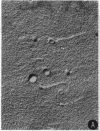
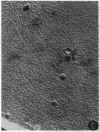
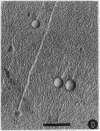
Abstract
The persistence of numerous pathogenic bacteria important in disease states, such as tuberculosis, in humans and domestic animals has been ascribed to an inhibition of fusion between the phagosomal vesicles containing the bacteria and lysosomes in the host cells [Elsbach, P. & Weiss, J. (1988) Biochim. Biophys. Acta 974, 29-52; Thoen, C. O. (1988) J. Am. Vet. Med. Assoc. 193, 1045-1048]. In tuberculosis this effect has been indirectly attributed to the production of cord factor (alpha,alpha-trehalose 6,6'-dimycolate). We show here that cord factor is extraordinarily effective at inhibiting Ca2(+)-induced fusion between phospholipid vesicles and suggest a mechanism by which cord factor confers this effect. These findings are likely to be important in our understanding of the pathogenesis and treatment of many diseases of bacterial etiology.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Cramp F. C., Fisher D., Howell J. I., Lucy J. A. Studies on chemically induced cell fusion. J Cell Sci. 1972 May;10(3):769–787. doi: 10.1242/jcs.10.3.769. [DOI] [PubMed] [Google Scholar]
- Anchordoguy T. J., Rudolph A. S., Carpenter J. F., Crowe J. H. Modes of interaction of cryoprotectants with membrane phospholipids during freezing. Cryobiology. 1987 Aug;24(4):324–331. doi: 10.1016/0011-2240(87)90036-8. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHN Z. A. The fate of bacteria within phagocytic cells. I. The degradation of isotopically labeled bacteria by polymorphonuclear leucocytes and macrophages. J Exp Med. 1963 Jan 1;117:27–42. doi: 10.1084/jem.117.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley A. C., Fuller N. L., Rand R. P., Parsegian V. A. Measurement of repulsive forces between charged phospholipid bilayers. Biochemistry. 1978 Jul 25;17(15):3163–3168. doi: 10.1021/bi00608a034. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Carpenter J. F., Rudolph A. S., Wistrom C. A., Spargo B. J., Anchordoguy T. J. Interactions of sugars with membranes. Biochim Biophys Acta. 1988 Jun 9;947(2):367–384. doi: 10.1016/0304-4157(88)90015-9. [DOI] [PubMed] [Google Scholar]
- Crowe J. H., Crowe L. M., Chapman D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984 Feb 17;223(4637):701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- Cullis P. R., Hope M. J. Effects of fusogenic agent on membrane structure of erythrocyte ghosts and the mechanism of membrane fusion. Nature. 1978 Feb 16;271(5646):672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- Davis-Scibienski C., Beaman B. L. Interaction of Nocardia asteroides with rabbit alveolar macrophages: association of virulence, viability, ultrastructural damage, and phagosome-lysosome fusion. Infect Immun. 1980 May;28(2):610–619. doi: 10.1128/iai.28.2.610-619.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. H+- and Ca2+-induced fusion and destabilization of liposomes. Biochemistry. 1985 Jun 18;24(13):3099–3106. doi: 10.1021/bi00334a005. [DOI] [PubMed] [Google Scholar]
- Ellens H., Bentz J., Szoka F. C. pH-induced destabilization of phosphatidylethanolamine-containing liposomes: role of bilayer contact. Biochemistry. 1984 Mar 27;23(7):1532–1538. doi: 10.1021/bi00302a029. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Weiss J. Phagocytosis of bacteria and phospholipid degradation. Biochim Biophys Acta. 1988 Feb 24;947(1):29–52. doi: 10.1016/0304-4157(88)90018-4. [DOI] [PubMed] [Google Scholar]
- Fry D. W., White J. C., Goldman I. D. Rapid separation of low molecular weight solutes from liposomes without dilution. Anal Biochem. 1978 Oct 15;90(2):809–815. doi: 10.1016/0003-2697(78)90172-0. [DOI] [PubMed] [Google Scholar]
- Goren M. B., D'Arcy Hart P., Young M. R., Armstrong J. A. Prevention of phagosome-lysosome fusion in cultured macrophages by sulfatides of Mycobacterium tuberculosis. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2510–2514. doi: 10.1073/pnas.73.7.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren M. B. Phagocyte lysosomes: interactions with infectious agents, phagosomes, and experimental perturbations in function. Annu Rev Microbiol. 1977;31:507–533. doi: 10.1146/annurev.mi.31.100177.002451. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra D., Düzgünes N., Wilschut J. Agglutination and fusion of globoside GL-4 containing phospholipid vesicles mediated by lectins and calcium ions. Biochemistry. 1985 Jan 29;24(3):565–572. doi: 10.1021/bi00324a004. [DOI] [PubMed] [Google Scholar]
- Hoekstra D., Düzgüneş N. Ricinus communis agglutinin-mediated agglutination and fusion of glycolipid-containing phospholipid vesicles: effect of carbohydrate head group size, calcium ions, and spermine. Biochemistry. 1986 Mar 25;25(6):1321–1330. doi: 10.1021/bi00354a020. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Walker D. C., Cullis P. R. Ca2+ and pH induced fusion of small unilamellar vesicles consisting of phosphatidylethanolamine and negatively charged phospholipids: a freeze fracture study. Biochem Biophys Res Commun. 1983 Jan 14;110(1):15–22. doi: 10.1016/0006-291x(83)91253-6. [DOI] [PubMed] [Google Scholar]
- Howell J. I., Lucy J. A. Cell fusion induced by lysolecithin. FEBS Lett. 1969 Aug;4(3):147–150. doi: 10.1016/0014-5793(69)80218-8. [DOI] [PubMed] [Google Scholar]
- Kaneda K., Sumi Y., Kurano F., Kato Y., Yano I. Granuloma formation and hemopoiesis induced by C36-48-mycolic acid-containing glycolipids from Nocardia rubra. Infect Immun. 1986 Dec;54(3):869–875. doi: 10.1128/iai.54.3.869-875.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M. Effect of anti-cord factor antibody on experimental tuberculosis in mice. Infect Immun. 1973 Jan;7(1):14–21. doi: 10.1128/iai.7.1.14-21.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M. Studies of a biochemical lesion in experimental tuberculosis in mice. IV. Effect of tubercle bacilli grown in vivo on respiratory chain enzymes. Am Rev Respir Dis. 1966 Sep;94(3):395–399. doi: 10.1164/arrd.1966.94.3.395. [DOI] [PubMed] [Google Scholar]
- Lemaire G., Tenu J. P., Petit J. F., Lederer E. Natural and synthetic trehalose diesters as immunomodulators. Med Res Rev. 1986 Jul-Sep;6(3):243–274. doi: 10.1002/med.2610060302. [DOI] [PubMed] [Google Scholar]
- Lepoivre M., Tenu J. P., Lemaire G., Petit J. F. Antitumor activity and hydrogen peroxide release by macrophages elicited by trehalose diesters. J Immunol. 1982 Aug;129(2):860–866. [PubMed] [Google Scholar]
- Marsh D. Water adsorption isotherms and hydration forces for lysolipids and diacyl phospholipids. Biophys J. 1989 Jun;55(6):1093–1100. doi: 10.1016/S0006-3495(89)82906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOLL H., BLOCH H., ASSELINEAU J., LEDERER E. The chemical structure of the cord factor of Mycobacterium tuberculosis. Biochim Biophys Acta. 1956 May;20(2):299–309. doi: 10.1016/0006-3002(56)90289-x. [DOI] [PubMed] [Google Scholar]
- Nir S., Düzgüneş N., Bentz J. Binding of monovalent cations to phosphatidylserine and modulation of Ca2+- and Mg2+-induced vesicle fusion. Biochim Biophys Acta. 1983 Oct 26;735(1):160–172. doi: 10.1016/0005-2736(83)90271-7. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Hui S., Vail W. J., Poste G. Studies on membrane fusion. I. Interactions of pure phospholipid membranes and the effect of myristic acid, lysolecithin, proteins and dimethylsulfoxide. Biochim Biophys Acta. 1976 Oct 5;448(2):254–264. [PubMed] [Google Scholar]
- Parant M., Audibert F., Parant F., Chedid L., Soler E., Polonsky J., Lederer E. Nonspecific immunostimulant activities of synthetic trehalose-6,6'-diesters (lower homologs of cord factor). Infect Immun. 1978 Apr;20(1):12–19. doi: 10.1128/iai.20.1.12-19.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V. A., Fuller N., Rand R. P. Measured work of deformation and repulsion of lecithin bilayers. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2750–2754. doi: 10.1073/pnas.76.6.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., Papahadjopoulos D. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 1979 Mar 6;18(5):780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- Rand R. P., Parsegian V. A. Physical force considerations in model and biological membranes. Can J Biochem Cell Biol. 1984 Aug;62(8):752–759. doi: 10.1139/o84-097. [DOI] [PubMed] [Google Scholar]
- Rudolph A. S., Crowe J. H. Membrane stabilization during freezing: the role of two natural cryoprotectants, trehalose and proline. Cryobiology. 1985 Aug;22(4):367–377. doi: 10.1016/0011-2240(85)90184-1. [DOI] [PubMed] [Google Scholar]
- Smaal E. B., Mandersloot J. G., Demel R. A., de Kruijff B., de Gier J. Consequences of the interaction of calcium with dioleoylphosphatidate-containing model membranes: calcium-membrane and membrane-membrane interactions. Biochim Biophys Acta. 1987 Feb 12;897(1):180–190. doi: 10.1016/0005-2736(87)90326-9. [DOI] [PubMed] [Google Scholar]
- Thoen C. O. Tuberculosis. J Am Vet Med Assoc. 1988 Nov 1;193(9):1045–1048. [PubMed] [Google Scholar]