Abstract
Fluorescent dyes that binds irreversibly to cellular amines, come in several available emission spectra, and do not poses health concerns were used to evaluate membrane integrity in fish sperm cells. The objectives of the present study were to determine: (1) a working dye concentration for fish sperm samples, and (2) if the traditional propidium iodide/SYBR-14 staining combination was comparable with the amine reactive dye (ARD) methods at identifying cell populations with intact and compromised membranes after sperm activation, refrigerated storage, and exposure to cryoprotectant and surfactant. Zebrafish (Danio rerio) sperm were obtained by stripping, and pooled samples (in triplicate) were used in all tests. Six dilutions of the amine dye (ranging from 0.625 to 0.02 μL/mL) were evaluated, and compared with the traditional staining protocol. A concentration of 0.5 μL/mL ARD was selected to be used in subsequent assays.
Sperm suspensions were activated with deionized water to simulate urine contamination. After 10 sec, osmolality was increased to stop activation, and the procedure was repeated in 10-sec intervals until the sperm remained activated for 120 consecutive sec; membrane integrity was analyzed at each time interval. For the storage assay, sperm suspensions were prepared in Hanks’ balanced salt solution at 302 mOsm/kg osmolality (HBSS302), HBSS354 and HBSS402, and evaluated every 2 hr for 8 hr, and every 24 hr for 72 hr. Cryoprotectant toxicity was tested by diluting sperm suspensions in HBSS340 with methanol at 5, 10 and 15% final concentrations. Surfactant toxicity was tested by diluting sperm suspensions in HBSS354 with Triton X-100 at 0.2, 0.15 and 0.1 mM final concentrations. In each toxicity assay, membrane integrity was tested every 20 min for 80 min. The number of membrane-intact cells significantly decreased across time in all treatments (p < 0.05). Significant differences between staining protocols were observed after activation and after exposure to methanol at 10 and 15%, and to Triton X-100 (p < 0.05). The average difference, however, was minor (between 1 and 6% in average) in relation to the typical values used for decision making based on this assay. Results showed that this method has the potential to contribute greatly to the standardization of cryopreservation in aquatic species.
Keywords: amine reactive dye, cell membrane integrity, fish, sperm, flow cytometry
1. Introduction
Increasing fish production while reducing husbandry costs, backing up lines and strains of species used for biomedical research, and protecting genetic resources and regenerating populations of imperiled species as part of conservation efforts provide examples of the need for cryopreservation of germplasm and the development of repositories for aquatic species. The increasing demand for cryopreserved germplasm goes along with an increasing demand for high quality in post-thaw motility, fertilization rates, and hatching rates (Horváth et al., 2008). Standardized quality control checkpoints along a given process are highly recommended to avoid lack of reproducibility (Baker, 2016). An assessment of membrane integrity, such as the proportion of membrane-intact cells (also known as “cell viability”), at each step of the cryopreservation process is an accepted indicator of sperm quality given that the sperm plasma membrane is one of the main structures that can be damaged during cryopreservation (Segovia et al., 2000).
Flow cytometry is a robust tool for identification and quantification of sperm cells with compromised membranes (Ogier De Baulny et al., 1997; Ogier De Baulny et al., 1999; Segovia et al., 2000). The fluorescent dyes propidium iodide (PI) and SYBR-14 have been used successfully for the past 20 yr, and the general protocol consists of adding both dyes to a sperm suspension. SYBR-14 is a permeant dye that will bind to the DNA of cells with intact membranes producing green fluorescence at 525 nm, whereas PI can only enter cells with impaired membranes, intercalating with their DNA and producing red fluorescence at 610 nm (Garner et al., 1994).
In addition to cell membrane integrity, other sperm cell characteristics can be evaluated using flow cytometry, such as: mitochondrial activity (Ogier De Baulny et al., 1997, Trigo et al., 2015), ATP content (Ogier De Baulny et al., 1999), apoptosis, and nuclear DNA fragmentation (Jenkins et al., 2014). However, in small fish, such as biomedical models, the volume of sperm that can be collected is minimal (≤ 2 ul), and the possibility of additional quality assessment is typically not possible. Amine reactive dyes (ARD), used previously with mammalian somatic cells, offer an alternative to the traditional method. To date, there are eight ARD stains available that cover most of the visible and UV spectrum (https://www.thermofisher.com/order/catalog/product/L34972?ICID=search-l34972, accessed on March 15, 2015), potentially allowing the evaluation of additional sperm quality characteristics by using other fluorescent markers simultaneously, optimizing the use of the limited sample. The ARD binds irreversibly to cellular amines present in the cell surface and in the interior of the cell. In cells with intact membranes, the ARD interact only with the surface amines. In cells with compromised membranes, ARD additionally reacts with intracellular free amines, resulting in intense fluorescent staining (Perfetto et al., 2010). Overall, the ARD are considered safe (Life Technologies, 2014), whereas concerns about PI include suspected mutagenic properties (Corliss and White, 1981; Fukunaga and Yielding, 1978) that require additional handling and disposal precautions (Sigma-Aldrich, 2014).
Using Zebrafish (Danio rerio) as our fish model, the goal of the present study was to evaluate if the ARD method was able to distinguish between sperm cells with impaired and intact cell membranes. The specific objectives were to determine: (1) a working dye concentration for fish sperm samples; and, (2) if the traditional PI/SYBR-14 and amine reactive dye methods were comparable at identifying the populations of cells with intact and compromised membranes after treating sperm with physical and chemical insults, such as those occurring during sample collection (e.g., urine contamination), refrigerated storage and equilibration (i.e., cryoprotectant addition). Finally, as a positive control, the performance of the two staining protocols was compared after exposing sperm cells to a non-ionic surfactant commonly used to lyse or permeabilize living cells. The amine reactive dyes proved to be an alternative method to analyze membrane integrity of fish sperm.
2. Methodology
2.1. Fish husbandry
Protocols for the use of animals in this study were reviewed and approved by the Louisiana State University Institutional Animal Care and Use Committee (Baton Rouge, LA). Adult Zebrafish (Danio rerio) were obtained from a commercial vendor (SunPet, GA, USA). Fish were maintained within a 638-L recirculating system. Water quality parameters were maintained at 28.5° C (temperature), 8.5 (pH) and 12-h light:12-h dark (photoperiod). Fish were fed to satiation twice daily with a dry food master mix (http://zebrafish.org/documents/protocols/pdf/Fish_Feeding/Flake_Food/Dry_Food_Recipes2015.pdf, accessed on May 10, 2015) in the morning and frozen brine shrimp (San Francisco Bay Brand®, Newark, CA) in the afternoon. Additional water quality parameters that were monitored weekly and kept at an acceptable range included: ammonia (0 – 1.0 mg/L), nitrites (0 – 0.8 mg/L) and nitrates (0 – 15 mg/L).
2.2. Sperm collection
Fish were anesthetized with 0.01% MS-222 (Tricaine methanesulfonate, Western Chemical, Inc. Ferndale, WA). Standard length and body mass were measured. Fish were placed ventral side up on a moist sponge and gently stripped. Sperm were collected into a glass capillary tube and mixed with Hanks’ balanced salt solution (HBSS, 0.137 M NaCl, 5.4 mM KCl, 1.3 mM CaCl2, 1.0 mM MgSO4, 0.25 mM Na2HPO4, 0.44 mM KH2PO4, 4.2 mM NaHCO3, and 5.55 mM glucose, pH 7.2) prepared at different osmolalities by adjusting the water volume. Given the limited volume of sperm collectable from Zebrafish (1 to 3 μl), pooled sperm samples from as many as 6 individuals were used.
2.3. Fluorescent staining and flow cytometry
Sperm plasma membrane integrity was determined using the amine reactive dye (ARD) (LIVE/DEAD® Fixable Red Dead Cell Stain Kit, for 488 nm excitation, cat. No. L-34972, Molecular Probes, Eugene, OR, USA) and the traditional fluorescent stains SYBR-14 and propidium iodide (PI) (PI/SYBR-14) (LIVE/DEAD® Sperm Viability Kit, cat. No. L-7011, Molecular Probes, Eugene, OR, USA). In all experiments described below (except for the titration assay, Section 2.4), the final concentration of theamine fluorescent dye was 0.5 μg/ml, samples were incubated in the dark for 20 to 25 min at room temperature (RT). For the PI/SYBR-14 protocol, the fluorescent dyes were prepared as described in Daly and Tiersch (2012), with the final concentration of SYBR 14 being 100 nM and of PI being 12 μM. Samples were incubated in the dark for 20 to 25 min at RT. Flow cytometry was performed with an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA) equipped with a 488-nm, 50 mW solid-state blue laser.
Flow cytometer performance was assessed daily using fluorescent validation beads (Spherotech, BD Accuri, BD Biosciences, San Jose, CA, USA) to ensure that coefficient of variation values were < 3.0% for the fluorescence detectors (BD Biosciences, 2012). Immediately before analysis, the microcentrifuge tubes containing treated samples were suspended with a vortex mixer, and 10,000 events were gated by forward scatter (FSC) and right-angle side-light scatter (SSC) at 35 μl/min rate using CFlow Plus analysis software (version 264.21, BD Accuri, BD Biosciences, San Jose, CA, USA). The FSC threshold was set at a default value of 80,000 to eliminate debris.
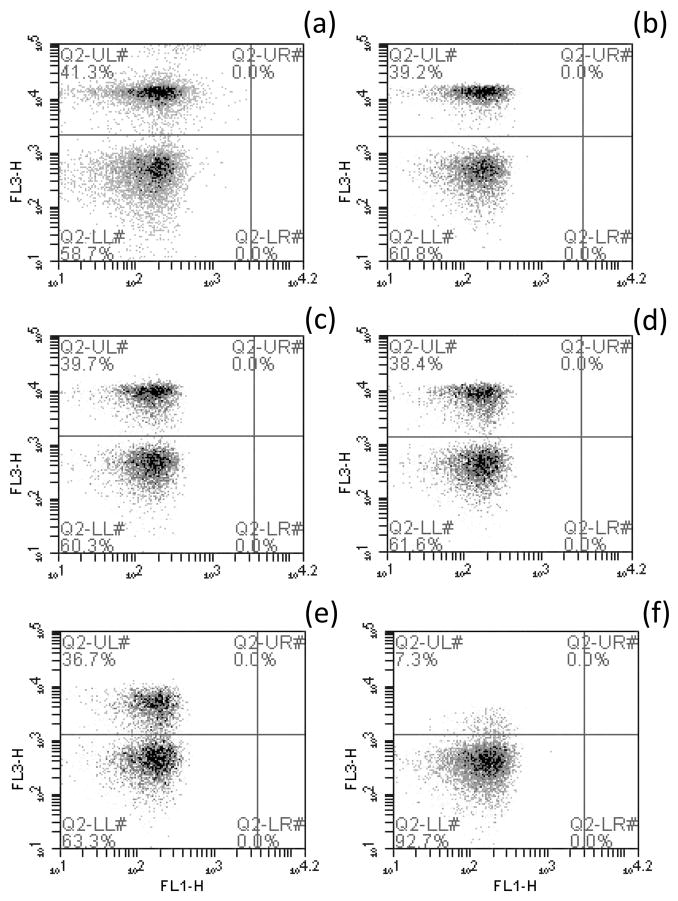
Gating settings for the sperm population (gated events) used to exclude non-sperm events (particles) were based on the FSC and SSC profile of Zebrafish sperm diluted at 2×106 cells/ml with HBSS and stained with either of the protocols described above (Figure 1). Gated events were viewed on a scatter plot showing the FL1 vs. the FL3 fluorescent detectors with fluorescence compensation based on the computed median fluorescence of single-dye control samples to reduce spectral overlap (Daly and Tiersch, 2012). The red amine reactive dye and the PI fluorescences were detected by the FL3 detector (> 670 nm), and SYBR-14 was detected by the FL1 detector (533/30 nm). The regions representing membrane-intact cell population (viable) and cells with compromised membrane (non-viable) were set manually. In the ARD protocol, cells with lower fluorescence values were classified as having intact membranes, whereas in the PI/SYBR-14 protocol, cells positive for SYBR-14 and negative for PI were classified as membrane-intact (Figure 1).
Figure 1.
Forward scatter vs. side scatter (FSC vs. SSC) and FL1 vs. FL3 fluorescent detectors scatter plots of Zebrafish sperm stained with (a) the PI/SYBR-14 and (b) the ARD protocol. The region designated as “P11” in the FSC vs. SSC plot is the gated sperm population, and the regions designated as (a) “P6” and (b) Q3-LL (lower left quadrant) in the FL1 vs. FL3 plot comprise the membrane-intact cell populations.
2.4. Titration
The concentration recommended by the manufacturer for the amine reactive dye is 0.5 μg/ml. A serial dilution of the dye was prepared in a range that included the recommended concentration, and the proportion of membrane-intact cell populations was estimated and compared to the PI/SYBR-14 protocol.
2.4.1. Sample preparation
Two pooled samples of sperm were collected from three fish each (standard length: 32.4 ± 0.4 mm; body mass: 0.618 ± 0.041 g) into 100 μl of HBSS348, with an average sperm concentration of 1.7 × 108 cells/ml. Each sample was diluted 1:50 with HBSS348 to a final volume of 3000 μl suspension (60 μl sperm + 2940 μl HBSS348). The diluted suspension was aliquoted to two equal parts. One aliquot was treated in a water bath at 60°C for 20 min (membrane-compromised). The second aliquot (membrane-intact) was held at RT. Membrane-compromised and membrane-intact suspensions from each sample were mixed in a 1:1 ratio.
2.4.2. Dye preparation and analysis
A vial of ARD (25 μg) was reconstituted with 50 μl of DMSO and a serial dilution was performed (as described in Perfetto et al., 2010) to generate 6 stock concentrations. To obtain the working concentrations, 1 μl of each stock solution was added to 39 μl of deionized water. Five μl of each dye working concentration were added to 95 μl of sperm suspension (Table 1). Samples were mixed and incubated in the dark for 20 min and immediately analyzed by flow cytometry. As reference, a sample of each sperm suspension was also treated with the PI/SYBR-14 protocol (as described in Daly and Tiersch, 2012). The percentage of the membrane-intact cells was calculated for each dilution. The deciding criteria used to help elucidate what ARD concentration was comparable to the results obtained with the PI/SYBR-14 protocol were: the degree of separation between cell populations, and the percentage of the membrane-intact cell population obtained with each dilution.
Table 1.
Six serial dilutions of the amine reactive dye were tested with aliquots of Zebrafish sperm suspension. Stock concentrations were obtained by dilution with dimethyl sulfoxide and working concentrations by further dilution with deionized water (Modified from Perfetto et al., 2010).
| Dilution | Stock conc. (μg/ml) | Working conc. (μg/ml) | Final conc. (μg/ml) |
|---|---|---|---|
| 1 | 500.00 | 12.50 | 0.625 |
| 2 | 250.00 | 6.25 | 0.313 |
| 3 | 125.00 | 3.12 | 0.156 |
| 4 | 62.50 | 1.56 | 0.078 |
| 5 | 31.25 | 0.78 | 0.039 |
| 6 | 15.62 | 0.39 | 0.020 |
2.5. Activation test
Three composite samples of sperm from two to three males each (standard length: 32.8 ± 0.8 mm; body mass: 0.626 ± 0.042 g) were collected into HBSS348, with an average concentration of 1.8 × 108 cells/ml. Sixteen μl of sperm suspension were activated with 34 μl of deionized water. At 10 sec after activation, 750 μl of HBSS348 were added and the suspension was mixed by vortexing. Immediately, two aliquots of 250 μl were obtained from the sample and were treated with each staining protocol. Membrane integrity was estimated using the same approach after activating the samples for 20, 30, 40 sec and so on, in 10-sec increasing intervals, through 120 sec.
2.6. Refrigerated storage
The sperm from one to four males (standard length: 31.5 ± 0.44 mm; body mass: 0. 647 ± 0.022 g) was pooled and collected into 100 μl of one of the buffer treatments (HBSS302, HBSS354 and HBSS402). Three sperm suspensions per treatment were obtained at an average concentration of 2.7 × 108 cells/ml. Membrane integrity of sperm was evaluated after 2, 4, 6, 8, 24, 48 and 72 hr of refrigerated storage (4° C). Two samples of 5 μl each were obtained from each treated replicate and added to 245 μl of each buffer treatment, and stained with either PI/SYBR-14 or ARD protocol.
2.7. Cryoprotectant exposure
The sperm from one to three males (standard length: 33.3 ± 0.3 mm; body mass: 0. 691 ± 0.017 g) was pooled and collected into 100 μl HBSS354, with an average concentration of 2.1 × 108 cells/ml. Three sperm suspensions were assigned to each of the three methanol (cat. no. A4121, Fisher Scientific, Hampton, New Hamshire, USA) final concentrations (5, 10 and 15%). Two samples of 5 μl each were obtained from each treated replicate at 0, 20, 40, 60 and 80 min of exposure. Each 5 μl sample was added to 245 μl of HBSS354, and stained with either PI/SYBR-14 or ARD protocols.
2.8. Surfactant exposure
The sperm from one to two males (standard length: 31.9 ± 0.5 mm; body mass: 0.611 ± 0.031 g) was pooled and collected into 100 μl HBSS354, with an average concentration of 2.9 × 108 cells/ml. Three sperm suspensions were assigned to each of the three Triton X-100 (cat. no. X100-5ML, Sigma Aldrich, St. Louis, MO, USA) final concentrations (0.2, 0.15 and 0.1 mM). Two samples of 5 μl were obtained from each treated replicate at 0, 20, 40, 60 and 80 min of exposure. Each 5 μl sample was added to 245 μl of HBSS354, and stained with either PI/SYBR-14 or ARD protocols.
2.9. Statistical analysis
Data were arcsine-square-root transformed prior to statistical analyses using SAS (SAS version 9.4, SAS Institute, Cary, North Carolina, USA). Significant differences between PI/SYBR-14 and the ARD procedures were assessed with a paired student t-test. One-way ANOVA with Tukey’s multiple comparisons test were used to identify significant differences in the concentration of membrane-intact cells among: time after activation, storage time within each extender treatment, exposure time within each cryoprotectant treatment, and exposure time within each surfactant concentration treatment. When normality was not achieved, the equivalent non-parametric tests – Wilcoxon signed rank sum test and Kruskal-Wallis with multiple comparisons – (Elliot and Hynan 2011; Zar 2010), were used. Results were considered statistically significant at p < 0.05. Data are presented as mean ± SEM.
3. Results
3.1. Titration
The percentage of sperm cells identified as having compromised membranes decreased with decreasing dye concentration (Figure 2), indicating that at lower dye concentrations not all of the sperm cells with compromised membranes were recognized. The percentage of membrane-intact cells was slightly higher by the ARD protocol when compared with the PI/SYBR-14 method; the difference was an average of 5% for the highest concentrations (between 0.625 and 0.156 μg/ml), and of 10% for the lowest (between 0.078 and 0.02 μg/ml). The scatter plots showed a clearer separation of sperm cell populations at higher dye concentrations (Figure 2), which included the concentration recommended by the manufacturer (0.5 μg/ml). As such, this concentration was used for the rest of assays. The dye was prepared using 5 μl of the stock solution (500 μg/ml) diluted into 45 μl DMSO (or HBSS), and 2.5 μl of the diluted dye were added to 250 μl sperm suspension for a final concentration of 0.5 μg/ml.
Figure 2.
Scatter plots of the FL1-H channel vs. the FL3-H channel portraying the gated populations of membrane-intact (Q2-LL, lower left quadrant) vs. membrane-compromised (Q2-UL, upper left quadrant) cells using the red amine reactive fluorescent dye dilutions, as follows: (a) 0.625 μg/ml, (b) 0.313 μg/ml, (c) 0.156 μg/ml, (d) 0.078 μg/ml, (e) 0.039 μg/ml, and (f) 0.020 μg/ml.
3.2. Activation testing
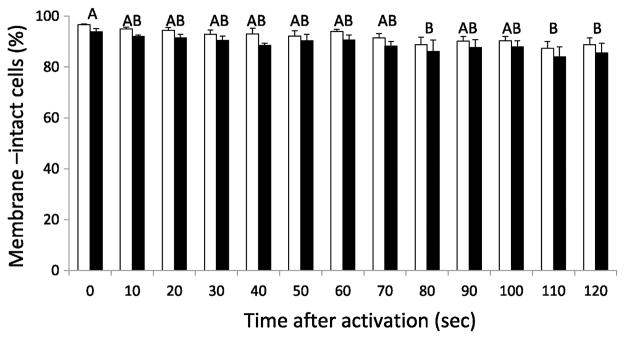
The proportion of membrane-intact cells decreased significantly after being activated for longer periods of time (ANOVA: F(12, 63) = 3.19, P = 0.0013). The ability of each staining procedure to identify sperm cells with intact or compromised membranes was significantly different (Paired-t(37) = 8.91, P < 0.0001), with the PI/SYBR-14 accounting for a higher proportion of cells with intact membranes at all times when compared with the ARD protocol (Figure 3). The average difference between protocols was 3% (± 0.3).
Figure 3.
Membrane integrity of Zebrafish sperm cells activated in 10-sec intervals, for 120 sec. Individual bars represent the means for each staining protocol (± SEM), white columns correspond to the PI/SYBR-14 protocol and black columns to the ARD protocol. Time intervals sharing superscript letters did not differ significantly. Significant differences between staining protocols were observed (P < 0.0001).
3.3. Refrigerated storage
The refrigerated storage of sperm in three different extenders led to significant differences in the proportion of membrane-intact cells during 72 hr in all of the extender treatments (Kruskal-Wallis: HBSS302, H(6) = 29.30, P < 0.0001; HBSS354, H(6) = 34.32, P < 0.0001; HBSS402, H(6) = 30.74, P < 0.0001). However, the ability of each staining procedure to identify sperm cells with intact or compromised membrane was not significantly different in any treatment (Signed Rank: HBSS302, S = −5.5, P = 0.8538; HBSS354, S = 30.5, P = 0.3003; HBSS402, S = −17, P = 0.5459) (Figure 4).
Figure 4.
Membrane integrity of Zebrafish sperm samples stored for 72 hr in HBSS at three different osmolalities: (a) 302, (b) 354 and (c) 402 mOsm/kg. Individual bars represent the means for each staining protocol (± SEM), white columns correspond to the PI/SYBR-14 protocol and black columns to the ARD protocol. Storage time intervals with common letters did not differ significantly. No significant differences between staining protocols were observed in any treatment: HBSS302 (P = 0.8538), HBSS354 (P = 0.3003) and HBSS402 (P = 0.5459).
3.4. Cryoprotectant exposure
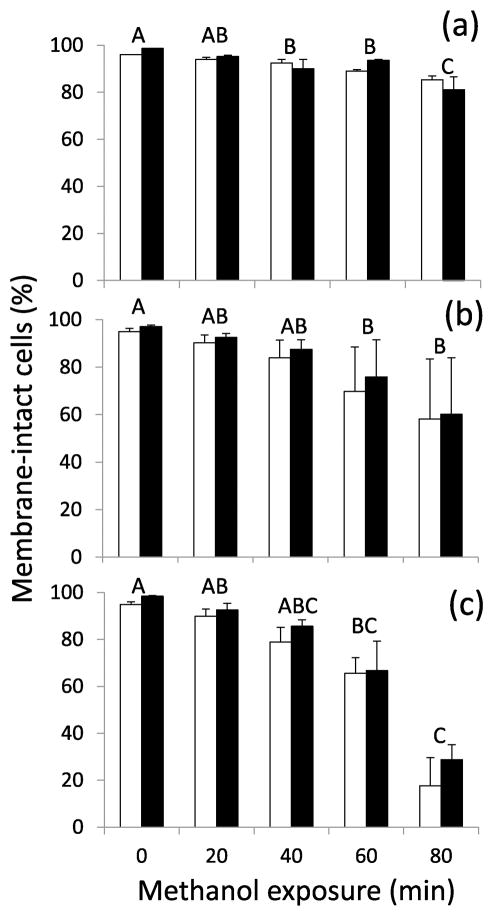
The proportion of membrane-intact cells decreased significantly as expected across time in the three methanol treatments (5% methanol, ANOVA: F(4,23) = 12.51, P <0.0001; 10% methanol, Kruskal-Wallis: H(4) = 14.34, P = 0.0063; 15% methanol, Kruskal-Wallis: H(4) = 25.63, P <0.0001). The ability of each staining procedure to identify sperm cells with intact or compromised membrane was not significantly different in the 5% methanol treatment (Paired-t(12) = −0.64, P = 0.5319), but it was significantly different for the 10% methanol (average difference of 3 ± 1%) (Signed Rank: S = −51.5, P = 0.0016) and for the 15% methanol (average difference of 6 ± 2%) (Signed Rank: S = −52, P = 0.0015) treatments, with the PI/SYBR-14 generally accounting for a lower proportion of cells with intact membrane when compared with the ARD protocol (Figure 5).
Figure 5.
Membrane integrity of Zebrafish sperm cells exposed to the cryoprotectant methanol at (a) 5%, (b) 10% and (c) 15% final concentrations. Individual bars represent the means for each staining protocol (± SEM), white columns correspond to the PI/SYBR-14 protocol and black columns to the ARD protocol. Acute toxicity time intervals with common letters did not differ significantly. Significant differences between staining protocols were observed for 10 (P = 0.0016) and 15% methanol concentrations (P = 0.0015).
3.5. Surfactant exposure
The proportion of membrane-intact cells decreased significantly as expected across time in the three Triton X-100 treatments (0.1 mM, Kruskal-Wallis: H(4) = 15.71, P = 0.0034; 0.15 mM, Kruskal-Wallis: H(4) = 21.32, P = 0.0003; 0.2 mM, ANOVA: F(4, 25) = 22.13, P <0.0001). The ability of each staining procedure to identify sperm cells with intact or compromised membrane was significantly different in all treatments (0.1 mM, Signed Rank: S = −43, P = 0.0125; 0.15 mM, Signed Rank: S = 41.5, P = 0.0017; 0.2 mM, Paired-t(14) = −4.27, P = 0.0008) (Figure 6). The average difference in the percentage of membrane-intact cells between the staining protocols was 1 (± 0%) for the 0.1 mM concentration, 3 (± 1%) for 0.15 mM, and 6 (± 2%) for 0.2 mM, with the PI/SYBR-14 generally accounting for a lower proportion of membrane-intact cells when compared with the ARD protocol.
Figure 6.
Membrane integrity of Zebrafish sperm cells exposed to Triton X-100 at (a) 0.1 mM, (b) 0.15 mM and (c) 0.2. mM final concentrations. Individual bars represent the means for each staining protocol (± SEM), white columns correspond to the PI/SYBR-14 protocol and black columns to the ARD protocol. Acute toxicity time intervals with common letters did not differ significantly. Significant differences between staining protocols were observed in all treatments: 0.1mM (P = 0.0125), 0.15 mM (P = 0.0017) and 0.2 mM (P = 0.0008).
4. Discussion
The assessment of membrane integrity is fundamental to evaluate sperm handling and cryopreservation protocols (He and Woods, 2004; Jenkins et al., 2011). Current methods are limited because they are not always sufficiently flexible to allow the use of additional fluorochromes to address other quality variables in the same sample, and they pose health concerns. Conversely, the proposed ARD method uses stains with a broad range of emission wavelengths that would facilitate quality evaluation of samples with less human health concerns.
In the present study, when sperm were exposed to a series of factors known to reduce membrane integrity, the PI/SYBR-14 and ARD methods led to equivalent results for the most part. Step-by-step along the cryopreservation pathway, from sperm collection to thawing and use, there are points where the integrity of sperm membranes is particularly vulnerable. In the present study, we focused on damage that can occur during sampling collection, refrigerated storage and equilibration (i.e., cryoprotectant addition).
One of the most common sources of contamination at the time of collection is by urine. In fish with external fertilization, sperm activation is generally triggered by an osmotic shift, and in most freshwater species this occurs when sperm cells are exposed to hypotonic solutions (Alavi and Cosson, 2006; Cosson, 2004), as is the case with urine. After sperm activation, reorganization of the lipid bilayer, subsequent changes in permeability (carp: Krasznai et al., 2000; Màriàn et al., 1993, 1997) and disruptions of the membrane (trout: Billard, 1978) have been observed due to osmotic damage. Being able to identify potentially damaged sperm in recently collected samples will allow the prompt identification of faulty procedures, and would influence the decision of keeping or discarding a particular sample. In other words, the early identification and removal of material that does not meet pre-established thresholds avoids subsequent waste and high variation in the process, assuring that the final products (i.e., thawed sperm) would meet basic quality requirements (i.e., fertilization success) (Hu et al., 2013). This is the basis of a quality assurance program and provides an extremely important quality control check point. In the present study, extended exposure to a hypotonic solution (deionized water) led to a significant decrease in the percentage of cells with intact membranes. This change was identified by each staining protocol, although their performances varied significantly by an average of 3%, with the PI/SYBR-14 consistently accounted for a higher proportion of viable cells when compared with the ARD protocol. The high degree of precision produced by both staining methods accounted for the small but significant differences (1 – 6%) between them. These differences in values are sufficiently small that they would not be biologically relevant when making quality control decisions.
After sperm samples are collected, they are typically mixed with an extender solution. Extenders, defined as solution of salts with certain osmolality and pH, are used to dilute sperm while keeping them immotile, lengthening their useable life (Renard et al., 1994; Strüssmann et al., 1994). Similarly, refrigerated storage of sperm suspended in extenders is vital to lengthen the time available between collection and use for cryopreservation or fertilization (Glenn et al., 2011; Tiersch, 2011), and typically is needed to transport samples. The extender osmolality is critical because, as mentioned earlier, this is one of the main factors known to trigger sperm activation (Alavi and Cosson, 2006; Cosson, 2004). Additionally, storage duration and temperature should be optimized to ensure the quality of the sperm (Glenn et al., 2011). In the present study, HBSS at three different osmolalities was used as an extender for refrigerated storage. The percentage of membrane-intact cells appeared similar among the three treatments during the first 8 hr of storage, and thereafter the mean percentage declined noticeably in the HBSS302 treatment followed by HBSS354 and HBSS402. The shift in the concentration of sperm with intact membranes was equally identified by both staining protocols, proving that the amine reactive dye can be a useful tool to compare the quality of fresh samples with stored (and potentially shipped) samples.
Following the mixing of sperm with the extender, sperm concentration should be estimated and adjusted (Dong et al., 2005), before the cryoprotectant is added. The concentration of cryoprotectant and the time allowed for its permeation inside the cell are critical for the preservation of the cell infrastructure and functionality (Schneider and Mazur, 1984). Given that cryoprotectants can also be toxic to cells, an acute toxicity test is recommended to choose an adequate cryoprotectant, its concentration, and a suitable equilibration time where minimal adverse effects are observed (Tiersch, 2011; Yang et al., 2007). In Zebrafish, methanol, a commonly used permeating cryoprotectant, functions to dehydrate the cell, minimizing osmotic shock and reducing the formation of intracellular ice (Meryman, 1966). Studies have shown that motility of Zebrafish sperm decreased with increasing methanol concentrations (5, 10 and 15%) and incubation time (Yang et al., 2007), observations that concur with the trends of membrane integrity observed in the present study. When sperm were exposed to 5% methanol, differences between staining procedures were not apparent, but also the decrease in the percentage of cells with impaired membranes was not as drastic (although statistically significant) as the decrease observed with the 10 and 15% methanol exposures. In all cases, the percentage of membrane-intact cells detected was mostly higher in the ARD protocol; however, the average difference between staining protocols was low (between 3% for the 10% methanol treatment, and 6% for the 15% methanol treatment).
Overall, the results suggest that the amine reactive dye was effective at identifying sperm cells with compromised membranes during critical steps of the cryopreservation process. In addition to the scenarios already described, a non-ionic surfactant commonly used to lyse or permeabilize living cells was used to further investigate the capabilities of the ARD protocol. Triton X-100 disrupts hydrogen bonds within the lipid bilayer, compromising the integrity of lipid membranes (Koley and Bard, 2010). When sperm cells were exposed to Triton X-100, a significant decrease in the percentage of viable cells was observed in all treatments, being most pronounced in the 0.15 and 0.2 mM concentrations. As was observed with the methanol exposure, ARD identified a higher percentage of membrane-intact cells as compared to PI/SYBR-14, and the average difference between protocols was statistically significant but low (between 1% for the 0.1 mM Triton X-100 treatment, and 6% for the 0.2 mM Triton X-100 treatment).
As described earlier, fish sperm cryopreservation comprises multiple steps. To assure the reproducibility of the process, a series of quality evaluation check points should be established (Hu et al., 2013; Baker, 2016). Some quality assessments, however, can be challenging. At the time of sample collection, for example, variables such as milt volume and sperm motility can be evaluated immediately. Others, such as membrane integrity, typically have to wait until the samples are transported to facilities with suitable equipment, and therefore they tend to be neglected, excluding an important piece of information. Another benefit of the ARD method – tested in somatic mammalian cells – is that it offers the possibility of cell fixation, in other words, after cells were labeled, they could be fixed and preserved for later analysis (Perfetto et al., 2010), without compromising the staining pattern (https://www.thermofisher.com/order/catalog/product/L34972?ICID=search-l34972, accessed on March 15, 2015). To our knowledge, the combination of ARD and cell fixation has not been tested with fish sperm. The potential of cell fixation when analyzing membrane integrity in fish sperm will not only provide crucial information about fresh samples, but it can also be an innovative approach to provide access to membrane integrity data for laboratories lacking their own flow cytometry capabilities. Therefore, this method can lead to a comprehensive evaluation of cryopreservation protocols, and to the strengthening and standardization of overall quality control strategies. Given the expanding emphasis that is being placed on strategies to assure reproducibility in science overall (Bandrowski and Martone, 2016; Nuzzo, 2015), the evaluation of cell fixatives suitable for fish sperm provides a valuable alternative to the current limited methods, especially when making comparisons among laboratories.
4.1. Conclusions
The present study demonstrated that the ability of the ARD method to assess membrane integrity in fish sperm was comparable to the commonly used PI/SYBR-14 method. Significant differences between the outcomes of both methods were observed when sperm were activated and when it was exposed to methanol and Triton X-100; the average differences in each case, however, were low (between 1 and 6%), and functionally irrelevant given that the criteria to accept or reject a freshly collected sample are generally based on much higher thresholds. The ARD method proved to be a feasible alternative to evaluate fish sperm membrane integrity, offering additional features such as a variety of stains that cover most of the visible and UV spectrum (allowing the analysis of additional quality parameters if needed), safety in handling, and potential use in combination with cell fixatives to preserve the cells for later analysis. Quality assessment of fresh sperm samples would contribute greatly to solve a critical flaw present in almost all cryopreservation studies, which is the lack of reproducibility (Torres et al., 2016). For all these characteristics, the ARD method has the potential to contribute greatly to the standardization of cryopreservation, and establishment of quality assurance programs in aquatic species.
Highlights.
This amine reactive dye method proved to be a feasible alternative to the propidium iodide and SYBR-14 method for evaluation of fish sperm membrane integrity.
Amine reactive dyes bind irreversibly to the cell amine groups (allowing a less strict incubation time), come in a variety of stains that cover most of the visible and UV spectrum, and are safe to handle.
The Amine reactive dye method has the potential to contribute greatly to the standardization of cryopreservation, and establishment of quality assurance for repository development in aquatic species.
Acknowledgments
This research was supported in part by the National Institutes of Health, Office of Research Infrastructure Programs (R24RR023998 and R24OD011120). We thank J. Jenkins for input on the preliminary validation of the flow cytometry method, and T. Lee for assistance in the laboratory. This manuscript was approved by the director of the Louisiana Agricultural Experiment Station as manuscript number 2015-241-24843.
Abbreviations
- ARD
amine reactive dye
- HBSS
Hanks’ balanced salt solution
- PI
propidium iodide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavi SMH, Cosson J. Sperm motility in fishes. II. Effects of ions and osmolality: a review. Cell Biol Int. 2006;30:1–14. doi: 10.1016/j.cellbi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Baker M. How quality control could save your science. Nature. 2016;529:456–458. doi: 10.1038/529456a. [DOI] [PubMed] [Google Scholar]
- Bandrowski AE, Martone ME. RRIDs: a simple step toward improving reproducibility through rigor and transparency of experimental methods. Neuron. 2016;90:434–436. doi: 10.1016/j.neuron.2016.04.030. http://dx.doi.org/10.1016/j.neuron.2016.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BD Biosciences. BD Accuri™ C6 Flow Cytometer Instrument Manual, Science is hard, flow cytometry should be easy. 2012 7820018-01 Rev-2. [Google Scholar]
- Billard R. Changes in structure and fertilizing ability of marine and fresh water fish spermatozoa diluted in media of various salinities. Aquaculture. 1978;14:187–198. doi: 10.1016/0044-8486(78)90094-7. [DOI] [Google Scholar]
- Corliss DA, White WE. Fluorescence of yeast vitally stained with ethidium bromide and propidium iodide. J Histochem Cytochem. 1981;29:45–48. doi: 10.1177/29.1.6162881. 45 0022-1554/81/01004504. [DOI] [PubMed] [Google Scholar]
- Cosson J. The ionic and osmotic factors controlling motility of fish spermatozoa. Aquacult Int. 2004;12:69–85. doi: 10.1023/B:AQUI.0000017189.44263.bc. [DOI] [Google Scholar]
- Daly J, Tiersch TR. Sources of variation in flow cytometric analysis of aquatic species sperm: The effect of cryoprotectants on flow cytometry scatter plots and subsequent population gating. Aquaculture. 2012;370–371:179–188. doi: 10.1016/j.aquaculture.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Q, Eudeline B, Huang C, Tiersch TR. Standardization of photometric measurement of sperm concentration from diploid and tetraploid Pacific oysters, Crassostrea gigas (Thunberg) Aquac Res. 2005;36:86–93. doi: 10.1111/j.1365-2109.2004.01188.x. [DOI] [Google Scholar]
- Elliot AC, Hynan LS. A SAS macro implementation of a multiple comparison post-hoc test for a Kruskal-Wallis analysis. Comput Meth Prog Bio. 2011;102:75–80. doi: 10.1016/j.cmpb.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Fukunaga M, Yielding KL. Propidium: induction of petites and recovery from ethidium mutagenesis in Saccharomyces cerevisiae. Biochem Bioph Res Co. 1978;84:501–507. doi: 10.1016/0006-291X(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Garner DL, Johnson LA, Yue ST, Roth BL, Haugland RP. Dual DNA staining assessment of bovine sperm viability using SYBR-14 and propidium iodide. J Androl. 1994;15:620–629. doi: 10.1002/j.1939-4640.1994.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Glenn DW, III, Lang RP, Tiersch TR. Evaluation of extenders for refrigerated storage of Koi Carp and Goldfish sperm. In: Tiersch TR, Green CC, editors. Cryopreservation in Aquatic Species. 2. World Aquaculture Society; Baton Rouge: 2011. pp. 107–124. [Google Scholar]
- He S, Woods LC., III Effects of dimethyl sulfoxide and glycine on cryopreservation induced damage of plasma membranes and mitochondria to Striped Bass (Morone saxatilis) sperm. Cryobiology. 2004;48:254–262. doi: 10.1016/j.crybiol.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Horváth Á, Wayman WR, Dean JC, Urbányi B, Tiersch TR, Mims SD, Johnson D, Jenkins JA. Viability and fertilizing capacity of cryopreserved sperm from three North American acipenseriform species: a retrospective study. J Appl Ichthyol. 2008;24:443–449. doi: 10.1111/j.1439-0426.2008.01134.x. [DOI] [Google Scholar]
- Hu E, Liao TW, Tiersch TR. A quality assurance initiative for commercial-scale production in high-throughput cryopreservation of Blue Catfish sperm. Cryobiology. 2013;67:214–224. doi: 10.1016/j.cryobiol.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins JA, Eilts BE, Guitreau AM, Figiel CR, Draugelis-Dale RO, Tiersch TR. Sperm quality assessments for endangered Razorback Suckers Xyrauchen texanus. Reproduction. 2011;141:55–65. doi: 10.1530/REP-10-0153. [DOI] [PubMed] [Google Scholar]
- Jenkins JA, Olivier HM, Draugelis-Dale RO, Eilts BE, Torres L, Patiño R, Nilsen E, Goodbred SL. Assessing reproductive and endocrine parameters in male largescale suckers (Catostomus macrocheilus) along a contaminant gradient in the lower Columbia River, USA. Sci Total Environ. 2014;484:365–378. doi: 10.1016/j.scitotenv.2013.09.097. [DOI] [PubMed] [Google Scholar]
- Koley D, Bard AJ. Triton X-100 concentration effects on membrane permeability of a single HeLa cell by scanning electrochemical microscopy (SECM) PNAS. 2010;107:16783–16787. doi: 10.1073/pnas.1011614107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasznai Z, Màriàn T, Izumi H, Damjanovich S, Balkay L, Tron L, Morisawa M. Membrane hyperpolarization removes inactivation of Ca2+ channels, leading to Ca2+ influx and subsequent initiation of sperm motility in the Common Carp. PNAS. 2000;97:2052–2057. doi: 10.1073/pnas.040558097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Life Technologies. Red fluorescent reactive dye *for 488 nm excitation*, SDS No. L34972A [Online] Life Technologies; Carlsbad, CA: 2014. [accessed November 8, 2015]. https://tools.thermofisher.com/content/sfs/msds/2014/L34972A_MTR-NALT_EN.pdf. [Google Scholar]
- Màriàn T, Krasznai Z, Balkay L, Balazs M, Emri M, Bene L, Tron L. Hypoosmotic shock induces an osmolality dependent permeabilization and structural changes in the membrane of carp sperm. J Histochem Cytochem. 1993;41:291–297. doi: 10.1177/41.2.8419464. [DOI] [PubMed] [Google Scholar]
- Màriàn T, Krasznai Z, Balkay L, Balazs M, Emri M, Tron L. Role of extracellular and intracellular pH in Carp sperm motility and modifications by hyperosmosis or regulation of the Na+/H+ exchanger. Cytometry. 1997;27:374–382. doi: 10.1002/(SICI)1097-0320(19970401)27:4<374::AID-CYTO9>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Meryman HT. Review of biological freezing. In: Meryman HT, editor. Cryobiology. Academic Press Inc; New York: 1966. pp. 1–114. [Google Scholar]
- Nuzzo R. How scientists fool themselves – and how they can stop. Nature. 2015;526:182–185. doi: 10.1038/526182a. [DOI] [PubMed] [Google Scholar]
- Ogier De Baulny B, Le Bern Y, Kerboeuf D, Maisse G. Flow cytometric evaluation of mitochondrial activity and membrane integrity in fresh and cryopreserved Rainbow Trout (Oncorhynchus mykiss) spermatozoa. Cryobiology. 1997;34:141–149. doi: 10.1006/cryo.1996.1992. [DOI] [Google Scholar]
- Ogier De Baulny B, Labbe C, Maisse G. Membrane integrity, mitochondrial activity, ATP content, and motility of the European Catfish (Silurus glanis) testicular spermatozoa after freezing with different cryoprotectants. Cryobiology. 1999;39:177–184. doi: 10.1006/cryo.1999.2200. [DOI] [PubMed] [Google Scholar]
- Perfetto SP, Chattopadhyay PK, Lamoreaux L, Nguyen R, Ambrozak D, Koup RA, Roederer M. Amine-reactive dyes for dead cell discrimination in fixed samples. Curr Protocol Cytom. 2010;9(34):1–20. doi: 10.1002/0471142956.cy0934s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard P, Strüssmann CA, Ling H, Takashima F. Evaluation of extenders for Pejerrey Odontesthes bonariensis sperm. Fisheries Sci. 1994;60:661–666. [Google Scholar]
- Schneider U, Mazur P. Osmotic consequences of cryoprotectant permeability and its relation to the survival of frozen-thawed embryos. Theriogenology. 1984;21:68–79. doi: 10.1016/0093-691X(84)90307-8. [DOI] [Google Scholar]
- Segovia M, Jenkins JA, Paniagua-Chavez C, Tiersch TR. Flow cytometric evaluation of antibiotic effects on viability and mitochondrial of refrigerated spermatozoa of Nile tilapia. Theriogenology. 2000;53:1489–1499. doi: 10.1016/S0093-691X(00)00291-0. [DOI] [PubMed] [Google Scholar]
- Sigma-Aldrich . Propidium iodide, SDS No. 81845 [Online] Sigma Aldrich; Saint Louis, MO: 2014. [accessed November 8 2015]. http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=81845&brand=SIAL&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Fterm%3D81845%26interface%3DAll%26N%3D0%26mode%3Dmatch%2520partialmax%26lang%3Den%26region%3DUS%26focus%3Dproduct. [Google Scholar]
- Strüssmann CA, Renard P, Ling H, Takashima F. Motility of Pejerrey Odontesthes bonariensis spermatozoa. Fisheries Sci. 1994;60:9–13. [Google Scholar]
- Tiersch TR. Process pathways for cryopreservation research, application and commercialization. In: Tiersch TR, Green CC, editors. Cryopreservation in Aquatic Species. 2. World Aquaculture Society; Baton Rouge: 2011. pp. 646–671. [Google Scholar]
- Torres L, Hu E, Tiersch TR. Cryopreservation in fish: current status and pathways to quality assurance and quality control in repository development. Reprod Fertil Dev. 2016 doi: 10.1071/RD15388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trigo P, Merino O, Figueroa E, Valdebenito I, Sanchez R, Risopatron J. Effect of short-term semen storage in salmon (Oncorhynchus mykiss) on sperm functional parameters evaluated by flow cytometry. Andrologia. 2015;47:407–411. doi: 10.1111/and.12276. [DOI] [PubMed] [Google Scholar]
- Yang H, Carmichael C, Varga ZM, Tiersch TR. Development of a simplified and standardized protocol with potential for high-throughput for sperm cryopreservation in zebrafish Danio rerio. Theriogenology. 2007;68:128–136. doi: 10.1016/j.theriogenology.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 5. Pearson Prentice Hall; Upper Saddle River: 2010. [Google Scholar]