Abstract
Introduction
Vemurafenib induces tumour regression in most patients with BRAFV600E-mutant melanoma; eventually, most experience progressive disease (PD). Long-term follow-up of patients with BRAFV600E melanoma treated in the phase 1 vemurafenib trial is reported.
Methods
Patients received vemurafenib 240–1120 mg (dose escalation cohort) or 960 mg (extension cohort) orally twice daily. Clinical response was evaluated every 8 weeks by RECIST. Patients with PD amenable to local therapy (surgery or radiotherapy) were allowed to continue vemurafenib after progression. Overall survival (OS) from time of treatment initiation and from PD was estimated. Sites of PD were recorded.
Results
Forty-eight patients (escalation cohort, n = 16; extension cohort, n = 32) received therapeutic doses of vemurafenib (≥240 mg twice daily). Forty-three patients had PD by the time of this analysis, and 5 remained progression free (follow-up time, 1.2–56.1 months). Median OS was 14 months (range, 1.2–56.1); 3- and 4-year melanoma-specific survival rate in the extension cohort was 26% and 19%, respectively. Median OS was 26.0 months (range, 7.7–56.1) among 20 patients who continued vemurafenib after local therapy. Median treatment duration beyond initial PD was 3.8 months (range, 1.1–26.6). In the extension cohort, 6 and 5 patients were alive after 3 and 4 years, respectively, on vemurafenib monotherapy.
Conclusions
Some patients with melanoma achieved long-term survival with vemurafenib monotherapy. Continuation of vemurafenib after PD might be beneficial in some patients because remaining disease might continue to respond to BRAF inhibition.
Keywords: vemurafenib, BRAF inhibitor, metastatic melanoma
1. Introduction
Oncogenic BRAF signalling is implicated in ~50% of melanomas, making BRAF a key therapeutic target [1–3]. BRAF inhibition blocks cell growth in most BRAF-mutant melanoma cell lines [4–9]. The most common oncogenic BRAF mutation involves substitution of glutamic acid for valine at codon 600 (V600E) in exon 15 [5,7]. Vemurafenib is a potent inhibitor of BRAFV600E [7,10].
The phase 1 trial of vemurafenib in patients with advanced solid tumours (ClinicalTrials.gov ID, NCT00405587) identified a biologically active serum concentration with twice-daily dosing of ≥240 mg [11]. A maximum tolerated dose (MTD) of 960 mg twice daily was identified, and unconfirmed, investigator-assessed partial response (PR) occurred in 24 of 32 patients in an extension cohort at this dose; complete response (CR) occurred in 2 patients [11]. Estimated median progression-free survival (PFS) in the extension cohort was >7 months; overall survival (OS) had not been reached at the time of the initial report [11]. A phase 2 study of vemurafenib in patients with metastatic melanoma harbouring BRAFV600 mutations showed a confirmed, independently reviewed overall response rate of 53%, median PFS of 6.8 months, and median OS of 15.9 months [12]. Results of the randomised phase 3 study (BRIM3) showed that vemurafenib improved OS compared with dacarbazine (hazard ratio for death, 0.70; 95% confidence interval [CI], 0.57–0.87; P = 0.0008) in patients with BRAFV600E [12–14]. Vemurafenib has been approved in more than 80 countries for the treatment of BRAFV600E-mutant metastatic melanoma.
Herein we report long-term survival of patients with BRAF-mutant melanoma treated with vemurafenib in the phase 1 study and evaluate long-term efficacy among patients who did and did not continue vemurafenib after confirmation of progressive disease (PD).
2. METHODS
Methodology of the phase 1 study of vemurafenib was reported previously [11].
2.1 Study design
There were 2 stages: dose escalation and extension. The dose escalation cohort identified a recommended phase 2 dose, defined as the highest dose at which ≤1 of 6 patients had dose-limiting toxicities (MTDs) [11]. An extension cohort evaluated response rate at the recommended phase 2 dose (960 mg twice daily) in patients with prospectively confirmed BRAFV600E-mutant melanoma [11]. Patients continued treatment until occurrence of unacceptable adverse events (AEs) or PD. Safety evaluations were conducted every 4 weeks and AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [11].
2.2 Treatment after progressive disease
PD was defined per Response Evaluation Criteria In Solid Tumors (RECIST), version 1.0 [15]. Patients with PD limited to sites suitable for local therapy (surgery or radiotherapy) could continue receiving vemurafenib after local therapy [11].
2.3 Study population
Patients ≥18 years of age with any histologically confirmed solid tumour refractory to standard therapy, or for which standard or curative therapy did not exist, were eligible [11]. Patients had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1; life expectancy ≥3 months [16]; adequate hematologic, hepatic, and renal function; and no progressive or unstable brain metastases.
Current analyses are restricted to patients with BRAFV600E-mutant melanoma, identified by certified BRAF sequencing analysis at each study centre in the dose escalation cohort (n = 16) or polymerase chain reaction–based assay (cobas 4800 BRAF V600 Mutation Test; Roche Molecular Systems, Pleasanton, CA, USA) in the extension cohort (n = 32). These patients received vemurafenib ≥240 mg twice daily.
2.4 Study assessments
This analysis describes long-term follow-up and clinical characteristics of patients who experienced durable clinical response and long-term survival. PD patterns in patients receiving vemurafenib and outcomes after local therapy for PD in patients continuing vemurafenib were assessed. Computed tomography (CT) was performed every 8 weeks during therapy. Tumour response was assessed according to RECIST v1.0 [15]. End points were objective response (CR or PR) confirmed ≥4 weeks after initial documentation, duration of objective response (defined as time from initial CR or PR to PD or death), and PFS.
PFS (defined as time from first treatment to first documentation of PD or death, whichever occurred first) and OS (defined as time from enrolment to death as a result of any cause) were estimated using the Kaplan-Meier method. Sub-group analyses were conducted based on PD pattern and subsequent therapy. Survival outcomes were analysed for patients in the extension cohort who survived >3 years and for 20 patients who received vemurafenib for >30 days after disease progression. The >30 days’ duration was chosen to distinguish between i) patients who continued with vemurafenib after progression but only until the results of the confirmatory biopsy for tissue analysis, and ii) patients who continued with vemurafenib after progression and local therapy. Median survival beyond initial PD (defined as time from PD to death as a result of any cause) and melanoma-specific survival were assessed.
2.4 Statistical analysis
Descriptive statistics (mean, standard deviation [SD], range) are presented. Kaplan-Meier survival curves were generated by SAS version 8.1 (SAS Institute, Cary, NC, USA). Data cutoff was March 6, 2014.
3. RESULTS
Between August 2008 and August 2009, 48 patients with BRAFV600E-metastatic melanoma were enrolled in the phase 1 trial; they received vemurafenib ≥240 mg twice daily (16 in the dose escalation cohort and 32 in the extension cohort) [11]. Patient demographics and key baseline characteristics are shown in Table 1. At the time of analysis, median follow-up duration was 13.8 months (range, 1.2–56.1) for all patients and 12.3 months (range, 1.2–56.1) for the extension cohort.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | Patients n = 48 (dose escalation [n = 16] dose extension [n = 32]) |
Vemurafenib treatment after PD N = 44 |
|
|---|---|---|---|
| >30 days (n = 20)a |
Discontinued (n = 24)b |
||
| Age, years, median (range) |
53 (22–88) | 52 (22–65) | 53 (23–88) |
| Male, n (%) | 27 (56) | 15 (75) | 11 (46) |
| Confirmed stage M1c disease, n (%) |
35 (73) | 12 (60) | 22 (92) |
| ECOG PS of 1, n (%) | 27 (56) | 12 (60) | 15 (63) |
| Received ≥2 previous systemic therapies, n (%) |
26 (54) | 10 (50) | 16 (67) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; PD, progressive disease.
All 20 patients experienced limited disease progression and continued vemurafenib after local therapy.
All 24 patients received <30 days of vemurafenib after disease progression only for the purpose of tumour biopsy at the time of disease progression.
3.1 Clinical outcome and OS
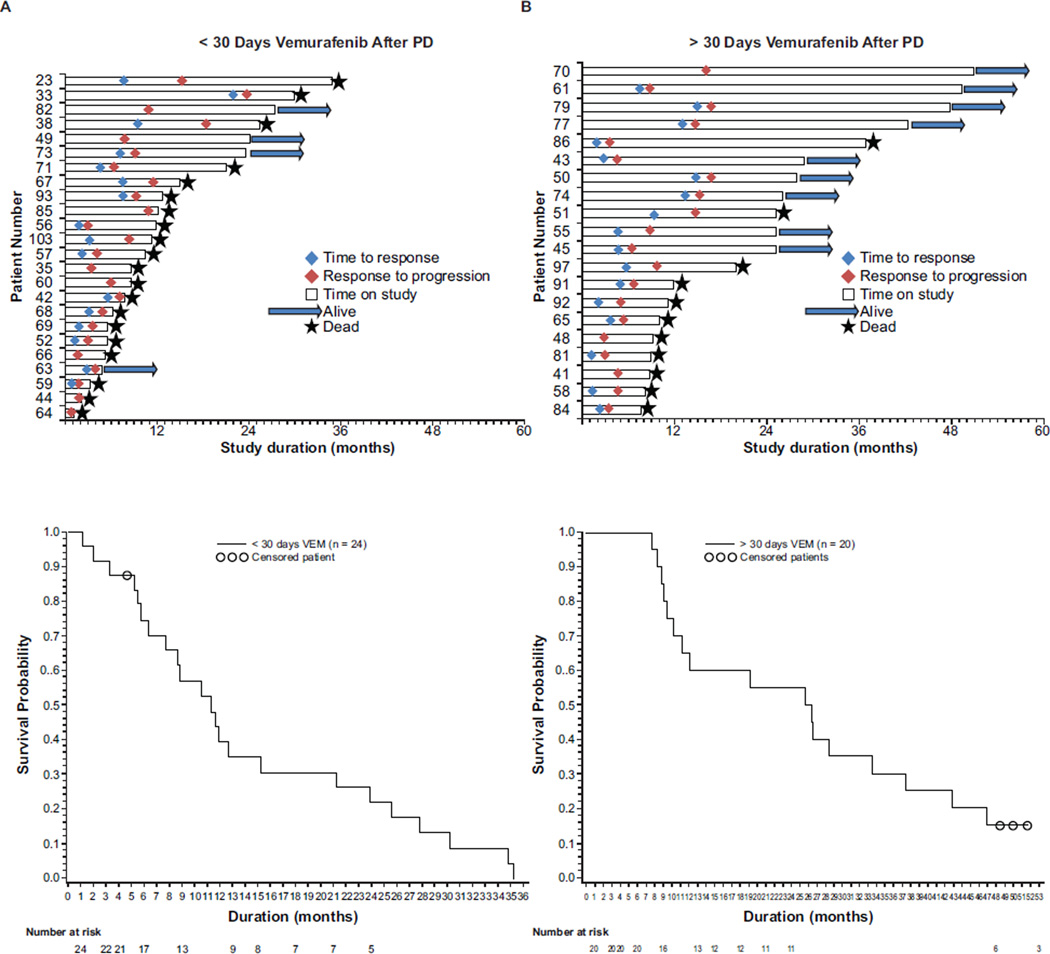
Median PFS for all patients was 7.2 months (range, 0.9–56.0). Median PFS was similar in patients who received vemurafenib for <30 or >30 days after PD (6.4 months versus 6.7 months) (Table 2).
Table 2.
Sites of disease progression, subsequent therapy, and outcomes (PFS and OS)
| All patientsa n = 48 |
Vemurafenib treatment after PD n = 43 |
||
|---|---|---|---|
| >30 days (n = 20)b |
Discontinued (n = 24)c |
||
| Median PFS, months (range) |
7.2 (0.9–56.0) |
6.7 (2.9–17.1) |
6.4 (0.9–24.2) |
| Median treatment duration beyond initial PD, months (range) |
— | 3.8 (1.1–26.6) |
— |
| Median OS beyond initial PD, months (range) |
6.1 (0–41.0)d |
10.0 (3.6–41.0) |
3.4 (0–26.9) |
| Median OS from initiation of vemurafenib, months (range) |
14.0 (1.2–56.1) |
26.0 (7.7–56.1) |
11.0 (1.2–35.4) |
| Site of progression, n (%)e | |||
| Skin/soft tissue | 18 (38) | 10 (53) | 8 (33) |
| Brain/CNS/spine | 13 (27) | 6 (32) | 7 (29) |
| Lungs/chest wall | 10 (21) | 5 (26) | 5 (21) |
| Liver | 6 (13) | 1 (5) | 5 (21) |
| Lymph nodes | 7 (15) | 5 (26) | 2 (8) |
| Gastrointestinal tract | 5 (10) | 1 (5) | 4 (17) |
| Spleen | 4 (8) | — | 4 (17) |
| Symptomatic PD only |
3 (6) | — | 3 (13) |
| Bone | 2 (4) | 1 (5) | 1 (4) |
| Adrenal glands | 1 (2) | 1 (5) | — |
Abbreviations: CNS, central nervous system; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
No progressive disease was recorded for 5 patients.
All 20 patients experienced limited disease progression and continued vemurafenib after local therapy for an overall clinical benefit. One patient (included in this group) did not meet strict RECIST criteria for progression; however, determination of progression was made based on positron emission/computed tomography.
All 24 patients received <30 days of vemurafenib after PD, only for the purpose of tumour biopsy at the time of disease progression.
This occurred among the 44 patients who experienced PD while receiving vemurafenib.
Only sites affected by the earliest recorded progression of disease are represented. Percentage exceeds 100% because some patients experienced multiple sites of disease progression. Data are unknown for 6 patients in the group receiving vemurafenib treatment after PD >30 days (n = 19).
Characteristics of patients who experienced long-term benefit from vemurafenib were determined in an exploratory analysis of survival, baseline characteristics, and post-progression treatment in subsets of patients with short (<6 months, n = 19) and prolonged (>12 months, n = 15) PFS. Assessed baseline characteristics were serum lactate dehydrogenase level, stage, mean sum of target lesions on CT, and ECOG PS. The only characteristics associated with PFS >12 months were mean sum of target lesions and ECOG PS (Table 3). Mean (±SD) sums of target lesions were 168 mm (±113 mm) and 65 mm (±37 mm) for patients with short and long PFS, respectively (P = 0.002).
Table 3.
Baseline characteristics of patients with short and long duration of PFS
| Characteristics | PFS <6 months 19 of 48 patients (40%) |
PFS >12 months 15 of 48 patients (31%) |
P |
|---|---|---|---|
| LDH >ULN, % | 42 | 40 | 0.11a |
| Stage M1c, % (95% CI) | 74 (49–91) | 52 (27–79) | 0.28a |
| Sum of baseline target lesions, mm, mean (±SD) |
168 (±113) | 65 (±37) | 0.002 |
| ECOG PS of 1, % (95% CI) | 74 (49–91) | 27 (7–55) | 0.01a |
Abbreviations: CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; PFS, progression-free survival; LDH, lactate dehydrogenase; SD, standard deviation; ULN, upper limit of normal.
P value determined by Fisher exact test.
Median OS from study initiation for all 48 patients was 14.0 months (range, 1.2–56.1) (Table 2). Median OS in patients who discontinued at first PD was 11.0 months (range, 1.2–35.4), whereas median OS in patients who continued vemurafenib for >30 days after PD was 26.0 months (range, 7.7–56.1). Figure 1 shows time to response and progression and patient status.
Fig 1.
Time to response, progression, OS, and individual status (deceased/living) for patients with PD during treatment with vemurafenib doses ≥240 mg twice daily. Kaplan-Meier plots show the number of patients alive over time. (A) Patients with disseminated PD received vemurafenib therapy for <30 days after progression. (B) Patients with localised PD continued vemurafenib therapy for >30 days after progression. OS, overall survival; PD, progressive disease; VEM, vemurafenib.
3.2 Long-term OS (years from treatment initiation)
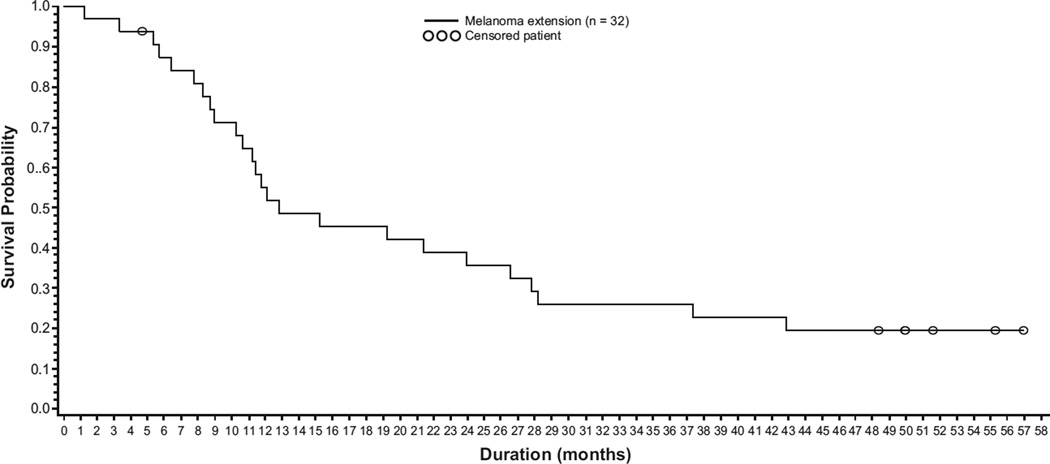
Eight patients treated with vemurafenib 960 mg twice daily were alive >3 years after treatment initiation; 6 patients survived ≥4 years. Three- and 4-year melanoma-specific survival (censoring patients who did not die of melanoma) duration for the 32 patients in the extension cohort was 26% (95% CI, 10.4–41.3) and 19% (95% CI, 5.5–33.3), respectively (Fig. 2). One additional patient experienced PR (per RECIST v1.0) but died 27.8 months after initiation (2 months after vemurafenib discontinuation) with no evidence of PD; therefore, death was considered unrelated to melanoma or vemurafenib. Clinical characteristics of these patients are in Table 4; 3 had CR and 6 had PR as investigator-assessed best overall response in target lesions after vemurafenib; 4 of these patients had stage M1c.
Fig. 2.
Kaplan-Meier estimate of melanoma-specific overall survival (n = 32).
Table 4.
Characteristics of patients who experienced long-term survivala
| Patient | Age, years |
Sex | Stage | Previous therapies |
Baseline ECOG PS |
Baseline serum LDH levelb |
Extent of disease |
Sites of disease progression |
Initial vemurafenib dose, mg/day |
Duration of vemurafenib treatment, months |
INV- assessed BOR |
Greatest decrease in target lesion size, % |
Pre-PD PFS duration. months |
OS duration, months |
Post-PD to last follow- up duration, months |
Subsequent systemic therapies after vemurafenib |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 61 | 41 | M | M1c | 0 | 1 | Normal | Lymph node, liver |
Lymph node | 1920 | 29.0 | PRg | 97.7 | 8.8 | 49.2 | 40.4 | None |
| 70c | 62 | M | M1c | 0 | 0 | Normal | Lymph node, skin |
Subcutaneous skin |
1920 | 42.4 | PRg | 28.1 | 16.2 | 50.9 | 34.7 | None |
| 75 | 37 | M | M1c | 1 | 0 | High | Lymph node, retroperitoneal, intra- abdominal mass, and abdomen |
None | 1,920 | 54.3 | PR | 43.8 | N/A | 54.3 | N/A | None |
| 76 | 58 | F | M1a | 1 | 0 | ND | Lymph node | None | 1920 | 53.9 | CR | 100 | N/A | 54.5 | N/A | None |
| 77d | 51 | M | M1a | 7 | 1 | Normal | Soft tissue | Soft tissue | 1920 | 34.1 | CR | 100 | 14.7 | 42.3 | 27.6 | None |
| 79 | 56 | M | M1c | 0 | 0 | High | Lymph node, adrenal |
Brain | 1920 | 18.7 | PR | 75.6 | 16.8 | 47.7 | 30.9 | PH1-0882; nivolumab, ipilimumab |
| 86 | 56 | M | M1b | 4 | 0 | Normal | Soft tissue | Soft tissue | 1920 | 7.8 | PR | 100h | 3.6 | 36.8 | 33.2 | AMG 208 (c- MET i), E7080 + TMZ, E7080 monotherapy, vemurafenib + ipilimumab + sirolimus |
| 105e | 59 | F | M1a | 1 | 0 | Normal | Lymph node | None | 1920 | 43.5 | CR | 100 | N/A | 49.3 | N/A | None |
| 62f | 68 | F | M1a | 0 | 0 | High | Pelvis, lymph node, skin |
None | 1920 | 25.0 | PR | 62.3 | N/A | 27.8 | N/A | None |
Abbreviations: BOR, best overall response; CR, complete response; ECOG PS, Eastern Cooperative Oncology Group performance status; FDG-PET, fluorodeoxyglucose positron emission tomography; INV, investigator; LDH, lactate dehydrogenase; Met, metformin; N/A, not applicable; ND, not determined; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria In Solid Tumors; TMZ, temozolomide.
Long-term survival was defined as >3 years.
Graded as high, normal, or low, based on institutional standards.
Patient had lymph nodes that converted from FDG-PET avid to FGD-PET non-responsive in September 2010 and was included in the PD group based on clinician experience. Patient’s target lesion growth did not meet RECIST criteria for PD.
Patient died of lymphoma, not melanoma.
Dose decreased from 960 mg twice daily to 720 mg twice daily.
Patient included because of death at 27.8 months not related to melanoma; patient experienced continued PR for duration and no evidence of melanoma relapse.
CR after surgery.
Patient classified as partial responder because of development of new non-target lesions.
As of March 2014, 3 of 9 patients were alive without PD (>49.3, >54.3 and >54.5 months after initiation, respectively). Median duration from vemurafenib initiation to initial PD in the 5 patients with PD was 14.7 months (range, 3.6–16.8). Median duration from initial PD to last follow-up was 33.2 months (range, 27.6–40.4). Among these 5 patients, 1 died of lymphoma, 2 underwent surgical resection of progressing lesions, 1 discontinued vemurafenib and subsequently received nivolumab then ipilimumab plus vemurafenib and 1 discontinued vemurafenib and subsequently received trametinib then ipilimumab before death because of PD. Six of 8 patients who survived >3 years received only vemurafenib therapy; among these patients, 5 survived >4 years and continued to receive only vemurafenib therapy.
3.3 Site and frequency of PD
Forty-four patients had PD at the time of analysis. PD sites included skin/soft tissue (38%), brain/central nervous system (CNS) (27%), lungs (21%), nodes (15%), liver (13%), gastrointestinal tract (10%), and bone (4%) (Table 2). Among those 44 patients, 20 (45%) experienced PD at ≥1 metastatic site, including an original lesion site, and 19 (43%) experienced it at new sites without progression at existing sites. Five patients had symptomatic progression reported as PD. Brain metastases developed in 12 of 28 patients (43%) with new metastases (with or without PD at target or non-target lesions) and in 9 of 19 patients (47%) with PD at only new sites.
Twenty patients (45.5%) received vemurafenib for >30 days after PD because of the overall clinical benefit they experienced from the treatment; most of these initial instances of PD were considered isolated by the Investigator, and the remainder were controlled (limited PD). Lesions were treated with local therapy (surgery or radiation). Median treatment duration beyond initial PD was 3.8 months (range, 1.1–26.6).
Median OS beyond initial progression was 6.1 months (range, 0–41.0) in all 44 patients with PD, 3.4 months (range, 0–26.9) in those who discontinued <30 days after progression (n = 24), and 10.0 months (range, 3.6–41.0) in those who received vemurafenib for >30 days after progression (n = 20) (Table 2).
Of the 48 patients in the total population, 23 (48%) received at least one subsequent treatment, including systemic therapy (n = 18), radiotherapy (n = 8), surgery (n = 2). Systemic therapies most commonly consisted of MEK inhibitors, combined BRAF inhibitor + MEK inhibitor, temozolomide, ipilimumab, carboplatin + paclitaxel, nab-paclitaxel, and other investigational agents.
3.4 Adverse events in patients monitored long term
The most common AEs reported by ≥20% of the total population (N = 48) included arthralgia (64.6%), fatigue (62.5%), rash (58.3%), alopecia (52.1%), photosensitivity (45.8%), nausea (45.8%), diarrhoea (37.5%), vomiting (33.3%), squamous cell carcinoma of the skin (33.3%), palmar–plantar erythrodysesthesia syndrome (31.3%), pyrexia (31.3%), myalgia (31.3%), anorexia (31.3%), hyperglycaemia (29.2%), headache (29.2%), cough (27.1%), extremity pain (27.1%), dry skin (27.1%), pruritus (27.1%), peripheral edema (27.1%), constipation (25.0%), hypokalaemia (25.0%), sunburn (22.9%), hypercholesterolemia (22.9%), hyperkeratosis (22.9%), weight decrease (22.9%), skin papilloma (20.8%), hypertriglyceridemia (20.8%) and hyponatremia (20.8%). AEs according to grade in the total population (supplementary Table S1) and AEs in patients who received vemurafenib for >30 days after progression, and in patients who discontinued vemurafenib after PD confirmation (supplementary Table S2), appear in the Supporting Information. Treatment-emergent adverse events leading to dose reductions occurred in 22 (45.8%) patients, and treatment with vemurafenib was discontinued because of adverse events in 3 (6.3%) patients.
4. DISCUSSION
Our data suggest that OS >3 years without PD may be possible for some patients with BRAFV600E-mutated metastatic melanoma receiving single-agent BRAF inhibitor treatment. For a subset of patients with PD at sites accessible to local therapy (20 of 44 patients), continuation of vemurafenib might be clinically beneficial after local therapy. In the total population, patients who had PFS >12 months had lower baseline tumour load and ECOG PS of 0. Characteristics common among patients with OS >3 years included non-CNS metastases and baseline ECOG PS of 0.
A recent single-centre retrospective analysis of patients in single-agent dabrafenib or vemurafenib clinical trials also showed benefit of treatment with BRAF inhibitors beyond progression. Median OS from initiation of BRAF inhibitor therapy was longer in patients who continued BRAF inhibitors after RECIST-defined PD than in those who did not (17.8 months versus 7.0 months; P < 0.001); OS from the date of PD was also longer (11.6 months versus 2.0 months; P < 0.001) [17].
Interestingly, our 3-year OS rate (26%) is similar to that reported for ipilimumab [18]. With combination targeted therapies (i.e. BRAF inhibitor and MEK inhibitor)—which are superior to single-agent BRAF inhibitor therapies—now available, it is possible to conjecture that subsets of patients with BRAFV600-mutant melanoma could achieve long-term survival approximating that possible with ipilimumab or other checkpoint inhibitors, such as anti-PD1 antibodies. It remains to be seen which patient and melanoma characteristics are predictive of long-term disease control with BRAF inhibitor-based therapy.
The observed heterogeneity in progression patterns suggests that the differential responses to continued BRAF inhibition beyond progression are attributed to differences in tumor biology and reflect limitations in the method of measuring tumor response by RECIST criteria [19]. Among described patterns of resistance, some may be associated with more widespread or rapid onset of resistance, and others with focal progression or more prolonged response [20–22]. Recent analyses of progressing tumours during BRAF inhibitor therapy provide evidence of divergent clonal evolution at different metastatic sites [20]. This could underlie the phenomenon of ongoing disease control and isolated progression at sites amenable to local therapy, as observed in this cohort.
Drugs targeting other escape pathways might potentially overcome resistance to BRAF inhibition. Recent phase 2 studies showed that patients previously exposed to BRAF inhibitors did not respond to single-agent trametinib [23], a selective MEK inhibitor, but 5 of 26 patients responded to sequential treatment with dabrafenib, another selective BRAF inhibitor, and trametinib. Recently, the combination of BRAF inhibitor and MEK inhibitor (dabrafenib and trametinib) was shown to provide superior response rates and PFS to dabrafenib alone [24,25] or vemurafenib alone [26], and is now approved for patients with BRAF-mutant advanced melanoma in the United States. Similarly, the combination of vemurafenib and the MEK inhibitor cobimetinib, currently under investigation in a phase 3 study (coBRIM), met its primary end point, significantly improving PFS compared with vemurafenib alone in patients with previously untreated BRAFV600-mutant advanced melanoma [27].
Analyses similar to those reported here, comparing PD patterns, therapy after progression, and long-term survival between BRAF/MEK combinations and BRAF-inhibitor monotherapy, are the next steps to identify possible differences in long-term outcomes. Recently, Hauschild et al presented long-term follow-up (median 16.9 months) of patients enrolled in the phase 3 BREAK-3 trial, which compared dabrafenib to dacarbazine in 250 patients with BRAFV600E metastatic melanoma [28]. Twenty-four patients originally randomized to dabrafenib and three patients originally randomized to dacarbazine and crossed over to dabrafenib remained on dabrafenib and 19/27 (70%) of these patients were without PD on dabrafenib. Median time on dabrafenib for the 19 patients was 31.0 months (range 26.8–33.6) [28]. However, identifying characteristics associated with long-term PFS on dabrafenib was complicated by the crossover design of BREAK-3 [28]. Difficulties were also encountered in BRIM3: the crossover design from dacarbazine to vemurafenib complicated sensitivity analyses exploring survival benefits of vemurafenib compared to dacarbazine [14]. Careful planning of future trials to incorporate the possibility of long-term administration and the potential for BRAF inhibitor administration after PD is warranted.
Limitations of the current study include the following: the study was not initially designed to explore long-term OS, the number of patients was small, the groups were not prospectively defined, selection bias might have skewed survival data, and a control population was lacking.
Novel treatment options now exist with the introduction and approval of anti-PD1 agents for patients with recurrent or progressive metastatic melanoma with BRAFV600 mutation after BRAF inhibitor and ipilimumab progression [29]. However, the observed response rates of 20–30% and the uncertain durability call for careful individualization of all the available therapies in order to maximize patient survival. Our analyses suggest that continued vemurafenib to control BRAF-sensitive clones is one option to manage limited PD after vemurafenib therapy. Additionally, we demonstrated that it is possible to achieve long-term OS in metastatic melanoma patients receiving vemurafenib.
Supplementary Material
Highlights.
We report long-term follow-up of the phase 1 vemurafenib trial.
Analyses were restricted to BRAFV600E melanoma patients.
Some patients achieved long-term survival with vemurafenib monotherapy.
Continuation of vemurafenib after progression might be beneficial in some patients.
Acknowledgments
This study was funded by F. Hoffmann-La Roche Ltd.
The authors thank Jennifer M. Kulak, PhD, and David Gibson, PhD, CMPP, of ApotheCom (Yardley, PA), who provided writing services on behalf of F. Hoffmann-La Roche Ltd.
F. Hoffmann-La Roche Ltd (Roche) provided access to the raw data and statistical support for the analyses as proposed by the authors. Roche was involved in the decision to publish inasmuch as Plexxikon Inc. co-authors reviewed data analyses, reviewed drafts of the manuscript, and approved the manuscript for submission. Plexxikon co-promotes vemurafenib with Genentech, Inc, a subsidiary of F. Hoffmann-La Roche Ltd, in the United States. In addition, Dr. Lin has a patent US 2014/0037617 pending. Consultant or Advisory Role: Bartosz Chmielowski: Genentech, Bristol Myers Squibb, Glaxo Smith Kline; Keith Flaherty: Roche, Genentech; Grant McArthur: Provectus; Keith Nolop: Kite Pharma, Inc.; Igor Puzanov: Hoffmann-La Roche, Genentech; Antoni Ribas: Amgen, Genentech, Merck, MedImmune, Novartis; Kevin B. Kim: F. Hoffmann-La Roche, Genentech. Stock Ownership: Keith Nolop: Kite Pharma, Inc.; Antoni Ribas: Kite Pharma, Inc. Honoraria: Bartosz Chmielowski: Genentech, Bristol Myers Squibb, Merck; Kevin B. Kim: F. Hoffmann-La Roche, Genentech; Antoni Ribas: Amgen, Genentech, Merck, MedImmune, Novartis. Research Funding: Ravi Amaravadi: Plexxikon, Roche, Genentech; Grant McArthur: Novartis, Pfizer, Celgene; Igor Puzanov: Hoffmann-La Roche, Genentech; Jeffrey A. Sosman: Bristol Myers Squibb; Antoni Ribas, Genentech; Paul B. Chapman: Roche/Genentech, Novartis; Kevin B. Kim: F. Hoffmann-La Roche, Genentech; Mark Shackleton: Pfizer Australia. Employment: Paul Lin: Plexxikon Inc. Leadership: Paul Lin: Plexxikon Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial number: ClinicalTrials.gov ID, NCT00405587
Conflict of Interest Disclosures
Nothing to disclose: Patrick Hwu.
Contributor Information
Igor Puzanov, Email: igor.puzanov@vanderbilt.edu.
Ravi K. Amaravadi, Email: Ravi.Amaravadi@uphs.upenn.edu.
Grant A. McArthur, Email: grant.mcarthur@petermac.org.
Keith T. Flaherty, Email: kflaherty@partners.org.
Paul B. Chapman, Email: chapmanp@mskcc.org.
Jeffrey A. Sosman, Email: jeff.sosman@vanderbilt.edu.
Antoni Ribas, Email: aribas@mednet.ucla.edu.
Mark Shackleton, Email: Mark.Shackleton@petermac.org.
Patrick Hwu, Email: phwu@mdanderson.org.
Bartosz Chmielowski, Email: BChmielowski@mednet.ucla.edu.
Paul S. Lin, Email: plin@plexxikon.com.
REFERENCES
- 1.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 2.Goydos JS, Mann B, Kim HJ, et al. Detection of B-RAF and N-RAS mutations in human melanoma. J Am Coll Surg. 2005;200:362–370. doi: 10.1016/j.jamcollsurg.2004.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Libra M, Malaponte G, Navolanic PM, et al. Analysis of BRAF mutation in primary and metastatic melanoma. Cell Cycle. 2005;4:1382–1384. doi: 10.4161/cc.4.10.2026. [DOI] [PubMed] [Google Scholar]
- 4.Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190–200. doi: 10.1111/j.1755-148X.2010.00685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee JT, Li L, Brafford PA, et al. PLX4032, a potent inhibitor of the B-Raf V600E oncogene, selectively inhibits V600E-positive melanomas. Pigment Cell Melanoma Res. 2010;23:820–827. doi: 10.1111/j.1755-148X.2010.00763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sala E, Mologni L, Truffa S, et al. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res. 2008;6:751–759. doi: 10.1158/1541-7786.MCR-07-2001. [DOI] [PubMed] [Google Scholar]
- 7.Sondergaard JN, Nazarian R, Wang Q, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Translational Med. 2010;8:39. doi: 10.1186/1479-5876-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H, Higgins B, Kolinsky K, et al. RG7204 (PLX4032), a selective BRAFV600E inhibitor, displays potent antitumor activity in preclinical melanoma models. Cancer Res. 2010;70:5518–5527. doi: 10.1158/0008-5472.CAN-10-0646. [DOI] [PubMed] [Google Scholar]
- 9.Tsai J, Lee JT, Wang W, et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci U S A. 2008;105:3041–3046. doi: 10.1073/pnas.0711741105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bollag G, Hirth P, Tsai J, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med. 2012;366:707–714. doi: 10.1056/NEJMoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF and BRAF mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol. 2000;15:323–332. doi: 10.1016/S1470-2045(14)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 17.Chan MM, Haydu LE, Menzies AM, et al. The nature and management of metastatic melanoma after progression on BRAF inhibitors: effects of extended BRAF inhibition. Cancer. 2014;120:3142–3153. doi: 10.1002/cncr.28851. [DOI] [PubMed] [Google Scholar]
- 18.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015 Feb 9; doi: 10.1200/JCO.2014.56.2736. pii: JCO.2014.56.2736. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tirkes T, Hollar MA, Tan M, et al. Response criteria in oncologic imaging: review of traditional and new criteria. RadioGraphics. 2013;33:1323–1341. doi: 10.1148/rg.335125214. [DOI] [PubMed] [Google Scholar]
- 20.Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discovery. 2014;4:80–93. doi: 10.1158/2159-8290.CD-13-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discovery. 2014;4:94–109. doi: 10.1158/2159-8290.CD-13-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wagle N, Van Allen EM, Treacy DJ, et al. MAP kinase pathway alterations in BRAF-mutant melanoma patients with acquired resistance to combined RAF/MEK inhibition. Cancer Discovery. 2014;4:61–68. doi: 10.1158/2159-8290.CD-13-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol. 2013;31:482–489. doi: 10.1200/JCO.2012.43.5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long GV, Stroyakovsky DL, Gogas H, et al. Combined BRAF and MEK Inhibition versus BRAF Inhibition Alone in Melanoma. N Engl J Med. 2014;371:1877–1888. doi: 10.1056/NEJMoa1406037. [DOI] [PubMed] [Google Scholar]
- 26.Robert C, Karaszewska B, Schachter J, et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 27.Larkin J, Ascierto PA, Dréno B, et al. Combined Vemurafenib and Cobimetinib in BRAF-Mutated Melanoma. N Engl J Med. 2014;371:1867–1876. doi: 10.1056/NEJMoa1408868. [DOI] [PubMed] [Google Scholar]
- 28.Hauschild A, Grobb J, Demidov L, et al. An update on overall survival (OS) and follow-on therapies in BREAK-3, a phase III, randomized trial: dabrafenib (D) vs. dacarbazine (DTIC) in patients (pts) with BRAF V600E mutation-positive metastatic melanoma (MM) Ann Oncol. 2014;25(suppl 4):iv378. [Google Scholar]
- 29.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther. 2015 Mar 27; doi: 10.1016/j.clinthera.2015.02.018. pii: S0149-2918(15)00088-0. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.