Abstract
Brain metastases (BMs) occur in 10% to 20% of adult patients with cancer, and with increased surveillance and improved systemic control, the incidence is likely to grow. Despite multimodal treatment, prognosis remains poor. Current evidence supports use of whole-brain radiation therapy when patients present with multiple BMs. However, its associated cognitive impairment is a major deterrent in patients likely to live longer than 6 months. In patients with oligometastases (one to three metastases) and even some with multiple lesions less than 3 to 4 cm, especially if the primary tumor is considered radiotherapy resistant, stereotactic radiosurgery is recommended; if the BMs are greater than 4 cm, surgical resection with or without postoperative whole-brain radiation therapy should be considered. There is increasing evidence that systemic therapy, including targeted therapy and immunotherapy, is effective against BM and may be an early choice, especially in patients with sensitive primary tumors. In patients with progressive systemic disease, limited treatment options, and poor performance status, best supportive care may be appropriate. Regardless of treatment goals, use of corticosteroids or antiepileptic medications is helpful in symptomatic patients. In this review, we provide a summary of current therapy, as well as developments in the treatment of BM from solid tumors.
INTRODUCTION
Brain metastases (BMs) occur in 10% to 20% of adult patients with cancer and are 10 times more common than primary brain tumors.1–3 BMs most commonly arise from lung and breast carcinoma and melanoma, but any malignancy can spread to the brain.4 With increased surveillance, improved control of systemic cancer, and prolonged survival, the incidence of patients with BM is growing, partially attributed to the brain being a sanctuary site. For example, more patients with human epidermal growth factor receptor 2 (HER2) –positive breast carcinoma5 and epidermal growth factor receptor (EGFR) –mutant non–small-cell lung cancer (NSCLC)6 have the brain as the initial site of relapse after successful systemic treatment with trastuzumab, a large molecule that does not cross the blood-brain barrier (BBB), or gefitinib, which has inadequate BBB penetration.
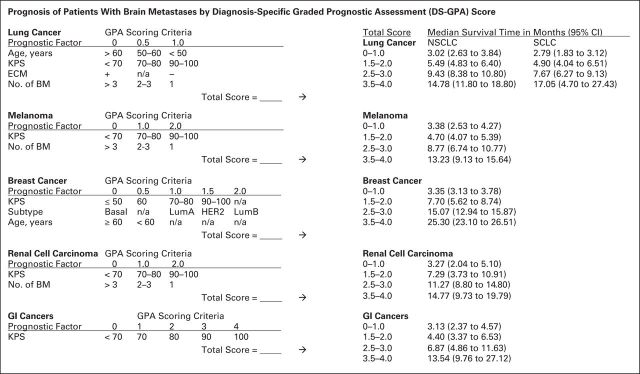
Historically, patients with BM had such a poor prognosis that little thought was given to determining each individual's prognosis and optimal treatment. The first effort came in the late 1990s, when the Radiation Therapy Oncology Group (RTOG) performed a recursive partitioning analysis (RPA).7–9 Using this approach, median survival ranged from 7.1 months in patients with the best prognostic score (RPA class 1) to 2.3 months in those with the worst (RPA class 3; Table 1). The RPA system can be applied to any patient with a BM, but a newer prognostic index, the Diagnosis-Specific Graded Prognostic Assessment score, provides a higher level of refinement where the median survival ranges from 2.79 to 25.30 months (Fig 1).10 The Diagnosis-Specific Graded Prognostic Assessment accounts for primary tumor type and unique features applicable to each primary tumor, making the system relevant to daily clinical practice.
Table 1.
Prognosis of Patients With Brain Metastases by RPA Class
| RPA Class | Median Survival (months) |
|---|---|
| RPA class 1 | |
| KPS ≥ 70, age < 65 years, controlled primary tumor | 7.1 |
| No extracranial disease | |
| Single brain metastasis | 13.5 |
| Multiple brain metastases | 6.0 |
| RPA class 2 | |
| All other situations | 4.2 |
| Single brain metastasis | 8.1 |
| Multiple brain metastases | 4.1 |
| RPA class 3 | |
| KPS < 70 | 2.3 |
NOTE. Reprinted with permission.69 Survival results for overall RPA classes are from Radiation Therapy Oncology Group trials,7 and those for single and multiple metastases subdivisions of class 1 and 2 are from Lutterbach et al.8
Abbreviations: KPS, Karnofsky performance score; RPA, recursive partitioning analysis.
Fig 1.
Prognosis of patients with brain metastases (BM) by Diagnosis-Specific Graded Prognostic Assessment (DS-GPA) score. Breast cancer subtypes are as follows: basal: triple negative; luminal A (LumA): estrogen receptor (ER)/progesterone receptor (PR) positive, human epidermal growth factor receptor 2 (HER2) negative; luminal B (LumB): triple positive; HER2: ER/PR negative, HER2 positive. ECM, extracranial metastases; KPS, Karnofsky performance score; n/a, not applicable; NSCLC, non–small-cell lung cancer; SCLC, small-cell lung cancer. Data adapted.10
Recognizing this marked heterogeneity in outcomes has changed the way patients with BM are managed. BMs are no longer considered a single entity across patients, but a special site of metastatic disease, assessed and managed in the context of the primary tumor and the patient's overall systemic options. Treatment is now individualized, with more emphasis placed on balancing treatment effectiveness against neurotoxicity. In patients with good prognosis, the goal of therapy has shifted from short-term palliation to long-term survival and quality of life (QOL). Hence, whole-brain radiation therapy (WBRT) is less preferable in situations where stereotactic radiosurgery (SRS)11,12 and systemic agents13–15 are reasonably effective.
DIAGNOSIS
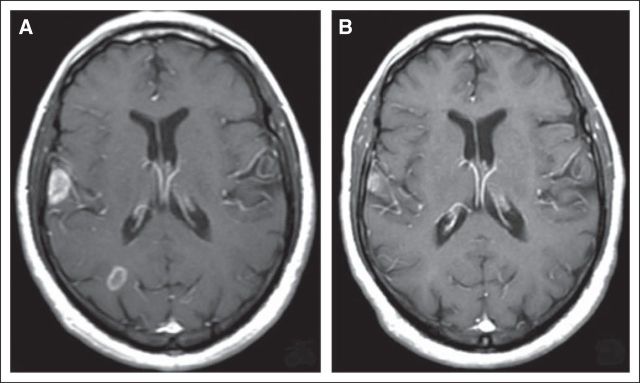
Magnetic resonance imaging (MRI) is the single most important diagnostic tool in evaluating presence, number, size, and location of BM. MRI usually establishes the diagnosis, but some patients with cancer have brain lesions as a result of other processes that are indistinguishable from metastases on MRI. In a prospective study,16 six (11%) of 48 patients with cancer with radiographic evidence of a presumed single metastasis were found to have nonmetastatic lesions on biopsy or resection. Although the study was conducted more than 20 years ago and may not reflect current imaging diagnostic accuracy, it was performed with MRI and highlights the radiologic limitation in distinguishing BM from other enhancing cerebral lesions. Hence, unusual clinical circumstances, such as a brain lesion detected in a patient with cancer in remission, or unusual imaging features, such as a large lesion crossing the corpus callosum, may dictate the need for pathologic confirmation (Fig 2).
Fig 2.
T1 postcontrast axial magnetic resonance image demonstrating a new large contrast-enhancing right frontal mass in a 40-year-old woman being treated for metastatic ovarian carcinoma. She was responding well systemically to chemotherapy when she developed a left hemiparesis. This large homogeneously enhancing right frontal lesion with associated vasogenic edema and central restricted diffusion was identified. Given atypical clinical and radiologic features, biopsy was performed and revealed lymphoma. This case highlights the need to consider other diagnoses for a brain lesion in a patient with known cancer, especially when clinical and radiologic features are unusual.
TREATMENT
Therapy for BM includes definitive treatment directed against the tumor itself and supportive treatment, including glucocorticoids, antiepileptic drugs (AEDs), and anticoagulants, to help reduce symptoms.
Corticosteroids
Glucocorticoids improve neurologic symptoms in up to 75% of patients with cerebral edema and are indicated in any symptomatic patient.17,18 Dexamethasone is generally considered the corticosteroid of choice because of its minimal mineralocorticoid effect and long half-life. One study suggested that 4 or 8 mg of dexamethasone is as effective as 16 mg19; hence, most guidelines support an initial dexamethasone dose of 4 to 8 mg per day in two divided doses.17,18 Higher doses are used in patients with marked mass effect or severe symptoms or who do not respond to treatment within 48 hours.18,19 There is no role for corticosteroids in asymptomatic patients, although the authors suggest initiation of 2 to 4 mg per day before initiation of radiotherapy if cerebral edema is evident on imaging.20 This will reduce or prevent acute radiation toxicity.
AEDs
AEDs are indicated in the approximately 25% of patients who present with seizures.21 In contrast, there is no evidence that prophylactic AEDs in patients who have not had seizures will prevent future seizures.22–25 In patients undergoing BM resection, short-term prophylactic AED use with rapid tapering off reduces the risk of seizures by 40% to 50% within the first postoperative week.26
Anticoagulation Therapy
Patients with cancer are at increased risk of venous thromboembolism (VTE), which is an important cause of cancer-related mortality.27,28 In a meta-analysis of 827 patients who underwent elective neurosurgery, heparin prophylaxis was effective in preventing VTE without excessive bleeding.29 For patients with BM and VTE, anticoagulation was safe and more effective than inferior vena cava filters.30 Even in patients with melanoma with hemorrhagic BM, anticoagulation did not significantly increase the risk of intracranial hemorrhage.28 These studies demonstrate that anticoagulation can be administered safely in patients with BM for VTE prophylaxis and treatment.
DEFINITIVE TREATMENT
The most appropriate definitive therapy is selected based on the number, size, and location of BM; the primary tumor type; extent and control of systemic disease; and a patient's performance status.
Chemotherapy and Biologic Agents
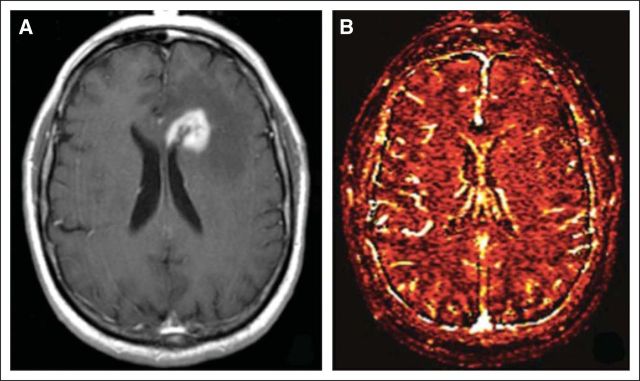
Currently, systemic therapy is not used routinely to treat BM.31 However, it may be the first therapeutic choice for BM from highly chemotherapy-sensitive primary tumors, such as germ cell tumors32 and small-cell lung carcinomas (SCLCs).33,34 For asymptomatic BM found on screening MRI in patients slated to receive chemotherapy for their systemic disease, it is reasonable to monitor the cerebral response to systemic therapy before initiating CNS-directed treatment (Fig 3, Table 2).13–15
Fig 3.
A 71-year-old man was found to have asymptomatic brain metastases on diagnosis of lung adenocarcinoma. (A) T1 postcontrast magnetic resonance imaging (MRI) demonstrating two brain metastases. Because of the presence of an EGFR mutation and lack of neurologic symptoms, erlotinib was initiated for treatment of his systemic and CNS disease. (B) T1 postcontrast MRI demonstrating a response after only 1 month of erlotinib.
Table 2.
Clinical Decision Making in Treatment of Brain Metastases
|
Abbreviations: BM, brain metastases; MRI, magnetic resonance imaging; OM, oligometastases; POD, progression of disease; PS, performance status; PT, primary tumor; SRS, stereotactic radiosurgery; WBRT, whole-brain radiation therapy.
Current data support use of SRS for up to three BMs. However, there is a trend toward using SRS in treatment of up to 10 BMs.
The two major predictive factors of chemotherapy response are the intrinsic chemotherapy sensitivity of the primary tumor and the BM and, less importantly, the BBB permeability of the agent. BMs typically respond best to agents effective against the primary cancer; however, BM cells may have substantial genetic differences from their primary tumor because the BM arose from a subpopulation in the primary tumor or acquired new mutations that conferred chemotherapy resistance.35,36 Hence, cytotoxic agents with good CNS penetration and proven benefit in primary tumors may not consistently be efficacious against BM. BBB permeability is the other potential obstacle. BMs have variable BBB breakdown, which is crudely assessed by contrast enhancement on MRI, causing unpredictable tumor drug concentrations.37,38 Thus, water-soluble drugs that do not ordinarily reach the brain have proven effective against BM.
Phase III trials examining topotecan and carboplatin given in combination with WBRT failed to show a survival advantage over WBRT alone in patients with NSCLC with BM.39,40 A trial of temozolomide in combination with thalidomide for BM from melanoma also failed to show any significant benefit.41 However, a combination of cisplatin and etoposide, both water-soluble agents that do not penetrate the BBB, was efficacious against BM from breast carcinoma and NSCLC.42,43 Other cytotoxic agents with activity in BM include capecitabine,44 high-dose intravenous methotrexate,45 and temozolomide.46–48 The critical variable is to choose the chemotherapeutic agent based on the primary tumor and not the expected BBB penetration.
There is increasing evidence that targeted therapies can prevent and treat BM. A phase III study conducted in patients with advanced HER2-positive breast cancer showed that lapatinib plus capecitabine was associated with a lower rate of CNS progression than capecitabine alone (4% v 13%, respectively; P = .045).49 It is unclear whether this reflects a direct effect on the CNS or better systemic control with fewer BM from the combination therapy. CEREBEL (ClinicalTrials.gov identifier: NCT00820222; A Randomized, Multicentre, Open-Label, Phase III Study of Lapatinib Plus Capecitabine Versus Trastuzumab Plus Capecitabine in Patients With Anthracycline- or Taxane-Exposed ErbB2-Positive Metastatic Breast Cancer) is a study of whether lapatinib is superior to trastuzumab in reducing BM frequency.
Although these studies seek to reduce the occurrence of BM, others try to identify the best treatment against established BM. Lapatinib, given alone or in combination with capecitabine, has limited activity against BM.50,51 Other anti-HER2 drugs in trials include neratinib (NCT01494662) and afatinib (NCT01441596). A phase II study of BKM120, a phosphatidylinositol-3-kinase (PI3K) inhibitor with CNS penetration, plus capecitabine for triple-negative breast cancer with BM is also ongoing (NCT02000882).
Gefitinib and erlotinib, inhibitors of EGFR tyrosine kinase, have shown activity in BM from NSCLC with EGFR mutations52,53 (Fig 3). In a phase II trial, erlotinib administered concurrently with WBRT demonstrated an 86% response rate without increased neurotoxicity.53 Isolated CNS progression in patients with controlled systemic disease on standard-dose erlotinib and gefitinib may respond to a change in the dose and schedule to enhance CNS penetration; high-dose weekly pulsatile dosing has proven effective.54 In BM from BRAF-mutant melanoma, dabrafenib55 and vemurafenib56 also showed good intracranial response of 30% to 39%. However, sunitinib, a multireceptor tyrosine kinase inhibitor, was ineffective against BM from renal cell cancer, despite its activity against systemic disease.57
There were initial concerns of bevacizumab, a vascular endothelial growth factor inhibitor, causing intracranial hemorrhage in BM, but it has been proven safe.58,59 Small prospective studies of bevacizumab, in combination with other systemic agents, demonstrated activity against BM from heavily pretreated HER2-positive breast cancer,60 NSCLC,61 melanoma,62 and SCLC.63 Currently, there is an ongoing phase III trial examining the efficacy of bevacizumab, in addition to cisplatin and pemetrexed, in patients with NSCLC with asymptomatic BM (NCT02162537). Phase II trials of bevacizumab in BM from breast cancer (NCT02185352), melanoma (NCT02065466), and any solid tumor (NCT01898130) are also under way.
In recent years, advances in immunotherapy have provided more therapeutic options for patients with melanoma. Ipilimumab, a monoclonal antibody that blocks the inhibitory molecule cytotoxic T-lymphocyte antigen-4 (CTLA-4) and stimulates T-cell–mediated antitumor immune response, achieved CNS disease control in 24% of patients with melanoma with asymptomatic BM and 10% of patients with symptomatic disease.64 Other immune checkpoint inhibitors have not been tested specifically in BM.
Several interesting agents have been designed to deliver non–brain-permeable drugs across the BBB and are currently in clinical trials. One such example is GRN1005, which consists of paclitaxel attached to a peptide that binds low-density lipoprotein receptor–related protein receptors at the BBB and facilitates transport of paclitaxel across the BBB (NCT02048059). Early studies in malignant glioma suggested efficacy compared with unmodified paclitaxel.65 Whether this is appropriate for BM remains to be determined.
Radiation Therapy
WBRT is the most frequently used treatment for multiple BM and improves neurologic symptoms and median survival, from 1 to 2 months without WBRT to 3 to 6 months with it.66–68 Some primary tumors such as breast and lung cancer, especially SCLC, are more radiotherapy sensitive than others, including melanoma and colon and renal cancers. Indications for WBRT include the presence of multiple BMs, oligometastases (one to three metastases) with poorly controlled systemic disease, oligometastases too large (> 4 cm) for SRS, reirradiation after late WBRT failure, and after surgery or SRS.69 In patients with systemic disease progression, few treatment options, and poor performance status, supportive care alone may be appropriate.70
The recommended dose of WBRT is 30 Gy in 10 daily fractions. A Cochrane review of eight published trials evaluating various WBRT dose fractionation schedules compared with the standard regimen showed no significant difference in response rates or median survival71; however, daily fractions of more than 3 Gy may cause acute radiation toxicity with neurologic deterioration in patients with extensive pretreatment edema.72
Several studies have examined the effects of adding radiotherapy sensitizers, including lonidamine,73 metronidazole,74 misonidazole,75 motexafin gadolinium (MGd),76 bromodeoxyuridine,77 and efaproxiral,78 to WBRT, but none improved overall survival (OS)73–77 or local response rates.73,74,77,78 The phase III trial of efaproxiral also had negative results but improved response rates and survival in a subgroup of patients with breast cancer78; patients who received efaproxiral also reported improved QOL and Karnofsky performance score (KPS). In the randomized study of WBRT with or without MGd, subgroup analysis demonstrated improved neurologic and cognitive function in patients with lung cancer who received MGd, which could be attributed to better CNS disease control (median time to neurologic progression, 4.3 v 3.8 months, respectively; P = .018).76 However, none of these randomized trials were sufficiently powered for the subgroup analyses.
WBRT may be associated with delayed, progressive, irreversible cognitive dysfunction in long-term survivors; however, tumor progression affects neurocognitive function adversely more frequently in patients with BM than radiotherapy-induced neurotoxicity.79,80 Regardless, attempts have been made to reduce WBRT-associated neurotoxicity. Neuroprotective agents, including donepezil81 and memantine,82 have been tried without convincing success. A phase III trial of WBRT plus memantine versus WBRT plus placebo showed that patients receiving memantine had less decline in delayed recall at 24 weeks; however, the study was underpowered to show a significant difference based on only 149 evaluable patients.82 Hippocampus-avoidance WBRT, a novel technique devised to spare critical hippocampal areas and reduce the effects of radiotherapy on neural stem cells, was safe and possibly effective in preserving memory and QOL in phase II trials.83,84 Two ongoing cooperative group studies, the National Surgical Adjuvant Breast and Bowel Project, Radiation Therapy Oncology Group, and Gynecologic Oncology Group–Cancer Control (NRG-CC) 001 and NRG-CC 003 trials, are evaluating hippocampus-avoidance WBRT in a randomized fashion.85
Seventy to 80% of patients with BM have one to three lesions.86 In the 1980s, SRS was introduced and studied extensively in patients with oligometastases. The first randomized trial evaluating the role of SRS boost to WBRT versus WBRT alone, in patients with two to four metastases, was stopped at an interim evaluation because of a significant benefit in local control for the SRS arm (1-year failure rate, 8% v 100%, respectively; P < .001; median time to failure, 36 v 6 months, respectively; P < .001).87 With only 27 patients, the study failed to show significant survival benefit. A subsequent large prospective trial, RTOG 9508, demonstrated improved median survival from 4.9 months to 6.5 months in patients with a single BM who received WBRT and SRS compared with WBRT alone (P = .0393).88 RPA class 1 patients with oligometastases who received the SRS boost had improved median survival from 9.6 months to 11.6 months (P = .0452); significantly more patients in the SRS boost group had a stable or improved KPS score at 6-month follow-up (43% v 27% with WBRT alone; P = .03). This study also demonstrated that the gamma knife and linear accelerator were equivalent in delivering SRS effectively.
In a separate study, patients with one to four BMs were randomly assigned to receive either WBRT plus SRS at 30% reduced dose or full-dose SRS alone; there was no significant survival or functional difference between the groups.89 However, there was an increased frequency of intracranial relapse in the SRS-alone group compared with WBRT plus SRS (12-month brain recurrence rate, 76.4% v 46.8%, respectively; P < .001). Relapse was significantly higher in patients with two to four BMs before treatment, active extracranial metastases, and lower KPS of 70 to 80. A subsequent prospective trial examined neurocognitive functions of patients with oligometastases randomly assigned to receive SRS plus WBRT or SRS alone.90 This study was stopped prematurely because an interim analysis showed a 96% probability that the SRS plus WBRT group was more likely to show learning and memory decline at 4 months. Similar to the previous study, there was also an increased frequency of 1-year intracranial relapse in the SRS-alone group (73% v 23% with SRS plus WBRT; P < .001). However, more patients in the SRS plus WBRT group died at 4 months (29% v 13% with SRS alone), raising concern that general disease-related deterioration could have contributed to the significant cognitive decline in this group. Despite controversies surrounding the trial results, the lack of survival benefit, risk of radiation necrosis, and potential worse cognitive outcome with SRS plus WBRT have made SRS alone the preferred initial treatment option in patients with oligometastases smaller than 4 cm (Table 2).91 A recent cost-effectiveness study also showed that first-line SRS alone delivers more cost-effective care despite an increased need for salvage therapy, further supporting this approach.92
The role of SRS in patients with more than three BMs is less clear. A recent Japanese multi-institutional prospective observational cohort study showed that SRS alone as initial treatment for patients with five to 10 BMs demonstrated noninferior survival compared with patients with two to four BMs93; however, patients with five to 10 BMs had an increased incidence of leptomeningeal dissemination (P = .035) and new BM (P = .067). Whether earlier brain progression translates to worse cognition compared with WBRT is unknown but was suggested in the MGd WBRT study76 and will be studied in the North American Gamma Knife Consortium trial (NCT01731704). Cost-effectiveness of SRS to more than three BMs also warrants further assessment before routine use can be advocated in this setting.
SRS has also been studied as salvage therapy. A phase I trial, RTOG 9005, evaluated the safety of SRS in recurrent previously irradiated primary brain tumors and BM.94 Maximum-tolerated doses were determined as 24, 18, and 15 Gy for tumors ≤ 20, 21 to 30, and 31 to 40 mm in maximum diameter, respectively. Larger tumor diameter, higher radiotherapy dose, and worse KPS were significantly associated with grade 3 to 5 neurotoxicity. A retrospective study assessed efficacy of salvage SRS in recurrent BM after WBRT and demonstrated good 1-year (76.6%) and 2-year (57.9%) metastasis local control rates.95 A more recent retrospective review of 106 patients who received salvage SRS showed a 1-year local control rate of 60.1%, a median progression-free survival time of 6.2 months, and a median OS time of 11.7 months.96 Older age, poor systemic disease control, and increased time from initial radiotherapy to salvage SRS correlated with worse survival.
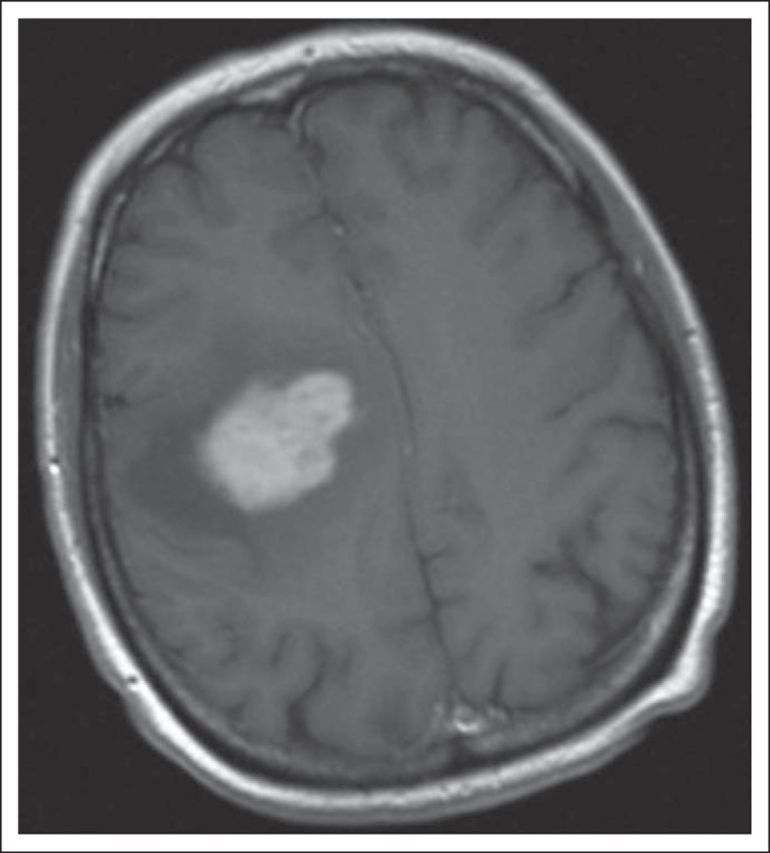
SRS leads to radiation necrosis in approximately 10% of patients, particularly when combined with or after WBRT.94,97,98 Radiation necrosis can be challenging to discriminate from recurrent BM on standard MRI. Perfusion MRI sequences may be useful because recurrent BMs are characterized by increased vascular perfusion, whereas radiation necrosis has decreased vascularity (Fig 4)99; however, these are relative changes with possible false-positive and false-negative results. Corticosteroids help reduce edema and improve neurologic function, but some patients with radiation necrosis become corticosteroid-dependent with the attendant toxicities. Surgical resection of radiation necrosis is the only definitive treatment; it usually provides symptomatic relief and allows reduction of corticosteroid dose but is not always feasible depending on the location. Antiplatelet agents, anticoagulation, and hyperbaric treatment have failed to show definite benefit.100 A retrospective study showed that bevacizumab reduced radiographic and clinical manifestations of radiation necrosis in patients with lung and breast carcinoma with BM.101 A small controlled trial also showed efficacy of bevacizumab in treatment of symptomatic CNS radiation necrosis in primary brain tumors and patients with head and neck carcinoma.102 This benefit remains to be validated in patients with BM.
Fig 4.
A 60-year-old man underwent surgical resection followed by stereotactic radiosurgery for an isolated left frontal metastasis secondary to lung adenocarcinoma. A year later, he developed an asymptomatic new contrast-enhancing lesion. (A) T1 postcontrast magnetic resonance imaging (MRI) demonstrating a heterogeneously contrast-enhancing left periventricular mass. (B) Dynamic contrast-enhanced perfusion MRI demonstrating lack of increased plasma volume in the contrast-enhancing left periventricular mass, strongly suggesting this is radiation necrosis. The patient was monitored with serial imaging with no significant growth of mass or new metastatic lesions.
Surgical Therapy
In the 1990s, three randomized controlled trials evaluated the benefit of surgical resection in addition to WBRT for single BM.16,103,104 Two trials showed survival and functionally independent survival benefits of surgery plus WBRT compared with WBRT alone.16,103 However, these benefits were not observed in a third trial, in part because the entire cohort in this study had a worse KPS and more extracranial metastases, and 22% of patients in the WBRT-alone group had surgery at progression.104 A Cochrane review of the three trials comprising 195 patients showed no significant OS difference,105 but there was a high degree of heterogeneity between the trials included in the meta-analysis (I2 = 83%). Thus, most view surgery as an effective treatment that can improve survival and outcome for patients with single BM.
Subsequent trials examined various postoperative therapies for single BM. A multicenter trial randomly assigned patients with single BM after resection to either WBRT (50.4 Gy in 28 fractions) or observation; WBRT reduced intracranial recurrence (18% v 70%, respectively; P < .001) and death as a result of neurologic cause (14% v 44%, respectively; P = .003) but did not improve OS.106 Despite enhanced intracranial control, the absence of a survival advantage and the risk of WBRT-associated neurotoxicity have resulted in continued controversy over the role of postoperative WBRT.
With growing interest in SRS, two studies, one retrospective107 and one prospective,108 examined SRS versus surgery plus WBRT for treatment of one or two BMs and showed no significant difference in OS or local control between the two groups. In the prospective study, more patients in the SRS group experienced distant brain recurrences, but SRS was associated with shorter hospital stay, less corticosteroid use, and lower toxicity.108
A retrospective, case-control study comparing surgery and WBRT versus SRS and WBRT in 133 patients showed no OS difference between the two groups.109 Multivariable analyses demonstrated a greater response of radiotherapy-resistant BM to SRS compared with surgery (P < .005). A more recent randomized noninferiority trial examining the same treatment arms was closed early as a result of slow accrual, with consequent low statistical power to detect any survival or local control differences.110 A subsequent trial, the European Organisation for Research and Treatment of Cancer 22952-26001 trial, assessed the efficacy of adjuvant WBRT in patients with one to three BMs treated with either SRS or surgery.111 OS and functional independence duration were similar in patients who did and did not receive WBRT (P < .001). WBRT improved 2-year local and distant cerebral control rates and reduced neurologic deaths. These studies indicate that SRS is an excellent treatment option for patients with oligometastases, especially when the primary tumor is considered radiotherapy resistant; the addition of WBRT may prolong CNS disease control but does not improve survival. Therefore, with careful surveillance, clinicians can use SRS alone with confidence and reserve WBRT for salvage if needed (Table 2).112 Cognitive function was not assessed in these studies, so detailed data on the differential cognitive consequences of WBRT versus an increased risk of intracranial relapse after SRS or surgery are not available.
In recent years, there has been a trend toward treating postsurgical cavities with SRS. A systematic review of 14 studies involving 629 patients with BM treated with SRS postoperatively demonstrated excellent 1-year local control (85%) and median OS (14 months)113 comparable to the postoperative WBRT study.106 A recent phase II trial also showed improved local control when a surgical cavity was treated with SRS114; large tumor size (≥ 3 cm) with superficial dural/pial involvement and infratentorial lesions were associated with worse local and regional control. With these data, close clinical monitoring with or without early SRS are reasonable alternatives to postoperative WBRT. The exception may be patients with large infratentorial BM with superficial dural/pial involvement; given their 40% incidence of subsequent leptomeningeal metastases, the authors recommend postoperative treatment (Table 2).115
Limited data exist from randomized trials comparing the efficacy of surgery alone with SRS alone for BM. An early study followed 13 patients and 62 matched patients treated with SRS and surgery, respectively, and demonstrated increased median survival time in the surgery group (7.5 v 16.4 months, respectively; P < .001).116 The higher mortality in the SRS group was a result of progression of SRS-treated BM and not poor systemic control. A subsequent retrospective review showed no significant survival difference when patients received either treatment.117 However, surgical resection resulted in increased local recurrence compared with SRS (58% v 0%, respectively; P = .02).
Pathologically, BMs have a relatively sharp border with normal surrounding brain, especially compared with malignant gliomas. However, new data suggest that BMs have an invasive edge that may account for their high local recurrence rate after surgical resection.118 Current surgical therapy involves conventional white-light microscopy-assisted microsurgical and circumferential stripping of BM from surrounding brain parenchyma.118 A recent study demonstrated that microscopic total resections, which included removal of adjacent brain parenchyma with pathologic confirmation of tumor-free resection margins, yielded better local control than gross total resections without removal of neighboring brain parenchyma (hazard ratio, 3.14; P = .003).119 Interestingly, there was no significant difference in local recurrence rate between microscopic total resection without radiotherapy and gross total resection with postoperative radiotherapy.
New or worsening perioperative neurologic deficit is always a potential concern, especially when metastases occur at critical sites. In a study of 206 patients with BM located in motor eloquent and noneloquent locations, 39 patients (19%) had improved neurologic status postoperatively, whereas 29 (14%) developed new deficits.120 Risk factors identified with postoperative motor deficits were prior radiotherapy, tumor located in eloquent cortex, and high RPA class. Thus, these factors must be considered before recommending surgery.
In conclusion, BMs are common, and their frequency is increasing. Current care involves radiotherapy, either SRS or WBRT, and/or surgery and depends on the number, size, and site of metastases, as well as overall systemic disease control and a patient's performance status. Systemic chemotherapeutic approaches are gaining traction and are increasingly efficacious options that are being used earlier in the course of the illness. Early vigorous treatment can enhance a patient's functional status and prolong CNS disease control and survival. Better understanding of the interactions between BM, the microenvironment, and the BBB may identify novel targets to prevent and treat BM.
Addendum
The North Central Cancer Treatment Group trial N0574 that was recently presented at the 2015 American Society of Clinical Oncology Annual Meeting examined patients with one to three brain metastases who received SRS with or without WBRT. The study showed that patients who received WBRT plus SRS had worse cognitive outcome (P < .001) despite improved local control (P < .001); there was no significant difference in overall survival between the two groups (P = .092).121 This further supports the use of SRS alone with close monitoring as up-front therapy for patients with newly diagnosed brain metastases.
Footnotes
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Disclosures provided by the authors are available with this article at www.jco.org.
AUTHOR CONTRIBUTIONS
Conception and design: Lisa M. DeAngelis
Collection and assembly of data: All authors
Data analysis and interpretation: Lisa M. DeAngelis
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Treatment of Brain Metastases
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Xuling Lin
No relationship to disclose
Lisa M. DeAngelis
Consulting or Advisory Role: Celgene, CarThera, BTG International
Patents, Royalties, Other Intellectual Property: Oxford University Press, royalties
REFERENCES
- 1.Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978;19:579–592. [PubMed] [Google Scholar]
- 2.Takakura K. Metastatic Tumors of the Central Nervous System. Tokyo, Japan: Igaku-Shoin; 1982. [Google Scholar]
- 3.Stewart BW, Wild C International Agency for Research on Cancer, et al. World Cancer Report 2014. Lyon, France: International Agency for Research on Cancer; 2014. [Google Scholar]
- 4.Nussbaum ES, Djalilian HR, Cho KH, et al. Brain metastases: Histology, multiplicity, surgery, and survival. Cancer. 1996;78:1781–1788. [PubMed] [Google Scholar]
- 5.Lai R, Dang CT, Malkin MG, et al. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101:810–816. doi: 10.1002/cncr.20418. [DOI] [PubMed] [Google Scholar]
- 6.Omuro AM, Kris MG, Miller VA, et al. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103:2344–2348. doi: 10.1002/cncr.21033. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar LE, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 8.Lutterbach J, Bartelt S, Ostertag C. Long-term survival in patients with brain metastases. J Cancer Res Clin Oncol. 2002;128:417–425. doi: 10.1007/s00432-002-0354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaal EC, Niël CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4:289–298. doi: 10.1016/S1474-4422(05)70072-7. [DOI] [PubMed] [Google Scholar]
- 10.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rwigema JC, Wegner RE, Mintz AH, et al. Stereotactic radiosurgery to the resection cavity of brain metastases: A retrospective analysis and literature review. Stereotact Funct Neurosurg. 2011;89:329–337. doi: 10.1159/000330387. [DOI] [PubMed] [Google Scholar]
- 12.Iorio-Morin C, Masson-Cote L, Ezahr Y, et al. Early Gamma Knife stereotactic radiosurgery to the tumor bed of resected brain metastasis for improved local control. J Neurosurg. 2014;121(suppl):69–74. doi: 10.3171/2014.7.GKS141488. [DOI] [PubMed] [Google Scholar]
- 13.Robinet G, Thomas P, Breton JL, et al. Results of a phase III study of early versus delayed whole brain radiotherapy with concurrent cisplatin and vinorelbine combination in inoperable brain metastasis of non-small-cell lung cancer: Groupe Francais de Pneumo-Cancérologie (GFPC) Protocol 95-1. Ann Oncol. 2001;12:59–67. doi: 10.1023/a:1008338312647. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Han JY, Kim HT, et al. Primary chemotherapy for newly diagnosed nonsmall cell lung cancer patients with synchronous brain metastases compared with whole-brain radiotherapy administered first: Result of a randomized pilot study. Cancer. 2008;113:143–149. doi: 10.1002/cncr.23526. [DOI] [PubMed] [Google Scholar]
- 15.Kim KH, Lee J, Lee JI, et al. Can upfront systemic chemotherapy replace stereotactic radiosurgery or whole brain radiotherapy in the treatment of non-small cell lung cancer patients with asymptomatic brain metastases? Lung Cancer. 2010;68:258–263. doi: 10.1016/j.lungcan.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 17.Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soffietti R, Cornu P, Delattre JY, et al. EFNS guidelines on diagnosis and treatment of brain metastases: Report of an EFNS Task Force. Eur J Neurol. 2006;13:674–681. doi: 10.1111/j.1468-1331.2006.01506.x. [DOI] [PubMed] [Google Scholar]
- 19.Vecht CJ, Hovestadt A, Verbiest HB, et al. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: A randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994;44:675–680. doi: 10.1212/wnl.44.4.675. [DOI] [PubMed] [Google Scholar]
- 20.Hempen C, Weiss E, Hess CF. Dexamethasone treatment in patients with brain metastases and primary brain tumors: Do the benefits outweigh the side-effects? Support Care Cancer. 2002;10:322–328. doi: 10.1007/s00520-001-0333-0. [DOI] [PubMed] [Google Scholar]
- 21.Oberndorfer S, Schmal T, Lahrmann H, et al. The frequency of seizures in patients with primary brain tumors or cerebral metastases: An evaluation from the Ludwig Boltzmann Institute of Neuro-Oncology and the Department of Neurology, Kaiser Franz Josef Hospital, Vienna [in German] Wien Klin Wochenschr. 2002;114:911–916. [PubMed] [Google Scholar]
- 22.Glantz MJ, Cole BF, Friedberg MH, et al. A randomized, blinded, placebo-controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology. 1996;46:985–991. doi: 10.1212/wnl.46.4.985. [DOI] [PubMed] [Google Scholar]
- 23.North JB, Penhall RK, Hanieh A, et al. Phenytoin and postoperative epilepsy: A double-blind study. J Neurosurg. 1983;58:672–677. doi: 10.3171/jns.1983.58.5.0672. [DOI] [PubMed] [Google Scholar]
- 24.Forsyth PA, Weaver S, Fulton D, et al. Prophylactic anticonvulsants in patients with brain tumour. Can J Neurol Sci. 2003;30:106–112. doi: 10.1017/s0317167100053361. [DOI] [PubMed] [Google Scholar]
- 25.Franceschetti S, Binelli S, Casazza M, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochir (Wien) 1990;103:47–51. doi: 10.1007/BF01420191. [DOI] [PubMed] [Google Scholar]
- 26.Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: Anticonvulsant prophylaxis in patients with newly diagnosed brain tumors—Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 27.Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: A population-based case-control study. Arch Intern Med. 2000;160:809–815. doi: 10.1001/archinte.160.6.809. [DOI] [PubMed] [Google Scholar]
- 28.Alvarado G, Noor R, Bassett R, et al. Risk of intracranial hemorrhage with anticoagulation therapy in melanoma patients with brain metastases. Melanoma Res. 2012;22:310–315. doi: 10.1097/CMR.0b013e328353efd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iorio A, Agnelli G. Low-molecular-weight and unfractionated heparin for prevention of venous thromboembolism in neurosurgery: A meta-analysis. Arch Intern Med. 2000;160:2327–2332. doi: 10.1001/archinte.160.15.2327. [DOI] [PubMed] [Google Scholar]
- 30.Schiff D, DeAngelis LM. Therapy of venous thromboembolism in patients with brain metastases. Cancer. 1994;73:493–498. doi: 10.1002/1097-0142(19940115)73:2<493::aid-cncr2820730240>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 31.Peak S, Abrey LE. Chemotherapy and the treatment of brain metastases. Hematol Oncol Clin North Am. 2006;20:1287–1295. doi: 10.1016/j.hoc.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Oechsle K, Bokemeyer C. Treatment of brain metastases from germ cell tumors. Hematol Oncol Clin North Am. 2011;25:605–613. doi: 10.1016/j.hoc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Kristensen CA, Kristjansen PE, Hansen HH. Systemic chemotherapy of brain metastases from small-cell lung cancer: A review. J Clin Oncol. 1992;10:1498–1502. doi: 10.1200/JCO.1992.10.9.1498. [DOI] [PubMed] [Google Scholar]
- 34.Grossi F, Scolaro T, Tixi L, et al. The role of systemic chemotherapy in the treatment of brain metastases from small-cell lung cancer. Crit Rev Oncol Hematol. 2001;37:61–67. doi: 10.1016/s1040-8428(00)00098-6. [DOI] [PubMed] [Google Scholar]
- 35.Shah SP, Morin RD, Khattra J, et al. Mutational evolution in a lobular breast tumour profiled at single nucleotide resolution. Nature. 2009;461:809–813. doi: 10.1038/nature08489. [DOI] [PubMed] [Google Scholar]
- 36.Campbell PJ, Yachida S, Mudie LJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467:1109–1113. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16:5664–5678. doi: 10.1158/1078-0432.CCR-10-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pitz MW, Desai A, Grossman SA, et al. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104:629–638. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neuhaus T, Ko Y, Muller RP, et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS-metastases due to lung cancer. Br J Cancer. 2009;100:291–297. doi: 10.1038/sj.bjc.6604835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerrieri M, Wong K, Ryan G, et al. A randomised phase III study of palliative radiation with concomitant carboplatin for brain metastases from non-small cell carcinoma of the lung. Lung Cancer. 2004;46:107–111. doi: 10.1016/j.lungcan.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 41.Krown SE, Niedzwiecki D, Hwu WJ, et al. Phase II study of temozolomide and thalidomide in patients with metastatic melanoma in the brain: High rate of thromboembolic events (CALGB 500102) Cancer. 2006;107:1883–1890. doi: 10.1002/cncr.22239. [DOI] [PubMed] [Google Scholar]
- 42.Cocconi G, Lottici R, Bisagni G, et al. Combination therapy with platinum and etoposide of brain metastases from breast carcinoma. Cancer Invest. 1990;8:327–334. doi: 10.3109/07357909009012049. [DOI] [PubMed] [Google Scholar]
- 43.Franciosi V, Cocconi G, Michiara M, et al. Front-line chemotherapy with cisplatin and etoposide for patients with brain metastases from breast carcinoma, nonsmall cell lung carcinoma, or malignant melanoma: A prospective study. Cancer. 1999;85:1599–1605. [PubMed] [Google Scholar]
- 44.Ekenel M, Hormigo AM, Peak S, et al. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85:223–227. doi: 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 45.Lassman AB, Abrey LE, Shah GD, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78:255–260. doi: 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 46.Antonadou D, Paraskevaidis M, Sarris G, et al. Phase II randomized trial of temozolomide and concurrent radiotherapy in patients with brain metastases. J Clin Oncol. 2002;20:3644–3650. doi: 10.1200/JCO.2002.04.140. [DOI] [PubMed] [Google Scholar]
- 47.Agarwala SS, Kirkwood JM, Gore M, et al. Temozolomide for the treatment of brain metastases associated with metastatic melanoma: A phase II study. J Clin Oncol. 2004;22:2101–2107. doi: 10.1200/JCO.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 48.Verger E, Gil M, Yaya R, et al. Temozolomide and concomitant whole brain radiotherapy in patients with brain metastases: A phase II randomized trial. Int J Radiat Oncol Biol Phys. 2005;61:185–191. doi: 10.1016/j.ijrobp.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 49.Cameron D, Casey M, Press M, et al. A phase III randomized comparison of lapatinib plus capecitabine versus capecitabine alone in women with advanced breast cancer that has progressed on trastuzumab: Updated efficacy and biomarker analyses. Breast Cancer Res Treat. 2008;112:533–543. doi: 10.1007/s10549-007-9885-0. [DOI] [PubMed] [Google Scholar]
- 50.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 51.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 52.Shimato S, Mitsudomi T, Kosaka T, et al. EGFR mutations in patients with brain metastases from lung cancer: Association with the efficacy of gefitinib. Neuro Oncol. 2006;8:137–144. doi: 10.1215/15228517-2005-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31:895–902. doi: 10.1200/JCO.2011.40.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grommes C, Oxnard GR, Kris MG, et al. “Pulsatile” high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol. 2011;13:1364–1369. doi: 10.1093/neuonc/nor121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13:1087–1095. doi: 10.1016/S1470-2045(12)70431-X. [DOI] [PubMed] [Google Scholar]
- 56.Dummer R, Goldinger SM, Turtschi CP, et al. Vemurafenib in patients with BRAF(V600) mutation-positive melanoma with symptomatic brain metastases: Final results of an open-label pilot study. Eur J Cancer. 2014;50:611–621. doi: 10.1016/j.ejca.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 57.Chevreau C, Ravaud A, Escudier B, et al. A phase II trial of sunitinib in patients with renal cell cancer and untreated brain metastases. Clin Genitourin Cancer. 2014;12:50–54. doi: 10.1016/j.clgc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Socinski MA, Langer CJ, Huang JE, et al. Safety of bevacizumab in patients with non-small-cell lung cancer and brain metastases. J Clin Oncol. 2009;27:5255–5261. doi: 10.1200/JCO.2009.22.0616. [DOI] [PubMed] [Google Scholar]
- 59.Khasraw M, Holodny A, Goldlust SA, et al. Intracranial hemorrhage in patients with cancer treated with bevacizumab: The Memorial Sloan-Kettering experience. Ann Oncol. 2012;23:458–463. doi: 10.1093/annonc/mdr148. [DOI] [PubMed] [Google Scholar]
- 60.Falchook GS, Moulder SL, Wheler JJ, et al. Dual HER2 inhibition in combination with anti-VEGF treatment is active in heavily pretreated HER2-positive breast cancer. Ann Oncol. 2013;24:3004–3011. doi: 10.1093/annonc/mdt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falchook GS, Naing A, Hong DS, et al. Dual EGFR inhibition in combination with anti-VEGF treatment: A phase I clinical trial in non-small cell lung cancer. Oncotarget. 2013;4:118–127. doi: 10.18632/oncotarget.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.González-Cao M, Viteri S, Díaz-Lagares A, et al. Preliminary results of the combination of bevacizumab and weekly paclitaxel in advanced melanoma. Oncology. 2008;74:12–16. doi: 10.1159/000138351. [DOI] [PubMed] [Google Scholar]
- 63.Mountzios G, Emmanouilidis C, Vardakis N, et al. Paclitaxel plus bevacizumab in patients with chemoresistant relapsed small cell lung cancer as salvage treatment: A phase II multicenter study of the Hellenic Oncology Research Group. Lung Cancer. 2012;77:146–150. doi: 10.1016/j.lungcan.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012;13:459–465. doi: 10.1016/S1470-2045(12)70090-6. [DOI] [PubMed] [Google Scholar]
- 65.Drappatz J, Brenner A, Wong ET, et al. Phase I study of GRN1005 in recurrent malignant glioma. Clin Cancer Res. 2013;19:1567–1576. doi: 10.1158/1078-0432.CCR-12-2481. [DOI] [PubMed] [Google Scholar]
- 66.Cairncross JG, Kim JH, Posner JB. Radiation therapy for brain metastases. Ann Neurol. 1980;7:529–541. doi: 10.1002/ana.410070606. [DOI] [PubMed] [Google Scholar]
- 67.Chatani M, Teshima T, Hata K, et al. Whole brain irradiation for metastases from lung carcinoma: A clinical investigation. Acta Radiol Oncol. 1985;24:311–314. doi: 10.3109/02841868509136057. [DOI] [PubMed] [Google Scholar]
- 68.Hoskin PJ, Crow J, Ford HT. The influence of extent and local management on the outcome of radiotherapy for brain metastases. Int J Radiat Oncol Biol Phys. 1990;19:111–115. doi: 10.1016/0360-3016(90)90142-7. [DOI] [PubMed] [Google Scholar]
- 69.DeAngelis LM, Posner JB, Posner JB. Neurologic Complications of Cancer (ed 2) New York, NY: Oxford University Press; 2009. [Google Scholar]
- 70.Gerrard GE, Prestwich RJ, Edwards A, et al. Investigating the palliative efficacy of whole-brain radiotherapy for patients with multiple-brain metastases and poor prognostic features. Clin Oncol (R Coll Radiol) 2003;15:422–428. doi: 10.1016/s0936-6555(03)00148-1. [DOI] [PubMed] [Google Scholar]
- 71.Tsao MN, Lloyd N, Wong RK, et al. Whole brain radiotherapy for the treatment of newly diagnosed multiple brain metastases. Cochrane Database Syst Rev. 2012;4:CD003869. doi: 10.1002/14651858.CD003869.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young DF, Posner JB, Chu F, et al. Rapid-course radiation therapy of cerebral metastases: Results and complications. Cancer. 1974;34:1069–1076. doi: 10.1002/1097-0142(197410)34:4<1069::aid-cncr2820340416>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 73.DeAngelis LM, Currie VE, Kim JH, et al. The combined use of radiation therapy and lonidamine in the treatment of brain metastases. J Neurooncol. 1989;7:241–247. doi: 10.1007/BF00172917. [DOI] [PubMed] [Google Scholar]
- 74.Eyre HJ, Ohlsen JD, Frank J, et al. Randomized trial of radiotherapy versus radiotherapy plus metronidazole for the treatment metastatic cancer to brain: A Southwest Oncology Group study. J Neurooncol. 1984;2:325–330. doi: 10.1007/BF00178115. [DOI] [PubMed] [Google Scholar]
- 75.Komarnicky LT, Phillips TL, Martz K, et al. A randomized phase III protocol for the evaluation of misonidazole combined with radiation in the treatment of patients with brain metastases (RTOG-7916) Int J Radiat Oncol Biol Phys. 1991;20:53–58. doi: 10.1016/0360-3016(91)90137-s. [DOI] [PubMed] [Google Scholar]
- 76.Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol. 2003;21:2529–2536. doi: 10.1200/JCO.2003.12.122. [DOI] [PubMed] [Google Scholar]
- 77.Phillips TL, Scott CB, Leibel SA, et al. Results of a randomized comparison of radiotherapy and bromodeoxyuridine with radiotherapy alone for brain metastases: Report of RTOG trial 89-05. Int J Radiat Oncol Biol Phys. 1995;33:339–348. doi: 10.1016/0360-3016(95)00168-X. [DOI] [PubMed] [Google Scholar]
- 78.Suh JH, Stea B, Nabid A, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106–114. doi: 10.1200/JCO.2004.00.1768. [DOI] [PubMed] [Google Scholar]
- 79.Li J, Bentzen SM, Renschler M, et al. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 80.Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68:1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 81.Jatoi A, Kahanic SP, Frytak S, et al. Donepezil and vitamin E for preventing cognitive dysfunction in small cell lung cancer patients: Preliminary results and suggestions for future study designs. Support Care Cancer. 2005;13:66–69. doi: 10.1007/s00520-004-0696-0. [DOI] [PubMed] [Google Scholar]
- 82.Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gondi V, Tome WA, Marsh J, et al. Estimated risk of perihippocampal disease progression after hippocampal avoidance during whole-brain radiotherapy: Safety profile for RTOG 0933. Radiother Oncol. 2010;95:327–331. doi: 10.1016/j.radonc.2010.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): A phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Suh JH. Hippocampal-avoidance whole-brain radiation therapy: A new standard for patients with brain metastases? J Clin Oncol. 2014;32:3789–3791. doi: 10.1200/JCO.2014.58.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Delattre JY, Krol G, Thaler HT, et al. Distribution of brain metastases. Arch Neurol. 1988;45:741–744. doi: 10.1001/archneur.1988.00520310047016. [DOI] [PubMed] [Google Scholar]
- 87.Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 88.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 89.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 90.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 91.Tsao M, Xu W, Sahgal A. A meta-analysis evaluating stereotactic radiosurgery, whole-brain radiotherapy, or both for patients presenting with a limited number of brain metastases. Cancer. 2012;118:2486–2493. doi: 10.1002/cncr.26515. [DOI] [PubMed] [Google Scholar]
- 92.Hall MD, McGee JL, McGee MC, et al. Cost-effectiveness of stereotactic radiosurgery with and without whole-brain radiotherapy for the treatment of newly diagnosed brain metastases. J Neurosurg. 2014;121(suppl):84–90. doi: 10.3171/2014.7.GKS14972. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 94.Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291–298. doi: 10.1016/s0360-3016(99)00507-6. [DOI] [PubMed] [Google Scholar]
- 95.Yomo S, Hayashi M. The efficacy and limitations of stereotactic radiosurgery as a salvage treatment after failed whole brain radiotherapy for brain metastases. J Neurooncol. 2013;113:459–465. doi: 10.1007/s11060-013-1138-y. [DOI] [PubMed] [Google Scholar]
- 96.Kurtz G, Zadeh G, Gingras-Hill G, et al. Salvage radiosurgery for brain metastases: Prognostic factors to consider in patient selection. Int J Radiat Oncol Biol Phys. 2014;88:137–142. doi: 10.1016/j.ijrobp.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 97.Marks JE, Wong J. The risk of cerebral radionecrosis in relation to dose, time and fractionation: A follow-up study. Prog Exp Tumor Res. 1985;29:210–218. doi: 10.1159/000411642. [DOI] [PubMed] [Google Scholar]
- 98.Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010;99:81–88. doi: 10.1007/s11060-009-0106-z. [DOI] [PubMed] [Google Scholar]
- 100.Glantz MJ, Burger PC, Friedman AH, et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020–2027. doi: 10.1212/wnl.44.11.2020. [DOI] [PubMed] [Google Scholar]
- 101.Boothe D, Young R, Yamada Y, et al. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro Oncol. 2013;15:1257–1263. doi: 10.1093/neuonc/not085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993;33:583–590. doi: 10.1002/ana.410330605. [DOI] [PubMed] [Google Scholar]
- 104.Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996;78:1470–1476. doi: 10.1002/(sici)1097-0142(19961001)78:7<1470::aid-cncr14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 105.Hart MG, Grant R, Walker M, et al. Surgical resection and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases. Cochrane Database Syst Rev. 2005;1:CD003292. doi: 10.1002/14651858.CD003292.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 107.Rades D, Bohlen G, Pluemer A, et al. Stereotactic radiosurgery alone versus resection plus whole-brain radiotherapy for 1 or 2 brain metastases in recursive partitioning analysis class 1 and 2 patients. Cancer. 2007;109:2515–2521. doi: 10.1002/cncr.22729. [DOI] [PubMed] [Google Scholar]
- 108.Muacevic A, Wowra B, Siefert A, et al. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: A randomized controlled multicentre phase III trial. J Neurooncol. 2008;87:299–307. doi: 10.1007/s11060-007-9510-4. [DOI] [PubMed] [Google Scholar]
- 109.Schoggl A, Kitz K, Reddy M, et al. Defining the role of stereotactic radiosurgery versus microsurgery in the treatment of single brain metastases. Acta Neurochir (Wien) 2000;142:621–626. doi: 10.1007/s007010070104. [DOI] [PubMed] [Google Scholar]
- 110.Roos DE, Smith JG, Stephens SW. Radiosurgery versus surgery, both with adjuvant whole brain radiotherapy, for solitary brain metastases: A randomised controlled trial. Clin Oncol (R Coll Radiol) 2011;23:646–651. doi: 10.1016/j.clon.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 111.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952-26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Linskey ME, Andrews DW, Asher AL, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gans JH, Raper DM, Shah AH, et al. The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery. 2013;72:317–325. doi: 10.1227/NEU.0b013e31827fcd60. [DOI] [PubMed] [Google Scholar]
- 114.Brennan C, Yang TJ, Hilden P, et al. A phase 2 trial of stereotactic radiosurgery boost after surgical resection for brain metastases. Int J Radiat Oncol Biol Phys. 2014;88:130–136. doi: 10.1016/j.ijrobp.2013.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.van der Ree TC, Dippel DW, Avezaat CJ, et al. Leptomeningeal metastasis after surgical resection of brain metastases. J Neurol Neurosurg Psychiatry. 1999;66:225–227. doi: 10.1136/jnnp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bindal AK, Bindal RK, Hess KR, et al. Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg. 1996;84:748–754. doi: 10.3171/jns.1996.84.5.0748. [DOI] [PubMed] [Google Scholar]
- 117.O'Neill BP, Iturria NJ, Link MJ, et al. A comparison of surgical resection and stereotactic radiosurgery in the treatment of solitary brain metastases. Int J Radiat Oncol Biol Phys. 2003;55:1169–1176. doi: 10.1016/s0360-3016(02)04379-1. [DOI] [PubMed] [Google Scholar]
- 118.Kamp MA, Dibue M, Santacroce A, et al. The tumour is not enough or is it? Problems and new concepts in the surgery of cerebral metastases. Ecancermedicalscience. 2013;7:306. doi: 10.3332/ecancer.2013.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoo H, Kim YZ, Nam BH, et al. Reduced local recurrence of a single brain metastasis through microscopic total resection. J Neurosurg. 2009;110:730–736. doi: 10.3171/2008.8.JNS08448. [DOI] [PubMed] [Google Scholar]
- 120.Obermueller T, Schaeffner M, Gerhardt J, et al. Risks of postoperative paresis in motor eloquently and non-eloquently located brain metastases. BMC Cancer. 2014;14:21. doi: 10.1186/1471-2407-14-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown PD, Asher AL, Ballman KV, et al. NCCTG N0574 (Alliance): A phase III randomized trial of whole brain radiation therapy (WBRT) in addition to radiosurgery (SRS) in patients with 1 to 3 brain metastases. J Clin Oncol. 2015;33(suppl):4s. abstr LBA4. [Google Scholar]