Abstract
In the current study, we sought to identify bone marrow-derived mononuclear cell (BM-MNC) subpopulations associated with a combined improvement in left ventricular ejection fraction (LVEF), left ventricular end-systolic volume (LVESV), and maximal oxygen consumption (VO2 max) in patients with chronic ischemic cardiomyopathy 6 months after receiving transendocardial injections of autologous BM-MNCs or placebo. For this prospectively planned analysis, we conducted an embedded cohort study comprising 78 patients from the FOCUS-Cardiovascular Cell Therapy Research Network (CCTRN) trial. Baseline BM-MNC immunophenotypes and progenitor cell activity were determined by flow cytometry and colony-forming assays, respectively. Previously stable patients who demonstrated improvement in LVEF, LVESV, and VO2 max during the 6-month course of the FOCUS-CCTRN study (group 1, n = 17) were compared to those who showed no change or worsened in one to three of these endpoints (group 2, n = 61) and to a subset of patients from group 2 who declined in all three functional endpoints (group 2A, n = 11). Group 1 had higher frequencies of B-cell and CXCR4+ BM-MNC subpopulations at study baseline than group 2 or 2A. Furthermore, patients in group 1 had fewer endothelial colony-forming cells and monocytes/macrophages in their bone marrow than those in group 2A. To our knowledge, this is the first study to show that in patients with ischemic cardiomyopathy, certain bone marrow-derived cell subsets are associated with improvement in LVEF, LVESV, and VO2 max at 6 months. These results suggest that the presence of both progenitor and immune cell populations in the bone marrow may influence the natural history of chronic ischemic cardiomyopathy—even in stable patients. Thus, it may be important to consider the bone marrow composition and associated regenerative capacity of patients when assigning them to treatment groups and evaluating the results of cell therapy trials.
Keywords: Bone marrow, Heart failure, Ischemic cardiomyopathy, Stem cells, Cell therapy
INTRODUCTION
Clinical investigations of heterogeneous bone marrow-derived mononuclear cells (BM-MNCs) to treat patients with acute myocardial infarction (MI) or chronic ischemic heart failure (HF) have not met early expectations for success1–3. These variable findings have highlighted the need for studies aimed at improving our understanding of whether the mechanisms and pathways associated with endogenous cardiac repair involve BM-MNCs. In our recently reported study of an acute MI cohort, we found that improved patient outcomes were associated with higher frequencies of CD31+ BM-MNCs and with higher growth rates in colony-forming assays4, suggesting that both cell number and function can contribute to patient outcomes. However, in that study, we examined associations between bone marrow (BM) cell populations and patient outcomes only in the cell-treated group and only shortly after acute MI. It is unknown whether there are specific cell populations in the BM that affect cardiac repair independently of treatment, especially in patients with HF. In the current study, we sought to identify BM-MNC subpopulations associated with a rigorous definition of improved cardiac function in patients with HF. The FOCUS-Cardiovascular Cell Therapy Research Network (FOCUS-CCTRN) clinical trial, which assessed the effects of BM-MNC therapy in patients with chronic ischemic HF, showed negative results for all three primary endpoints [left ventricular end-systolic volume (LVESV), maximal oxygen consumption (VO2 max), and reversibility on single-photon emission tomography (SPECT)], but a 2.7% difference in the exploratory endpoint left ventricular ejection fraction (LVEF) between the cell- and placebo-treated patients at 6 months1,5,6. We regrouped FOCUS-CCTRN patients according to outcome using a combined criterion of improved LVEF, LVESV, and VO2 max with the goal of identifying associations between the BM-MNC profile and functional outcomes. Although a reversible defect by SPECT was originally one of the three primary endpoints chosen for the FOCUS study, it became obvious during the study that signal noise exceeded the expected bounds; accordingly, SPECT measurements were not included in our multiple parameter determination of functional improvement for this analysis.
It is rare to see HF patients in whom LVEF, LVESV, and VO2 max improve at the same time; thus, by requiring favorable changes in all three of these cardiac functional parameters, we set a rigorous criterion for identifying patients who improved in the FOCUS-CCTRN trial. We reasoned that applying this criterion and comparing the BM profile of patients who improved to the BM profile of patients who did not could contribute to a better understanding of BM factors associated with positive functional changes. Accordingly, we identified the subgroup of participants in the FOCUS-CCTRN trial who showed improvement in LVEF, LVESV, and VO2 max at the 6-month follow-up and then performed a cohort analysis to determine whether the composition of their BM-MNC samples at study onset differed from that of patients who did not improve in all three functional outcomes over the same period.
MATERIALS AND METHODS
Participants and Procedures
The FOCUS-CCTRN trial1 was conducted at five clinical sites, and institutional review board approval was obtained from each site. The study complied with the Declaration of Helsinki, and informed consent was obtained for all patients. Of the 92 patients who were randomized and eligible for participation in this analysis, 78 had complete follow-up data and consented to have a portion of their BM-MNC product analyzed.
BM Collection and Analysis
BM samples were obtained and processed according to previously published methods1. Briefly, the BM-MNC concentration was adjusted to produce a fixed dose of autologous cells, as described by Gee et al.7. The target dose of BM-MNCs was 100 × 106. The treatment (BM-MNCs or placebo) was administered to the patient in 15 separate injections (0.2 ml each) to left ventricular (LV) endocardial regions identified as viable by electromechanical mapping on the day of BM collection within 12 h of aspiration1. The excess fresh BM-MNC product was shipped to the Biorepository Core, where the functional activity (via colony-forming assays) and immunophenotype (via flow cytometry) were determined, as described previously8.
To characterize the hematopoietic stem cells, endothelial progenitor cells (EPCs), and immune cells in the BM, 1–5 × 106 viable cells were labeled with anti-CD45-PerCP-Cy5.5, anti-CD34-PE-Cy7, anti-CD133-PE (Miltenyi Biotec, Auburn, CA, USA), anti-CD306 vascular endothelial growth factor receptor (VEGFR2/KDR)-APC (R&D Systems, Minneapolis, MN, USA), anti-CD31-FITC, anti-CD3-PE-Cy7, anti-CD19-APC-H7, anti-CD11b-APC, anti-CXCR4-PE, and anti-CD14-FITC or an isotype-matched control antibody. Cell surface markers were analyzed using FlowJo software 7.6.5 (Tree Star, Ashland, OR, USA). Data analysis was performed by gating the individual lymphocyte, monocyte, and granulocyte populations on the basis of their forward scatter (FSC-A) versus side scatter (SSC-A) properties. The frequencies of single-, double-, and triple-labeled cell populations (Table 1) and subsets were determined as a function of CD45 expression. Unless otherwise indicated, single-marker expression included CD45+ and CD45− cells. Expression of CD14 and CD11b and secondary markers were assessed in the monocyte fraction, whereas the expression of CD19, CD3, CD31, CD34, KDR, and CXCR4 and secondary markers were assessed in the lymphocyte fraction. A modified version9 of the International Society of Hematotherapy and Graft Engineering (ISHAGE) sequential gating strategy10 was used to analyze the CD34+, CD133+, and KDR+ EPCs in the total nucleated cell fraction.
Table 1.
Cell Surface Marker Expression Used to Report Phenotypes of Bone Marrow-Derived Mononuclear Cells (BM-MNCs)
| Single-Positive Cell Surface Marker Profiles | Double-Positive Cell Surface Marker Profiles | Triple-Positive Cell Surface Marker Profiles |
|---|---|---|
| CD3+ | CD45+CD31bright | CD133+CD34+KDR+ |
| CD31dim | CD31+CD34− | |
| KDR+ | CD45+CD11b+ | |
| CD133+ | CD45+CXCR4− | |
| CD14+ | CD45+CD3+ | |
| CD11b+ | CD45+CXCR4dim | |
| CD45+ | CD45+CD14− | |
| CD31+ | CD45+CD11b− | |
| CXCR4+ | CD34+CD133+ | |
| CD19+ | CD45+CD133+ | |
| CD45+CD14+ | ||
| CD34+CD31+ | ||
| CD34+KDR+ | ||
| CD45brightCXCR4+ | ||
| CD45+CD11b− | ||
| CD45+CD31+ | ||
| CD45+CD31dim | ||
| CD45+CXCR4interm | ||
| CD19+CD11b+ | ||
| CD45+CD19+ | ||
| CD19+CXCR4+ |
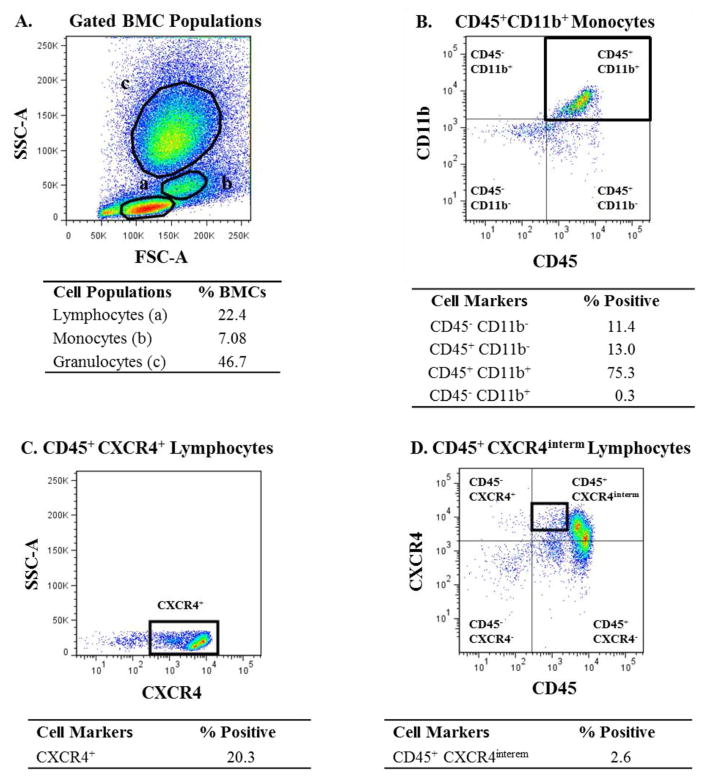
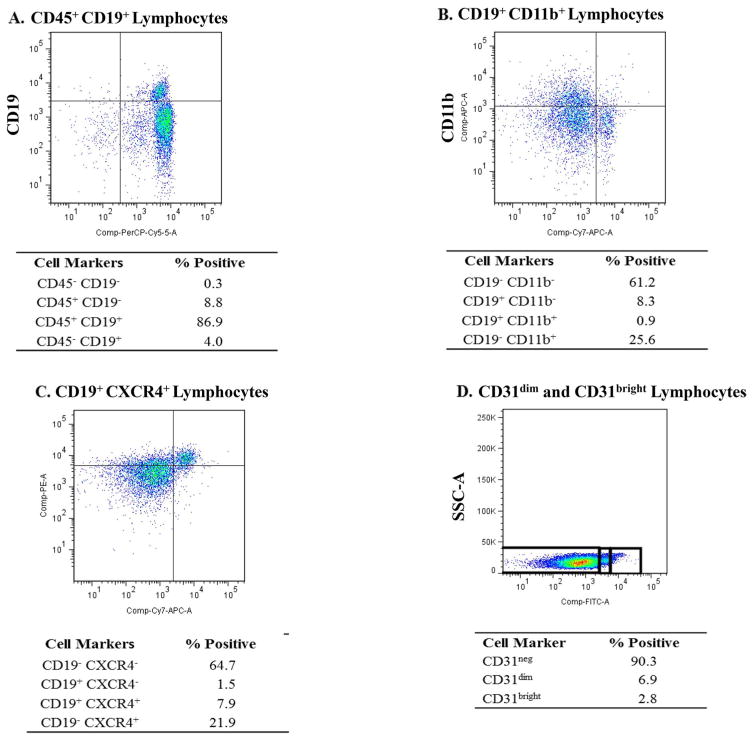
The gating strategies for the cell populations found to be significant are shown in Figures 1 and 2. In addition to cell populations expressing a given marker, certain subsets within those populations were also gated [e.g., negative(neg), dim(dim), intermediate(intermed), or bright(bright)] as indicated (Fig. 2D). The negative gate was always placed on the basis of the isotype control negative signal.
Figure 1.
Gating strategy used for analyzing bone marrow-derived mononuclear cell (BM-MNC) populations that showed significant differences between group 1 and groups 2 and 2A. (A) Representative dot plot showing the gates used to identify BM-MNC populations based on forward scatter (FSC-A) versus side scatter (SSC-A). (B) Representative dot plot showing CD45+CD11b+ cells within the monocyte gate. (C) Representative dot plot showing CXCR4+ cells within the lymphocyte gate. (D) Representative dot plot showing CD45+CXCR4interm cells within the lymphocyte gate.
Figure 2.
Gating strategy used for analyzing BM-MNC populations that showed significant differences between group 1 and groups 2 and 2A. (A) Representative dot plot showing CD45+CD19+ cells within the lymphocyte gate. (B) Representative dot plot showing CD19+CD11b+ cells within the lymphocyte gate. (C) Representative dot plot showing CD19+CXCR4+ cells within the lymphocyte gate. (D) Representative dot plot showing CD31neg, CD31dim, and CD31bright cells within the lymphocyte gate.
Endothelial cell function was quantified by using the endothelial colony-forming cell (ECFC) assay (reported as ECFC per 108 cells) and the CFU-Hill assay (reported as CFU-EC) by using commercial kits according to the manufacturers’ instructions (STEMCELL Technologies, Vancouver, Canada), as previously described8.
Embedded Cohort Analysis
The purpose of this analysis was to determine whether patients with a favorable cardiac functional outcome at 6 months had specific BM characteristics at study baseline that differed from those who did not improve. This evaluation of the putative relationships between BM-MNC immunophenotypes and LV function was prespecified in the FOCUS-CCTRN protocol. In the FOCUS-CCTRN trial, patients were randomized 2:1 to cell treatment or placebo. In the cell-treated patients, no significant beneficial effects of therapy were observed for LVESV or VO2 max, whereas a small improvement in LVEF (2.7%, p = 0.03) occurred. We combined the BM-MNC and placebo groups into a single cohort characterized by chronic ischemic cardiomyopathy and HF, which increased the statistical power for the current analyses. A cohort approach was used to compare the baseline BM-MNC profiles of patients who improved in three available cardiac function indicators (LVEF, LVESV, and VO2 max) to those of patients who did not improve. Left ventricular end-diastolic volume (LVEDV) was not an endpoint of this study and was not considered.
Patients in the placebo and cell-treated groups were reassigned to three different groups based on their 6-month functional outcomes: (1) group 1 (n = 17): patients who showed improvement in all three endpoints (i.e., an increase in LVEF, a decrease in LVESV, and an increase in VO2 max), (2) group 2 (n = 61): all other patients, and (3) group 2A (n = 11): a subset of group 2 that included only individuals who showed a decline in all three endpoints. We then determined whether the baseline BM-MNC immunophenotypes and functions of the three groups differed at study onset. It is important to note that we grouped patients based on functional endpoints rather than treatment; therefore, the composition of the three groups differed in regard to treatment received. Group 1 (triple-positive functional outcomes) included 16 BM-MNC-treated and 1 placebo-treated patients, group 2 (all others) included 35 BM-MNC-treated and 26 placebo-treated patients, and group 2A (triple-negative functional outcomes) included 7 BM-MNC-treated and 4 placebo-treated patients.
Statistical Methods
Measures of central tendency and dispersion were compared. Patient demographic and clinical data were compared by using the Student’s t-test for continuous variables and were reported by mean and standard error of the mean (SEM). Wilcoxon two-sample testing was used for the non-parametric evaluations [brain natriuretic peptide BNP(reg) and BNP(pro)]. Fisher’s exact test was used to compare dichotomous variables. Differences in the New York Heart Association classification and the Canadian Cardiovascular Society classification were assessed by using the chi-square test. Differences in phenotype and function data were assessed by using the Student’s t-test. Statistical comparisons with a value of p < 0.05 were considered significant with no corrections for multiplicity in this wide-ranging evaluation.
Heat Map
To visualize the distribution of cell phenotypes and changes present in group 1 versus groups 2 and 2A, we generated a heat map showing the relative change in frequencies of a given cell population or function. The signal-to-noise ratio (SNR), calculated as the delta percent difference in group 1 versus groups 2 and 2A divided by the standard error, was plotted for each cell population and function. Green represents a positive SNR (i.e., an absolute higher cell percentage or function), black represents no difference, and red represents a negative SNR (i.e., an absolute lower cell percentage or function).
RESULTS
Comparisons Between Group 1 and Group 2 Patients
By definition, at the 6-month follow-up, group 1 showed greater improvement in LVEF, LVESV, and VO2 max, as compared to group 2 (Table 2). Other baseline demographic and clinical data for groups 1 and 2 are shown in Table 3. Patients in group 1 had significantly lower BNP levels and a significantly smaller percentage who underwent coronary artery bypass graft (CABG) surgery than in group 2. The age in groups 1 and 2 did not statistically differ, but there was a trend (p = 0.067) toward a lower age in group 1.
Table 2.
Change in Contractile Function (Baseline to 6 Months) in Groups 1, 2, and 2A
| Group 1
|
Group 2
|
Group 2A
|
||||
|---|---|---|---|---|---|---|
| Change | n | Change | n | Change | n | |
| LVEF | 3.8 ± 3.0*† | 17 | 0.3 ± 4.3 | 49 | −6.8 ± 3.7 | 11 |
| VO2 max | 2.8 ± 2.5*† | 17 | −0.5 ± 2.7 | 47 | −2.0 ± 2.1 | 11 |
| LVESV | −16.5 ± 10.2*† | 17 | −1.6 ± 22.4 | 49 | 26.1 ± 22.3 | 11 |
Data are presented as mean ± standard deviation (SD).
Significant compared to group 2.
Significant compared to group 2A.
Table 3.
Baseline Characteristics of Groups 1, 2, and 2A
| Group 1 (n = 17) | Group 2 (n = 61) | Group 2A (n = 11) |
p Value
|
||
|---|---|---|---|---|---|
| Group 1 Versus Group 2 | Group 1 Versus Group 2A | ||||
| Age in years [mean (SD)] | 60.4 (12.0) | 65.2 (8.7) | 69.53 (9.04) | 0.067 | 0.022 |
| Female | 3 (17.7) | 5 (8.2) | 2 (18.18) | 0.362 | 1.000 |
| White | 17 (100.0) | 57 (93.4) | 11 (100.00) | 0.571 | 1.000 |
| BMI [mean (SD)] | 30·3 (5.5) | 30.5 (5.9) | 30.02 (7.29) | 0.872 | 0.520 |
| NYHA classification | |||||
| Class I | 3 (17.7) | 5 (8.2) | 2 (18.18) | 0.198 | 0.083 |
| Class II | 10 (58.8) | 28 (45.9) | 2 (18.18) | ||
| Class III | 4 (23.5) | 28 (45.9) | 7 (63.64) | ||
| Class IV | 0 | 0 | 0 (0.00) | ||
| CCS classification* | |||||
| Class I | 5 (29.4) | 12 (23.5) | 2 (20.00) | 0.878 | 0.315 |
| Class II | 8 (47.1) | 23 (45.1) | 3 (30.00) | ||
| Class III | 4 (23.5) | 15 (29.4) | 4 (40.00) | ||
| Class IV | 0 (0·0) | 1 (2.0) | 1 (10.00) | ||
| BP in mmHg [mean (SD)] | |||||
| Systolic | 120.0 (23.9) | 122.8 (17.1) | 120.55 (16.82) | 0.587 | 0.898 |
| Diastolic | 73.4 (14.9) | 72.4 (10.3) | 72.64 (10.38) | 0.737 | 0.918 |
| Laboratory evaluations | |||||
| BNP [median (interquartile range)]† | 68.5 (51.0–128.1) | 132.0 (81.0–278.0) | 258.89 (185.11) | 0.032 | 0.029 |
| ProBNP [median (interquartile range)]‡ | 606.0 (171.0–2187.0) | 863.5 (443.0–5334.0) | 941.00 (384.67) | 0.401 | 0.806 |
| Medications at time of randomization | |||||
| ACEi/ARB | 13 (76.5) | 38 (62.3) | 5 (45.45) | 0.39 | 0.108 |
| Aldosterone inhibitor | 3 (17.7) | 11 (18.3) | 1 (9.09) | 1 | 0.626 |
| Antiarrhythmics | 3 (17.7) | 14 (23.0) | 2 (18.18) | 0.751 | 1.000 |
| Aspirin | 13 (76.5) | 53 (86.9) | 10 (90.91) | 0.282 | 0.372 |
| β-Blockers | 16 (94.1) | 59 (96.7) | 11 (100.00) | 0.527 | 1.000 |
| Calcium channel blockers | 1 (5.9) | 10 (16.6) | 1 (9.09) | 0.439 | 1.000 |
| Digitalis | 1 (5.9) | 5 (8.2) | 2 (18.18) | 1 | 0.611 |
| Diuretics | 11 (64.7) | 44 (72.1) | 7 (63.64) | 0.56 | 1.000 |
| Nitrates | 8 (47.1) | 41 (67.2) | 8 (72.73) | 0.16 | 0.260 |
| P2 Y12 inhibitors | 11 (64.7) | 38 (62.3) | 8 (72.73) | 1 | 0.702 |
| Medical history | |||||
| Diabetes | 5 (29.4) | 26 (42.6) | 3 (27.27) | 0.407 | 1.000 |
| Hypertension | 14 (82.4) | 48 (78.7) | 10 (90.91) | 1 | 0.626 |
| Hyperlipidemia | 17 (100.0) | 57 (93.4) | 10 (90.91) | 0.389 | 0.367 |
| Angina | 5 (29.4) | 21 (34.4) | 3 (27.27) | 0.778 | 0.702 |
| Obesity | 7 (41.2) | 18 (29.5) | 3 (27.27) | 0.559 | 0.443 |
| History of myocardial infarction | 15 (93.8) | 55 (93.2) | 11(100.00) | 1 | 1.000 |
| Prior revascularization | 15 (88.2) | 52 (85.3) | 9 (81.82) | 1 | 0.611 |
| Prior CABG | 7 (41.2) | 53 (86.9) | 9 (81.82) | <0.001 | 0.121 |
| Prior hospitalization for CHF | 12 (70.6) | 38 (62.3) | 3 (27.27) | 0.582 | 1.000 |
| History of TIA | 1 (5.9) | 7 (11.5) | 2 (18.18) | 0.678 | 0.126 |
| History of stroke | 0 (0.00) | 5 (8.2) | 2 (18.18) | 0.58 | 0.672 |
| Peripheral vascular disease | 3 (17.7) | 11 (18.0) | 2 (18.18) | 1 | 1.000 |
| History of arrhythmia | 10 (58.8) | 29 (52.7) | 5 (50.00) | 0.783 | 1.000 |
| Cardiac pacemaker | 12 (70.6) | 48 (78.7) | 10 (90.91) | 0.522 | 0.215 |
| Smoking (former or current) | 13 (76.5) | 44 (72.1) | 9 (81.82) | 1 | 0.672 |
| Cardiac function (baseline) | |||||
| LVEF (%) | 35.3 (10.9) | 33.5 (8.3) | 35.8 (8.7) | 0.97 | 0.870 |
| LVESV (ml) | 116.4 (53.2) | 127.8 (45.7) | 120.6 (46.2) | 0.301 | 0.806 |
| VO2 max (ml/kg/min) | 15.1 (4.1) | 14.7 (4.2) | 15.6 (4.9) | 0.999 | 0.74 |
Data presented as n (%), unless otherwise specified. ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blockers; BMI, body mass index; BNP, B-type natriuretic peptide; BP, blood pressure; CABG, coronary artery bypass graft; CCS, Canadian Cardiovascular Society; CHF, congestive heart failure; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; NYHA, New York Heart Association; TIA, transient ischemic attack; VO2 max, maximal oxygen consumption.
Group 2: n = 51.
Group 1: n = 10, group 2: n = 47, group 2A: n = 11; results reported as median (first quartile–third quartile).
Group 1: n = 7, group 2: n = 14, group 2A: n = 2; results reported as median (first quartile–third quartile).
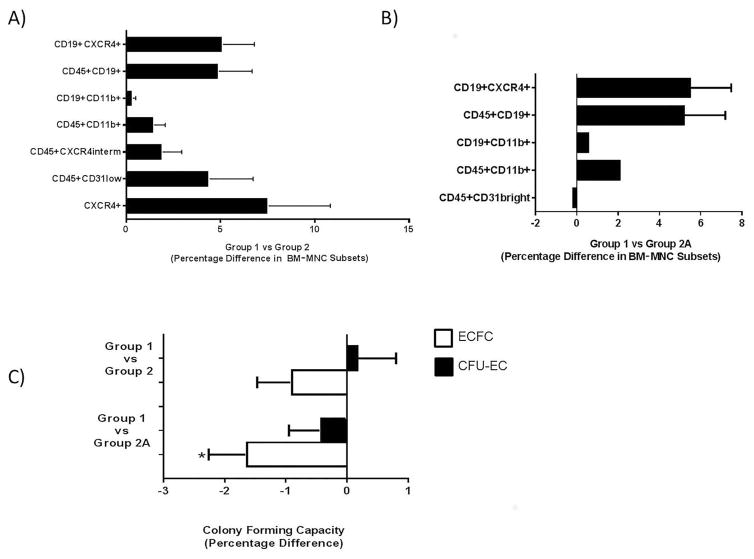
Flow cytometry showed that the frequencies of eight cell populations were significantly elevated in the BM of patients in group 1 when adjusted for age, as compared to the frequencies in group 2 patients (Table 4). The elevated populations included CD11b+, CD31dim, CD19+, and CXCR4+ cells and subsets thereof. In terms of absolute percentages, these cell populations were increased by 1–7% (Fig. 3A). It was not possible to adjust the results for baseline BNP since BNP was not measured in the same manner across sites. When the data were adjusted for CABG, the CXCR4+ and CXCR4intermed cell populations were no longer significantly different between groups 1 and 2.
Table 4.
Significant Differences in Bone Marrow Cell Phenotype for Group 1 Versus Group 2
| Cell Surface Marker Expression | Group 1 (n = 17) | Group 2 (n = 61) | p Value |
|---|---|---|---|
| CXCR4+ | 57.0 (11.3) | 49.5 (13.8) | 0.042 |
| CD45+CD31dim* | 30.9 (8.7) | 26.5 (7.0) | 0.033 |
| CD45+CXCR4interm* | 6.8 (3.9) | 4.9 (2.9) | 0.028 |
| CD45+CD11b+* | 6.2 (2.0) | 4.7 (2.5) | 0.028 |
| CD19+CD11b+† | 1.2 (0.6) | 0.9 (0.5) | 0.018 |
| CD19+ | 15.0 (7.2) | 10.0 (5.0) | 0.001 |
| CD45+CD19+ | 15.8 (6.8) | 10.9 (4.7) | 0.001 |
| CD19+CXCR4+ | 14.4 (6.4) | 9.30 (4.3) | <0.001 |
Data presented as mean (SD). Cell phenotype data shown as the cell frequency in the lymphocyte fraction of the bone marrow (BM), except for the CD45+CD11b+ population, which is shown as the cell frequency in the monocyte fraction of the BM.
Group 2: n = 60.
Group 2: n = 58.
Figure 3.
Significant differences in BM-MNC phenotype and function. Significant differences in BM-MNC subpopulation frequencies between (A) group 1 versus group 2 and (B) group 1 versus group 2A. (C) Percentage differences in BM-MNC function for group 1 versus groups 2 and 2A. *p=0.016.
Comparisons Between Group 1 and Group 2A Patients
As a more constrained approach, we compared the patients with the best functional outcomes (triple-positive patients, group 1) to those with the worst outcomes (triple-negative patients, group 2A). Group 1 and group 2A membership was defined on the basis of changes in LVEF, VO2 max, and LVESV and, therefore, had the expected differences in these variables (Table 2). The differences in LVEF, VO2 max, and LVESV were greater when group 1 was compared with group 2A than when it was compared with group 2. A comparison of other baseline demographic and clinical variables showed that the patients in group 1 were significantly younger than those in group 2A and had significantly lower BNP levels (Table 3). Four hematopoietic/ immune cell populations (CD45+CD19+, CD19+CD11b+, CD19+CXCR4+, and CD45+CD11b+ cells) were significantly higher in the BM of group 1 than in group 2A (Table 5 and Fig. 3B), whereas both the percentage of CD45+CD31bright cells and the number of ECFCs per 108 cells were lower (Table 5 and Fig. 3C). This remained true after adjustment for age.
Table 5.
Significant Differences in Bone Marrow Cell Phenotypes and Function for Group 1 Versus Group 2A
| Variable | Group 1 (n = 17) | Group 2A (n = 11) | p Value |
|---|---|---|---|
| Cell phenotype | |||
| CD45+CD31bright | 0.2 (0.1) | 0.4 (0.3) | 0.046 |
| CD45+CD11b+ | 6.2 (2.0) | 4.1 (2.0) | 0.012 |
| CD19+CD11b+ | 1.2 (0.6) | 0.6 (0.2) | 0.007 |
| CD45+ CD19+ | 15.8 (6.8) | 10.6 (5.4) | 0.043 |
| CD19+CXCR4+ | 14.4 (6.4) | 9.0 (4.9) | 0.024 |
| Cell function | |||
| ECFC per dose* | 85.0 (87.2) | 248.9 (227.8) | 0.016 |
Data presented as mean (SD). Cell phenotype data shown as the cell frequency in the lymphocyte fraction of the BM, except for the CD45+CD11b+ population, which is shown as the cell frequency in the monocyte fraction of the BM. ECFC, endothelial colony-forming cells.
Group 1: n = 16, group 2A: n = 9.
BM-MNC Characteristics Associated With Positive Functional Outcomes
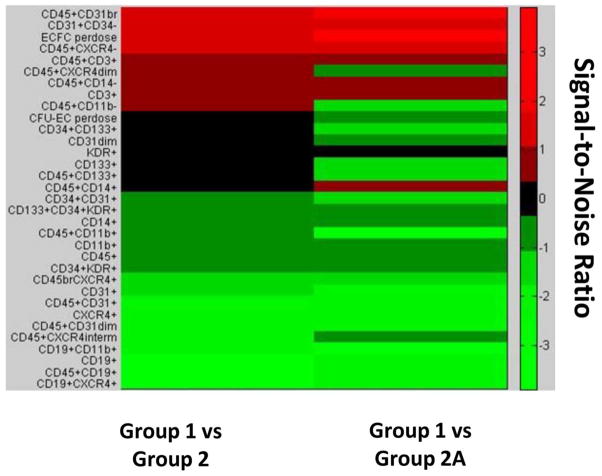
The heat map shown in Figure 4 was designed to enable high-level comparisons between group 1 and groups 2 and 2A. These comparisons are shown for a total of 33 variables: 31 BM-MNC frequencies and 2 cell functions (CFU-EC and ECFC). A positive SNR was observed for 48% of these variables in the group 1 versus group 2 comparison and for 69% in the group 1 versus group 2A comparison. In the comparison of group 1 versus group 2, the primary cell populations with a positive SNR were hematopoietic and immune cells. However, approximately twice as many progenitor cell populations (12 vs. 7) showed a positive SNR in the comparison of group 1 versus group 2A. This difference suggests that higher endogenous levels of progenitor cells may predict a beneficial cardiac outcome.
Figure 4.
Heat map illustrating signal-to-noise ratios of bone marrow cell variables for group 1 versus groups 2 and 2A. Relative differences are shown for BM-MNC subpopulation frequencies and functions of group 1 versus group 2 (left) and group 1 versus group 2A (right). Green = higher relative levels in group 1, black = no difference in relative levels, and red = lower relative levels.
DISCUSSION
The current study was designed to determine whether improvements in three important functional endpoints (LVEF, LVESV, and VO2 max) in patients with ischemic cardiomyopathy were associated with the frequency of certain cell types in the patient’s BM. The effect of treatment was outside the scope of this study, but of the 17 patients in group 1 (i.e., those who showed improvement for all three endpoints), 16 were treated with BM-MNCs. We found that HF patients who improved in a combination of LVEF, LVESV, and VO2 max at the 6-month follow-up had higher levels of CD19+ B cells, CD11b+ monocytes, CD31dim subsets, and CXCR4+ migratory cells and lower levels of CD31bright lymphocyte subsets in their BM at baseline than patients who improved in fewer endpoints or whose condition worsened.
Our results suggest that B cells may either play a beneficial role in repair, or their numbers may serve as predictors of a favorable functional outcome in chronic ischemic cardiomyopathy patients. The increased frequencies of B-cell subsets (CD19+CXCR4+ and CD19+CD11b+) in the BM may be due to either an increase in B-cell production (for potential cardiac repair) or a decrease in B-cell mobilization from the BM. The latter possibility seems less likely, given the increase in markers associated with migratory capability (CD11b and CXCR4) in the B-cell subsets.
Cell subsets expressing CXCR4, including CD19+ B cells, were also elevated in the BM-MNCs of patients who had superior functional outcomes. Several studies have identified CXCR4 and its ligand, stromal cell-derived factor-1 (SDF-1), as key molecular contributors to myocardial repair11,12. Although the present study was not designed to, and therefore did not directly, assess cell recruitment or the incorporation of cells at cardiac injury sites, the association we found between higher numbers of CXCR4-expressing cells and functional improvement suggests that better cell migratory capacity in response to tissue injury or ischemia could contribute to improved outcome.
Several reports have suggested that monocytes and macrophages play a critical role both in cardiac repair and in angiogenesis and arteriogenesis13–15. In this study, we found that the BM frequency of CD45+CD11b+ monocytes was higher in group 1 than in groups 2 and 2A. Other studies have shown contradictory results about the role of CD11b on cardiac function. For instance, Frantz et al.16 demonstrated increased mortality in macrophage- and CD11b+Ly6G+ monocyte-depleted mice after MI compared with controls. However, Cogle et al.13 reported that increased levels of BM CD11b+ cells are associated with reductions in LVEF after acute MI, and Maier et al.15 showed that continuous CD11b+ cell infiltration is associated with chronic inflammation, fibrosis, and myocyte atrophy. Although the reason for these conflicting results is unclear, differences in disease severity, the fact that CD11b is expressed on a diverse array of immune cell populations (e.g., monocytes, granulocytes, natural killer, and B cells)17–19, and the recently described heterogeneity within monocyte/macrophage populations14,20 may be contributing factors.
Associations between cardiovascular disease outcomes and the frequency of CD31+ cells have been reported previously4,21. The frequency of CD31dim lymphocytes has been shown to be inversely correlated with cardiovascular risk factors, such as age, male sex, and CRP level, whereas CD31bright monocytes have been found to be positively associated with cardiovascular risk factors22. We observed that the frequency of CD31dim cells in the BM was significantly higher in group 1 than in group 2. In contrast, the frequency of CD45+CD31bright cells in the BM was significantly lower in group 1 than in group 2A. It is currently unknown whether these BM CD31bright and CD31dim cells are positively associated with cardiovascular risk factors. However, the BM frequency and function of CD31dim and CD31bright BM subsets, as well as their relationship to cardiovascular risk factors, may warrant future study in candidates for stem cell therapy.
An unexpected finding in this study was the lower levels of BM ECFCs in group 1, as compared to the levels in group 2A. Peripheral blood ECFC levels have been reported to be low (0–3 ECFCs per 108 mononuclear cells) in healthy individuals23,24 but to increase approximately 10-fold in patients with acute MI at 3 h after symptom onset, indicating increased mobilization of ECFCs to the circulation and ischemic tissue25–27. Our finding of lower ECFC levels in the BM of chronic HF patients is consistent with these previous studies in acute MI patients and may indicate greater mobilization of these cells to the ischemic myocardium.
A heat map analysis, which focused on cell population profiles, showed a high number of progenitor cell populations with a positive SNR in the group 1 versus group 2A comparison. This association, which occurred regardless of treatment type, supports the concept that the functional outcome is influenced by the patient’s underlying progenitor cell profile/activity.
It is unknown why patients who were previously stable showed an improvement or decline in LV function. It is interesting to note that 16 of the 17 patients in group 1 received BM-MNCs. Furthermore, the difference in LVEF between group 1 and group 2A was greater than 10%, which is larger than the LVEF changes seen with many approved therapies in these patients. In addition, the fact that patients who showed improvement in LVEF, LVESV, and VO2 max had a different BM profile than those who did not suggests that the underlying physiology of BM and its activation should not be ignored.
It has long been recognized that patient responses to treatments may differ substantially in a variety of clinical settings. Personalized medicine is based on the concept that optimal medical treatments may be tailored to the individual patient. In oncology, personalized therapy is already being used to select the most effective treatment option, including cell therapies, on the basis of the characteristics of the individual patient28. A similarly personalized approach may be possible for cardiovascular cell therapy, if the specific variables influencing outcomes can be identified. The current findings suggest that both progenitor and nonprogenitor (e.g., hematopoietic and immune) cells are associated with increased endogenous regenerative capacity. Moreover, these results provide further rationale for identifying BM cell populations that enhance the repair process with the eventual hope of using this information as a tool to improve the stratification and randomization of patients for cell therapy trials.
Study Limitations
The analyses in this study only show associations between BM cell phenotype and functional outcome and do not identify a causal relationship. Potential confounders in this study were the BM harvest and the transendocardial route of administration. Previous studies have shown that both BM-derived stem cells and resident tissue stem cells actively participate in tissue repair in response to different types of injury27,29–31. A consideration in cell therapy trials is that the harvest and delivery processes may themselves stimulate this pool of resident stem/progenitor cells, even in placebo-treated subjects, thereby contributing to repair independent of treatment type.
CONCLUSIONS
In this cohort of patients from the FOCUS-CCTRN trial, increased frequencies of B-cell subsets and migratory cells and decreased frequencies of both monocytes/ macrophages and ECFCs in the BM at baseline were associated with improvements in LVEF, LVESV, and VO2 max, independent of treatment group. This study suggests that both progenitor and immune cell populations in the BM influence the natural history of chronic ischemic cardiomyopathy. When assessing a new therapy, all study groups should have the same baseline characteristics so that the treatment effects can be interpreted accurately. When designing and interpreting the results of cardiovascular disease studies, potential confounding variables, such as age, disease severity, and current comorbidities, must be taken into account. Although we adjusted our findings based on age and CABG, other confounding variables such as BNP could not be addressed. Our study findings suggest that BM composition may be another important factor to consider, as it may affect the endogenous regenerative capacity of the patients.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute under cooperative agreement 5 UM1 HL087318, and also, in part, by the National Heart, Lung, and Blood Institute by contracts N01 HB 37164 and HHSN268201000008C, which were awarded to the Molecular and Cellular Therapeutics Facility, University of Minnesota, and by contracts N01 HB 37163 and HHSN268201000007C, which were awarded to the Cell Processing Facility, Baylor College of Medicine. Further funding was provided by the National Center for Research Resources CTSA grant UL1 TR000064 awarded to the University of Florida. Funding from the Texas State Legislature was also used to assist investigators at the Texas Heart Institute, Houston, Texas. The CCTRN acknowledges its industry partners, Biosafe, Biologics Delivery System Group, and Cordis Corporation, for their contributions of equipment and technical support during the conduct of the trial. We also acknowledge Dr. Sonia Skarlatos (1953–2013) for her insight, expertise, and support of the CCTRN, which continues to propel the cell therapy field forward. In addition, we thank Dr. Ke Li of the Texas Heart Institute for creation of the heat map. The opinions expressed in this report do not necessarily reflect those of the National Heart, Lung, and Blood Institute, the NIH, or the US Department of Health and Human Services. The authors declare no conflicts of interest.
References
- 1.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, et al. Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The FOCUS-CCTRN trial. JAMA. 2012;307(16):1717–26. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, et al. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The Late TIME randomized trial. JAMA. 2011;306(19):2110–9. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, et al. Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The TIME randomized trial. JAMA. 2012;308(22):2380–9. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schutt RC, Trachtenberg BH, Cooke JP, Traverse JH, Henry TD, Pepine CJ, Willerson JT, Perin EC, Ellis SG, Zhao DX, Bhatnagar A, Johnstone BH, Resende M, Ebert RF, Wu JC, Sayre SL, Orozco A, Zierold C, Simari RD, Moyé L, Cogle CR, Taylor DA. Bone marrow characteristics associated with changes in infarct size after STEMI: A biorepository evaluation from the CCTRN TIME trial. Circ Res. 2015;116(1):99–107. doi: 10.1161/CIRCRESAHA.116.304710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simari RD, Moye LA, Skarlatos SI, Ellis SG, Zhao DX, Willerson JT, Henry TD, Pepine CJ. Development of a network to test strategies in cardiovascular cell delivery: The NHLBI-sponsored cardiovascular cell therapy research network (CCTRN) J Cardiovasc Transl Res. 2010;3(1):30–6. doi: 10.1007/s12265-009-9160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willerson JT, Perin EC, Ellis SG, Pepine CJ, Henry TD, Zhao DX, Lai D, Penn MS, Byrne BJ, Silva G, Gee A, Traverse JH, Hatzopoulos AK, Forder JR, Martin D, Kronenberg M, Taylor DA, Cogle CR, Baraniuk S, Westbrook L, Sayre SL, Vojvodic RW. Intramyocardial injection of autologous bone marrow mononuclear cells for patients with chronic ischemic heart disease and left ventricular dysfunction (first mononuclear cells injected in the US [FOCUS]): Rationale and design. Am Heart J. 2010;160(2):215–23. doi: 10.1016/j.ahj.2010.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gee AP, Richman S, Durett A, McKenna D, Traverse J, Henry T, Fisk D, Pepine C, Bloom J, Willerson J, Prater K, Zhao D, Koc JR, Ellis S, Taylor D, Cogle C, Moyé L, Simari R, Skarlatos S. Multicenter cell processing for cardiovascular regenerative medicine applications: The cardiovascular cell therapy research network (CCTRN) experience. Cytotherapy. 2010;12(5):684–91. doi: 10.3109/14653249.2010.487900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zierold C, Carlson MA, Obodo UC, Wise E, Piazza VA, Meeks MW, Vojvodic RW, Baraniuk S, Henry TD, Gee AP, Ellis SG, Moyé L, Pepine CJ, Cogle CR, Taylor DA. Developing mechanistic insights into cardiovascular cell therapy: Cardiovascular cell therapy research network biorepository core laboratory rationale. Am Heart J. 2011;162(6):973–80. doi: 10.1016/j.ahj.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt-Lucke C, Fichtlscherer S, Aicher A, Tschope C, Schultheiss HP, Zeiher AM, Dimmeler S. Quantification of circulating endothelial progenitor cells using the modified ISHAGE protocol. PLoS One. 2010;5(11):e13790. doi: 10.1371/journal.pone.0013790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. international society of hematotherapy and graft engineering. J Hematother. 1996;5(3):213–26. doi: 10.1089/scd.1.1996.5.213. [DOI] [PubMed] [Google Scholar]
- 11.Penn MS. Importance of the SDF-1:CXCR4 axis in myocardial repair. Circ Res. 2009;104(10):1133–5. doi: 10.1161/CIRCRESAHA.109.198929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104(10):1209–16. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cogle CR, Wise E, Meacham AM, Zierold C, Traverse JH, Henry TD, Perin EC, Willerson JT, Ellis SG, Carlson MA, Zhao DX, Bolli R, Cooke JP, Anwaruddin S, Bhatnagar A, da Graca Cabreira-Hansen M, Grant MB, Lai D, Moyé L, Ebert RF, Olson RE, Sayre SL, et al. Detailed analysis of bone marrow from patients with ischemic heart disease and left ventricular dysfunction: BM CD34, CD11b and clonogenic capacity as biomarkers for clinical outcomes. Circ Res. 2014;115(10):867–74. doi: 10.1161/CIRCRESAHA.115.304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lundahl J, Hallden G, Hallgren M, Skold CM, Hed J. Altered expression of CD11b/CD18 and CD62L on human monocytes after cell preparation procedures. J Immunol Methods. 1995;180(1):93–100. doi: 10.1016/0022-1759(94)00303-e. [DOI] [PubMed] [Google Scholar]
- 15.Maier HJ, Schips TG, Wietelmann A, Kruger M, Brunner C, Sauter M, Klingel K, Bottger T, Braun T, Wirth T. Cardiomyocyte-specific IkappaB kinase (IKK)/ NF-kappaB activation induces reversible inflammatory cardiomyopathy and heart failure. Proc Natl Acad Sci USA. 2012;109(29):11794–9. doi: 10.1073/pnas.1116584109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frantz S, Hofmann U, Fraccarollo D, Schafer A, Kranepuhl S, Hagedorn I, Nieswandt B, Nahrendorf M, Wagner H, Bayer B, Pachel C, Schön MP, Kneitz S, Bobinger T, Weidemann F, Ertl G, Bauersachs J. Monocytes/macrophages prevent healing defects and left ventricular thrombus formation after myocardial infarction. FASEB J. 2013;27(3):871–81. doi: 10.1096/fj.12-214049. [DOI] [PubMed] [Google Scholar]
- 17.Kawai K, Tsuno NH, Matsuhashi M, Kitayama J, Osada T, Yamada J, Tsuchiya T, Yoneyama S, Watanabe T, Takahashi K, Nagawa H. CD11b-mediated migratory property of peripheral blood B cells. J Allergy Clin Immunol. 2005;116(1):192–7. doi: 10.1016/j.jaci.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 18.Pahl HL, Rosmarin AG, Tenen DG. Characterization of the myeloid-specific CD11b promoter. Blood. 1992;79(4):865–70. [PubMed] [Google Scholar]
- 19.Solovjov DA, Pluskota E, Plow EF. Distinct roles for the alpha and beta subunits in the functions of integrin alphaMbeta2. J Biol Chem. 2005;280(2):1336–45. doi: 10.1074/jbc.M406968200. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka M, Honda J, Imamura Y, Shiraishi K, Tanaka K, Oizumi K. Surface phenotype analysis of CD16+ monocytes from leukapheresis collections for peripheral blood progenitors. Clin Exp Immunol. 1999;116(1):57–61. doi: 10.1046/j.1365-2249.1999.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, Kim TY, Kim JY, Kang HJ, Chae IH, Oh BH, Park YB, Kim HS. Identification of a novel role of T cells in postnatal vasculogenesis: Characterization of endothelial progenitor cell colonies. Circulation. 2007;116(15):1671–82. doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 22.Ge Y, Cheng S, Larson MG, Ghorbani A, Martin RP, Klein RJ, O’Donnell CJ, Vasan RS, Shaw SY, Wang TJ, Cohen KS. Circulating CD31+ leukocyte frequency is associated with cardiovascular risk factors. Atherosclerosis. 2013;229(1):228–33. doi: 10.1016/j.atherosclerosis.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estes ML, Mund JA, Ingram DA, Case J. Identification of endothelial cells and progenitor cell subsets in human peripheral blood. Curr Protoc Cytom. 2010;Chapter 9(Unit 9.33.1–11) doi: 10.1002/0471142956.cy0933s52. [DOI] [PubMed] [Google Scholar]
- 25.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104(9):2752–60. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 26.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewestein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109(4):428–36. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105(1):199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 28.De Mattos-Arruda L, Rodon J. Pilot studies for personalized cancer medicine: Focusing on the patient for treatment selection. Oncologist. 2013;18(11):1180–8. doi: 10.1634/theoncologist.2013-0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox A, Smythe J, Fisher N, Tyler MP, McGrouther DA, Watt SM, Harris AL. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008;95(2):244–51. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 30.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;97(7):663–73. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 31.Wan M, Li C, Zhen G, Jiao K, He W, Jia X, Wang W, Shi C, Xing Q, Chen YF, Jan De Beur S, Yu B, Cao X. Injury-activated transforming growth factor beta controls mobilization of mesenchymal stem cells for tissue remodeling. Stem Cells. 2012;30(11):2498–511. doi: 10.1002/stem.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]