Abstract
It is generally acknowledged that reactive oxygen species (ROS) play crucial roles in a variety of natural processes in cells. If increased to levels which cannot be neutralized by the defense mechanisms, they damage biological molecules, alter their functions, and also act as signaling molecules thus generating a spectrum of pathologies. In this review, we summarize current data on oxidative stress markers associated with human immunodeficiency virus type-1 (HIV-1) infection, analyze mechanisms by which this virus triggers massive ROS production, and describe the status of various defense mechanisms of the infected host cell. In addition, we have scrutinized scarce data on the effect of ROS on HIV-1 replication. Finally, we present current state of knowledge on the redox alterations as crucial factors of HIV-1 pathogenicity, such as neurotoxicity and dementia, exhaustion of CD4+/CD8+ T-cells, predisposition to lung infections, and certain side effects of the antiretroviral therapy, and compare them to the pathologies associated with the nitrosative stress.
1. Introduction
Reactive oxygen species (ROS) is a general term of oxygen intermediates with high reactive capacity towards various biological molecules. They include hydroxyl radical (HO•), singlet oxygen (1O2), superoxide anion (O2 •−), hydrogen peroxide (H2O2), and other reactive species [1, 2]. ROS are produced in various cellular processes and organelles: electron leakage from the mitochondrial electron transport chain (ETC), degradation of lipids, amino acids, and biogenic polyamines, protein folding in the lumen of endoplasmic reticulum (ER), and so forth [3–7]. The most reactive type of ROS is the hydroxyl radical. It is produced from hydrogen peroxide that oxidizes divalent iron cations via the Fenton reaction
| (1) |
or as a result of the Haber-Weiss cycle that involves a reduction of ferric ions by superoxide anions into ferrous ions followed by the Fenton reaction:
| (2) |
Thus, the net reaction of the Haber-Weiss cycle can be described as
| (3) |
Superoxide anions have several sources in cells. First, they are generated in mitochondria. Electron transport through the ETC during oxidative phosphorylation is generally accompanied by escape of up to 1-2% of electrons that are trapped by molecular oxygen [7]. Alteration of mitochondrial bioenergetics by various factors usually gives rise to superoxide anion production. Secondly, superoxide anion is produced by a family of NADPH oxidases (NOX/DUOX), comprised of seven isoforms: NOX1–NOX5 and DUOX1-DUOX2 [6]. They transport electrons across the membranes and generate superoxide with the exception of NOX4 that produces hydrogen peroxide [8]. Activation of NOX-mediated ROS production can be achieved by various mechanisms. For example, NOX4 is controlled only at the level of transcription since this enzyme is constitutively active [6]. NOX1–NOX3 are generally induced on the transcriptional level and activated by a controlled assembly of the multisubunit complexes. Finally, several isoforms including NOX5 and DUOX1-DUOX2 possess calcium-binding domains that mediate additional level of ROS production. Third, superoxide anions are generated by cytochromes P450 (CYP) which catabolize various endogenous compounds and xenobiotics [9]. Hydrogen peroxide is mainly formed as a stoichiometric by-product in catabolic reactions and through formation of disulfide bonds during protein folding in the ER [5, 10]. Finally, reactive oxygen species can derive from the activity of xanthine oxidoreductase (XOR) [11, 12]. XOR is widely distributed throughout various organs including the liver, gut, lung, kidney, heart, and brain as well as the plasma. It is generally accepted that the enzyme is normally present in vivo as an NAD-dependent cytosolic dehydrogenase (XDH), incapable of ROS production. However, sulfhydryl oxidation or limited proteolysis converts the XDH into xanthine oxidase (XO) which produces superoxide and hydrogen peroxide, with the latter being the major product under physiological conditions [11]. Furthermore, both XO and XDH can oxidize NADH, with the concomitant formation of the reactive oxygen species [11, 12].
Different types of ROS are characterized by their varying ability to react with biological molecules. The most reactive ROS is the hydroxyl radical, HO•, the one-electron oxidized form of the hydroxide ion (HO−) [1, 13]. It can oxidize almost any molecule in its proximity including DNA, phospholipids, and proteins [13, 14]. Oxidation results in the accumulation of 8-oxoguanine (8-oxoG) and other oxidized nucleic bases, malondialdehyde (MDA), and 4-hydroxynonenal (HNE) as typical lipid peroxidation products and in protein damage manifested in the increase of the protein carbonyl content [15]. Much less active is the superoxide anion: its reactivity is hampered by a negative charge of the species; however, its protonation generates the perhydroxyl radical (HO2 •) with a higher oxidizing potential [16]. The reaction potential of H2O2 is also very low; however, it is converted into the hydroxyl radical with a much higher oxidizing capacity [17]. H2O2 also possesses a unique (for ROS) capacity to cross biological membranes which turns it into a classical signaling molecule [17, 18].
Eukaryotic cells have developed multiple mechanisms of ROS neutralization (“scavenging”) in order to protect themselves against oxidation of biological molecules. First, ROS can be neutralized directly by the low molecular weight compounds referred to as antioxidants, such as vitamins C and E and glutathione (GSH) [19], and a wide set of ROS-converting enzymes [20] including NAD(P)H:quinone oxidoreductase 1 (Nqo1) that scavenges superoxide anion [21] and superoxide dismutases (SODs) that convert O2 •− into H2O2 [22]. SODs exist in three isoforms expressed in different cellular compartments: SOD1 (Cu/Zn-SOD) is mostly localized in the cytoplasm; SOD2, (MnSOD) in the mitochondrial matrix; and SOD3 (EC-SOD), at the cell surface. Neutralization of H2O2 is performed by multiple enzymes such as catalase (CAT), glutathione peroxidases (GPx, eight isoforms), and peroxiredoxins (Prdx, six isoforms) [23, 24]. Of these enzymes, GPx4 and 1-Cys peroxiredoxins are responsible for scavenging lipid peroxides thus protecting lipids from the oxidative damage [25–27]. Additional protection from ROS is mediated by heme oxygenase, the rate-limiting enzyme of heme catabolism which leads to the release of free iron, which in turn offers protection against oxidative stress [28]. Other antioxidant proteins include enzymes that mediate biosynthesis of glutathione and proteins that recycle oxidized glutathione, peroxiredoxins, and glutathione peroxidases (glutaredoxins and thioredoxins) [20, 29]. Noteworthily, expression of a wide set of antioxidant enzymes is controlled by NF-E2-related factor 2 (Nrf2), a transcription factor that recognizes a common short sequence, referred to as Antioxidant Response Elements (ARE), in the promoters of genes encoding ROS-converting enzymes [20]. Components of antioxidant defense systems differ in their capacity to neutralize ROS. Hydrogen peroxide is much more efficiently neutralized by peroxiredoxins and glutathione peroxidases, while classical antioxidants such as glutathione have a much lower potential [16, 30]. The actual levels of ROS are defined by the balance between the activities of ROS-generating and ROS-scavenging molecules, being different for different cellular compartments [31].
Several techniques are currently used to analyze the redox status of the cell and to determine the levels of ROS. Firstly, oxygen radicals can be detected by the electron paramagnetic resonance (EPR) using a spin-trapping technique; however, this method requires highly specialized equipment [32]. Secondly, ROS levels can be quantified indirectly using low molecular weight compounds (sensors) that are oxidized by ROS into fluorophores. They include 2′,7′-dichlorodihydrofluorescein (DCFH2DA), dihydroethidium (DHE) and its mitochondrially targeted derivative MitoSOX, and boronate probes [33, 34]. Protein sensors such as HyPER or roGFP can be introduced as genes which makes them suitable for measuring ROS levels in almost any organelle [35–37]. Thirdly, the oxidative stress can be assessed indirectly by evaluating the levels of oxidative stress biomarkers, such as stable (by-)products generated under conditions of oxidative stress which enter the tissues, cells, or circulation, such as oxidized glutathione (GSSG), MDA, and HNE (for lipids) and 8-oxoG (DNA) and protein carbonyls [15, 38]. In addition, the cellular redox state can be quantified by estimating the capacity of blood/serum/tissue samples to oxidize/reduce some standard compounds that mimic cellular targets of ROS (e.g., see [39, 40]).
Oxidative stress accompanies a wide variety of viral infections including those induced by hepatitis B (HBV) [41], C (HCV) [42], and delta (HDV) [43] viruses, herpes [44, 45], respiratory [46, 47], and other viruses. In this review, we will summarize data on the mechanisms by which HIV triggers massive ROS production in the host cell and deregulates the antioxidant defense system. We will also present current concepts on the role of HIV-induced oxidative stress in the development of HIV-associated pathologies.
2. HIV-1 Biology
The human immunodeficiency virus type-1 (HIV-1) is a lentivirus that infects and by various mechanisms kills vital cells of human immune system, such as T-helper cells, macrophages, and dendritic cells, thus causing immunodeficiency [48, 49]. The acquired immunodeficiency syndrome (AIDS) is a condition in humans in which the progressive failure of the immune system undermined by HIV-1 infection allows life-threatening opportunistic infections and cancers to thrive. Without treatment, the survival time of HIV-1 infected individuals is estimated to be 9 to 11 years. However, during the three decades since its discovery, 27 antiretroviral drugs have been approved for HIV therapy [50]. Current antiretroviral therapy (ART), based on combinations of 3-4 drugs, now allows us to efficiently suppress HIV viral load and to prolong life of HIV/AIDS patients almost to the one of the general population, at least in high-income countries [51].
HIV is a single-stranded, positive-sense, enveloped RNA virus. The genome carries nine genes (gag, pol, env, tat, rev, nef, vif, vpr, and vpu) that encode 19 proteins; the coding sequence is framed by the long terminal repeats (LTRs) [48, 49]. Three of these genes, gag, pol, and env, contain information needed to make new viral particles. Processing of pol gene results in formation of three enzymes: reverse transcriptase (RT), integrase, and protease. Translation of env gene produces glycoprotein 160 (Gp160) that further is processed to give Gp120 and Gp41. Gag gene ensures production of matrix (MA), capsid (CA), nucleocapsid (NC), and P6 proteins as well as spacer peptides 1 (SP1) and 2 (SP2). The six remaining genes, tat, rev, nef, vif, vpr, and vpu (or vpx in the case of HIV-2), are regulatory genes for proteins that control the ability of HIV to infect cells, produce new copies of virus (replicate), or cause the disease [52]. Upon entry into the target cell, HIV reverse-transcribes the RNA genome into the double-stranded DNA, transports it into the cell nucleus, and integrates into the chromosomes, the activities mediated by virus-encoded enzymes reverse transcriptase and integrase, and cellular cofactors [48]. Once integrated, the virus may become latent, which allows the infected cells to avoid detection by the immune system. Alternatively, the virus may be transcribed and translated, producing new RNA genomes and viral proteins that are packaged and released from the cell as new virus particles to start the new infection cycle.
3. Oxidative Stress during HIV Infection
To date, numerous lines of evidence show that HIV infection triggers pronounced oxidative stress in both laboratory models and the context of in vivo infection. HIV-infected individuals exhibit enhanced ROS production in monocytes [53] and severely elevated levels of oxidized nucleic bases such as 8-oxoG and lipid peroxidation products, including MDA in plasma and alkanes in the breath output [54–63].
Compensation of the pathogenic effects of HIV-1 replication requires intact functions of ROS detoxifying enzymes. Parsons et al. showed that HIV-1 individuals with a null-allele polymorphism in gstm1 gene, associated with a loss of function of the Phase II detoxifying enzyme glutathione S-transferase [64], exhibit lower count of CD4 T-cells, increased HIV viral load, and increased 8-oxoG in mitochondrial DNA [65]. However, HIV-infected individuals demonstrate a reduction of total antioxidant capacity [59], decreased GSH/GSSG ratio in epithelial lung fluid [3], and decreased GSH content in blood [56, 58, 61, 63, 66–69]. Marked elevation of ROS levels was also detected in the HIV-infected cell cultures [70, 71]. The most profound decrease of the total antioxidant capacity was detected in subsets of CD4+ and CD8+ T-lymphocytes [66], with low CD4 T-cell counts correlating with more severe oxidative stress [59, 60, 62, 72]. Corroborating these observations, the number of CD4+ cells positively correlates with the total levels of ROS scavengers such as glutathione [56]. Noteworthily, these changes are more pronounced in treatment-naive patients than in patients under ART [58, 61] since ART restores the numbers of CD4+ T-cells but at the same time augments the imbalance of the redox status [73]. In HIV/HCV-coinfected patients, the levels of oxidative stress markers are generally higher than in individuals with HIV monoinfection, as indicated by MDA and GSSG plasma levels [74, 75].
Elevated levels of the oxidative stress markers are also detected in other tissues and body fluids. Brain tissues (brain frontal cortex collected from autopsia) of HIV-infected individuals are characterized by the increased levels of 8-oxoG in the nuclear DNA [76] and increased HNE levels [69]. Elevated levels of superoxide radical and HNE are also detected in the cerebrospinal fluid [69, 76]. Interestingly, similar effects are observed in the NL4-3Δ transgenic rat model expressing HIV proteome devoid of the Gag-Pol polypeptide. In these animals, high levels of superoxide anion can be indirectly detected by electron spin resonance spectroscopy/ESR using CMH probe in the aortas [77] and by fluorescent microscopy with DHE dye in the lungs [78]. Altogether, this indicates that HIV-1 actively interferes with the development of oxidative stress response.
4. Mechanisms of ROS Production during HIV Infection
HIV-1 induces oxidative stress by deregulation of oxidative stress pathways with escalation of ROS production and by inducing mitochondrial dysfunction [70, 71]. The enhancement of ROS production is mediated by the envelope protein Gp120 [79–85], Tat [83, 84, 86–88], Nef [89–91], Vpr [71, 92, 93], and reverse transcriptase (RT) [94].
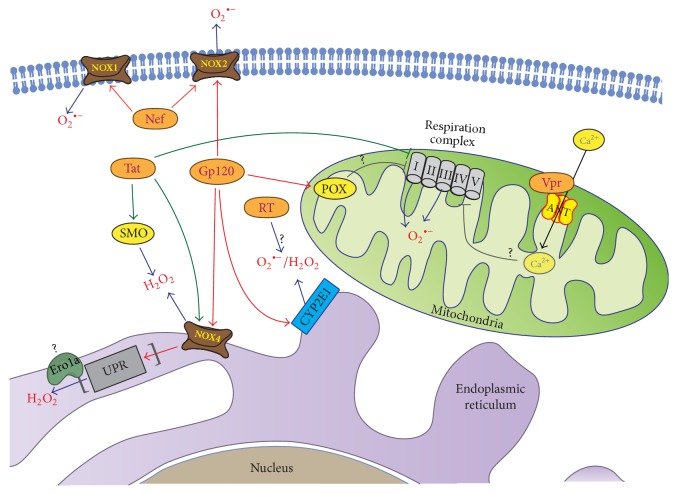
The envelope protein Gp120 enhances ROS production in various cell lines of lymphoid origin [82], in endothelial brain cells [83], microglia cells, neurons, and astrocytes [79, 80]. In astrocytes, it enhances ROS production by several parallel mechanisms: via cytochrome P450 2E1 (CYP2E1), NOX2 and NOX4, and the Fenton-Weiss-Haber reaction (Figure 1) [79, 95]. The effect of Gp120 on CYP2E1 is mediated through upregulation of CYP2E1 expression. Interestingly, however, EPR analysis of the HIV-1 infected monocyte-derived macrophages revealed no increase in the production of either hydroxyl or other oxygen radicals [96]. In neuroblastoma cells, Gp120 was shown to induce proline oxidase (POX) that produces pyrroline-5-carboxylate with a concomitant generation of ROS (Figure 1) [85].
Figure 1.
Cellular sources of reactive oxygen species in HIV infection. Several HIV proteins enhance ROS production by different mechanisms. These viral proteins include amongst others the envelope protein Gp120, Tat, Nef, Vpr, and RT. The envelope protein Gp120 enhances ROS production via upregulation of cytochrome P450 2E1 (CYP2E1), proline oxidase (POX), and activation of NOX2 and NOX4. Tat protein induces spermine oxidase (SMO), an enzyme involved in catabolism of biogenic polyamines, and may impact mitochondrial function. Tat also activates NADPH (but not xanthine) oxidases and in particular Nox4, which in turn may induce other peroxide-generating enzymes involved in unfolded protein response (UPR) such as ER oxidoreductin 1α (Ero1α). Vpr protein interacts with adenine nucleotide translocator (ANT, a component of mitochondrial permeability transition pore (PTP)) that is implicated in Ca2+ influx into mitochondria. Nef protein can directly interact with the p22phox subunit of NADPH oxidases without affecting NOX expression. Finally, RT triggers ROS production by yet undiscovered mechanism(s).
The regulatory Tat protein triggers ROS production via several independent mechanisms (Figure 1). The first involves the NADPH (but not xanthine) oxidases [86]. The second implies the induction of spermine oxidase (SMO), an enzyme involved in the catabolism of biogenic polyamines [88, 97]. The third relies on mitochondrial dysfunction [98] but was questioned in a later study [86]. A detailed analysis of the levels of ROS in different subcellular compartments of the HIV-1 infected cells revealed no significant increase in the content of H2O2 in either cytoplasm or mitochondria but a strong increase in the ER [99]. ER is the primary “residence” for NOX4 that produces hydrogen peroxide [8, 100]. An increase in H2O2 levels in ER of HIV-1 infected cells was demonstrated using a genetically encoded ratiometric HyPER sensor [99]. Moreover, in these cells, NOX4 mediated the induction of unfolded protein response (UPR). In concordance with these data, an elegant study demonstrated that the suppression of NOX4 by RNAi in Tat-expressing cells results in a significant reduction of H2O2 levels in the ER [99]. The results generated using HyPER sensor could be questioned. Such sensors have been used in a number of studies (such as [4, 99]) that demonstrated that the dynamic range of its signal is small [101] to negligible [102], with changes in the HyPERER fluorescence reflecting not so much the changes in peroxide levels but rather the influence of other factors such as proline disulphide isomerases (PDI) [103]. Also, one cannot rule out that NOX4 contributes to the induction of oxidative stress indirectly, through the induction of other peroxide-generating enzymes involved in UPR. If so, one could propose a component of the protein-folding machinery which could be involved, namely, ER oxidoreductin 1α (Ero1α) [104] which is upregulated within the PERK branch of UPR (Figure 1) [105]. Intriguingly, the elevation of hydrogen peroxide levels in the ER contradicts the existing concept on the efficient neutralization of hydrogen peroxide in the ER by scavenging enzymes, including peroxiredoxin 4 [106] and glutathione peroxidases 7/8 [101, 107]. This may not be widely accepted, which leaves open the actual mechanism of the Tat-mediated oxidative stress in the ER.
HIV-1 Nef protein has shown a prooxidant activity in microglial cells and in neutrophils [89–91]. The activity is related to the ability of Nef to interact with Vav protein (Figure 1) [89]. Vav is a nucleotide exchange factor for Rac1 that is recruited to the NOX1–NOX3 complexes [6], with the p22phox subunit of NADPH oxidases, but without affecting NOX expression [91]. These interactions are in perfect concordance with the absence of changes in the expression of NOX1, NOX2, and NOX4 in NL4-3Δ gag-pol transgenic rats compared to the wild-type animals [77].
Viral protein R (Vpr) is another important regulator of ROS production [108]. In yeast, Vpr expression induces an oxidative stress leading first to the decreased levels of superoxide anion and hydroxyl radical as well as glutathione and significantly decreased activities of catalase, glutathione peroxidase, glutathione reductase, glucose-6-phosphate dehydrogenase, and glutathione S-transferase and later on to elevated levels of superoxide anion and peroxides and increased activities of most of antioxidant enzymes [108]. It was shown that Vpr triggers oxidative stress by causing mitochondrial dysfunction [92, 109, 110] and ROS production in mitochondria (Figure 1) [71]. Mitochondrial dysfunction is promoted by binding of Vpr to the adenine nucleotide translocase (ANT) [110], a protein that forms an inner channel of the mitochondria permeability transition pore (PTP) [110]. This indicates the propensity of Vpr to unbalance the redox state of the cells contributing to the HIV-1 pathology.
Mitochondrial dysfunction is a general mechanism of ROS production common for most viral infections [111–113]. NADPH oxidases and CYP2E1 serve as the major sources of ROS in infections with human hepatitis C, influenza, and respiratory syncytial viruses [114–121]. The overview of the field demonstrates that sources of ROS operational in HIV-1 infection follow similar trends.
5. HIV and Antioxidant Defense Pathways
The effect of HIV-1/HIV-1 proteins on the cellular antioxidant defense system is debatable. Several groups reported a decrease in SOD (SOD3 in particular), CAT, and GPx activities in plasma of the HIV-infected individuals [61, 63, 75, 122]. The data on Gp120 is controversial; it was shown to either enhance [123] or not affect the expression of sod2 gene [82]. However, the individual Tat protein causes an opposite effect: it suppresses the expression of MnSOD through inhibition of binding of Sp1 and Sp3 transcription factors to sod2 gene promoter and binding to its mRNA [124, 125]. In addition, studies done in HIV-1 NL4-3Δ transgenic rats demonstrate a decrease in the Cu/Zn-SOD expression, whereas the expression of MnSOD remains unaltered [77].
Overall, both Gp120 and Tat suppress expression of the glutathione synthesizing and metabolizing enzymes. Both downregulate the expression of glutathione synthase (GSS), glutathione reductase (GR), and GPx, leading to a decrease in the total glutathione content and an increase of the GSSG/GSH ratio [83, 84, 123, 126]. Gp120 also shows a strong ROS-dependent inhibitory effect on the expression of glutamine synthase (GS) [127]. Interestingly, Tat exhibits a stronger inhibitory effect on glutathione than Gp120 [83]. In addition to the inhibition of GSH biosynthesis pathways, Tat induces the expression of glutathione peroxidase isoform GPx4 [126], which scavenges lipid peroxides. At the same time, Tat has no effect on the expression of thioredoxin reductase [126], an enzyme that reduces thioredoxin, which in turn reduces glutathione peroxidases and peroxiredoxins [29]. Vpr is yet another virus protein that triggers a decrease in the GSH levels [128]. The latter is caused by the suppression of ATP biosynthesis in mitochondria [128] (two molecules of ATP are required for biosynthesis of every glutathione moiety [129]).
A majority of glutathione metabolizing genes are controlled by the Nrf2 transcription factor [20]. In vivo, HIV-1 appears to suppress the Nrf2/ARE pathway. Indeed, brain cortex tissues of HIV-1 infected individuals demonstrate the decreased levels of heme oxygenase 1 [130]. This effect is not mediated by Tat, Nef, or Vpr proteins but is apparently due to the replication of the viral genome. HO-1 protein expression correlates negatively with HIV replication levels. In vitro analysis of HO-1 expression in HIV-infected macrophages, a primary central nervous system (CNS) HIV reservoir along with microglia, demonstrated a decrease in HO-1 as HIV replication increased; HO-1 repression was mediated by high levels of IFN-γ concomitant with virus replication in the CNS [131]. While HIV replication seems to (indirectly) suppress the Nrf2/ARE pathway, the effects of the individual viral proteins are the opposite. HIV reverse transcriptase activates Nrf2 and upregulates the transcription of both HO-1 and Nqo1, at least in the cell culture system [94]. An ability to activate the Nrf2/ARE pathway was recently reported also for Tat [88]. It is mediated through the induction of spermine oxidase and concomitant production of hydrogen peroxide. Gp120 induces yet another classical Nrf2-dependent gene, multidrug resistant protein 1 (Mrp1) [81]. Such discrepancy between the factual data from in vitro studies and the status of Nrf2/ARE signaling during HIV infection has been observed also for other viruses such as HCV [132–135]. The actual (also long-term) effects of HIV-1 on the Nrf2/ARE pathway and their outcomes for the pathogenesis of HIV-1 infection remain to be elucidated.
6. ROS in HIV's Life Cycle
Hypoxia induces oxidative stress via an overgeneration of ROS [136]. A crucial role in the mammalian response to oxygen levels is played by the transcription factor Hypoxia-Inducible Factor-1 (HIF-1). Increased expression of HIF-1α contributes to the mitochondrial activity and ROS formation during the hypoxia [137]. HIV-1 protein Vpr induces HIF-1 resulting in the ROS-dependent activation of HIV LTR [71]. HIV-1 LTR is activated even by low concentrations of H2O2 [138] (whereas antioxidants inhibit viral transcription [139]). Further enhancement of the transcription is triggered by proinflammatory cytokines, including TNF-α [140, 141] induced through the redox-dependent NF-κB pathway [142]. Additional influence of elevated ROS levels on HIV life cycle is achieved through the redox-sensitive transcription factors AP-1 and p53 [143]. Interestingly, transcription activation by exogenous hydrogen peroxide takes place only after the prolonged treatment, allowing us to hypothesize that virus-induced oxidative stress can play a crucial role in activation of the latent viral infection [138]. Supporting this, activation of the latent infection was triggered by modest changes in the cell redox potential (25 mV) [144]. Such changes can be induced either directly by HIV proteins or indirectly through the induction of proinflammatory cytokines such as TNF-α [145].
Oxidative stress may be also beneficial for the late stages of the HIV life cycle, since glutathione treatment of chronically infected cells leads to the abrogation of virion budding and release [146] preventing the infection of new T-cells [147]. The addition of GSH or GSH analogues is able to block late steps of viral replication [148], possibly by inhibiting the proper folding of glycosylated surface viral proteins in the ER, as was demonstrated for influenza virus [149]. The inhibitory effects of antioxidant treatment could also be attributed to the ability of ROS to induce CXCR4 receptor [150] as well as the glucose transporter Glut1 [151]. Enhanced expression of Glut1 was observed in both the infected cell cultures [152] and the neuronal tissues of the patients [153]. It leads to the elevation of glucose influx into the lymphocytes, monocytes, and epithelial cells. Enhanced glucose flux is known to promote infection with oncogenic viruses [154, 155]. In case of HIV-1, it also leads to augmented ROS production and enhanced infection of target cells [147, 156, 157]. However, opposite data were also reported: overexpression of peroxide-scavenging enzyme, GPx1, enhances production of HIV virions, whereas treatment of such cells with buthionine sulfoximine (BSO) that inhibits glutathione biosynthesis inhibits such increase [158].
7. ROS in HIV-1 Related Pathologies
HIV-induced oxidative stress plays an important role in the development of a wide spectrum of virus-associated pathologies. Among them are neurotoxicity and dementia and immune imbalance with the exhaustion of the pool of CD4 T-lymphocytes, as well as lung and cardiovascular disorders.
7.1. CNS Toxicity
Neurotoxicity and dementia are believed to be the direct consequences of HIV-1 infection: a majority of the cases with these neurological symptoms below 60 years of age are AIDS patients. HIV-1 affects the microglial cells; progressive infection leads to damage to astrocytes and neurons [159]. Levels of oxidative stress markers such as mitochondrial 8-oxoG in serum inversely correlate with the volume of the grey substance from selected brain areas (hippocampus, pallidum, etc.) [160]. Moreover, an increase in 8-oxoG in the nuclear DNA is accompanied by a decrease in the mitochondrial DNA content observed in the frontal cortex of the patients, altogether pointing at a direct link between ROS and neurological pathologies in AIDS patients [160]. The accumulated data points at the neurotoxicity being triggered by Gp120, Tat, and Vpr proteins which can penetrate the blood-brain barrier (BBB) (Figure 2) [161, 162]. Penetration is likely due to the disruption of BBB through several redox-regulated processes, including the induction of matrix metalloproteinases (MMP) 2 and 9 that target BBB tight junction receptors ZO-1, laminin, claudin 5, and occludin ([84, 163, 164], see also a comprehensive review by Toborek et al. [165]).
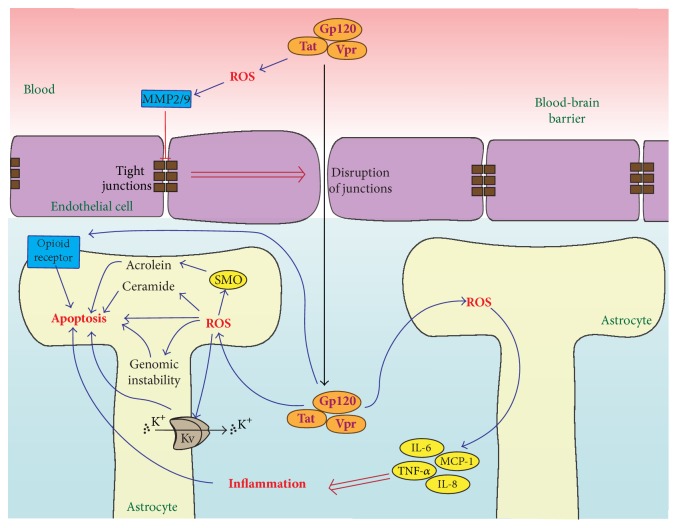
Figure 2.
Mechanisms of HIV neurotoxicity. Enhanced ROS production, triggered by gp120, Tat, and Vpr proteins that circulate in the blood, results in alteration of blood-brain barrier (BBB) through matrix metalloproteinase 2/9- (MMP2/9-) mediated disruption of tight junction receptors ZO-1, laminin, claudin 5, and occludin. Gp120, Tat, and Vpr proteins activate a consequence of proapoptotic events. They include (i) oxidation of DNA and consequent genomic and mitochondrial DNA instability, (ii) increased lipid peroxidation and accumulation of ceramide that aggravates toxicity, (iii) induction of spermine oxidase (SMO) augmenting oxidative stress and producing toxic acrolein, (iv) stimulation of A-type transient outward K+ currents by Kv channels, and (v) induction of proinflammatory cytokines. In addition, it upregulates expression of opioid receptors that contribute to neurotoxicity in HIV-infected drug addicts.
Gp120, Tat, and Vpr proteins contribute to the CNS pathology by both direct and indirect mechanisms (Figure 2). The direct mechanism involves induction of ROS production, which leads to the exhaustion of the antioxidant defense system and decreased cell viability [79, 98, 128, 166, 167]. Elevated levels of ROS result in the enhanced oxidation of DNA nucleic bases in both the nucleus and mitochondria, while their removal and DNA reparation are inhibited through suppression of DNA glycosylase 1 (enzyme the function of which is the removal of 8-oxoG; OGG1) [76]. This scenario leads to DNA instability, particularly to the deletion of the D-loop in mitochondrial DNA. Significantly, contribution to neurotoxicity of Gp120 and Tat is made by an increased lipid peroxidation and accumulation of ceramide [69].
An indirect promotion of CNS pathology is believed to be mediated by the enhanced production of the inflammatory cytokines and chemokines in astrocytes and microglia [159]. Gp120 and Vpr induce TNF-α, IL-6, IL-8, and MCP-1 in the ROS-dependent fashion (Figure 2) [80, 87, 167, 168]. In addition, Gp120 stimulates A-type transient outward K+ currents that contribute to the cell death [169]. Notably, this effect is also ROS-dependent [80]. An additional contribution to the pathogenic effects could come from the induction of spermine oxidase, an enzyme that mediates one of the two alternative pathways of polyamine metabolism [5]. It catalyses a reaction that yields H2O2 and acrolein as stoichiometric by-products. The latter compound is implicated in the brain pathology during ischemia-reperfusion [170, 171]. The induction of SMO may therefore represent an important mechanism of the HIV-induced brain damage. Finally, recent data of Pandhare et al. revealed that Gp120-mediated induction of proline oxidase leads to autophagy that at least partially alleviates neurotoxicity [85].
Interestingly, certain regions of AIDS patient brain are characterized by an increased expression of the opioid receptors [172]. In line with this, drugs such as morphine and amphetamine can per se trigger ROS production and dysregulate the antioxidant defense system, augmenting the pathogenic properties of Gp120 (Figure 2) [79, 123]. This may account for a more severe progression of the disease in the intravenous drug users.
7.2. Redox Associated Cardiovascular and Lung Pathologies
HIV-1 infection is accompanied by an increased risk of various cardiovascular diseases including arterial hypertension [173], atherosclerosis [174, 175], injury to coronary arteries [176], vasculitis [177], pericarditis, and myocarditis [173]. HIV-associated lung pathologies include increased susceptibility to infections, emphysema, and lung cancer [178, 179]. Their development is believed to be promoted by virus-induced oxidative stress. Oxidative stress in the lung leads to a decreased expression of the tight junction receptors, disrupting the epithelium and rendering lungs more susceptible to the microbes [180]. Moreover, treatment with lipopolysaccharide aggravates the redox imbalance in HIV-infected cells [78]. It may be speculated that these redox perturbations can trigger the inflammatory response, resulting in the tissue damage, as well as causing the genomic instability.
7.3. Effects of the Oxidative Stress on the Immune System
Very recent vivid example of the effects of oxidative stress on retroviral infection was provided by the study of Brundu et al. in a murine model [181]. Infection with the murine leukemia virus LP-BM5 causes murine AIDS, a disease characterized by many dysfunctions of the immunocompetent cells. Mice infected with LP-BM5 murine leukemia have a marked redox imbalance reflected by GSH and/or cysteine depletion in multiple immune organs/tissues. Significant decrease in cysteine and GSH levels was measured also in pancreas and in the brain, respectively [181]. Mice demonstrated a predominance of T-helper 2 (Th2) responses manifested by the expression of Th2 cytokines. Their peritoneal macrophages expressed the genetic markers of the alternative M2 macrophage polarization as Fizz1, Ym1, and Arginase 1 [181]. Conversely, macrophages capable of expressing iNOS (a marker of classical activation of macrophages) produced predominantly T-helper 1 (Th1) cytokines [181]. Restoration of the GSH/cysteine levels in the infected mouse organs (done with a N-acetyl-cysteine supplier) reduced the expression of M2 macrophage markers and increased the production of IFN-γ, while decreasing the production of Th2-cytokines as IL-4 and IL-5 [181]. Interestingly, this is not the first report of the association between the Th2 polarization and alteration of the redox status by retroviral infection and/or retroviral proteins. We have earlier shown that HIV-1 reverse transcriptase induces potent oxidative stress and, when expressed in mice as DNA immunogen, induces potent strongly Th2-polarized type of specific immune response [94]. Thus, HIV-1 infection and even expression of HIV-1 antigens induce an immune imbalance marked by M2-shift of the macrophage response and Th2-shift of the T-cell profiles which together promote the continuation of viral replication.
A separate set of immune abnormalities in HIV-1 infection is linked to the abnormalities in the tryptophan metabolism. In HIV-1 infected individuals, these abnormalities correlate with the enhanced oxidative kynurenine pathway of tryptophan catabolism [182, 183]. This pathway generates quinolinic acid, 3-hydroxykynurenine, and 3-hydroxyanthranilic acid, all of which are known to have the ability to generate free radicals [184]. Indoleamine 2,3-dioxygenase (IDO) is an intracellular enzyme involved in the first step of tryptophan catabolism [185]. Increased IDO expression occurs during human [186] and simian [187] retroviral infections. The data on the murine LP-BM5 immunodeficiency-causing retroviral infection is contradictory [188, 189]. In HIV-1 infection, increased IDO mRNA correlates with increased viral loads, while ART decreases IDO expression, which may be anticipated as a proof of the direct correlation between IDO and HIV virus propagation [190].
Catabolism of tryptophan by IDO leads to the reduction in tryptophan levels [191]. Th1 cell clones are more sensitive to changes in tryptophan levels than Th2 cell clones, resulting in a selective immunosuppression with the shift of the immune response towards the Th2-type [192]. Besides, IDO activity results in the increased levels of toxic downstream metabolites and generation of free radicals which contribute to Th1-cell suppression [191]. Furthermore, IDO activity causes even more imbalance in the T-cell subsets by increasing the proportion of T-regulatory [193] and decreasing the proportion of T-helper 17 cells [194]. In chronically infected hosts, the dysregulated activation/alterations in the immune regulatory mechanisms involving IDO lead to a compromised antiviral response and enhancement of viral replication [195–197]. In short, chronic IDO activation leads to the immune impairment, whereas IDO inhibition represses viral replication. Thus, the effects of IDO on the viral replication may in fact be indirect, being modulated by the disturbances in the virus-specific immune response.
These series of studies demonstrate that longitudinal (chronic) oxidative stress has detrimental consequences to the HIV-1 specific immune response, impairing the capacity of the body to control viral replication. On the contrary, suppression of the chronic oxidative stress with restoration of the antioxidant levels can reestablish the disturbed Th1/Th2 balance and open a possibility to control retroviral infection.
7.4. T-Cell Exhaustion
ROS levels correlate inversely with the CD4+ cell counts [59, 60, 62, 72, 198]. This may relate to a decrease in the reduced glutathione pool and the exhaustion of ROS-scavenging systems of the host cells [68, 70, 199]. It could also be due to the accumulation of DNA damage in these cells due to both increased production of ROS and the suppression of the respective DNA repair enzymes [62]. The molecular interrelations between HIV-induced oxidative stress and CD4+/CD8+ cell exhaustion remain to be investigated.
7.5. Pathological Consequences of the Nitrosative Stress
HIV is capable of infiltrating the brain and infecting brain cells. In the years following HIV infection, patients show signs of various levels of neurocognitive problems termed HIV-associated neurocognitive disorders (HAND) which afflict about half of HIV-infected patients. In Section 7.1, we described multiple links between neurological pathologies in AIDS patients and ROS. It is important to note that the latter are attributed not only to the oxidative but also to nitrosative stress and overproduction of nitrosative species during neuroinflammation [200]. Both processes occur due to the early direct and indirect effects of the viral proteins and through the late effects on mitochondrial integrity during apoptosis. There is clear experimental and clinical evidence linking the CNS symptoms of HIV with the effects of reactive nitrogen species (RNS), specifically nitric oxide (NO).
Mammalian cells generate NO as a by-product of NO synthase (NOS) activity. Neurons express neuronal NOS (nNOS), a constitutive isoform that synthesizes moderate amounts of NO; glial cells express inducible NOS (iNOS), which generates major NO amounts [201]. The nitrosative species are involved in the posttranslational modification of the brain proteome. NO is required for regular neuronal function, is produced by neuronal (nNOS), endothelial (eNOS), and inducible (iNOS) nitric oxide synthases, and is an important neurotransmitter in the brain.
At the same time, NO is the main mediator of mitochondrial dysfunction associated with HIV central nervous system symptoms, with an increased production of NO related to HIV-associated dementia. Nitrosative stress in microglia and astrocytes can be promoted by the individual viral proteins, such as Tat [202, 203]. Another viral protein Gp41 (its N-terminus) induces iNOS protein activity [204]. HIV-1 Gp120 is also involved in the induction of iNOS leading to the nitrosative stress [205]. Recent study by Mangino et al. in the murine model suggests a potential role in the promotion of the neuronal injury of the extracellular Nef which upregulates the expression of iNOS and production of NO [206].
The data on the effects of RNS outside of the brain is less “homogenous.” The overproduction of NO and the reduction of mitochondrial transmembrane potential correlate with the level of apoptosis in PBMCs of HIV-1 patient [200, 207]. This may be explained by the inhibitory effects of NO on the electron transport chain in the mitochondria as well as by the amino acid modifications. Amino acid modifications ascribed to NO are associated with the S-nitrosylation of cysteine and nitration of tyrosine and tryptophan (resulting in 6-nitro tryptophan or nitrohydroxytryptophan). The latter may be, at least in part, responsible for the abnormalities in the tryptophan pathway in HIV-1 infected individuals with neurological or psychiatric complications [182]. S-Nitrosylation of phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) has been demonstrated both in the brains of HIV-1 patients with HAND and in the HIV-Gp120 transgenic mouse model, leading to decreased Akt activity [208].
Another RNS-centered hypothesis for the mechanism of neuronal damage following HIV infection involves the downstream effects of nitrosative radicals produced during the immune response [209]. Proinflammatory factors (as iNOS) are released in the astrocytes by the HIV-infected macrophages [210, 211]. The severity of HIV-related dementia is correlated with the levels of iNOS expression [212]. This confirms a link between nitrosative stress and the neuroinflammatory environment in the HIV-1 infected brain [212–214].
HIV-1-infected children with high viral load exhibited higher NO blood levels than those with viral load below this threshold [215]. This and other studies in AIDS patients point at the involvement of NO in the apoptosis and functional impairment of the lymphocytes [215–217]. Pathophysiological significance of these findings was demonstrated by showing an enhanced effect of NO on HIV-1 replication in vitro [215]. This study has shown that the addition of NO donors together with TNF-alpha to mitogen-activated HIV-1-infected human peripheral blood mononuclear cell (PBMC) cultures produces a significant increase in viral replication, whereas the addition of iNOS specific inhibitors suppresses replication [215]. Altogether, these results suggest that NO promotes HIV-1 replication, especially in proinflammatory settings [215], which lines up with similar effects of ROS (as depicted in Section 6).
However, NO donor compounds present in the human circulatory system, such as S-nitrosothiols (RSNOs), can inhibit HIV-1 replication in acutely infected human PBMCs demonstrating an additive inhibitory effect on HIV-1 replication with 3′-azidothymidine (AZT) [218]. One of the explanatory mechanisms might be the inhibition of HIV-1 protease subjected to S-nitrosation [219, 220]. Thus, in acute HIV-1 infection, RNS may inhibit viral replication. Indeed, a study done with the fluorescent probes with an enhanced sensitivity to NO demonstrated that low NO and iNOS levels in PBMC from HIV-infected patients correlate with enhanced viral replication [221]. Interestingly, HIV-1 transgenic rats are also characterized by low NO-hemoglobin, serum nitrite, serum S-nitrosothiols, and the aortic tissue NO levels [77]. The latter indicates that the decreased levels of NO and its downstream products are linked to the direct effects of the viral proteins [77]. Their propensity to downregulate levels of RNS may create a microenvironment favouring (acute) viral infection. These considerations are in line with findings that HIV can be targeted by the compounds that affect oxidative status of the central and transitional memory T-cells: the major cellular reservoirs for HIV [222] (see Section 8 for an overview).
This set of somewhat contradictory data indicates that, in HIV-1 infection, the predominant tissue exposed to the effects of RNS is apparently the brain, while the effects of RNS on other tissues and organs of HIV-1 infected individuals may be positive and/or negative depending on RNS levels and duration of exposure.
8. Oxidative Stress and Antiretroviral Therapy
One of the milestone findings in the redox biology of HIV-1 was the induction of oxidative stress during antiretroviral therapy (ART). To date, numerous reports show that nucleoside and nonnucleoside RT inhibitors, as well as inhibitors of the viral protease, trigger massive ROS production in various cell types (e.g., [223–229]). Series of studies reported an increase in oxidative stress additional to the persistent redox imbalance associated with HIV-1 infection manifested by an increase in oxidants and a decrease in antioxidant serum levels [73, 230, 231]. Specifically, a study done in 84 HIV-infected patients during a 6-month period of ART demonstrated a significant increase in serum peroxidation potential, total hydroperoxide, MDA, and advanced oxidation protein product levels as well as a decrease in glutathione level, compared to their levels before the treatment and to healthy controls [232]. Ngondi et al. as well registered an aggravation of the oxidative stress by certain ART regimens in the form of a significant decrease in the levels of GSH (sulfhydryl group) [233]. Again, in patients receiving nonnucleoside reverse-transcriptase inhibitors, peroxide concentrations were significantly lower than in those treated with protease inhibitors [185]. This could be attributed to an enhancement in GSH utilization or/and to the limited intracellular reduction of its oxidized form [234]. It is generally acknowledged that the components of ART may contribute to the development of cardiovascular diseases and CNS pathologies. Some of the antiretroviral drugs, such as 2′,3′-dideoxycytidine (ddC), can penetrate the BBB and trigger oxidative stress also in the brain [235]. Experiments in the ART-exposed cell lines and laboratory animals demonstrated that the enhanced production of the oxidized metabolites occurs through the mitochondrial interference ([225]; reviewed in [73]). Mitochondrial dysfunction under ART arises from the altered replication of mitochondrial DNA and inhibited oxidative phosphorylation [236]. Some of the abovementioned dysfunctions correlate with the duration of antiretroviral therapy [237, 238]. The exact impact of oxidative stress on the efficacy of ART and HIV-1/AIDS progression and the molecular mechanisms of the redox imbalance in ART-treated HIV-infected individuals are still obscure and require further comprehensive studies.
Although ART is able to clear viremia and improve the immunological condition of HIV-infected individuals for prolonged time, the virus rebounds to levels comparable to those observed before treatment initiation shortly after treatment withdrawal due to intactness of the major cellular reservoirs for HIV, central and transitional memory T-cells (TCM and TTM, resp.) which harbour the transcriptionally silent form of viral DNA not affected by classical antiretroviral drug regimens. Interestingly, novel oxidative stress-based therapies are arising that target these major cellular HIV reservoirs that are inaccessible to classical ART. A candidate anti-HIV reservoir compound dubbed auranofin (AF) is a prooxidant gold-based drug that inhibits thioredoxin reductases thus affecting cellular/protein redox status [239]. Auranofin was shown to exert a selective “antimemory” effect by exploiting the baseline oxidative status of lymphocytes [222]. A study by Chirullo et al. [240] explored the molecular bases of the effects of auranofin. TCM and TTM lymphocytes were shown to have lower baseline antioxidant defenses as compared with their naive counterparts. AF was able to exert a prodifferentiating and proapoptotic effect preferentially in these memory subsets. Namely, AF induced redox-sensitive cell death pathways initiated by an early activation of the p38 mitogen-activated protein kinase (p38 MAPK) followed by the mitochondrial depolarization and finalized by the burst in intracellular peroxides [240]. AF-induced apoptosis was inhibited by pyruvate, a well-known peroxide scavenger. Proapoptotic and prodifferentiating effects involved different pathways. Similar effect of AF was described for simian immunodeficiency virus (SIV) in monkey model [241, 242]. Additional effect on T-lymphocyte can be achieved by combining AF with drugs that inhibit glutathione biosynthesis and lower its level such as buthionine sulfoximine (BSO) [243]. Using a combination of AF, BSO, and standard ART drugs, Shytaj et al. achieved complete clearance of SIV viremia in macaques with a 100% AIDS-free survival for at least 2 years after discontinuation of the therapy [242, 244]. This data indicates that AF and other drugs inducing redox-sensitive cell death pathways can be recruited to restrict viral reservoirs in vivo, limit the “stem-cell-ness” of the TCM and TTM pools, and turn these cells into the short-lived lymphocytes [240].
9. Conclusions and Future Perspectives
In this review, we summarized current knowledge on the fact that HIV infection leads to a pronounced oxidative stress, described the mechanisms by which the virus triggers ROS production, and discussed the impact of HIV on antioxidant defense systems. In addition, we presented an analysis of HIV-driven oxidative stress on the associated pathology. All these data clearly show that reactive oxygen species underlie a wide spectrum of events in infected cells and tissues. At the same time, there are still notable gaps in the field that might become targets for future studies. As such, we can propose the following subjects. First, many of the multiple sources of ROS that are activated by HIV may undergo common regulation and, hence, common prohibitive or stimulative treatment. Second, the current data on the status of antioxidant defense systems are rather contradictory, and many efforts are still required to understand the actual effect of the virus in acute versus chronic infection, not only in in vitro and animal model systems. Third, HIV-induced oxidative stress might impact susceptibility towards other viral infections, and this question has not yet been properly addressed. Fourth, virus-triggered ROS production is a strong modulator of the immune system, a property which needs to be controlled and that can be targeted for immune suppression of viral replication. Continuation of these studies would contribute to the development of efficient antiretroviral treatments and HIV vaccines.
Acknowledgments
This manuscript was prepared with the support from the Russian Science Foundation (Projects 14-14-01021, Sections 1, 4, 5, and 9; 14-50-00060, Section 6; and 15-15-30039, Sections 7.3, 7.4, and 8). Interaction of the coauthors was supported by the thematic partnership grant from the Swedish Institute (09272_2013). In addition, Maria G. Isaguliants (Sections 3, 7.1, 7.2, and 7.5) was supported by EU Grants BALTINFECT #316275 and VACTRAIN #692293. Birke Bartosch (Section 2) was supported by grants from the French National Agency for AIDS and Viral Hepatitis Research (14370), Comité de Saône-et-Loire de la Ligue Contre le Cancer, Agence Nationale de Recherche, the Region Rhone-Alpes, DevWeCan French Laboratories of Excellence Network (Labex, Grant ANR-10-LABX-61), PHC “Kolmogorov,” and the Opera IHU Program (Grant ANR-10-IBHU-004). Mr. Patrick Hort is gratefully acknowledged for the language editing.
Competing Interests
The authors declare that there are no competing interests regarding the publication of this paper.
References
- 1.Gutowski M., Kowalczyk S. A study of free radical chemistry: their role and pathophysiological significance. Acta Biochimica Polonica. 2013;60(1):1–16. [PubMed] [Google Scholar]
- 2.Augusto O., Miyamoto S. Oxygen radicals and related species. In: Pantopoulos K., Schipper H. M., editors. Principles of Free Radical Biomedicine. Nova Science Publishers; 2011. pp. 1–23. [Google Scholar]
- 3.Bindoli A., Rigobello M. P. Principles in redox signaling: from chemistry to functional significance. Antioxidants & Redox Signaling. 2013;18(13):1557–1593. doi: 10.1089/ars.2012.4655. [DOI] [PubMed] [Google Scholar]
- 4.Enyedi B., Várnai P., Geiszt M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxidants & Redox Signaling. 2010;13(6):721–729. doi: 10.1089/ars.2009.2880. [DOI] [PubMed] [Google Scholar]
- 5.Casero R. A., Jr., Pegg A. E. Polyamine catabolism and disease. Biochemical Journal. 2009;421(3):323–338. doi: 10.1042/BJ20090598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bedard K., Krause K.-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiological Reviews. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Figueira T. R., Barros M. H., Camargo A. A., et al. Mitochondria as a source of reactive oxygen and nitrogen species: from molecular mechanisms to human health. Antioxidants and Redox Signaling. 2013;18(16):2029–2074. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
- 8.Nisimoto Y., Diebold B. A., Constentino-Gomes D., Lambeth J. D. Nox4: a hydrogen peroxide-generating oxygen sensor. Biochemistry. 2014;53(31):5111–5120. doi: 10.1021/bi500331y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhattacharyya S., Sinha K., Sil P. C. Cytochrome P450s: mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Current Drug Metabolism. 2014;15(7):719–742. doi: 10.2174/1389200215666141125121659. [DOI] [PubMed] [Google Scholar]
- 10.Görlach A., Klappa P., Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxidants and Redox Signaling. 2006;8(9-10):1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 11.Cantu-Medellin N., Kelley E. E. Xanthine oxidoreductase-catalyzed reactive species generation: a process in critical need of reevaluation. Redox Biology. 2013;1(1):353–358. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pacher P., Nivorozhkin A., Szabó C. Therapeutic effects of xanthine oxidase inhibitors: renaissance half a century after the discovery of allopurinol. Pharmacological Reviews. 2006;58(1):87–114. doi: 10.1124/pr.58.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Halliwell B., Gutteridge J. M. C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemical Journal. 1984;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayala A., Muñoz M. F., Argüelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Medicine and Cellular Longevity. 2014;2014:31. doi: 10.1155/2014/360438.360438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayne S. T. Antioxidant nutrients and chronic disease: use of biomarkers of exposure and oxidative stress status in epidemiologic research. The Journal of Nutrition. 2003;133(supplement 3):933S–940S. doi: 10.1093/jn/133.3.933S. [DOI] [PubMed] [Google Scholar]
- 16.Winterbourn C. C. Reconciling the chemistry and biology of reactive oxygen species. Nature Chemical Biology. 2008;4(5):278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 17.D'Autréaux B., Toledano M. B. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nature Reviews Molecular Cell Biology. 2007;8(10):813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 18.Dynowski M., Schaaf G., Loque D., Moran O., Ludewig U. Plant plasma membrane water channels conduct the signalling molecule H2O2 . Biochemical Journal. 2008;414(1):53–61. doi: 10.1042/bj20080287. [DOI] [PubMed] [Google Scholar]
- 19.Forman H. J., Davies K. J. A., Ursini F. How do nutritional antioxidants really work: nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radical Biology and Medicine. 2014;66:24–35. doi: 10.1016/j.freeradbiomed.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aleksunes L. M., Manautou J. E. Emerging role of Nrf2 in protecting against hepatic and gastrointestinal disease. Toxicologic Pathology. 2007;35(4):459–473. doi: 10.1080/01926230701311344. [DOI] [PubMed] [Google Scholar]
- 21.Siegel D., Gustafson D. L., Dehn D. L., et al. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Molecular Pharmacology. 2004;65(5):1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 22.Zelko I. N., Mariani T. J., Folz R. J. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radical Biology and Medicine. 2002;33(3):337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 23.Perkins A., Nelson K. J., Parsonage D., Poole L. B., Karplus P. A. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling. Trends in Biochemical Sciences. 2015;40(8):435–445. doi: 10.1016/j.tibs.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brigelius-Flohé R., Maiorino M. Glutathione peroxidases. Biochimica et Biophysica Acta—General Subjects. 2013;1830(5):3289–3303. doi: 10.1016/j.bbagen.2012.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Fisher A. B., Dodia C., Manevich Y., Chen J.-W., Feinstein S. I. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. The Journal of Biological Chemistry. 1999;274(30):21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- 26.Imai H., Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radical Biology and Medicine. 2003;34(2):145–169. doi: 10.1016/S0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- 27.Manevich Y., Sweitzer T., Pak J. H., Feinstein S. I., Muzykantov V., Fisher A. B. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(18):11599–11604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi A. M. K., Alam J. Heme oxygenase-1: function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. American Journal of Respiratory Cell and Molecular Biology. 1996;15(1):9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 29.Hanschmann E.-M., Godoy J. R., Berndt C., Hudemann C., Lillig C. H. Thioredoxins, glutaredoxins, and peroxiredoxins-molecular mechanisms and health significance: From cofactors to antioxidants to redox signaling. Antioxidants and Redox Signaling. 2013;19(13):1539–1605. doi: 10.1089/ars.2012.4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox A. G., Winterbourn C. C., Hampton M. B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochemical Journal. 2010;425(2):313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 31.Aoyama K., Nakaki T. Glutathione in cellular redox homeostasis: association with the Excitatory Amino Acid Carrier 1 (EAAC1) Molecules. 2015;20(5):8742–8758. doi: 10.3390/molecules20058742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hawkins C. L., Davies M. J. Detection and characterisation of radicals in biological materials using EPR methodology. Biochimica et Biophysica Acta—General Subjects. 2014;1840(2):708–721. doi: 10.1016/j.bbagen.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 33.Kalyanaraman B., Darley-Usmar V., Davies K. J. A., et al. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Biology and Medicine. 2012;52(1):1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zielonka J., Sikora A., Hardy M., Joseph J., Dranka B. P., Kalyanaraman B. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chemical Research in Toxicology. 2012;25(9):1793–1799. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lukyanov K. A., Belousov V. V. Genetically encoded fluorescent redox sensors. Biochimica et Biophysica Acta—General Subjects. 2014;1840(2):745–756. doi: 10.1016/j.bbagen.2013.05.030. [DOI] [PubMed] [Google Scholar]
- 36.Bilan D. S., Belousov V. V. HyPer family probes: state of the art. Antioxidants & Redox Signaling. 2016;24(13):731–751. doi: 10.1089/ars.2015.6586. [DOI] [PubMed] [Google Scholar]
- 37.Morgan B., Van Laer K., Owusu T. N., et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nature Chemical Biology. 2016;12(6):437–443. doi: 10.1038/nchembio.2067. [DOI] [PubMed] [Google Scholar]
- 38.Emerit I. Clastogenic factors as potential biomarkers of increased superoxide production. Biomark Insights. 2007;2:429–438. [PMC free article] [PubMed] [Google Scholar]
- 39.Alam M. N., Bristi N. J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal. 2013;21(2):143–152. doi: 10.1016/j.jsps.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erel O. A new automated colorimetric method for measuring total oxidant status. Clinical Biochemistry. 2005;38(12):1103–11111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Higgs M. R., Chouteau P., Lerat H. ‘Liver let die’: oxidative DNA damage and hepatotropic viruses. Journal of General Virology. 2014;95(5):991–1004. doi: 10.1099/vir.0.059485-0. [DOI] [PubMed] [Google Scholar]
- 42.Ivanov A. V., Bartosch B., Smirnova O. A., Isaguliants M. G., Kochetkov S. N. HCV and oxidative stress in the liver. Viruses. 2013;5(2):439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Williams V., Brichler S., Khan E., et al. Large hepatitis delta antigen activates STAT-3 and NF-κB via oxidative stress. Journal of Viral Hepatitis. 2012;19(10):744–753. doi: 10.1111/j.1365-2893.2012.01597.x. [DOI] [PubMed] [Google Scholar]
- 44.Ma Q., Cavallin L. E., Leung H. J., Chiozzini C., Goldschmidt-Clermont P. J., Mesri E. A. A role for virally induced reactive oxygen species in Kaposi's sarcoma herpesvirus tumorigenesis. Antioxidants and Redox Signaling. 2013;18(1):80–90. doi: 10.1089/ars.2012.4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamranvar S. A., Masucci M. G. The Epstein-Barr virus nuclear antigen-1 promotes telomere dysfunction via induction of oxidative stress. Leukemia. 2011;25(6):1017–1025. doi: 10.1038/leu.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garofalo R. P., Kolli D., Casola A. Respiratory syncytial virus infection: mechanisms of redox control and novel therapeutic opportunities. Antioxidants & Redox Signaling. 2013;18(2):186–217. doi: 10.1089/ars.2011.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strengert M., Jennings R., Davanture S., Hayes P., Gabriel G., Knaus U. G. Mucosal reactive oxygen species are required for antiviral response: role of duox in influenza a virus infection. Antioxidants & Redox Signaling. 2014;20(17):2695–2709. doi: 10.1089/ars.2013.5353. [DOI] [PubMed] [Google Scholar]
- 48.Freed E. O., Martin M. A. HIVs and their replication. In: Knipe D. M., Howley P. M., editors. Fields Virology. Philadelphia, Pa, USA: Lippincott, Williams & Wilkins; 2007. pp. 2107–2185. [Google Scholar]
- 49.Kuritzkes D. R., Walker B. D. HIV-1 pathigenesis, clinical manifestations and treatment. In: Knipe D. M., Howley P. M., editors. Fields Virology. Philadelphia, Pa, USA: Williams & Wilkins; 2007. pp. 2187–2214. [Google Scholar]
- 50. http://www.fda.gov/ForPatients/Illness/HIVAIDS/Treatment/ucm118915.htm.
- 51.Samji H., Cescon A., Hogg R. S., et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0081355.e81355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volberding P. Sande's HIV/AIDS Medicine. Global Care. 2nd 2012. [Google Scholar]
- 53.Elbim C., Pillet S., Prevost M. H., et al. Redox and activation status of monocytes from human immunodeficiency virus-infected patients: relationship with viral load. Journal of Virology. 1999;73(6):4561–4566. doi: 10.1128/jvi.73.6.4561-4566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sönnerborg A., Carlin G., Åkerlund B., Jarstrand C. Increased production of malondialdehyde in patients with HIV infection. Scandinavian Journal of Infectious Diseases. 1988;20(3):287–290. doi: 10.3109/00365548809032453. [DOI] [PubMed] [Google Scholar]
- 55.Revillard J.-P., Vincent C. M. A., Favier A. E., Richard M.-J., Zittoun M., Kazatchkine M. D. Lipid peroxidation in human immunodeficiency virus infection. Journal of Acquired Immune Deficiency Syndromes. 1992;5(6):637–638. [PubMed] [Google Scholar]
- 56.Wanchu A., Rana S. V., Pallikkuth S., Sachdeva R. K. Short communication: Oxidative stress in HIV-infected individuals: a cross-sectional study. AIDS Research and Human Retroviruses. 2009;25(12):1307–1311. doi: 10.1089/aid.2009.0062. [DOI] [PubMed] [Google Scholar]
- 57.Allard J. P., Aghdassi E., Chau J., Salit I., Walmsley S. Oxidative stress and plasma antioxidant micronutrients in humans with HIV infection. The American Journal of Clinical Nutrition. 1998;67(1):143–147. doi: 10.1093/ajcn/67.1.143. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe L. M., Barbosa Júnior F., Jordão A. A., Navarro A. M. Influence of HIV infection and the use of antiretroviral therapy on selenium and selenomethionine concentrations and antioxidant protection. Nutrition. 2016;32(11-12):1238–1242. doi: 10.1016/j.nut.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 59.Teto G., Kanmogne G. D., Torimiro J. N., et al. Lipid peroxidation and total cholesterol in HAART-Naïve Patients infected with circulating recombinant forms of human immunodeficiency virus type-1 in cameroon. PLoS ONE. 2013;8(6) doi: 10.1371/journal.pone.0065126.e65126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalinowska M., Bazdar D. A., Lederman M. M., Funderburg N., Sieg S. F. Decreased IL-7 responsiveness is related to oxidative stress in hiv disease. PLoS ONE. 2013;8(3) doi: 10.1371/journal.pone.0058764.e58764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Awodele O., Olayemi S. O., Nwite J. A., Adeyemo T. A. Investigation of the levels of oxidative stress parameters in HIV and HIV-TB co-infected patients. Journal of Infection in Developing Countries. 2012;6(1):79–85. doi: 10.3855/jidc.1906. [DOI] [PubMed] [Google Scholar]
- 62.Aukrust P., Luna L., Ueland T., et al. Impaired base excision repair and accumulation of oxidative base lesions in CD4+ T cells of HIV-infected patients. Blood. 2005;105(12):4730–4735. doi: 10.1182/blood-2004-11-4272. [DOI] [PubMed] [Google Scholar]
- 63.Gil L., Martínez G., González I., et al. Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacological Research. 2003;47(3):217–224. doi: 10.1016/S1043-6618(02)00320-1. [DOI] [PubMed] [Google Scholar]
- 64.Strange R. C., Spiteri M. A., Ramachandran S., Fryer A. A. Glutathione-S-transferase family of enzymes. Mutation Research—Fundamental and Molecular Mechanisms of Mutagenesis. 2001;482(1-2):21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 65.Parsons M., Campa A., Lai S., et al. Effect of GSTM1-polymorphism on disease progression and oxidative stress in HIV infection: modulation by HIV/HCV co-infection and alcohol consumption. Journal of AIDS and Clinical Research. 2013;4, article 237 doi: 10.4172/2155-6113.1000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Staal F. J. T., Roederer M., Israelski D. M., et al. Intracellular glutathione levels in T cell subsets decrease in HIV-infected individuals. AIDS Research and Human Retroviruses. 1992;8(2):305–311. doi: 10.1089/aid.1992.8.305. [DOI] [PubMed] [Google Scholar]
- 67.Staal F. J. T., Ela S. W., Roederer M., Anderson M. T., Herzenberg L. A., Herzenberg L. A. Glutathione deficiency and human immunodeficiency virus infection. The Lancet. 1992;339(8798):909–912. doi: 10.1016/0140-6736(92)90939-z. [DOI] [PubMed] [Google Scholar]
- 68.Aukrust P., Svardal A. M., Muller F., et al. Increased levels of oxidized glutathione in CD4+ lymphocytes associated with disturbed intracellular redox balance in human immunodeficiency virus type 1 infection. Blood. 1995;86(1):258–267. [PubMed] [Google Scholar]
- 69.Haughey N. J., Cutler R. G., Tamara A., et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Annals of Neurology. 2004;55(2):257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- 70.Banki K., Hutter E., Gonchoroff N. J., Perl A. Molecular ordering in HIV-induced apoptosis. Oxidative stress, activation of caspases, and cell survival are regulated by transaldolase. The Journal of Biological Chemistry. 1998;273(19):11944–11953. doi: 10.1074/jbc.273.19.11944. [DOI] [PubMed] [Google Scholar]
- 71.Deshmane S. L., Mukerjee R., Fan S., et al. Activation of the oxidative stress pathway by HIV-1 Vpr leads to induction of hypoxia-inducible factor 1α expression. The Journal of Biological Chemistry. 2009;284(17):11364–11373. doi: 10.1074/jbc.m809266200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jareño E. J., Bosch-Morell F., Fernández-Delgado R., Donat J., Romero F. J. Serum malondialdehyde in HIV-seropositive children negatively correlates with CD4+ lymphocytes count. BioFactors. 1998;8(1-2):129–132. doi: 10.1002/biof.5520080121. [DOI] [PubMed] [Google Scholar]
- 73.Elias A., Nelson B., Oputiri D., Geoffrey O.-B. P. Antiretroviral toxicity and oxidative stress. American Journal of Pharmacology and Toxicology. 2013;8(4):187–196. doi: 10.3844/ajptsp.2013.187.196. [DOI] [Google Scholar]
- 74.Huang X., Liang H., Fan X., Zhu L., Shen T. Liver damage in patients with HCV/HIV coinfection is linked to HIV-related oxidative stress. Oxidative Medicine and Cellular Longevity. 2016;2016:11. doi: 10.1155/2016/8142431.8142431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baum M. K., Sales S., Jayaweera D. T., et al. Coinfection with hepatitis C virus, oxidative stress and antioxidant status in HIV-positive drug users in Miami. HIV Medicine. 2011;12(2):78–86. doi: 10.1111/j.1468-1293.2010.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Y., Wang M., Li H., et al. Accumulation of nuclear and mitochondrial DNA damage in the frontal cortex cells of patients with HIV-associated neurocognitive disorders. Brain Research. 2012;1458:1–11. doi: 10.1016/j.brainres.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 77.Kline E. R., Kleinhenz D. J., Liang B., et al. Vascular oxidative stress and nitric oxide depletion in HIV-1 transgenic rats are reversed by glutathione restoration. American Journal of Physiology—Heart and Circulatory Physiology. 2008;294(6):H2792–H2804. doi: 10.1152/ajpheart.91447.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacob B. A., Porter K. M., Elms S. C., Cheng P.-Y., Jones D. P., Sutliff R. L. HIV-1-induced pulmonary oxidative and nitrosative stress: exacerbated response to endotoxin administration in HIV-1 transgenic mouse model. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2006;291(4):L811–L819. doi: 10.1152/ajplung.00468.2005. [DOI] [PubMed] [Google Scholar]
- 79.Shah A., Kumar S., Simon S. D., Singh D. P., Kumar A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death and Disease. 2013;4(10, article e850) doi: 10.1038/cddis.2013.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guo L., Xing Y., Pan R., et al. Curcumin protects microglia and primary rat cortical neurons against HIV-1 gp120-mediated inflammation and apoptosis. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0070565.e70565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ronaldson P. T., Bendayan R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. Journal of Neurochemistry. 2008;106(3):1298–1313. doi: 10.1111/j.1471-4159.2008.05479.x. [DOI] [PubMed] [Google Scholar]
- 82.Shatrov V. A., Ratter F., Gruber A., Dröge W., Lehmann V. HIV type 1 glycoprotein 120 amplifies tumor necrosis factor-induced NF-κB activation in Jurkat cells. AIDS Research and Human Retroviruses. 1996;12(13):1209–1216. doi: 10.1089/aid.1996.12.1209. [DOI] [PubMed] [Google Scholar]
- 83.Price T. O., Ercal N., Nakaoke R., Banks W. A. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Research. 2005;1045(1-2):57–63. doi: 10.1016/j.brainres.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 84.Banerjee A., Zhang X., Manda K. R., Banks W. A., Ercal N. HIV proteins (gp120 and Tat) and methamphetamine in oxidative stress-induced damage in the brain: potential role of the thiol antioxidant N-acetylcysteine amide. Free Radical Biology and Medicine. 2010;48(10):1388–1398. doi: 10.1016/j.freeradbiomed.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pandhare J., Dash S., Jones B., Villalta F., Dash C. A novel role of proline oxidase in HIV-1 envelope glycoprotein-induced neuronal autophagy. The Journal of Biological Chemistry. 2015;290(42):25439–25451. doi: 10.1074/jbc.m115.652776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gu Y., Wu R. F., Xu Y. C., Flores S. C., Terada L. S. HIV Tat activates c-Jun amino-terminal kinase through an oxidant-dependent mechanism. Virology. 2001;286(1):62–71. doi: 10.1006/viro.2001.0998. [DOI] [PubMed] [Google Scholar]
- 87.Toborek M., Lee Y. W., Pu H., et al. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. Journal of Neurochemistry. 2003;84(1):169–179. doi: 10.1046/j.1471-4159.2003.01543.x. [DOI] [PubMed] [Google Scholar]
- 88.Mastrantonio R., Cervelli M., Pietropaoli S., et al. HIV-Tat induces the Nrf2/ARE pathway through NMDA receptor-elicited spermine oxidase activation in human neuroblastoma cells. PLoS ONE. 2016;11(2) doi: 10.1371/journal.pone.0149802.e0149802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vilhardt F., Plastre O., Sawada M., et al. The HIV-1 Nef protein and phagocyte NADPH oxidase activation. The Journal of Biological Chemistry. 2002;277(44):42136–42143. doi: 10.1074/jbc.m200862200. [DOI] [PubMed] [Google Scholar]
- 90.Olivetta E., Pietraforte D., Schiavoni I., Minetti M., Federico M., Sanchez M. HIV-1 Nef regulates the release of superoxide anions from human macrophages. Biochemical Journal. 2005;390(2):591–602. doi: 10.1042/BJ20042139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Salmen S., Colmenares M., Peterson D. L., Reyes E., Rosales J. D., Berrueta L. HIV-1 Nef associates with p22-phox, a component of the NADPH oxidase protein complex. Cellular Immunology. 2010;263(2):166–171. doi: 10.1016/j.cellimm.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 92.Arunagiri C., Macreadie I., Hewish D., Azad A. A C-terminal domain of HIV-1 accessory protein Vpr is involved in penetration, mitochondrial dysfunction and apoptosis of human CD4+ lymphocytes. Apoptosis. 1997;2(1):69–76. doi: 10.1023/a:1026487609215. [DOI] [PubMed] [Google Scholar]
- 93.Gazdag Z., Stromájer-Rácz T., Belagyi J., et al. Regulation of unbalanced redox homeostasis induced by the expression of wild-type HIV-1 viral protein R (NL4-3Vpr) in fission yeast. Acta Biologica Hungarica. 2015;66(3):326–338. doi: 10.1556/018.66.2015.3.8. [DOI] [PubMed] [Google Scholar]
- 94.Isaguliants M., Smirnova O., Ivanov A. V., et al. Oxidative stress induced by HIV-1 reverse transcriptase modulates the enzyme's performance in gene immunization. Human Vaccines and Immunotherapeutics. 2013;9(10):2111–2119. doi: 10.4161/hv.25813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foga I. O., Nath A., Hasinoff B. B., Geiger J. D. Antioxidants and dipyridamole inhibit HIV-1 gp120-induced free radical- based oxidative damage to human monocytoid cells. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1997;16(4):223–229. doi: 10.1097/00042560-199712010-00001. [DOI] [PubMed] [Google Scholar]
- 96.Pietraforte D., Tritarelli E., Testa U., Minetti M. gp120 HIV envelope glycoprotein increases the production of nitric oxide in human monocyte-derived macrophages. Journal of Leukocyte Biology. 1994;55(2):175–182. doi: 10.1002/jlb.55.2.175. [DOI] [PubMed] [Google Scholar]
- 97.Capone C., Cervelli M., Angelucci E., et al. A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radical Biology and Medicine. 2013;63:99–107. doi: 10.1016/j.freeradbiomed.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 98.Perry S. W., Norman J. P., Litzburg A., Zhang D., Dewhurst S., Gelbard H. A. HIV-1 transactivator of transcription protein induces mitochondrial hyperpolarization and synaptic stress leading to apoptosis. The Journal of Immunology. 2005;174(7):4333–4344. doi: 10.4049/jimmunol.174.7.4333. [DOI] [PubMed] [Google Scholar]
- 99.Wu R.-F., Ma Z., Liu Z., Terada L. S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local ras activation. Molecular and Cellular Biology. 2010;30(14):3553–3568. doi: 10.1128/mcb.01445-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Helmcke I., Heumüller S., Tikkanen R., Schröder K., Brandes R. P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxidants and Redox Signaling. 2009;11(6):1279–1287. doi: 10.1089/ars.2008.2383. [DOI] [PubMed] [Google Scholar]
- 101.Ramming T., Hansen H. G., Nagata K., Ellgaard L., Appenzeller-Herzog C. GPx8 peroxidase prevents leakage of H2O2 from the endoplasmic reticulum. Free Radical Biology and Medicine. 2014;70:106–116. doi: 10.1016/j.freeradbiomed.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 102.Malinouski M., Zhou Y., Belousov V. V., Hatfield D. L., Gladyshev V. N. Hydrogen peroxide probes directed to different cellular compartments. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0014564.e14564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Birk J., Ramming T., Odermatt A., Appenzeller-Herzog C. Green fluorescent protein-based monitoring of endoplasmic reticulum redox poise. Frontiers in Genetics. 2013;4, article 108 doi: 10.3389/fgene.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Laurindo F. R., Pescatore L. A., Fernandes Dde C. Protein disulfide isomerase in redox cell signaling and homeostasis. Free Radical Biology & Medicine. 2012;52(9):1954–1969. doi: 10.1016/j.freeradbiomed.2012.02.037. [DOI] [PubMed] [Google Scholar]
- 105.Li G., Mongillo M., Chin K.-T., et al. Role of ERO1-α-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. Journal of Cell Biology. 2009;186(6):783–792. doi: 10.1083/jcb.200904060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tavender T. J., Bulleid N. J. Peroxiredoxin IV protects cells from oxidative stress by removing H2O2 produced during disulphide formation. The Journal of Cell Science. 2010;123(15):2672–2679. doi: 10.1242/jcs.067843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang L., Zhang L., Niu Y., Sitia R., Wang C.-C. Glutathione peroxidase 7 utilizes hydrogen peroxide generated by Ero1α to promote oxidative protein folding. Antioxidants and Redox Signaling. 2014;20(4):545–556. doi: 10.1089/ars.2013.5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stromájer-Rácz T., Gazdag Z., Belágyi J., Vágvölgyi C., Zhao R. Y., Pesti M. Oxidative stress induced by HIV-1 F34IVpr in Schizosaccharomyces pombe is one of its multiple functions. Experimental and Molecular Pathology. 2010;88(1):38–44. doi: 10.1016/j.yexmp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 109.Macreadie I. G., Thorburn D. R., Kirby D. M., Castelli L. A., de Rozario N. L., Azad A. A. HIV-1 protein Vpr causes gross mitochondrial dysfunction in the yeast Saccharomyces cerevisiae . FEBS Letters. 1997;410(2-3):145–149. doi: 10.1016/s0014-5793(97)00542-5. [DOI] [PubMed] [Google Scholar]
- 110.Halestrap A. P., Brenner C. The adenine nucleotide translocase: a central component of the mitochondrial permeability transition pore and key player in cell death. Current Medicinal Chemistry. 2003;10(16):1507–1525. doi: 10.2174/0929867033457278. [DOI] [PubMed] [Google Scholar]
- 111.Okuda M., Li K., Beard M. R., et al. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122(2):366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 112.McClain S. L., Clippinger A. J., Lizzano R., Bouchard M. J. Hepatitis B virus replication is associated with an HBx-dependent mitochondrion-regulated increase in cytosolic calcium levels. Journal of Virology. 2007;81(21):12061–12065. doi: 10.1128/JVI.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim H. J., Kim C.-H., Ryu J.-H., et al. Reactive oxygen species induce antiviral innate immune response through IFN-λ regulation in human nasal epithelial cells. American Journal of Respiratory Cell and Molecular Biology. 2013;49(5):855–865. doi: 10.1165/rcmb.2013-0003oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ivanov A. V., Smirnova O. A., Petrushanko I. Y., et al. HCV core protein uses multiple mechanisms to induce oxidative stress in human hepatoma Huh7 cells. Viruses. 2015;7(6):2745–2770. doi: 10.3390/v7062745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smirnova O. A., Ivanova O. N., Bartosch B., et al. Hepatitis C virus NS5A protein triggers oxidative stress by inducing NADPH oxidases 1 and 4 and cytochrome P450 2E1. Oxidative Medicine and Cellular Longevity. 2016;2016:10. doi: 10.1155/2016/8341937.8341937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amatore D., Sgarbanti R., Aquilano K., et al. Influenza virus replication in lung epithelial cells depends on redox-sensitive pathways activated by NOX4-derived ROS. Cellular Microbiology. 2015;17(1):131–145. doi: 10.1111/cmi.12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Selemidis S., Seow H. J., Broughton B. R. S., et al. Nox1 oxidase suppresses influenza a virus-induced lung inflammation and oxidative stress. PLoS ONE. 2013;8(4) doi: 10.1371/journal.pone.0060792.e60792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.To E. E., Broughton B. R. S., Hendricks K. S., Vlahos R., Selemidis S. Influenza A virus and TLR7 activation potentiate NOX2 oxidase-dependent ROS production in macrophages. Free Radical Research. 2014;48(8):940–947. doi: 10.3109/10715762.2014.927579. [DOI] [PubMed] [Google Scholar]
- 119.Boudreau H. E., Emerson S. U., Korzeniowska A., Jendrysik M. A., Leto T. L. Hepatitis C virus (HCV) proteins induce NADPH oxidase 4 expression in a transforming growth factor β-dependent manner: a new contributor to HCV-induced oxidative stress. Journal of Virology. 2009;83(24):12934–12946. doi: 10.1128/jvi.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Mochel N. S. R., Seronello S., Wang S. H., et al. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52(1):47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fink K., Duval A., Martel A., Soucy-Faulkner A., Grandvaux N. Dual role of NOX2 in respiratory syncytial virus- and sendai virus-induced activation of NF-κB in airway epithelial cells. The Journal of Immunology. 2008;180(10):6911–6922. doi: 10.4049/jimmunol.180.10.6911. [DOI] [PubMed] [Google Scholar]
- 122.Gwarzo M. Y., Muhammad S. A. Extracellular superoxide dismutase activity and plasma malondialdehyde in human immunodeficiency virus subjects of kano state as surrogate markers of CD4 status. International Journal of Biomedical Science. 2010;6(4):294–300. [PMC free article] [PubMed] [Google Scholar]
- 123.Samikkannu T., Ranjith D., Rao K. V. K., et al. HIV-1 gp120 and morphine induced oxidative stress: role in cell cycle regulation. Frontiers in Microbiology. 2015;6, article 614 doi: 10.3389/fmicb.2015.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Marecki J. C., Cota-Gomez A., Vaitaitis G. M., et al. HIV-1 Tat regulates the SOD2 basal promoter by altering Sp1/Sp3 binding activity. Free Radical Biology and Medicine. 2004;37(6):869–880. doi: 10.1016/j.freeradbiomed.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 125.Flores S. C., Marecki J. C., Harper K. P., Bose S. K., Nelson S. K., McCord J. M. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(16):7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Richard M.-J., Guiraud P., Didier C., Seve M., Flores S. C., Favier A. Human immunodeficiency virus type 1 Tat protein impairs selenoglutathione peroxidase expression and activity by a mechanism independent of cellular selenium uptake: consequences on cellular resistance to UV-A radiation. Archives of Biochemistry and Biophysics. 2001;386(2):213–220. doi: 10.1006/abbi.2000.2197. [DOI] [PubMed] [Google Scholar]
- 127.Visalli V., Muscoli C., Sacco I., et al. N-acetylcysteine prevents HIV gp 120-related damage of human cultured astrocytes: correlation with glutamine synthase dysfunction. BMC Neuroscience. 2007;8, article 106 doi: 10.1186/1471-2202-8-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Monroy N., Herrero L., Carrasco L., González M. E. Influence of glutathione availability on cell damage induced by human immunodeficiency virus type 1 viral protein R. Virus Research. 2016;213:116–123. doi: 10.1016/j.virusres.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 129.Mehdi K., Penninckx M. J. An important role for glutathione and γ-glutamyltranspeptidase in the supply of growth requirements during nitrogen starvation of the yeast Saccharomyces cerevisiae . Microbiology. 1997;143(6):1885–1889. doi: 10.1099/00221287-143-6-1885. [DOI] [PubMed] [Google Scholar]
- 130.Gill A. J., Kovacsics C. E., Vance P. J., Collman R. G., Kolson D. L. Induction of heme oxygenase-1 deficiency and associated glutamate-mediated neurotoxicity is a highly conserved HIV phenotype of chronic macrophage infection that is resistant to antiretroviral therapy. Journal of Virology. 2015;89(20):10656–10667. doi: 10.1128/JVI.01495-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gill A. J., Kovacsics C. E., Cross S. A., et al. Heme oxygenase-1 deficiency accompanies neuropathogenesis of HIV-associated neurocognitive disorders. The Journal of Clinical Investigation. 2014;124(10):4459–4472. doi: 10.1172/jci72279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burdette D., Olivarez M., Waris G. Activation of transcription factor Nrf2 by hepatitis C virus induces the cell-survival pathway. Journal of General Virology. 2010;91(3):681–690. doi: 10.1099/vir.0.014340-0. [DOI] [PubMed] [Google Scholar]
- 133.Ivanov A. V., Smirnova O. A., Ivanova O. N., Masalova O. V., Kochetkov S. N., Isaguliants M. G. Hepatitis C virus proteins activate NRF2/ARE pathway by distinct ROS-dependent and independent mechanisms in HUH7 cells. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0024957.e24957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jiang Y., Bao H., Ge Y., et al. Therapeutic targeting of GSK3β enhances the Nrf2 antioxidant response and confers hepatic cytoprotection in hepatitis C. Gut. 2015;64(1):168–179. doi: 10.1136/gutjnl-2013-306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Carvajal-Yepes M., Himmelsbach K., Schaedler S., et al. Hepatitis C virus impairs the induction of cytoprotective Nrf2 target genes by delocalization of small maf proteins. The Journal of Biological Chemistry. 2011;286(11):8941–8951. doi: 10.1074/jbc.m110.186684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pialoux V., Mounier R., Brown A. D., Steinback C. D., Rawling J. M., Poulin M. J. Relationship between oxidative stress and HIF-1α mRNA during sustained hypoxia in humans. Free Radical Biology and Medicine. 2009;46(2):321–326. doi: 10.1016/j.freeradbiomed.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 137.Qutub A. A., Popel A. S. Reactive oxygen species regulate hypoxia-inducible factor 1α differentially in cancer and ischemia. Molecular and Cellular Biology. 2008;28(16):5106–5119. doi: 10.1128/mcb.00060-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kurata S.-I. Sensitization of the HIV-1-LTR upon long term low dose oxidative stress. The Journal of Biological Chemistry. 1996;271(36):21798–21802. doi: 10.1074/jbc.271.36.21798. [DOI] [PubMed] [Google Scholar]
- 139.Staal F. J. T., Roederer M., Raju P. A., et al. Antioxidants inhibit stimulation of HIV transcription. AIDS Research and Human Retroviruses. 1993;9(4):299–306. doi: 10.1089/aid.1993.9.299. [DOI] [PubMed] [Google Scholar]
- 140.Dong Q., Kelkar S., Xiao Y., Joshi-Barve S., McClain C. J., Barve S. S. Ethanol enhances TNF-α-inducible NFκB activation and HIV-1-LTR transcription in CD4+ Jurkat T lymphocytes. Journal of Laboratory and Clinical Medicine. 2000;136(5):333–343. doi: 10.1067/mlc.2000.110104. [DOI] [PubMed] [Google Scholar]
- 141.Duh E. J., Maury W. J., Folks T. M., Fauci A. S., Rabson A. B. Tumor necrosis factor α activates human immunodeficiency virus type 1 through induction of nuclear factor binding to the NF-κB sites in the long terminal repeat. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(15):5974–5978. doi: 10.1073/pnas.86.15.5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Staal F. J. T., Roederer M., Herzenberg L. A., Herzenberg L. A. Intracellular thiols regulate activation of nuclear factor kappa B and transcription of human immunodeficiency virus. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(24):9943–9947. doi: 10.1073/pnas.87.24.9943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pereira L. A., Bentley K., Peeters A., Churchill M. J., Deacon N. J. A compilation of cellular transcription factor interactions with the HIV-1 LTR promoter. Nucleic Acids Research. 2000;28(3):663–668. doi: 10.1093/nar/28.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bhaskar A., Munshi M., Khan S. Z., et al. Measuring glutathione redox potential of HIV-1-infected macrophages. The Journal of Biological Chemistry. 2015;290(2):1020–1038. doi: 10.1074/jbc.m114.588913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Palmer C. S., Cherry C. L., Sada-Ovalle I., Singh A., Crowe S. M. Glucose metabolism in T cells and monocytes: new perspectives in HIV pathogenesis. EBioMedicine. 2016;6:31–41. doi: 10.1016/j.ebiom.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Palamara A. T., Perno C.-F., Aquaro S., Buè M. C., Dini L., Garaci E. Glutathione inhibits HIV replication by acting at late stages of the virus life cycle. AIDS Research and Human Retroviruses. 1996;12(16):1537–1541. doi: 10.1089/aid.1996.12.1537. [DOI] [PubMed] [Google Scholar]
- 147.Lan X., Cheng K., Chandel N., et al. High glucose enhances HIV entry into T cells through upregulation of CXCR4. Journal of Leukocyte Biology. 2013;94(4):769–777. doi: 10.1189/jlb.0313142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Garaci E., Palamara A. T., Ciriolo M. R., et al. Intracellular GSH content and HIV replication in human macrophages. Journal of Leukocyte Biology. 1997;62(1):54–59. doi: 10.1002/jlb.62.1.54. [DOI] [PubMed] [Google Scholar]
- 149.Sgarbanti R., Nencioni L., Amatore D., et al. Redox regulation of the influenza hemagglutinin maturation process: a new cell-mediated strategy for anti-influenza therapy. Antioxidants and Redox Signaling. 2011;15(3):593–606. doi: 10.1089/ars.2010.3512. [DOI] [PubMed] [Google Scholar]
- 150.Chetram M. A., Hinton C. V. ROS-mediated regulation of CXCR4 in cancer. Frontiers in Biology. 2013;8(3):273–278. doi: 10.1007/s11515-012-1204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Andrisse S., Koehler R. M., Chen J. E., et al. Role of GLUT1 in regulation of reactive oxygen species. Redox Biology. 2014;2(1):764–771. doi: 10.1016/j.redox.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sorbara L. R., Maldarelli F., Chamoun G., et al. Human immunodeficiency virus type 1 infection of H9 cells induces increased glucose transporter expression. Journal of Virology. 1996;70(10):7275–7279. doi: 10.1128/jvi.70.10.7275-7279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kovitz C. A., Morgello S. Cerebral glucose transporter expression in HIV infection. Acta Neuropathologica. 1997;94(2):140–145. doi: 10.1007/s004010050685. [DOI] [PubMed] [Google Scholar]
- 154.Bartosch B. Hepatitis C virus and its complex interplay with hepatic glucose and lipid metabolism. Journal of Hepatology. 2009;50(5):845–847. doi: 10.1016/j.jhep.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 155.Lévy P., Bartosch B. Metabolic reprogramming: a hallmark of viral oncogenesis. Oncogene. 2016;35(32):4155–4164. doi: 10.1038/onc.2015.479. [DOI] [PubMed] [Google Scholar]
- 156.Wu C.-H., Wu C.-F., Huang H.-W., Jao Y.-C., Yen G.-C. Naturally occurring flavonoids attenuate high glucose-induced expression of proinflammatory cytokines in human monocytic THP-1 cells. Molecular Nutrition and Food Research. 2009;53(8):984–995. doi: 10.1002/mnfr.200800495. [DOI] [PubMed] [Google Scholar]
- 157.Yao K., Ge J.-B., Sun A.-J., et al. Effects and mechanism of hyperglycemia on development and maturation and immune function of human monocyte derived dendritic cells. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34(1):60–64. [PubMed] [Google Scholar]
- 158.Sandstrom P. A., Murray J., Folks T. M., Diamond A. M. Antioxidant defenses influence HIV-1 replication and associated cytopathic effects. Free Radical Biology and Medicine. 1998;24(9):1485–1491. doi: 10.1016/S0891-5849(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 159.Kaul M., Garden G. A., Lipton S. A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- 160.Kallianpur K. J., Gerschenson M., Mitchell B. I., et al. Oxidative mitochondrial DNA damage in peripheral blood mononuclear cells is associated with reduced volumes of hippocampus and subcortical gray matter in chronically HIV-infected patients. Mitochondrion. 2016;28:8–15. doi: 10.1016/j.mito.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Galicia O., Sanchez-Alavez M., Mendez Diaz M., Navarro L., Prospero-Garcia O. HIV glycoprotein 120: possible etiological agent of AIDS-associated dementia. Revista de Investigación Clínica. 2002;54(5):437–452. [PubMed] [Google Scholar]
- 162.Brenneman D. E., McCune S. K., Mervis R. F., Hill J. M. gp120 as an etiologic agent for NeuroAIDS: neurotoxicity and model systems. Advances in Neuroimmunology. 1994;4(3):157–165. doi: 10.1016/s0960-5428(06)80252-4. [DOI] [PubMed] [Google Scholar]
- 163.Louboutin J.-P., Agrawal L., Reyes B. A. S., Van Bockstaele E. J., Strayer D. S. HIV-1 gp120-induced injury to the blood-brain barrier: role of metalloproteinases 2 and 9 and relationship to oxidative stress. Journal of Neuropathology and Experimental Neurology. 2010;69(8):801–816. doi: 10.1097/nen.0b013e3181e8c96f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu R., Feng X., Xie X., Zhang J., Wu D., Xu L. HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Research. 2012;1436:13–19. doi: 10.1016/j.brainres.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 165.Toborek M., Lee Y. W., Flora G., et al. Mechanisms of the blood-brain barrier disruption in HIV-1 infection. Cellular and Molecular Neurobiology. 2005;25(1):181–199. doi: 10.1007/s10571-004-1383-x. [DOI] [PubMed] [Google Scholar]
- 166.Ferrucci A., Nonnemacher M. R., Cohen É. A., Wigdahl B. Extracellular human immunodeficiency virus type 1 viral protein R causes reductions in astrocytic ATP and glutathione levels compromising the antioxidant reservoir. Virus Research. 2012;167(2):358–369. doi: 10.1016/j.virusres.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ferrucci A., Nonnemacher M. R., Wigdahl B. Extracellular HIV-1 viral protein R affects astrocytic glyceraldehyde 3-phosphate dehydrogenase activity and neuronal survival. Journal of NeuroVirology. 2013;19(3):239–253. doi: 10.1007/s13365-013-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hoshino S., Konishi M., Mori M., et al. HIV-1 Vpr induces TLR4/MyD88-mediated IL-6 production and reactivates viral production from latency. Journal of Leukocyte Biology. 2010;87(6):1133–1143. doi: 10.1189/jlb.0809547. [DOI] [PubMed] [Google Scholar]
- 169.Chen L., Liu J., Xu C., Keblesh J., Zang W., Xiong H. HIV-1gp120 induces neuronal apoptosis through enhancement of 4-aminopyridine-senstive outward K+ currents. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0025994.e25994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tomitori H., Usui T., Saeki N., et al. Polyamine oxidase and acrolein as novel biochemical markers for diagnosis of cerebral stroke. Stroke. 2005;36(12):2609–2613. doi: 10.1161/01.str.0000190004.36793.2d. [DOI] [PubMed] [Google Scholar]
- 171.Wood P. L., Khan M. A., Moskal J. R., Todd K. G., Tanay V. A. M. I., Baker G. Aldehyde load in ischemia-reperfusion brain injury: neuroprotection by neutralization of reactive aldehydes with phenelzine. Brain Research. 2006;1122(1):184–190. doi: 10.1016/j.brainres.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 172.Dever S. M., Xu R., Fitting S., Knapp P. E., Hauser K. F. Differential expression and HIV-1 regulation of μ-opioid receptor splice variants across human central nervous system cell types. Journal of NeuroVirology. 2012;18(3):181–190. doi: 10.1007/s13365-012-0096-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sudano I., Spieker L. E., Noll G., Corti R., Weber R., Lüscher T. F. Cardiovascular disease in HIV infection. American Heart Journal. 2006;151(6):1147–1155. doi: 10.1016/j.ahj.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 174.Spieker L. E., Karadag B., Binggeli C., Corti R. Rapid progression of atherosclerotic coronary artery disease in patients with human immunodeficiency virus infection. Heart and Vessels. 2005;20(4):171–174. doi: 10.1007/s00380-004-0790-8. [DOI] [PubMed] [Google Scholar]
- 175.Bonnet D., Aggoun Y., Szezepanski I., Bellal N., Blanche S. Arterial stiffness and endothelial dysfunction in HIV-infected children. AIDS. 2004;18(7):1037–1041. doi: 10.1097/00002030-200404300-00012. [DOI] [PubMed] [Google Scholar]
- 176.Paton P., Tabib A., Loire R., Tete R. Coronary artery lesions and human immunodeficiency virus infection. Research in Virology. 1993;144(3):225–231. doi: 10.1016/s0923-2516(06)80033-6. [DOI] [PubMed] [Google Scholar]
- 177.Joshi V. V., Pawel B., Connor E., et al. Arteriopathy in children with acquired immune deficiency syndrome. Fetal and Pediatric Pathology. 1987;7(3):261–275. doi: 10.1080/15513818709177129. [DOI] [PubMed] [Google Scholar]
- 178.Cadranel J., Garfield D., Lavolé A., Wislez M., Milleron B., Mayaud C. Lung cancer in HIV infected patients: facts, questions and challenges. Thorax. 2006;61(11):1000–1008. doi: 10.1136/thx.2005.052373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Beck J. M., Rosen M. J., Peavy H. H. Pulmonary complications of HIV infection. Report of the fourth NHLBI workshop. American Journal of Respiratory and Critical Care Medicine. 2001;164(11):2120–2126. doi: 10.1164/ajrccm.164.11.2102047. [DOI] [PubMed] [Google Scholar]
- 180.Fan X., Staitieh B. S., Jensen J. S., et al. Activating the Nrf2-mediated antioxidant response element restores barrier function in the alveolar epithelium of HIV-1 transgenic rats. American Journal of Physiology—Lung Cellular and Molecular Physiology. 2013;305(3):L267–L277. doi: 10.1152/ajplung.00288.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brundu S., Palma L., Picceri G. G., et al. Glutathione depletion is linked with th2 polarization in mice with a retrovirus-induced immunodeficiency syndrome, murine AIDS: role of proglutathione molecules as immunotherapeutics. Journal of Virology. 2016;90(16):7118–7130. doi: 10.1128/jvi.00603-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Davies N. W. S., Guillemin G., Brew B. J. Tryptophan, neurodegeneration and HIV-associated neurocognitive disorder. International Journal of Tryptophan Research. 2010;3(1):121–140. doi: 10.4137/ijtr.s4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zilberman-Schapira G., Zmora N., Itav S., Bashiardes S., Elinav H., Elinav E. The gut microbiome in human immunodeficiency virus infection. BMC Medicine. 2016;14(1, article 83) doi: 10.1186/s12916-016-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Forrest C. M., Mackay G. M., Stoy N., et al. Tryptophan loading induces oxidative stress. Free Radical Research. 2004;38(11):1167–1171. doi: 10.1080/10715760400011437. [DOI] [PubMed] [Google Scholar]
- 185.Baban B., Hansen A. M., Chandler P. R., et al. A minor population of splenic dendritic cells expressing CD19 mediates IDO-dependent T cell suppression via type I IFN signaling following B7 ligation. International Immunology. 2005;17(7):909–919. doi: 10.1093/intimm/dxh271. [DOI] [PubMed] [Google Scholar]
- 186.Schröcksnadel K., Wirleitner B., Winkler C., Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clinica Chimica Acta. 2006;364(1-2):82–90. doi: 10.1016/j.cca.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 187.Bosinger S. E., Li Q., Gordon S. N., et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. The Journal of Clinical Investigation. 2009;119(12):3556–3572. doi: 10.1172/jci40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hoshi M., Saito K., Hara A., et al. The absence of IDO upregulates type I IFN production, resulting in suppression of viral replication in the retrovirus-infected mouse. The Journal of Immunology. 2010;185(6):3305–3312. doi: 10.4049/jimmunol.0901150. [DOI] [PubMed] [Google Scholar]
- 189.O'Connor M. A., Green W. R. The role of indoleamine 2,3-dioxygenase in LP-BPM5 murine retroviral disease progression. Virology Journal. 2013;10, article 154 doi: 10.1186/1743-422x-10-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Boasso A., Herbeuval J.-P., Hardy A. W., et al. HIV inhibits CD4+ T-cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109(8):3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Soliman H., Mediavilla-Varela M., Antonia S. Indoleamine 2,3-dioxygenase: is it an immune suppressor? Cancer Journal. 2010;16(4):354–359. doi: 10.1097/ppo.0b013e3181eb3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mellor A. L., Munn D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Reviews Immunology. 2004;4(10):762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 193.Boasso A., Vaccari M., Hryniewicz A., et al. Regulatory T-cell markers, indoleamine 2,3-dioxygenase, and virus levels in spleen and gut during progressive simian immunodeficiency virus infection. Journal of Virology. 2007;81(21):11593–11603. doi: 10.1128/JVI.00760-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Favre D., Mold J., Hunt P. W., et al. Tryptophan catabolism by indoleamine 2, 3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Science Translational Medicine. 2010;2(32) doi: 10.1126/scitranslmed.3000632.32ra36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Boasso A., Vaccari M., Nilsson J., et al. Do regulatory T-cells play a role in AIDS pathogenesis? AIDS Reviews. 2006;8(3):141–147. [PubMed] [Google Scholar]
- 196.Boasso A., Shearer G. M. How does indoleamine 2,3-dioxygenase contribute to HIV-mediated immune dysregulation. Current Drug Metabolism. 2007;8(3):217–223. doi: 10.2174/138920007780362527. [DOI] [PubMed] [Google Scholar]
- 197.Nzowa L. K., Teponno R. B., Tapondjou L. A., et al. Two new tryptophan derivatives from the seed kernels of Entada rheedei: effects on cell viability and HIV infectivity. Fitoterapia. 2013;87:37–42. doi: 10.1016/j.fitote.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Malorni W., Rivabene R., Lucia B. M., et al. The role of oxidative imbalance in progression to AIDS: effect of the thiol supplier N-acetylcysteine. AIDS Research and Human Retroviruses. 1998;14(17):1589–1596. doi: 10.1089/aid.1998.14.1589. [DOI] [PubMed] [Google Scholar]
- 199.Aukrust P., Svardal A. M., Müller F., Lunden B., Berge R. K., Frøland S. S. Decreased levels of total and reduced glutathione in CD4+ lymphocytes in common variable immunodeficiency are associated with activation of the tumor necrosis factor system: possible immunopathogenic role of oxidative stress. Blood. 1995;86(4):1383–1391. [PubMed] [Google Scholar]
- 200.Uzasci L., Nath A., Cotter R. Oxidative stress and the HIV-infected brain proteome. Journal of Neuroimmune Pharmacology. 2013;8(5):1167–1180. doi: 10.1007/s11481-013-9444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Esplugues J. V. NO as a signalling molecule in the nervous system. British Journal of Pharmacology. 2002;135(5):1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Polazzi E., Levi G., Minghetti L. Human immunodeficiency virus type 1 Tat protein stimulates inducible nitric oxide synthase expression and nitric oxide production in microglial cultures. Journal of Neuropathology and Experimental Neurology. 1999;58(8):825–831. doi: 10.1097/00005072-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 203.Liu X., Jana M., Dasgupta S., et al. Human immunodeficiency virus type 1 (HIV-1) tat induces nitric-oxide synthase in human astroglia. The Journal of Biological Chemistry. 2002;277(42):39312–39319. doi: 10.1074/jbc.m205107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Adamson D. C., Kopnisky K. L., Dawson T. M., Dawson V. L. Mechanisms and structural determinants of HIV-1 coat protein, gp41-induced neurotoxicity. The Journal of Neuroscience. 1999;19(1):64–71. doi: 10.1523/JNEUROSCI.19-01-00064.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yu Q.-R., Zhang Z.-P., Zhang H., et al. Inducible nitric oxide synthase is involved in the oxidation stress induced by HIV-1 gp120 in human retina pigment epithelial cells. Chinese Medical Journal. 2008;121(24):2578–2583. [PubMed] [Google Scholar]
- 206.Mangino G., Famiglietti M., Capone C., et al. HIV-1 myristoylated nef treatment of murine microglial cells activates inducible nitric oxide synthase, NO2 production and neurotoxic activity. PLoS ONE. 2015;10(6) doi: 10.1371/journal.pone.0130189.e0130189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.García M., Del Llano A. M., Cruz-Colón E., Saavedra S., Lavergne J. A., Lavergne J. A. Characteristics of nitric oxide-induced apoptosis and its target cells in mitogen-stimulated peripheral blood mononuclear cells from HIV+ subjects. Immunological Investigations. 2001;30(4):267–287. doi: 10.1081/imm-100108163. [DOI] [PubMed] [Google Scholar]
- 208.Banerjee S., Liao L., Russo R., et al. Isobaric tagging-based quantification by mass spectrometry of differentially regulated proteins in synaptosomes of HIV/gp120 transgenic mice: implications for HIV-associated neurodegeneration. Experimental Neurology. 2012;236(2):298–306. doi: 10.1016/j.expneurol.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Steiner J., Haughey N., Li W., et al. Oxidative stress and therapeutic approaches in HIV dementia. Antioxidants and Redox Signaling. 2006;8(11-12):2089–2100. doi: 10.1089/ars.2006.8.2089. [DOI] [PubMed] [Google Scholar]
- 210.Hori K., Burd P. R., Furuke K., Kutza J., Weih K. A., Clouse K. A. Human immunodeficiency virus-1-infected macrophages induce inducible nitric oxide synthase and nitric oxide (NO) production in astrocytes: astrocytic NO as a possible mediator of neural damage in acquired immunodeficiency syndrome. Blood. 1999;93(6):1843–1850. [PubMed] [Google Scholar]
- 211.Hu S., Ali H., Sheng W. S., Ehrlich L. C., Peterson P. K., Chao C. C. Gp-41-mediated astrocyte inducible nitric oxide synthase mRNA expression: involvement of interleukin-1beta production by microglia. The Journal of Neuroscience. 1999;19(15):6468–6474. doi: 10.1523/JNEUROSCI.19-15-06468.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Adamson D. C., McArthur J. C., Dawson T. M., Dawson V. L. Rate and severity of HIV-associated dementia (HAD): correlations with Gp41 and iNOS. Molecular Medicine. 1999;5(2):98–109. [PMC free article] [PubMed] [Google Scholar]
- 213.Li W., Malpica-Llanos T. M., Gundry R., et al. Nitrosative stress with HIV dementia causes decreased L-prostaglandin D synthase activity. Neurology. 2008;70(19, part 2):1753–1762. doi: 10.1212/01.wnl.0000282761.19578.35. [DOI] [PubMed] [Google Scholar]
- 214.Uzasci L., Bianchet M. A., Cotter R. J., Nath A. Identification of nitrated immunoglobulin variable regions in the HIV-infected human brain: implications in HIV infection and immune response. Journal of Proteome Research. 2014;13(3):1614–1623. doi: 10.1021/pr401117m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.González-Nicolás J., Resino S., Jiménez J. L., Alvarez S., Fresno M., Muñoz-Fernández M. A. Tumor necrosis factor-alpha and nitric oxide in vertically HIV-1-infected children: implications for pathogenesis. European Cytokine Network. 2001;12(3):437–444. [PubMed] [Google Scholar]
- 216.Mossalayi M. D., Becherel P.-A., Debré P. Critical role of nitric oxide during the apoptosis of peripheral blood leukocytes from patients with aids. Molecular Medicine. 1999;5(12):812–819. [PMC free article] [PubMed] [Google Scholar]
- 217.Jiménez J. L., González-Nicolás J., Alvarez S., Fresno M., Muñoz-Fernández M. A. Regulation of human immunodeficiency virus type 1 replication in human T lymphocytes by nitric oxide. Journal of Virology. 2001;75(10):4655–4663. doi: 10.1128/JVI.75.10.4655-4663.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mannick J. B., Stamler J. S., Teng E., et al. Nitric oxide modulates HIV-1 replication. Journal of Acquired Immune Deficiency Syndromes. 1999;22(1):1–9. doi: 10.1097/00042560-199909010-00001. [DOI] [PubMed] [Google Scholar]
- 219.Persichini T., Colasanti M., Lauro G. M., Ascenzi P. Cysteine nitrosylation inactivates the HIV-1 protease. Biochemical and Biophysical Research Communications. 1998;250(3):575–576. doi: 10.1006/bbrc.1998.9350. [DOI] [PubMed] [Google Scholar]
- 220.Sehajpal P. K., Basu A., Ogiste J. S., Lander H. M. Reversible S-nitrosation and inhibition of HIV-1 protease. Biochemistry. 1999;38(40):13407–13413. doi: 10.1021/bi9912995. [DOI] [PubMed] [Google Scholar]
- 221.Cairoli E., Scott-Algara D., Pritsch O., Dighiero G., Cayota A. HIV-1 induced decrease of nitric oxide production and inducible nitric oxide synthase expression during in vivo and in vitro infection. Clinical Immunology. 2008;127(1):26–33. doi: 10.1016/j.clim.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 222.Badley A. D., Sainski A., Wightman F., Lewin S. R. Altering cell death pathways as an approach to cure HIV infection. Cell Death and Disease. 2013;4, article e718 doi: 10.1038/cddis.2013.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Wang X., Chai H., Lin P. H., Yao Q., Chen C. Roles and mechanisms of human immunodeficiency virus protease inhibitor ritonavir and other anti-human immunodeficiency virus drugs in endothelial dysfunction of porcine pulmonary arteries and human pulmonary artery endothelial cells. The American Journal of Pathology. 2009;174(3):771–781. doi: 10.2353/ajpath.2009.080157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Nagiah S., Phulukdaree A., Chuturgoon A. Mitochondrial and oxidative stress response in HepG2 cells following acute and prolonged exposure to antiretroviral drugs. Journal of Cellular Biochemistry. 2015;116(9):1939–1946. doi: 10.1002/jcb.25149. [DOI] [PubMed] [Google Scholar]
- 225.Manda K. R., Banerjee A., Banks W. A., Ercal N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radical Biology and Medicine. 2011;50(7):801–810. doi: 10.1016/j.freeradbiomed.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mondal D., Pradhan L., Ali M., Agrawal K. C. HAART drugs induce oxidative stress in human endothelial cells and increase endothelial recruitment of mononuclear cells: exacerbation by inflammatory cytokines and amelioration by antioxidants. Cardiovascular Toxicology. 2004;4(3):287–302. doi: 10.1385/ct:4:3:287. [DOI] [PubMed] [Google Scholar]
- 227.Hurwitz B. E., Klimas N. G., Llabre M. M., et al. HIV, metabolic syndrome X, inflammation, oxidative stress, and coronary heart disease risk: role of protease inhibitor exposure. Cardiovascular Toxicology. 2004;4(3):303–315. doi: 10.1385/ct:4:3:303. [DOI] [PubMed] [Google Scholar]
- 228.Chandra S., Mondal D., Agrawal K. C. HIV-1 protease inhibitor induced oxidative stress suppresses glucose stimulated insulin release: protection with thymoquinone. Experimental Biology and Medicine. 2009;234(4):442–453. doi: 10.3181/0811-rm-317. [DOI] [PubMed] [Google Scholar]
- 229.Weiß M., Kost B., Renner-Müller I., Wolf E., Mylonas I., Brüning A. Efavirenz causes oxidative stress, endoplasmic reticulum stress, and autophagy in endothelial cells. Cardiovascular Toxicology. 2016;16(1):90–99. doi: 10.1007/s12012-015-9314-2. [DOI] [PubMed] [Google Scholar]
- 230.Masiá M., Padilla S., Bernal E., et al. Influence of antiretroviral therapy on oxidative stress and cardiovascular risk: a prospective cross-sectional study in HIV-infected patients. Clinical Therapeutics. 2007;29(7):1448–1455. doi: 10.1016/j.clinthera.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 231.Mandas A., Iorio E. L., Congiu M. G., et al. Oxidative imbalance in HIV-1 infected patients treated with antiretroviral therapy. Journal of Biomedicine and Biotechnology. 2009;2009:7. doi: 10.1155/2009/749575.749575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Gil L., Tarinas A., Hernández D., et al. Altered oxidative stress indexes related to disease progression marker in human immunodeficiency virus infected patients with antiretroviral therapy. Biomedicine & Aging Pathology. 2011;1(1):8–15. doi: 10.1016/j.biomag.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 233.Ngondi J. L., Oben J., Forkah D. M., Etame L. H., Mbanya D. The effect of different combination therapies on oxidative stress markers in HIV infected patients in Cameroon. AIDS Research and Therapy. 2006;3(1, article 19) doi: 10.1186/1742-6405-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sen C. K. Cellular thiols and redox-regulated signal transduction. Current Topics in Cellular Regulation. 2001;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- 235.Opii W. O., Sultana R., Abdul H. M., Ansari M. A., Nath A., Butterfield D. A. Oxidative stress and toxicity induced by the nucleoside reverse transcriptase inhibitor (NRTI)—2′,3′-dideoxycytidine (ddC): relevance to HIV-dementia. Experimental Neurology. 2007;204(1):29–38. doi: 10.1016/j.expneurol.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Day B. J., Lewis W. Oxidative stress in NRTI-induced toxicity: evidence from clinical experience and experiments in vitro and in vivo. Cardiovascular Toxicology. 2004;4(3):207–216. doi: 10.1385/ct:4:3:207. [DOI] [PubMed] [Google Scholar]
- 237.Sevastianova K., Sutinen J., Westerbacka J., Ristola M., Yki-Järvinen H. Arterial stiffness in HIV-infected patients receiving highly active antiretroviral therapy. Antiviral Therapy. 2005;10(8):925–935. [PubMed] [Google Scholar]
- 238.van Vonderen M. G. A., Smulders Y. M., Stehouwer C. D. A., et al. Carotid intima-media thickness and arterial stiffness in HIV-infected patients: the role of HIV, antiretroviral therapy, and lipodystrophy. Journal of Acquired Immune Deficiency Syndromes. 2009;50(2):153–161. doi: 10.1097/qai.0b013e31819367cd. [DOI] [PubMed] [Google Scholar]
- 239.Becker K., Gromer S., Schirmer R. H., Müller S. Thioredoxin reductase as a pathophysiological factor and drug target. European Journal of Biochemistry. 2000;267(20):6118–6125. doi: 10.1046/j.1432-1327.2000.01703.x. [DOI] [PubMed] [Google Scholar]
- 240.Chirullo B., Sgarbanti R., Limongi D., et al. A candidate anti-HIV reservoir compound, auranofin, exerts a selective ‘anti-memory’ effect by exploiting the baseline oxidative status of lymphocytes. Cell Death and Disease. 2013;4, article e944 doi: 10.1038/cddis.2013.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Lewis M. G., DaFonseca S., Chomont N., et al. Gold drug auranofin restricts the viral reservoir in the monkey AIDS model and induces containment of viral load following ART suspension. AIDS. 2011;25(11):1347–1356. doi: 10.1097/qad.0b013e328347bd77. [DOI] [PubMed] [Google Scholar]
- 242.Shytaj I. L., Chirullo B., Wagner W., et al. Investigational treatment suspension and enhanced cell-mediated immunity at rebound followed by drug-free remission of simian AIDS. Retrovirology. 2013;10, article 71 doi: 10.1186/1742-4690-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Vint I. A. M., Chain B. M., Foreman J. C. The interaction of auranofin and buthionine sulfoximine blocks activation of human peripheral T lymphocytes. Cellular Immunology. 1993;152(1):152–161. doi: 10.1006/cimm.1993.1275. [DOI] [PubMed] [Google Scholar]
- 244.Shytaj I. L., Nickel G., Arts E., et al. Two-year follow-up of macaques developing intermittent control of the human immunodeficiency virus homolog simian immunodeficiency virus SIVmac251 in the chronic phase of infection. Journal of Virology. 2015;89(15):7521–7535. doi: 10.1128/jvi.00396-15. [DOI] [PMC free article] [PubMed] [Google Scholar]