Abstract
An increased aspartate aminotransferase-to-alanine aminotransferase ratio (AAR) has been widely used as a marker of advanced hepatic fibrosis. Increased AAR was also shown to be significantly associated with the risk of developing cardiovascular (CV) disease. The aim of this study was to assess the relationship between the AAR and mortality risk in a well-characterized cohort of patients with type 2 diabetes.
A cohort of 2529 type 2 diabetic outpatients was followed-up for 6 years to collect cause-specific mortality. Cox regression analyses were modeled to estimate the independent association between AAR and the risk of all-cause and CV mortality.
Over the 6-year follow-up period, 12.1% of patients died, 47.5% of whom from CV causes. An increased AAR, but not its individual components, was significantly associated with an increased risk of all-cause (adjusted-hazard risk 1.83, confidence interval [CI] 95% 1.14–2.93, P = 0.012) and CV (adjusted-hazard risk 2.60, CI 95% 1.38–4.90, P < 0.003) mortality after adjustment for multiple clinical risk factors and potential confounding variables.
The AAR was independently associated with an increased risk of both all-cause and CV mortality in patients with type 2 diabetes. These findings suggest that an increased AAR may reflect more systemic derangements that are not simply limited to liver damage. Further studies are needed to elucidate the pathophysiological implications of an increased AAR.
Keywords: AST/ALT ratio, liver diseases, mortality, type 2 diabetes
1. Introduction
The aspartate aminotransferase (AST)-to-alanine aminotransferase (ALT) ratio (AAR) is also referred to as the De Ritis ratio from the author who first described it.[1] The release of AST and ALT from hepatocytes is largely due to hepatocellular damage or death.[1 2] These 2 enzymes are normally released at a constant rate with their usual levels in health being the equilibrium between the normal turnover of hepatocytes due to programmed cell death and the clearance of these enzymes from plasma.[3] ALT is present prevalently in the hepatocyte cytoplasm, whereas AST is present in both hepatocyte cytoplasm and mitochondria.[4 5] Functionally, AST and ALT are 2 important metabolic links between carbohydrate and protein metabolism.[6] In particular, AST is vital for the aerobic glycolysis by allowing the nicotinamide adenine dinucleotide–hydrogen (NADH) that has been generated in the cytoplasm to be effectively relocated within the mitochondria through the shuffling of malate (as well as α-ketoglutarate, aspartate, and glutamate).[1 6] Although aminotransferase activity is important in all cells with high metabolic activity, it is more relevant for the liver and muscle cells.[6] With a hepatic proportion of AST/ALT approximating 2.5:1, a higher level of AST would be expected in serum. However, due to different removal rates, the resulting levels of AST and ALT are fairly similar, leading to an AAR of about 1 in healthy subjects.[7] Importantly, in healthy subjects the circulating AST levels consist mainly of cytoplasmic AST, which probably originates from cytoplasmic leakage.[7 8] Conversely, whenever hepatic cellular death is increased beyond the usual “background” levels, the circulating AST values compared with those of ALT tend to reflect more accurately the hepatic proportion where AST is more than twice as prevalent as ALT.[9] Therefore, the use of AAR through the reciprocal modification of both these enzymes may reflect a certain degree of damage to hepatocytes, including mitochondrial sufferance.[10]
Clinically, as reported by Angulo,[11] an AAR >1 confers a 4.3-fold increased risk of severe liver fibrosis. A retrospective study involving patients with chronic hepatitis C virus demonstrated that an increased AAR was correlated with the histologic and clinical severity of disease.[12] Importantly, progressive liver impairment may be reflected by an increased AAR.[13] However, not all studies are in agreement and the prognostic information of an increased AAR seems to be similar to that provided by other established prognostic scores in cirrhotic patients.[14–16] In a sample of patients with non-alcoholic steatohepatitis, an AAR of ≥1 strongly suggested the presence of cirrhosis.[17] Recently, an increased AAR was also found to be independently associated with the risk of developing cardiovascular (CV) diseases, especially in men.[18]
To our knowledge, no data are currently available about the relationship between the AAR and risk of all-cause and CV mortality in patients with type 2 diabetes. Therefore, the main aim of this prospective study was to evaluate the association between the AAR and mortality risk in a well-characterized cohort of outpatients with type 2 diabetes.
2. Materials and methods
In this study, we enrolled a cohort of 2529 outpatients with type 2 diabetes (mean age 70 years; 1373 men), who had prospective data on the rates of mortality and its specific causes as derived from anonymized electronic medical records. Patients were classified as having type 2 diabetes when the diagnosis of this disease was made after the age of 35 years, irrespective of diabetes treatment, or when the patients were treated with diet or oral hypoglycemic drugs, irrespective of the age at diagnosis. The inclusion criteria of the study were to have data on serum aminotransferase levels in the electronic database. Patients with serum aminotransferase levels higher than 3 times the upper limit of the reference range for our local laboratory were excluded from the analysis (in order to exclude patients with acute hepatitis or muscular lesions). Patients with a known history of drug-induced liver injury, viral hepatitis, cirrhosis of any aetiology, and hemochromatosis were also excluded from the study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Informed consent for this study was not obtained (and was exempted by the local ethics committee) because de-identified data were analyzed.
Information on diabetes history, diabetes duration, medication use, previous diseases, and clinical variables was obtained by the electronic database. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Blood pressure was measured with a standard mercury manometer.
In all patients venous blood was withdrawn in the morning after an overnight fast for standard biochemical testing. Serum aminotransferases, creatinine, triglycerides, total and high density lipoprotein (HDL) cholesterol were determined by standard reference procedures (DAX 96; Siemens Diagnostics, Milan, Italy). More specifically, both AST and ALT were measured using the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) recommended methods, with pyridoxal phosphate at 37 °C.[19 20] The upper limit of the reference range, as for local technique and instrumentation, was 41 U/L for AST and 54 U/L for ALT, respectively. Low-density lipoprotein (LDL)-cholesterol was calculated using the Friedewald's equation. Hemoglobin A1c was measured by an automatic high-performance liquid chromatography analyzer (Bio-Rad Diamat, Milan, Italy), with an upper limit of normality fixed at 5.8%. Glomerular filtration rate was estimated (eGFR) from serum creatinine values using the chronic kidney disease epidemiology collaboration (CKD-EPI) equation.[21] Urinary albumin was measured by an immuno-nephelometric method (BN, Siemens Diagnostics, Milan, Italy) on a 24-hour sample.[22]
Patients were considered to have hypertension if systolic blood pressure was >140 mm Hg and/or diastolic blood pressure was >90 mm Hg, or if they were taking anti-hypertensive agents.
Vital status was ascertained by examining the electronic database of the Social Health Unit of the Veneto Region, which included all records of mortality that occurred within the Veneto Region as well as the individual causes of mortality. The ascertainment at the end of the 6-year follow-up period was 96% complete. Subjects who moved and could not be traced were lost at follow-up, so that they were not included in the final statistical analysis. Causes of death were identified by reviewing death certificates, which were available in 100% of those who died. Death certificates were coded by trained nosologists using the International Classification of Diseases, Ninth Revision (ICD-9). The death was attributed to cardiovascular causes when ICD-9 codes were 390 to 459.
2.1. Statistical analysis
Patients were stratified in three groups according to the distribution of the AAR (Fig. 1), as follows: first group AAR = 1; second group AAR <1 and third group AAR >1. Prior to analyses, skewed variables (i.e., AST, ALT, and albuminuria) were logarithmically transformed to improve normality. One-way analysis of variance, Student t test and chi-squared test with Yates correction for continuity were used to compare clinical and biochemical features. Survival analysis was performed by Kaplan–Meier curves and the statistical significance estimated by the log-rank test. Cox proportional-hazards model was used to assess the independent association of the AAR (included as either continuous or categorical variable) with risk of both all-cause and CV mortality. Three forced entry multivariate Cox regression models were performed. The first one unadjusted; the second model adjusted for sex, age, and BMI; and, finally, a fully adjusted regression model that included AAR, age (years), sex (man vs. woman), BMI (kg/m2), hemoglobin A1c (%), diabetes duration (years), hypertension (yes/no), LDL-cholesterol (mmol/L), e-GFREPI (mL/min/1.73 m2), albuminuria (mg/24 hour), retinopathy (yes/no), and use of medications (anti-platelet drugs and insulin) (yes/no). The covariates included in these regression models were chosen as potential confounding factors based on their biological plausibility or according to their statistical association with mortality in univariate analyses. Results are presented as hazard ratios (HR) with 95% confidence intervals (CI) and statistical significance was evaluated by the likelihood-ratio test. Statistical analyses were performed with the statistical package SPSS 14.0. P values <0.05 were considered statistically significant.
Figure 1.

In the upper part of the figure is reported the distribution of the AST/ALT ratio (AAR), showing that most of our type 2 diabetic patients had a ratio =1. In the lower part of the figure are reported the dispersion graphs of the relationship between the AAR and either ALT or AST levels. AAR = aspartate aminotransferase-to-alanine aminotransferase ratio, ALT = alanine aminotransferase, AST = aspartate aminotransferase.
3. Results
3.1. AAR distribution
During the 6 years of follow-up 305 (12.1%) patients died, 145 (47.5%) of whom from CV causes. Given the AAR distribution in our cohort (Fig. 1), characterized by a large group of patients with AAR equal to 1 and 2 other groups with AAR below and above 1, we decided to stratify the study cohort in 3 groups: a first group with AAR equal to 1, a second group with AAR <1, and a third group with AAR >1. The other 2 panels in Fig. 1 show the mutual variations of both AST and ALT generating the AAR.
3.2. Clinical and demographic characteristics
The baseline clinical and biochemical characteristics of patients stratified by the AAR cutoff points are shown in Table 1. Patients with AAR <1 were younger, had higher BMI, higher HbA1c, and shorter duration of diabetes than those with AAR >1. Moreover, patients with AAR <1 had higher levels of eGFR, triglycerides, and lower HDL-cholesterol. Albuminuria, prevalence of retinopathy, and the main pharmacological treatments did not significantly differ among the 3 groups of patients.
Table 1.
Baseline clinical and biochemical characteristics of type 2 diabetic outpatients (n = 2529) stratified according to the AST/ALT ratio categories.

As reported in Table 2, at baseline the decedents were more likely to be older, lean, and to have a longer duration of diabetes compared with survivors. In addition, they also had lower eGFR, higher albuminuria, higher HbA1c, and a greater prevalence of diabetic retinopathy. Notably, decedents also had higher AAR and lower ALT values. Finally, oral hypoglycemic drugs and insulin were more frequently used in decedents, while lipid-lowering drugs were less frequently used.
Table 2.
Baseline clinical and biochemical characteristics of type 2 diabetic outpatients (n = 2529) stratified according to their vital status.
3.3. Comparisons of AAR with its single components on mortality
We addressed the question of how the AAR retained independent significance compared with its single components (i.e., ALT and AST). As reported in Table 3, the AAR was positively associated with an increased risk of all-cause and CV mortality in univariate analysis and even after adjustment for age, sex, and BMI (HR 2.05, CI 95% 1.56–2.67, P < 0.001; HR 3.11, CI 95% 2.34–4.19, P < 0.001, respectively), while serum ALT was inversely associated with the risk of all-cause and cardiovascular mortality only in univariate analysis (HR 0.48, CI 95% 0.37–0.62, P < 0.001; HR 0.34, CI 95% 0.22–0.46, P < .001). On the contrary, serum AST was not associated with mortality risk neither in univariate nor in multivariate analyses (Table 3).
Table 3.
Cox multivariate models of the effect of the AST/ALT ratio on the risk of all-cause mortality in outpatients with type 2 diabetes (n = 2529).
3.4. Survival analysis
As shown in Fig. 2, the cumulative survival probability of both all-cause mortality and CV mortality was significantly worse in patients with AST/ALT ratio higher than 1. The global statistical significance was estimated by log-rank test (P < 0.001 for both analyses).
Figure 2.
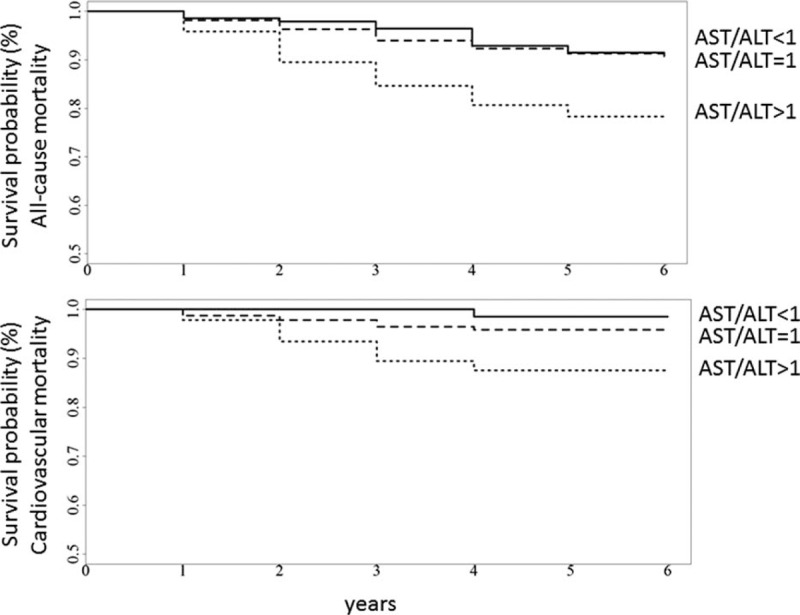
Kaplan–Meier curves according to AAR categories in 2529 patients with type 2 diabetes. In the upper panel is reported the cumulative survival probability of all-cause mortality, whereas in the lower panel is reported the cumulative survival probability of CV mortality. The global statistical significance was estimated by log-rank test (P < 0.001 for both analyses). AAR = aspartate aminotransferase-to-alanine aminotransferase ratio, CV = cardiovascular.
3.5. Multivariate Cox regression analyses
As the AAR maintained a statistical significance with mortality risk beyond its single components, we further tested the independent association of the AAR with the risk of both all-cause and CV mortality in multivariate regression models that included many clinical risk factors and potential confounding factors as shown in Table 4. Notably, increased AAR remained a significant predictor of all-cause (HR 1.83, CI 95% 1.14–2.93, P = 0.012) and CV mortality (HR 2.60, CI 95% 1.38–4.90, P < 0.003) even after adjustment for multiple risk factors. When the AAR was also modeled as a categorical variable in the Cox regression model adjusted for age, sex, and BMI (with AAR =1 as the reference category), the ARR <1 showed an HR value of 1.52 (CI 95%, 0.82–2.80) for all-cause mortality, while the AAR >1 showed an HR value of 2.50 (CI 95%, 1.34–4.64) that was highly significant (P < 0.001).
Table 4.
Cox multivariate models of the effect of the AST/ALT ratio on the risk of all-cause and cardiovascular mortality in outpatients with type 2 diabetes (n = 2529).
4. Discussion
The main finding of our study was the positive and independent association of the AAR, beyond its single components, with the risk of both all-cause and CV mortality in patients with type 2 diabetes. The significant association between increased AAR and the risk of both all-cause and CV mortality persisted even after adjustment for multiple potential confounders in the Cox multivariate models. An increase in 1 unit of AAR was associated with an approximately 2-fold increased risk of all-cause and CV mortality. Moreover, when the AAR was modeled as categorical variable, a J shaped curve delineated the relationship between the AAR and mortality risk with patients with AAR >1 being at the highest risk of mortality. Although the relationship between serum transaminase levels and mortality has been extensively evaluated,[23] the AAR has been mainly used as a marker of the severity of chronic liver diseases, including alcoholic and non-alcoholic liver diseases and chronic viral hepatitis C,[24–30] but not of autoimmune hepatitis where the AAR was also associated with an increased risk of mortality.[31] As suggested by some studies, increasing AAR may be considered a reliable marker of the severity of liver fibrosis. Accordingly, an increased AAR has been demonstrated as a marker of hepatic fibrosis also in patients with type 2 diabetes.[32] However, a recent prospective cohort of 29,316 primary care patients reported that increased AAR was significantly associated with an increased risk of developing cardiovascular disease, especially in men.[18] In this study, 17% of subjects were diabetics.[18] Therefore, our findings indicate that an increased AAR may be a marker of increased risk of cardiovascular mortality also in type 2 diabetic patients. This finding may have clinical implications as in this era of stratified medicine, patients with AAR >1 may represent a subgroup of individuals at higher risk of mortality who deserve closer monitoring.
The putative mechanisms underlying the observed relationship between increased AAR and mortality are not entirely understood. We postulate that different mechanisms can exist. In general, the AAR, as a marker of severity of chronic liver diseases, may aggregate patients more complicated and with a higher likelihood of death. However, the AAR showed to be positively associated with the risk of both all-cause and CV mortality even after adjustment for many clinical risk factors, thus implying a specific role for AAR. The serum aminotransferase levels should not be considered as simple biomarkers of underlying liver damage, but as key players in more systemic conditions such as metabolic syndrome, sarcopenia, and enhanced oxidative stress, which are all risk factors for mortality.[6 33] Therefore, an AAR >1 may reflect systemic alterations, beyond liver diseases, which are associated with an increased risk of mortality, especially for CV causes. ALT is mainly located in the cytoplasm, while AST is present both in the cytoplasm and in the mitochondria.[1] It may be speculated that the AAR may somehow reflect a dysfunction at the mitochondrial level, generating or reflecting an increased oxidative stress. A recent study performed in patients with hepatitis C showed a significant relationship between liver oxidative balance and serum aminotransferase levels.[34] Of interest, rats with fructose-induced liver damage showed a higher AAR coupled with a reduced aerobic capacity compared with control rats. In these animals, markers of oxidative stress were also increased.[35] Similar findings have been reported in subjects with alcoholic hepatitis, who showed a parallel increase both in the AAR and in oxidative stress markers. In this study, AST levels were increased compared with ALT, and this is likely attributable to the release of AST by mitochondria.[36] Therefore, it is plausible to speculate that increased AAR may be related to an increased oxidative stress that is potentially responsible for the increased risk of CV mortality.
Another possible interpretation of our results could be a selective reduction of serum ALT levels in the elderly. A previous study in 455 70-year-old ambulatory individuals who were followed-up for 12 years showed an association between decreased serum ALT levels and risk of mortality, especially in men. However, in multivariate regression analyses the strongest predictors of mortality were the presence of diabetes, chronic kidney failure, and malignancies.[37] In our study, patients with AAR >1 were older and, as shown in Fig. 1, the reduction in serum ALT levels might be responsible for the increased AAR, thus reflecting hepatic ageing. Again, it is known that hepatic ageing is associated with a higher generation of free radicals, so leading to a greater oxidative stress. Nevertheless, it should be noted that in our study the AAR, not ALT alone, was independently associated with the risk of mortality, thus implying that the AAR may provide more prognostic information respect to its individual components.
Finally, in our study the AAR was found to be negatively correlated to BMI (r = −0.113, P < 0.001), that is, the higher AAR the lower the BMI of patients. Lower BMI may be indicative of both worse nutritional state and/or sarcopenia in this age group, both conditions strictly associated with predisposition to increased mortality.
Collectively, therefore, the results of our study suggest that the AAR could be considered a systemic marker of different conditions that may concur to increase the risk of all-cause and CV mortality, possibly through oxidative damages. Aminotransferase activity may be important not only as a marker of liver diseases but also as an indicator of cardiovascular health.[38]
Our study has several strengths, including its prospective design, the large number of participants, the relatively long duration of the follow-up period, and the ability to adjust for multiple important risk factors and potential confounders. However, there are some important limitations that should be noted. Firstly, our Cox regression models are based on single measurements of serum aminotransferase levels at baseline. Since all participants were outpatients with type 2 diabetes, the results are not generalizable to other groups of patients. Moreover, we could not stratify our patients for the severity of steatosis. Finally, measurements of oxidative stress biomarkers were not available. Future studies are needed to better elucidate the mechanistic pathways underlying the association between AAR and mortality risk.
In conclusion, the results of our study suggest that increased AAR is independently associated with an increased risk of both all-cause and CV mortality in patients with type 2 diabetes. The AAR may underline more systemic alterations, such as increased oxidative stress, that may be responsible for the observed increased risk of mortality in this particularly high-risk patient population.
Footnotes
Abbreviations: AAR = aspartate aminotransferase-to-alanine aminotransferase ratio, BMI = body mass index, CI = confidence intervals, CKD-EPI = chronic kidney disease epidemiology collaboration, CV = cardiovascular, eGFR = glomerular filtration rate was estimated, HCV = hepatitis C virus, ICD-9 = International Classification of Diseases-Ninth Revision, IFCC = International Federation of Clinical Chemistry and Laboratory Medicine, NADH = nicotinamide adenine dinucleotide–hydrogen.
Authors Contributions: GZ, GT, conception, design and analysis and interpretation of data; GZ, drafting the manuscript; GT, GL, EB, revising critically the manuscript for important intellectual content; VC, CN, VS, they contributed to the analysis and interpretation of data.
The authors have no conflicts of interest to disclose.
References
- 1. Botros M, Sikaris KA. The de Ritis ratio: the test of time. Clin Biochem Rev 2013; 34:117–130. [PMC free article] [PubMed] [Google Scholar]
- 2. Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 2000; 342:1266–1271. [DOI] [PubMed] [Google Scholar]
- 3. Horiuchi S, Kamimoto Y, Morino Y. Hepatic clearance of rat liver aspartate aminotransferase isozymes: evidence for endocytotic uptake via different binding sites on sinusoidal liver cells. Hepatology 1985; 5:376–382. [DOI] [PubMed] [Google Scholar]
- 4. Glinghammar B, Rafter I, Lindström AK, et al. Detection of the mitochondrial and catalytically active alanine aminotransferase in human tissues and plasma. Int J Mol Med 2009; 23:621–631. [DOI] [PubMed] [Google Scholar]
- 5. Jiang X, Chang H, Zhou Y. Expression, purification and preliminary crystallographic studies of human glutamate oxaloacetate transaminase 1 (GOT1). Protein Expr Purif 2015; 113:102–106. [DOI] [PubMed] [Google Scholar]
- 6. Sookoian S, Pirola CJ. Liver enzymes, metabolomics and genome-wide association studies: from systems biology to the personalized medicine. World J Gastroenterol 2015; 21:711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kamimoto Y, Horiuchi S, Tanase S, et al. Plasma clearance of intravenously injected aspartate aminotransferase isozymes: evidence for preferential uptake by sinusoidal liver cells. Hepatology 1985; 5:367–375. [DOI] [PubMed] [Google Scholar]
- 8. Lindblom P, Rafter I, Copley C, et al. Isoforms of alanine aminotransferases in human tissues and serum—differential tissue expression using novel antibodies. Arch Biochem Biophys 2007; 466:66–77. [DOI] [PubMed] [Google Scholar]
- 9. Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology 2003; 125:437–443. [DOI] [PubMed] [Google Scholar]
- 10. Kojima H, Sakurai S, Uemura M, et al. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res 2007; 31:S61–S66. [DOI] [PubMed] [Google Scholar]
- 11. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002; 346:1221–1231. [DOI] [PubMed] [Google Scholar]
- 12. Sheth SG, Flamm SL, Gordon FD, et al. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998; 93:44–48. [DOI] [PubMed] [Google Scholar]
- 13. Giannini E, Botta F, Fasoli A, et al. Progressive liver functional impairment is associated with an increase in AST/ALT ratio. Dig Dis Sci 1999; 44:1249–1253. [DOI] [PubMed] [Google Scholar]
- 14. Reedy DW1, Loo AT, Levine RA. AST/ALT ratio > or =1 is not diagnostic of cirrhosis in patients with chronic hepatitis C. Dig Dis Sci 1998; 43:2156–2159. [DOI] [PubMed] [Google Scholar]
- 15. Imperiale TF, Said AT, Cummings OW, et al. Need for validation of clinical decision aids: use of the AST/ALT ratio in predicting cirrhosis in chronic hepatitis C. Am J Gastroenterol 2000; 95:2328–2332. [DOI] [PubMed] [Google Scholar]
- 16. Giannini E, Botta F, Testa E, et al. The 1-year and 3-month prognostic utility of the AST/ALT ratio and model for end-stage liver disease score in patients with viral liver cirrhosis. Am J Gastroenterol 2002; 97:2855–2860. [DOI] [PubMed] [Google Scholar]
- 17. Angulo P, Keach JC, Batts KP, et al. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology 1999; 30:1356–1362. [DOI] [PubMed] [Google Scholar]
- 18. Weng SF, Kai J, Guha IN, et al. The value of aspartate aminotransferase and alanine aminotransferase in cardiovascular disease risk assessment. Open Heart 2015; 2:e000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 4. Reference procedure for the measurement of catalytic activity concentration of alanine aminotransferase [L-alanine: 2-oxoglutarate aminotransferase (ALT), EC 2. 6. 1. 2]. Clin Chem Lab Med 2002; 40:718–724. [DOI] [PubMed] [Google Scholar]
- 20. Schumann G, Bonora R, Ceriotti F, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 °C. Part 5. Reference procedure for the measurement of catalytic activity concentration of aspartate-aminotransferase [L-aspartate: 2-oxoglutarate-aminotransferase (AST), EC 2. 6. 1. 1]. Clin Chem Lab Med 2002; 40:725–733. [DOI] [PubMed] [Google Scholar]
- 21. Inal BB, Oguz O, Emre T, et al. Evaluation of MDRD, Cockcroft-Gault, and CKD-EPI formulas in the estimated glomerular filtration rate. Clin Lab 2014; 60:1685–1694. [DOI] [PubMed] [Google Scholar]
- 22. Martin H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin Biochem Rev 2011; 32:97–102. [PMC free article] [PubMed] [Google Scholar]
- 23. Kunutsor SK, Apekey TA, Seddoh D, et al. Liver enzymes and risk of all-cause mortality in general populations: a systematic review and meta-analysis. Int J Epidemiol 2014; 43:187–201. [DOI] [PubMed] [Google Scholar]
- 24. McPherson S, Henderson E, Burt AD, et al. Serum immunoglobulin levels predict fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol 2014; 60:1055–1062. [DOI] [PubMed] [Google Scholar]
- 25. Deghady A, Abdou A, El-Neanaey WA, et al. Association of genetic polymorphism −670A>G in the Fas gene and serum markers AST platelet ratio index, AST/ALT with significant fibrosis and cirrhosis in chronic hepatitis C. Genet Test Mol Biomarkers 2012; 16:531–535. [DOI] [PubMed] [Google Scholar]
- 26. Nyblom H, Björnsson E, Simrén M, et al. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int 2006; 26:840–845. [DOI] [PubMed] [Google Scholar]
- 27. Nyblom H, Berggren U, Balldin J, et al. High AST/ALT ratio may indicate advanced alcoholic liver disease rather than heavy drinking. Alcohol Alcohol 2004; 39:336–339. [DOI] [PubMed] [Google Scholar]
- 28. Gurung RB, Purbe B, Gyawali P, et al. The ratio of aspartate aminotransferase to alanine aminotransferase (AST/ALT): the correlation of value with underlying severity of alcoholic liver disease. Kathmandu Univ Med J (KUMJ) 2013; 11:233–236. [DOI] [PubMed] [Google Scholar]
- 29. Nyblom H, Nordlinder H, Olsson R. High aspartate to alanine aminotransferase ratio is an indicator of cirrhosis and poor outcome in patients with primary sclerosing cholangitis. Liver Int 2007; 27:694–699. [DOI] [PubMed] [Google Scholar]
- 30. Di Lorenzo L, Corfiati M, Bulfaro D, et al. Aspartate aminotransferase (AST) to alanine aminotransferase (ALT) ratio in health surveillance of workers exposed to vinyl chloride monomer: preliminary results. G Ital Med Lav Ergon 2003; 25:109–111. [PubMed] [Google Scholar]
- 31. Hino T, Kumashiro R, Ide T, et al. Autoimmune Hepatitis Study Group. Predictive factors for remission and death in 73 patients with autoimmune hepatitis in Japan. Int J Mol Med 2003; 11:749–755. [PubMed] [Google Scholar]
- 32. Morling JR, Fallowfield JA, Guha IN, et al. Edinburgh Type 2 diabetes study investigators. Using non-invasive biomarkers to identify hepatic fibrosis in people with type 2 diabetes mellitus: the Edinburgh type 2 diabetes study. J Hepatol 2014; 60:384–391. [DOI] [PubMed] [Google Scholar]
- 33. Sookoian S, Pirola CJ. Alanine and aspartate aminotransferase and glutamine-cycling pathway: their roles in pathogenesis of metabolic syndrome. World J Gastroenterol 2012; 18:3775–3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vendemiale G, Grattagliano I, Portincasa P, et al. Oxidative stress in symptom-free HCV carriers: relation with ALT flare-up. Eur J Clin Invest 2001; 31:54–63. [DOI] [PubMed] [Google Scholar]
- 35. Botezelli JD, Cambri LT, Ghezzi AC, et al. Fructose-rich diet leads to reduced aerobic capacity and to liver injury in rats. Lipids Health Dis 2012; 11:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sripradha R, Sridhar MG, Agrawal A. Can protein carbonyl/glutathione ratio be used as a potential biomarker to assess oxidative stress in alcoholic hepatitis? Ind J Med Sci 2010; 64:476–483. [PubMed] [Google Scholar]
- 37. Elinav E, Ackerman Z, Maaravi Y, et al. Low alanine aminotransferase activity in older people is associated with greater long-term mortality. J Am Geriatr Soc 2006; 54:1719–1724. [DOI] [PubMed] [Google Scholar]
- 38. Targher G, Byrne CD. Circulating markers of liver function and cardiovascular disease risk. Arterioscler Thromb Vasc Biol 2015; 35:2290–2296. [DOI] [PubMed] [Google Scholar]


