ABSTRACT
Long synthetic peptides and CpG-containing oligodeoxynucleotides are promising components for cancer vaccines. In this phase I trial, 19 patients received a mean of 8 (range 1–12) monthly vaccines s.c. composed of the long synthetic NY-ESO-179–108 peptide and CpG-B (PF-3512676), emulsified in Montanide ISA-51. In 18/18 evaluable patients, vaccination induced antigen-specific CD8+ and CD4+ T-cell and antibody responses, starting early after initiation of immunotherapy and lasting at least one year. The T-cells responded antigen-specifically, with strong secretion of IFNγ and TNFα, irrespective of patients' HLAs. The most immunogenic regions of the vaccine peptide were NY-ESO-189–102 for CD8+ and NY-ESO-183–99 for CD4+ T-cells. We discovered a novel and highly immunogenic epitope (HLA-DR7/NY-ESO-187–99); 7/7 HLA-DR7+ patients generated strong CD4+ T-cell responses, as detected directly ex vivo with fluorescent multimers. Thus, vaccination with the long synthetic NY-ESO-179–108 peptide combined with the strong immune adjuvant CpG-B induced integrated, robust and functional CD8+ and CD4+ T-cell responses in melanoma patients, supporting the further development of this immunotherapeutic approach.
KEYWORDS: CpG-B, HLA-DR7, long synthetic peptide, malignant melanoma, NY-ESO-1
Introduction
Over the past years, several vaccines consisting of exactly fitting MHC class-I binding peptides have been evaluated for therapeutic efficacy in different cancer types.1 Although induction of specific CD8+ T-cell responses was observed,2-4 absence of concomitant CD4+ T-cell activation may have been a reason that clinical benefit remained minimal. In agreement with this notion, inclusion of MHC class-II peptides in the vaccine formulation showed superior CD8+ T-cell responses in both pre-clinical models and clinical trials.5 More recently, the use of long synthetic peptides (LSPs) harboring both CTL and T helper epitopes has demonstrated induction of strong immune responses.6,7 The additional advantages of LSPs are that they need professional APCs for efficient MHC I epitope presentation, and that their use is not limited to patients with defined HLA molecules.8 In various Phase I/II clinical trials using LSPs, we and others showed potent CD8+ and CD4+ T-cell responses in patients with different solid cancers, as well as in pre-malignant lesions as vulvar intraepithelial neoplasia.9-14
The cancer germ line antigen New York esophageal squamous cell carcinoma-1 (NY-ESO-1) was discovered in 1997,15 and the NY-ESO-1 protein is aberrantly overexpressed in malignant transformed cells of different histological types.15 During the past years, it has emerged as a potential target for cancer immunotherapy since it is highly immunogenic and includes both humoral and T-cell epitopes.16 Interestingly, among all currently known NY-ESO-1 T-cell epitopes, approximately half of them are present within the region 80–111,17-20 making it an attractive protein stretch to be used for patient's immunization using LSPs.
Synthetic oligodeoxynucleotides (ODNs) containing unmethylated CpG (CpG–ODNs) are TLR-9 agonists. Class B CpG–ODNs directly induce activation and maturation of plasmacytoid dendritic cells and promote B cells differentiation.21 Various results in mice demonstrated the improvement of therapeutic responses of DC-based vaccines, short and long peptide immunizations and protein vaccines with this adjuvant.22 Similarly, short peptide-based clinical trials that included CpG-B in melanoma patients showed the generation of a stronger and more rapid Melan-A-specific CD8+ T-cell response compared to the vaccine alone.3 In another vaccination trial with recombinant NY-ESO-1 protein supplemented with CpG-B and Montanide, results showed a significant augmentation of tumor-specific antibodies as well as the detection of NY-ESO-1-specific CD8+ T-cells.23
To date, no clinical trial evaluated vaccination with CpG-B in combination with LSPs. Some recent studies assessed the safety and in vivo immunogenicity of synthetic overlapping long NY-ESO-1 peptides in combination with diverse adjuvants. In an initial study, 91% of patients in the cohort receiving the vaccine supplemented with the TLR-3 agonist Poly-ICLC showed T-cell responses, as compared to the modest specific T-cell induction in the absence of Poly-ICLC. The cellular response correlated in these patients with an acceleration of seroconversion and a significant increase in specific antibody titers.14 Similar results were obtained by Tsuji et al., who characterized NY-ESO-1–specific vaccine-induced CD4+ T-cell lines to investigate the effect of both Montanide and Poly-ICLC adjuvants.24 While Montanide promoted a Th2 polarization and an expansion of high avidity vaccine induced CD4+ T-cells through a better protein recognition, the addition of Poly-ICLC abrogated IL-4, IL-13, and IL-10 secretion, resulting in a more prominent Th1 polarization. As comparison, only half of the patients had CD8+ T-cell responses when vaccinated with full NY-ESO-1 protein supplemented with Montanide and CpG-B (CpG-7909/ PF-3512676).23
In this study, we evaluated safety and immunogenicity of the combination of the 30 amino acid LSP NY-ESO-179–108, administered in combination with CpG-B (CpG-7909/ PF-3512676) and Montanide ISA-51 subcutaneously, accompanied or not by low dose interleukin-2, in patients with advanced malignant melanoma.
Results
Patients' characteristics
In this clinical trial, 19 patients with resected cutaneous melanoma of stage III or IV were enrolled in 2 groups, as summarized in Table 1 and Tables S1 and 2 [1.1; number labeling based on the MIATA checklist is indicated in square brackets throughout the manuscript]. Ten patients were in group A (without IL-2) and nine patients in group B (with IL-2). Six (60%) and seven (78%) patients from groups A and B, respectively, discontinued the study treatment prior to completion of the three vaccination cycles (Table 1 and Tables SI and II), mainly due to disease progression. Patient LAU 1408 received only one vaccine and was thus not evaluable for immune response. The treatment schedule is shown in Fig. 1.
Table 1.
Patients' characteristics.
| Tumor outcome |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study vaccination groups | Patient ID | Sex (F/M) | Age at study entry | AJCC tumor staging at study entry | Vaccines received | Study entry | Study end | Best response | Disease-free survival (months) | Overal survival (months) | Discontinuation reason |
| Group A (no IL-2) | |||||||||||
| LAU 986 | M | 35 | IIIA | 12 | NED | NED | NED | 63.8 | 63.8 | ||
| LAU 205 | M | 36 | IV | 2 | ED | PD | PD | 73.6 | 8.5 | PD | |
| LAU 331 | M | 45 | IV | 18 | NED | NED | NED | 80.5 | 80.5 | ||
| LAU 518 | M | 65 | IV | 9 | ED | PD | PD | 5.8 | 33.1 | PD | |
| LAU 1280 | M | 59 | IIIB | 8 | NED | PD | NED | 9.4 | 11.4 | PD | |
| LAU 1330 | M | 61 | IIIC | 8 | NED | PD | PD | 3.5 | 11.3 | PD | |
| LAU 1293 | F | 65 | IV | 12 | NED | NED | NED | 49.9 | 49.9 | ||
| LAU 1286 | F | 31 | IIIC | 10 | NED | NED | NED | 71.6 | 71.6 | Patient's choice | |
| LAU 1352 | M | 65 | IIIC | 12 | NED | PD | NED | 14.0 | 24.5 | ||
| LAU 1350 | M | 61 | IV | 6 | ED | PD | PD | 3.9 | 34.6 | PD | |
| all patients | 2/8 | 60 (31–65) | 10 (2–18) | 32.0 | 33.8 | ||||||
| Group B (IL-2) | |||||||||||
| LAU 1357 | M | 58 | IIIB | 12 + IL-2 | NED | NED | NED | 6.7 | 68.4 | ||
| LAU 1397 | F | 36 | IIIC | 8 + IL-2 | NED | PD | NED | 8.7 | 61.2 | PD | |
| LAU 1408 | M | 64 | IIIB | 1 | NED | PD | PD | 1.1 | 2.2 | Degraded health status | |
| LAU 1415 | M | 46 | IV | 5 + IL-2 | NED | PD | NED | 5.1 | 12.4 | PD | |
| LAU 1417 | M | 67 | IIIA | 8 + IL-2 | NED | PD | NED | 8.0 | 55.9 | PD | |
| LAU 466 | F | 65 | IV | 2 + IL-2 | NED | NED | NED | 41.3 | 41.3 | Patient's choice | |
| LAU 1394 | M | 64 | IIIC | 4 + IL-2 | NED | PD | PD | 8.8 | 56.8 | PD | |
| LAU 1402 | F | 59 | IIIA | 4 + IL-2 | NED | NED | NED | 49.1 | 49.1 | Patient's choice | |
| LAU 1504 | M | 37 | IV | 12 | NED | NED | NED | 20.2 | 20.2 | ||
| all patients | 3/6 | 59 (36–67) | 6 (1–12) | 8.7 | 49.1 | ||||||
| All groups | |||||||||||
| all patients | 5/146 | 59 (31–67) | 3-IIIA | 8 (2–18) | 16- ED | 8 - NED | 13-NED | 9.4 | 41.3 | ||
| 3-IIIB | 3-ED | ||||||||||
| 5-IIIC | 0-PD | 11-D | 6-PD | ||||||||
| 8-IV | |||||||||||
Abbreviations: AJCC, American Joint Committee on Cancer; NED, no evidence of disease; ED, evidence of disease; PD, progressive disease.
The patient was rendered tumor-free by resection of a left axillary metastasis, after V6.
Figure 1.
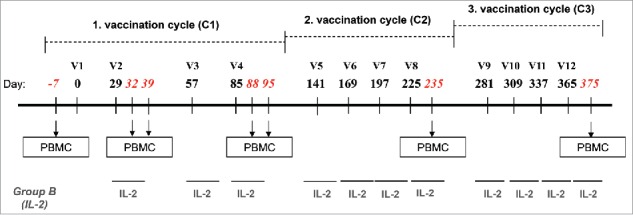
Study design. Vaccinations (V) consisted of three cycles (C1–C3) of four monthly subcutaneous (s.c.) injections of 0.5 mg of NY-ESO-179–108 long peptide. HLA-A2+ patients were also vaccinated with 0.1 mg of Melan-A26–35 native peptide and 20 μg of Mage-A10254–262 peptide in the first cycle, followed by 0.1 mg Melan-A26–35(A27L) analog peptide and 0.1 mg of Mage-A10254–262 peptide in the following cycles. In addition, Group B patients were treated with low dose rh-IL-2. Peptides for HLA-A2+ patients were emulsified in 1 mL Montanide® ISA-51 and 2 mg CpG-7909/PF-3512676, peptides for HLA-A2− patients were emulsified in 0.5 mL Montanide® ISA-51 and 1 mg CpG-7909/PF-3512676. The three vaccines of the cycle 3 were formulated without Montanide. Blood samples were withdrawn and PBMC were prepared at baseline (100 mL), after two vaccinations (two samples at 7 d interval: 30 and 100 mL) and after four vaccinations (two samples at 7 d interval: 30 and 100 mL) for the assessment of immune responses.
Expression of Melan-A, and NY-ESO-1/LAGE-1, and MAGE-A/MAGE-A10 was assessed for each patient. Table 1 shows TAA expression as assessed either by immunohistochemical analysis or qPCR depending on material availability [1.1]. For IHC detection of MAGE-A expression, we used clone 6C1 specific for MAGE-A1/A2/A3/A4/A6/A10 and A12.25 Unfortunately, there is no MAGE-A10 mono-specific antibody available.
Safety and tolerability
The vaccine was generally well tolerated. Among all patients enrolled in groups A and B there were a total of two events of Grade 3 (10%) for those who were definitely, probably and possibly related to the study treatment, while no Grade 4 (life-threatening) or Grade 5 (death) adverse events were observed during the study (Table S3).
The most commonly reported adverse events were general disorders and conditions of mild intensity mainly represented by injection site reactions (rash/erythema, skin induration, pain, and warmth) and systemic reactions (chills, myalgia, arthralgia, asthenia, and headache). There were no severe adverse events related to the study drugs.
As expected, the low dose IL-2 treatment (group B) induced frequent side effects, with inflammatory reactions at s.c. injection sites, and systemic effects (chills, fever, asthenia, headache, arthralgia, myalgia, nausea, diarrhea, and insomnia). Many patients required IL-2 dose reductions and/or stopped IL-2 treatment prematurely. Instead of the 45 intended injections for each patient on average, patients received 33 injections on average, resulting in an average of 73.8% of the intended cumulated dose (Table S4).
Monitoring of NY-ESO-1-specific CD8+ T-cell responses
A total of 18 patients were analyzed for immune responses by intracellular cytokine staining, separately for CD8+ and CD4+ T-cells. As shown in representative flow cytometry examples in Fig. 2A and 3A, significant responses were observed both in CD8+ and CD4+ T-cells. Baseline frequencies of NY-ESO-1-specific CD8+ T-cells were undetectable or very low in the majority of the patients (Figs. 2B and C, Fig. S2A and B). However, six patients showed significant NY-ESO-1-specific natural responses, reaching frequencies of IFNγ and TNFα positive cells of 1.23% of total CD8+ T-cells before vaccination (Figs. 2B–D and Fig. 4). All patients mounted significant responses upon vaccination, as shown by the patient's individual longitudinal curves (Fig. 2B, summary in Figs. 2C, D and 4). Frequencies of cytokine+ CD8+ T-cells readily increased after 2–4 immunizations in the majority of the patients. Total cytokine+ cells reached high levels, accounting for one third of total CD8+ T-cells (total cytokine+ CD8+ T-cells ranging from 0.05 to 33.1%). Some patients displayed a more delayed kinetics and showed initial responses only during cycle 2. Specific CD8+ T-cell responses were sustained, as assessed by cytokine measurement during the third cycle of immunization (Fig. 2D, Fig. S2C). No significant differences in CD8+ T-cell responses were observed when Group A and Group B patients were compared.
Figure 2.
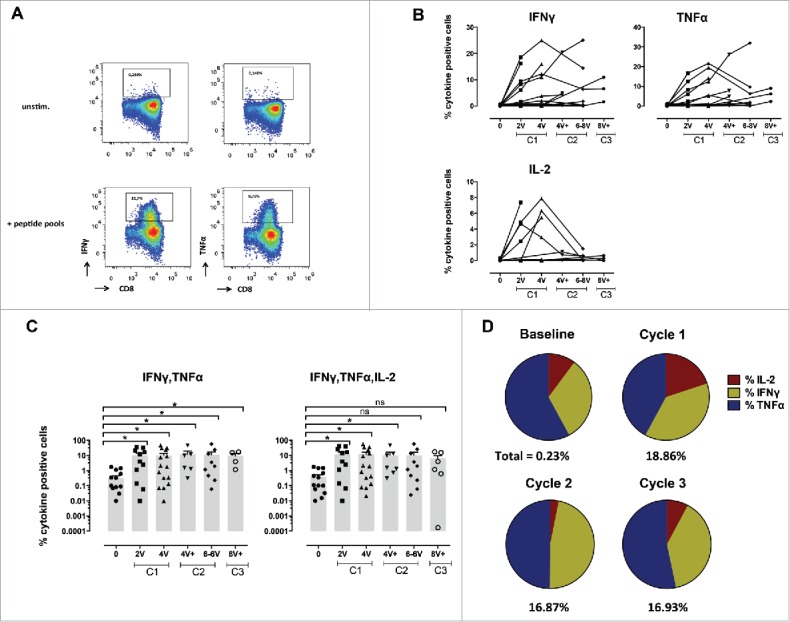
Specific CD8+ T-cell responses before and after vaccination with NY-ESO-1 LSP. (A) Representative example of a NY-ESO-1-specific CD8+ T-cell response 14 d after IVS. Cytokine secreting cells are enumerated after 6-hchallenging of the expanded cells with the NY-ESO-1 pool of overlapping peptides, or without any peptide as control. (B) Details of longitudinal NY-ESO-1-specific CD8+ T-cell responses (IFNγ, TNFα, and IL-2) measured individually in each patient before and during vaccination. (C) Polyfunctionality of NY-ESO-1-specific CD8+ T-cell responses assessed as IFNγ+TNFα+ or IFNγ+TNFα+IL-2+ cells, measured individually in each patient before and during vaccination. (D) Quantification of the contribution of each individual cytokine (IFNγ, TNFα, and IL-2) to the NY-ESO-1-specific CD8+ T-cell response, before and during vaccination. The mean of the response for each cytokine is shown for all patients grouped as % of the total response (that is defined as 100%). The magnitude (mean for all patients grouped) of the total response at each time point is indicated on the bottom of each pie.
Figure 3.
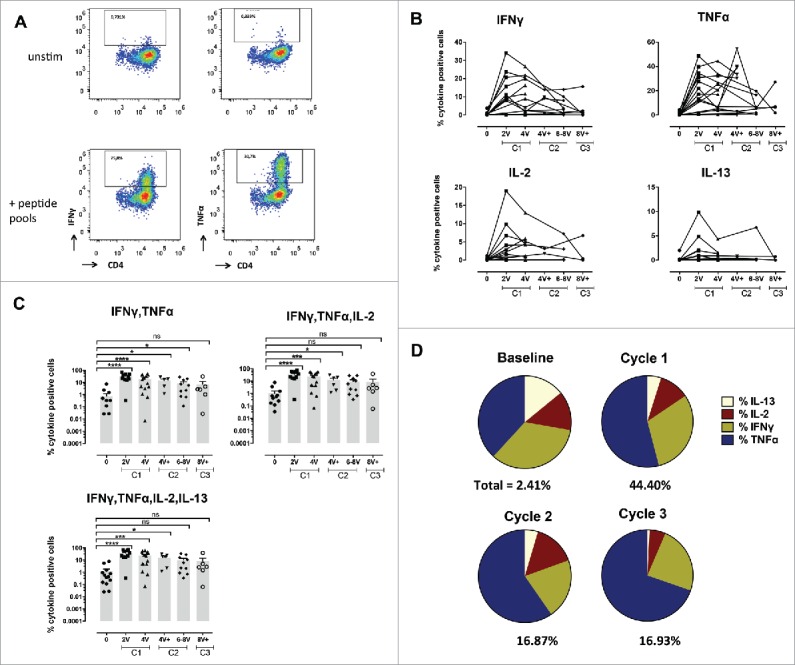
Specific CD4+ T-cell responses before and after vaccination with NY-ESO-1 LSP. (A) Representative example of a NY-ESO-1-specific CD4+ T-cell response 14 d after IVS. Cytokine secreting cells are enumerated after 6-h challenging of the expanded cells with the NY-ESO-1 pool of overlapping peptides, or without any peptide as control. (B) Details of longitudinal NY-ESO-1-specific CD4+ T-cell responses (IFNγ, TNFα, IL2, and IL-13) measured individually in each patient before and during vaccination. (C) Polyfunctionality of NY-ESO-1-specific CD4+ T-cell responses assessed as IFNγ+TNFα+, or IFNγ+TNFα+IL-2+, or IFNγ+TNFα+IL-2+IL-13+ cells, measured individually in each patient before and during vaccination. (D) Quantification of the contribution of each individual cytokine (IFNγ, TNFα, IL-13, and IL-2) to the NY-ESO-1-specific CD4+ T-cell responses, before and during vaccination. The mean of the response for each cytokine is shown for all patients grouped as % of the total response (that is defined as 100%). The magnitude (mean for all patients grouped) of the total response at each time point is indicated on the bottom of each pie.
Figure 4.
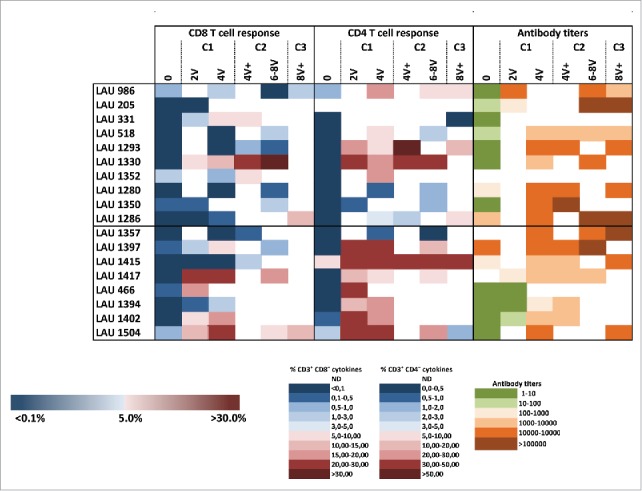
Summary of NY-ESO-1-specific CD8+ and CD4+ T-cell responses, and antibody responses. Cellular responses were measured 14 d after IVS and humoral responses were analyzed by ELISA against the NY-ESO-1 protein in plasma collected from enrolled patients' pre- treatment and during treatment as indicated.
Monitoring of NY-ESO-1-specific CD4+ T-cell responses and specific antibody responses
Similar to CD8+ T-cell responses (Fig. 2), 4 out of 18 patients already showed baseline detectable frequencies of IVS specific CD4+ T-cells (ranging from 0.1–5.77%). Nevertheless, in all patients NY-ESO-1-specific CD4+ T-cells were highly significantly increased by immunization (Figs. 3B–D, Figs. S3A and B). Responses were observed as early as after two vaccinations, and reached 70% of specific cells after IVS. They were long-lasting, since high frequencies of cytokine+ cells were detectable one year after the initiation of the trial (Fig. 3D, Fig. S3C). In general, CD4+ T-cell expansions occurred earlier than the ones of CD8+ T-cells, suggesting that CD4+ T-cell help might be necessary for specific CD8+ T-cell induction (Fig. 4). Responses with higher magnitude were detected at earlier time points in Group B, however long-term no difference was observed between the two groups. Importantly, not only Type I, but also Type II cytokines were detectable, arguing for the capacity of the vaccine to also impact on T-cell polarization. Type II cytokines might also have contributed to the generation of specific antibodies. We measured a significant increase in NY-ESO-1-specific antibodies in the majority of the patients, with also a discrete increase of antibodies specific for other tumor antigens, in particular Melan-A, arguing for induction of antigen spreading (Fig. 4 and Fig. S4).
Determination of immunodominant regions for CD8+ and CD4+ T-cells, and identification of a novel HLA-DR7 specific epitope
Results using individual overlapping peptides showed that the a.a. regions 90–102 and 87–99 were the most immunogenic sequences for CD8+ and CD4+ T-cells, respectively (Fig. 5A). However, the analysis of fine specificity of recognition using the set of overlapping nonapeptides revealed individual patterns (Fig. S5A), and suggests that each patient focuses CD8+ T-cell responses on distinct portions of the LSP. EC50 of peptide recognition for specific CD8+ T-cells ranged from micro- to nanomolar concentrations (Fig. S5B). Using HLA-B35 multimers loaded with NY-ESO-194–104, we identified specific CD8+ T-cells in HLA-B35 patients not only after IVS, but also directly ex vivo (Fig. 5B). For CD4+ T-cells, the contribution of individual MHC class II was evaluated using blocking antibodies and HLA class II typing (Table S1). In 8/9 patients that could be included in these analyses, we observed a partial or complete abrogation of NY-ESO-1-specific CD4+ T-cell responses in the presence of pan-HLA-DR blocking antibodies (Fig. 5C). In in vitro peptide competition assays, we identified the peptide NY-ESO-187–99 as a strong binder to HLA-DR7 (data not shown). We generated DR7/NY-ESO-187–99 multimers and stained IVS cultures from the seven HLA-DR7+ patients included in our study. We identified specific cells in 7/7 HLA-DR7+ patients. As shown in a representative example in Fig. 5D and as summarized in Table 2 multimer+ cells accounted for a large proportion of the overall response induced by vaccination. Interestingly, in all seven HLA-DR7+ patients, multimer+ cells could be detected in samples collected before immunization. Their frequency significantly increased during time and was maintained until completion of the trial. Notably, in one patient that was previously recruited in another vaccination trial consisting of MAGE-A1 immunizations, high baseline DR7/NY-ESO-187–99 multimer+ cells were observed (e.g., 19.6%). This data suggest that natural CD4+ T-cell responses to the novel NY-ESO-1 epitope might have been induced in this patient by antigen spreading upon vaccination with MAGE-A1 peptide.
Figure 5.
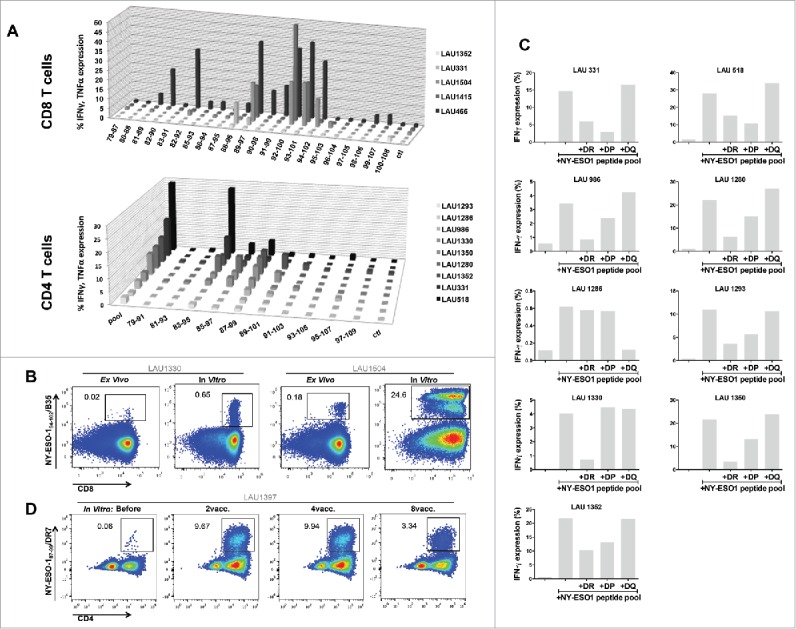
Mapping of NY-ESO-1-specific CD8+ and CD4+ T-cell responses. (A) Using individual overlapping peptides covering the entire NY-ESO-1 LSP sequence, NY-ESO-1-specific CD8+ T-cell responses (n = 5 patients) and CD4+ T-cell responses (n = 9 patients) were mapped, by monitoring IFNγ + TNFα (CD8+ T-cells) and IFNγ (CD4+ T-cells) production after 6-h peptide challenge. (B) Representative example of NY-ESO-194–104/B35 multimer staining directly ex vivo and after IVS of CD8+ T-cells from HLA-B35+ patients. (C) MHC class II restriction of NY-ESO-1-specific CD4+ T-cell responses was assessed in a 6-h peptide challenge in the absence or presence of blocking anti-DR, -DP, or -DQ antibodies. Specific responses were measured by quantification of IFNγ production. (D) Representative example of NY-ESO-187–99/DR7 multimer staining of IVS CD4+ T-cells obtained from HLA-DR7+ patients, before and during immunization.
Table 2.
Summary of frequencies of NY-ESO-1/DR7-specific CD4+ T-cells, detected after one round of IVS in HLA-DR7+ patients.
| Patient ID | Before | 2vacc. | 4vacc. | 6vacc. | 8vacc. | 12vacc. |
|---|---|---|---|---|---|---|
| LAU 331 | 19.60 | ND | ND | 6.50 | 1.38 | 6.74 |
| LAU 1293 | 2.94 | 2.52 | 5.00 | ND | ND | ND |
| LAU 1352 | 1.72 | ND | 1.54 | ND | 4.91 | 8.62 |
| LAU 1350 | 0.13 | 0.26 | ND | 6.80 | ND | ND |
| LAU 1357 | 0.18 | 0.55 | ND | 1.41 | 1.44 | ND |
| LAU 1397 | 0.08 | 9.67 | 9.94 | ND | 3.35 | ND |
| LAU 466 | 0.10 | 3.69 | ND | ND | ND | ND |
Notes: ND, not done.
Polyfunctionality and cytolytic activity of HLA-DR7/NY-ESO-187–99-specific CD4+ T-cell clones
We generated HLA-DR7/NY-ESO-187–99-specific CD4+ T-cell clones and lines from four HLA-DR7+ patients (Fig. 6A, upper panel). By functional characterization we defined the peptide 87–99 as the minimal epitope inducing maximal responses in 15/19 of the clones (data not shown). Clones responded by secreting both Th1 and Th2-prototypic cytokines, albeit with a different EC50 (Fig. 6A, lower panel). Then, we assessed whether DR7/NY-ESO-187–99-specific cells are able to recognize the newly identified NY-ESO-1 epitope when presented by tumor cells. We used HLA-DR7+/− melanoma cell lines, pre-treated or not with IFNγ, and co-cultured them with specific CD4 T-cell clones. We observed that specific clones secreted significant amounts of cytokines in response to NY-ESO-1/DR7 peptide presented by tumor cells (Fig. 6B, left panel). We also assessed whether NY-ESO-187–99 CD4+ T-cell clones could directly kill target cells. When co-cultured with three different HLA-DR7+ melanoma cell lines, displaying either endogenous or IFNγ-induced MHC II expression, the clones induced significant tumor cell lysis of the HLA-DR7+ tumor cells, pulsed with the specific peptide (Fig. 6B, right panel). As expected, HLA-DR7− tumor cells were not susceptible to killing.
Figure 6.
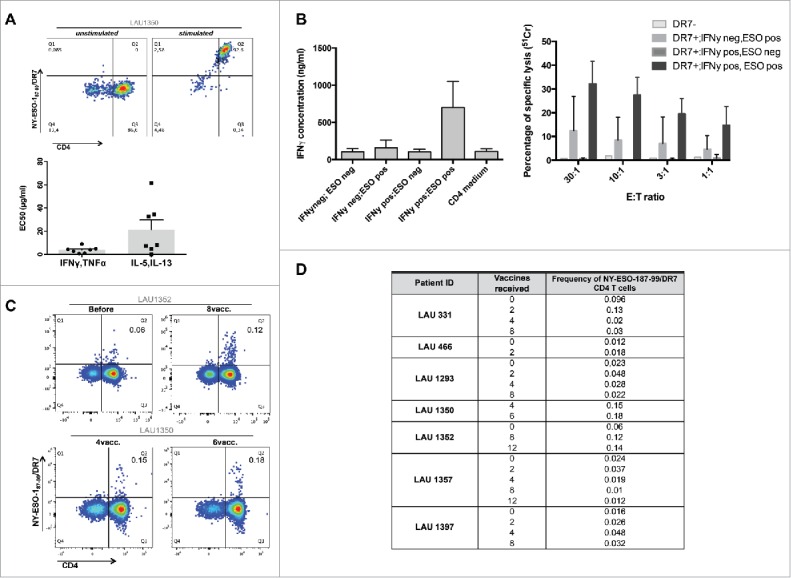
NY-ESO-187–99 peptide represents a novel MHC class II epitope. A. NY-ESO-187–99-specific CD4+ T-cell clones were generated and stained with NY-ESO-1/DR7 multimers (upper panels). Reactivity to specific peptide was tested and EC50 was calculated for both Type1 and Type2 cytokines (lower panels). (B) NY-ESO-187–99-specific-CD4+ T-cell clones were assessed for their capacity to secrete IFNγ or kill HLA-DR7+ target T-cells, in the presence or absence of specific peptide. (C) Representative example of direct ex vivo multimer staining of NY-ESO-187–99-specific-CD4+ T-cells in HLA-DR7+ patients. (D) Summary of frequencies of direct ex vivo detectable NY-ESO-187–99-specific-CD4+ T-cells in HLA-DR7+ patients.
Direct ex vivo visualization of HLA-DR7/NY-ESO-187–99-specific CD4+ T-cells
Finally, we performed multicolor flow cytometry analyses directly ex vivo (without prior in vitro T-cell expansion) from HLA-DR7+ patients. Remarkably, in 7/7 patients we were able to detect multimer+ cells without prior in vitro stimulation (representative examples in Fig. 6C, summary in Fig. 6D). Their frequencies varied between 0.01 and 0.18% of total CD4+ T-cells, and their phenotype corresponded to antigen-experienced, memory cells (data not shown).
Follow-up and clinical observations
The median follow-up time was 63.8 mo for group A (ranging from 8.5 to 80.5 mo) and 55.9 mo for group B (ranging from 2.2 to 68.4 mo) at the time of analysis (December 8th, 2015). Overall, the median follow-up time was 56.8 mo (with a range from 2.2 to 80.5 mo). At the last follow-up, 12 patients were alive (five of group A and seven of group B), whereas seven patients died (five of group A and two of group B) due to progressive disease. Eight patients (four patients of each of the two groups) remained without evidence of disease (Table 1). Patients with above median levels of IFNγ+ NY-ESO-1-specific CD4+ T-cells showed tendencies for longer overall and progression-free survival than patients with below median levels, but the differences were not statistically significant (Fig. 7). These clinical results are relatively favorable, but not conclusive as expected for a phase I study.
Figure 7.
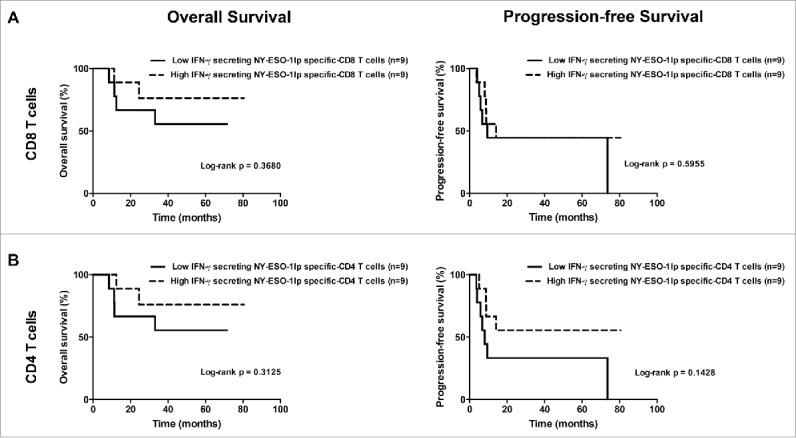
Overall survival and progression-free survival depending on the maximal level of IFNγ+ NY-ESO-1-specific CD8+ T-cell (A) and CD4+ T-cell (B) frequencies reached during the study after IVS. (A). Overall survival (left panel) and progression-free survival (right panel) in patients with low frequencies of IFNγ+ NY-ESO-1-specific CD8+ T-cells (lower than the median, n = 9) and in patients with high frequencies of IFNγ+ NY-ESO-1-specific CD8+ T-cells (higher than the median, n = 9). (B) Overall survival (left panel) and progression-free survival (right panel) in patients with low frequencies of IFNγ+ NY-ESO-1-specific CD4+ T-cells (lower than the median, n = 9) and in patients with high frequencies of IFNγ+ NY-ESO-1-specific CD4+ T-cells (higher than the median, n = 9).
Discussion
Montanide in combination with TLR3 agonists and LSPs has been shown to elicit both humoral and cellular responses in cancer patients.14,24 Here, we assessed for the first time the combination of Montanide, CpG-B, and LSP in advanced melanoma patients. We report on the induction of strong and long-lasting polyspecific CD8+ and CD4+ T-cell responses, with T-cells able to recognize and kill tumor cells, and the generation of specific antibodies. By in-depth characterization of the induced CD4+ T-cell responses, we identified a novel, immunodominant HLA-DR7 restricted NY-ESO-1 epitope, that triggers CD4+ T-cells detectable directly ex vivo in all evaluable patients. Hence, combination of LSP and CpG-B represents an attractive immunotherapy strategy in cancer patients, beyond virus-driven tumors.
DNA-containing CG repeats, mimicked by CpG-ODN, ligate TLR9 and induce production of TNFα (CpG-B) or IFNα (CpG-A). Thus, CpG-ODN are considered the most advanced danger signals for the development of adjuvants for immunotherapy. CpG-A ODNs spontaneously assembly in nanoparticles26 and have been shown to induce tumor specific CD8+ T-cell responses in VLP based vaccines.27 Yet, current evidence suggests that CpG-B might be superior, as previously reported by us and others in adjuvanted short peptide- and protein-based trials.2,3, 23,28-30
Here, we show that the combination of CpG-B and a 30-amino acid long peptide is safe and well tolerated by the majority of the patients. Short peptide-based vaccines have the disadvantage to be limited for use in selected cohorts of HLA-compatible patients, and harbor the potential hazard to directly bind to MHC molecules and induce tolerance.31 In contrast, full protein-based vaccines depend on processing for epitope generation, lead to a broad spectrum of T-cell epitopes and induce antibody responses; however, they are expensive, might lead to the generation of poorly immunogenic epitopes, and are often suboptimal in inducing CD8+ T-cell responses.32 Therefore, selection of strong and immunogenic LSP has proven interesting. Hence, patients with HPV-driven pre-malignant and malignant tumors treated with E6/E7 LSP show immunological,10,11, 33 pre-clinical and clinical benefits,12,34 and combination of a TLR3 agonist with overlapping LSP from NY-ESO-1 also resulted in significant T-cell induction in ovarian cancer patients.14,24 Here, we observe early onset, highly significant, sustained NY-ESO-1-specific CD4+ T-cell responses, followed by very high frequencies of specific CD8+ T-cells, in all patients. Importantly, kinetics of CD8+ T-cell responses are delayed compared to CD4+ T-cells, suggesting that helper CD4+ T-cells35 and CpG induced DC-activation might play a critical role in activating and sustaining the effector phase of CD8+ T-cells. Moreover, by deconvoluting the specific T-cell responses using individual overlapping peptides, we show that CD8+ T-cells recognize different regions of the LSP, whereas CD4+ T-cell responses are confined to a common stretch of the LSP (aa 83–97). These data suggest that in contrast to short peptide and protein vaccination, the use of a LSP allows generation of multiple MHC class I epitopes,36 likely favored by the presence of CpG. Immunodominant regions for CD8+ T-cells in the LSP sequence are heterogeneous and probably linked to the MHC class I of the patients. HLA-B35+ patients showed directly ex vivo detectable CD8+ T-cell responses to a known NY-ESO-1-B35 epitope, while HLA-B35− patients mounted responses to other regions of the LSP, including putative novel MHC class I epitopes. In contrast, different MHC class II molecules might efficiently bind peptides processed from the region 83–97 and promiscuously present the same peptide, as previously shown by us and others for other epitopes from tumor-associated antigens.17,37 In that regard, numerous publications have reported on the immunogenicity of NY-ESO-1, and in particular of the protein region 87–111.17,18, 20,38 Mandic et al. previously reported on the characterization of a CD4 T-cell clone recognizing the epitope 87–101 presented by HLA-DR7 transfected cells,17 By fine mapping of specific CD4+ T-cell responses in our cohort of HLA-DR7+ patients, we identified NY-ESO-1 87–99 as a minimal, immunogenic epitope presented by HLA-DR7. Importantly, the presence of directly ex vivo detectable DR7-restricted NY-ESO-1-specific CD4+ T-cells in patients even before immunization convincingly show the endogenous generation of this epitope. Given the abundance of this HLA in the Caucasian population (25%) further evaluation of directly ex vivo and in vitro expanded DR7/NY-ESO-1-specific CD4+ T-cells is warranted. By comparing frequencies of total cytokine+ CD4+ T-cells to those of multimer+ CD4+ T-cells after IVS, our initial quantification points toward a dominant contribution of the DR7-restricted response to the total specific T-cell response. Furthermore, phenotypic and functional characterization of DR7/NY-ESO-1 T-cells indicates a predominant Th1 polarization. Yet, by stimulating specific clones with high peptide doses, we observed secretion of Type-2 cytokines, arguing for plasticity and polyfunctionality of these cells. In that regard, it was previously reported both in mice and primates that TLR9 agonists induce potent antitumor effects, through induction of adaptive Th1 cellular responses. Inversely, Montanide seems to favor Th2 differentiation of vaccine-induced TAA-specific CD4+ T-cells,39 suggesting that a careful evaluation of adjuvant and peptide doses are needed to optimize vaccinations based on LSP with CpG-B/Montanide combination, as compared to other adjuvants. In addition, caution will be needed when LSP and potent molecularly defined immune adjuvants are used, in order to avoid life threatening immune responses and vaccine toxicity, as reported in a murine study using HY-LSP combined with CpG.40 Nevertheless, an advantage in the utilization of TLR9 agonists, but not TLR4, TLR5, or TLR7-agonists,41 is the ability of CpG to safely tilt the immunologic balance toward effector rather than regulatory T-cells,29,42 thus favoring the overcoming of immune tolerance. The direct ex vivo phenotypic characterization of DR7/NY-ESO-1 CD4+ T-cells showed very low levels of regulatory T-cells upon immunization (data not shown), in line with our previous results.29
Finally, beside Type 1 cytokine secretion upon co-culture with HLA-matched tumor cells, DR7/NY-ESO-1 CD4+ T-cells were also able to directly kill targets. Importantly, beside implications of killer specific CD4+ T-cells against viruses, recent reports on killer CD4+ T-cells in solid tumors have emerged.43 It was recently reported on the potent rejection of melanoma in lymphopenic mice after transfer of small numbers of naive CD4+ T-cells in combination with CTLA-4 blockade.44 Similarly, co-culture of antigen-specific CD4+ T-cells obtained from patients treated with anti-CTLA-4 antibodies specifically recognized and killed tumors.45 In parallel, an increased killing capacity of specific CD4+ T-cells was triggered via OX40/OX40L in combination with chemotherapy.46 Additional work is needed to define the exact contribution of these cells to tumor eradication and the potential involvement of CpG-B in their generation. Moreover, it will be of interest to determine antigen recognition of endogenously processed antigens by tumor cells as compared to professional APCs. In this context, it has recently been shown that in addition to endosomal/lysosomal proteases that are typically involved in MHC class II antigen processing, other pathways usually used for MHC class I presentation, could also be involved in the presentation of intracellular NY-ESO-1 on MHC class II by ovarian tumor cells.47 Thus, these observations suggest that inclusion of multiple LSP from different TAA in optimized vaccine formulations might exploit these novel pathways, favor the induction of epitope spreading and promote the generation of robust and combined CD8+ and CD4+ T-cell, and humoral responses.
We did not observe significant differences in the results from patients without vs. with IL-2 treatment, even though a trend for stronger CD4+ T-cell responses was observed in the patients receiving IL-2, at the early immunomonitoring time points. This contradicts previous studies showing that frequencies of tumor-antigen specific T-cells are reduced in the blood of patients treated with low dose IL-2, likely due to T-cell emigration into peripheral tissues.48,49 However, the IL-2 doses delivered in our study were very low, and therefore perhaps without significant consequences for T-cell functions. Despite the very low doses, patients experienced many adverse events typical for IL-2 treatment. The maximally tolerated dose was as low as 1 Mio UI/m2/day, thus significantly lower than what is conventionally regarded as low dose IL-2 therapy. We suspect that the concomitant treatment with CpG-B may have contributed to this relatively high toxicity, because previous peptide/Montanide vaccination studies without CpG showed lower toxicity despite higher IL-2 doses.50 However, a direct comparison is required to determine a potential role of CpG-B in IL-2 toxicity.
Finally, we observed a trend for longer overall and progression-free survival in patients with above median levels of IFNγ+ NY-ESO-1-specific CD4+ T-cells. However, beside the eight patients that remained without evidence of disease throughout the study, the others experienced progressive disease. The discrepancy between strong immunological and only modest clinical responses might be due to the fact our trial was performed in advanced melanoma patients (stage III/IV). In addition, we monitored T-cell responses in the circulation, but not at tumor site. We and others previously showed that local tumor-derived factors might block efficient immune responses in tumors.2 Future studies on tumor-infiltrating lymphocytes in patients receiving LSP combined with CpG will provide additional information on T-cell fitness directly at tumor site.
In conclusion, the high immunogenicity power of NY-ESO-179–108 LSP combined with CpG-B, the relatively low synthesis costs and the relative ease of production defines this vaccine formulation as a great candidate to be explored for cancer immunotherapy.
Materials and methods
Study design, patients, and treatment
This is a phase I vaccination study of stage III and IV (American Joint Committee on Cancer-AJCC) malignant melanoma patients [1.1]. The vaccines were composed of clinical-grade antigenic peptides, CpG-B 7909/PF-3512676 (Pfizer Inc.) and Montanide ISA-51 (Seppic SA) and were administered subcutaneously (s.c.). Antigenic peptides were the 30-amino acid long NY-ESO-179–108 peptide (for all patients), and the short HLA-A2-restricted peptides Melan-A26–35 (native EAAGIGILTV), Melan-A26–35(A27L) (analog ELAGIGILTV) and MAGE-A10254–262 (GLYDGMEHL) (only for HLA-A2 positive patients). Vaccinations were administered in cycles of 4 mo vaccines with intervals of 2 mo between the cycles (Fig. 1).
The primary objectives of the study were safety and specific cellular immune responses to NY-ESO-1, Melan-A, and MAGE-A10. The secondary endpoints were tumor responses and disease status. Nineteen patients were enrolled in this study and first assigned to group A (without IL-2), followed by group B with supplementary daily low dose IL-2 (Novartis) s.c. for 10 d after each vaccination, starting the day of the second vaccination up to the end of cycle 3. IL-2 was administered following a dose escalation scheme (three doses injected: 0.5, 1 or 2 Mio UI/m2/day). For inclusion, tumors had to express either NY-ESO-1 (LAGE-2) or LAGE-1, and Melan-A in HLA-A2 positive patients (HLA haplotype analysis and immunohistochemistry/PCR for TAA expression was performed on tumor biopsies, after given written informed consent [1.1]).
Administered vaccines were composed of 0.5 mg NY-ESO-179–108 peptide, 1 mg CpG-B 7909/PF-3512676 and 0.5 mL Montanide (syringe 1). HLA-A2+ patients received a second injection (syringe 2), with the short Melan-A26–35 and MAGE-A10254–262 peptides, 1 mg CpG-B and 0.5 mL Montanide. The three vaccines of the cycle 3 were formulated without Montanide. The short peptides were given in a “prime-boost” approach, as follows: for the first cycle 0.1 mg Melan-A26–35 natural peptide and 0.02 mg MAGE-A10254–262 peptide, and for the following cycles 0.1 mg Melan-A26–35(A27L) analog peptide and 0.1 mg MAGE-A10254–262 peptide. A total of 19 immunocompetent patients (5 female and 14 male) with median age of 59 y old were vaccinated [1.1].
Disease status was assessed every 3 mo for patients with measurable disease and every 6 mo for patients with no measurable disease. The study (NCT00112242) sponsored by the Ludwig Center for Cancer Research was approved by the Lausanne University Hospital Ethics Committee and written informed consent was obtained from patients prior to enrolment. Safety was evaluated according to the National Cancer Institute CTC Scale (Version 2.0; April 30, 1999).
This study was performed under GLP conditions [5.1], following SOP developed in the laboratory [5.4] and using investigative assays [5.5].
Blood collection and PBMCs isolation
Heparinized blood samples were withdrawn by venipuncture [1.2, 1.3, 1.4] at baseline, after two and four vaccinations during the first cycle and at the end of each of the following cycles. Peripheral blood mononuclear cells were isolated by Lymphoprep centrifugation gradient [1.6] from blood kept at room temperature [1.5] no longer than 4 h following blood drawn [1.7]. Isolated PBMCs were immediately stored in cryovials at 10 × 106 cells in 1ml of cold 50% RPMI (Gibco), 40% FCS (PAA laboratories), and 10% DMSO freezing medium (Sigma Aldrich) [1.9, 1.12] at −80°C, and further into liquid nitrogen until use [1.10, 1.11]. Before freezing cells were counted using trypan blue [1.20] and viability was >95% [1.15, 1.16].
Cryopreserved cells were thawed at 37°C, washed once and re-suspended at the desired concentration in RPMI supplemented with 10% FCS, 1.15% nonessential amino acids (Sigma), 1% penicillin‐streptomycin (Sigma), 1% Hepes buffer (Gibco, Life Tchnologies) [2.3]. Cells were re-suspended in RPMI (Gibco), 8% Human Serum (pooled human sera from healthy donors' blood from the local blood bank), 1% non-essential amino acids, 1% penicillin-streptomycin, 1% L. glutamine (Gibco, Life Technologies), 1% sodium pyruvate (Gibco, Life Technologies), and 0.1% 2-mercaptoethanol (Sigma) [2.1] and counted using trypan blue [1.20] with viability >80% [1.15, 1.17].
Cell media supplemented with serum were negative from previous tests for extracellular contamination sources [2.2].
Peptide/MHC multimers
Fluorescent mulitmers were: HLA-B35/NY-ESO-194–104, HLA-DR*0701/NY-ESO-187–99. All multimers were provided by TCMetrix [2.4].
In vitro peptide stimulation (IVS) of CD8+ and CD4+ T-cells
Patients' CD8+ T-cells were purified by positive selection using MACS isolation microbeads (Miltenyi), followed by CD4+ T-cell positive selection starting from the CD8 negative fraction. Positive T-cells were stimulated in vitro (IVS) and CD4+/CD8+ T-cells depleted PBMCs were irradiated (30 Gy) and used as feeders for stimulation of the cultures [2.4]. T-cells and autologous APCs were mixed at 1:1 ratio and co-cultured for 14 d with three 18-mers (NY-ESO-179–96, NY-ESO-185–102, NY-ESO-191–108) spanning the entire vaccine NY-ESO-179–108 sequence, with 12 amino acids overlaps, at 2 μM, in RPMI 1640 medium supplemented with 8% heat inactivated, pooled human serum. In parallel, a CD4+ T-cell blast culture was set up using 1 μg/mL PHA. At day 2, 100 U/mL IL-2 was added and cultured until day 14 [2.1, 2.4].
NY-ESO-179–108-specific T-cell responses were evaluated after IVS in a 6-h re-challenge experiment using overlapping peptide pools (2 μM final concentration) in the presence of Brefeldin A (10 μg/mL): For evaluation of CD4+ T-cell responses 10 13-mer peptides overlapping by 11 amino acids were used, while for CD8+ T-cell responses 22 9-mer peptides were pulsed on autologous PHA CD4+ T-cell blasts for 1 h, before addition to the CD8+ T-cells [2.4]. As negative control, cells from the same cultures were left unchallenged and as positive control, two wells for CD8+ and CD4+ T-cells were stimulated with PMA/Ionomycin in the presence of Brefeldin A (Fig. 2) [2.5].
NY-ESO-1 restimulated T-cells were evaluated for IFNγ, TNFα, and IL-2 production and analyzed by flow cytometry. Additionally, CD4+ T-cells were concomitantly analyzed for IL-5 and IL-13 production. Cells were first stained for CD3-APC AF750 (BD Biosciences), CD4+-PB (BD PharMingen), and CD8+-ECD (Beckman Coulter) and Live/Dead Aqua (Invitrogen), followed by a fixation step. Cells were washed with buffer (PBS, 0.2% BSA, 0.2% azide, 5 μM EDTA) and permeabilized with 0.1% saponin for staining for IL-2-FITC (BD PharMingen), IFNγ-PECy7 (BD PharMingen), TNFα-AF700 (BD PharMingen) for read out of CD8+, while CD4+ T-cells were additionally stained for IL-5 and IL-13-APC (BD Biosciences) [2.4]. Samples were acquired on a Gallios flow cytometer (Beckman Coulter) and data were analyzed using FlowJo software (TreeStar) [3.1, 3.2]. The PMT voltages were adjusted for each fluorescence channel using unstained PBMCs and compensations were set using PBMCs stained with single antibodies according to a local SOP [3.2]. The analysis was performed on living, singlets, CD3+CD4+ or CD3+CD8+ lymphocytes [3.3]. A representative example of the full gating strategy is shown on Fig. S6 [3.4]. Dot plots can be provided per request [4.3]. Stainings were considered as positive for each measured cytokine if the stimulated responses were at least three times higher than the unstimulated control [4.4] as defined during the study design [4.6].
Generation of HLA-DR7-restricted NY-ESO-187–99-specific CD4+ T-cell clones and lines
Polyclonal cultures from HLA-DR7 patients containing NY-ESO-187–99-specific CD4+ T-cells were sorted using NY-ESO-187–99/HLA-DR7 multimers by fluorescence activated cell sorting following the staining panel: anti-CD4+ and anti-CD3 antibodies, DAPI and PE-conjugated NY-ESO-187–99/HLA-DR7 multimer [4.6]. Clones were obtained by limiting dilution (0.5 cell/well) in Terasaki plates and cultured in RPMI medium with 8% HS and 100 U/mL IL-2, 10,000 irradiated allogenic feeder cells per well and 1 μg/mL PHA [2.4].
Direct ex vivo enumeration of HLA-DR7-restricted NY-ESO-187–99-specific CD4+ T-cells
PBMCs from HLA-DR7+ patients were stained directly ex vivo using a combination of PE-conjugated NY-ESO-187–99/HLA-DR7 multimer, followed by staining using PerCP-Cy5.5-conjugated anti-CD3 (Biolegend), AF700-conjugated anti-CD45RA (Biolegend), APC-H7 conjugated anti-CD4 (BD Bioscience), and Vivid Aqua (Invitrogen). Unstained cultures from the same patients were used as controls [2.5].
Killing assay
The specific lytic activity of the NY-ESO-187–99 CD4+ T-cell lines was assessed against HLA-DR7+ (T331A; GEF I; GEF II) or HLA-DR7− (T1415A) melanoma cell lines, pre-treated or not with hrIFNγ (50 U/mL, Peprotech) for 48 h [2.4]. Cells were labeled with 51Chromium (Amersham Biosciences), loaded or not with peptides, and washed [2.4]. Labeled target cells were incubated with effectors at the indicated ratio for 4 h at 37°C [2.4]. The supernatants were harvested and radioactivity was counted in an automatic gamma-counter [3.1]. The percentage of specific lysis was determined using the formula: (experimental-spontaneous release)/(maximum-spontaneous) × 100. Internal controls were included in each assay to measure the spontaneous release (target cells alone) and the total release (target cells with 1 M HCl) [2.5].
IFNγ ELISA
NY-ESO-187–99 CD4 T-cells were co-cultured at 1:1 ratio with HLA-DR7+ (T331A; GEF I; GEF II) or HLA-DR7– (T1415A) melanoma cell lines, pre-treated or not with hrIFNγ (50 U/mL, Peptrotech) for 48 h, pulsed or not with peptides [2.4]. Supernatants from stimulated conditions and unstimulated controls [2.5] were harvested after 24 h and IFNγ ELISA was performed using the Human BD OptEIA ELISA set (BD Biosciences) [2.4, 3.1].
IFNγ ELISPOT
The Elispot was performed using the ELISpotPRO kit for Human IFNγ from MABTECH (3420-2APT-10), following the standard supplier instructions. CD8+ T-cells after IVS were thawed and rested in presence of 100 U/mL IL-2 for two days before use. The cell number per well was adjusted to have maximum 3% of cytokine producing cells per well to avoid saturation of the membrane (values taken from the previous immune monitoring). For the analysis, the values had been normalized to equal percentages of spot forming units (SFU) [2.4].
Serology
Recombinant NY-ESO-1 protein and control dihydrofolate reductase (DHFR) proteins were used to coat plates and measure specific serum antibody levels in ELISA as previously described14 [2.4, 2.5]. A reciprocal titer was estimated from optical density readings of serially diluted plasma samples. Negative control sera from healthy individual and positive control sera for each antigen from patients with cancer were always included [2.5]. The anti-human immunoglobulin antibodies used as secondary reagents were: alkaline phosphatase (AP)-labeled goat-anti-human IgG (polyclonal antisera; Southern Biotech), biotinylated mouse-anti-human IgG1 (Clone JDC-1; BD Pharmingen), AP-labeled mouse anti-human IgG2 (clone HP6002; Southern Biotech), AP-labeled mouse anti-human IgG3 (clone HP6050; Southern Biotech), AP-labeled mouse anti-human IgG4 (clone HP6023; Southern Biotech), AP-labeled goat-anti-human IgA (polyclonal antisera; Southern Biotech), AP-labeled mouse-anti-human IgD-AP (clone IADB6; Southern Biotech), AP-labeled goat-anti-human IgE (Clone HP6029; Southern Biotech) [2.4], and AP-labeled goat-anti-human IgM (polyclonal antisera; Southern Biotech). To be considered significant, reciprocal titers had to be more than 100 [4.4].
Tumor responses
In patients with measurable disease, tumor responses were classified as follows: complete response as disappearance of all the tumor signs for at least 4 weeks, partial response as decrease of at least 50% of all tumor lesions for at least 4 weeks, minor response as decrease of all the lesions by at least 25% for the same minimum period of time, stable disease as no more than 25% changes in size of previous lesions for the same minimum period of time, progressive disease as appearance of new lesions or increased lesions by at least 25% in size, and major progressive disease as tumor progression requiring other standard therapy comprising chemotherapy and/or radiotherapy [4.6].
Statistical analyses
In IVS, baseline values from the same unstimulated CD8+ and CD4+ T-cell cultures were used as negative control and excluded from the peptide challenged responses [2.5]. For each patient and each time point at least six individual cultures were analyzed [2.8]. Vaccination effects on tumor specific CD8+ and CD4+ T-cell responses were analyzed according to the vaccination schedule and relative to the same results at study entry. For statistical analysis unpaired Kruskal–Wallis test was used. For all analyses, a p value less than 0.05 was considered as statistically significant and labeled with *, very significant less than 0.01 with **, strongly significant less than 0.001 with *** and less than 0.0001 with **** [4.4]. Not significant differences were labeled with ns.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to the patients for their dedicated collaboration, and Pfizer and Ludwig Cancer Research for providing CpG-7909/PF-3512676 and clinical-grade peptides, respectively. We gratefully acknowledge L.J. Old, J. O'Donnell-Tormey, L. Harmer, J. Skipper, R. Venhaus, L. Pan, M. Matter, C. Brignone, S. Leyvraz, P.O. Gannon, J. Schmidt, P. Guillaume, I. Luescher, E. Devêvre, N. Montandon, L. Leyvraz, M. van Overloop, C. Geldhof, P. Werffeli, A. Wilson, D. Labbes, S. Winkler, A. Digklia, K. Homicsko, S. Badel, H. Bouchaab, G. Buss, A. Cristinat, F. Claude, A. Auteri, A. Daccord, X. Durando, M. Frigeri, M. Gavillet, A. Stravodimou, D. Taylor, E. Tzika, J.-P. Zuercher, C. Sedrak, A. Orcurto, and B. Martins-Moura for essential support, collaboration, and advice. We are also thankful for the support and assistance of the CHUV physicians, nurses, and staff of the Medical Oncology Service, Institute of Pathology, Clinical Investigation Units, and Blood Bank Donor Room.
Funding
This work was supported in part by the Cancer Research Institute (USA), Ludwig Cancer Research (USA), the Cancer Vaccine Collaborative (USA), Atlantic Philanthropies (USA), the Wilhelm Sander-Foundation (Germany), Swiss Cancer Research (3507-08-2014), the Swiss National Science Foundation (CRSII3_160708, 320030_152856, 31003A_163204 and 310030-130812), and SwissTransMed (KIP 18).
References
- 1.Slingluff CL., Jr The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J 2011; 17:343-50; PMID:21952285; http://dx.doi.org/15696196 10.1097/PPO.0b013e318233e5b2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appay V, Jandus C, Voelter V, Reynard S, Coupland SE, Rimoldi D, Lienard D, Guillaume P, Krieg AM, Cerottini JC et al.. New generation vaccine induces effective melanoma-specific CD8+ T cells in the circulation but not in the tumor site. J Immunol 2006; 177:1670-8; PMID:16849476; http://dx.doi.org/15696196 10.4049/jimmunol.177.3.1670 [DOI] [PubMed] [Google Scholar]
- 3.Speiser DE, Lienard D, Rufer N, Rubio-Godoy V, Rimoldi D, Lejeune F, Krieg AM, Cerottini JC, Romero P. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Investig 2005; 115:739-46; PMID:15696196; http://dx.doi.org/ 10.1172/JCI23373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karbach J, Gnjatic S, Bender A, Neumann A, Weidmann E, Yuan J, Ferrara CA, Hoffmann E, Old LJ, Altorki NK et al.. Tumor-reactive CD8+ T-cell responses after vaccination with NY-ESO-1 peptide, CpG 7909 and Montanide ISA-51: association with survival. Int J Cancer 2010; 126:909-18; PMID:19728336; http://dx.doi.org/ 10.1002/ijc.24850. [DOI] [PubMed] [Google Scholar]
- 5.Ossendorp F, Mengede E, Camps M, Filius R, Melief CJ. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med 1998; 187:693-702; PMID:9480979; http://dx.doi.org/ 10.1084/jem.187.5.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol 2007; 179:5033-40; PMID:17911588; http://dx.doi.org/ 10.4049/jimmunol.179.8.5033 [DOI] [PubMed] [Google Scholar]
- 7.Melief CJ, van der Burg SH. Immunotherapy of established (pre)malignant disease by synthetic long peptide vaccines. Nat Rev Cancer 2008; 8:351-60; PMID:18418403; http://dx.doi.org/ 10.1038/nrc2373 [DOI] [PubMed] [Google Scholar]
- 8.Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, van der Burg SH, Offringa R. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol 2008; 38:1033-42; PMID:18350546; http://dx.doi.org/ 10.1002/eji.200737995 [DOI] [PubMed] [Google Scholar]
- 9.Braun M, Jandus C, Maurer P, Hammann-Haenni A, Schwarz K, Bachmann MF, Speiser DE, Romero P. Virus-like particles induce robust human T-helper cell responses. Eur J Immunol 2012; 42:330-40; PMID:22057679; http://dx.doi.org/ 10.1002/eji.201142064 [DOI] [PubMed] [Google Scholar]
- 10.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ et al.. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008; 14:169-77; PMID:18172268; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1881 [DOI] [PubMed] [Google Scholar]
- 11.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, Berends-van der Meer DM, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J et al.. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 2008; 14:178-87; PMID:18172269; http://dx.doi.org/ 10.1158/1078-0432.CCR-07-1880 [DOI] [PubMed] [Google Scholar]
- 12.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW et al.. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009; 361:1838-47; PMID:19890126; http://dx.doi.org/ 10.1056/NEJMoa0810097 [DOI] [PubMed] [Google Scholar]
- 13.Kakimi K, Isobe M, Uenaka A, Wada H, Sato E, Doki Y, Nakajima J, Seto Y, Yamatsuji T, Naomoto Y et al.. A phase I study of vaccination with NY-ESO-1f peptide mixed with Picibanil OK-432 and Montanide ISA-51 in patients with cancers expressing the NY-ESO-1 antigen. Int J Cancer 2011; 129:2836-46; PMID:21448901; http://dx.doi.org/ 10.1002/ijc.25955 [DOI] [PubMed] [Google Scholar]
- 14.Sabbatini P, Tsuji T, Ferran L, Ritter E, Sedrak C, Tuballes K, Jungbluth AA, Ritter G, Aghajanian C, Bell-McGuinn K et al.. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 2012; 18:6497-508; PMID:23032745; http://dx.doi.org/ 10.1158/1078-0432.CCR-12-2189 [DOI] [PubMed] [Google Scholar]
- 15.Chen YT, Scanlan MJ, Sahin U, Tureci O, Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M, Old LJ. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A 1997; 94:1914-8; PMID:9050879; http://dx.doi.org/ 10.1073/pnas.94.5.1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caballero OL, Chen YT. Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Sci 2009; 100:2014-21; PMID:19719775; http://dx.doi.org/ 10.1111/j.1349-7006.2009.01303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mandic M, Castelli F, Janjic B, Almunia C, Andrade P, Gillet D, Brusic V, Kirkwood JM, Maillere B, Zarour HM. One NY-ESO-1-derived epitope that promiscuously binds to multiple HLA-DR and HLA-DP4 molecules and stimulates autologous CD4+ T cells from patients with NY-ESO-1-expressing melanoma. J Immunol 2005; 174:1751-9; PMID:15661941; http://dx.doi.org/ 10.4049/jimmunol.174.3.1751 [DOI] [PubMed] [Google Scholar]
- 18.Eikawa S, Kakimi K, Isobe M, Kuzushima K, Luescher I, Ohue Y, Ikeuchi K, Uenaka A, Nishikawa H, Udono H et al.. Induction of CD8 T-cell responses restricted to multiple HLA class I alleles in a cancer patient by immunization with a 20-mer NY-ESO-1f (NY-ESO-1 91-110) peptide. Int J Cancer 2013; 132:345-54; PMID:22729530; http://dx.doi.org/ 10.1002/ijc.27682 [DOI] [PubMed] [Google Scholar]
- 19.Chen Q, Jackson H, Parente P, Luke T, Rizkalla M, Tai TY, Zhu HC, Mifsud NA, Dimopoulos N, Masterman KA et al.. Immunodominant CD4+ responses identified in a patient vaccinated with full-length NY-ESO-1 formulated with ISCOMATRIX adjuvant. Proc Natl Acad Sci U S A 2004; 101:9363-8; PMID:15197261; http://dx.doi.org/ 10.1073/pnas.0403271101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mizote Y, Taniguchi T, Tanaka K, Isobe M, Wada H, Saika T, Kita S, Koide Y, Uenaka A, Nakayama E. Three novel NY-ESO-1 epitopes bound to DRB1*0803, DQB1*0401 and DRB1*0901 recognized by CD4 T cells from CHP-NY-ESO-1-vaccinated patients. Vaccine 2010; 28:5338-46; PMID:20665979; http://dx.doi.org/ 10.1016/j.vaccine.2010.05.044 [DOI] [PubMed] [Google Scholar]
- 21.Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R, Koretzky GA, Klinman DM. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995; 374:546-9; PMID:7700380; http://dx.doi.org/ 10.1038/374546a0 [DOI] [PubMed] [Google Scholar]
- 22.Krieg AM. Development of TLR9 agonists for cancer therapy. J Clin Investig 2007; 117:1184-94; PMID:17476348; http://dx.doi.org/ 10.1172/JCI31414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valmori D, Souleimanian NE, Tosello V, Bhardwaj N, Adams S, O'Neill D, Pavlick A, Escalon JB, Cruz CM, Angiulli A et al.. Vaccination with NY-ESO-1 protein and CpG in Montanide induces integrated antibody/Th1 responses and CD8 T cells through cross-priming. Proc Natl Acad Sci U S A 2007; 104:8947-52; PMID:17517626; http://dx.doi.org/ 10.1073/pnas.0703395104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuji T, Sabbatini P, Jungbluth AA, Ritter E, Pan L, Ritter G, Ferran L, Spriggs D, Salazar AM, Gnjatic S. Effect of Montanide and poly-ICLC adjuvant on human self/tumor antigen-specific CD4+ T cells in phase I overlapping long peptide vaccine trial. Cancer Immunol Res 2013; 1:340-50; PMID:24777970; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0089 [DOI] [PubMed] [Google Scholar]
- 25.Carrel S, Schreyer M, Spagnoli G, Cerottini JC, Rimoldi D. Monoclonal antibodies against recombinant-MAGE-1 protein identify a cross-reacting 72-kDa antigen which is co-expressed with MAGE-1 protein in melanoma cells. Int J Cancer 1996; 67:417-22; PMID:8707418; http://dx.doi.org/ 10.1002/(SICI)1097-0215(19960729)67:3%3c417::AID-IJC17%3e3.0.CO;2-4 [DOI] [PubMed] [Google Scholar]
- 26.Kerkmann M, Costa LT, Richter C, Rothenfusser S, Battiany J, Hornung V, Johnson J, Englert S, Ketterer T, Heckl W et al.. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J Biol Chem 2005; 280:8086-93; PMID:15591070; http://dx.doi.org/ 10.1074/jbc.M410868200 [DOI] [PubMed] [Google Scholar]
- 27.Goldinger SM, Dummer R, Baumgaertner P, Mihic-Probst D, Schwarz K, Hammann-Haenni A, Willers J, Geldhof C, Prior JO, Kundig TM et al.. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8(+) T-cell responses in melanoma patients. Eur J Immunol 2012; 42:3049-61; PMID:22806397; http://dx.doi.org/ 10.1002/eji.201142361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumgaertner P, Jandus C, Rivals JP, Derre L, Lovgren T, Baitsch L, Guillaume P, Luescher IF, Berthod G, Matter M et al.. Vaccination-induced functional competence of circulating human tumor-specific CD8 T-cells. Int J Cancer 2012; 130:2607-17; PMID:21796616; http://dx.doi.org/ 10.1002/ijc.26297 [DOI] [PubMed] [Google Scholar]
- 29.Jandus C, Bioley G, Dojcinovic D, Derre L, Baitsch L, Wieckowski S, Rufer N, Kwok WW, Tiercy JM, Luescher IF et al.. Tumor antigen-specific FOXP3+ CD4 T cells identified in human metastatic melanoma: peptide vaccination results in selective expansion of Th1-like counterparts. Cancer Res 2009; 69:8085-93; PMID:19808957; http://dx.doi.org/ 10.1158/0008-5472.CAN-09-2226 [DOI] [PubMed] [Google Scholar]
- 30.Karbach J, Neumann A, Atmaca A, Wahle C, Brand K, von Boehmer L, Knuth A, Bender A, Ritter G, Old LJ et al.. Efficient in vivo priming by vaccination with recombinant NY-ESO-1 protein and CpG in antigen naive prostate cancer patients. Clin Cancer Res 2011; 17:861-70; PMID:21163871; http://dx.doi.org/ 10.1158/1078-0432.CCR-10-1811 [DOI] [PubMed] [Google Scholar]
- 31.Toes RE, van der Voort EI, Schoenberger SP, Drijfhout JW, van Bloois L, Storm G, Kast WM, Offringa R, Melief CJ. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol 1998; 160:4449-56; PMID:9574550 [PubMed] [Google Scholar]
- 32.Sabado RL, Pavlick A, Gnjatic S, Cruz CM, Vengco I, Hasan F, Spadaccia M, Darvishian F, Chiriboga L, Holman RM et al.. Resiquimod as an immunologic adjuvant for NY-ESO-1 protein vaccination in patients with high-risk melanoma. Cancer Immunol Res 2015; 3:278-87; PMID:25633712; http://dx.doi.org/ 10.1158/2326-6066.CIR-14-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Vos van Steenwijk PJ, Ramwadhdoebe TH, Lowik MJ, van der Minne CE, Berends-van der Meer DM, Fathers LM, Valentijn AR, Oostendorp J, Fleuren GJ, Hellebrekers BW et al.. A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer Immunol Immunother 2012; 61:1485-92; PMID:22684521; http://dx.doi.org/ 10.1007/s00262-012-1292-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zwaveling S, Ferreira Mota SC, Nouta J, Johnson M, Lipford GB, Offringa R, van der Burg SH, Melief CJ. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol 2002; 169:350-8; PMID:12077264; http://dx.doi.org/ 10.4049/jimmunol.169.1.350 [DOI] [PubMed] [Google Scholar]
- 35.Kast WM, Bronkhorst AM, de Waal LP, Melief CJ. Cooperation between cytotoxic and helper T lymphocytes in protection against lethal Sendai virus infection. Protection by T cells is MHC-restricted and MHC-regulated; a model for MHC-disease associations. J Exp Med 1986; 164:723-38; PMID:3018121; http://dx.doi.org/ 10.1084/jem.164.3.723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnjatic S, Atanackovic D, Matsuo M, Jager E, Lee SY, Valmori D, Chen YT, Ritter G, Knuth A, Old LJ. Cross-presentation of HLA class I epitopes from exogenous NY-ESO-1 polypeptides by nonprofessional APCs. J Immunol 2003; 170:1191-6; PMID:12538675; http://dx.doi.org/ 10.4049/jimmunol.170.3.1191 [DOI] [PubMed] [Google Scholar]
- 37.Bioley G, Jandus C, Tuyaerts S, Rimoldi D, Kwok WW, Speiser DE, Tiercy JM, Thielemans K, Cerottini JC, Romero P. Melan-A/MART-1-specific CD4 T cells in melanoma patients: identification of new epitopes and ex vivo visualization of specific T cells by MHC class II tetramers. J Immunol 2006; 177:6769-79; PMID:17082590; http://dx.doi.org/ 10.4049/jimmunol.177.10.6769 [DOI] [PubMed] [Google Scholar]
- 38.Gnjatic S, Nagata Y, Jager E, Stockert E, Shankara S, Roberts BL, Mazzara GP, Lee SY, Dunbar PR, Dupont B et al.. Strategy for monitoring T cell responses to NY-ESO-1 in patients with any HLA class I allele. Proc Natl Acad Sci U S A 2000; 97:10917-22; PMID:11005863; http://dx.doi.org/ 10.1073/pnas.97.20.10917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yip HC, Karulin AY, Tary-Lehmann M, Hesse MD, Radeke H, Heeger PS, Trezza RP, Heinzel FP, Forsthuber T, Lehmann PV. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol 1999; 162:3942-9; PMID:10201913 [PubMed] [Google Scholar]
- 40.Kitamura H, Sedlik C, Jacquet A, Zaragoza B, Dusseaux M, Premel V, Sastre-Garau X, Lantz O. Long peptide vaccination can lead to lethality through CD4+ T cell-mediated cytokine storm. J Immunol 2010; 185:892-901; PMID:20543102; http://dx.doi.org/ 10.4049/jimmunol.1000933 [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Dominguez AL, Lustgarten J. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol 2006; 177:8348-55; PMID:17142731; http://dx.doi.org/ 10.4049/jimmunol.177.12.8348 [DOI] [PubMed] [Google Scholar]
- 42.Perret R, Sierro SR, Botelho NK, Corgnac S, Donda A, Romero P. Adjuvants that improve the ratio of antigen-specific effector to regulatory T cells enhance tumor immunity. Cancer Res 2013; 73:6597-608; PMID:24048821; http://dx.doi.org/ 10.1158/0008-5472.CAN-13-0875 [DOI] [PubMed] [Google Scholar]
- 43.Tsuji T, Matsuzaki J, Caballero OL, Jungbluth AA, Ritter G, Odunsi K, Old LJ, Gnjatic S. Heat shock protein 90-mediated peptide-selective presentation of cytosolic tumor antigen for direct recognition of tumors by CD4(+) T cells. J Immunol 2012; 188:3851-8; PMID:22427632; http://dx.doi.org/ 10.4049/jimmunol.1103269 [DOI] [PubMed] [Google Scholar]
- 44.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, Blasberg R, Yagita H, Muranski P, Antony PA et al.. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010; 207:637-50; PMID:20156971; http://dx.doi.org/ 10.1084/jem.20091918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kitano S, Tsuji T, Liu C, Hirschhorn-Cymerman D, Kyi C, Mu Z, Allison JP, Gnjatic S, Yuan JD, Wolchok JD. Enhancement of tumor-reactive cytotoxic CD4+ T cell responses after ipilimumab treatment in four advanced melanoma patients. Cancer Immunol Res 2013; 1:235-44; PMID:24396833; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hirschhorn-Cymerman D, Budhu S, Kitano S, Liu C, Zhao F, Zhong H, Lesokhin AM, Avogadri-Connors F, Yuan J, Li Y et al.. Induction of tumoricidal function in CD4+ T cells is associated with concomitant memory and terminally differentiated phenotype. J Exp Med 2012; 209:2113-26; PMID:23008334; http://dx.doi.org/ 10.1084/jem.20120532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsuzaki J, Tsuji T, Luescher I, Old LJ, Shrikant P, Gnjatic S, Odunsi K. Nonclassical antigen-processing pathways are required for MHC class II-restricted direct tumor recognition by NY-ESO-1-specific CD4(+) T cells. Cancer Immunol Res 2014; 2:341-50; PMID:24764581; http://dx.doi.org/ 10.1158/2326-6066.CIR-13-0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med 2003; 9:540-7; PMID:12692546; http://dx.doi.org/ 10.1038/nm866 [DOI] [PubMed] [Google Scholar]
- 49.Kammula US, Lee KH, Riker AI, Wang E, Ohnmacht GA, Rosenberg SA, Marincola FM. Functional analysis of antigen-specific T lymphocytes by serial measurement of gene expression in peripheral blood mononuclear cells and tumor specimens. J Immunol 1999; 163:6867-75; PMID:10586088 [PubMed] [Google Scholar]
- 50.Lienard D, Rimoldi D, Marchand M, Dietrich PY, van Baren N, Geldhof C, Batard P, Guillaume P, Ayyoub M, Pittet MJ et al.. Ex vivo detectable activation of Melan-A-specific T cells correlating with inflammatory skin reactions in melanoma patients vaccinated with peptides in IFA. Cancer Immunity 2004; 4:4; PMID:15149168 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


