring the population impact of knee pain and disability with the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) Pain. 2002;100:55–64. doi: 10.1016/s0304-3959(02)00239-7. [DOI] [PubMed] [Google Scholar]
- 44.Joels CS, Mostafa G, Matthews BD, Kercher KW, Sing RF, Norton HJ, Heniford BT. Factors affecting intravenous analgesic requirements after colectomy. Journal of the American College of Surgeons. 2003;197:780–785. doi: 10.1016/S1072-7515(03)00671-9. [DOI] [PubMed] [Google Scholar]
- 45.Kaiko RF, Wallenstein SL, Rogers AG, Houde RW. Sources of variation in analgesic responses in cancer patients with chronic pain receiving morphine. Pain. 1983;15:191–200. doi: 10.1016/0304-3959(83)90018-0. [DOI] [PubMed] [Google Scholar]
- 46.Kayser V, Berkley KJ, Keita H, Gautron M, Guilbaud G. Estrous and sex variations in vocalization thresholds to hindpaw and tail pressure stimulation in the rat. Brain Res. 1996;742:352–354. doi: 10.1016/s0006-8993(96)01108-0. [DOI] [PubMed] [Google Scholar]
- 47.King CD, Wong F, Currie T, Mauderli AP, Fillingim RB, Riley JL., 3rd Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain. 2009;143:172–178. doi: 10.1016/j.pain.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krzanowska EK, Bodnar RJ. Morphine antinociception elicited from the ventrolateral periaqueductal gray is sensitive to sex and gonadectomy differences in rats. Brain Res. 1999;821:224–230. doi: 10.1016/s0006-8993(98)01364-x. [DOI] [PubMed] [Google Scholar]
- 49.Krzanowska EK, Ogawa S, Pfaff DW, Bodnar RJ. Reversal of sex differences in morphine analgesia elicited from the ventrolateral periaqueductal gray in rats by neonatal hormone manipulations. Brain Res. 2002;929:1–9. doi: 10.1016/s0006-8993(01)03350-9. [DOI] [PubMed] [Google Scholar]
- 50.Lee YC, Wang HP, Chiu HM, Lin CP, Huang SP, Lai YP, Wu MS, Chen MF, Lin JT. Factors determining post-colonoscopy abdominal pain: prospective study of screening colonoscopy in 1000 subjects. Journal of gastroenterology and hepatology. 2006;21:1575–1580. doi: 10.1111/j.1440-1746.2006.04145.x. [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Duckles SP. Influence of gender on vascular reactivity in the rat. J Pharmacol Exp Ther. 1994;268:1426–1431. [PubMed] [Google Scholar]
- 52.Li Z, Krause DN, Doolen S, Duckles SP. Ovariectomy eliminates sex differences in rat tail artery response to adrenergic nerve stimulation. Am J Physiol. 1997;272:H1819–1825. doi: 10.1152/ajpheart.1997.272.4.H1819. [DOI] [PubMed] [Google Scholar]
- 53.Liem MS, van Duyn EB, van der Graaf Y, van Vroonhoven TJ, Coala Trial G. Recurrences after conventional anterior and laparoscopic inguinal hernia repair: a randomized comparison. Annals of surgery. 2003;237:136–141. doi: 10.1097/00000658-200301000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache. 2001;41:646–657. doi: 10.1046/j.1526-4610.2001.041007646.x. [DOI] [PubMed] [Google Scholar]
- 55.Liu NJ, Gintzler AR. Prolonged ovarian sex steroid treatment of male rats produces antinociception: identification of sex-based divergent analgesic mechanisms. Pain. 2000;85:273–281. doi: 10.1016/s0304-3959(99)00278-x. [DOI] [PubMed] [Google Scholar]
- 56.Liu NJ, Gintzler AR. Spinal Endomorphin 2 Antinociception and the Mechanisms That Produce It Are Both Sex- and Stage of Estrus Cycle-Dependent in Rats. J Pain. 2013;14(11):1522–1530. doi: 10.1016/j.jpain.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu NJ, Schnell S, Wessendorf MW, Gintzler AR. Sex, pain and opioids: inter-dependent influences of sex and pain modality on dynorphin-mediated antinociception in rats. J Pharmacol Exp Ther. 2013;344:522–530. doi: 10.1124/jpet.112.199851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu NJ, Schnell SA, Schulz S, Wessendorf MW, Gintzler AR. Regulation of Spinal Dynorphin 1–17 Release by Endogenous Pituitary Adenylyl Cyclase-Activating Polypeptide in the Male Rat: Relevance of Excitation via Disinhibition. J Pharmacol Exp Ther. 2011;336:328–335. doi: 10.1124/jpet.110.173039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu NJ, von Gizycki H, Gintzler AR. Sexually dimorphic recruitment of spinal opioid analgesic pathways by the spinal application of morphine. J Pharmacol Exp Ther. 2007;322:654–660. doi: 10.1124/jpet.107.123620. [DOI] [PubMed] [Google Scholar]
- 60.Loyd DR, Morgan MM, Murphy AZ. Morphine preferentially activates the periaqueductal gray-rostral ventromedial medullary pathway in the male rat: a potential mechanism for sex differences in antinociception. Neuroscience. 2007;147:456–468. doi: 10.1016/j.neuroscience.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loyd DR, Murphy AZ. The neuroanatomy of sexual dimorphism in opioid analgesia. Exp Neurol. 2014 doi: 10.1016/j.expneurol.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Madia PA, Navani DM, Yoburn BC. [(35)S]GTPgammaS binding and opioid tolerance and efficacy in mouse spinal cord. Pharmacology, biochemistry, and behavior. 2012;101:155–165. doi: 10.1016/j.pbb.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 63.Marks HE, Hobbs SH. Changes in stimulus reactivity following gonadectomy in male and female rats of different ages. Physiology & behavior. 1972;8:1113–1119. doi: 10.1016/0031-9384(72)90206-5. [DOI] [PubMed] [Google Scholar]
- 64.Martin-Schild S, Gerall AA, Kastin AJ, Zadina JE. Differential distribution of endomorphin 1- and endomorphin 2-like immunoreactivities in the CNS of the rodent. J Comp Neurol. 1999;405:450–471. [PubMed] [Google Scholar]
- 65.Meyer PJ, Fossum EN, Ingram SL, Morgan MM. Analgesic tolerance to microinjection of the micro-opioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology. 2007;52:1580–1585. doi: 10.1016/j.neuropharm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mogil JS, Chanda ML. The case for the inclusion of female subjects in basic science studies of pain. Pain. 2005;117:1–5. doi: 10.1016/j.pain.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 67.Mogil JS, Sternberg WF, Kest B, Marek P, Liebeskind JC. Sex differences in the antagonism of swim stress-induced analgesia: effects of gonadectomy and estrogen replacement. Pain. 1993;53:17–25. doi: 10.1016/0304-3959(93)90050-Y. [DOI] [PubMed] [Google Scholar]
- 68.Moguilewsky M, Bouton MM. How the study of the biological activities of antiandrogens can be oriented towards the clinic. Journal of steroid biochemistry. 1988;31:699–710. doi: 10.1016/0022-4731(88)90021-0. [DOI] [PubMed] [Google Scholar]
- 69.Nayebi AR, Ahmadiani A. Involvement of the spinal serotonergic system in analgesia produced by castration. Pharmacology, biochemistry, and behavior. 1999;64:467–471. doi: 10.1016/s0091-3057(99)00113-6. [DOI] [PubMed] [Google Scholar]
- 70.Nelson NR, Bird IM, Behan M. Testosterone restores respiratory long term facilitation in old male rats by an aromatase-dependent mechanism. The Journal of physiology. 2011;589:409–421. doi: 10.1113/jphysiol.2010.198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Neri R, Florance K, Koziol P, Van Cleave S. A biological profile of a nonsteroidal antiandrogen, SCH 13521 (4′-nitro-3′ trifluoromethylisobutyranilide) Endocrinology. 1972;91:427–437. doi: 10.1210/endo-91-2-427. [DOI] [PubMed] [Google Scholar]
- 72.Nielsen CS, Stubhaug A, Price DD, Vassend O, Czajkowski N, Harris JR. Individual differences in pain sensitivity: genetic and environmental contributions. Pain. 2008;136:21–29. doi: 10.1016/j.pain.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Peckham EM, Barkley LM, Divin MF, Cicero TJ, Traynor JR. Comparison of the antinociceptive effect of acute morphine in female and male Sprague-Dawley rats using the long-lasting mu-antagonist methocinnamox. Brain Res. 2005;1058:137–147. doi: 10.1016/j.brainres.2005.07.060. [DOI] [PubMed] [Google Scholar]
- 74.Peckham EM, Traynor JR. Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther. 2006;316:1195–1201. doi: 10.1124/jpet.105.094276. [DOI] [PubMed] [Google Scholar]
- 75.Peng X, Knapp BI, Bidlack JM, Neumeyer JL. Pharmacological properties of bivalent ligands containing butorphan linked to nalbuphine, naltrexone, and naloxone at mu, delta, and kappa opioid receptors. J Med Chem. 2007;50:2254–2258. doi: 10.1021/jm061327z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–382. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- 77.Ritter MA, Wing JT, Berend ME, Davis KE, Meding JB. The clinical effect of gender on outcome of total knee arthroplasty. The Journal of arthroplasty. 2008;23:331–336. doi: 10.1016/j.arth.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 78.Robinson-White A. Mechanisms of action of anesthetics on inositol phospholipid hydrolysis in vascular endothelial cells and rat basophilic leukemia cells in tissue culture. Advances in experimental medicine and biology. 1991;301:271–287. doi: 10.1007/978-1-4684-5979-1_25. [DOI] [PubMed] [Google Scholar]
- 79.Rollman GB, Lautenbacher S. Sex differences in musculoskeletal pain. Clin J Pain. 2001;17:20–24. doi: 10.1097/00002508-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 80.Rosseland LA, Solheim N, Stubhaug A. Pain and disability 1 year after knee arthroscopic procedures. Acta anaesthesiologica Scandinavica. 2008;52:332–337. doi: 10.1111/j.1399-6576.2007.01541.x. [DOI] [PubMed] [Google Scholar]
- 81.Sarton E, Olofsen E, Romberg R, den Hartigh J, Kest B, Nieuwenhuijs D, Burm A, Teppema L, Dahan A. Sex differences in morphine analgesia: an experimental study in healthy volunteers. Anesthesiology. 2000;93:1245–1254. doi: 10.1097/00000542-200011000-00018. discussion 1246A. [DOI] [PubMed] [Google Scholar]
- 82.Soignier RD, Vaccarino AL, Brennan AM, Kastin AJ, Zadina JE. Analgesic effects of endomorphin-1 and endomorphin-2 in the formalin test in mice. Life Sci. 2000;67:907–912. doi: 10.1016/s0024-3205(00)00689-5. [DOI] [PubMed] [Google Scholar]
- 83.Stevens CW, Lacey CB, Miller KE, Elde RP, Seybold VS. Biochemical characterization and regional quantification of mu, delta and kappa opioid binding sites in rat spinal cord. Brain Res. 1991;550:77–85. doi: 10.1016/0006-8993(91)90407-m. [DOI] [PubMed] [Google Scholar]
- 84.Sufrin G, Coffey DS. Flutamide. Mechanism of action of a new nonsteroidal antiandrogen. Investigative urology. 1976;13:429–434. [PubMed] [Google Scholar]
- 85.Tague SE, Smith PG. Vitamin D receptor and enzyme expression in dorsal root ganglia of adult female rats: modulation by ovarian hormones. J Chem Neuroanat. 2011;41:1–12. doi: 10.1016/j.jchemneu.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Takeshima T, Ishizaki K, Fukuhara Y, Ijiri T, Kusumi M, Wakutani Y, Mori M, Kawashima M, Kowa H, Adachi Y, Urakami K, Nakashima K. Population-based door-to-door survey of migraine in Japan: the Daisen study. Headache. 2004;44:8–19. doi: 10.1111/j.1526-4610.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 87.Thomas E, Silman AJ, Croft PR, Papageorgiou AC, Jayson MI, Macfarlane GJ. Predicting who develops chronic low back pain in primary care: a prospective study. BMJ (Clinical research ed) 1999;318:1662–1667. doi: 10.1136/bmj.318.7199.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thornton SR, Smith FL. Characterization of neonatal rat fentanyl tolerance and dependence. J Pharmacol Exp Ther. 1997;281:514–521. [PubMed] [Google Scholar]
- 89.Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, Borges GL, Bromet EJ, Demytteneare K, de Girolamo G, de Graaf R, Gureje O, Lepine JP, Haro JM, Levinson D, Oakley Browne MA, Posada-Villa J, Seedat S, Watanabe M. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9:883–891. doi: 10.1016/j.jpain.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 90.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–167. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 91.Verzillo V, Madia PA, Liu NJ, Chakrabarti S, Gintzler AR. Mu-opioid receptor splice variants: sex-dependent regulation by chronic morphine. J Neurochem. 2014;130:790–796. doi: 10.1111/jnc.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vierck CJ, Acosta-Rua AJ, Rossi HL, Neubert JK. Sex differences in thermal pain sensitivity and sympathetic reactivity for two strains of rat. J Pain. 2008;9:739–749. doi: 10.1016/j.jpain.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113:331–339. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 94.Walker JS, Carmody JJ. Experimental pain in healthy human subjects: gender diferences in nociception and in response to ibuprofen. Anesthesia & Analgesia. 1998;86:1257–1262. doi: 10.1097/00000539-199806000-00023. [DOI] [PubMed] [Google Scholar]
- 95.Wang L, Gintzler AR. Bimodal opioid regulation of cAMP formation: implications for positive and negative coupling of opiate receptors to adenylyl cyclase. J Neurochem. 1994;63:1726–1730. doi: 10.1046/j.1471-4159.1994.63051726.x. [DOI] [PubMed] [Google Scholar]
- 96.Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wijnhoven HA, de Vet HC, Picavet HS. Prevalence of musculoskeletal disorders is systematically higher in women than in men. Clin J Pain. 2006;22:717–724. doi: 10.1097/01.ajp.0000210912.95664.53. [DOI] [PubMed] [Google Scholar]
- 98.Xu H, Gintzler AR. Opioid enhancement of evoked [Met5]enkephalin release requires activation of cholinergic receptors: possible involvement of intracellular calcium. Proc Natl Acad Sci USA. 1992;89:1978–1982. doi: 10.1073/pnas.89.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu H, Smolens I, Gintzler AR. Opioids can enhance and inhibit the electrically evoked release of methionine-enkephalin. Brain Research. 1989;504:36–42. doi: 10.1016/0006-8993(89)91594-1. [DOI] [PubMed] [Google Scholar]
- 100.Yunus MB. Gender differences in fibromyalgia and other related syndromes. J Gend Specif Med. 2002;5:42–47. [PubMed] [Google Scholar]
- 101.Zabka AG, Mitchell GS, Behan M. Conversion from testosterone to oestradiol is required to modulate respiratory long-term facilitation in male rats. The Journal of physiology. 2006;576:903–912. doi: 10.1113/jphysiol.2006.114850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zadina JE, Hackler L, Ge LJ, Kastin AJ. A potent and selective endogenous agonist for the mu-opiate receptor [see comments] Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]
- 103.Zhu J, Xue JC, Law PY, Claude PA, Luo LY, Yin J, Chen C, Liu-Chen LY. The region in the mu opioid receptor conferring selectivity for sufentanil over the delta receptor is different from that over the kappa receptor. FEBS Lett. 1996;384:198–202. doi: 10.1016/0014-5793(96)00312-2. [DOI] [PubMed] [Google Scholar]
- 104.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, Meyer CR, Koeppe RA, Stohler CS. mu-opioid receptor-mediated antinociceptive responses differ in men and women. J Neurosci. 2002;22:5100–5107. doi: 10.1523/JNEUROSCI.22-12-05100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]