Abstract
Objectives. Cigarette smoking is a major risk factor for RA and has been associated with increased disease severity and lower rates of disease remission. We hypothesized that inflammation and disease activity would be associated with smoking status and this would be related to levels of ACPA.
Methods. RA patients from the Veterans Affairs RA registry were studied (n = 1466): 76.9% anti-CCP2 positive, 89% male, median age 63 years (interquartile range 57–72), median disease duration 8.45 years (interquartile range 2.8–18). Baseline serum samples were evaluated for levels of anti-CCP2, RF, 19 distinct ACPAs and 17 cytokines. Smoking status at baseline was recorded as current, former or never. The association of smoking status with cytokines, autoantibodies and disease activity (DAS28) was evaluated.
Results. Among anti-CCP-positive RA patients, RA-associated cytokines (false-discovery rates q < 0.1%) and DAS28 (P < 0.01) were higher in current smokers compared with former or never smokers. DAS28 and cytokine levels were similar between former and never smokers. In contrast, ACPA concentrations were higher among both current and former smokers compared with never smokers, and levels of ACPA were not associated with DAS28 or cytokine levels.
Conclusion. Among anti-CCP2-positive RA patients, current smoking status is associated with elevations in pro-inflammatory cytokines and increased RA disease activity. Similar levels of inflammation and disease activity among former and never smokers suggests that the detrimental effects of smoking could be ameliorated through tobacco cessation. The effect of tobacco cessation on RA disease activity should be evaluated prospectively.
Keywords: rheumatoid arthritis, smoking, inflammation, disease activity
Rheumatology key messages
In ACPA-positive RA, current smoking is associated with increased RA disease activity.
The association between smoking and disease activity does not appear related to level of ACPA.
The longitudinal effects of smoking cessation on RA disease activity should be further explored.
Introduction
Multiple studies have demonstrated that tobacco use, usually in the form of cigarette smoking, is a major risk factor for the development of seropositive RA [1, 2], and a model for smoking driving the development of ACPA has been previously proposed [3–5].
However, less well studied is the contribution of smoking in established RA with respect to disease severity or outcomes. Several studies have reported that any tobacco exposure was associated with increased radiographic severity and worse functional outcomes compared with non-smokers [6–9]. However, such studies do not indicate whether the potential effect of smoking on the RA disease course is imparted prior to or at the onset of clinical disease and, more importantly, whether its effects might be reversible with smoking cessation. It is also unknown whether any effects on the disease course or severity are limited to seropositive individuals as has been demonstrated with disease risk. Such information could provide a highly actionable intervention in the education of current smokers as to yet another health benefit of smoking cessation and, potentially, the formal institution of smoking cessation programmes as part of routine care in RA patients.
We sought to evaluate the association of current, former and never smoking status with RA disease activity and, indirectly, to explore whether smoking cessation might negate this risk and return patients to disease activity states corresponding to never smoking status. Additionally, we attempted to dissect potential mechanisms by which smoking may contribute to disease burden. We hypothesized that current smoking, referent to former and never smoking status, would be associated with increased disease activity as reflected in circulating concentrations of pro-inflamamtory cytokines and clinical assessments. Furthermore, we hypothesized that this difference would be most evident among seropositive patients.
Methods
Patient samples and clinical measures
Study participants included US Veterans enrolled in the Veterans Affairs RA (VARA) registry from sites across the USA [10]. The registry has received Institutional Review Board approval at each site, and the Stanford Institutional Review Board approved the biomarker measurements performed on RA samples. All patients satisfied the 1987 ACR classification criteria for RA [11] and provided informed written consent. The VARA Scientific Ethics Advisory Committee also approved the study, and all patients provide informed consent for participation. Subjects in this study include a cohort of 1488 Veterans with RA; 1130 (76%) of whom were positive for anti-CCP2 antibodies and for whom detailed demographics are presented in Table 1. Banked serum was available for a representative population of 1466 subjects (1127 of 1130 anti-CCP positive) and was used for multiplex cytokine and autoantibody analysis.
Table 1.
Characteristics of anti-CCP2-positive RA patients at enrolment
| Characteristic | Never smokers (n = 210) | Former smokers (n = 599) | Current smokers (n = 321) | P-value |
|---|---|---|---|---|
| Sociodemographics and co-morbidity | ||||
| Age, mean (s.d.), years | 61 (13) | 66 (10) | 58 (10) | <0.001 |
| Sex, male, % | 79 | 94 | 94 | <0.001 |
| Race/ethnicity, % | 0.081 | |||
| Caucasian | 72 | 80 | 75 | |
| African American | 20 | 15 | 18 | |
| Other | 9 | 5 | 7 | |
| ≥High school education, % | 92 | 81 | 83 | 0.002 |
| Co-morbidity count, mean (s.d.) a | 1.6 (1.4) | 2.2 (1.4) | 1.7 (1.4) | <0.001 |
| Detectable cotinine, % | 2 | 11 | 92 | <0.001 |
| RA factors | ||||
| RF positive, % | 87 | 90 | 97 | <0.001 |
| Age at diagnosis, mean (s.d.), years | 49 (12) | 53 (14) | 50 (12) | <0.001 |
| Disease duration, mean (s.d.), years | 13 (12) | 13 (12) | 9 (9) | <0.001 |
| Prednisone use, % | 42 | 43 | 50 | 0.087 |
| MTX use, % | 59 | 51 | 48 | 0.033 |
| Biologics use, % | 25 | 21 | 18 | 0.139 |
| Nodules, % | 27 | 34 | 41 | 0.006 |
aCo-morbidity was examined using a count of prevalent diabetes mellitus, coronary artery disease, cerebrovascular disease, hypertension, hyperlipidaemia, depression, chronic obstructive and interstitial lung disease and chronic kidney disease (range of scores 0–9).
In addition to banked sera, VARA collects clinical data including a DAS28 using the ESR at enrolment, as well as the measurement of ACPA using the second-generation anti-CCP2 antibody ELISA (Diastat, Axis-Shield Diagnostics Ltd, Dundee UK, positivity ⩾5 U/ml). RF (positivity ⩾15 IU/ml) and high-sensitivity CRP (hs-CRP) were measured on banked serum by nephelometry (Siemens, Germany). While VARA is a multicentre project, standardized autoantibodies are measured in a single investigator’s laboratory (G.M.T.).
Measures of disease activity collected at enrolment include tender and swollen joint counts (0–28), ESR (in millimetres per hour), pain (0–10), multidimensional HAQ (MD-HAQ, 0–3) [12], patient and provider global assessments (0–100) and treatments. A co-morbidity count (0–9) was calculated for each patient using administrative codes [13].
Multiplex cytokine analysis
Multiplex analysis of cytokines and chemokines in human serum was performed by using the 17 cytokine Bio-Plex ProHuman Cytokine Assay (BioRad, Hercules, CA) run on the Luminex 200 system (Luminex, Austin, Tx) according to the manufacturer’s instructions with the exception that a proprietary BioRad assay dilution buffer was modified to contain reagents demonstrated to reduce the effects of heterophilic antibodies, such as RF, in multiplex immunoassays, as previously described [14]. To examine both the depth and the breadth of cytokine expression, we calculated both a cytokine score and the number of cytokines and/or chemokine analytes that were present in high concentrations (number positive), the latter defined as > 2 s.d. above the mean in the entire RA cohort. Cytokine score was calculated as the sum of normalized fluorescence values (normalized as the cytokine value divided by the mean value of each cytokine across the cohort). Data processing was performed by using Bio-Plex Manager 5.0, and analyte concentrations were interpolated from standard curves.
Multiplex ACPA arrays
Antibodies targeting 37 putative RA-associated autoantigens were measured using a custom bead-based immunoassay on the BioPlex platform as previously described [14, 15]. Of the 37 antigens, 30 are citrullinated and 7 are native controls for their citrullinated counterparts. Briefly, serum was diluted and mixed with spectrally distinct florescent beads conjugated with putative RA-associated autoantigens, followed by incubation with anti-human phycoerythrin antibody and analysis on a Luminex 200 instrument. As detailed above for cytokines and chemokines, both an ACPA score (estimating depth of the ACPA response; calculated as above for cytokine score) and the number ACPA positive (representing breadth of the ACPA response) were also examined by patient group.
Data analysis
Our primary analyses focused on anti-CCP2 antibody-positive patients (n = 1130), whereas anti-CCP2-negative subjects (n = 339) were analysed and reported separately (supplementary Table S1, available at Rheumatology Online). Patients were categorized based on self-reported smoking status at the time of enrolment, including current smokers (n = 321), former smokers (n = 599) or never smokers (n = 210). We have previously shown that among VARA participants reporting current smoking, 94% had detectable levels of serum cotinine [16], a metabolite of nicotine and a reliable biomarker of recent tobacco exposure [17]. Among those reporting former or never smoking, 84% had no detectable serum cotinine [16]. Cross-sectional comparisons of patient characteristics were examined by smoking subgroup using analysis of variance (ANOVA). Unadjusted comparisons of the eight continuous disease activity measures assessed at enrolment were examined using one-way ANOVA with Scheffe’s post hoc test to compare each of the three subgroups. The association of smoking status with DAS28 level was examined as the primary measure of disease activity, with other measures examined, including separate DAS28 components, examined as secondary outcomes. CRP was log-transformed prior to analysis to render a more normal distribution. With highly skewed distributions, levels of serum cytokines/chemokines and ACPA (as calculated by a weighted score and number positive) were compared between current, former and never smokers using the non-parametric Kruskal–Wallis test with Dunn’s post hoc test. We examined whether the associations observed between smoking status and disease activity at enrolment were independent of other covariates, including age, sex, disease duration, co-morbidity score, race/ethnicity and treatments, including use of MTX, prednisone or biologics. For multivariable analyses, never smokers served as the referent population. Analyses were completed using STATA v12 (StataCorp, College Station, TX, USA), SAS v9.3 (SAS Institute, Cary, NC, USA) and Prism GraphPad v5 (GraphPad Software, La Jolla, CA, USA).
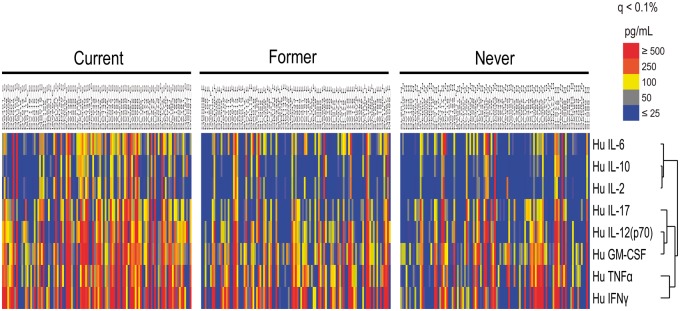
To examine the relationship of smoking status with individual inflammatory parameters, multiplex cytokine comparisons were performed using significance analysis of microarrays (SAM) version 3.08 [18]. Owing to difficulties with multivariate adjustment of multiplex data, a subset of current, former and never smokers (all anti-CCP+; n = 107 per group) was matched by age (0.5 years), sex and disease duration (0.5 years). Output was sorted based on false-discovery rates in order to identify the cytokines or chemokines with the greatest differences in concentrations between smoking subgroups. The use of false-discovery rates obviates the need to adjust for multiple comparisons. Hierarchical clustering was performed using Cluster® 3.0 (Cluster, Berkeley, CA) to arrange the SAM results according to similarities among cytokine specificities, and results were displayed using Java Treeview® (Version 1.1.3) (Treeview, Berkeley, CA).
Results
Smoking status in the VARA cohort
Enrolment characteristics of the 1130 anti-CCP2-positive RA patients examined are summarized in Table 1. A majority were men (89%), with a mean age of ∼63 years. Roughly one in four (27.1%) were never smokers, 52.4% former smokers and 20.5% current smokers. Women were less likely to be never smokers, and more never smokers reported a higher level of education. Current and former smokers had a higher proportion of patients positive for RF and an increased prevalence of rheumatoid nodules. Current smokers were younger at enrolment and tended to have shorter disease duration, increased prednisone use and slightly less MTX use compared with former and never smokers.
Current smoking status is associated with increased inflammatory cytokines
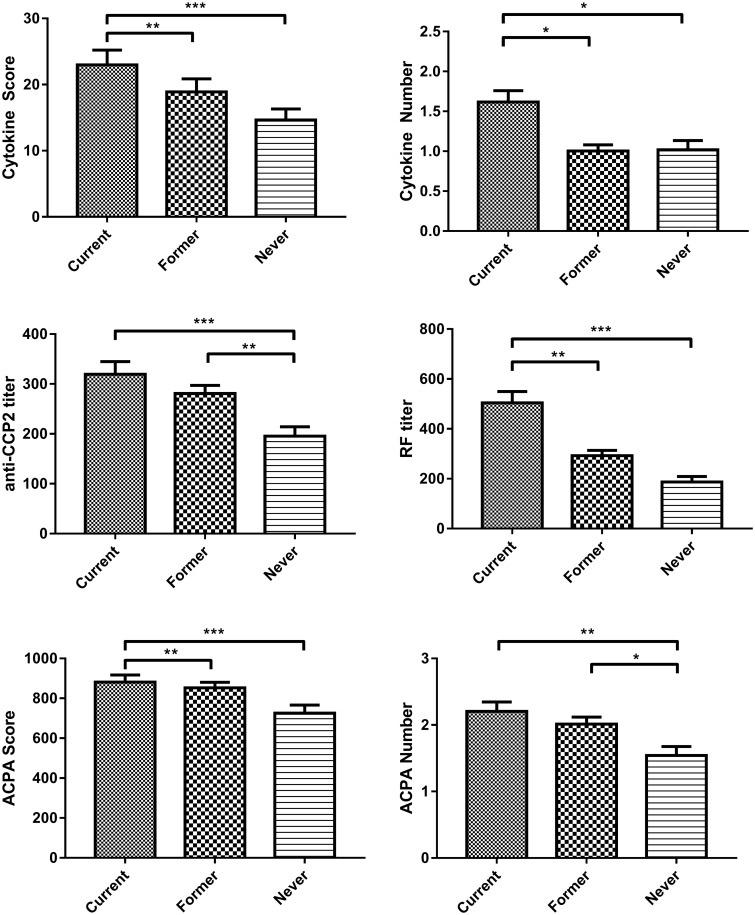
Anti-CCP2-positive current smokers exhibited a significantly higher cytokine score (overall ANOVA, P < 0.0001; current vs former and current vs never, P < 0.001) and number of cytokines positive compared with former or never smokers (Fig. 1A and B). SAM analysis using samples from current, former and never smokers matched for age, sex and disease duration demonstrated that circulating concentrations of multiple circulating inflammatory cytokines/chemokines, including IL-2, IL-6, IL-12, IL-12p70, IFNγ, GM-CSF, MCP-1 and TNF-α, were significantly increased in current smokers compared with former and never smokers (q < 0.1%; Fig. 2). Notably, the cytokines/chemokines differentially elevated among current smokers were many of those strongly implicated in RA disease pathogenesis, whereas several other cytokines/chemokines, including IL-4, IL-5, IL-7, IL-8, IL-10 and MIP1β, were not differentially expressed between current and former/never smokers.
Fig. 1.
Differential associations of serum cytokines, ACPAs and RF with smoking status
Anti-CCP2-positive patients were categorized into subgroups including current (n = 321), former (n = 599) or never (n = 210) smokers. Levels of serum cytokines were measured by multiplex immunoassay, levels of ACPA were assessed by measurement of autoantibodies against 37 putative targets of the RA immune response, and levels of IgM RF were measured by nephelometry. Comparisons between groups were performed using the Kruskal–Wallis test with Tukey’s multiple comparison post hoc test. The cytokine score (A) and number of cytokines (B) were higher among current smokers, whereas levels of anti-CCP2 (C), ACPA score (E) and ACPA number (F) were higher in both current and former smokers compared with never smokers. (D) RF titre was significantly higher among current smokers than former and never smokers.
Fig. 2.
Increased RA-associated cytokines are identified among current compared with former and never smokers
Levels of 17 cytokines were compared between age- and sex-matched subgroups of current, former and never smokers (n = 107 subjects per group) using a bead-based multiplex cytokine assay. Using significance analysis of microarrays, output was sorted based on false-discovery rates in order to identify cytokines with the greatest differences between subgroups.
Current smoking status is associated with increased RA disease activity
Among anti-CCP2-positive patients, current smokers exhibited significantly higher levels of RA disease activity [DAS28 4.6 (1.7)] than never smokers [DAS28 3.7 (1.6); overall ANOVA, P < 0.001; current vs never, P < 0.001; Table 2]. Former smokers demonstrated levels of RA disease activity [DAS28 3.9 (1.5)] significantly lower than current smokers (current vs former, P < 0.001) and similar to never smokers. These results persisted following post hoc adjustment for comparisons across three groups as well as when adjusted for multivariable associations including age, sex, disease duration, co-morbidity score, race/ethnicity and treatments, including methotrexate, prednisone or biologics (Table 3).
Table 2.
Measures of disease at study enrolment by smoking status
| Measure | Never smokers (n = 210) | Former smokers (n = 599) | Current smokers (n = 321) | P-value |
|---|---|---|---|---|
| DAS28 | 3.7 (1.6) | 3.9 (1.5) | 4.6 (1.7) | <0.001 |
| CvF***, CvN*** | ||||
| Provider global (0–100) | 31.4 (22.4) | 34.2 (22.3) | 43.1 (23.2) | <0.001 |
| CvF**, CvN** | ||||
| Log-CRP | 1.7 (1.5) | 1.7 (1.3) | 1.9 (1.2) | 0.044 |
| Pain score (0–10) | 4.0 (2.8) | 4.3 (2.7) | 5.7 (2.9) | <0.001 |
| CvF***, CvN*** | ||||
| MD-HAQ (0–3) | 0.8 (0.6) | 0.9 (0.6) | 1.1 (0.6) | <0.001 |
| CvF**, CvN***, FvN* |
*P < 0.05,
**P<0.01 and
***P<0.001, using Scheffe’s method for multiple comparisons for each set of three follow-up comparisons. All values are given as the mean (s.d.). CvF: current vs former; CvN: current vs never; FvN: former vs never; MD-HAQ: multidimensional HAQ.
Table 3.
Multivariable associations of smoking status with measures of disease activity a
| Measure | Never smokers | Former smokers |
Current smokers |
||
|---|---|---|---|---|---|
| β-Coefficient | P-value | β-Coefficient | P-value | ||
| DAS28 | Reference | 0.25 | 0.116 | 0.83 | <0.001 |
| Provider global (0–100 mm) | Reference | 5.61 | 0.070 | 10.38 | 0.004 |
| Log-CRP | Reference | −0.03 | 0.830 | 0.25 | 0.057 |
| Pain score (0–10) | Reference | 0.55 | 0.054 | 1.72 | <0.001 |
| MD-HAQ (0–3) | Reference | 0.14 | 0.033 | 0.30 | <0.001 |
aMultivariable models adjusted for age, sex, disease duration, co-morbidity score, race/ethnicity and treatments, including the use of MTX, prednisone or biologics. MD-HAQ: multidimensional HAQ.
A similar pattern of increased disease activity/severity among current smokers was observed for nearly all components of the DAS28 score, including tender and swollen joint counts and patient global assessment, although there was no association with ESR (data not shown). Similar associations of smoking status were observed with MD-HAQ, pain, provider global and log-CRP values (Table 2). Notably, these differences were observed only among subjects positive for anti-CCP2 antibody and were attenuated and non-significant among anti-CCP2-negative individuals (supplementary Table S1, available at Rheumatology Online). Although a smaller population than the seropositive subjects, formal power analysis (based on the effect size observed in the anti-CCP2-positive subjects) suggested that this substudy was powered to identify a signficant difference in DAS28 if one existed.
Association of autoantibody levels among current and former smokers
We hypothesized that the reduced disease activity seen in former smokers would be associated with reduction in anti-CCP2 and/or ACPA titres. We observed significantly increased anti-CCP2 ELISA titres among both current and former smokers, relative to never smokers (Fig. 1C). Likewise, by antigen microarray, we observed increased depth (ACPA score; P = 0.019) and breadth of the ACPA response (number of ACPA positive; P = 0.004) in current smokers compared with never smokers (Fig. 1D and E). Notably, although both anti-CCP2 and ACPA scores were numerically higher in current smokers compared with former smokers, this small difference was not statistically significant, and thus, reduced levels of inflammation and disease activity were not associated with a reduction in circulating ACPA. In contrast to the lack of differences in ACPA titres between current and former smokers, compared with current smokers we did observe a significantly lower titre of RF among former smokers and never smokers (both P < 0.001), with a much smaller but significant reduction among never smokers compared with former smokers (P = 0.04; Fig. 1F).
Discussion
The association between smoking and the development of RA is well established. The fact that smoking-associated RA risk is limited to the seropositive RA population [5] implicates a role for smoking in the generation of RA-associated autoantibodies or, potentially, the generation of cognate antigen, both of which have been observed [4, 19]. Whether such an effect can also contribute to RA disease activity remains less well defined.
We observed significantly higher levels of multiple serum cytokines as well as as clinical measures of RA disease activity among current smokers compared with both former and never smokers. Notably, the cytokines found to be elevated in current smokers were many of the molecules that have been most strongly implicated in RA disease pathogenesis (including TNF-α, IL-2, IL-6, IL-12, IL-12p70 and IFNγ), whereas several other cytokine/chemokines were not found to be different by smoking status. Interestingly, another cytokine elevated among current smokers was GM-CSF, a mediator implicated in osteoclastogenesis [20], perhaps providing insight into earlier reports linking smoking with increased structural damage [8, 9, 21].
We initially hypothesized that reduced disease activity among both never and former smokers would be correlated with a reduction in the breadth and depth of the ACPA response. However, levels of ACPA were relatively similar between both former and current smokers, even after stratification to the anti-CCP-positive population. Thus, although ACPA levels seem imprinted in current or former smokers, measures of inflammation and disease activity appear to fall in the setting of smoking cessation. Interestingly, we observed a corresponding decrement in RF titre among former smokers (relative to current smokers) to a level approaching never smokers, an observation supporting prior studies associating the presence and titre of RF with smoking status [8, 19, 22–25]. However, this is the first study, to our knowledge, to demonstrate meaningful differences in RF concentration between current and former cigarette smokers as manifested by a corresponding reduced level of disease activity. Thus, the correlation of RF titre with smoking status suggests it to be a potential driver of autoantibody-mediated inflammation and supports recent work by our group and others demonstrating the capacity for RF to potentiate the inflammatory capacity of ACPA immune complexes [26, 27].
Another possible explanation is an antibody-independent effect of smoking cessation. Supporting this assertion is evolving evidence that smokers with axial spondyloarthritis have more severe spinal damage than non-smokers [28, 29]. However, in our cohort, the increased disease activity among smokers was not observed in the anti-CCP-negative population, suggesting that other factors interact with ACPA upon tobacco exposure. We propose that although levels of APCA are determined early and remain relatively stable over time, smoking continues to effect the production of citrullinated antigen and thus unfavourably alters the dynamics of ACPA–immune complex formation. Such fuelling of the fire [30] is supported by studies which have identified production of citrullinated proteins in both the lungs [4, 31] and periodontium of smokers relative to non-smokers [32].
Similar to our results, several studies have associated current smoking with increased measures of disease activity [9, 21, 23], whereas others did not observe differences in levels of disease activity but did report increased disease damage [8, 33, 34] among current smokers. Our results are unique in the inclusion of current and former smokers, thus enabling separation of the effect of current smoking from ever smoking. Notably, our results differ slightly from two recent cohort studies that studies examined the impact of smoking cessation (127 and 333 patients, respectively) on clinical measures of disease activity, and neither of these (relatively small) studies demonstrated a significant reduction in disease activity during the duration of clinical observation.
Interestingly, most of the studies identifying higher levels of disease activity among current smokers included longer periods of observation than the shorter prospective studies, perhaps suggesting that benefits related to smoking cessation may require longer smoke-free periods. As such, in our study, the suggested effects of smoking cessation may have occurred prior to the time of enrolment in the VARA cohort. Also in contrast to our study, many earlier studies did not stratify analyses by anti-CCP2 status nor did any include multiplex biomarker assessments of inflammation, which might have demonstrated dynamic change during even a brief smoke-free period of observation.
Strengths of our study include the size of the cohort, the extent of clinical and serological data, as well as the measurement of multiplex cytokines, which provide a surrogate of disease amelioration among former and never smokers. An important limitation is the cross-sectional nature of our study. With only one time point for assessment, we cannot be sure that a reduction in disease activity was directly correlated with smoking cessation. Likewise, data relevant to the quantity of current or former tobacco exposure (i.e. pack-years or packs per day) and time from smoking cessation among former smokers were not available in this cohort. Two previous studies have reported a discrepancy in results regarding the association of cumulative smoking with RA disease severity [23, 34]. Perhaps more so than other patient-reported data, it can be challenging to confirm genuine smoking status, let alone quantitative exposure. However, a sensitivity analysis performed using levels of serum cotinine, a surrogate for use of nicotine-containing products, reinforced the observed association of increased disease activity with current smoking (data not shown). Finally, our results demonstrating similar levels of ACPA between current and former smokers were limited to the IgG isotype and may not reflect the well-demonstrated relationship between smoking and IgA ACPA. It is possible that reduced levels of IgA ACPA, the presence of which has been associated with a more severe disease course, could have been associated with the reduced RA disease activity observed in former (and never) smokers [37–39].
In conclusion, we observed that among anti-CCP-positive RA patients, current smoking status is associated with higher levels of disease activity and levels of multiple RA-associated inflammatory serum cytokines. Patients who report discontinuation in tobacco use have less disease activity, lower levels of serum cytokines and lower RF titres, whereas ACPA levels in former smokers remain relatively constistant with current smokers. Thus, in the anti-CCP-positive RA population, previous smoking cessation followed by sustained abstinence appears to be associated with decreased serum cytokines and decreased disease activity, an effect that appears independent of ACPA levels but potentially associated with RF titre. Based on these observations, the effect of smoking cessation on RA disease activity should be evaluated in a prospective manner, and serum cytokines may provide a supporting and/or surrogate biomarker of its efficacy.
Supplementary Material
Acknowledgements
The VARA registry has received research support from the Health Services Research & Development Program of the Veterans Health Administration in addition to unrestricted research funds from Abbott Laboratories and Bristol-Myers Squibb. J.S. received support from a VA Career Development Award [IK2 BX001301], the Rheumatology Research Foundation and National Institutes of Health [HL122773]; W.H.R. received support from the National Institutes of Health [AR0636763 and U01-AI101981].
Funding: This work was supported by the Nebraska Artiritis Outcomes Research Centre and the Rheumatology Research Foundation.
Disclosure statement: The authors have declared no conflicts of interest.
Supplementary data
Supplementary data are available at Rheumatology Online.
References
- 1. Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis. Results from a nationwide study of disease-discordant twins. Arthritis Rheum 1996;39:732–5. [DOI] [PubMed] [Google Scholar]
- 2. Uhlig T, Hagen KB, Kvien TK. Current tobacco smoking, formal education, and the risk of rheumatoid arthritis. J Rheumatol 1999;26:47–54. [PubMed] [Google Scholar]
- 3. Klareskog L, Padyukov L, Lorentzen J, Alfredsson L. Mechanisms of disease: Genetic susceptibility and environmental triggers in the development of rheumatoid arthritis. Nat Clin Pract Rheumatol 2006;2:425–33. [DOI] [PubMed] [Google Scholar]
- 4. Klareskog L, Stolt P, Lundberg K. et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006;54:38–46. [DOI] [PubMed] [Google Scholar]
- 5. Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene–environment interaction between smoking and shared epitope genes in HLA–DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum 2004;50:3085–92. [DOI] [PubMed] [Google Scholar]
- 6. Nyhall-Wahlin BM, Petersson IF, Nilsson JA, Jacobsson LT, Turesson C. High disease activity disability burden and smoking predict severe extra-articular manifestations in early rheumatoid arthritis. Rheumatology 2009;48:416–20. [DOI] [PubMed] [Google Scholar]
- 7. Harrison BJ, Silman AJ, Wiles NJ, Scott DG, Symmons DP. The association of cigarette smoking with disease outcome in patients with early inflammatory polyarthritis. Arthritis Rheum 2001;44:323–30. [DOI] [PubMed] [Google Scholar]
- 8. Wolfe F. The effect of smoking on clinical, laboratory, and radiographic status in rheumatoid arthritis. J Rheumatol 2000;27:630–7. [PubMed] [Google Scholar]
- 9. Papadopoulos NG, Alamanos Y, Voulgari PV. et al. Does cigarette smoking influence disease expression, activity and severity in early rheumatoid arthritis patients? Clin Exp Rheumatol 2005;23:861–6. [PubMed] [Google Scholar]
- 10. Mikuls TR, Kazi S, Cipher D. et al. The association of race and ethnicity with disease expression in male US veterans with rheumatoid arthritis. J Rheumatol 2007;34:1480–4. [PubMed] [Google Scholar]
- 11. Arnett F, Edworthy S, Bloch D. et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- 12. Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum 1999;42:2220–30. [DOI] [PubMed] [Google Scholar]
- 13. Mikuls TR, Padala PR, Sayles HR. et al. Prospective study of posttraumatic stress disorder and disease activity outcomes in US veterans with rheumatoid arthritis. Arthritis Care Res 2013;65:227–34. [DOI] [PubMed] [Google Scholar]
- 14. Sokolove J, Bromberg R, Deane KD. et al. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS ONE 2012;7: e35296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sokolove J, Lindstrom TM, Robinson WH. Development and deployment of antigen arrays for investigation of B-cell fine specificity in autoimmune disease. Front Biosci (Elite Ed) 2012;4:320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maska LB, Sayles HR, O'Dell JR. et al. Serum cotinine as a biomarker of tobacco exposure and the association with treatment response in early rheumatoid arthritis. Arthritis Care Res 2012;64:1804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Florescu A, Ferrence R, Einarson T. et al. Methods for quantification of exposure to cigarette smoking and environmental tobacco smoke: focus on developmental toxicology. Ther Drug Monit 2009;31:14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 2001;98:5116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goodson NJ, Farragher TM, Symmons DP. Rheumatoid factor, smoking, and disease severity: associations with mortality in rheumatoid arthritis. J Rheumatol 2008;35:945–9. [PubMed] [Google Scholar]
- 20. Lee MS, Kim HS, Yeon JT. et al. GM-CSF regulates fusion of mononuclear osteoclasts into bone-resorbing osteoclasts by activating the Ras/ERK pathway. J Immunol 2009;183:3390–9. [DOI] [PubMed] [Google Scholar]
- 21. Mattey DL, Hutchinson D, Dawes PT. et al. Smoking and disease severity in rheumatoid arthritis: association with polymorphism at the glutathione S-transferase M1 locus. Arthritis Rheum 2002;46:640–6. [DOI] [PubMed] [Google Scholar]
- 22. Jónsson T, Thorsteinsson J, Valdimarsson H. Does smoking stimulate rheumatoid factor production in non-rheumatic individuals? APMIS 1998;106: 970–4. [DOI] [PubMed] [Google Scholar]
- 23. Masdottir B, Jónsson T, Manfredsdottir V. et al. Smoking, rheumatoid factor isotypes and severity of rheumatoid arthritis. Rheumatology 2000;39:1202–5. [DOI] [PubMed] [Google Scholar]
- 24. Mattey DL, Dawes PT, Clarke S. et al. Relationship among the HLA-DRB1 shared epitope, smoking, and rheumatoid factor production in rheumatoid arthritis. Arthritis Rheum 2002;47:403–7. [DOI] [PubMed] [Google Scholar]
- 25. Korpilähde T, Heliövaara M, Knekt P. et al. Smoking history and serum cotinine and thiocyanate concentrations as determinants of rheumatoid factor in non-rheumatoid subjects. Rheumatology 2004;43:1424–8. [DOI] [PubMed] [Google Scholar]
- 26. Sokolove J, Johnson DS, Lahey LJ. et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol 2014;66:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Laurent L, Anquetil F, Clavel C. et al. IgM rheumatoid factor amplifies the inflammatory response of macrophages induced by the rheumatoid arthritis-specific immune complexes containing anticitrullinated protein antibodies. Ann Rheum Dis 2015;74:1425–31. [DOI] [PubMed] [Google Scholar]
- 28. Chung HY, Machado P, van der Heijde D, D’Agostino MA, Dougados M. Smokers in early axial spondyloarthritis have earlier disease onset, more disease activity, inflammation and damage, and poorer function and health-related quality of life: results from the DESIR cohort. Ann Rheum Dis 2012;71:809–16. [DOI] [PubMed] [Google Scholar]
- 29. Rudwaleit M, Haibel H, Baraliakos X. et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum 2009;60:717–27. [DOI] [PubMed] [Google Scholar]
- 30. Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis. Arthritis Res Ther 2004;6:107–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Makrygiannakis D, Hermansson M, Ulfgren AK. et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in BAL cells. Ann Rheum Dis 2008;67:1488–92. [DOI] [PubMed] [Google Scholar]
- 32. Nesse W, Westra J, van der Wal JE. et al. The periodontium of periodontitis patients contains citrullinated proteins which may play a role in ACPA (anti-citrullinated protein antibody) formation. J Clin Periodontol 2012;39:599–607. [DOI] [PubMed] [Google Scholar]
- 33. de Rooy DP, van Nies JA, Kapetanovic MC. et al. Smoking as a risk factor for the radiological severity of rheumatoid arthritis: a study on six cohorts. Ann Rheum Dis 2014;73:1384–7. [DOI] [PubMed] [Google Scholar]
- 34. Lu B, Rho YH, Cui J. et al. Associations of smoking and alcohol consumption with disease activity and functional status in rheumatoid arthritis. J Rheumatol 2014; 41:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Andersson ML, Bergman S, Söderlin MK. The effect of stopping smoking on disease activity in rheumatoid arthritis (RA). Data from BARFOT, a multicenter study of early RA. Open Rheumatol J 2012;6:303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fisher MC, Hochberg MC, El-Taha M. et al. Smoking, smoking cessation, and disease activity in a large cohort of patients with rheumatoid arthritis. J Rheumatol 2012;39:904–9. [DOI] [PubMed] [Google Scholar]
- 37. Verpoort KN, Papendrecht-van der Voort EA, van der Helm-van Mil AH. et al. Association of smoking with the constitution of the anti-cyclic citrullinated peptide response in the absence of HLA-DRB1 shared epitope alleles. Arthritis Rheum 2007;56:2913–8. [DOI] [PubMed] [Google Scholar]
- 38. Svärd A, Kastbom A, Reckner-Olsson A, Skogh T. Presence and utility of IgA-class antibodies to cyclic citrullinated peptides in early rheumatoid arthritis: the Swedish TIRA project. Arthritis Res Ther 2008;10:R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Svärd A, Kastbom A, Söderlin MK, Reckner-Olsson Å, Skogh T. A comparison between IgG- and IgA-class antibodies to cyclic citrullinated peptides and to modified citrullinated vimentin in early rheumatoid arthritis and very early arthritis. J Rheumatol 2011;38:1265–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.