Abstract
The rodent dorsal medial prefrontal cortex (PFC), specifically the prelimbic cortex (PL), regulates the expression of conditioned fear and behaviors interpreted as reward-seeking. Meanwhile, the ventral medial PFC, namely the infralimbic cortex (IL), is essential to extinction conditioning in both appetitive and aversive domains. Here we review evidence that supports, or refutes, this “PL-go/IL-stop” dichotomy. We focus on the extinction of conditioned fear and the extinction and reinstatement of cocaine- or heroin-reinforced responding. We then synthesize evidence that the PL is essential for developing goal-directed response strategies, while the IL supports habit behavior. Finally, we propose that some functions of the orbital PFC parallel those of the medial PFC in the regulation of response selection. Integration of these discoveries may provide points of intervention for inhibiting untethered drug seeking in drug use disorders, failures in extinction in Post-traumatic Stress Disorder, or co-morbidities between the two.
Keywords: cerebral cortex, striatum, amygdala, operant, cue, orbitofrontal, addiction, review
The inhibition of aberrant fear in Post-traumatic Stress Disorder (PTSD) and drug-seeking behavior in addiction represent major hurdles in treating these conditions. Furthermore, co-morbidities are commonly reported: For example, cocaine use is associated with anxiety, anxiety attacks, and PTSD1, suggestive of common or interactive biological etiologies. A better understanding of the overlapping (and non-overlapping) behavioral, cellular, and molecular mechanisms underlying the successful suppression of reward- and fear-related behaviors may result in novel intervention strategies.
This review begins with a brief overview of the neuroanatomy of the mPFC. We then review evidence that the PL prefrontal cortex serves as a “go” structure, energizing the expression of fear- and reward-related behaviors, while the IL compartment guides “stopping”2. We next attempt to reconcile the “PL-go/IL-stop” model with evidence that the PL is essential to goal-directed decision making, which can include response inhibition, while the IL supports stimulus-response habits. In the interest of scope, we emphasize studies using Pavlovian fear conditioning and cocaine- and food-reinforced conditioning, in particular those using lesions, stimulation, and inactivation (pharmacological, optogenetic, and chemogenetic) techniques in rodents. We also address the reinstatement of heroin-reinforced behaviors, but we note that a large number of significant reports have been neglected due to length restrictions. Accordingly, we aim to complement, rather than replace, excellent recent reviews on these topics3–10.
Part 1. Structures and functions of the mPFC
The mPFC has long been considered part of a mesocorticolimbic system involved in both reward- and threat-related conditioning. For example, the dorsal mPFC is essential for maintaining instrumental responding for food when reinforcement availability is uncertain11,12, while the ventral mPFC is associated with response inhibition following extinction conditioning in both aversive and appetitive domains, as discussed below. The mPFC receives input from the amygdala and hippocampus, particularly the ventral subregion, as well as other limbic structures, positioning it to integrate information regarding salience, value, and contextual cues associated with appetitive and aversive outcomes13–15. The mPFC in turn innervates amygdalar and striatal structures to regulate, for example, motor output.
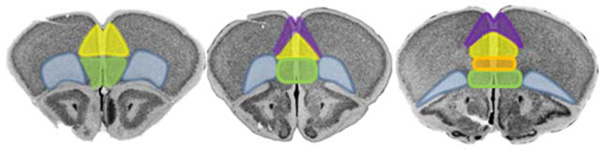
The mPFC can be separated into multiple different subregions including the ACC, PL, IL, and the medial oPFC10 (fig.1), which are differentiated based on efferent and afferent projection patterns. For example, within the ventral striatum, the PL largely innervates the NAC core, while the IL preferentially innervates the shell compartment10. The PL also projects to both the basolateral and lateral nuclei of the amygdala, while the IL targets the basal, central, and medial compartments, as well as the GABAergic intercalated cell masses that separate these regions2,16,17.
Figure 1. Subregions of the rodent prefrontal cortex.
The rodent prefrontal cortex includes the PL (yellow), medial oPFC (green), oPFC (blue; highlighting areas commonly referred to as the ventral and lateral oPFC), anterior cingulate cortex (purple), and IL (orange). Outlines of these regions are transposed onto coronal sections from the Mouse Brain Library126.
These medial wall structures can also be grouped according to their functional outputs and their positioning along the dorsoventral axis, with the dorsomedial PFC containing the ACC and dorsal PL, and a ventral compartment containing the ventral portion of the PL, the IL, and the medial oPFC2. Behaviorally, these dorsal/ventral mPFC networks have been termed “go” and “stop” systems, respectively, that guide behavior2.
PL/IL dichotomies in conditioned fear: Going and stopping
In several domains, the PL and IL exert distinct, sometimes opposing, influences over behavior. Perhaps the most commonly cited example of this phenomenon pertains to the expression and extinction of conditioned fear. In fear conditioning experiments, an innocuous stimulus, such as an auditory tone, is paired with an unconditioned stimulus, such as a foot shock. This once-innocuous stimulus, the conditioned stimulus (CS), takes on fear-eliciting properties, which can be inferred in the rodent by conditioned startle or freezing. The term “conditioned fear” most often refers to conditioned freezing, a defensive response to threat. This term is widely used in the field, but complicated by the implication that the experimenter can infer the subjective state of the animal (fear)18. We will nonetheless use this imperfect phraseology in the present review, in line with current practice.
While the tone-shock association is thought to be stored within the amygdala, the retrieval of this memory and consequent expression of conditioned fear is PL-dependent. For example, PL lesions and inactivation interfere with conditioned fear expression19–24. Additionally, reducing the activity of PL inhibitory interneurons disinhibits PL output to the BLA and accordingly, enhances the expression of conditioned fear25. Optogenetic studies further indicate that the PL is required for conditioned fear expression and have interestingly revealed that the retrieval of “new” fear memory requires PL-BLA interactions, but “old” fear memory requires PL-thalamic interactions26. Thus, fear retrieval circuits shift with time, but the PL remains a key cortical node for conditioned fear expression (fig.2a).
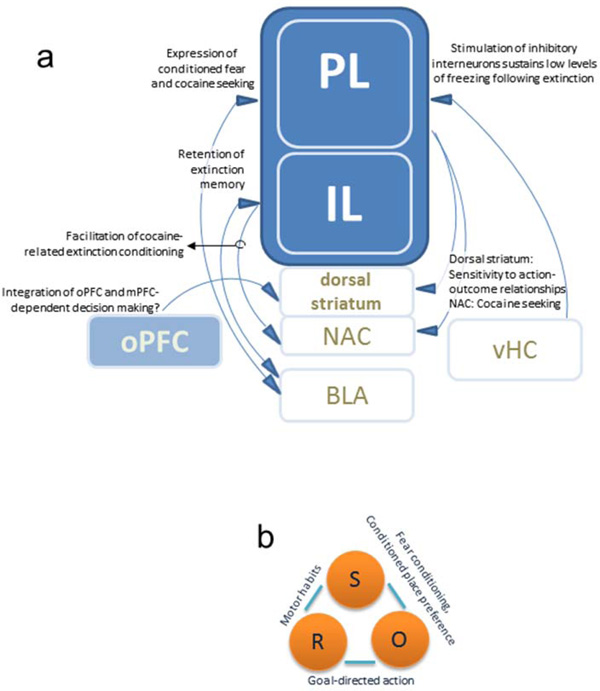
Figure 2. Connections and functions of the PL and IL.
(a) Connections and functions of the PL and IL are discussed in this review. For example, interactions between the PL and BLA support the expression of conditioned fear, as well as the reinstatement of cocaine-reinforced behaviors following extinction. PL innervation of the NAC additionally promotes the reinstatement of cocaine-reinforced responding, and interactions with the dorsal striatum are associated with goal-directed response selection. The dorsal striatum may also be a site of integration of oPFC- and mPFC-dependent learning and memory. Meanwhile, the IL is essential to fear extinction, particularly the retention of extinction memory in both appetitive and aversive domains, as well as habit formation. PL-dependent fear expression can be blunted by vHC innervation of inhibitory interneurons, sustaining low levels of conditioned fear following extinction conditioning. The authors note that this figure highlights the connections and models discussed in this manuscript and do not represent all anatomical connections between these regions. (b) These interactions are essential to several types of conditioning – response-outcome, stimulus-outcome, and stimulus-response. Examples are provided. (R = response; O = outcome; S = stimulus.)
In fear extinction conditioning, repeated presentation of the CS in the absence of foot shock leads to a reduction, or decay, in startle or freezing. This process is thought to reflect new learning rather than memory erasure8,27. Early studies indicated that lesions of the mPFC that included the IL do not impair the initial acquisition of extinction conditioning, but rather, extinction retention, resulting in aberrantly high levels of conditioned fear despite extinction training28,29. This phenomenon has since been replicated using pharmacological and optogenetic inactivation – i.e., IL inactivation during extinction conditioning blocks subsequent extinction memory retrievale.g.,22,30,31 (and see21,23 in which IL inactivation impaired both extinction conditioning and retrieval). Conversely, optogenetic stimulation of the IL in conjunction with extinction conditioning reduces conditioned freezing both during training and also in subsequent retrieval tests, mirroring the effects of electrical stimulation of the IL31;cf.,32,33. Additionally, the retention of extinction memory is dependent on new gene transcription and protein synthesis in the IL34,35.
These and other findings indicate that extinction-induced IL neuroplasticity is necessary for the retention of fear extinction36. This plasticity is rapid, given that IL inactivation immediately following extinction training has failed in some reports to modify subsequent retrieval22,23. In counter-conditioning procedures, conditioned fear can be blunted by the co-presentation of a separate CS that is not paired with an aversive outcome; for example, this CS can be paired with positive reinforcement. In this case, inactivation of the IL, but not PL, blocks the ability of reward-related cues to mitigate conditioned freezing following extinction conditioning24. Early models suggested that the IL facilitates fear extinction by stimulating inhibitory intercalated interneurons in the amygdala to suppress amygdala output; however, this model is evolving with evidence that a key functional target of the IL is instead the BLA (reviewed9) (fig.2a).
Interestingly, whether these general principles translate to avoidance behaviors is unclear. The expression of active avoidance (escaping a foot shock signaled by a CS) can be blocked by reversible inactivation of either the IL or PL, and surprisingly, PL inactivation in this setting can leave conditioned freezing intact37,38. By contrast, IL inactivation increases conditioned freezing even in the absence of extinction training and also blocks extinction retention37,38. In other reports, lesions containing both the PL and ACC interfered with the expression of avoidance, but lesions selective to either structure alone had no effects38,39. In ref.39, lesions selective to the PL were, in contrast, sufficient to reduce conditioned lick suppression, in general accordance with the conditioned fear studies discussed above, and suggesting that the expression of avoidance, specifically, uniquely recruits multiple structures, complicating a simple “PL-go/IL-stop” model.
From fear to reward: Convergence in the PL
As in the context of aversive conditioning, the acquisition, expression, and extinction of “reward-seeking” behaviors can be dissociated in rodentssee,6. For example, in conditioned place preference (CPP) testing, cocaine, for example, is repeatedly paired with a context. Following several pairings, the experimental animal prefers the cocaine-paired context, evidence of knowledge of the context-cocaine association. This stimulus-outcome conditioning parallels classical fear conditioning in that an initially innocuous stimulus, the context, is paired with an experimenter-delivered outcome, cocaine (fig.2b).
In cocaine self-administration studies, mice or rats are placed in conditioning chambers in which they can generate an operant response (e.g., nose poke, lever press, chain pull) reinforced with cocaine. Cocaine is delivered most commonly via intravenous catheter. Often, stimuli such as lights or tones signal delivery. Thus, multiple associations may be formed – that a stimulus predicts an outcome (as in CPP and classical fear conditioning), and that a response produces an outcome (unlike in CPP and classical fear conditioning) (fig.2b). From a translational perspective, an appealing aspect of self-administration paradigms is that laboratory animals will, like humans, volitionally self-administer drugs of abuse. Moreover, relapse-like behavior can be assessed – in this case, the cocaine-reinforced response is first extinguished, or alternatively, the animal may simply undergo forced abstinence. Then, the animal is returned to the drug-associated chamber. The degree to which responding is energized by re-exposure to the drug-associated context, cues, a drug prime, or other stimuli such as stressors models the relapse of drug seeking following abstinence in humans and is termed “reinstatement.”
The mPFC regulates behavioral sensitivity to positive reinforcement. Laboratory animals will self-administer electrical stimulation to the mPFC; cocaine energizes this behavior; and further, stimulation of the mPFC induces CPP, while lesions or inactivation of the mPFC can attenuate cocaine-CPP (reviewed6). A large body of research has also been devoted to identifying factors regulating reinstatement, since understanding this process could elucidate the mechanisms driving relapse in clinical populations. Much of this work has implicated the PL as a critical node in driving reinstatement behavior. For example, lesions or inactivation of the PL generally decrease responding in cocaine (though possibly not mephamphetamine40) reinstatement tests. These include reinstatement elicited by stressors, which can also be blocked by PL-targeted infusions of a dopamine D1 receptor antagonist41,42. PL-targeted infusions of a D1 antagonist also interfere with the reinstatement of heroin-reinforced responding43. PL inactivation reduces behavioral sensitivity to a cocaine prime41,44–47 and cocaine-associated cues48–51, occluding the reinstatement of cocaine-reinforced behaviors. Notably, PL inactivation failed in at least two reports to impact responding when rats were exposed to drug abstinence but not extinction conditioning40,52, suggesting a more nuanced role for PL in the absence of extinction conditioning.
Cocaine, amphetamine, and dopamine infusion into the PL reinstates drug-related responding following extinction conditioning44,53. Moreover, following prolonged withdrawal, cocaine-associated cue presentation preferentially activates PL, and not IL, neurons54. Blockade of new protein synthesis in the PL, but not the dorsally-situated ACC, interferes with cocaine-induced reinstatement following the reconsolidation of a cocaine-related memory55,56. Notably, rats subjected to extinction conditioning or abstinence, and not reconsolidation training (in which a previously consolidated memory is recalled and again consolidated), were unaffected by protein synthesis blockade in one report56. These findings could suggest that the mechanisms driving the reinstatement of cocaine seeking following the reconsolidation of a cocaine-associated memory distinctively involve de novo protein synthesis within the PL.
Cocaine-induced reinstatement augments synaptic glutamate release from PL terminals in the NAC core57,58. Inactivation of PL terminals in the NAC decreases reinstatement and interferes with reinstatement-induced modifications in dendritic spines in the NAC46,59. Meanwhile, inhibition of BLA-PL interactions or BLA projections to the PL also reduces reinstatement50,60, and in fear conditioning contexts, BLA silencing occludes the electrophysiological responsiveness of PL neurons to shock-associated tones61. Thus, PL projections to, and innervation by, the BLA appear to facilitate both conditioned fear expression and cocaine-reinforced responding (fig.2a). Notably, blockade of neuroplasticity in the PL mitigates cocaine-induced reinstatement even in rats considered cocaine-vulnerable, i.e., rats that self-administer at high frequencies62. This may be because high rates of cocaine self-administration are associated with immediate-early gene activation in the mPFC-NAC pathway. Meanwhile, resilience is instead associated with immediate-early gene expression in BLA-NAC pathways62.
Does the IL impact drug seeking?
Relapse is a major challenge in the successful treatment of cocaine abuse, hence a strong focus in the field on the regulatory mechanisms associated with the reinstatement of cocaine-reinforced behaviors. This is as opposed to their extinction. Nonetheless, some investigations suggest that the IL is involved in the extinction of cocaine seeking. Following cocaine-CPP, optogenetic stimulation of the IL enhances, while inhibition occludes, the extinction of place preference63. Stimulation of AMPA or β2-adrenergic receptor systems immediately following extinction training in cocaine self-administering rats enhances the subsequent retrieval of extinction memory64. Meanwhile, inactivation of the IL or β2-adrenergic inhibition interferes with extinction retrieval64, paralleling effects in conditioned fear extinction experiments35. Manipulations delayed by 3 hours have no consequences, suggesting that IL plasticity is essential to the consolidation of extinction conditioning64. Additionally, these manipulations in the PL have no effects, supporting a “PL-go/IL-stop” model.
Importantly, this “go/stop” model does not negate influences of other regulatory structures. The partial NMDA receptor agonist D-Cycloserine enhances the extinction of drug-seeking behavior in response to cocaine-associated cues, but these actions are attributable to activity in the NAC, rather than IL65. Further, the blockade of new protein synthesis in the IL failed in one report to deter extinction conditioning, while inhibition in the subiculum and BLA instead disrupted extinction66. This report utilized very few training sessions, and similarly, IL inactivation did not impact the extinction of a cocaine-reinforced response when delivered in conjunction with a single extinction training session in another report67. These findings are parsimonious with experiments in which single-unit recordings in the IL of fear-conditioned rats revealed sensitivity to “extinguished” CSs only following successful extinction68, and a general model in which extinction-induced IL plasticity is essential for the retention of extinction memory36.
Thus, unlike the PL, the IL does not appear to facilitate responding in reinstatement tests41,69,44,42. Instead, IL stimulation following extinction conditioning enhances extinction retention, mitigating reinstatement67,70. Conversely, IL inactivation following extinction conditioning exaggerates the subsequent reinstatement, as well as the spontaneous recovery, of cocaine-reinforced behaviors67,71. This can be blocked by simultaneous inactivation of the PL, re-establishing response inhibition67. Interestingly, the timing of infusions in this report67 implicates the IL in the retrieval of the extinction memory, whereas the IL may not be necessary for the retrieval of Pavlovian fear extinction memory, only retention31. This report also suggests that PL and IL systems are competitive in some contexts. In another example, mice with selective reduction of the pro-plasticity protein Brain-derived Neurotrophic Factor (BDNF) in the PL have blunted cocaine-CPP and conditioned fear expression, and in the absence of reinforcement, they more rapidly inhibit responding for food than control mice, favoring a presumably IL-dependent extinction strategy72,73.
Interactions between the IL and NAC shell are thought to be necessary for extinction training to inhibit subsequent cocaine reinstatement behavior67 (fig.2a). During a drug-free period following cocaine exposure, silent (immature) synapses linking IL projection neurons to medium spiny neurons in the NAC mature. Interference with this process exaggerates the subsequent reinstatement of cocaine-directed responding, suggesting that synapse maturation in this network facilitates the inhibition of drug-seeking behaviors74.
Despite the studies discussed above, several lines of evidence challenge an “IL-stop” model as it pertains to the reinstatement of drug seeking in general. For example, inactivating a subset of IL neurons stimulated by heroin-associated cues, the IL in general (via muscimol/baclofen infusions), or local cannabinoid receptors reduces, rather than disinhibits, the reinstatement of heroin-reinforced responding75–78. One interpretation may be that the IL promotes the retention of cocaine-extinction memory, but inhibits the retention of heroin-extinction memory. However, blocking GluR2 endocytosis in the IL (but not PL) decreases cue-induced reinstatement of heroin responding79, and IL inactivation induces the expression of heroin-CPP80, suggesting that IL stimulation, by contrast, can mitigate heroin seeking under some circumstances.
In other reports, IL inactivation reduced, rather than exaggerated, the reinstatement of cocaine-45, methamphetamine-81, and nicotine-directed82 responding, and also cocaine-directed responding after abstinence52. The reinstatement of alcohol-directed responding can be either disinhibited or delayed by IL inactivation83,84. Willcocks and McNally84 propose, as an alternative to the “IL-stop” model, that the IL gates sensitivity to extinction-associated contextual cues. In another report, selective inactivation of alcohol-cue-stimulated IL neurons exaggerated reinstatement behaviors, while global inactivation did not83. Thus, a focus on context-extinction associations and cell-type-specific influences may lead to a more nuanced understanding of the regulation of reinstatement by the IL. Novel information could reinforce the convergences between fear- and reward-related regulatory systems highlighted in fig.2a, or alternatively, give rise to models that cannot generalize across appetitive and aversive domains, or across distinct drugs of abuse.
Part 2. Actions, habits, and mPFC structures
Reward-related behaviors can be characterized by the associations that support them. For example, responding directed toward obtaining reinforcement can be maintained by the predictive relationship between the response and expected outcome85 – a process referred to as goal-directed action selection – while a habit system is instead supported by stimulus-response associations (fig.2b). The actual reinforcer plays a reinforcing function in habitual behaviors, serving to strengthen the stimulus-response association, but it is not encoded as a goal. The incentive salience of drugs of abuse on the one hand, and drug-induced biases towards habit-based response strategies on the other, are both considered factors in the development and maintenance of addiction86–90. Given that the PL and IL are key regulators in toggling between goal-directed actions and habits3,7,91, these structures likely regulate both goal-directed and habit-based drug seeking.
The most common way to test whether instrumental responding occurs according to outcome-based (goal-directed) vs. stimulus-response (habit) contingencies is by assessing responding following some alteration in reinforcer value. This can be accomplished by pairing a reinforcer with, for example, lithium chloride-induced malaise. If responding persists despite this devaluation, then responding is independent of outcome value and interpreted as being under the control of a stimulus-response habit. Response inhibition by contrast reflects goal-directed action selection.
Instrumental contingency degradation can also be used to classify response strategies. Here, organisms are typically trained to generate two distinct reinforced responses, such as a nose poke and a lever press. Then, the likelihood that one response will be reinforced is reduced, or “degraded.” Rodents that are sensitive to the predictive relationship between actions and their outcomes – that are goal-directed – will selectively inhibit responding, evidence of knowledge of the response-outcome relationship. By contrast, equivalent engagement of both responses is considered habitual.
The PL regulates reward-related decision making
PL lesions interfere with response-outcome conditioning, resulting in insensitivity to reinforcer value and instrumental contingency degradation92–94,11. The PL appears necessary for encoding the value of reinforcement, but not necessarily expressing this knowledge, given that inactivation following training or reinforcer devaluation leaves behavioral sensitivity to devaluation intact95,96,12.
In recent studies aimed at recapitulating the neurobiological effects of early-life stressor exposure, PFC GABAAα1 expression was chronically reduced via viral-mediated silencing of Gabra1. Gene knockdown decreased synaptic marker expression, and knockdown in the PL interfered with the acquisition, but not extinction, of a cocaine-reinforced response97,98, evidence that, as with food, the healthy PL may encode the incentive value of cocaine. Other studies have reported that large lesions of the mPFC that include the PL increase cocaine-reinforced responding during self-administration acquisition, an apparently contradictory effect99. The ability to selectively manipulate particular genes in discrete brain regions may help to elucidate disease mechanisms in future investigations.
As implied in the prior paragraph, cocaine seeking can be considered goal-directed in nature, for example, sensitive to disruptions in seeking-taking response chains100,101, as well as absence or punishment. Inactivation studies indicate that the PL is essential to the (goal-directed) inhibition of cocaine seeking under these circumstances102–104. Accordingly, PL stimulation can inhibit cocaine-reinforced responding when responding also results in foot shock104. Interestingly, another report indicated that PL-targeted lesions – rather than stimulations – also inhibited cocaine-reinforced responding in response to foot shock105. In this report, lesions extended to the medial oPFC, however. This is relevant because inactivation of the medial oPFC reduces cocaine-reinforced behaviors106, and PL and medial oPFC lesions can have opposite effects in food-reinforced tasks12. Thus, we argue that the PL can be considered a “go” structure, but because PL function is responsive to outcome value, “going” may include the inhibition of seeking behaviors, i.e., when reinforcers lose value.
The IL supports habit behavior – an adaptive function?
Unlike the PL, lesions of the IL in rats maintain goal-directed behavior despite extensive response training that otherwise causes habits93. Furthermore, IL inactivation following extensive response training reinstates goal-directed behavior after habits have formed107. In other words, the IL is essential to the acquisition and expression of habit behavior, and this could presumably include cocaine-reinforced habits. Meanwhile, the IL is also essential to extinction memory retention, as discussed above, resulting in the inhibition of conditioned fear and cocaine-reinforced behaviors.
How might we reconcile these findings? It may be that the IL suppresses behavioral sensitivity to previously-learned associations when this behavioral sensitivity is no longer advantageouscf.,3. For example, in the case of fear conditioning, freezing deprives the animal of valuable opportunities to instead seek food, mates, etc. Thus, when a CS no longer predicts threat, the IL promotes the retention of extinction, allowing for exploratory behavior. In appetitive contexts, habits free attentional resources to attend to other events when a familiar behavior is highly likely to be reinforced. Investigations using T-maze tasks indicate that IL activity is essential for both the acquisition and expression of habit-based response strategies, and even orchestrates toggling back and forth between old and new habits108,109. These findings suggest that the IL actively promotes behaviorally-advantageous response strategies, rather than simply driving habit- or extinction-based behaviors irrespective of context.
Synergies with oPFC function and final considerations
Within the PFC, the oPFC is positioned both ventrally and laterally to the PL and IL (fig.1). It is inter-connected with the mPFC, as well as amygdalar and hippocampal structures, and across species, it receives information from all sensory modalities, a unique property within the PFC10,16,14,110–114. The connectional networks of the oPFC and mPFC are considered distinct, but notably, none of the focal projections of the various PFC subregions are fully segregated within the striatum115. For instance, PL innervation occupies a large territory of dorsal striatum that overlaps significantly with oPFC innervation115. This provides possible points of convergence in PL- and oPFC-dependent decision making.
The oPFC plays a role in determining reinforcer value and integrating available information regarding outcome features, magnitude, and other characteristics in the service of reward-related decision making90,113,116. Although the oPFC is largely associated with stimulus-outcome conditioning (again, fig.2b), oPFC neurons encode both stimulus-outcome and response-outcome associative contingencies in non-human primates117. In rodents, several manipulations of the oPFC can occlude response-outcome conditioning – these include lesions114; CaMKII-driven Gi-coupled Designer Receptors Exclusively Activated by Designer Drugs (DREADDs)118; site-selective knockdown of the plasticity-associated neurotrophin Bdnf118,119, the GABAAα1 receptor subunit97, and Fragile X Mental Retardation Protein (FMRP)120; asymmetric lesions disconnecting the oPFC from the dorsal striatum119; and asymmetric oPFC Bdnf knockdown and BLA lesions118. These findings suggest that some of the functions of the oPFC parallel those of the PL, specifically to support response-outcome learning and memory.
Prolonged exposure to stress hormones causes dendritic spine elimination in the oPFC121. Spine deficiencies are durable, detectable after a time point when spine modifications have recovered in the mPFC. If the oPFC influences mPFC-dependent behavior, the long-term loss of synaptic contacts in this region could conceivably contribute to durable stress-related failures in, for example, response-outcome goal-directed action selection122,123. Further characterization of how (and where) mPFC- and oPFC-dependent learning and memory systems intersect could increase knowledge regarding the long-term consequences of stress, drugs, and trauma on aberrant decision-making processes and mental health.
An additional consideration is that the PFC matures considerably throughout postnatal life, with substantial developmental modifications occurring well into adolescence. For example, vHC innervation of the mPFC develops during adolescence, and disruptions in this process impair the ability of these projections to gate competing excitatory projections from the BLA124. This would be expected to promote fear expression via disinhibition of the PL61, and also cue-induced cocaine seeking, given that stimulation of BLA-PL interactions energizes drug-reinforced behaviors60 (fig.2a). The protracted developmental trajectory of the PFC may also open a window of opportunity for insults, such as cocaine and traumatic stressors, to cross-sensitize125.
Better understanding the circuit-level sequelae, as well as the extracellular and intracellular signaling factors and structural and neurodevelopmental dynamics that determine vulnerability to cocaine, stressors, and trauma may lead to novel approaches to mental illness. Identification of common etiologies between drug- and fear-related disorders may elucidate novel strategies that could treat these illnesses or symptom comorbidities, or that could serve as adjuncts to behavioral intervention therapies. We argue that the “Pl-go/IL-stop” model, while imperfect (for example, unable to fully account for the role of the IL in the reinstatement of drug-seeking or avoidance behaviors), provides a conceptual framework for these future investigations, in which new evidence favoring the model, as well as challenging it, will be instructive.
Box 1. Anatomical abbreviations.
ACC, anterior cingulate cortex; BLA, basolateral amygdala; DLS, dorsolateral striatum; DMS, dorsomedial striatum; IL, infralimbic prefrontal cortex; mPFC, medial prefrontal cortex (a term referring to the structures positioned along the medial wall of the prefrontal cortex including the prelimbic and infralimbic cortices); NAC, nucleus accumbens; oPFC, orbitofrontal prefrontal cortex; PL, prelimbic prefrontal cortex; vHC, ventral hippocampus
Box 2. Does neural remodeling in the PFC correspond with changes in fear-related behaviors?
Dendritic spines are the primary sites of excitatory plasticity in the brain. Do changes in spine density or structure parallel modifications in IL-dependent behaviors? Following fear extinction conditioning, axospinous synapses and dendritic spines in the IL indeed proliferate128. The heads of spines on excitatory IL neurons are thin, suggestive of the proliferation of new, immature spines, which could subsequently stabilize and form synapses.
Failures in fear extinction following stressor exposure are conversely associated with dendrite retraction in the IL and dendritic spine loss in a mouse model of Amyotrophic Lateral Sclerosis129,130, see also131,121 for stress-related failures in extinction and IL neural structure. Further, impairments in fear extinction following early-life antipsychotic treatment are associated with abnormally high PL dendritic spine densities132. In this case, aberrant fear expression may reflect a structural imbalance between PL/IL systems. Accordingly, failures in typical fear expression have been associated with reduced PL dendritic spine density133.
Box 3. Acute cocaine impacts dendritic spines and PL function.
Abundant evidence indicates that cocaine induces dendritic spine proliferation in the mPFC134,135. Although these studies historically focused on the ACC, experiments using in vivo multiphoton imaging suggest that cocaine induces spine proliferation in the PL, and it is most robust following the first exposure to the drug136. Could these rapid changes serve as a neuroanatomical substrate for the encoding of response-outcome associations (e.g., subserving fruitful drug acquisition strategies), or simply disorganize goal-directed response choice? We have previously paired a single cocaine injection with instrumental contingency degradation in mice. Cocaine disrupted new learning regarding the predictive relationship between actions and their consequences, resulting in a deferral to familiar habit-based strategies when mice were subsequently tested drug-free127 (fig.3a–c). Injections delayed by 4 hours had no effects, suggesting that cocaine interfered with the consolidation of new response-outcome memory (fig.3c). In a conceptually similar experiment, pairing an instrumental behavior with cocaine accelerated the development of habitual control over that behavior137. Thus, cocaine seeking can be goal-directed and PL-dependent, but at the same time, cocaine exposure weakens goal-directed response selection strategies, resulting in a bias towards habit-based behavior.
Under typical circumstances, PL interactions with the DMS are thought to support response-outcome conditioning7. By contrast, the DLS regulates habit-based response strategies via interactions with sensorimotor cortex138,139. Methamphetamine and cocaine decrease dendritic spine density, synaptic marker expression, and activity of the cytoskeletal regulatory element cortactin in the DMS, while spines in the DLS proliferate140,127 (fig.3d). These patterns of synaptic marker and dendritic spine changes could contribute to drug-induced biases in response strategies that favor stimulus-dependent habit-based systems [see also141–147 (fig.3e)].
Box 4. IL Dopamine D2: Diverging roles in motor habits and conditioned fear extinction.
Multiple research groups, using multiple techniques, report that a history of repeated exposure to cocaine or amphetamine induces habit-based responding for food when rats or mice are tested drug-free (reviewed box 3 and135). Systemic treatment with dopamine D1 receptor antagonists blocks amphetamine-induced habits, while D2 blockade accelerates habit formation143. These effects might be attributable to IL dopamine receptor systems, given that IL-specific infusion of D1 inhibitors, D2 agonists, or dopamine itself also occlude habit formation148,149, potentially by decreasing the excitability of IL pyramidal neurons3. Interestingly, Mueller et al. reported that the D2 antagonist raclopride blocks, rather than enhances, the retention of fear extinction memory150. With the caveat the limited pharmacological dose ranges have been applied (presumably for feasibility), IL dopamine D2 thus appears to be necessary for extinction-induced IL plasticity on the one hand, but stimulation interferes with IL-dependent habits on the other hand.
Summary.
The prefrontal cortex supports the expression and inhibition of fear- and reward-related behaviors. These dualities are attributable to discrete functional domains making up this brain region, which allow it to stimulate or inhibit behavior, depending on an organism’s experiences. The authors review evidence that supports, or refutes, this “go/stop” function.
Acknowledgments
The authors thank L. Shapiro, A. Allen, L. DePoy, and E. Pitts for valuable feedback and contributions to figures 1 and 3. This work was supported by PHS DA011717, DA027844 (JRT), MH101477, DA034808, and DA036737 (SLG), and the Connecticut Department of Mental Health and Addiction Services (JRT). The Yerkes National Primate Research Center is supported by the Office of Research Infrastructure Programs/OD P51OD011132.
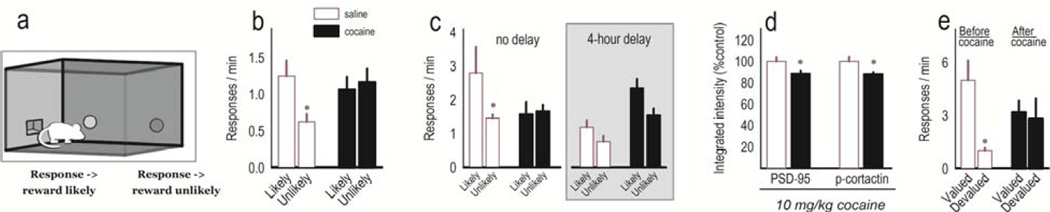
Figure 3. Acute cocaine dysregulates PL-dependent action selection.
(a) In instrumental contingency degradation tasks, mice can generate two responses, here two nose poke responses. Then, the likelihood that one response will be reinforced is greatly reduced. Goal-directed action selection is reflected by preferential performance of the remaining response and is PL-dependent. (b) Acute cocaine delivered in conjunction with contingency degradation training causes habit behavior, indicated by non-selective responding during a subsequent drug-free probe test. (c) If injections are delayed by contrast, response preference is intact, suggesting that cocaine disrupts the consolidation of response-outcome learning and memory. (d) The same behaviorally-active dose of cocaine also decreases PSD-95 and phospho-cortactin, a cytoskeletal regulatory factor, in the DMS, part of a “goal-directed” response network7. (e) Repeated cocaine exposure also induces biases towards habit-based responding. Here, mice were sensitive to reinforcer devaluation prior to cocaine exposure, but insensitive to reinforcer devaluation following repeated daily cocaine exposure for 1 week. Graphs were compiled from127 and unpublished experiments associated with that report. Bars and symbols represent group means+SEMs,*p<0.05.
Footnotes
Author Contributions: SLG and JRT together prepared the manuscript.
Competing Financial Interests Statement: The authors report no conflicts of interest.
References
- 1.Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn. Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidbreder CA, Groenewegen HJ. The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci. Biobehav. Rev. 2003;27:555–579. doi: 10.1016/j.neubiorev.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Barker JM, Taylor JR, Chandler LJ. A unifying model of the role of the infralimbic cortex in extinction and habits. Learn. Mem. 2014;21:441–448. doi: 10.1101/lm.035501.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herry C, Johansen JP. Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci. 2014;17:1644–1654. doi: 10.1038/nn.3869. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald PJ, Seemann JR, Maren S. Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res. Bull. 2014;105:46–60. doi: 10.1016/j.brainresbull.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moorman DE, James MH, McGlinchey EM, Aston-Jones G. Differential roles of medial prefrontal subregions in the regulation of drug seeking. Brain Res. 2014 doi: 10.1016/j.brainres.2014.12.024. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hart G, Leung BK, Balleine BW. Dorsal and ventral streams: the distinct roles of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol. Learn. Mem. 2014;108:104–118. doi: 10.1016/j.nlm.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd TP, Vurbic D, Bouton ME. Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiol. Learn. Mem. 2014;108:52–64. doi: 10.1016/j.nlm.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.180. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 11.Corbit LH, Balleine BW. The role of prelimbic cortex in instrumental conditioning. Behav. Brain Res. 2003;146:145–157. doi: 10.1016/j.bbr.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 12.Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of instrumental action within mouse prefrontal cortex. Eur. J. Neurosci. 2010;32:1726–1734. doi: 10.1111/j.1460-9568.2010.07438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 1991;313:574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 14.McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- 15.Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 16.Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- 17.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 18.LeDoux JE. Coming to terms with fear. Proc. Natl. Acad. Sci. USA. 2014;111:2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum S, Hebert AE, Dash PK. A role for the prefrontal cortex in recall of recent and remove memories. Neuroreport. 2006;17:341–344. doi: 10.1097/01.wnr.0000201509.53750.bc. [DOI] [PubMed] [Google Scholar]
- 20.Corcoran KA, Quirk GJ. Activity in the prelimbic cortex is necessary for the expression of learned, but not innate, fears. J. Neurosci. 2007;37:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn. Mem. 2009;16:520–529. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- 22.Sierra-Mercado D, Jr, Corcoran KA, Lebrón-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur. J. Neurosci. 2006;24:1715–1718. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- 23.Sierra-Mercado D, Padillo-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sangha S, Robinson PD, Greba Q, Davies DA, Howland JG. Alterations in reward, fear and safety cue discrimination after inactivation of the rat prelimbic and infralimbic cortices. Neuropsychopharmacology. 2014;39:2405–2413. doi: 10.1038/npp.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Courtin J, Chaudun F, Rozeske RR, Karalis N, Gonzalez-Campo C, Wurtz H, Abdi A, Baufreton J, Bienvenu TC, Herry C. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature. 2014;505:92–96. doi: 10.1038/nature12755. [DOI] [PubMed] [Google Scholar]
- 26.Do-Monte FH, Quinones-Laracuente K, Quirk GJ. A temporal shift in the circuits mediating retrieval of fear memory. Nature. 2015;519:460–463. doi: 10.1038/nature14030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim. Behav. Process. 1975;1:88–96. [PubMed] [Google Scholar]
- 28.Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci. Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 29.Quirk GJ, Russo GK, Barron JL, Lebron K. The role of the ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgos-Robles A, Videl Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Do-Monte FH, Manzano-Nieves G, Quinones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J. Neurosci. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 2006;13:728–733. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav. Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- 34.Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J. Neurosci. 2008;28:369–375. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bravo-Rivera C, Roman-Ortiz C, Brignoni-Perez E, Sotres-Bayon F, Quirk GJ. Neural structures mediating expression and extinction of platform-mediated avoidance. J. Neurosci. 2014;34:9636–9742. doi: 10.1523/JNEUROSCI.0191-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moscarello JM, LeDoux JE. Active avoidance learning requires prefrontal suppression of amygdala-mediated defensive reactions. J. Neurosci. 2013;33:3815–3823. doi: 10.1523/JNEUROSCI.2596-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joel D, Tarrasch R, Feldon J, Weiner I. Effects of electrolytic lesions of the medial prefrontal cortex or its subfields on 4-arm baited, 8-arm radial maze, two-way active avoidance and conditioned fear tasks in the rat. Brain Res. 1997;765:37–50. doi: 10.1016/s0006-8993(97)00334-x. [DOI] [PubMed] [Google Scholar]
- 40.Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2015;40:1297–1306. doi: 10.1038/npp.2014.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- 42.McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J. Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.See RE. Dopamine D1 receptor antagonism in the prelimbic cortex blocks the reinstatement of heroin-seeking in an animal model of relapse. Int. J. Neuropsychopharmacol. 2009;12:431–436. doi: 10.1017/S1461145709000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vassoler FM, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine reinstatement through local and antidromic activation. J. Neurosci. 2013;33:14446–14454. doi: 10.1523/JNEUROSCI.4804-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefanik MT, et al. Optogenetic inhibition of cocaine seeking in rats. Addict. Biol. 2013;18:50–53. doi: 10.1111/j.1369-1600.2012.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen HW, Gipson CD, Huits M, Kalivas PW. Prelimbic cortex and ventral tegmental area modulate synaptic plasticity differentially in nucleus accumbens during cocaine-reinstated drug seeking. Neuropsychopharmacology. 2014;39:1169–1177. doi: 10.1038/npp.2013.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- 49.Di Pietro NC, Black YD, Kantak KM. Context-dependent prefrontal cortex regulation of cocaine self-administration and reinstatement behaviors in rats. Eur. J. Neurosci. 2006;24:3285–3298. doi: 10.1111/j.1460-9568.2006.05193.x. [DOI] [PubMed] [Google Scholar]
- 50.Mashhoon Y, Wells AM, Kantak KM. Interaction of the rostral basolateral amygdala and prelimbic prefrontal cortex in regulating reinstatement of cocaine-seeking behavior. Pharmacol. Biochem. Behav. 2010;96:347–353. doi: 10.1016/j.pbb.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gipson CD, et al. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koya E, Uejima JL, Wihbey KA, Bossert JM, hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56:177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Park WK, et al. Cocaine administered into the medial prefrontal cortex reinstates cocaine-seeking behavior by increasing AMPA receptor-mediated glutamate transmission in the nucleus accumbens. J. Neurosci. 2002;22:2916–2925. doi: 10.1523/JNEUROSCI.22-07-02916.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.West EA, Saddoris MP, Kerfoot EC, Carelli RM. Prelimbic and infralimbic cortical regions differentially encode cocaine-associated stimuli and cocaine-seeking before and following abstinence. Eur. J. Neurosci. 2014;39:1891–1902. doi: 10.1111/ejn.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramirez DR, Bell GH, Lasseter HC, Xie X, Traina SA, Fuchs RA. Dorsal hippocampal regulation of memory reconsolidation processes that facilitate drug context-induced cocaine-seeking behavior in rats. Eur. J. Neurosci. 2009;30:901–912. doi: 10.1111/j.1460-9568.2009.06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sorg BA, Todd RP, Slaker M, Churchill L. Anisomycin in the medial prefrontal cortex reduces reconsolidation of cocaine-associated memories in the rat self-administration model. Neuropharmacology. 2015;92:25–33. doi: 10.1016/j.neuropharm.2014.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McFarland K, Lapish CC, Kalivas PW. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003;23:3531–3537. doi: 10.1523/JNEUROSCI.23-08-03531.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 59.Stefanik MT, Kupchik YM, Kalivas PW. Optogenetic inhibition of cortical afferents in the nucleus accumbens simultaneously prevents cue-induced synaptic potentiation and cocaine-seeking behavior. Brain Struct. Funct. 2015 doi: 10.1007/s00429-015-0997-8. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stefanik MT, Kalivas PW. Optogenetic dissection of basolateral amygdala projections during cue-induced reinstatement of cocaine-seeking. Front. Behav. Neurosci. 2013;7:213. doi: 10.3389/fnbeh.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ. Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron. 2012;76:804–812. doi: 10.1016/j.neuron.2012.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin-Garcia E, et al. Frequency of cocaine self-administration influences drug seeking in the rat: optogenetic evidence for a role of the prelimbic cortex. Neuropsychopharmacology. 2014;39:2317–2330. doi: 10.1038/npp.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Oever MC, et al. Ventromedial prefrontal cortex pyramidal cells have a temporal dynamic role in recall and extinction of cocaine-associated memory. J. Neurosci. 2013;33:18225–1833. doi: 10.1523/JNEUROSCI.2412-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn. Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Torregrossa MM, Sanchez H, Taylor JR. D-Cycloserine reduces the context specificity of pavlovian extinction of cocaine cues through actions in the nucleus accumbens. J. Neurosci. 2010;30:10526–10533. doi: 10.1523/JNEUROSCI.2523-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Szalay JJ, Jordan CJ, Kantak KM. Neural regulation of the time course for cocaine-cue extinction consolidation in rats. Eur. J. Neurosci. 2013;37:269–277. doi: 10.1111/ejn.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peters J, LaLumiere RT, Kalivas PW. Infralimbic prefrontal cortex is responsible for inhibiting cocaine seeking in extinguished rats. J. Neurosci. 2008;28:6046–6053. doi: 10.1523/JNEUROSCI.1045-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 69.Fuchs RA, et al. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- 70.LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur. J. Neurosci. 2012;35:14–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peters J, Vallone J, Laurendi K, Kalivas PW. Opposing roles for the ventral prefrontal cortex and the basolateral amygdala on the spontaneous recovery of cocaine-seeking in rats. Psychopharmacology. 2008;197:319–326. doi: 10.1007/s00213-007-1034-2. [DOI] [PubMed] [Google Scholar]
- 72.Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl. Psychiatry. 2012;2:e205. doi: 10.1038/tp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR. Prelimbic cortex bdnf knockdown reduces instrumental responding in extinction. Learn. Mem. 2009;16:756–760. doi: 10.1101/lm.1547909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma YY, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;84:1344–1345. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bossert JM, Stern AL, Theberge FR, Cifani C, Koya E, Hope BT, Shaham Y. Ventral medial prefrontal cortex neuronal ensembles mediate context-induced relapse to heroin. Nat. Neurosci. 2011;14:420–422. doi: 10.1038/nn.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bossert JM, Stern AL, Theberge FR, Marchant NJ, Wang HL, Morales M, Shaham Y. Role of projections from ventral medial prefrontal cortex to nucleus accumbens shell in context-induced reinstatement of heroin seeking. J. Neurosci. 2012;32:4982–4991. doi: 10.1523/JNEUROSCI.0005-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rogers JL, Ghee S, See RE. The neural circuitry underlying reinstatement of heroin-seeking in an animal model of relapse. Neuroscience. 2008;151:579–588. doi: 10.1016/j.neuroscience.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alvarez-Jaimes L, Polis I, Parsons LH. Attenuation of cue-induced heroin-seeking behavior by cannabinoid CB1 antagonist infusions into the nucleus accumbens core and prefrontal cortex, but not basolateral amygdala. Neuropsychopharmacology. 2008;33:2483–2493. doi: 10.1038/sj.npp.1301630. [DOI] [PubMed] [Google Scholar]
- 79.van den Oever MC, et al. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat. Neurosci. 2008;11:1053–1058. doi: 10.1038/nn.2165. [DOI] [PubMed] [Google Scholar]
- 80.Ovari J, Leri F. Inactivation of the ventromedial prefrontal cortex mimics re-emergence of heroin seeking caused by heroin reconditioning. Neurosci. Lett. 2008;444:52–55. doi: 10.1016/j.neulet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 81.Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur. J. Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lubbers BR, van Mourik Y, Schetters D, Smit AB, De Vries TJ, Spijker S. Prefrontal gamma-aminobutyric acid type A receptor insertion controls cue-induced relapse to nicotine seeking. Biol. Psychiatry. 2014;76:750–758. doi: 10.1016/j.biopsych.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Pfarr S, et al. Losing control: Excessive alcohol seeking after selective inactivation of cue-responsive neurons in the infralimbic cortex. J. Neurosci. 2015;35:10750–10761. doi: 10.1523/JNEUROSCI.0684-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Willcocks AL, McNally GP. The role of medial prefrontal cortex in extinction and reinstatement of alcohol-seeking in rats. Eur. J. Neurosci. 2013;37:259–268. doi: 10.1111/ejn.12031. [DOI] [PubMed] [Google Scholar]
- 85.Dickinson A. Contemporary Animal Learning Theory. Cambridge Univ. Press; 1980. [Google Scholar]
- 86.Robinson TE, Berridge KC. Addiction. Annu. Rev. Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 87.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 88.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat. Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 89.Torregrossa MM, Corlett PR, Taylor JR. Aberrant learning and memory in addiction. Neurobiol. Learn. Mem. 2011;96:609–623. doi: 10.1016/j.nlm.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lucantonio F, Caprioli D, Schoenbaum G. Transition from ‘model-based’ to ‘model-free’ behavioral control in addiction: involvement of the orbitofrontal cortex and dorsolateral striatum. Neuropharmacology. 2014;76:407–415. doi: 10.1016/j.neuropharm.2013.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Balleine BW, O’Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Balleine BW, Dickinson A. Goal-directed instrumental action: contingency and incentive learning and their cortical substrates. Neuropharmacology. 1998;37:407–419. doi: 10.1016/s0028-3908(98)00033-1. [DOI] [PubMed] [Google Scholar]
- 93.Killcross S, Coutureau E. Coordination of actions and habits in the medial prefrontal cortex of rats. Cereb. Cortex. 2003;13:400–408. doi: 10.1093/cercor/13.4.400. [DOI] [PubMed] [Google Scholar]
- 94.Dutech A, Coutureau E, Marchand AR. A reinforcement learning approach to instrumental contingency degradation in rats. J. Physiol. Paris. 2011;105:36–44. doi: 10.1016/j.jphysparis.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 95.Ostlund SB, Balleine BW. Lesions of medial prefrontal cortex disrupt the acquisition but not the expression of goal-directed learning. J. Neurosci. 2005;25:7763–7770. doi: 10.1523/JNEUROSCI.1921-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tran-tu-Yen DA, Marchand AR, Pape JR, Di Scala G, Coutureau E. Transient role of the rat prelimbic cortex in goal-directed behaviour. Eur. J. Neurosci. 2009;30:464–471. doi: 10.1111/j.1460-9568.2009.06834.x. [DOI] [PubMed] [Google Scholar]
- 97.Swanson AM, Allen AG, Shapiro LP, Gourley SL. GABAAα1-mediated plasticity in the orbitofrontal cortex regulates context-dependent action selection. Neuropsychopharmacology. 2015;40:1027–1036. doi: 10.1038/npp.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Butkovich LM, et al. Adolescent-onset GABAAα1 silencing regulates reward-related decision-making. Eur. J. Neurosci. 2015;42:2114–2121. doi: 10.1111/ejn.12995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Weissenborn R, Robbins TW, Everitt BJ. Effects of medial prefrontal or anterior cingulate cortex lesions on responding for cocaine under fixed-ratio and second-order schedules of reinforcement in rats. Psychopharmacology. 1997;134:242–257. doi: 10.1007/s002130050447. [DOI] [PubMed] [Google Scholar]
- 100.Olmstead MC, Lafond MV, Everitt BJ, Dickinson A. Cocaine seeking by rats is a goal-directed action. Behav. Neurosci. 2001;115:394–402. [PubMed] [Google Scholar]
- 101.Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J. Neurosci. 2010;30:15467–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Limpens JH, Damsteegt R, Broekhoven MH, Voorn P, Vanderschuren LJ. Pharmacological inactivation of the prelimbic cortex emulates compulsive reward seeking in rats. Brain Res. 2014 doi: 10.1016/j.brainres.2014.10.045. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 103.Mihindou C, Guillem K, Navailles S, Vouillac C, Ahmed SH. Discriminative inhibitory control of cocaine seeking involves the prelimbic prefrontal cortex. Biol. Psychiatry. 2013;73:271–279. doi: 10.1016/j.biopsych.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 104.Chen BT, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 105.Pelloux Y, Murray JE, Everitt BJ. Differential roles of the prefrontal cortical subregions and basolateral amygdala in compulsive cocaine seeking and relapse after voluntary abstinence in rats. Eur. J. Neurosci. 2013 doi: 10.1111/ejn.12289. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fuchs RA, Evans KA, Parker MP, See RE. Differential involvement of the orbitofrontal cortex subregions in conditioned cue-induced and cocaine-primed reinstatement of cocaine seeking in rats. J. Neurosci. 2004;24:6600–6610. doi: 10.1523/JNEUROSCI.1924-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coutureau E, Killcross S. Inactivation of the infralimbic prefrontal cortex reinstates goal-directed responding in over-trained rats. Behav. Brain Res. 2003;146:167–174. doi: 10.1016/j.bbr.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 108.Smith KS, Virkud A, Deisseroth K, Graybiel AM. Reversible online control of habitual behavior by optogenetic perturbation of medial prefrontal cortex. Proc. Natl. Acad. Sci. 2012;109:18932–18937. doi: 10.1073/pnas.1216264109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Smith KS, Graybiel AM. A dual operator view of habitual behavior reflecting cortical and striatal dynamics. Neuron. 2013;79:361–374. doi: 10.1016/j.neuron.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morecraft RJ, Geula C, Mesulam MM. Cytoarchitecture and neural afferents of orbitofrontal cortex in the brain of the monkey. J. Comp. Neurol. 1992;323:341–358. doi: 10.1002/cne.903230304. [DOI] [PubMed] [Google Scholar]
- 111.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J. Comp. Neurol. 1996;371:179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 112.Schilman EA, Uylings HB, Galis-de Graaf Y, Joel D, Groenewegen HJ. The orbital cortex in rats topographically projects to central parts of the caudate-putamen complex. Neurosci. Lett. 2008;432:40–45. doi: 10.1016/j.neulet.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 113.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat. Neurosci. 2012;15:13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gremel CM, Costa RM. Orbitofrontal and striatal circuits dynamically encode the shift between goal-directed and habitual action. Nat. Commun. 2013;4:2264. doi: 10.1038/ncomms3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mailly P, Aliane V, Groenewegen HJ, Haber SN, Deniau J-M. The rat prefrontostriatal system analyzed in 3D: Evidence for multiple interacting functional units. J. Neurosci. 2013;33:5718–5727. doi: 10.1523/JNEUROSCI.5248-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rudebeck PH, Murray EA. The orbitofrontal oracle: cortical mechanisms for the prediction and evaluation of specific behavioral outcomes. Neuron. 2014;84:1143–1156. doi: 10.1016/j.neuron.2014.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luk C-H, Wallis JD. Choice coding in frontal cortex during stimulus-guided or action-guided decision-making. J. Neurosci. 2013;33:1864–1871. doi: 10.1523/JNEUROSCI.4920-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zimmermann KS, Yamin JA, Rainnie DG, Kessler KJ, Gourley SL. Connections of the mouse orbitofrontal cortex and regulation of goal-directed action selection by BDNF-trkB. Biol Psychiatry. doi: 10.1016/j.biopsych.2015.10.026. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gourley SL, et al. The orbitofrontal cortex regulates outcome-based decision-making via the lateral striatum. Eur. J. Neurosci. 2013;38:2382–2388. doi: 10.1111/ejn.12239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gross C, Raj N, Molinar G, Allen AG, Whyte AJ, Gibson JR, Huber KM, Gourley SL, Bassell GJ. Selective role of the catalytic PI3K subunit p110beta in impaired higher order cognition in fragile x syndrome. Cell Rep. 2015;11:681–688. doi: 10.1016/j.celrep.2015.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gourley SL, Swanson AM, Koleske AJ. Corticosteroid-induced neural remodeling predicts behavioral vulnerability and resilience. J. Neurosci. 2013;33:3107–3112. doi: 10.1523/JNEUROSCI.2138-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerqueira JJ, Costa RM, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- 123.Gourley SL, et al. Action control is mediated by prefrontal BDNF and glucocorticoid receptor binding. Proc. Natl. Acad. Sci. 2012;109:20714–20719. doi: 10.1073/pnas.1208342109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Thomases DR, Cass DK, Meyer JD, Caballero A, Tseng KY. Early adolescent MK-801 exposure impairs the maturation of ventral hippocampal control of basolateral amygdala drive in the adult prefrontal cortex. J. Neurosci. 2014;34:9059–9066. doi: 10.1523/JNEUROSCI.1395-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology. 2014;231:1557–1580. doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rosen G, et al. The mouse brain library @ www.Mbl.Org . International Mouse Genome Conference. 2000;14:166. [Google Scholar]
- 127.Gourley SL, Olevska, Gordon J, Taylor JR. Cytoskeletal determinants of stimulus-response habits. J. Neurosci. 2013;33:11811–11816. doi: 10.1523/JNEUROSCI.1034-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vetere G, et al. Extinction partially reverts structural changes associated with remote fear memory. Learn. Mem. 2011;18:554–557. doi: 10.1101/lm.2246711. [DOI] [PubMed] [Google Scholar]
- 129.Izquierdo A, Wellman CL, Holmes A. Brief uncontrollable stress causes dendritic retraction in infralimbic cortex and resistance to fear extinction in mice. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sgobio C, et al. Abnormal medial prefrontal cortex connectivity and defective fear extinction in the presymptomatic G93A SOD1 mouse model of ALS. Genes Brain Behav. 2008;7:427–434. doi: 10.1111/j.1601-183X.2007.00367.x. [DOI] [PubMed] [Google Scholar]
- 131.Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–716. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Milstein JA, Einabawi A, Vinish M, Swanson T, Enos JK, Bailey AM, Kolb B, Frost DO. Olanzapine treatment of adolescent rats causes enduring specific memory impairments and alters cortical development and function. PLoS One. 2013;8:e57308. doi: 10.1371/journal.pone.0057308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Iafrati J, et al. Reelin, an extracellular matrix protein linked to early onset psychiatric diseases, drives postnatal development of the prefrontal cortex via GluN2B–NMDARs and the mTOR pathway. Mol. Psychiatry. 2014;19:417–426. doi: 10.1038/mp.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kolb B, Muhammad A. Harnessing the power of neuroplasticity for intervention. Front. Hum. Neurosci. 2014 doi: 10.3389/fnhum.2014.00377. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.DePoy LM, Gourley SL. Synaptic cytoskeletal plasticity in the prefrontal cortex following psychostimulant exposure. Traffic. 2015;16:919–940. doi: 10.1111/tra.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Munoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat. Neurosci. 2013;16:1367–1369. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schmitzer-Torbert N, Apostolidis S, Amoa R, O’Rear C, Kaster M, Stowers J, Ritz R. Post-training cocaine administration facilitates habit learning and requires the infralimbic cortex and dorsolateral striatum. Neurobiol. Learn Mem. 2015;118:105–112. doi: 10.1016/j.nlm.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yin HH, Ostlund SB, Balleine BW. Reward-guided learning beyond dopamine in the nucleus accumbens: the integrative functions of cortico-basal ganglia networks. Eur. J. Neurosci. 2008;28:1437–1448. doi: 10.1111/j.1460-9568.2008.06422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yin HH, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jedynak JP, Uslaner JM, Esteban JA, Robinson TE. Methamphetamine-induced structural plasticity in the dorsal striatum. Eur. J. Neurosci. 2007;25:847–853. doi: 10.1111/j.1460-9568.2007.05316.x. [DOI] [PubMed] [Google Scholar]
- 141.Schoenbaum G, Setlow B. Cocaine makes actions insensitive to outcomes but not extinction: implications for altered orbitofrontal-amygdalar function. Cereb. Cortex. 2005;15:1162–1169. doi: 10.1093/cercor/bhh216. [DOI] [PubMed] [Google Scholar]
- 142.Nelson A, Killcross S. Amphetamine exposure enhances habit formation. J. Neurosci. 2006;26:3805–3812. doi: 10.1523/JNEUROSCI.4305-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nelson A, Killcross S. Accelerated habit formation following amphetamine exposure is reversed by D1, but enhanced by D2, receptor antagonists. Front. Neurosci. 2013;7:76. doi: 10.3389/fnins.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nordquist RE, Voorn P, de Mooji-van Malsen JG, Joosten RN, Pennartz CM, Vanderschuren LJ. Augmented reinforcer value and accelerated habit formation after repeated amphetamine exposure. Eur. Neuropsychopharmacol. 2007;17:532–540. doi: 10.1016/j.euroneuro.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 145.LeBlanc KH, Maidment NT, Ostlund SB. Repeated cocaine exposure facilitates the expression of incentive motivation and induces habitual control in rats. PLoS One. 2013;8:e61355. doi: 10.1371/journal.pone.0061355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hinton EA, Wheeler MG, Gourley SL. Early-life cocaine interferes with BDNF-mediated plasticity. Learn. Mem. 2014;21:253–257. doi: 10.1101/lm.033290.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Corbit LH, Chieng BC, Balleine BW. Effects of repeated cocaine exposure on habit learning and reversal by N-acetylcysteine. Neuropsychopharmacology. 2014;39:1894–1901. doi: 10.1038/npp.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hitchcott PK, Quinn JJ, Taylor JR. Bidirectional modulation of goal-directed actions by prefrontal cortical dopamine. Cereb. Cortex. 2007;17:2820–2827. doi: 10.1093/cercor/bhm010. [DOI] [PubMed] [Google Scholar]
- 149.Barker JM, Torregrossa MM, Taylor JR. Bidirectional modulation of infralimbic dopamine D1 and D2 receptor activity regulates flexible reward seeking. Front. Neurosci. 2013;7:126. doi: 10.3389/fnins.2013.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol. Psychiatry. 2010;68:1055–1060. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]