Congenital myasthenic syndrome (CMS) is genetically and clinically heterogeneous.1 Despite a considerable number of causal genes discovered, many patients are left without a specific diagnosis after genetic testing. The presumption is that novel genes yet to be discovered will account for the majority of such patients. However, it is also possible that we are neglecting a type of genetic variation: copy number changes (>50 bp) as causal for some of these patients. Next-generation sequencing (NGS) can simultaneously screen all known causal genes2 and is increasingly being validated to have a potential to identify copy number changes.3 We present a CMS case who did not receive a genetic diagnosis from previous Sanger sequencing, but through a novel copy number analysis algorithm integrated into our targeted NGS panel, we discovered a novel copy number mutation in the COLQ gene and made a genetic diagnosis. This discovery expands the genotype-phenotype correlation of CMS, leads to improved genetic counsel, and allows for specific pharmacologic treatment.1
Case report.
The patient is a 16-year-old girl of Han Chinese descent. She had progressive muscle weakness in a limb-girdle pattern and neck weakness since age 6. The muscle weakness appeared more severe after prolonged activity and would partly resolve after minutes of rest. She was seen at the neurology clinic of China-Japan Friendship Hospital in July 2013 and was noted to have a waddling gait and could not walk more than ∼600 feet. No ptosis or double vision was present. She had normal sensory testing and was without pain or other sensory symptoms. She had weakness of neck muscles, proximal upper limbs 4/5 (Medical Research Council), and proximal lower limbs 3/5, with normal ankle, hand strengths, and deep tendon reflexes. Her parents and a younger brother did not have any symptoms.
Laboratory testing was unremarkable for creatine kinase, serum lactic acid, anti–acetylcholine receptor, anti-acetylcholinesterase, and anti-Musk antibody. On her EMG, 2 Hz stimulation demonstrated a >10% decrement without facilitation. Biceps muscle biopsy was without vacuoles, dystrophic changes, or tubular aggregates. Sanger sequencing for GFPT1, DOK-7, and COLQ showed only a heterozygous variant (IVS16+3A→G) in the COLQ gene, a previously reported mutation but only deleterious in homozygous state.4 This variant was inherited from her father. No genetic diagnosis could be made at that time (figure).
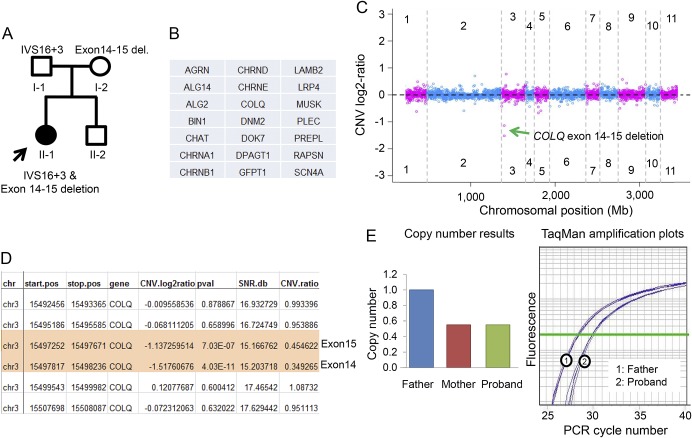
Figure. Novel compound heterozygous mutation in the COLQ gene causes congenital myasthenic syndrome.
(A) Pedigree of the proband (II-1). Point mutation (IVS16+3A→G) in the COLQ gene was inherited from the father (I-1), and multiexon deletion from the mother (I-2). (B) Gene list of 21 known congenital myasthenic syndrome (CMS) genes included in the targeted CMS panel. (C) Genome view of PatternCNV analysis shows decreased copy number variation (CNV) log2 ratio for the COLQ gene in chromosome 3. (D) Exon-level CNV summary table shows the start and end position of deletion (984 base pairs) in the COLQ gene, which indicates 1 copy deletion of exons 14 and 15. SNR.db: signal noise ratio expressed in decibels; CNV.ratio: copy number ratio converted from CNV.log2ratio. CNV.ratio of 1 indicates no copy number change. (E) TaqMan Copy Number Assay results confirm exon deletions in the COLQ gene in the proband, which is also found in her mother (data not shown). Longer PCR cycle number (X-axis) denotes 1 less copy after normalization using RNasP.
We recently developed a targeted NGS panel including 21 known CMS genes and applied it to this patient. Because a copy number variation (CNV) analysis algorithm (PatternCNV) is incorporated in the bioinformatics evaluation, this panel has a capability of detecting nucleotide changes, small insertion/deletions, and CNVs.3 The result from this panel confirmed the IVS16+3A→G variant and identified a heterozygous copy number deletion encompassing exons 14 and 15 of the COLQ gene (∼1 kb) (figure, A–C). This copy number change was subsequently confirmed using TaqMan Copy Number Assay (figure, D) and also found in her mother. TaqMan Copy Number Assay was performed using a probe targeting exon 15 of COLQ (Hs01393787_cn; Applied Biosystems, Foster City, CA) and housekeeping gene RNasP (Applied Biosystems) as copy number control with delta-delta Ct method for copy number calculation. The discovery of this novel copy number mutation of exons 14 and 15 led to the genetic diagnosis of this patient: compound heterozygous COLQ mutation, which allowed for more effective genetic counseling in her family. We are initiating drug therapies which have been shown beneficial for patients with COLQ mutations.1
Discussion.
Traditional genetic testing often misses large genomic deletions encompassing multiexon (a type of CNV) unless a separate microarray assay is performed. However, comparative genome hybridization arrays would have missed this COLQ exons 14 and 15 deletion as the resolution is often inadequate for less than 1 kb deletions. This report underscores the importance of including a validated copy number evaluation in targeted NGS testing in CMS. To date, mutation discovery has been largely focusing on nucleotide changes or small deletion/insertions, mainly due to technology limitations. Current studies estimate that ∼10% of the human genome has copy number changes,5 a magnitude higher than single nucleotide variants, underlining the importance of understanding copy number changes as we move forward in this molecular diagnostic era. A COLQ multiexon deletion (exon 2–3) was first reported once back in 1998.6 This is the second multiexon deletion of COLQ discovered in almost 20 years. With qPCR method, multiexon deletions of RAPSN were recently reported in 15% of previously undiagnosed patients with CMS,7 emphasizing the pathogenic role of copy number changes. Validating and providing copy number evaluation in targeted NGS will considerably improve the efficacy of genetic testing and is predicted to reduce the overall cost for CMS and possibly for other genetic neuromuscular disorders.
Footnotes
Author contributions: Dr. Wei Wang: study concept and design, acquisition of data, analysis and interpretation, drafting the manuscript, and critical revision of the manuscript. Dr. Yanhong Wu: acquisition of data, analysis and interpretation, and critical revision of the manuscript. Dr. Chen Wang: analysis and interpretation of data and critical revision of the manuscript. Dr. Jinsong Jiao: study concept and design, acquisition of data, analysis and interpretation, and critical revision of the manuscript. Dr. Christopher J. Klein: study concept and design; acquisition of data, analysis and interpretation, drafting the manuscript, critical revision of the manuscript, study supervision, and laboratory funding and support.
Study funding: No targeted funding reported.
Disclosure: Dr. W. Wang, Dr. Wu, Dr. C. Wang, and Dr. Jiao report no disclosures. Dr. Klein has served on the editorial board of the Journal of Peripheral Nerve Society and has received research support from a Mayo Clinic research grant. Go to Neurology.org/ng for full disclosure forms. The Article Processing Charge was paid by the authors.
References
- 1.Engel AG, Shen XM, Selcen D, Sine SM. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 2015;14:461. [DOI] [PubMed] [Google Scholar]
- 2.Abicht A, Dusl M, Gallenmuller C, et al. Congenital myasthenic syndromes: achievements and limitations of phenotype-guided gene-after-gene sequencing in diagnostic practice: a study of 680 patients. Hum Mutat 2012;33:1474–1484. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Wang C, Dawson DB, et al. Target-enrichment sequencing and copy number evaluation in inherited polyneuropathy. Neurology 2016;86:1762–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno K, Brengman JM, Felice KJ, Cornblath DR, Engel AG. Congenital end-plate acetylcholinesterase deficiency caused by a nonsense mutation and an A-->G splice-donor-site mutation at position +3 of the collagenlike-tail-subunit gene (COLQ): how does G at position +3 result in aberrant splicing? Am J Hum Genet 1999;65:635–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarrei M, MacDonald JR, Merico D, Scherer SW. A copy number variation map of the human genome. Nat Rev Genet 2015;16:172–183. [DOI] [PubMed] [Google Scholar]
- 6.Ohno K, Brengman J, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like tail subunit (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA 1998;95:9654–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gaudon K, Penisson-Besnier I, Chabrol B, et al. Multiexon deletions account for 15% of congenital myasthenic syndromes with RAPSN mutations after negative DNA sequencing. J Med Genet 2010;47:795–796. [DOI] [PubMed] [Google Scholar]